Abstract
The recent focus and development of nanotechnology in medicine is countless, which involves diagnostic, therapeutic and preventive systems for various diseases. Nanoparticles have received much attention due to their uses in cancer therapy. The current study focused on the synthesis of baicalein loaded iron oxide nanoparticles and their efficacy against triple negative breast cancer (TNBC) MDA-MB-231. The electron microscopic analysis reveals that the particles were internalized with the various sub cellular regions of selected cancer cells. Further flow cytometric analysis of mitochondrial membrane potential using JC-1 staining showed that significant aggregates were found in the cells treated with baicalein loaded iron oxide nanoparticles, which, in turn, implies momentous mitochondrial membrane potential loss that occurs. Similarly apoptotic and anti-apoptotic gene expression pattern showed that baicalein loaded iron oxide nanoparticles were upregulates the apoptotic genes like Bad, Bax, GADD45 and PARP cleavage in a dose dependant manner. Detailed kit based flowcytometric analysis also reveals that the above findings were significant in the focused field, it is apparent that nano conjugates have the ability to induce apoptosis, DNA damage and cell cycle arrest and decrease the rate of cell proliferation in TNBC cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer diagnosed among women, constituting 23% of all cancer cases in the World. 1,685,210 new cases of breast cancer are diagnosed in 2016. The incidence of breast cancer in developing countries is on the increase due to many underlying factors such as birth control measures, sliding to western culture, lifestyle and genetic mutations [31]. The treatments currently used for breast cancer are surgery, radiation therapy, hormone therapy, chemotherapy, and targeted therapy. Surgery and radiation therapy are types of local therapy, which removes or destroys cancer in the breast. Hormone therapy, chemotherapy, and targeted therapy are types of systemic therapy employed to manage disease progression [11]. The existing drug enters into the bloodstream and destroys or controls cancer leads to common side effects. Nanotechnology have the potential to modernize a wide range of clinical and environmental applications, including drug delivery, diagnostics, imaging, sensing, gene delivery, artificial implants, tissue engineering, parasitology, and pest management. Biomaterials are of particular interest for many such applications due to their unique optical, chemical and photo-electrochemical properties [8].
Iron oxide nanoparticles are widely used as the core of magnetic nanocarriers. The surfaces of the synthesized nanoparticles are modified by using specific surfactant to enhance their stability, to improve the biocompatibility and to avoid the agglomeration of the particles [28]. The most commonly used biocompatible magnetic nanoparticle coatings are natural polymeric coating materials consisting of dextran, chitosan, gelatin, starch, poly ethylene glycol (PEG) and poly vinyl pyrrolidone (PVP), poly(ethylene-co-vinyl acetate), poly(lactic-co-glycolic acid) (PLGA), and poly vinyl alcohol (PVA). However, the surface of the magnetic nanoparticles need to be coated to prevent the formation of large aggregates and provide functional groups (amines or carboxylic acid) for bio conjugation of anticancer drugs and also avoiding the access of cytotoxic agents to non-target tissues during the delivery process [24].
Drug loaded nanoparticles based cancer therapy can be employed to overcome the toxicity of the drug. The drugs used for cancer therapy have many side effects, which usually arise due to non-specificity of the drug actions. Precise and targeted delivery of drugs to the tumor have gained much importance in order to address the limitations of traditional therapies, and therefore, search for better drug delivery methods has been prompted in last few years [26]. The selective targeting of chemotherapeutic agents to the tumor can provide more effective means of cancer therapy. The problems associated with conventional chemotherapy can be avoided by using magnetic drug delivery systems. The nanodrugs have distinctive characters to enhance and improve the drug with poor solubility, increase the retention and permeability, and deliver the drug to the target, which has helped to overcome the disadvantages of standard drug system used in cancer therapy [30].
Numerous studies have been conducted to see the effect of drugs namely doxorubicin, tamoxifen etc., loaded iron oxide nanoparticles in breast cancer cell lines. Our previous studies have reported about the anticancer activity of baicalein loaded iron oxide nanoparticles in triple negative breast cancer cell lines [20], but, since, not many reports are available regarding the effectiveness of baicalein loaded iron oxide nanoparticles in triple negative breast cancer cells especially evaluation of gene regulation. With this background, the main goal and novelty of the present work is to evaluate the baicalein conjugated iron oxide nanoparticles internalization into breast cancer cells and induction of apoptosis, regulating cancer related genes against triple negative breast cancer cells (MDA-MB-231).
Materials and Methods
Synthesis and Characterization Baicalein Loaded Fe2O3 Nanoparticles
Preparations of baicalein loaded Fe2O3 nanoparticles followed by our previously described method [20]. The particle size range of the nanoparticles along with their polydispersity was determined using a particle size analyzer (90 Plus Particle Size Analyzer, Brookhaven Instruments Corporation). Particle size was derived based on measuring the time dependent fluctuation of laser light scattering by nanoparticles undergoing Brownian motion. The samples were placed on a polycarbonate substrate and the excess water was left to dry at room temperature. They were then dried in a critical point dryer using carbon dioxide, and sputter coated with gold in a metallizer, and examined under a scanning electron microscope (JSM5600LV, JEOL, Japan) operated at an accelerating voltage of 20 kV. The particle size and morphology of Fe2O3 nanoparticles were examined using a Philips CM120 transmission electron microscope at a voltage of 80 kV. An aqueous dispersion of the particles was drop-cast onto a carbon-coated copper grid and the grid was air dried at room temperature before viewing under the microscope.
Cellular Internalization of Baicalein Loaded Iron Oxide Nanoparticles by TEM
Triple negative breast cancer cells (MDA-MB-231) were treated with baicalein loaded iron oxide nanoparticles for 24 h followed by wash with phosphate buffer to remove any unbound nanoparticles. The cells were then fixed in 2.5% glutaraldehyde for 30 min at 4 ◦C. Fixed cells were collected, washed thrice with phosphate buffer and dehydrated by increasing the concentrations of acetone (40, 60, 80 and 100%) followed by treatment with spurr’s low viscosity resin (Sigma Aldrich, USA) in gradients. Acetone and spurr’s low viscosity resin were used in different ratios namely 3:1, 1:1 and 1:3. Finally, 100% spur resin was added and the beam capsule was incubated for 18 h at 70 °C. The cell sections of 60 nm thicknes were cut using RMC ultra-microtome, stained with 0.5% uranyl acetate and examined by TEM to observe the distribution of baicalein loaded iron oxide nanoparticles.
Nuclear Localization of Baicalein Loaded Iron Oxide Nanoparticles by Confocal Microscopy
MDA-MB-231 cells were treated with baicalein loaded iron oxide nanoparticles for 24 h, after the treatment, 3% paraformaldehyde (50 μl) was added and left for 10 min at room temperature to fix the cells. After fixation, the cells were washed with PBS twice to remove the excess fixative. The cells were incubated with Mitotracker Green for 10 min at room temperature. Then the cells were labeled with Rhodamine-123 and were visualized under confocal microscope to identify the nuclear localization and distribution of synthesized baicalein loaded iron oxide nanoparticles in triple negative breast cancer cell. Nanoparticle complex excited at 496, 546 and 565 nm and emitted at 578 nm. The green emission from Mitotracker Green was excited at 488 nm and emitted at 505–525 nm. Rhodamine-123 excited at 511 nm and emitted at 534 nm.
Detection of Mitochondrial Membrane Potential (ΔΨ) by—JC-1
The treated cells were trypsinized and centrifuged at 400×g for 5 min and discarded the supernatant. Added 0.5 ml of JC-1 working solution and incubated the cells for 10–15 min at 37º C in a CO2 incubator. Then the cells were washed with 2 ml of 1X assay buffer and centrifuged at 400×g for 5 min. Repeated the above step with 1 ml of 1X assay buffer and resuspended the pellet with 0.5 ml of 1X assay buffer and analyzed within hour using BD FACSverse.
Gene Expression Studies by Semi Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) in Triple Negative Breast Cancer Cells
The MDA-MB-231 cells were treated with baicalein iron nanoconjugates and an untreated control was also set up. After 24 h treatment, the medium was completely removed from the flask. TriZol reagent was used to isolate total RNA, and the RNA was reverse transcribed. The cDNA was isolated by using the cDNA synthesis kit and amplified according to the instruction. The RT-PCR for apoptotic genes were performed in 20 μl reaction mixture containing random primer pairs (forward and reverse 0.5 μl + 0.5 μl) 1.0 μL, 10X reaction buffer containing master mix (25 mM/l MgCl2, 10 mM/l dNTPs, Taq polymerase 2.5 U) 10 μl, cDNA as template 2 μl and remaining volume is nuclease free dH2O 7 μl. Amplification cycles consisted of denaturation at 94 °C for 1 min, primer annealing at 55 °C for 40 s and extension at 72 °C or 1 min, for a total of 32 cycles followed by final extension at 72 °C for 10 min.
Effect of Baicalein Iron Nanoconjugates on Apoptosis, DNA Damage, Cell Proliferation and Cell Cycle Analysis in Triple Negative Breast Cancer Cells
BrdU solution (10 μl of 1 mM BrdU in PBS) was added directly to each ml of tissue culture medium. The BrdU treated cells were incubated at 37 °C for 4 h. These BrdU-pulsed cells were trypsinized and centrifuged at 300g for 5 min. To the pellet, 50 μl of staining buffer was added. It was then incubated on ice for 15 min. After incubation, the cells were washed with 1 ml of staining buffer for each tube. The tubes were centrifuged at 300g for 5 min and the supernatant was discarded. The pellet was resuspended in 100 μl of BD Cytofix/Cytoperm Fixation/Permeabilization solution and incubated for 15–30 min on ice. The permeabilized cells were washed with 1 ml of 1X BD Perm/Wash Buffer and centrifuged. The washed cells were resuspended in 100 μl of BD Cytofix/Cytoperm Plus Permeabilization Buffer and incubated on ice for 5 min. The fixed cells were washed with 1 ml of 1X BD Perm/Wash Buffer as done earlier. The washed cells were resuspended in 100 μl of diluted DNase (30 μg of DNase in 70 μl of PBS) and incubated at 37 °C for 1 h and washed as earlier. The washed cells were treated with 20 μl BD Perm/Wash buffer and 5 μl of PerCP-Cy™5.5 Anti-BrdU, 5 μl of Alexa Fluor® 647 Mouse Anti-H2AX (pS139), 5 μl of PE Anti-Cleaved PARP (Asp214) antibodies and incubated at room temperature for 20 min. The antibody treated cells were washed and resuspended in 1 ml of DAPI solution (1 μg/ml of PBS). The stained cells were analyzed with a flow cytometer (BD FACSVerse, USA). The extent of Apoptosis, DNA damage, cell cycle analysis and effect on cell proliferation of control and treated cells were observed.
Results
Characterization of Materials
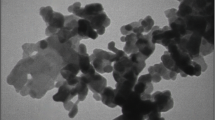
The hydrodynamic size distribution of the pure Fe2O3 nanoparticles, baicalein coated nanoparticles determined by a dynamic laser scattering (DLS) analysis. The size distributions of the baicalein Fe2O3 nanoparticles were found to be 90 nm in diameter. SEM analysis was used to confirm the surface morphology of the synthesized particles and then transmission electron microscopic studies reveals that synthesized baicalein loaded iron oxide nanoparticles are spherical in shape shown in Fig. 1a, b.
Cellular Internalization of Baicalein Iron Nanoconjugates by Transmission Electron Microscope
The cellular internalization of baicalein loaded iron oxide nanoparticles was confirmed by TEM analysis. The MDA-MB-231 cells were treated with the baicalein loaded iron oxide nanoparticles and internalization was observed by TEM. The results showed the internalization of nanoparticles in nucleus, cytoplasm and mitochondrial region of the treated cell. Figure 2 clearly indicates that localization of nanoparticles in mitochondrial region, further it indicates the internalized iron oxide nanoparticles were found in the cytoplasmic region especially vacuolar compartment of the cell. Further it confirms that particle has the efficiency to move across the plasma and nuclear membrane as well.
Nuclear Localization of Baicalein Iron Nanoconjugates by Confocal Microscopy
The nuclear localization of baicalein loaded iron oxide nanoparticles was further confirmed by confocal microscopy. The baicalein iron nanoconjugate treated MDA-MB-231 cells were analyzed by confocal microscopy under different fields. The results are depicted in Fig. 3. The baicalein coated iron oxide nanoparticles were loaded with the rhodamine-123 dye. Rhodamine-123 is cell-permeant, cationic dye and is readily sequestered by active mitochondria without cytotoxic effects. The results showed that in control group it doesn’t show any nuclear localization, whereas in MDA-MB-231 cells baicalein treated with iron nanoconjugates showed nuclear localization of synthesized nanoparticles. The red colour spots clearly indicated the nuclear localizations of baicalein iron nanoconjugates with rhodamine 123 tracker dye.
Mitochondrial Membrane Potential (ΔΨ) Detection-JC-1 by Flowcytometry in Triple Negative Breast Cancer Cells
Mitochondria play an important role in apoptotic process. Mitochondrial control of apoptosis has been described at the level of maintenance of ATP production and mitochondrial membrane potential (ΔΨm) and mitochondrial membrane permeability for the release of certain apoptogenic factors from the inter membrane space into the cytosol. The cyanine dye JC-1 has become widely used for microscopic and cytometric estimation and measurement of ΔΨm because it forms J-aggregates spectrally distinguishable from dye monomers when higher concentrations are reached in energised number of cells exposed to nanoparticles. Our aim was to evaluate the use of optical flow cytometry for detecting JC-1 aggregates (J-aggregates) in triple negative breast cancer cells (MDA-MB-231). The results revealed that in untreated group more number of cells existed in the form of monomers Fig. 4. However, baicalein coated iron oxide nanoparticles treated group, significant numbers of cells were found in the form of J-aggregates when compared to untreated control group.
Gene Expression Studies by Semi Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) in Triple Negative Breast Cancer Cell Line (MDA MB 231)
Bax and Bad are the pro-apoptotic genes that induce the release of cytochrome C and leads to the caspase activation in the apoptotic pathway. GADD45 is a DNA damage inducible gene and it plays a vital role in DNA repair, cell cycle control and senescence. Along with cytochrome C and PARP cleavage products, Bax, Bad and GADD 45 were also selected for gene expression studies in triple negative breast cancer cells.
The results showed that in the control group there is no expression of selected genes. Whereas in case of baicalein iron nano conjugate treated group the selected pro-apoptotic genes namely cytochrome C, Bax, Bad, PARP cleavage and GADD 45 showed up-regulation and down regulation of anti-apoptotic gene Bcl-2 was seen (Fig. 5). The expression profiles were further confirmed by densitometry analysis.
Apoptosis, DNA Damage, Cell Cycle and Cell Proliferation in Triple Negative Breast Cancer Cells
The important cellular events that occur during the manifestation of anticancer effect of an agent will encompass a profound influence on the extent of DNA damage and cell proliferation. This will, in turn, influence the cell cycle operation and, ultimately, result in cell death—either by apoptosis or by other types of cell death. Following this, the extent of DNA damage (using anti-γH2AX antibodies), the extent of cell proliferation (using anti-BrdU antibodies), the cell cycle events (using DAPI) and apoptosis (using anti-cleaved PARP antibodies) were also followed using flow cytometry in TNBC cells.
The histograms of the results are shown in Fig. 6. The extent of cell cycle operation was measured using DAPI, the results of which are presented as A1, A2, A3 and A4, corresponding to the control, iron oxide alone, baicalein alone and baicalein loaded iron oxide nanoparticles respectively. The control group showed active cell cycle, as seen by the number of cells in the lower left (LL) quadrant of A1. Treatment with iron oxide, baicalein and baicalein iron nano conjugate, decreased the intensity drastically, indicating that cell division was drastically arrested in MDA-MB-231 cells. This was also reflected in the extent of proliferation (upper left quadrants of A1–A4), which was increased in the control group, but drastically decreased in the other groups. This was again seen in the extent of proliferation (upper left quadrants of A1–A4), which was increased in the control group, but drastically decreased in the other groups of TNBC cells.
The patterns in B1-B4 represent the extent of DNA damage in correlation with cell proliferation. The control cells exhibited a lower intensity corresponding to DNA damage (UL quadrant) and higher proliferation (LR quadrant). In the groups treated with baicalein loaded iron oxide nanoparticles, the extent of DNA damage increased markedly, while the extent of proliferation decreased. C1-C4 depicts the extent of proliferation in relation to the extent of apoptosis. The scatter patterns once again prove that there was a decreased proliferation, which is a consequence of increased apoptosis. The 2D-scattergrams represented as D1-D4 present the extent of DNA damage versus apoptotic death. Both these processes showed high correlation, reflecting that the increased apoptosis had resulted in a high level of DNA fragmentation.
Thus, the results of the flow cytometry studies strongly support our earlier results, wherein increased apoptotic death was observed upon treatment with baicalein iron nano conjugates. The flow cytometric analysis using specific antibodies revealed that the baicalein iron nanoconjugates increases the extent of apoptosis and the extent of DNA damage, while decreasing cell proliferation and cell cycle operation in MDA-MB-231 cells.
Discussion
Cellular internalization of baicalein iron nanoconjugates were studied in triple negative breast cancer cells (MDA-MB-231) by TEM analysis. In the present study, internalization of baicalein iron nanoconjugates were found in mitochondrial region and some were found in cytoplasmic vacuolar region of the cell. Reports are available similarly, where internalization of glucose-coated superparamagnetic iron oxide nanoparticles in BxPC3 (pancreatic adenocarcinoma cell line) showed that glc-IONP were localized in vesicles confined to the cytosol, and some amount of glc-IONP were found in cellular organelles and in the nucleus [6]. Cellular internalization of dimercaptosuccinic acid-coated superparamagnetic iron oxide nanoparticles (DMSA-SPION) after 30 min in MCF-7 cells showed some clusters of nanonybrids in the cytoplasm and after 3 h the clusters were seen in vesicles. After longer incubation times (6, 12 and 24 h) they were accumulated close to the nuclei [12].
The internalization of iron oxide nanoparticles in MCL-5 cells (human lymphoblastoid cell line) showed that encapsulated nanoparticles were seen in vesicles and cytoplasm [10]. Cabeza et al. [11], reported that internalization of doxorubicin loaded poly (butylcyanoacrylate) nanoparticles in the nucleus and mitochondria after 12 h of incubation. Our results are in agreement with these reports where, in the present study, baicalein nanoconjugates moved across the nuclear membrane and were localized in the nuclear compartment.
Mitochondrial dysfunction has been shown to participate in the induction of apoptosis and has even been suggested to be central to the apoptotic pathway. The mitochondrion is an important organelle involved in apoptosis. The loss of mitochondrial membrane potential (ΔΨ) is putatively the initial event leading to apoptosis. The cyanine dye JC-1(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide) a generally used for microscopic and cytometry estimation and measurement of ΔΨm because it forms J-aggregates spectrally distinguishable from dye monomers at the high concentrations [14].
The results of mitochondrial membrane potential detection by JC-1 revealed that baicalein iron nanoconjugates treated MDA-MB-231 cells showed significant increase in the percentage of J-aggregates when compared to the iron oxide and baicalein alone treated cells. The present study is supported by the findings of various researchers. The loss of mitochondrial membrane is a key indicator of intrinsic apoptosis. Mitochondrial membrane potential of curcumin loaded magnetic nanoparticles treated with curcumin (5 µM) alone showed significant decrease in tetramethyl rhodamine ethyl ester stain in MDA-MB-231 cells. Whereas, the curcumin loaded magnetic nanoparticles showed decreased stain, indicating effective mitochondrial membrane damage and thus increased anticancer potential [36]. A dose dependent increased level of mitochondrial membrane potential was observed when treated with copper ferrite nanoparticles in MCF-7 cells [2]. Similar results were observed when BEL-7402 cells were treated with carbon coated magnetic nanoparticles [19].
Remarkable loss of mitochondrial membrane potential in A549 cells was observed after exposure to increasing concentrations of Iron oxide nanoparticles [21]. He et al. [18], reported that superparamagnetic nanoparticles treated HepG2 cells showed increased percentage of aggregates when compared to monomers. Similar results were observed in our study. A concentration-dependent decrease in mitochondrial membrane potential was observed in A-549 cells after the exposure to iron oxide nanoparticles for 24 h [15]. Dose-dependent mitochondrial depolarization was found in Jurkat cells treated with quercetin and menadione compounds [5]. Mitochondrial membrane potential of bare and passivated silica coated iron oxide nanoparticles using JC-1 showed the perturbation of mitochondrial membrane potential was more evident in cells treated with bare nanoparticles, whereas passivated nanoparticles induced only a slight significant variation in both A549 and HeLa cell lines [25].
In the light of these literatures, it was clear that baicalein loaded iron oxide nanoparticles induced apoptosis in mitochondria and led to the activation of intrinsic pathway. This was ascertained by visualising the proteins involved by Western blotting. The activation of pro-apoptotic and anti-apoptotic protein expression induced by baicalein iron nanoconjugates were studied by Western blotting.
Bax is an important pro-apoptotic protein of the Bcl-2 family members. Bax promotes apoptosis by forming a heterodimer with Bcl-2 to inhibit Bcl-2 activity. In addition, Bax can accelerate the opening of the mitochondrial voltage-dependent anion channel, which leads to decreases in the mitochondrial membrane potential, cytochrome C release, and caspase-3 activation before triggering apoptosis. GADD45 encodes a ubiquitously expressed protein that is often induced by DNA damage and other stress signals associated with growth arrest and apoptosis [23].
Results of present study showed that baicalein iron nanoconjugates caused increased expression of pro-apoptotic gene (cytochrome C, Bax, Bad and PARP cleavage) and down regulation of Bcl-2. The results of the present study also correlated with several scientific reports. Dose dependent changes were noticed when MDA-MB-231 cells were treated with chitosan–silver–phycoerythrin nanocomposites. The treated cells showed the upregulation of pro-apoptotic genes (Cytochrome C, caspase-9, p53 and PARP cleavage) and anti-apoptotic gene Bcl-2 [33]. Effect of daunorubicin and 5-bromotetrandrin loaded magnetic iron oxide nanoparticles showed the upregulation of Bax and caspase-3. In contrast, Bcl-2 was decreased in human leukemia K562/A02 cells [35].
A study on three types of magnetic nanoparticles induced apoptosis in human hepatoma BEL-7402 cells revealed the Bax upregulation indicating intrinsic pathway of apoptosis [19]. Superparamagnetic nanoparticles increased the expression of caspase-3 in HepG2 cells [18]. Exposure of K562 cells to CuO nanoparticles showed the up regulation of p53 and down regulation of Bcl-2 and did not show any alteration in the expression of Bax [32]. Gold nanoparticles induced apoptosis in myoblast cancer cells (C2C12) indicating the upregulation of caspase-3 and caspase-7 [34]. Aluminium doped zinc oxide nanoparticles showed increased mRNA levels of pro-apoptotic genes namely p53, Bax, caspase-3 and caspase-9, while the expression of anti-apoptotic gene Bcl-2 was down-regulated in treated cells as compared to controls in MCF-7 cells [3]. Another study on, folate-decorated albumin-stabilized AgNPs showed the up regulation of pro-apoptotic gene like caspase- 3, Bax, Bad, c-myc, p53. Contrary to this, while the expression of anti-apoptotic gene Bcl-xl was found to be down-regulated in both MCF-7 and A549 cells [9].
In tune with the above results, in the present study, the pro-apoptotic genes cytochrome C, Bax, Bad, PARP cleavage and GADD45 expression were significantly enhanced and anti-apoptotic Bcl-2 gene expression was reduced. This clearly shows that the baicalein iron nanoconjugates acts as an anticancer agent to inhibit cell growth and trigger apoptosis. The results of the experiments done so far clearly showed that baicalein iron nanoconjugates were able to suppress cell division and also regulated expression of apoptosis inducing genes and showed differential response in cancer cells and normal cells.
Multiparameter flow cytometry provides a powerful tool for understanding the mechanism by which cells maintain viability, enter and progress through cell cycle or undergo cell death. For this purpose Apoptosis, DNA damage, Cell cycle and Cell proliferation (ADDCP) kit (BD Biosciences) was used to study these effects in MDA-B-231 cells. Apoptosis plays a major role in the regulation of tissue homeostasis and elimination of abnormal cells. Apoptosis is believed to be deregulated in cancer. Most of the antitumour drugs kill the cancer cells by stimulating the apoptotic pathway [17]. Further the apoptosis in liver cancer cells was induced by nanoparticle treatment at doses of 30 μg/ml, the apoptotic percentage liver cancer cells increased significantly, which elucidate the activation of pro-apoptotic genes and proteins leads to cell death signaling pathways. [7]
The arrest of the cells in various phases of the cell cycle, ultimately results in triggering apoptotic death, which was reported by several researchers. Cell cycle analysis of dimercaptosuccinic acid modified iron oxide nanoparticles combined with non toxic concentration of bortezomib and gambogic acid in multiple myeloma (MM) RPMI-8226 cells showed increased amount of the cell population were seen in the G2/M phase in the nanoconjugates treated group when compared with the other groups [38]. In another study, the treatment with a metallin nanoparticles arrested the GM07492 cells at sub G0/G1 phase of the cell cycle [16].
Dose-dependent accumulation of cells in the G0/G1 phase and a corresponding decrease in S phase was observed in both breast cancer cell lines MCF-7 and MDA-MB-231 treated with S-allyl mercaptocysteine (SAMC) and the accumulation of sub-G1 phase, a hallmark of apoptosis, was also noted at higher concentrations of the drug [37]. These studies lend strong support to our results, wherein the baicalein iron nanoconjugates blocked the progression of cell cycle at the sub-G0 phase.
Apart from cell cycle arrest, the ADDCP kit was also used to analyze the extent of cell proliferation and DNA damage, in relation to apoptosis. Our observations showed that the baicalein iron nanoconjugates caused decreased cell proliferation, increased the extent of DNA damage and increased the extent of apoptosis (as seen in the scattergram patterns obtained using anti-BrdU antibodies, anti-γH2AX antibodies and anti-PARP antibodies respectively) when compared to the untreated MDA-MB-231 cells. Similar results have been reported by Angelopoulos et al. [4], using BrdU incorporation where treatment with superparamagnetic iron oxide nanoparticles decreased the proliferation of smooth muscle cells. A dose-dependent decrease in BrdU incorporation was observed with flavokawain A in MCF-7 and MDA-MB-231 breast cancer cells, reflecting reduction in the extent of cell proliferation [1].
Superparamagnetic iron oxide nanoparticles labelled with bromodeoxyuridine (BrdU) inhibited the cell proliferation of activated macrophages [27]. In the present study, BrdU incorporation, measured using anti-BrdU antibodies, showed that the baicalein iron nanoconjugates exposure caused a marked decrease in proliferation, coupled with an increase in apoptosis and cell cycle arrest.
The extent of DNA damage, measured using the phosphorylated H2AX (histone) protein antibody, showed more DNA damage when MDA-MB-231 cells were exposed to baicalein iron nanoconjugates compared to iron oxide and baicalein alone exposure. Similarly, epidermal growth factor receptor (EGFR)-targeted hybrid plasmonic magnetic nanoparticles (225-NP) increased DNA damage and triggered apoptosis in HCC827 cells [22]. Chang et al. [13], reported that the apoptotic pathway was triggered by hypoxia drug with DNA damage, while exposure to topoisomerase resulted in apoptosis that started with cell cycle arrest in the G2/M-phase. In the present study, the exposure to iron oxide nanoparticles caused cell cycle arrest in G2/M phase. Thus, it is inferable that baicalein coated iron oxide nanoparticles triggers apoptosis in the MDA-MB-231 cells by increasing DNA damage.
In accordance to our results, Romoser et al. [29], also showed increased DNA damage when treated with cerium dioxide, titanium dioxide and zinc oxide in human skin cells. Thus, the results of the flow cytometry analysis using specific antibodies revealed that the baicalein iron nano conjugates increased the extent of apoptosis and the extent of DNA damage, while decreasing cell proliferation and cell cycle operation in MDA-MB-231 cells.
Conclusion
The current findings elucidate the mechanism of apoptotic induction, DNA damage, Cell cycle arrest and Cell proliferation inhibition upon baicalein iron nanoconjugates using various antibodies by flow cytometry. The mitochondrial membrane potential, which is an important parameter affected during cell death was seen in triple negative breast cancer cells treated with baicalein iron nanoconjugates by JC-1 staining. The flow cytometric analysis using specific antibodies revealed that the baicalein iron nano conjugates increased the extent of apoptosis and the extent of DNA damage, while decreasing cell proliferation and cell cycle operation in MDA-MB-231 cells. Similarly apoptotic and anti-apoptotic gene expression pattern showed that baicalein loaded iron oxide nanoparticles upregulated the apoptotic genes like Bad, Bax, GADD45 and PARP cleavage. It also showed that the baicalein loaded iron oxide nanoparticles caused upregulation of the apoptotic process and inhibited the expression of anti-apoptotic genes. Further it showed a profound influence on the breast cancer associated genes. Taken together, our findings suggest that the strategy to use the iron oxide nanoparticles as a carrier of baicalein can be a highly efficient way to treat the triple negative breast cancer.
References
N. Abu, M. A. Akhtar, K. S. Yeap, K. L. Lim, W. Y. Ho, A. J. Zulfadli, A. R. Omar, M. R. Sulaiman, M. P. Abdullah, and N. B. Alitheen (2014). Flavokawain A induces apoptosis in MCF-7 and MDA-MB-231 and inhibits the metastatic process in vitro. PLoS one, 9, e105244.
M. Ahamed, M. J. Akhtar, H. A. Alhadlaq, and A. Alshamsan (2016). Copper ferrite nanoparticle-induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids Surf. B Biointerfaces 142, 46–54.
N. Akhtar, G. Ihsan-ul-Haq, and B. Mirza (2015). Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem.. doi:10.1016/j.aranjc.2015.01.013.
I. Angelopoulos, P. Southern, Q. A. Pankhurst, and R. M. Day (2016). Superparamagnetic iron oxide nanoparticles regulate smooth muscle cell phenotype. J. Biomed. Mater. Res. Part A 1, 20–25.
I. Baran, D. Ionescu, A. Filippi, M. M. Mocanu, A. Iftime, R. Babes, I. T. Tofolean, R. Irimia, A. Goicea, V. Popescu, A. Dimancea, A. Neagu, and C. Ganea (2014). Novel insights into the antiproliferative effects and synergism of quercetin and menadione in human leukemia Jurkat T cells. Leuk. Res. 38, 836–849.
D. Barbaro, L. Di Bari, V. Gandin, C. Evangelisti, G. Vitulli, E. Schiavi, C. Marzano, A. M. Ferretti, and P. Salvadori (2015). Glucose-coated superparamagnetic iron oxide nanoparticles prepared by metal vapour synthesis are electively internalized in a pancreatic adenocarcinoma cell line expressing GLUT1 transporter. PLoS ONE. doi:10.1371/journal.pone.0123159.
G. Benelli (2016). Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer: a brief review. Enzyme Microb. Technol. 95, 58–68.
G. Benelli and C. M. Lukehart (2017). Special issue: applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J. Clust. Sci. 28, 1–2.
B. Bhushan and P. Gopinath (2015). Tumor-targeted folate-decorated albuminstabilised silver nanoparticles induce apoptosis at low concentration in human breast cancer cells. RSC Adv. 5, 86242–86253.
A. P. Brown, R. M. D. Brydson, and N. S. Hondow (2014). Measuring in vitro cellular uptake of nanoparticles by transmission electron microscope. J. Phys. Conf. Ser.. doi:10.2088/1742-6596/522/1/012058.
L. Cabeza, R. Ortiz Quesada, J. L. Arias Mediano, A. Ruiz Martínez, J. M. Entrena Fernández, R. Luque Caro, and C. Melguizo Alonso (2015). Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly (butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 10, 1291–1306.
M. Calero, M. Chiappi, A. Lazaro-Carrillo, M. J. Rodríguez, F. J. Chichón, K. Crosbie-Staunton, A. Prina-Mello, Y. Volkov, A. Villanueva, and J. L. Carrascosa (2015). Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 13, 1–15.
L. Chang, X. Liu, D. Wang, J. Ma, T. Zhou, Y. Chen, R. Sheng, Y. Hu, Y. Du, Q. He, B. Yang, and H. Zhu (2015). Hypoxia-targeted drug Q6 induces G2-M arrest and apoptosis via poisoning topoisomerase II under hypoxia. PLoS ONE 10, 1–16.
B. Chertok, A. E. David, and V. C. Yang (2011). Brain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topography. J. Control. Release 155, 393–399.
S. Dwivedi, M. A. Siddiqui, N. N. Farshori, M. Ahamed, J. Musarrat, and A. A. Al-Khedhairy (2014). Synthesis, characterization and toxicological evaluation of iron oxide nanoparticles in human lung alveolar epithelial cells. J. Colloid Interface Sci. 122, 209–215.
L. P. Franchi, B. B. Manshian, T. A. Souza, S. J. Soenen, E. Y. Matsubara, J. M. Rosolen, and C. S. Takahashi (2015). Cyto-and genotoxic effects of metallic nanoparticles in untransformed human fibroblast. Toxicol. In Vitro 29, 1319–1331.
G. Ganesh, T. Abhishek, M. Saurabh, and N. C. Sarada (2014). Cytotoxic and apoptosis induction potential of Mimusops elengi L. in human cervical cancer (SiHa) cell line. J. King Saud. Univ. Sci. 26, 333–337.
C. He, S. Jiang, H. Jin, S. Chen, G. Lin, H. Yao, X. Wang, P. Mi, Z. Ji, Z. Lin, Y. Lin, and G. Liu (2016). Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials 83, 102–114.
W. Kai, X. Xiaojun, P. Ximing, H. Zhenqing, and Z. Qiqing (2011). Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 6, 1–19.
K. Kavithaa, S. Sumathi, M. Paulpandi, and Palghat Raghunathan Padma (2016). Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv. 2016, (6), 64531.
M. I. Khan, A. Mohammad, G. Patil, S. A. H. Naqvi, L. K. S. Chauhan, and I. Ahmad (2012). Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33, 1477–1488.
S. Kuroda, J. Tam, J. A. Roth, and R. Ramesh (2014). EGFR-targeted plasmonic magnetic nanoparticles suppress lung tumor growth by abrogating G2/M cell-cycle arrest and inducing DNA damage. Int. J. Nanomed. 9, 3825–3839.
Y. K. Lee, E. J. Choi, T. J. Webster, S. H. Kim, and D. Khang (2014). Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 10, 97–113.
M. Malekigorji, A. D. Curtis, and C. Hoskins (2014). The use of iron oxide nanoparticles for pancreatic cancer therapy. J. Nanomed Res. 1, 1–12.
M. A. Malvindi, V. De Matteis, A. Galeone, V. Brunetti, G. C. Anyfantis, A. Athanassiou, R. Cingolani, and P. P. Pompa (2014). Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 9, 1–10.
M. Nadeem, M. Ahmad, M. S. Akhtar, A. Shaari, S. Riaz, S. Naseem, M. Masood, and M. A. Saeed (2016). Magnetic properties of polyvinyl alcohol and doxorubicin loaded iron oxide nanoparticles for anticancer drug delivery applications. PLoS ONE. doi:10.1371/journal.pone.0158084.
E. Pawelczyk, A. S. Arbab, A. Chaudhry, A. Balakumaran, P. G. Robey, and J. A. Frank (2008). In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells 26, 1366–1375.
J. Rahul (2015). Importance of nanoparticles in targeted drug delivery system for treatment of cancer: a brief review. Res. Rev. J. Pharm. Nanotechnol. 3, 1–8.
A. A. Romoser, M. F. Criscitiello, and C. M. Sayes (2014). Engineered nanoparticles induce DNA damage in primary human skin cells, even at low doses. Nano Life 4, 1–13.
M. Rui, C. Ma, Y. Hao, J. Guo, Y. Rui, X. Tang, Q. Zhao, X. Fan, Z. Zhang, T. Hou, and S. Zhu (2016). Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant. Sci.. doi:10.3389/fpls.2016.00815.
C. Saha, A. Kaushik, A. Das, S. Pal, and D. Majumder (2016). Anthracycline drugs on modified surface of quercetin-loaded polymer nanoparticles: a dual drug delivery model for cancer treatment. PLoS ONE. doi:10.1371/journal.pone.0155710.
M. Shafagh, F. Rahmani, and N. Delirezh. (2015). CuO nanaparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and p53. Iran. J. Basic Med. Sci. 18, 993–1000.
R. Thangam, S. Sundarraj, R. Vivek, V. Suresh, S. Sivasubramanian, M. Paulpandi, S. V. Karthick, S. Ragavi, and S. Kannan (2015). Theranostic potentials of multifunctional chitosan–silver–phycoerythrin nanocomposites against triple negative breast cancer cells. RSC Adv. 5, 12209–12223.
R. Wahab, S. Dwivedi, F. Khan, Y. K. Mishra, I. H. Hwang, H. Y. Shin, J. Musarrat, and A. A. Khedhairy (2014). Statistical analysis of gold nanoparticle-induced oxidative stress and apoptosis in myoblast (C2C12) cells. Colloids Surf. B 123, 664–672.
J. Wang, B. Chen, J. Cheng, X. Cai, G. Cai, R. Liu, and X. Wang (2011). Apoptotic mechanism of human leukemia K562/A02 cells induced by magnetic iron oxide nanoparticles co-loaded with daunorubicin and 5-bromotetrandrin. Int. J. Nanomed. 6, 1027–1034.
M. M. Yallapu, S. F. Othman, E. T. Curtis, N. Chauhan, N. A. Bauer, M. Jaggi, and S. C. Chauhan (2012). Curcumin loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Cancer Res. 72, 2893.
H. Zhang, K. Wang, G. Lin, and Z. Zhao (2014). Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer therapy. BMC Complement. Altern. Med. 14, (1), 270.
W. Zhang, L. Qiao, X. Wang, R. Senthilkumar, F. Wang, and B. Chen (2015). Inducing cell cycle arrest and apoptosis by dimercaptosuccinic acid modified Fe3O4 magnetic nanoparticles combined with nontoxic concentration of bortezomib and gambogic acid in RPMI-8226 cells. Int. J. Nanomed. 10, 3275–3289.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kavithaa, K., Sumathi, S. & Padma, P.R. Intracellular Uptake of PEG-Funtionalized Baicalein Loaded Iron Oxide Nanoparticles Regulates Apoptotic Genes in Triple Negative Breast Cancer Cells: Mitochondrial Pathway Targeted Therapy for Breast Cancer. J Clust Sci 28, 2057–2073 (2017). https://doi.org/10.1007/s10876-017-1204-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1204-2