Abstract
Cancer is a group of around 100 diseases that has been tormenting mankind since ancient time. Due to cancer, estimated 8.2 million people died globally in 2012, and the toll is expected to reach 13 million in 2030. Despite the improvement of conventional therapeutic modalities, the outcome of cancer patients has not improved significantly. So, alternative therapeutic modalities and new effective anticancer drugs are highly sought for. Different parts of plants and their extracts have been used to cure many diseases and relive from physical agony since ancient times. In the traditional system of medicine, herbal products have been used for treating different types of diseases and alignments globally. Active compounds from herbal medicine, such as curcumin, are found to be effective against cancer. Despite their excellent therapeutic ability, the potential of these herbal compounds or phytochemicals is limited due to their low water solubility and poor bioavailability.
Advances in nanomedicines are revolutionizing the healthcare sector. Significant progresses have been made in development of nanocarriers in recent decades. Therapeutic efficacies of conventional drugs are reported to enhance by many folds using these novel nanocarriers through the intervention of nanotechnology. Application of nanotechnology may be effective in overcoming limitations of herbal drugs such as low water solubility, poor bioavailability, toxicity, and poor therapeutic efficacy of the drugs. It greatly helps in achieving higher efficiency of the drugs compared to its molecular form. Development of herbal-based nanocarriers like polymeric nanoparticles, dendrimers, liposomes, and micelles is reported to be more effective in treatment and managements of cancer. Loading of herbal compounds within these nanodrug delivery systems changes their pharmacokinetics profile and increases their bioavailability and therapeutic efficacy.
In this review, a comprehensive effort has been made on discovery of herbal drugs, herbal nanocarriers, and their application for cancer therapy. The coverage of this review will also extend to its current status and future prospects with elaborative and graphical examples.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
2.1.1 Cancer Overview
Cancer represents a diverse group of life-threatening diseases that causes abnormal and uncontrolled growth of malignant cells. These malignant cells are highly unorganized, irregular in shape and size, and capable of invading neighboring healthy tissues and organs. The characteristics of cancer cells are: ability of tissue invasion and metastasis, sustain angiogenesis, self-sufficiency in growth signals, limitless replicative potential, evasion of apoptosis, and insensitivity to anti-growth signals (Hanahan and Weinberg 2000; Gogoi et al. 2016). Cancer has been affecting mankind since ancient times. There are more than 100 types of cancers reported till date, and their subtypes are found within specific organs (Gogoi et al. 2016). With time, the tumor cells disintegrate from the primary tumor and migrate through the blood vessels and lymphatic streams to form their colonies at different sites of the patient’s body. This process is called metastasis and it leads to the death of the host.
The exact causes and the ways of initiation and spreading of cancer are still not well understood, but both external factors (e.g., tobacco smoking, infections, exposure to retroviruses, chemicals, and radiations) and internal factors (e.g., inherited metabolism mutations, hormones, and immune conditions) are believed to be the reasons for cancer formation and growth (Feng and Chien 2003). These factors may act together or in sequential manners to initiate and promote cancer. Till date, no complete curing procedure for cancer is available, only remission or palliation is possible with the current treatment procedures. A cancer is said to be in remission state when all clinical evidence of cancer has been disappeared and the microscopic foci of cancer cells may still remain (Feng and Chien 2003).
2.1.2 Limitation of Conventional Therapeutic Modalities
The most common and effective cancer treatment modalities are surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy. All these modalities have their own advantages as well as disadvantages and usually combination of two or more modalities gives the best result (Feng and Chien 2003). Surgery is one of major treatment procedures for treating tumor; but, erroneous or inadequately margined resection of tumor cells may lead to faster metastasis (Feng and Chien 2003; Gogoi et al. 2017). Moreover, tumors at metastasis cannot be treated with either surgery or radiotherapy. Radiotherapy is not selective to cancer cells, and it kills both malignant and healthy cells. Success of chemotherapeutic agents in treating cancer is limited by their severe side effects and development of multidrug resistance by the cancer cells. A schematic representation of how chemotherapy kills cancer cells is shown in Fig. 2.1. Chemotherapeutic drugs which are effective against rapidly dividing cells cannot kill large portion of dormant tumor cells. Thus the chemotherapy is compromised (Gogoi et al. 2017; Paszek et al. 2005; Tannock 2001). Hormone therapy is applicable to only hormone-sensitive cancers like breast cancer, prostate cancer, ovarian cancer, etc. Hormone therapy inhibits the growth of cancer cell by blocking the action of hormones such as estrogen receptor-α responsible for the tumor growth (Hayashi and Kimura 2015). But, almost all patients with metastatic breast and prostate cancer that initially respond to hormone therapy develop resistance to hormone therapy, and it leads to progression of the disease (Abraham and Staffurth 2016). Despite having number of therapeutic options, cancer is posing as a big menace to mankind. In 2008, 7.6 million people died of cancer, and toll is expected to reach 13.2 million by 2030 (Global cancer facts and figures, 2015, 2nd ed.). So, search for new effective and developed therapy is still going on.
Outline of chemotherapy (Subramanian et al. 2016)
2.1.3 Importance of Nanomedicine
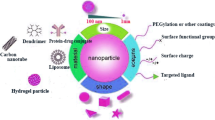
Application of nanotechnology especially nanomedicines opens up a window of opportunities to enhance the efficacy of anticancer drugs. According to the National Institute of Health, nanomedicine is referred as application of nanotechnology for treatment diagnosis, monitoring, and control of biological systems. Research into nanodrug delivery systems and diagnostic agents come within the preview of nanomedicine (Moghimi et al. 2005). The size of nanocarriers is generally in the range of 1 to 100 nm (Subramanian et al. 2016). But, for the purposes of this chapter, we are considering all the drug delivery systems below size 1000 nm as “nanodrug delivery systems” or “nanocarriers.” Nowadays a lot of herbal nanocarrier-based nanomedicines are being investigated globally and showing promising results for the holistic treatment of cancer disease. Herbal compound-loaded nanocarriers can overcome the problems like aqueous solubility and permeability through biological membrane due to their size and modified surface properties as faced by herbal bioactive compounds. Encapsulation of herbal drugs in nanocarriers improves the pharmacological activity and biodistribution of drugs, ensures their solubility and stability, and helps in maintaining sustained delivery (Jain et al. 2011). Moreover, application of nanodrug delivery systems may help in (i) achieving enhanced and targeted delivery of phytochemicals; (ii) crossing the tight epithelial and endothelial barriers and delivering large molecules to intracellular sites of action; and (iii) co-delivering of two or more phytomedicines or therapeutic modalities for combined therapy and imaging the site of drug action (Lambert 2010; Liong et al. 2008; Gunasekaran et al. 2014). Targeted delivery herbal bioactive molecules to the tumor site(s) reduces the side effects caused by off targeted delivery and increases the therapeutic efficacy of the nanoformulations.
Herein, we are reviewing the different types of herb-based nanocomposite existing in the literatures along with illustrative figures and explanation, which have been specially applied for the cancer treatment. The current development and future prospects in this direction have also been discussed.
2.2 Bioactive Herbal Compounds: History and Discovery Strategies
Historically, plants and their products have been playing an important role in curing many diseases and reliving from different physical agonies. Plants are important sources of traditional medicines (Bhattacharjya and Borah 2008; Newman et al. 2000; Buss et al. 2003). Herbal medicines were reported to use in different civilizations around the world since ancient times. In Mesopotamia, approximately 1000 plant-derived substances were reported to use as medicine in around 2600 BCE. Egyptians had been using herbal medicines since 2900 BCE; but, the Ebers Papyrus only properly reported use of over 700 drugs of mostly plant origin in 1550 BCE. The Indian Ayurvedic system is dated prior to 1000 BCE. Charaka Samhita and Sushruta Samhita documented use of 341 and 516 drugs, respectively (Kapoor 1990; Dev 1999).The Chinese materia medica also documented use of large number of herbal medicine for treating different diseases. The Greco–Roman knowledge on their traditional herbal medicine was dated back to the first century AD, and large amount of this knowledge bases were preserved by the Arabs during the dark and middle ages during the fifth to twelfth centuries (Cragg and Newman 2013). Much later, numbers of German books on herbal medicines were compiled during the period of the fourteenth to seventeenth century (Atanasov et al. 2015).
During all those periods, herbal medicines were used to treat different diseases or alignments without the in-depth knowledge of pharmacological activity or active components of the herbs (Atanasov et al. 2015). But, rational clinical investigation on medicinal herbs was laid down in the eighteenth century, when Anton von Störck had studied the properties of poisonous herbs like aconite and colchicum and William Withering had studied foxglove for the treatment of edema (Sneader 2005).
At the beginning of the nineteenth century, rational drug discovery from plants started when the German apothecary assistant Friedrich Sertürner had successfully isolated analgesic and sleep-inducing agent from opium named morphium (morphine). Later numbers of papers were published based on this discovery. This led to successful isolation and study of numerous natural drugs from herbs and followed by chemical synthesis of these drugs (Kaiser 2008). As per the world health organization (WHO) report, 80% of rural people of world’s population especially in developing countries depend on the herbal medicine (World Health Organization Guideline 2001). Till today, substantial portion of therapeutic agents are comprised of natural products and their derivatives; e.g., 61% of anticancer compounds and 49% anti-infectives approved during the period of 1981 to 2010 are derived from nature (Newman and Cragg 2012). However, the pharmaceutical companies have been avoiding investigation on natural product discovery processes since 1990, due to difficulties in supply, screening, characterization, and increase in rate of rediscovering the known compounds (Li and Vederas 2009). Still, research on fast, inexpensive next-generation genome sequence technology and the discovery of natural product is flourishing at academic level (Luo et al. 2014).
The natural product discovery processes are broadly classified into two categories, namely, top-down and bottom-up approaches (Fig. 2.2). In top-down approaches, system level information are utilized to generate the new natural products without having prior knowledge of genes and enzymes involved in the biosynthesis. These approaches don’t require complicated genome sequencing and sophisticated genetic manipulation.
Overview of the recent strategies applied for the discovery of natural products (Luo et al. 2014)
In these approaches, biological samples are collected from diverse environments either for extraction or laboratory cultivation. The extracts are then screened for a desired bioactivity, and the “hits” are isolated for structural characterization. New innovation in sampling and screening has mitigated the risk of rediscovering new chemical entities and allowing this approach to remain a viable means of natural product discovery (Luo et al. 2014). On the other hand, in the bottom-up approaches, the gene cluster of interest is identified, manipulated using transcription and translation processes, and then the corresponding natural product is synthesized (Luo et al. 2014). Plant-derived natural products are generally nontoxic to the normal cells and well tolerated by our body (Singh et al. 2016).
The plant-derived marketed anticancer compounds can be divided into four important classes, the vinca alkaloids (vinblastine, vincristine, and vindesine), the epipodophyllotoxins (etoposide and teniposide), the taxanes (paclitaxel and docetaxel), and the camptothecin derivatives (camptothecin and irinotecan) (Desai et al. 2008). Apart from these, the plants have tremendous potential to provide newer drugs, and search for new medicinal plants with potential anticancer compounds is going on.
Vinca alkaloids are herbal compounds extracted from Madagascar periwinkle plant, Catharanthus roseus G. Don., and they have the potential to treat diabetes and cancer (Moudi et al. 2013). Vinca alkaloids inhibit microtubule assembly and hence disrupt the cellular division process of tumor cells (Duflos et al. 2002). Moreover, disruption of microtubules function affects the cellular functions like intracellular organelle transport, cell migration, cell signaling, and mitosis (Perez 2009). Herbal compounds derived from vinca alkaloid are used to treat breast cancer, Hodgkin’s lymphoma and Kaposi’s sarcoma, severe lymphoblastic leukemia, non-Hodgkin leukemia, William’s tumor, and non-small cell lung cancer (Safarzadeh et al. 2014).
Epipodophyllotoxins or podophyllotoxins are extracted from the root of the Indian podophyllum plant (Podophyllum peltatum). Etoposide and teniposide are two active and semisynthetic compounds belonging to this family. These compounds arrest the proliferation of tumor cells by inhibiting topoisomerase II, which causes breakdown of DNA double strands (Damayanthi and Lown 1998; Safarzadeh et al. 2014).
Taxanes such as paclitaxel, docetaxel, and other taxane homologs are considered as the most effective antitumor agents and effective against wide range of cancers such as breast, ovary, lung, and other metastatic cancers. Paclitaxel is derived from Pacific yew bark (Taxus brevifolia). These taxanes inhibit the polymerization of microtubules and thereby prevent proliferation of tumor cells (Hagiwara and Sunada 2004).
Camptothecins are natural cytotoxic drugs isolated from Camptotheca accuminata of the Nyssaceae family. These are strong inhibitor of nucleic acid in mammalian cells and induce strand breaks in chromosomal DNA topoisomerase I (Hsiang et al. 1985).
Apart from these four groups of drugs, a large number of herbal drugs have been tried/investigated for their anticancer properties. These drugs from herbs or spices reveal their anticancer properties either by direct cytotoxic effects or modulating the immune system (Kitagishi et al. 2012). There are at least 2,50,000 species of plants out of which more than 1000 plants have been found to possess significant anticancer properties (Mukherjee et al. 2001). Active phytochemicals and their derivatives are found in leaf, root, flower, stem, and bark, and they perform number of pharmacological activities in human body (Singh et al. 2016). The search of novel bioactive compounds from natural sources continues with botanists, marine biologists, and microbiologists teaming up with chemists, pharmacologists, toxicologists, and clinicians. A comprehensive list of phytochemicals investigated for treatment of different cancers is shown in Table 2.1.
2.2.1 Structures of Important Herbal Compounds
Though a large number of plant-derived chemicals are investigated for their anticancer activity, only a few chemical entities were able to get approved for clinical applications due to stringent evaluation processes of pharmaceutical agents. The plant-derived anticancer agents approved for therapeutic use in the last 30 years (1984–2014) are summarized in Table 2.2.
2.3 Cancer Targeting Strategies and Herbal Nanostructures
Despite discovery of large numbers of plant-derived drugs, success in treating solid tumor is limited due to the severe side effects of chemotherapeutic agents and the development of multidrug resistance. Moreover, highly acidic and oxygen-deprived hypoxic environments within the tumor mass reduce the effectiveness of drugs that are basic in nature and/or utilize oxygen-free radicals for anticancer action (Kellen 1993). In solid tumor, a substantial portion of tumor cells present in dormant state, and they do not divide in the early stage of tumor formation (Rockwell and Hughes 1994). Therefore, chemotherapeutic agents effective against rapidly dividing cells could not kill them (Slingerland and Tannock 1998; Gogoi et al. 2017). Under this circumstances, the intervention of nanotechnology into the herbal drugs start playing an enhancing factor of its therapeutic efficacy towards the targeted diseases. Herbal drug-loaded nanoformulations can be prepared using methods such as high pressure homogenization, complex coacervation, co-precipitation, salting out, nanoprecipitation or solvent displacement, solvent emulsification–diffusion, supercritical fluid method and self-assembly method, etc. (Gunasekaran et al. 2014). Some of the common herbal nanodrug delivery systems are liposomes, emulsions, solid lipid nanoparticles, micelles, polymeric nanoparticles, dendrimers, carbon nanotube, inorganic nanoparticles (silica, ZnO), etc. These nanoparticles deliver drug to the cancer site(s) by two strategies, i.e., active and passive targeting.
2.3.1 Active Targeting
In active targeting, nanocarriers are channeled to tumor sites with the help of targeting ligands specific against receptors overexpressed on tumor cells or tumor vasculature, which are not expressed by normal cells. In this process, chemotherapeutic agent-loaded nanocarriers are conjugated with targeting ligands or moieties such as folic acid, monoclonal antibody, integrin, etc. which can target (i) receptors preferentially expressed on endothelial cells of tumor blood vessels (e.g., integrin-αv β3 and negatively charged phospholipids) (Li et al. 2004; Nisato et al. 2003); (ii) receptors overexpressed on tumor cells, e.g., HER2 and folate receptor (Chen et al. 2008; Pradhan et al. 2010); and (iii) lineage-specific targets that are expressed at the same level on both tumor and normal cells (e.g., CD19) (Cheng and Allen 2008) and kill tumor cells. These targeting ligands or moieties tagged effectively internalized by the tumor cells through receptor-mediated endocytosis. For effective deployment of active targeting strategy, the following issues need to be addressed: (i) liposome prepared for active targeting extravasated and bound to the first line of targeted tumor cells in the interstitial compartment and reported to obstruct the way for more liposomes to accumulate (Barenholz 2001), (ii) immunoliposomes prepared for active targeting were found to be cleared rapidly (Koning et al. 2002), and (iii) nanocarriers prepared for active targeting were reported to internalize via endocytosis process and end up with degradation in endosomes/lysosomes. Moreover, drug loading methods need to be devised properly so that the encapsulated drug does not form aggregate and degrade instantly for effective cancer treatment (Barenholz 2001). A schematic representation of active and passive targeting strategies of nanocarriers is demonstrated in Fig. 2.3.
Schematic representation of different mechanisms through which nanocarriers can deliver drugs at tumor sites. Polymeric nanoparticles are shown as representative nanocarriers (circles). Passive tissue targeting is achieved by extravasation of nanoparticles through enhanced permeability and retention (EPR) effect. Active cellular targeting (inset) can be achieved by functionalizing the surface of nanoparticles with ligands/moieties specific to the receptors/biomolecules expressed on the surface of the cancer cells (Peer et al. 2007)
2.3.2 Passive Targeting: Enhanced Permeability and Retention (EPR) Effect
In passive targeting process, nanocarriers/molecules are guided into the tumor interstitium or tissue through leaky tumor vasculature with the help of molecular movement within fluids (i.e., convection) or passive diffusion (Haley and Frenkel 2008). Conventional force mostly transports the larger molecules, whereas diffusion helps in transportation of low molecular weight compounds. It is well-known that tumor vasculatures are highly chaotic and complex structures, and they have the ability of extensive angiogenesis or forming hyperbranched defective vasculatures, impaired lymphatic drainage systems, and ability to generate number of vasculature permeability factors such as bradykinin, nitric oxide (NO) (Maeda et al. 1988; Matsumura et al. 1988; Maeda et al. 1994), and peroxynitrite (ONOO−) (Maeda et al. 2000); and hence, tumor vasculatures are highly porous. The pore size in the tumor vasculature is in the range of 100–780 nm (Yuan et al. 1995) which is much larger than normal tissue junctions, i.e., less than 6 nm (Drummond et al. 1999). So, nanocarriers circulating in the blood selectively enter into the interstitial spaces of tumor tissues and get accumulated there due to impaired lymphatic drainage system. This effect is called enhanced permeability and retention (EPR) effect. But, the pore size of endothelium tissues of kidney glomerulus is in the range of 40–60 nm size; sinusoidal endothelium of liver and spleen have pores of size up to 150 nm (Seymour 1992). Nanocarriers like liposomes can avoid accumulation in the kidney due to their bigger size, but macrophages present in the liver and spleen can remove them from blood circulation. PEG coating onto surface of nanocarriers prevents their clearance by macrophages due to steric hindrance offered by PEG coating, increases their blood circulation time, and hence helps in selective accumulation of the nanocarriers in tumor through passive diffusion (Andresen et al. 2005).
A large numbers of nanocarriers have been investigated for treatment of cancer exploiting the active and passive targeting strategies. Surface functionalization of nanoparticles using PEG or similar molecules has been reported to improve the bioavailability of drugs at tumor sites in different preclinical animal models. However, clinical translation of the nanocarriers from bench to bedside is a huge challenge due to stochastic nature of ligand–receptor interactions and difficulties in controlling release of drug at diseased sites (Gogoi et al. 2017). In order to improve the therapeutic index of drugs, drug release at tumor sites is essential as it prevents their rapid metabolization and clearance from the patient’s body. Drug release from nanocarriers can be triggered using either exogenous stimuli such as temperature, ultrasound, light, and electric fields or endogenous stimuli like change in pH, enzyme, redox potential, etc.
2.3.3 Herbal Nanostructures for Cancer Treatment
A large number of nanocarriers with herbal compounds have been investigated for treatment of various types of cancer. These nanocarriers target cancer cells either by active targeting or passive targeting strategy. A lot of herbal compounds are poorly soluble in aqueous solubility and resulting in poor bioavailability following oral administration (Bansal et al. 2011). Delivery of these poorly aqueous soluble drugs through nanocarriers reduces their systemic toxicity, improves pharmacokinetic properties, enhances their delivery at tumor sites, and hence improves the therapeutic indices of the drugs (Aqil et al. 2013).The following section discusses the application of herbal nanocarriers in treatment of cancer.
2.3.3.1 Liposomes and Other Lipid Carriers
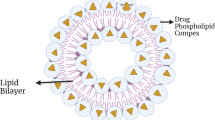
Liposomes
Liposomes are spherical vesicles made up of phospholipids which have a hydrophilic head and a hydrophobic tail. These phospholipids self-assemble under given conditions to form a bilayered structure called liposome. These liposomes have the ability to carry both hydrophobic and hydrophilic payload together. They have the advantages of high biocompatibility, biodegradability, ease of preparation, chemical versatility, and the ability to modulate the pharmacokinetic properties by changing the chemical composition and the components of the bilayers (Terreno et al. 2008). Dhule et al. (2014) investigated the combined antitumor effect of curcumin and C6 ceramide (C6) against osteosarcoma (OS) cell lines. They prepared three liposomal formulations, i.e., curcumin liposomes, C6 liposomes, and C6-curcumin liposomes. Curcumin in combination with C6 was found to be effective against MG-63 and KHOS OS cell lines, in comparison with curcumin liposomes alone. The therapeutic efficacy of the preparations was tested in vivo using a human osteosarcoma xenograft assay. PEGylated and folate tagged liposomes prepared for targeted delivery of curcumin and C6 significantly reduce the tumor volume in vivo. Recently, Gogoi et al. (2017) investigated the therapeutic efficacy of paclitaxel-loaded magnetic liposomes in vitro and in vivo under self-controlled hyperthermic condition. Results showed that the combined thermochemotherapy was effective in treating cancer in comparison to the drug and heat alone. Similar results were demonstrated by Gharib et al. (2015) who treated breast cancer using artemisinin and transferrin-loaded magnetic liposomes under AC magnetic field. In another study, berberine derivatives and doxorubicin-loaded long-circulating liposomes were studied for their ability to target mitochondria of drug-resistant cancer cells. Results demonstrated the superiority of these liposomes over regular doxorubicin-loaded liposomes and free doxorubicin (Tuo et al. 2016).
Solid Lipid Nanoparticles (SLNs)
Solid lipid nanoparticles have generated tremendous attention in last few decades due to their good release profile and targeted drug delivery with excellent physical stability. Good deals of studies on SLNs have been done for improvising the delivery of phytochemicals with anticancer properties in recent decades. Phytochemicals like berberine (Xue et al. 2015), resveratrol (Teskac and Kristl 2010), and paclitaxel (Pooja et al. 2016) were encapsulated in SLNs and studied their therapeutic properties. Teskac and Kristl (2010) demonstrated that encapsulation of resveratrol within SLNs enhances the bioavailability of drug and hence increases the therapeutic efficacy of the drug.
2.3.3.2 Micelles
Polymeric micelles have been drawing attention due to their ability of site-specific delivery of therapeutic agents, reducing off-target toxicity, and improving pharmacokinetics (Biswas et al. 2016). Tea epigallocatechin gallate and Herceptin loaded polymeric micelles were reported to use for cancer therapy. These nanomicelles demonstrated better tumor selectivity and growth reduction, as well as longer blood half-life, than free Herceptin (Chung et al. 2014). Micelles have been used for delivery of poorly water-soluble anticancer agent quercetin. Tan et al. (2012) reported development of quercetin-loaded micelles for treatment of lung cancer. Nanomicelles made from the diblock copolymer and polyethylene glycol (PEG)-derivatized phosphatidylethanolamine (PE) were found to enhance peroral anticancer activity and no apparent toxicity to the intestinal epithelium.
2.3.3.3 Polymeric Nanoparticles
Polymeric nanoparticles are drawing huge attention in cancer drug delivery due to their stability, ease of conjugating functional moieties, and ease of surface modification. Yallapu et al. (2012b) developed curcumin-loaded cellulose nanoparticles for targeting prostate cancer. They investigated and compared cellular uptake and cytotoxicity of these curcumin-loaded cellulose nanoparticles with β-cyclodextrin (CD), hydroxypropyl methylcellulose (cellulose), poly(lactic-co-glycolic acid) (PLGA), magnetic nanoparticles (MNP), and dendrimer-based curcumin nanoformulations in prostate cancer cells. Results demonstrated the superiority of curcumin-loaded cellulose nanoparticles in comparison to the other nanoformulations in inducing apoptosis in cancer cells. Recently, paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy were evaluated in lung cancer both in vitro and in vivo. Results suggested that these paclitaxel-loaded nanoparticles exhibit greater anti-tumor activity against A549 cells, in comparison with paclitaxel. The anti-tumor effect at 15 h after light onset (HALO) administration was reported to be the best in all groups (Hu et al. 2017). Curcumin-loaded PLGA nanoparticles were reported to enhance the aqueous solubility of curcumin and increase the antitumor potential of curcumin (Nair et al. 2012).
2.3.3.4 Nanoemulsions
Nanoemulsions are colloidal nanoparticles known for their stability and high loading efficiency. These carriers are solid spheres, and their surface is amorphous and lipophilic with a negative charge. Recently, a good deal of works has been done on herbal agent-loaded nanoemulsions for cancer therapy. Anuchapreeda et al. (2012) studied therapeutic efficacy of curcumin-loaded nanoemulsion in number of different cancer cell lines. Results showed high encapsulation of curcumin, physical stability of these nanocarriers, and their preserved toxicity. In another study, Pool et al. (2013) studied the feasibility of encapsulating hydrophobic quercetin in nanoemulsion. In a recent study, camptothecin-loaded polymer stabilized nanoemulsion was investigated for the in vitro cytotoxicity as well as their potential to target breast cancer in vivo. Results showed the possibility of targeting breast cancer using these nanocarriers (Sugumaran et al. 2017) (Fig. 2.4).
Phytochemical loaded different types of nanocarriers (Subramanian et al. 2016)
2.3.3.5 Nanocapsules
Nanocapsules consist of a liquid/solid core in which the drug is placed into a cavity, which is surrounded by a distinctive polymer membrane made up of natural or synthetic polymers. They have been drawing huge attention due to the protective coating which can be tuned to achieve sustain and controlled release of active ingredients (Kothamasu et al. 2012). Artemisinin crystals were encapsulated using nanocapsules composed of chitosan, gelatin, and alginate. This investigation showed the possibility of achieving prolonged drug release through self-assembly of polyelectrolytes on natural drug crystals (Chen et al. 2009). In another study, anticancer drug quercetin was encapsulated in nanocapsules prepared for passive and active targeting to tumors. The investigators prepared nanocapsules from folic acid conjugated to poly(lactide-co-glycolide) (PLGA) polymer to facilitate active targeting to cancer cells and PEGylated PLGA for passive targeting. Comparative in vitro studies on the cytotoxicity and cellular uptake of the different formulations were carried out using MTT assay and confocal laser scanning microscopy, respectively. Results confirmed the selective uptake and cytotoxicity of the folic acid targeted nanocapsules to the folate enriched cancer cells in a folate-dependent manner. Finally, in vivo experiments were done to evaluate the passive tumor accumulation and the active targeting of the nanocapsules to folate-expressing cells in HeLa or IGROV-1 tumor-bearing mice. The developed nanocapsules provide a system for targeted delivery of a range of hydrophobic anticancer drugs in vivo (El-Gogary et al. 2014). Recently, Boissenot et al. (2016) developed a paclitaxel-loaded nanocapsule formulation composed of poly(lactide-co-glycolide)-polyethylene glycol shell and perfluorooctyl bromide (PFOB) core for cancer theranostic application. PFOB was used as imaging agent. This nanocapsule formulation was tested in vitro and in vivo. Results demonstrated that the formulation could be applied as a cancer theranostic agent.
2.3.3.6 Dendrimers
Dendrimers are hyperbranched polymeric architectures widely investigated these days due to their versatility in drug delivery and high functionality. These nanostructured macromolecules have the abilities to entrap and/or conjugate the high molecular weight hydrophilic/hydrophobic entities by host–guest interactions and covalent bonding (prodrug approach), respectively. Moreover, due to high ratio of surface groups to molecular volume, they are extensively studied for gene delivery (Madaan et al. 2014). Fox et al. (2009) prepared a PEGylated poly(l-lysine) (PLL) dendrimer formulation by covalently binding polymer conjugates of camptothecin to improve solubility, increase blood circulation time, enhance tumor uptake, and hence significantly improve efficacy of the drug. The reported formulation was found to be effective in treating HT-29 tumor-bearing mice. Therapeutic efficacy of hydrophilic paclitaxel-conjugated polyamidoamine (PAMAM) dendrimers was studied cancer cells. Combination of ensemble and single microtubule imaging techniques were used to determine the mechanism of action of these dendrimers in vitro. Results provided mechanistic insights into the cytotoxicity of paclitaxel-conjugated PAMAM dendrimers and uncovered unexpected risks of using such conjugates therapeutically (Cline et al. 2013). Anticancer agent berberine (BBR) was attempted to deliver using G4-PAMAM dendrimers by conjugation (BPC) as well as encapsulation (BPE) approach. The entrapment efficiency in BPE was found to be 29.9%, whereas the percentage conjugation in BPC was found to be 37.49% indicating high drug payload in conjugation. In vitro results showed significantly higher anticancer activity for the PAMAM-BBR (p < 0.01) against MCF-7 and MDA-MB-468 breast cancer cells. In vivo results showed that the formulation was safer and biocompatible with very least but insignificant (p > 0.05) effects. The study demonstrated that conjugated formulation (BPC) was found to be more prominent than the encapsulated one (BPE) (Gupta et al. 2017).
2.3.3.7 Inorganic Nanoparticles
Inorganic nanoparticles including gold, oxides of iron, zinc, silicon, etc. were extensively investigated in both preclinical and clinical setting for delivering different anticancer phytochemicals. Poorly water-soluble curcumin was encapsulated in PMMA-PEG/ZnO bionanocomposite, and therapeutic potential and cellular uptake were studied in gastric cancer cell line (Dhivya et al. 2017). Results showed that curcumin-loaded PMMA-PEG/ZnO can induce the apoptosis of cancer cells through a cell cycle-mediated apoptosis corridor. In another study, cellular uptake and phototoxic potential of curcumin organically modified silica nanoparticle complexes and free curcumin were reported to investigate in multicellular spheroids of human oral cancer cells. Results showed accumulation of nanoformulated curcumin was higher in cancer cells, and hence cell death in the spheroids was more following irradiation of blue light in comparison to free curcumin. Results suggested that nanoformulated curcumin was able to improve the phototoxic effects of curcumin in spheroids in comparison to free curcumin (Singh et al. 2015). In another study, Janus magnetic mesoporous silica (Fe3O4-mSiO2) nanoparticles consisting of a Fe3O4 head for magnetic targeting and a mesoporous SiO2 body was reported to develop for berberine delivery. This pH responsive nanoformulation was designed for magnetic targeting of berberine to hepatocellular carcinoma. Results suggested that Janus nanocarriers driven by the magnetic field might be use for effective and safe delivery of berberine to against hepatocellular carcinoma (Wang et al. 2016).
Apart from these studies, a host of nanoparticles with different shape, size, architecture, materials, and inherent properties were studied for improvising delivery of anticancer agent in recent decades. These studies were tried to summarize with the help of Table 2.3.
In recent years a wide range of herbal compound-loaded nanocarriers with heterogeneous structures are developed and investigated their efficacy in various cancer cell lines. These nanocarriers are internalized by the cancer cells via phagocytosis or endocytosis processes depending upon their size, shape, and surface treatment (Zhang et al. 2015). These bioactive natural compounds inhibit the growth of cancer cells by inducing apoptosis or programmed cell death. Initifvtion of cell death indicated by the significant changes in DNA structure (Wei et al. 2009); ROS generation (Wei et al. 2009; Das et al. 2013); cytochrome C release (Guo et al. 2010; Mulik et al. 2010); activation of caspases 3/7 (Zheng et al. 2011; Guo et al. 2010; Zhang et al. 2013a); cell cycle arrest (Kumar et al. 2014); activation of NF-κB (Bisht et al. 2007); and downregulation of MMP, BaX, Cyclin D, and VEGF (Subramanian et al. 2016) along with visible morphological changes (Merlina et al. 2012). The different targets of bioactive compounds inside the cancer cell are demonstrated in Fig. 2.5.
Molecular targets of herbal compounds loaded nanocarriers against cancer cell (Subramanian et al. 2016)
2.4 Challenges and Future Prospects
Though a large number of nanomedicines are investigated for treatment of different types of cancers, only few nanoformulations reached the market today. A nanocarrier formulation has to go through a host of evaluation processes before it reaches the market. Though most of the nanocarriers are developed based on EPR effect, the EPR effect is unlikely to be present and equal in all the tumors nor the sole driver for efficacy of nanocarriers. Moreover, the pathological heterogeneity among different types of tumors and within the same type of tumor possesses a big challenge in the nanomedicine development process (Hare et al. 2017).The success rate of nanomedicine can be improved by adopting a specific decision-making framework, such as AstraZeneca’s 5Rs principle: right target/efficacy, right tissue/exposure, right safety, right patient, and right commercial potential. The following points need to be addressed for development of cost-effective superior therapies for the patients, i.e., (i) should have a clear cut understanding about the heterogeneity of clinical cancers and the biological factors influencing the behavior of nanomedicines in patients’ tumors; (ii) transition from formulation-driven research to disease-driven development; (iii) adaptation of more relevant animal models and testing protocols; and (iv) preselection of the patients most likely to respond to nanomedicine therapies.
Nanocarriers offer novel efficient strategies to treat cancer; nanotoxicity is a major area of concern as potentially high reactivity arising from the large surface-to-volume ratio of nanoparticles compared to bulk systems. Besides these, biodegradability of nanoparticles, side effects from by-products and bioaccumulation, and change in physicochemical characteristics of material at nanoscale are few apprehensions related to the nanomedicine. Moreover, distribution of nanocarriers in the body following systemic administration; development of mathematical and computer models to predict risk and benefits of nanoparticles; safe processes of nanoparticle manufacturing; and disposal and detrimental effects of nanoparticles to environment are few issues related to the nanomedicine to be addressed. Limited work has been done in scaling up laboratory or pilot technologies of nanodrug delivery for commercialization due to high cost of materials and challenges associated to maintain size and composition of nanomaterials at large scale.
2.5 Conclusions
Cancer has been tormenting the mankind from ancient times. Despite improvement in different therapeutic modalities, the number of deaths due to cancer is on rise. Therefore, a large number of herbs and their parts or extracts have been used to treat cancer. Nowadays, bioactive compounds from herbs have been extracted for effective treatment of different types of cancer. Due to the side effects of conventional therapies, herbal compounds or their derivatives have been loaded in different nanocarriers and investigated. Herbal compound-loaded nanocarriers have been able to effectively deliver drugs to the tumor site(s), reduce the side effects associated with the therapy, and kill the tumor cells more effectively. These nanocarriers can target tumor either by passive targeting or active targeting strategy. Though a host of nanocarriers have been investigated for cancer therapy, due to stringent preclinical evaluation and regulatory processes, only few nanoformulations have reached the market. The success rate of the nanocarriers in reaching market can be improved by adapting efficient decision-making strategies like AstraZeneca’s 5Rs framework, implementing new validation method and preselection of patients, etc. Moreover, issues like nanotoxicity, prior prediction of nanoparticles distribution in the body, and risk–benefit analysis are to be addressed.
References
Abdalla MO, Aneja R, Dean D, Rangari V, Russell A, Jaynes J, Yates C, Turner T (2010) Synthesis and characterization of noscapine loaded magnetic polymeric nanoparticles. J Magn Magn Mater 322(2):190–196
Abraham J, Staffurth J (2016) Hormonal therapy for cancer. Medicine 44(1):30–33
Adekenov SM, Muchametzhanov MN, Kagarlitskii AD, Kuprianov AN (1982) Arglabin, a new sesquiterpene lactone from Artemisia glabella. Chem Nat Compd 18(5):623–624
Aditya NP, Shim M, Lee I, Lee Y, Im MH, Ko S (2013) Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem 61:1878–1883
Al Sinani SS, Eltayeb EA, Coomber BL, Adham SA (2016) Solamargine triggers cellular necrosis selectively in different types of human melanoma cancer cells through extrinsic lysosomal mitochondrial death pathway. Cancer Cell Int 16:11. https://doi.org/10.1186/s12935-016-0287-4
Andresen TL, Jensen SS, Jørgensen K (2005) Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res 44:68–97
Anitha A, Deepa N, Chennazhi KP, Lakshmanan VK, Jayakumar R (2014) Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim Biophys Acta 1840(9):2730–2743
Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H (2012) Preparation of lipid nanoemulsions incorporating Curcumin for cancer therapy. J Nanotechnol 2012:11 pages. https://doi.org/10.1155/2012/270383
Aqil F, Munagala R, Jeyabalan J, Vadhanam MV (2013) Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett 334(1):133–141
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM et al (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33:1582–1614
Aziz MY, Omar AR, Subramani T, Yeap SK, Ho WY, Ismail NH et al (2014) Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF7 breast cancer cells. Oncol Lett 7:1479–1484. https://doi.org/10.3892/ol.2014.1898
Banerjee I, De K, Mukherjee D, Dey G, Chattopadhyay S, Mukherjee M, Mandal M, Bandyopadhyay AK, Gupta A, Ganguly S, Misra M (2016) Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy. Acta Biomater 38:69–81
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC (2011) Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prevent Res 4:1158–1171
Barenholz Y (2001) Liposome application: problems and prospects. Curr Opin Colloid Interface Sci 6:66–77
Bhattacharjya DK, Borah PC (2008) Medicinal weeds of crop fields and role of women in rural health and hygiene in Nalbari district, Assam. Indian J Tradit Knowl 7(3):505–510
Bhouri W, Boubaker J, Skandrani I, Ghedira K, Ghedira LC (2012) Investigation of the apoptotic way induced by digallic acid in human lymphoblastoid TK6 cells. Cancer Cell Int 12:26. https://doi.org/10.1186/1475-2867-12-26
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C (2007) Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5:3
Biswas S, Kumari P, Lakhani PM, Ghosh B (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83:184–202
Boissenot T, Fattal E, Bordat A, Houvenagel S, Valette J, Chacun H, Gueutin C, Tsapis N (2016) Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur J Pharm Biopharm 108:136–144
Buss AD, Cox B, Waigh RD (2003) Natural products as leads for new pharmaceuticals. In: Abraham DJ (ed) Burger’s medicinal chemistry and drug discovery 1: drug discovery, 6th edn. Wiley, Hoboken, pp 847–900
Chan LL, George S, Ahmad I, Gosangari SL, Abbasi A, Cunningham BT, Watkin KL (2011) Cytotoxicity Effects of Amoora rohituka and chittagonga on Breast and Pancreatic Cancer Cells. Evid. Based Complement. Alternat. Med. 2011:1–8. https://doi.org/10.1155/2011/860605
Chen H, Gao J, Lu Y et al (2008) Preparation and characterization of PE38KDEL-loaded anti-HER2 nanoparticles for targeted cancer therapy. J Control Release 128(3):209–216
Chen Y, Lin X, Park H, Greever R (2009) Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomedicine 5:316–322
Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L (2012) Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 17(5):5972–5987
Cheng WW, Allen TM (2008) Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J Control Release 126(1):50–58
Chung JE, Tan S, Gao SJ, Yongvongsoontorn N, Kim SH, Lee JH, Choi HS, Yano H, Zhuo L, Kurisawa M, Ying JY (2014) Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol 9(11):907–912
Cline EN, Li M-H, Choi SK, Herbstman JF, Kaul N, Meyhöfer E, Skiniotis G, Baker JR, Larson RG, Walter NG (2013) Paclitaxel-conjugated PAMAM dendrimers adversely affect microtubule structure through two independent modes of action. Biomacromolecules 14(3):654–664
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695
Dai L, Cao X, Liu K-F, Li C-X, Zhang G-F, Deng L-H, Si C-L, He J, Lei J-D (2015) Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol–betulinic acid nanoparticles for co-delivery of anticancer drugs. J Mater Chem B 3:3754–3766
Damayanthi Y, Lown JW (1998) Podophyllotoxins: current status and recent developments. Curr Med Chem 5(3):205–252
Das J, Das S, Samadder A, Bhadra K, Khuda-Bukhsh AR (2012) Poly (lactide-co-glycolide) encapsuled extract of Phytolacca decandra demonstrates better intervention against induced lung adenocarcinoma in mice and on A549 cells. Eur J Pharm Sci 47(2):313–324
Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh AR (2013) Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol Lett 223(2):124–138
Das J, Samadder A, Das S, Paul A, Khuda-Bukhsh AR (2016) Nanopharmaceutical approach for enhanced anti-cancer activity of Betulinic acid in lung-cancer treatment via activation of PARP: interaction with DNA as a target: anti-cancer potential of nano-betulinic acid in lung Cancer. J Pharmacopunct 19(1):37–44
Dasaroju S, Gottumukkala KM (2014) Current trends in research of Emblica officinalis (Amla): a pharmacological perspectives. Int J Pharma Sci Rev Res 24:150–159
de Pace RC, Liu X, Sun M, Nie S, Zhang J, Cai Q, Gao W, Pan X, Fan Z, Wang S (2013) Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J Liposome Res 23:187–196
Desai AG, Qazi GN, Ganju RK et al (2008) Medicinal plants and cancer chemoprevention. Curr Drug Metab 9(7):581–591
Detoni CB, Souto GD, da Silva AL, Pohlmann AR, Guterres SS (2012) Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem Photobiol 88:913–921
Dev S (1999) Ancient-modern concordance in Ayurvedic plants: some examples. Environ Health Prespect 107:783–789
Dhivya R, Ranjani J, Bowen PK, Rajendhran J, Mayandi J, Annaraj J (2017) Biocompatible curcumin loaded PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in human gastric cancer cells. Mater Sci Eng C Mater Biol Appl 80:59–68
Dhule SS, Penfornis P, He J, Harris MR, Terry T, John V, Pochampally R (2014) The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm 11(2):417–427
Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51:691–743
Du GJ, Wang CZ, Qi LW, Zhang ZY, Calway T, He TC et al (2013) The synergistic apoptotic interaction of panaxadiol and epigallocatechin gallate in human colorectal cancer cells. Phytother Res 27:272–277. https://doi.org/10.1002/ptr.4707
Duflos A, Kruczynski A, Barret JM (2002) Novel aspects of natural and modified vinca alkaloids. Curr Med Chem Anticancer Agents 2(1):55–70
Dwivedi A, Mazumder A, du Plessis L, du Preez JL, Haynes RK, du Plessis J (2015) In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomedicine 11:2041–2050
El-Gogary RI, Rubio N, Wang JT, Al-Jamal WT, Bourgognon M, Kafa H, Naeem M, Klippstein R, Abbate V, Leroux F, Bals S, Van Tendeloo G, Kamel AO, Awad GA, Mortada ND, Al-Jamal KT (2014) Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 8(2):1384–1401
Elisa P, Elisa D, Fiorenza O, Guendalina L, Beatrice B, Paolo L et al (2015) Antiangiogenic and antitumor activities of berberine derivative NAX014compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis 36:1169–1179. https://doi.org/10.1093/carcin/bgv103
Elshamy HA, Aboul-Soud MA, Nassr-Allah AA, Aboul-Enein KM, Kabash A, Yagi A (2010) Antitumor properties and modulation of antioxidant enzymes by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr Med Chem 17:129–138
Fakhoury I, Saad W, Bauhadir K, Nygren P, Stock RS, Muhtasib HG (2016) Uptake, delivery and anticancer activity of thymoquinone nanoparticles in breast cancer cells. J Nanopart Res 18:210. https://doi.org/10.1007/s11051-016-3517-8
Feng SS, Chien S (2003) Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci 58(18):4087–4114
Fox ME, Guillaudeu S, Fréchet JMJ, Jerger K, Macaraeg N, Szoka FC (2009) Synthesis and in vivo antitumor efficacy of PEGylated poly(L-lysine) dendrimer-camptothecin conjugates. Mol Pharm 6(5):1562–1572
Gach K, Dluqosz A, Janecka A (2015) The role of anticancer activity of sesquiterpene lactones. Naunyn Schmiedeberg’s Arch Pharmacol 388:477–486. https://doi.org/10.1007/s00210-015-1096-3
Ganta S, Amiji M (2009) Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm 6(3):928–939
Garg P, Deep A (2015) Anticancer potential of boswellic acid: a mini review. Hygeia J Drugs Med 7:18–27. https://doi.org/10.15254/H.J.D.Med.7.2015.147
Geethangili M, Rao YK, Fang SH, Tzeng YM (2008) Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother Res 22:1336–1341
Gharib A, Faezizadeh Z, Mesbah-Namin SAR, Saravani R (2015) Experimental treatment of breast cancer-bearing BALB/c mice by artemisinin and transferrin-loaded magnetic nanoliposomes. Pharmacogn Mag 11(Suppl 1):S117–S122
Ghasemzadeh A, Jaafar HZE, Rahmat A (2015) Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med 15:258. https://doi.org/10.1186/s12906-015-0718-0
Global cancer facts and figures, 2015. 2nd edn. Available from: http://oralcancerfoundation.org/wp-content/uploads/2016/03/acspc-027766.pdf. Viewed on 12.10.2017
Gogoi M, Sarma HD, Bahadur D, Banerjee R (2014) Biphasic magnetic nanoparticles – nanovesicle hybrids for chemotherapy and self-controlled hyperthermia. Nanomedicine (London) 9(7):955–970
Gogoi M, Kumar N, Patra S (2016) Multifunctional magnetic liposomes for cancer imaging and therapeutic applications. In: Holban AM, Grumezescu AM (eds) Nanoarchitectonics for smart delivery and drug targeting. Elsevier, Amsterdam, pp 743–772
Gogoi M, Jaiswal MK, Sarma HD, Bahadur D, Banerjee R (2017) Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy. Integr Biol 9(6):555–565
Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD (2014) Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed 4(Suppl 1):S1–S7. https://doi.org/10.12980/APJTB.4.2014C980
Guo L, Peng Y, Yao J, Sui L, Gu A, Wang J (2010) Anticancer activity and molecular mechanism of resveratrol–bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in nude mice. Cancer Biother Radiopharm 25(4):471–477
Gupta L, Sharma AK, Gothwal A, ShahidKhan M, PrasadKhinchi M, Qayum A, Singh SK, Gupta U (2017) Dendrimer encapsulated and conjugated delivery of berberine: a novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int J Pharm 528(1–2):88–99
Hagiwara H, Sunada Y (2004) Mechanism of taxane neurotoxicity. Breast Cancer 11(1):82–85
Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26:57–64
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST (2017) Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 108:25–38
Hayashi S, Kimura M (2015) Mechanisms of hormonal therapy resistance in breast cancer. Int J Clin Oncol 20(2):262–267
Householder KT, DiPerna DM, Chung EP, Wohlleb GM, Dhruv HD, Berens ME, Sirianni RW (2015) Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int J Pharm 479(2):374–380
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260(27):14873–14878
Hsu PM, Tien HJ (1974) Studies on the components of Formosan Solanum species. J Taiwan Pharm Assoc 26:28
Hu J, Fu S, Peng Q, Han Y, Xie J, Zan N, Chen Y, Fan J (2017) Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: In vitro and in vivo evaluation. Int J Pharm 516(1–2):313–322
Huang RY, Chu YL, Jiang ZB, Chen XM, Zhang X, Zeng X (2014) Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell Physiol Biochem 33:375–388. https://doi.org/10.1159/000356677
Jain D, Raturi R, Jain V, Bansal P, Singh R (2011) Recent technologies in pulsatile drug delivery systems. Biomatter 1:57–65
Kaiser H (2008) Von der Pflanze zur Chemie – Die Frühgeschichte der “Rheumamittel”. Z Rheumatol 67:252–262
Kapoor LD (1990) CRC handbook of Ayurvedic medicinal plants. CRC Press, Boca Raton
Karthikeyan S, Prasad NR, Ganamani A, Balamurugan E (2013) Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed Prev Nutr 3(1):64–73
Karthikeyan S, Hoti SL, Prasad NR (2015) Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed Pharmacother 70:274–282
Kavithaa K, Sumathi S, Padma PR (2017) Intracellular uptake of PEG-funtionalized baicalein loaded Iron oxide nanoparticles regulates apoptotic genes in triple negative breast Cancer cells: mitochondrial pathway targeted therapy for breast cancer. J Clust Sci 28(4):2057–2073
Keglevich P, Hazai L, Kalaus G, Szantay C (2012) Modifications of basic skeleton of vinblastin and vincristine. Molecules 17:5893–5914. https://doi.org/10.3390/molecules17055893
Kellen JA (1993) Multidrug resistance in human malignancies. In: Kellen JA (ed) Reversal of multidrug resistance in cancer. CRC Press, London, pp 69–91
Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, Mousa SA, Mukhtar H (2013) Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 35(2):415–423
Kim J-E, Park Y-L (2017) Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer. Int J Nanomedicine 12:645–658
Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S et al (2011) Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm 403:285–291
Kitagishi Y, Kobayashi M, Matsuda S (2012) Protection against cancer with medicinal herbs via activation of tumor suppressor. J Oncol 2012:236530. https://doi.org/10.1155/2012/236530
Koning GA, Kamps JAAM, Scherphof GL (2002) Interference of macrophages with immunotargeting of liposomes. J Liposome Res 12:107–119
Kothamasu P, Kanumur H, Ravur N, Maddu C, Parasuramrajam R, Thangavel S (2012) Nanocapsules: the weapons for novel drug delivery systems. Bioimpacts 2(2):71–81
Król SK, Kiełbus M, Rivero-Müller AR, Stepulak A (2015) Comprehensive review on betulin as a potential anticancer agent. Biomed Res Int 2015:584189. https://doi.org/10.1155/2015/584189
Kulshrestha P, Gogoi M, Bahadur D, Banerjee R (2012) In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf B Biointerfaces 96:1–7
Kumar SS, Mahadevan S, Vijayaraghavan R, Mandal AB, MacFarlane DR (2014) Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid – in vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur J Pharm Sci 51:34–44
Lambert WJ (2010) Considerations in developing a target product profile for parenteral pharmaceutical products. AAPS Pharm Sci Tech 11:1476–1481
Lee IC, Choi BY (2016) Withaferin a: a natural anticancer agent with pleiotropic mechanisms of action. Int J Mol Sci 17:290. https://doi.org/10.3390/ijms17030290
Li JW, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165
Li L, Wartchow CA, Danthi SN et al (2004) A novel antiangiogenesis therapy using an integrin antagonist or anti-Flk-1 antibody coated 90Y-labeled nanoparticles. Int J Radiat Oncol Biol Phys 58(4):1215–1227
Li QS, Li CY, Li ZL, Zhu HL (2012) Genistein and its synthetic analogs as anticancer agents. Anti Cancer Agents Med Chem 12:271–281. https://doi.org/10.2174/187152012800228788
Li K, Zhang H, Gao L, Zhai Y, Shi M, Li J, Xiu C, Cao J, Cheng S, Jiang L, Di H (2016a) Preparation and characterization of baicalein-loaded nanoliposomes for antitumor therapy. J Nanomater 2016 2861915, 9 pages, https://doi.org/10.1155/2016/2861915
Li X, Wu M, Pan L, Shi J (2016b) Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int J Nanomedicine 11:93–105
Liljegren D (1971) Glucosylation of solasodine by extracts from Solanum laciniatum. Phytochemistry 10:3061–3064
Lin X, Peng Z, Su C (2015) Potential anticancer activities and mechanisms of costunolide and dehydrocostuslactone. Int J Mol Sci 16:10888–10906. https://doi.org/10.3390/ijms160510888
Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE et al (2008) Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2:889–896
Liu YQ, Tian J, Qian K, Zhao XB, Susan LM, Yang L et al (2015) Recent progress on c-4 modified podophyllotoxin analogs as potent antitumor agents. Med Res Rev 35:1–62. https://doi.org/10.1002/med.21319
Lu J, Liong M, Li Z, Zink JI, Tamanoi F (2010) Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 6(16):1794–1805
Luo J, Chuang T, Cheung J, Quan J, Tsai J, Sullivan C et al (1998) Masoprocol (nordihydroguaiaretic acid): a new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur J Pharmacol 346:77–79
Luo Y, Cobb RE, Zhao H (2014) Recent advances in natural product discovery. Curr Opin Biotechnol 30:230–237
Madaan K, Kumar S, Poonia N, Lather V, Pandita D (2014) Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci 6(3):139–150
Madan J, Dhiman N, Sardana S, Aneja R, Chandra R, Katyal A (2011) Long-circulating poly(ethylene glycol)-grafted gelatin nanoparticles customized for intracellular delivery of noscapine: preparation, in-vitro characterization, structure elucidation, pharmacokinetics, and cytotoxicity analyses. Anti-Cancer Drugs 22(6):543–555
Maeda H, Matsumura Y, Kato H (1988) Purification and identification of [hydroxypropyl] bradykinin in ascitic fluid from a patient with gastric cancer. J Biol Chem 263:16051–16054
Maeda H, Noguchi Y, Sato K, Akaike T (1994) Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J Cancer Res 85:331–334
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK (2007) Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330(1–2):155–163
Matsumura Y, Kimura M, Yamamoto T, Maeda H (1988) Involvement of the kinin-generating cascade and enhanced vascular permeability in tumor tissue. Jpn J Cancer Res 79:1327–1334
Merlina JPJ, Prasada NR, Shibli SMA, Sebeel M (2012) Ferulic acid loaded Poly-d,l-lactide-co-glycolide nanoparticles: systematic study of particle size, drug encapsulation efficiency and anticancer effect in non-small cell lung carcinoma cell line in vitro. Biomed Prev Nutr 2(1):69–76
Min KH, Park K, Kim YS, Bae SM, Lee S, Jo HG et al (2008) Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release 127(3):208–218
Mitrus I, Sochanik A, Cichoń T, Szala S (2009) Combination of combretastatin A4 phosphate and doxorubicin-containing liposomes affects growth of B16-F10 tumors. Acta Biochim Pol 56(1):161–165
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. FASEB J 19(3):311–330
Moudi M, Go R, Yien CYS, Nazre M (2013) Vinca alkaloids. Int J Prev Med 4(11):1231–1235
Mukerjee A, Vishwanatha JK (2009) Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res 29(10):3867–3875
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8(12):1467–1486
Mukhija M, Singh MP, Dhar KL, Kalia AN (2015) Cytotoxic and antioxidant activity of Zanthozylum alatum stem bark and its flavonoid constituents. J Pharmacogn Phytochem 4:86–92
Mulik RS, Mönkkönen J, Juvonen RO, Mahadik KR, Paradkar AR (2010) ApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol Pharm 7(3):815–825
Mullauera FB, van Blooisb L, Daalhuisena JB, Brinka MST, Stormb G, Medemaa JP, Schiffelersb RM, Kesslera JH (2011) Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anti-Cancer Drugs 22:223–233
Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS (2012) Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm 425:44–52
Nallamothu R, Wood GC, Pattillo CB, Scott RC, Kiani MF, Moore BM, Thoma LA (2006) A tumor vasculature targeted liposome delivery system for combretastatin A4: design, characterization, and in vitro evaluation. AAPS Pharm Sci Tech 7(2):E7–E16
Narayanan NK, Nargi D, Randolph C, Narayanan BA (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 125(1):1–8
Natesan S, Ponnusamy C, Sugumaran A, Chelladurai S, Shanmugam Palaniappan S, Palanichamy R (2017) Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int J Biol Macromol 104(Pt B):1853–1859
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Newman DJ, Cragg GM, Snader KM (2000) The influence of natural products upon drug discovery. Nat Prod Rep 17:215–234
Nisato RE, Tille JC, Jonczyk A et al (2003) alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 6(2):105–119
Pahari P, Saikia UP, Das TP, Damodaran C, Rohr J (2016) Synthesis of psoralidin derivatives and their anticancer activity: first synthesis of lespeflorin l1. Tetrahedron 72:3324–3334. https://doi.org/10.1016/j.tet.2016.04.066
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Paul S, Bhattacharyya SS, Boujedaini N, Khuda-Bukhshi AR (2011) Anticancer potentials of root extract of Polygala senega and its PLGA nanoparticles-encapsulated form. Evid Based Complement Alternat Med 2011:517204
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751–760
Perez EA (2009) Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity and resistance. Mol Cancer Ther 8(8):2086–2095
Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Russo LL et al (2015) Biological and therapeutic activities and anticancer properties of curcumin. Exp Ther Med 10:1615–1623. https://doi.org/10.3892/etm.2015.2749
Pooja D, Kulhari H, Kuncha M, Rachamalla SS, Adams DJ, Bansal V, Sistla R (2016) Improving efficacy, oral bioavailability, and delivery of paclitaxel using protein-grafted solid lipid nanoparticles. Mol Pharm 13:3903–3912
Pool H, Mendoza S, Xiao H et al (2013) Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: impact of physical form on quercetin bioaccessibility. Food Funct 4(1):162–174
Powell RG, Rogovin SP, Smith CR Jr (1974) Isolation of antitumor alkaloids from Cephalotaxus harringtonia. Ind Eng Chem Prod Res Dev 13:129–132
Pradhan P, Giri J, Rieken F et al (2010) Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release 142:108–121
Preethi R, Padma PR (2016) Anticancer activity of silver nanobioconjugates synthesized from Piper betle leaves extract and its active compound eugenol. Int J Pharm Pharm Sci 8:201–205. https://doi.org/10.22159/ijpps.2016.v8i9.12993
Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P (2012) Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin 33:823–831
Rajan M, Krishnan P, Pradeepkumar P, Jeyanthinath M, Jeyaraj M, Ling MP, Arulselvan P, Higuchi A, Munusamy MA, Arumugam R, Benelli G, Muruganmo K, Kumar SS (2017) Magneto-chemotherapy for cervical cancer treatment with camptothecin loaded Fe3O4 functionalized b-cyclodextrin nanovehicle. RSC Adv 7:46271
Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B (2015) Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf B Biointerfaces 125:65–72
Ranjani R, Ayya RM (2012) Anticancer properties of Allium sativum – a review. Asian J Biochem Pharm Res 3:190–196
Rastogi N, Duggal S, Singh SK, Porwal K, Srivastava VK, Maurya R et al (2015) Proteosome inhibition mediates p53 reactivation and anticancer activity of 6-gingerol in cervical cancer cells. Oncotarget 6:43310–43325. https://doi.org/10.18632/oncotarget.6383
Rocha S, Generalov R, Pereira MC, Peres I, Juzenas P, Coelho MAN (2011) Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine (Lond) 6(1):79–87
Rockwell S, Hughes CS (1994) Effects of mitomycin C and porfiromycin on exponentially growing and plateau phase cultures. Cell Prolif 27:153–163
Safarzadeh E, Shotorbani SS, Baradaran B (2014) Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull 4(Suppl 1):421–427
Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, Rowen E, Yu J, Kerr RO (2010) Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Investig 28(2):186–194
Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M (2011) Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem 54:1321–1332
Sanna V, Siddiqui IA, Sechi M (2013) Resveratrol-loaded nanoparticles based on poly (epsilon-caprolactone) and poly (d, l-lactic-co-glycolic acid)–poly (ethylene glycol) blend for prostate cancer treatment. Mol Pharm 10(10):3871–3881
Sebak S, Mirzaei M, Malhotra M, Kulamarva A, Prakash S (2010) Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysis. Int J Nanomedicine 5:525–532
Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R (2005) Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 436:568–572
Seymour LW (1992) Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst 9:135–187
Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui H, Mousa SA, Mukhtar H (2009) Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res 69:1712–1716
Singh SP, Sharma M, Gupta PK (2015) Evaluation of phototoxic effects of curcumin loaded in organically modified silica nanoparticles in tumor spheroids of oral cancer cells. BioNanoScience 5(1):10–21
Singh S, Sharma B, Kanwar SS, Kumar A (2016) Lead phytochemicals for anticancer drug development. Front Plant Sci 7:1667
Slingerland JM, Tannock IF (1998) Cell proliferation and cell death. In: Tannock IF, Hill RP (eds) The basic science of oncology. McGraw-Hill, Toronto, pp 134–165
Sneader W (2005) Drug discovery: a history. Wiley, Hoboken
Subramanian AP, Jaganathan SK, Manikandan A, Pandiaraj KN, Gomathi N, Supriyanto E (2016) Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy. RSC Adv 6:48294–48314
Sugumaran A, Ponnusamy C, Kandasamy P, Krishnaswami V, Palanichamy R, Kandasamy R, Lakshmanan M, Natesan S (2017) Development and evaluation of camptothecin loaded polymer stabilized nanoemulsion: targeting potential in 4T1-breast tumour xenograft model. Eur J Pharm Sci S0928-0987(17):30548–30551
Sulaiman GM (2016) Molecular structure and antiproliferative effect of galangin in HCT116 cells: In vitro study. Food Sci Biotechnol 25:247–252. https://doi.org/10.1007/s10068-016-0036-4
Tan BJ, Liu Y, Chang KL, Lim BK, Chiu GN (2012) Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int J Nanomedicine 7:651–761
Tang S, Gao D, Zhao T, Zhou J, Zhao X (2013) An evaluation of the anti-tumor efficacy of oleanolic acid-loaded PEGylated liposomes. Nanotechnology 24:235102
Tannock IF (2001) Tumor physiology and drug resistance. Cancer Metastasis Rev 20(1–2):123–132
Teong B, Lin CY, Chang SJ, Niu GC, Yao CH, Chen IF, Kuo SM (2015) Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J Mater Sci Mater Med 26(1):5357. https://doi.org/10.1007/s10856-014-5357-3
Terreno E, Delli Castelli D, Cabella C, Dastru W et al (2008) Paramagnetic liposomes as innovative contrast agents for magnetic resonance (MR) molecular imaging applications. Chem Biodivers 5:1901–1912
Teskac K, Kristl J (2010) The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm 390:61–69
Thangapazham RL, Sharad S, Maheshwari RK (2016) Phytochemicals in wound healing. Adv Wound Care 5:230–241. https://doi.org/10.1089/wound.2013.0505
Tokudome Y, Oku N, Doi K, Namba Y, Okada S (1996) Antitumor activity of vincristine encapsulated in glucuronide-modified long-circulating liposomes in mice bearing Meth A sarcoma. Biochim Biophys Acta 1279:70–74
Tsai MJ, Wu PC, Huang YB, Chang JS, Lin CL, Tsai YH, Fang JY (2012) Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int J Pharm 423(2):461–470
Tu LY, Pi J, Jin H, Cai JY, Deng SP (2016) Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. Bioorgan Med Chem Lett 26:2730–2734. https://doi.org/10.1016/j.bmcl.2016.03.091
Tuo J, Xie Y, Song J, Chen Y, Guo Q, Liu X, Ni X, Xu D, Huang H, Yin S, Zhu W, Wu J, Hu H (2016) Development of a novel berberine-mediated mitochondria-targeting nano-platform for drug-resistant cancer therapy. J Mater Chem B 4:6856–6864
Vergaro V, Lvov YM, Leporatti S (2012) Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol Biosci 12(9):1265–1271
Wal A, Srivastava RS, Wal P, Rai A, Sharma S (2015) Lupeol as a magic drug. Pharm Biol Eval 2:142–151
Wang Z, Ho PC (2010) Self-assembled core–shell vascular-targeted nanocapsules for temporal antivasculature and anticancer activities. Small 6(22):2576–2583
Wang XX, Li YB, Yao HJ, Ju RJ, Zhang Y, Li RJ, Yu Y, Zhang L, Lu WL (2011a) The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cell. Biomaterials 32(24):5673–5687
Wang Y, Hong C, Qu H (2011b) Screening of antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L seeds. Evid Based Complement Alternat Med 2011:363052. https://doi.org/10.1093/ecam/nen087
Wang G, Wang JJ, Yang GY, Du SM, Zeng N, Li DS, Li RM, Chen JY, Feng JB, Yuan SH, Ye F (2012) Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int J Nanomedicine 7:271–280
Wang W, Xi M, Duan X, Wang Y, Kong F (2015) Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int J Nanomedicine 10:3737–3750
Wang L, Phan DD, Zhang J, Ong PS, Thuya WL, Soo RA et al (2016) Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget 7(28):44790–44802. https://doi.org/10.18632/oncotarget.8316
Wang Z, Wang YS, Chang ZM, Li L, Zhang Y, Lu MM, Zheng X, Li M, Shao D, Li J, Chen L, Dong WF (2017) Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem Biol Drug Des 89(3):464–469
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Wei XW, Chang YG, Shia S et al (2009) Self-assembled honokiol-loaded micelles based on poly(ɛ-caprolactone)-poly(ethylene glycol)-poly(ɛ-caprolactone) copolymer. Int J Pharm 369:170–175
World Health Organization (2001) General guidelines for methodologies on research and evaluation of traditional medicine. WHO, Geneva, p 1
Wozniak L, Skapska S, Marszalek K (2015) Ursolic acid – a pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 20:20614–20641. https://doi.org/10.3390/molecules201119721
Wu Z, Zou X, Yang L, Lin S, Fan J, Yang B, Sun X, Wan Q, Chen Y, Fu S (2014) Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids Surf B Biointerfaces 122:90–98
Xiong M, Wang L, Yu HL, Han H, Mao D, Chen J et al (2016) Ginkgetin exerts growth inhibitory and apoptotic effects on osteosarcoma cells through inhibition of STAT3 and activation of caspase-3/9. Oncol Rep 35:1034–1040. https://doi.org/10.3892/or.2015.4427
Xue M, Zhang L, Yang M, Zhang W, Li X, Ou Z, Li Z, Liu S, Li X, Yang S (2015) Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine 10:5049–5057
Yadav B, Bajaj A, Saxena AK (2010) In vitro anticancer activity of the root, stem and leaves of Withania somnifera against various human cancer cell lines. Indian J Pharm Sci 72:659–663. https://doi.org/10.4103/0250-474X.78543
Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, Kumar D, Jaggi M, Chauhan SC (2012a) Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine 7:1761–1779
Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi M, Chauhan SC (2012b) Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metab 13(1):120–128
Yan CH, Li F, Ma YC (2015) Plumbagin shows anticancer activity in human osteosarcoma (MG-63) via the inhibition of s-phase checkpoints and downregulation of c-myc. Int J Clin Exp Med 8:14432–14439
Yu F, Ao M, Zheng X, Li N, Xia J, Li Y, Li D, Hou Z, Qi Z, Chen XD (2017) PEG–lipid–PLGA hybrid nanoparticles loaded with berberine–phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv 24(1):825–833
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK (1995) Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 55:3752–3756
Zhan Y, Jia G, Wu D, Xu Y, Xu L (2009) Design and synthesis of a gossypol derivative with improved antitumor activities. Arch Pharm Chem Life Sci 342:223–229. https://doi.org/10.1002/ardp.200800185
Zhang H, Li X, Ding J, Xu H, Dai X, Hou Z, Zhang K, Sun K, Sun W (2013a) Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int J Pharm 441:261–268
Zhang YJ, Gallis B, Taya M, Wang S, Ho RJY, Sasaki T (2013b) pH-responsive artemisinin derivatives and lipid nanoparticle formulations inhibit growth of breast cancer cells in vitro and induce down-regulation of HER family members. PLoS One 8(3):e59086. https://doi.org/10.1371/journal.pone.0059086
Zhang S, Gao H, Gang Bao G (2015) Physical principles of nanoparticle cellular endocytosis. ACS Nano 9(9):8655–8671
Zhang YY, Huang CT, Liu SM, Wang B, Guo J, Bai JQ et al (2016) Licochalcone A exerts antitumor activity in bladder cancer cell lines and mouse models. Tropic J Pharm Res 15:1151–1157. https://doi.org/10.4314/tjpr.v15i6.6
Zheng S, Löw K, Wagner S, Yang X, von Briesen H, Zou S (2011) Cytotoxicity of triptolide and triptolide loaded polymeric micelles in vitro. Toxicol In Vitro 25(8):1557–1567
Zhigaltsev IV, Maurer N, Akhong QF, Leone R, Leng E, Wang J, Semple SC, Cullis PR (2005) Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release 104:103–111
Acknowledgments
Authors acknowledge DST-SERB, Govt. of India, for financial support through two no. of SERB projects (No. EMR/2016/002634 dated 21/03/2017 and EMR/2016/004219 dated 05/06/2017) sanctioned to Dr. Mrityunjoy Mahato (PI) and Dr. Manashjit Gogoi (Co-PI). MM also would like to thank Department of BSSS, NEHU, Shillong, for providing laboratory working facility. MG also would like to thank Department of BME, NEHU, Shillong, for providing laboratory working facility.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mahato, M., Patra, S., Gogoi, M. (2021). Herbal Nanocarriers for Cancer Therapy. In: Yata, V., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanopharmaceuticals: Principles and Applications Vol. 2. Environmental Chemistry for a Sustainable World, vol 47. Springer, Cham. https://doi.org/10.1007/978-3-030-44921-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-44921-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44920-9
Online ISBN: 978-3-030-44921-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)