Abstract
Patients with Chiari I malformations may present again after a foramen magnum decompression for two reasons: either they are unsatisfied with the result or new neurological symptoms have appeared. This chapter provides a systematic approach to these patients. As a general rule, revision surgery should be reserved for patients with progressive neurological symptoms. Arachnoid scarring causing obstructions of cerebrospinal fluid (CSF) flow was the commonest intraoperative finding in such revisions. Craniocervical instability in patients with basilar invagination or Klippel-Feil syndromes is the other potential mechanism leading to postoperative deterioration after a foramen magnum decompression. In such patients, a revision has to include stabilization. Apart from these foramen magnum-related mechanisms, degenerative diseases of the cervical spine may lead to signs of a cervical myelopathy requiring early surgery. With revision surgeries, no major postoperative improvements should be expected. Stabilization of the neurological state is the realistic outlook.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Foramen magnum decompression
- Chiari I
- Secondary surgery
- Arachnoid scarring
- Craniocervical instability
- Cervical myelopathy
- Management, Pain
- Dysesthesia
Introduction
Foramen magnum decompression is widely recognized as the procedure of choice for treatment of patients with Chiari I malformation (CM I), with short-term success rates well above 80% reported in numerous reports in the literature. On the other hand, few publications deal specifically with treatment concepts for patients who develop new neurological problems early after such a decompression or in the long term. Furthermore, there still exists considerable disagreement as to how a foramen magnum decompression should be performed: Is it necessary to open both layers of the dura? Should the arachnoid be opened and dissected? How should we deal with the cerebellar tonsils? Should a duraplasty be performed and if yes, what kind of material should be used for grafting? An analysis of patients with failed decompressions may provide some answers to these questions. On the other hand, a neurological deterioration may not always be related to an insufficient decompression or an unrecognized craniospinal instability but caused by another pathology such as a degenerative problem of the cervical spine [1].
Diagnosis and Decision-Making
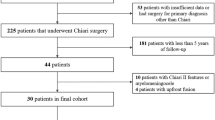
Among a series of 856 patients presenting with CM I, 158 patients had already undergone a foramen magnum decompression. Of these, 36 had an additional syrinx shunt implanted. Ninety-two of these 158 patients were not operated: In 64 patients a revision was not recommended because the neurological status was stable or considered unlikely to be stabilized by another intervention, while 28 refused another operation. The majority of patients, in whom a revision was not recommended, presented because they were disappointed by the result of their decompression. Burning-type dysesthesias were the commonest complaint of these patients. Despite postoperative improvements of other symptoms and a reduction of syrinx size, this type of neuropathic pain may persist and turn out to be notoriously difficult to treat. It is important to inform a patient before surgery that burning-type dysesthesias may not respond to an otherwise successful decompression. Bernard Williams even observed postoperative aggravations in a few patients despite regression of a syrinx (personal communication). However, this was not observed in this series. No surgery at the foramen magnum was recommended for most patients with a history of bacterial meningitis or after multiple procedures at the foramen magnum, considering the increased risks of another intervention and the reduced chances for success under such circumstances.
Sixty-six patients underwent a total of 73 surgical procedures. The decision was based on a detailed clinical and neuroradiological analysis. Once hydrocephalus was ruled out, the evaluation started with the clinical history before the previous decompression and how preoperative symptoms responded to it. Was the neurology unchanged or improved, or did symptoms progress further without a free interval of stable neurology? If symptoms progressed immediately, such a course suggested an insufficient decompression. In most instances, this was related to persistent cerebrospinal fluid (CSF) flow obstruction at the foramen magnum or untreated features such as an associated basilar invagination with craniocervical instability.
In the majority of patients, however, the clinical history revealed a free interval of an improved or unchanged neurological condition before new symptoms of medullary compression developed. The only clinical symptoms, which clearly pointed to a new foramen magnum problem, were occipital headaches and swallowing dysfunctions. If these did not progress or reappeared, the clinical history often provided no clue whether a foramen magnum-related cause or another pathology was responsible for the clinical deterioration. As a general rule, the longer the free interval, the less likely this cause was related to the foramen magnum.
Next, a careful neuroradiological assessment was essential for these patients. The area of the previous operation was evaluated comparing preoperative and postoperative magnetic resonance imaging (MRI) scans. Was there any evidence for an insufficient decompression or recurrent compression? It has been reported that new bone formation may cause recurrent compression, particularly in children [2,3,4]. However, this was not observed in this series. No example of cerebellar ptosis [5] due to an oversized craniectomy [6] resulting in medullary compression was found either.
Was there a basilar invagination with persistent anterior compression of the odontoid? Was there an indication of craniocervical instability such as an assimilated atlas to the occiput, a Klippel-Feil syndrome of the upper cervical spine, or pannus formation around the odontoid (Fig. 44.1) [7]? Was there a cisterna magna of sufficient size (Figs. 44.1, 44.2, and 44.3)? Was there a pseudomeningocele pushing the dura anteriorly (Figs. 44.1, 44.2, and 44.3) [8]?
(a) This sagittal T2-weighted magnetic resonance image (MRI) was performed 8 years after decompression of the foramen magnum in another institution in a 46-year-old patient with Chiari I malformation, basilar invagination, and syringomyelia. The syrinx appears of small caliber; a small pseudomeningocele is apparent. C1 is assimilated to the occiput and C2/C3 is fused, i.e., Klippel-Feil syndrome. The patient complained about severe neck pain, dysesthesias, and a slight gait ataxia. (b, c) The cine MRI shows no flow signals in the foramen magnum region. (d, e) Functional X-rays of the cervical spine demonstrate the laminectomy of C2 and C3 and instability at C3/C4. Revision surgery incorporated a revision at the foramen magnum with arachnoid dissection and a new duraplasty followed by occipitocervical fusion C0–C5 with lateral mass screws. (f) The postoperative MRI demonstrates a large cisterna magna. (g) The control X-ray 7 years later shows the correct positions of all implants with a good sagittal profile. Postoperatively, the patient has remained neurologically stable for 7 years with some improvement of her neck pain
(a) This sagittal T2-weighted magnetic resonance image (MRI) shows a classical Chiari I malformation without syringomyelia in a 15-year-old boy with occipital headaches. (b) After decompression of the foramen magnum with resection of both tonsils in another institution, the postoperative scan demonstrates a large pseudomeningocele. There appears to be a membrane obstructing the foramen of Magendie. (c, d) The cine MRI shows no flow signals in the area of the foramen magnum. The patient no longer complained about occipital headaches but reported quite severe local discomfort. At reoperation 2 years later, a large defect in the suture line for the duraplasty was evident. After removal of the duraplasty, profound scarring at both tonsillar stumps was detected. Both posterior inferior cerebellar arteries were embedded in this scar tissue, which also obstructed the foramen of Magendie. The foramen was not opened to avoid any vascular injuries, and a new duraplasty was inserted. (e) The postoperative scan shows a free cerebrospinal fluid (CSF) passage across the foramen magnum with normal soft tissue healing. The patient made a full recovery
(a) This sagittal T2-weighted magnetic resonance image (MRI) shows a Chiari I malformation with a substantial syrinx and scoliosis in a 5-year-old girl. (b) After decompression, a pseudomeningocele had formed pushing the duraplasty anteriorly obstructing cerebrospinal fluid (CSF) flow. Consequently, the syrinx did not resolve. Seven years after the first operation, the scoliosis deteriorated, and the decision was made to revise the foramen magnum. (c) After this revision, which included arachnoid dissection and insertion of a new duraplasty, the CSF pathway is free and the syrinx has started to decrease. There has been no further progress of her scoliosis
Another important aspect was the postoperative course of a syrinx. If the syrinx decreased after surgery and remained so, it was unlikely that new symptoms were related to the foramen magnum with one exception: Craniocervical instability still had to be ruled out.
If all these points were excluded by conventional MRI scans, a cardiac-gated cine MRI was performed to evaluate the CSF passage at the foramen magnum. This modality is the most sensitive method to detect or exclude arachnoid scarring and adhesions that may have reformed or may not have been addressed sufficiently during the previous decompression [9,10,11,12]. If such a study demonstrated CSF flow at the foramen magnum and the neuroradiological evaluation had excluded all the other possibilities mentioned above, then the clinical deterioration had to be caused by a process unrelated to the previous decompression.
In patients with syrinx shunts, the shunt catheter could lead to a tethering of the nerve roots or spinal cord [13] leading to radicular or myelopathic symptoms, which were often provoked by neck or arm movements. The MRIs in these patients showed adherence of the cord to the dura at the level of the shunt.
If that had been excluded as well, degenerative changes of the cervical spine were evaluated next (Fig. 44.4). Many patients with a well-treated Chiari malformation and a collapsed syrinx demonstrated a considerable amount of spinal cord atrophy as the result of the long-standing syringomyelia. Therefore, MRI scans often gave the impression that a slight or moderate degree of cervical stenosis may not be clinically relevant. However, this is a very dangerous assumption. Such patients have very little functional reserve in their spinal cord as a consequence of their former syringomyelia. Any additional affection—even a minor one—may be enough to cause significant new deficits. It has even been suggested that Chiari patients may be particularly prone to degenerative problems of the cervical spine [14]. Signs of hypermobility of cervical segments should be looked for in particular by X-rays in ante- and retroflexion [1, 15].
(a) This sagittal T2-weighted, upright magnetic resonance image (MRI) was taken 9 years after decompression of the foramen magnum in inclined position and demonstrates profound spinal cord atrophy, a collapsed syrinx, a free cerebrospinal fluid (CSF) passage at the foramen magnum, and multilevel osteochondrosis in his cervical spine with a kyphotic deformity in a patient now 74 years of age. He suffered a progressive tetraparesis confining him to a wheelchair with increasing weakness of his respiratory muscles and loss of upper extremity functions. (b) The sagittal computed tomography (CT) reconstruction shows the multiple osteochondroses and the swan neck deformity. The patient underwent a combined decompression with corpectomies C4–C6, reconstruction and ventral fusion C3–C7, followed by posterior decompression C3–C6, and fixation with lateral mass screws C3–C7. The postoperative CT reconstruction (c), lateral X-ray (d), and the MRI 4 years later (e) demonstrate an improved sagittal profile with decompression of the cervical cord. Postoperatively he made a slow recovery. Four months after surgery, he was able to walk for about 20 m with improved respiratory functions, power, and coordination skills in his hands
Management
Table 44.1 gives an overview on symptoms at presentation for unoperated patients and patients operated again at the foramen magnum or elsewhere in the spinal canal. The percentage of patients suffering from neuropathic or occipital pain was similar in all three groups. For the remaining symptoms, unoperated patients were less severely affected compared to the surgical groups. Patients undergoing a foramen magnum revision presented occipital pain and swallowing problems more commonly, whereas sensory disturbances were more common in patients undergoing surgery for cervical disc diseases. Otherwise, the neurological courses of patients with either a new foramen magnum problem or a cervical myelopathy were indistinguishable.
Patients undergoing a foramen magnum revision rather than surgery for cervical disc disease were significantly younger (40 ± 16 years vs. 51 ± 15 years; t-test, p = 0.03), with trends for a shorter interval between previous decompression and onset of new symptoms (31 ± 39 months vs. 100 ± 161 months; t-test, p = 0.12) and a longer history before the secondary operation (48 ± 89 months vs. 32 ± 65 months; t-test, p = 0.5). Eight patients underwent multiple operations: Two patients each underwent foramen magnum revisions followed by removal of syrinx catheters or foramen magnum revisions followed by decompressions for cervical disc diseases or two surgeries on the cervical spine or two foramen magnum revisions in separate operations, respectively.
Secondary Surgeries in the Cervical Spine
In 18 patients, a mechanism independent from the foramen magnum region had caused new neurological symptoms (Table 44.2 and Fig. 44.4). In all these patients, the Chiari malformation had been adequately treated with collapse of the syrinx and a free CSF passage at the foramen magnum. They underwent a total of 20 operations. One patient required a ventriculoperitoneal shunt for hydrocephalus. Four syrinx shunt catheters were removed to release a postoperative tethering of either the nerve roots or spinal cord. In each of these patients, pain and dysesthesias were provoked with certain body movements. The tethering had not caused reappearance of the syrinx in any of them.
For removal of a syrinx catheter, the sharp microsurgical dissection concentrated on untethering the nerve roots and spinal cord first. Once this was achieved, the catheter could be removed in most instances. If it was stuck in the cord, it was transected right at the entry point into the spinal cord. Three of these four patients reported improvement after syrinx catheter removal.
For patients with degenerative disc disease, it was the general policy to restrict one- or two-level ventral fusions to patients with radicular symptoms, whereas multilevel posterior decompressions and fusions were preferred for patients with a progressive myelopathy. This strategy was based on observations that patients with a progressive cervical myelopathy almost always displayed a profound spinal cord atrophy due to the former syringomyelia and often demonstrated multilevel hypermobilities of the cervical spine. The intention was to prevent future deteriorations from adjacent levels in patients with a significantly reduced functional reserve (Fig. 44.4).
Nine patients underwent ten ventral fusions for single- or two-level disc disease of the cervical spine. One of these underwent an additional posterior cervical decompression and fusion 10 years later when she developed a progressive myelopathy. One patient underwent a combined anterior and posterior decompression and fusion for a swan neck deformity (Fig. 44.4). Finally, three patients received a posterior decompression and fusion only. Posterior decompressions consisted of laminectomies C3 to C6 with lateral mass fixation (Table 44.2).
Apart from one patient with a postoperative pneumonia, no complications were observed in this group. One patient undergoing a posterior decompression and lateral mass fixation experienced a permanent worsening of his severe preoperative myelopathy.
Looking at individual symptoms, a trend for improvements of pain, sensory disturbances, and dysesthesias was observed at 3 months after surgery. Other neurological signs such as motor weakness or gait problems tended to remain unchanged. All but one ventral fusion of the cervical spine resulted in some clinical improvement at that time [15].
In the long term, a progression-free survival for at least 5 years was observed for 77% of patients undergoing surgery for degenerative diseases of the cervical spine [15]. Two patients developed adjacent-level disease after ventral fusions and underwent another ventral or posterior operation, respectively, which stabilized the clinical status.
After catheter removal, one patient experienced another deterioration due to postoperative scar formation 4 months after surgery. Her past history had been complicated by meningitis after the initial foramen magnum procedure, and no further operation was undertaken.
Secondary Surgeries at the Foramen Magnum
The 51 patients in this subgroup demonstrated either an untreated or new instability of the craniocervical junction, an insufficient decompression or an obstruction of CSF flow at this level (Figs. 44.1, 44.2, and 44.3). CSF flow obstructions were related to arachnoid scarring or compression of the cisterna magna by a pseudomeningocele. Combinations of these different mechanisms were common (Table 44.3).
In a previous publication, the lack of effect of syrinx shunts in patients with a Chiari I malformation and syringomyelia was demonstrated [16]. Therefore, such shunts were never considered for patients after a failed decompression. If a syrinx had not regressed or reappeared, the reason had to be looked for and treated at the foramen magnum. This required a revision with opening of the dura exchanging the duraplasty, arachnoid dissection with establishment of a free outflow from the foramen of Magendie, and insertion of a new duraplasty using alloplastic rather than autologeous material [1]. Several authors have mentioned the importance of opening this foramen during foramen magnum decompressions [2, 17, 18] and especially in revisions [18, 19].
Fifty-three revisions at the foramen magnum were performed in 51 patients (Figs. 44.2 and 44.3), of which 13 were combined with posterior craniocervical fusion (Fig. 44.1), where one revision included transoral resection of the odontoid and posterior decompression and fusion in a second operation. In four instances, the revision was restricted to craniocervical fusion only, as no CSF flow obstruction was detectable on preoperative imaging and intraoperatively using ultrasound (Table 44.2). The occiput was fused to C2 or subxial levels in patients with assimilation of the atlas only.
Severe arachnoid scarring was the commonest feature in patients demonstrating a CSF flow obstruction [20,21,22,23,24] and detected in 36 instances (73%) in the form of adhesions either between the dura graft and cerebellum or spinal cord, with obstruction of the foramen of Magendie in 33 of these (Table 44.3). Whereas the adherence of the dura graft to the underlying nervous tissue was due to either pseudomeningocele formation pushing the dura graft anteriorly, [8] the suture material, autologeous graft material, or insufficient arachnoid dissection at the first operation, the most severe arachnoid scarring at the foramen of Magendie was encountered after obex plugging, resection of tonsils (Fig. 44.2), or in patients with a history of meningitis [16, 20, 25].
The major problem of preoperative evaluation was to determine the severity of arachnoiditis. The more extensive and dense the arachnoid pathology is, the less the probability that a revision may produce a lasting benefit and the higher the risk of surgery. Unless there was a history of meningitis or a clear description of severe arachnoid changes in the operation notes, it was almost impossible to foresee exactly what would be discovered after opening of the dura. Thus, it is difficult to judge the prognosis for a patient before revision surgery. This needs to be discussed with the patient. Reexploration of the foramen magnum is to some degree a diagnostic procedure in order to find out why the first operation did not provide the desired result. Depending on the intraoperative findings, a surgical strategy has to be adopted to improve CSF flow but to minimize the risk of postoperative arachnoid scarring, which may again lead to CSF flow obstruction and prevent a long-term benefit. Limiting the arachnoid dissection to the midline with sharp transection of arachnoid adhesions obstructing the foramen of Magendie and the posterior spinal subarachnoid space is all that is required. Blunt dissection or preparation of arachnoid adhesions laterally carries the risk of damage to small perforating arteries and caudal cranial nerves and should be avoided. Finally, a spacious dura graft using alloplastic material provides reasonable protection against postoperative arachnoid scarring, which may lead to neurological deterioration once again.
Complications were encountered in 26% of foramen magnum revisions with CSF fistulas, hemorrhages, and hydrocephalus occurring after two revisions each. Surgical morbidity was observed after four revisions (7.5%) and encountered exclusively among patients who had undergone their first decompression at other institutions. Compared to patients undergoing the first foramen magnum decompression, the overall complication rate for foramen magnum revisions was slightly higher [26, 27], while permanent surgical morbidity was significantly lower (0.9%) after first decompressions [26].
A postoperative improvement after 3 months was reported after 62% of operations, while 26% resulted in no postoperative change, and neurological worsening was evident after 11% of revisions. Looking at individual symptoms in the first postoperative year, improvements for pain, sensory disturbances, and gait had been revealed. The remainder of symptoms tended to be left unchanged. Improvements tended to be marginal and of little functional significance. Similar experiences have been made for patients with severe foramen magnum arachnoiditis of other causes [28]. The realistic outlook for patients undergoing a foramen magnum revision was clinical stabilization of the previously progressive course [1].
Long-term results determined by Kaplan-Meier statistics revealed rates for progression-free survival of 71% for 5 years and 63% for 10 years, respectively.
Conclusion
Patients presenting with progressive neurological symptoms after a foramen magnum decompression for Chiari I malformation require a detailed clinical and radiological workup to identify the responsible mechanism. Not only the foramen magnum area needs a careful analysis, but degenerative diseases of the cervical spine should also be taken into account. Particularly important are signs of instabilities, as the often atrophic spinal cords of these patients may be extremely vulnerable to hypermobile segments. Multilevel decompressions and fusions may stabilize the course for 77% of such patients for at least 5 years. Foramen magnum revisions are indicated in patients with evidence of CSF flow obstruction, cord compression, or instabilities at this level. They carry a higher surgical morbidity and are less likely to produce significant neurological improvements compared to a primary decompression. However, still about 63% can be stabilized with such a revision for at least 10 years.
References
Klekamp J. Neurological deterioration after foramen magnum decompression for Chiari malformation type I: old or new pathology? J Neurosurg Pediatr. 2012;10(6):538–47.
Zerah M. [Syringomyelia in children]. Neurochirurgie. 1999;45(Suppl 1):37–57.
Aoki N, Oikawa A, Sakai T. Spontaneous regeneration of the foramen magnum after decompressive suboccipital craniectomy in Chiari malformation: case report. Neurosurgery. 1995;37(2):340–2.
Hudgins RJ, Boydston WR. Bone regrowth and recurrence of symptoms following decompression in the infant with Chiari II malformation. Pediatr Neurosurg. 1995;23(6):323–7.
Holly LT, Batzdorf U. Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. J Neurosurg. 2001;94(1):21–6.
Williams B. Surgery for hindbrain related syringomyelia. Adv Tech Stand Neurosurg. 1993;20:107–64.
Smith JS, Shaffrey CI, Abel MF, Menezes AH. Basilar invagination. Neurosurgery. 2010;66(3 Suppl):39–47.
Pare LS, Batzdorf U. Syringomyelia persistence after Chiari decompression as a result of pseudomeningocele formation: implications for syrinx pathogenesis: report of three cases. Neurosurgery. 1998;43(4):945–8.
Armonda RA, Citrin CM, Foley KT, Ellenbogen RG. Quantitative cine-mode magnetic resonance imaging of Chiari I malformations: an analysis of cerebrospinal fluid dynamics. Neurosurgery. 1994;35(2):214–2; discussion 23-43.
Bhadelia RA, Bogdan AR, Wolpert SM, Lev S, Appignani BA, Heilman CB. Cerebrospinal fluid flow waveforms: analysis in patients with Chiari I malformation by means of gated phase-contrast MR imaging velocity measurements. Radiology. 1995;196(1):195–202.
Hofkes SK, Iskandar BJ, Turski PA, Gentry LR, McCue JB, Haughton VM. Differentiation between symptomatic Chiari I malformation and asymptomatic tonsillar ectopia by using cerebrospinal fluid flow imaging: initial estimate of imaging accuracy. Radiology. 2007;245(2):532–40.
McGirt MJ, Atiba A, Attenello FJ, Wasserman BA, Datoo G, Gathinji M, et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for Chiari I malformation. Childs Nerv Syst. 2008;24(7):833–40.
Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89(3):382–8.
Takeuchi K, Yokoyama T, Ito J, Wada K, Itabashi T, Toh S. Tonsillar herniation and the cervical spine: a morphometric study of 172 patients. J Orthop Sci. 2007;12(1):55–60.
Klekamp J. Surgical treatment of multilevel cervical spondylosis in patients with or without a history of syringomyelia. Eur Spine J. 2017;26(4):948–57.
Klekamp J, Batzdorf U, Samii M, Bothe HW. The surgical treatment of Chiari I malformation. Acta Neurochir. 1996;138(7):788–801.
Menezes AH, Greenlee JD, Donovan KA. Honored guest presentation: lifetime experiences and where we are going: Chiari I with syringohydromyelia--controversies and development of decision trees. Clin Neurosurg. 2005;52:297–305.
Tubbs RS, Beckman J, Naftel RP, Chern JJ, Wellons JC 3rd, Rozzelle CJ, et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J Neurosurg Pediatr. 2011;7(3):248–56.
Sacco D, Scott RM. Reoperation for Chiari malformations. Pediatr Neurosurg. 2003;39(4):171–8.
Sakamoto H, Nishikawa M, Hakuba A, Yasui T, Kitano S, Nakanishi N, et al. Expansive suboccipital cranioplasty for the treatment of syringomyelia associated with Chiari malformation. Acta Neurochir. 1999;141(9):949–60; discussion 60-1.
Ellenbogen RG, Armonda RA, Shaw DW, Winn HR. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurg Focus. 2000;8(3):E6.
Mazzola CA, Fried AH. Revision surgery for Chiari malformation decompression. Neurosurg Focus. 2003;15(3):E3.
Rosen DS, Wollman R, Frim DM. Recurrence of symptoms after Chiari decompression and duraplasty with nonautologous graft material. Pediatr Neurosurg. 2003;38(4):186–90.
Yanni DS, Mammis A, Ebersole K, Roonprapunt C, Sen C, Perin NI. Revision of Chiari decompression for patients with recurrent syrinx. J Clin Neurosci. 2010;17(8):1076–9.
Vanaclocha V, Saiz-Sapena N, Garcia-Casasola MC. Surgical technique for cranio-cervical decompression in syringomyelia associated with Chiari type I malformation. Acta Neurochir. 1997;139(6):529–39; discussion 39-40.
Klekamp J. Surgical treatment of Chiari I malformation--analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery. 2012;71(2):365–80; discussion 80.
Klekamp J, Samii M. Syringomyelia - diagnosis and treatment. Heidelberg: Springer Verlag; 2001. 195 p.
Klekamp J, Iaconetta G, Batzdorf U, Samii M. Syringomyelia associated with foramen magnum arachnoiditis. J Neurosurg Spine. 2002;97(3):317–22.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Klekamp, J. (2020). Secondary Interventions for Chiari I Malformation. In: Tubbs, R., Turgut, M., Oakes, W. (eds) The Chiari Malformations. Springer, Cham. https://doi.org/10.1007/978-3-030-44862-2_44
Download citation
DOI: https://doi.org/10.1007/978-3-030-44862-2_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44861-5
Online ISBN: 978-3-030-44862-2
eBook Packages: MedicineMedicine (R0)