Abstract
Scars not only are an aesthetic problem, but may also cause annoying symptoms, functional alterations, and psychological problems and may worsen a patient’s quality of life. Scars are considered an inevitable consequence to surgical interventions. However, they are often the only visible part and the only memory of the intervention for the patient, and that is why scars should be given the attention they deserve.
The best treatment will always be prevention, using proper surgical technique and judicious postoperative care. Basic prophylactic treatments for scars (moisturizing, sun avoidance, massage, pressure therapy, silicone dressings, etc.) are revised, as well as more sophisticated techniques to treat high-risk scars (laser and light therapies, botulinum toxin, etc.).
The progressive knowledge of the wound healing process has allowed the development of new treatments for hypertrophic scars and keloids, as well as the development of multiple techniques for minimizing scar formation and correcting unaesthetic scars. Treatment of hypertrophic scars and keloids continues to be a persisting challenge, though good outcomes are being obtained with combination treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Take-Home Points-
Prevention, based on proper surgical technique and judicious postoperative care, is the best treatment for scars.
-
Recent research suggests that hypertrophic scars and keloids are caused by the same fibroproliferative pathology and that their different clinical and pathological features largely reflect the degree of inflammation in the healing wound.
-
Treatment combinations are typically the best option when treating hypertrophic scars and keloids.
-
Every scar should be considered unique, and each scar may require a customized approach.
-
An early diagnosis and adequate treatment of surgical wound complications are part of the prophylactic treatment of scars.
1 Introduction
Cutaneous scarring following surgical procedures is unavoidable, and increasingly patients request specialists to avoid visible scars whenever possible. What patients do not usually imagine is that the management of scars is a therapeutic challenge.
This chapter discusses the techniques used to prevent and treat scars, specifically those associated with pregnancy (episiotomy and cesarean section) and elective post-pregnancy surgeries (mastopexy, abdominoplasty, liposuction, etc.).
Consequences associated with scars are not only cosmetic; abnormal scarring may also cause the patient to have functional disabilities or symptoms of pain, tightness, and pruritus. Moreover, scars may induce distress and decreased quality of life because of their aesthetically unpleasant appearance.
1.1 Skin Anatomy
The skin is the largest organ of the body. It receives 1/3 of the body’s blood volume. The human skin consists of cells and extracellular matrices, and its thickness varies from 0.5 to 6.0 mm.
Our skin layer has many crucial functions [1]:
-
Protective barrier: physical, mechanical, and immunological
-
Social function (appearance)
-
Synthesis of vitamin D
-
Thermoregulation
-
Touch, proprioception, pain
The skin is composed of three layers that, from outer to inner, are epidermis, dermis, and hypodermis (Fig. 12.1).
Skin anatomy. (a) Human skin is composed of three layers: epidermis, dermis, and hypodermis. (b) The major cellular components of the dermis are fibroblasts, which interact with the external extracellular matrix of collagen, fibronectin, and elastic fibers. (c) The epidermis layer is composed of five layers: basal, spinous, granular, lucid, and corneum layer. Reprinted from Limbert G. Skin Biophysics. From Experimental Characterisation to Advanced Modelling. Switzerland: Springer; 2019
1.1.1 Epidermis
The epidermis is a stratified squamous keratinized epithelium. It is an avascular layer, receiving blood supply from the dermis across the semipermeable basement membrane. The thickness of the epidermis varies in different types of skin. It is the thinnest on the eyelids at 0.05 mm and the thickest on the palms and soles at 1.5 mm.
Epidermis contains four layers, described according to the morphological aspect of the keratinocytes, although plants and soles contain five layers. The layers are named, from bottom to top, as stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum [1, 2].
-
The stratum basale (basal layer) is the deepest layer and consists of a single layer of epidermal cells (keratinocytes). It forms the dermal-epidermal junction (basement membrane zone), which separates the epidermis from the dermis. It is the only layer of the epidermis in which cells undergo mitosis.
-
The stratum spinosum (spinous layer) consists of several rows of more mature keratinocytes.
-
The stratum granulosum (granular layer) contains 3–5 flattened cell rows comprising a higher concentration of keratin.
-
The stratum lucidum (lucid layer) is only present in palms and soles. It is a thin and clear layer of dead skin cells.
-
The stratum corneum (corneum layer) is the most superficial layer and consists of dead keratinocytes (corneocytes) and keratin.
Several types of cells can be found in epidermis:
-
Keratinocytes: They are major cells of the epidermis, making up approximately 90% of epidermal cells. They originate at the basal layer, mature, lose their nucleus, and flatten as they move upward. They produce keratin and form the basic component of hair, skin, and nails.
-
Melanocytes: They represent 1–8% of epidermal cells. They are responsible for melanogenesis, and they are found only in the basal layer. Variation in normal skin color is not determined by the number or density of melanocytes but the number, size, and distribution of melanosomes (organelles that produce melanin pigment). Melanin is transferred from melanocytes to keratinocytes, where they form a protective cap over the keratinocyte nucleus, protecting the nuclear DNA from the effects of ultraviolet radiation.
Pearls and Pitfalls
Although melanin is principally responsible for skin color, it is also influenced by hemoglobin, elastin, dermic collagen, and some components of the diet, such as carotenes.
-
Langerhans cells: They represent 2–8% of epidermal cells. They are antigen-presenting cells that protect the body against infection.
-
Merkel cells: They represent approximately 1% of epidermal cells. They are neuroendocrine cells. They are high-sensitivity mechanoreceptors that provide information on light touch sensation.
1.1.2 Dermis
The dermis is a thick layer of connective tissue, composed of many cells and blood and lymph vessels. Its main functions are structural support and nourishment of the epidermis.
Dermis is composed of two layers; the upper layer is named papillary dermis and the lower one is named reticular dermis [1, 2].
-
Papillary dermis: It represents 20% of dermis, and most of the cells of the dermis are located in this layer. It is named for its fingerlike projections called papillae, wherein fibers of collagen (predominantly type III) and elastin prevail. It contains capillaries for skin nourishment and pain touch receptors, such as Meissner and Pacinian corpuscles.
-
Reticular dermis: It represents 80% of dermis. In this layer prevail fibers of collagen (predominantly type I), and glycosaminoglycans. It is thicker than papillary dermis, so its main function is supportive. Epidermal appendages can be found in this layer, including sebaceous glands, eccrine and apocrine sweat glands, hair, and nails.
Important
Hair follicles are a source of multipotent stem cells, which have the capacity to restore epidermis [3, 4].
Principal cells that can be found in dermis are the following:
-
Fibroblasts: They are the main cells of the dermis, which produce collagen, elastin, granulation tissue, and cytokines (including growth factors). They are more numerous and larger in the papillary dermis.
-
Macrophages and white blood cells: Their function is defensive, helping fight infection.
-
Mast cells: They are immune cells that help initiate inflammation through secretion of histamine, enzymes, and chemical mediators.
1.1.3 Hypodermis
It is also named subcutis or subcutaneous tissue. Hypodermis is composed of adipocytes, which are grouped together in lobules of fat, and connective tissue, and contains larger blood vessels and nerves. Its functions are thermoregulation, structural support, energy storage, and mechanic absorption (as in soles). It represents about 10% of the body weight, although the size of this layer varies widely from person to person [1, 2].
2 Wound Healing
A wound is a disruption of the normal structure and function of the skin and underlying soft tissue, though the severity and depth may vary widely. This chapter focuses on surgical wounds, which are a controlled form of acute wounds that are created in the operating room environment.
Types of wound healing:
-
Primary healing (first intention): It consists of direct apposition of skin edges of acute surgical or traumatic wounds after appropriate wound preparation with sutures and/or staples.
-
Secondary healing (second intention): A wound is deliberately left open and fills in with granulation tissue, and eventually epithelization occurs over a period of time.
-
Delayed primary closure (tertiary): A wound is initially left open and, following an interval of wound management, the skin edge apposition is performed.
2.1 Phases of Wound Healing
The wound healing process is a complex process. The progressive knowledge of the pathophysiological events that occur in each type of scars will help us to better understand and treat abnormal scarring.
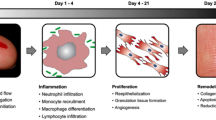
Classically, four phases have been described in the wound healing process, which are hemostasis, inflammation, proliferation, and maturation and remodeling [3,4,5,6,7,8].
2.1.1 Hemostasis
The hemostasis phase starts immediately after skin injury. Damaged small vessels contract for 5–10 min after injury. Fibrin and platelets form blood clots, which minimize blood loss, act as a physiologic barrier against bacterial infection and dehydration, serve as a provisional scaffold, and trigger clotting cascade.
The alpha granules of platelets contain essential growth factors and cytokines, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), or epidermal growth factor (EGF). These substances activate fibroblasts, vascular endothelial cells, and macrophages and allow the initiation of the wound healing process.
Key Point
Platelets are more than just a passive coagulation factor. They are the first responders to a wound site, actively secreting molecules that regulate and control the healing process.
2.1.2 Inflammation
After hemostasis, inflammatory phase starts, which is completed within the first 48–72 h. Main factors of inflammatory phase are secondary vasodilation, with an increase in vascular permeability, and cellular recruitment.
Neutrophils are the first leukocytes to arrive at the wound site, attracted by chemotactic agents (bacterial proteins containing C5a, N-formyl-methionyl-leucyl-phenylalanine (FMLP), leukotriene B4, etc.), and reach their peak number at 24 h. Chemotactic agents increase neutrophil adherence to vascular endothelial cells, where they cause acute inflammation. Neutrophils prevent infection from local resident bacteria, secrete proteases (elastase, collagenase, …), and remove damaged or degenerated extracellular matrices and dead tissue.
Within a few days, monocytes are attracted to the wound by bacterial products and fibronectin. Circulating monocytes are activated as they enter the wound site and are eventually converted into macrophages, becoming the dominant cell type about 48–72 h after injury. Macrophages phagocytize foreign bodies and bacteria, as well as secrete additional cytokines and growth factors that promote fibroblast proliferation, angiogenesis, and keratinocyte migration.
Key Point
Macrophages have the most important role in the inflammatory phase, because they release cytokines and growth factors that are essential for the development of the wound healing process.
2.1.3 Proliferation
The proliferative phase of wound healing is estimated to begin 3 days after injury and last for a few weeks. It is the stage when the wound actually closes, and this happens through different processes that occur simultaneously: collagen deposition, granulation tissue formation, angiogenesis, epithelialization, and wound contraction.
In this phase, the provisional matrix is dissolved by proteases and is converted into granulation tissue, which consists of a vascularized extracellular matrix formed of fibrin, fibronectin, collagens, proteoglycans, glycosaminoglycans (GAGs), and other glycoproteins.
Collagen synthesis and accumulation are directly associated with fibroblast migration into the wound, starting around 2–3 days after injury and gradually increasing for 2–3 weeks. Among the many growth factors involved in collagen synthesis, TGF-β has the strongest influence. Non-collagen proteins are also included in the extracellular matrix, including elastin that gives skin the physiologic property of pliability.
Angiogenesis, the formation of new vessels from the existing ones, is the basis for all wound healing, as new vessels supply oxygen and nutrients to the regenerated tissue.
Important
Angiogenesis and vasculogenesis are not the same process. Angiogenesis is the formation of new blood vessels from existing ones, whereas vasculogenesis is the process of blood vessel formation de novo.
The formation of granulation tissue facilitates epithelization, which is stimulated by EGF, FGF, TGF-β, and multiple cytokines. Epithelialization is initiated a few hours after injury, by keratinocytes present on the wound edge as well as from dermal appendages. It has several stages, including formation of epithelial cells, cell migration, proliferation, and cell differentiation.
Lastly, wound contraction normally stars 5 days after injury. Wound contraction is a dynamic phenomenon in which the surface area of the open wound gradually decreases and contracts toward its center. The amount of dermis influences wound contraction; for example, there is more wound contraction when less dermis is present.
Key Point
Epithelialization and wound contraction also play an important role in scar formation. Earlier epithelialization and controlled wound contraction result in less scarring.
2.1.4 Maturation and Remodeling
The last and longest phase of wound healing is the maturation phase, which begins about 3 weeks after injury. The basic processes of the maturation phase are collagen restructuring and formation of a mature scar.
During this phase, the cellular components of the healed wound do not increase. There is a delicate balance between apoptosis of existing cells and production of new cells. In addition, the granulation tissue previously produced is rearranged, and type III collagen is replaced by type I. These structural changes of the newly accumulated collagen gradually increase the tensile strength of the wound.
The decrease in metabolic demand during this phase initiates the regression of immature vessels created during the proliferation phase.
Although fibroblasts have a primary role in the synthesis of extracellular matrix components, such as collagen, elastin, and proteoglycans, they are also an important source of matrix-dissolving matrix metalloproteinases (MMPs). Therefore, fibroblasts are the major contributors to not only quantitative but also qualitative changes in the extracellular matrix.
Unfortunately, healed and regenerated tissue can never fully recover the highly organized structure displayed by uninjured normal dermis.
2.2 Factors Affecting Wound Healing
Acute wounds in healthy individuals heal through the orderly sequence of phases related previously. Nevertheless, some individuals may have one or more factors that contribute to impaired wound healing and complicate the regular surgical course.
Some of the more important risk factors [8] associated with impaired wound healing are the following:
-
Ischemia and hypoxia: Wound healing is a complex process that represents a high energetic demand, requiring glucose and oxygen. Moreover, low oxygen levels increase the risk of development of wound infection because it decreases the functioning of neutrophils and fibroblasts. Finally, collagen deposition is directly related to wound oxygen tension and tissue perfusion [4].
Pearls and Pitfalls
While tissue perfusion is vital to wound healing, normal hemoglobin levels are not. Low hemoglobin levels do not necessarily diminish oxygen supply. Oxygen supply is related to arterial partial pressure of oxygen, which can be maintained by modifications in vasodilation, cardiac output, and capillary permeability.
-
Infection: The presence of infection impairs several steps of the wound healing process. Among other actions, it decreases oxygen tension, retards epithelialization and angiogenesis, and prolongs inflammation and edema [9].
-
Smoking: The detrimental effect of smoking on wound healing is multifactorial, and it is not only due to nicotine. There are other constituents of tobacco (carbon monoxide, hydrogen cyanide, nitrogen oxides, N-nitrosamines, …) that have a greater impact too. Some of the effects of smoking on wound healing are vasoconstriction, a reduced inflammatory response, impaired bactericidal mechanisms, and alterations of collagen metabolism [4, 9, 10].
Attention
Postoperative healing complications occur significantly more often in former smokers compared with those who never smoked.
-
Aging: Some physiologic changes associated with aging contribute to slowed or impaired wound healing in older adults, such as loss of collagen, diminished ability to produce more collagen, and decrease of blood supply [9, 11].
-
Diabetes: Numerous factors contribute to impaired wound healing in patients with diabetes. Among other factors, diabetes produces microvascular and macrovascular disease, as well as disorders in growth factor production, macrophage function, collagen accumulation, or keratinocyte and fibroblast migration and proliferation [4, 9, 12].
-
Nutritional deficiencies: Surgery increases metabolic demand, so it can increase borderline deficiencies. Protein malnutrition and deficiency in the amino acids arginine and glutamine are of particular importance in wound healing. Vitamins most closely associated with wound healing are vitamins C and A. Of the micronutrients, the key players in wound healing are zinc and magnesium. Zinc is a cofactor for RNA and DNA polymerase, and its deficiency decreases wound strength and epithelialization. Magnesium also functions as a cofactor in enzymes required for protein and collagen synthesis [4, 13].
Tip
Benefits of supplementation of arginine and vitamin A have been demonstrated in wound healing. Specifically, vitamin A is used to reverse the detrimental effects of corticosteroids on wound healing.
-
Drugs: Many drugs can interfere in the wound healing process. The most frequently associated are corticosteroids, antineoplastic agents, and anti-inflammatories. Steroids decrease inflammation, inhibit epithelialization, and decrease collagen production. Antineoplastic agents specially affect vascular endothelial growth factor (VEGF) and increase the risk of development of wound infection. Lastly, anti-inflammatories may decrease collagen synthesis [9].
-
Radiation therapy: Surgical incisions located in radiated areas are more likely to develop a wound complication. Chronic damage, fibrosis, atrophy, and occlusion of small vessels generate bad perfusion in radiated tissues [4, 14].
-
Genetics: Recent research indicates that the formation and growth of hypertrophic scars and keloids are clearly associated with genetics [15].
2.3 Scarring
Human tissue is repaired either by scar formation or by regeneration of the original tissue. Scar formation consists of the substitution of a different cellular matrix as a patch to immediately re-establish a physical and physiological continuity to the injured organ, whereas regeneration is a recapitulation of the developmental processes that initially created the injured organ. Ideally, all defects made by wounds should be restored by regeneration, but the skin is an organ that repairs itself through scarring rather than regeneration.
Furthermore, wound repair and scar formation are a dynamic process, and if the normal wound healing response is altered, the result may be an undesirable scar. Abnormal scarring is usually classified into hypertrophic, keloid, or atrophic scars.
2.3.1 Hypertrophic Scars and Keloids
Recent research suggests that hypertrophic scars (Figs. 12.2 and 12.3) and keloids (Fig. 12.4) are caused by the same fibroproliferative pathology and that their different clinical and pathological features largely reflect the degree of inflammation in the healing wound [16].
The diagnosis of hypertrophic scars and keloids is usually clinical, based upon history, scar shape, size, and growth pattern (Table 12.1). Firstly, hypertrophic scars usually respect borders of the original scar, while keloids grow outside original borders. Secondly, hypertrophic scars usually present a rapid growth phase, followed by possible regression over the following 12–18 months. On the other hand, the beginning of development of keloids is very variable (from months to years) and they do not regress spontaneously (even they enlarge progressively over time) [16, 17].
Hypertrophic scars and keloids also differ in their histology characteristics. Hypertrophic scars present dermal nodules only, and keloids present hypocellular dermal nodules plus multiple thick eosinophilic collagen bundles called keloidal collagen.
The risk of formation of keloids and hypertrophic scars has been associated with genetic, epigenetic, and systemic and local risk factors (particularly skin tension around scars, delayed wound healing [17], and deep wounds). A genetic predisposition is suggested by the fact that keloids are more common in dark Africans, Americans, and Asians, and patients often have a family history of these scars. They also present endocrine influences, as their growth increases in puberty and pregnancy.
Hypertrophic scars affect 5–15% of wounds. They can be classified as linear or widespread. Linear scars usually result from surgery or trauma and widely spread from burn injuries or extensive soft-tissue trauma or infections (for example necrotizing fasciitis).
Keloids are less frequent than hypertrophic scars, and they occur predominantly on the upper chest, shoulders, upper back, and head and neck (especially on the ear). Pain and pruritus are frequently associated symptoms. Keloids can be classified according to their size: minor or major (with the latter being more than 0.5 cm).
2.3.2 Atrophic Scars
Atrophic scars usually develop after an inflammatory process and can be the result of collagen loss and dermal atrophy. These tend to develop after insults to the skin such as acne, varicella, or trauma; therefore, they are not common consequences of cesarean section (C-section), episiotomy, or elective surgeries.
2.3.3 Other Scarring Disorders
-
Widened scars: They look wide and depressed from wound tension perpendicular to wound and mobility during maturation phase. For example, periareolar and medial horizontal inframammary fold scars, associated to mastopexy, are proper to wide (Fig. 12.5).
Important
One of the principal reasons for patient dissatisfaction after mastopexy, associated or not with breast reduction surgery, is unaesthetic scars.
-
Depressed scars: They are the consequence of fibrosis and adherence to deep planes. As a result, the scar function may be impaired, and patients may suffer from scar stiffness and limited range of motion. For example, depressed and adherent scars can be observed associated with C-section (Fig. 12.6).
-
Pigmented scars: Pigmented scars are not only caused by sun exposure; they are usually caused by post-inflammatory hyperpigmentation and neovascularization. In fact, post-inflammatory hyperpigmentation can appear on sun-exposed skin or sun-protected skin, although it is proved that ultraviolet (UV) exposure usually worsens post-inflammatory hyperpigmentation.
3 Surgical Technique
3.1 Physiology and Biomechanics of Skin Flaps
A flap is a tissue with its own vascular supply, unlike for skin grafts, which does not have its own vascular supply.
This chapter focuses on skin flaps that are typically composed of skin and subcutaneous tissue, because they are the ones involved in abdominoplasty and mastopexy. However, flaps can also be composed of other types of tissues, such as fascia (fasciocutaneous flaps), muscle (myocutaneous flaps), or even bone (bone flaps).
According to their vascularization (Figs. 12.7 and 12.8), flaps can be classified into random pattern flaps (based on unnamed smaller vessels) and axial pattern flaps (based on an identified pedicle) [18, 19]. Flaps in abdominoplasty and mastopexy are random pattern flaps, so their vascularization is based on the longitudinal dermal-subdermal plexus. For example, at mastopexy, the thickness of the skin flaps, preserving some subcutaneous tissue and respecting the dermal-subdermal plexus, is essential to avoid flap necrosis.
3.1.1 Flap Physiology
When a flap is raised, the normal vessels supplying that skin are cut and the flap depends on decreased circulation from the collateral vessels. At that moment, flap survival is dependent on various factors [20, 21]:
-
Blood flow: Sufficient blood flow through the base of the flap is essential in the first 24–48 h. In random pattern flaps, the flow recovery is progressive for up to 4 weeks, and it happens from proximal to distal.
-
Angiogenesis and vascularization: The flap receives blood supply not only from its base, but also from the wound bed.
-
Edema: It affects flap blood supply.
-
Wound healing tension: Suturing wounds under excessive tension produces vascular stress on the tip of the flap and risk of necrosis and dehiscence (Fig. 12.9).
-
Postoperative complications (hematoma, seroma, infection): Hematoma and seroma increase tension and impair adhesion to the wound bed; in addition, infection produces important edema, vessel thrombosis, and releasing of toxic free radicals, which can facilitate flap necrosis.
3.1.2 Flap Biomechanics
Understanding the biomechanical properties of the skin is essential when managing skin flaps.
Biomechanical properties [18]
-
Stress: It is the force applied per cross-sectional area.
-
Strain: It is the change in length divided by the original length of the tissue on which a given force is applied.
-
Creep: It is the increase in strain seen when the skin is under constant stress.
-
Stress relaxation: It is related to creep, and it means that the amount of stress required to maintain the tension decreases with time when the skin is held under constant tension.
Although skin is considered an elastic structure, the stress-strain relationship of skin shows that it is not totally true. If a small amount of stress is applied, the skin length changes; however, it has a limit, and at certain point, even a large amount of applied stress will not result in further incremental skin length. This is caused by the relationship between collagen and elastin. In relaxed skin, collagen is randomly oriented and elastin is loosely wrapped around, but when a force is applied, the fibers stretch and there is a point when they cannot distend more.
If a constant force is applied for several minutes (approximately 5–10 min) to the skin, the reorganization of collagen and elastic fibers enables to exceed the stretch limit (creep), and some days later, the skin is able to relax (stress relaxation).
Therefore, it is important to know that there is a limit for skin elasticity and that it is necessary to avoid excessive stress on the flap edges and minimize tension on the wound closure. High tension may lead to wound edge necrosis, wound dehiscence, and unaesthetic scars.
Important
Aging and sun exposure decrease the capacity of stretching of skin because of collagen and elastic fiber damage.
3.2 Suture Techniques
Proper surgical technique has been found to reduce scar width and hypertrophy, so surgeons have to know how the surgical technique, the suture techniques, and the suture material influence scarring (Figs. 12.10 and 12.11). Furthermore, the position and length of the incision line should be carefully considered, and if possible, it should always be parallel to the relaxed skin tension lines.
3.2.1 Wound Apposition
It is proved that contributing factors to pathological scarring are tension, melanin, and inflammation. Some factors are not modifiable, such as melanin; in contrast, inflammation and tension are more controllable.
Inflammation can be reduced by gentle tissue handling, debridement of dead tissue, reducing the risk of infection through rinsing and disinfection, decreasing thermal energy spread, and proper suture selection.
Tension can be reduced by undermining (dissecting under the flaps and its surrounding area to allow tissue movement), but the possible detrimental consequences must be estimated. Undermining may compromise vascularization and increase dead space, which can lead to surgical complications.
3.2.2 Suture Material
Sutures can present different physical characteristics (elasticity, memory, knot security, tissue reactivity, visibility, etc.) that provide them different properties. Some of the most essential classifications are according to the suture configuration, monofilament, or multifilament (Table 12.2), and its absorption, absorbable or nonabsorbable (Table 12.3). In addition, the caliber of the suture is important, which is related to the strength of the suture [13]
The surgeon should choose the suture according to the tension of the wound. The smallest caliber suture that provides sufficient strength should be chosen, and if an absorbable suture is used, it has to lose strength comparable to the timing of wound strength recovery.
Finally, the alternative to sutures is stainless steel staples (Fig. 12.12). Although it is a time-saver closure method and there are some locations where they are recommended (for example hair-bearing scalp), we do not recommend it in C-section, episiotomy, or post-pregnancy surgeries. However, staples can be useful to position a skin closure temporarily before suturing. Some of the disadvantages of this skin closure technique are that they are inelastic, produce imprecise epidermal approximation, and pose the risk of skin marks if they are not removed early.
Important
When using staples, wound edges must be everted with forceps to prevent inverted skin edges.
3.2.3 Common Suture Techniques
The choice of closure technique should be based on wound and patient characteristics, but it should always be directed to the minimization of tension on the closure and meticulous closure with wound edge eversion.
Wound eversion is more likely to achieve a fine and flat scar than planar repair, which tends to result in a depressed scar. It is best achieved using deep dermal sutures.
Key Point
Deep dermal sutures relieve tension off the epidermis and produce skin edge eversion, whereas subcuticular sutures do not support much tension and their main function is approximating epidermis.
Suture techniques most used in C-section, episiotomy (Fig. 12.13), and post-maternity surgeries are the following:
-
Simple interrupted suture (Fig. 12.14): It is the most commonly employed suture. The suture must be placed at the same depth on each side of the incision; otherwise, the edges will overlap. Their main advantages are that it provides wound eversion and allows high-low correction and individual sutures may be removed without disturbing others. The disadvantages are the risk of skin marks, and it may increase closure time (in comparison with continuous sutures).
-
Simple continuous suture (Fig. 12.15): It is a time-saver closure technique, but it is not early as precise as interrupted sutures, there is a risk of leaving skin marks if they are not removed early, and integrity depends only on knots on either end.
-
Deep dermal suture (Fig. 12.16): They are essential to decrease wound tension. Sutures must also be placed at the same depth on each side of the incision.
-
Vertical mattress suture (Fig. 12.17): They are indicated in high-tension areas, and it helps in eversion of the skin edges. However, there is a risk of leaving skin marks if they are not removed early.
-
Horizontal mattress suture (Fig. 12.18): It has the same indications as vertical mattress suture, and it is used in tight situations when vertical mattress suture is not possible. In addition, although it may help in hemostasis, it increases tissue ischemia.
-
Subcuticular continuous suture (Fig. 12.19): This suture is used to approximate epidermis, but it is not able to support wound tension. Its principal benefit is that there is no risk of skin marks. It is usually the chosen technique for epidermis layer closure in elective surgeries.
-
Three-point-U suture (Fig. 12.20): This suture is especially important in “T” area at mastopexy (the point when vertical and horizontal scars join), but could also be necessary if the horizontal suprapubic scar of abdominoplasty has to be prolonged with a small vertical scar at midline. It approximates and relieves tension from the different flaps.
4 Postoperative Care: Scar Management and Prevention of Pathological Scarring
Although scars can never be completely removed, appropriate surgical technique and judicious postoperative care can minimize their appearance. With patients demanding less and less visible scars, prevention and improvement of scars should be one of the main goals in any surgical procedure.
Key Point
The best treatment of scars is prevention.
4.1 Scar Management
Prevention of pathological scarring should start as soon as surgery begins. Two important components of scar prevention, immediately after wound closure, are tension relief and taping [22]. There is a large list of surgical dressings that have been designed to provide coverage of sutured wounds. The most commonly used traditional dressing materials are gauze and adhesive dressings. However, in our opinion, there are dressings that provide greater benefits. Two dressings that should be highlighted are adhesive strips and paper tape (Fig. 12.21). Adhesive strips may be applied immediately after wound closure to provide wound coverage and relieve tension, but also after removal of the suture to help keep the wound edges approximated. Paper tape (Fig. 12.22) is a basic, hypoallergenic, breathable, and economic dressing, and additionally, it usually contains zinc oxide that contributes to supporting wound healing and preventing infection.
In high-tension wound or high-risk patients, there are some more sophisticated devices that can be used, such as negative pressure therapy (NPT) [23]. NPT dressings are typically applied in an operating room under sterile conditions. They ideally remain intact over the suture line for 5–7 days postoperatively. It can be beneficial in procedures such as abdominoplasty and breast reduction.
As soon as the suture is removed and the wound is completely epithelialized, the most basic principles are massage, moisturizing, and sun protection until the scar has matured. Studies have shown that, after wound healing, water still evaporates more rapidly through scar tissue and may take over a year to recover to pre-wound levels [22]. Moreover, avoiding exposure to sunlight and continued use of sunscreens with a high sun protection factor (>50 SPF) reduce the risk of hyperpigmentation of the scars. Finally, scar massage is an innocuous procedure that may be effective in decreasing pain and increasing a sense of well-being.
4.2 Prevention of Pathological Scarring
Basic procedures that are compulsory in postoperative care have already been described. However, many over-the-counter topical products and dressings have been marketed as tools to prevent scars or to improve their appearance or symptoms. Specialists should know their properties and indications in order to recommend them to their patients, according to the appearance of the scar and the risk of development of a pathological or unaesthetic scar.
Attention
Patient’s expectations should be discussed and managed prior to the initiation of any treatment.
4.2.1 Topical Products and Dressings
Silicone is universally considered as the first-line prophylactic and treatment option for hypertrophic scars and keloids [24]. It can be applied to scars in the form of sheets or gel. Gel can be preferred because of ease of application, and its use is compatible with other topical agents, for example sunscreen. It is a noninvasive and safe therapy, which may produce minimal side effects such as pruritus, contact dermatitis, or dry skin. Its effectiveness is believed to be based on occlusion and subsequent hydration of the scar tissue. Silicone therapy is recommended in high-risk scars or when patients are concerned about the outcome of their scars. The treatment should start once the wound is healed and at least for 3 months; however, if there is further scar maturation once that period of time has passed, it should be continued for as long as necessary.
Recent data has also shown that silicone sheeting can be used in combination with pressure therapy producing better improvements in hypertrophic scars than either therapy alone. The two treatments have complementary modes of action, with the silicone therapy acting on the erythema and pliability of the scar, whereas the pressure therapy prevents scar thickening. In this respect, some mechanomodulatory therapies have been developed to use on high-tension wounds, such us embrace Advanced Scar Therapy device® (Neodyne Biosciences, Inc., Menlo Park, Calif.) [25]. The device includes a simple disposable applicator that transfers a predetermined level of strain to a single-use adhesive silicone sheet, which is then adhered over the closed scar. This mechanism of action provides a uniform compressive strain, or a stress shield around a closed scar, which can minimize collagen proliferation and formation of scar tissue. This therapy has demonstrated to significantly reduce scarring following abdominoplasty surgery.
Steroid tape is recommended as a prophylactic treatment for patients at high risk of scar hypertrophy [26]. The application can start once the surgical wound is completely healed, approximately 1 month after epithelialization, and it should be changed every 24–48 h. Nevertheless, it is not an innocuous treatment, and it has to be strictly controlled to avoid overuse. In addition, it is important to cut the tape to the size and shape of the scar to decrease the risk of atrophy in the surrounding skin.
Other products described in the literature for the treatment of scars are onion extract and vitamin E [27]. However, though onion extract may have beneficial molecular effects on scars, its clinical efficacy in scar prevention and treatment has not been demonstrated. In the same way, the efficacy of vitamin E remains unproven.
4.2.2 Pressure Therapy
Pressure therapy, usually performed with pressure garments, bandages, or special devices for certain locations, has been a commonly used treatment modality for hypertrophic scars and keloids. Specifically, it is widely used for the prevention of pathological scarring in burn victims. However, its benefit has not been proved in uncomplicated surgical scar as a preventive treatment [28].
On the other hand, although its function is not specifically prevention of pathological scarring, specific pressure garments are recommended after abdominoplasty or liposuction to prevent some complications, such as seroma.
4.2.3 Light and Laser Therapies
Light and laser therapies are able to modify the different wound healing phases; therefore, they potentially affect scar formation and can be used both as a prophylactic measure or as a treatment in established scars [29].
Laser treatment is based on the principle of selective thermolysis. This means that the targeted tissue may be modified by chromophore absorption of laser light without significant thermal damage to surrounding normal tissues. The chromophores of skin used frequently as target are hemoglobin, melanin, and water.
The underlying mechanism for laser therapy as a prophylaxis for excessive scar formation is based on several facts. First, early use of vascular lasers produces scar tissue hypoxia, which leads to cell catabolism, prevention of abnormal collagen deposition, shortening of the duration of acute inflammatory response, and acceleration of scar maturation. There is not an established consensus regarding the timing of the treatment, the number of sessions, and the energy used. However, it is clear that it should be an early treatment, even starting on the day of suture removal.
In the last decades, the most common laser therapy used in the early treatment of scars has been pulsed dye laser (PDL). PDL laser selectively targets hemoglobin; therefore, it coagulates the microvasculature and decreases inflammation of neovascularized tissue [24]. The main effect is an improvement in erythema, although it may also improve texture and pliability and reduce scar volume. Wavelengths usually used to treat scars are 585 and 595 nm [30]. Common complications of PDL therapy include transient purpura, and mild-to-moderate erythema or edema that resolves in some days. More infrequent adverse effects are skin blistering or crusting, pigmentation disorders, or ulceration.
More recently, fractional non-ablative and ablative lasers have also been introduced as prophylactic and therapeutic treatment for scars. They are able to influence and modify the development, deposition, quality, and distribution of collagen, aiming toward that found in normal skin. Some of the most common adverse effects found in ablative laser treatment include prolonged erythema, delayed wound healing, ulceration, and post-inflammatory hyperpigmentation. Hypopigmentation, pain, or herpes simplex virus infection are infrequent side effects. Most common fractional ablative lasers used in the treatment of scars are carbon dioxide (CO2) laser (10,600 nm) and erbium:yttrium aluminum garnet (Er-YAG) laser (2940 nm). In relation to fractional non-ablative lasers, some of the most used are neodymium:yttrium aluminum garnet (Nd: YAG) laser (1064 nm, 1320 nm, 1340 nm, …) and diode laser (1455 nm) [31, 32].
Important
According to the wavelength, the same type of laser can be used as ablative or non-ablative treatment.
Finally, light devices can also be used as a prophylactic treatment for scars, including intense pulsed light (IPL), light-emitting diodes (LEDs), or photodynamic therapy (PDT). Most frequent light therapy used is IPL [32, 33]. (remove 36). This light differs from the laser because it is polychromatic, which means that the emitted radiation includes multiple wavelengths (ranging from 400 to 1800 nm), so it can act over different chromophores. IPL treatment may improve scar erythema, texture, and overall appearance of scars. It is a safe therapy, which has not been associated to long-term side effects. However, its learning curve is longer and is very user dependent.
4.2.4 New Treatments
As in other areas of medicine, nowadays research is focused on molecular therapies. Although some molecules have shown promising results in preclinical and clinical trials in animals, such as TGF-β3, human clinical trials have failed to produce effective results. In the same way, some preclinical experiments have demonstrated the therapeutic potential of mesenchymal stem cell-conditioned medium (MSC-CM) in wound healing and inhibition of hypertrophic scar formation [4, 20].
Important
Generally, all scars should be re-evaluated 4–8 weeks after surgery to determine whether additional scar management interventions are required or whether preventive therapy can be finished.
5 Treatment of Pathological Scarring
Hypertrophic and keloid scars can significantly affect the patient’s quality of life due to the appearance, the functional alterations, or the symptoms associated. However, these two scarring disorders are not the only cause of patient’s complaints. Specialists should know how to treat some other minor alterations, such as pigmentation disorders, widened scars, or depressed scars.
Therefore, the goals when scars are treated may include one or more of the following:
-
Relief of symptoms
-
Reduction of the scar volume
-
Functional improvement
-
Cosmetic improvement
Nowadays, most current clinical treatment strategies continue to focus on decreasing inflammatory processes. However, recent research suggests that molecular and cellular approaches may be promising scar therapies. Further research into genetics, epigenetics, and mechanobiology is needed, in order to find more effective prophylactic and clinical treatments.
5.1 Hypertrophic Scars
Silicone is universally considered as the first-line treatment option for hypertrophic scars, whereas pressure therapy may be an alternative first-line treatment. Pressure therapy can also provide benefits as symptomatic treatment, such as alleviation of edema, itchiness, and pain [34].
Second-line therapies include intralesional injections, light and laser therapies, botulinum toxin, and surgical excision [35]. Usually, silicone or pressure therapy is used as adjunctive therapies to second-line treatments. Steroid tape can also be applied as adjunctive therapy.
5.1.1 Intralesional Injections
Most frequent injected substances are corticosteroids and 5-fluorouracil.
-
Corticosteroids: They are the most frequently used drugs in hypertrophic scars (Fig. 12.23). The corticosteroid most commonly used is triamcinolone acetonide 10e40mg/mL. It should be injected into the papillary dermis every 2–4 weeks until the scar is flattened. Corticosteroids decrease collagen synthesis and limit fibroblast proliferation. Common adverse effects are subcutaneous atrophy, telangiectasias, and pigment changes [24].
-
5-Fluorouracil (5-FU): 5-FU is an antimetabolite that inhibits fibroblast proliferation. It is usually used when the treatment with corticosteroids is insufficient, as monotherapy or in combination with them. Some studies have proved that the combination of 5-fluorouracil and triamcinolone acetonide may be more effective in treating scars than the individual treatments. Some of the possible adverse effects are wound ulceration, hyperpigmentation, and pain [36].
Some more infrequent substances, usually reserved for resistant hypertrophic scars, are bleomycin and verapamil. Bleomycin is an antitumor agent that induces cell apoptosis and reduces fibroblast activity, whereas verapamil is a calcium channel antagonist that decreases collagen synthesis and increases collagen breakdown.
5.1.2 Light and Laser Therapies
Laser and light therapies are also interesting therapeutic tools used in the treatment of linear hypertrophic surgical scars. However, it is important to adapt the device to the stage of scar maturation.
PDL is more effective in immature hypertrophic scars (usually erythematous and younger than 1 year), because it only penetrates 1 mm and thick hypertrophic scars may not respond. The application of PDL in the early stages of the hypertrophic scars may decrease inflammation, erythema, and pruritus. Frequently, the treatment with PDL is supplemented with intralesional injections of corticosteroids, combined or not with intralesional 5-FU [37]. Immature hypertrophic scars refractory to PDL can be treated with fractional laser therapies. The combination of lasers, indeed, may also function in a synergistic manner. Firstly, PDL can be applied to reduce vascularization; later, fractional CO2 laser can be used to prevent the scar’s continued growth as this laser deals primarily with inhibiting proliferating fibroblasts and the deposition of abnormal collagen.
Moreover, immature scars can also be treated with IPL. Its application may reduce height and erythema and produces an overall clinical improvement in the appearance of hypertrophic scars. The treatment with IPL is also supplemented with intralesional injections of corticosteroids, combined or not with intralesional 5-FU.
In matured hypertrophic scars, fractional ablative lasers are frequently used to remodel irregular scar contour and improve stiffness and pliability. In addition to the effect of decreasing and remodeling of the scars, fractional ablative systems have allowed what is called the “laser-assisted drug delivery.” These lasers open channels through the epidermis, which can be used as a system of direct communication with the dermis. Therefore, when ablative lasers are used, it is recommended to apply topical corticosteroids (combined or not with intralesional 5-FU) instead of intralesional injection [38].
5.1.3 Botulinum Toxin
Botulinum toxin type A is a potent neurotoxin, derived from Clostridium botulinum, which indirectly blocks neuromuscular transmission. Therefore, it can be used as a prophylactic treatment for hypertrophic scars, by preventing muscle and skin contraction during wound healing. Moreover, it is also a therapeutic tool in the management of hypertrophic scars and keloids, because it has been demonstrated that the administration of botulinum toxin type A inhibits the proliferation of fibroblasts. And finally, it can be used to treat symptoms associated with scars, such as pruritus and pain [37,38,41].
5.1.4 Surgical Excision
If the patient develops a permanent hypertrophic scar (approximately 1 year), surgical scar revision may be considered. Aesthetic correction of linear hypertrophic scars is usually done by simple resection and primary closure, being necessary sometimes to apply some type of tension-releasing technique. After that, postoperative care will be essential to prevent the recurrence of a new hypertrophic scar.0
5.2 Keloids
Many treatments, such as steroid injections, topical dressings (silicone, steroids, …), pressure therapy, or laser treatment, have been shown to be successful treatment modalities for keloids. The problem is that they have high rate of recurrence rates when they are used as monotherapy, so the basis of the treatment of keloids lies in the combination therapy [42]. Furthermore, keloids manifest themselves in a wide variety of forms and etiologies and thus require tailored therapies.
First-line therapy could be intralesional corticosteroids, which can be combined with intralesional 5-FU and other adjunctive therapies (for example occlusion dressings and pressure therapy). Ablative laser could also be used in keloids, but it must be combined with intralesional injections of corticosteroids or 5-FU because laser-induced injury can often start the process of keloid recurrence [43].
Second-line therapy could be surgical excision, which must always be combined with post-excision adjuvant therapies. In addition to those previously commented, more specific adjuvant therapies that are reserved for aggressive keloids are the following:
-
Radiation therapy: The effectiveness of post-excision radiation therapy in reducing keloid size has been demonstrated by multiple studies [44, 45]. Radiation inhibits new vessel formation and proliferation of fibroblasts, which results in decreased collagen production. The most commonly used forms of radiation therapy for keloids are brachytherapy and electron beam radiation. Although radiation therapy can be associated with some adverse effects, such as carcinogenesis, it is regarded as a safe option when carefully applied in selected patients. In fact, it has been applied immediately after cesarean section in postpartum patients, with confirmed keloids resulting from previous C-sections, with good cosmetic results [46].
-
Cryotherapy: It produces cellular injury and necrosis of the keloidal tissue. It can be administered as spray or intralesional. Some adverse effects may be hypopigmentation, hyperpigmentation, blisters, and local pain.
Other emergent therapies, such as interferon (IFN), imiquimod, bleomycin [47], TGF-β, or tacrolimus, still need further studies to evaluate their role in the prevention and treatment of keloid scars [48].
Attention
All patients with keloids should be aware of the risks of recurrence with procedures. Nevertheless, the best treatment is prevention, avoiding nonessential surgeries or procedures.
5.3 Other Unaesthetic Scars
-
Depressed scars: Treatment consists of releasing the scar from the underlying tissues (subcision) and subsequently filling with fat grafting or other filler substances (for example hyaluronic acid). Moreover, neoangiogenesis induced by adipose-derived stem cells contained within the fat graft may play a role in scar improvement [49, 50].
-
Widened scars: The best treatment is debridement of the scar and direct closure. Scar revisions can be performed about 1 year after initial surgery, when the final result of the scar is usually visible and there would be less tension in the surrounding tissues (Fig. 12.24).
-
Pigmented scars: Hyperpigmentation can be treated essentially with depigmented creams (hydroquinone, kojic acid, retinoic acid, topical steroids, azelaic acid, tranexamic acid, …), laser therapies, or IPL. Topical hydroquinone, 2–5% concentration, is regarded as the gold standard treatment for hyperpigmentation in the last 50 years. Some of the adverse effects of hydroquinone are rebound hyperpigmentation, photosensitivity or phototoxicity, tolerance, resistance, and exogenous ochronosis. Furthermore, some creams based on the combination of depigmented agents, such as Kligman cream or modified Kligman cream, can lead to good results too.
-
Hypopigmented scars: Hypopigmentation is generally treated with non-ablative fractional lasers with positive outcomes, although good results in treatments with ablative fractional lasers have been proved too [51]. In addition, micropigmentation is also a good therapeutic tool to treat hypopigmentation.
Finally, some minor alterations may also be treated with laser too. For example, telangiectasias can be treated with any laser or light source that targets hemoglobin (PDL, Nd: YAG, IPL, …), whereas some small textural alterations are mostly treated with fractional ablative lasers.
Key Point
Treatment combinations are typically the best option when treating hypertrophic scars and keloids.
6 Treatment of Wound Healing Complications
During the early postoperative period, wounds are susceptible to suffer some complications, such as necrosis of surrounding tissues, dehiscence, or surgical site infection.
Not all surgeries commented in this chapter have the same risk of developing wound healing complications [52, 53]. While minimal liposuction scars rarely suffer any complication, mastopexy and abdominal surgery scars are more prone to necrosis and dehiscence, in most cases because of excessive tension. If surgical technique and postoperative care are adequate, surgical wound infections are rare in these procedures.
Attention
If a wound suffers a dehiscence without an excess of tension or another known cause, infection should always be considered.
In mastopexy, wound healing complications are more frequent in inverted T-procedures. T-zone is the most vulnerable region because of excess of tension, and it may suffer necrosis and dehiscence (Fig. 12.25). Another complication associated with mastopexy is necrosis of skin flaps. A proper surgical technique is essential to prevent this complication, as well as making sure to respect the subdermal plexus. And finally, another possible complication is partial or total necrosis of nipple-areola complex (Fig. 12.26).
Likewise, horizontal scar at abdominoplasty is also susceptible to skin necrosis and dehiscence, especially in midline because it is the area that supports most tension (Fig. 12.27). On the contrary, this kind of complications are infrequent at cesarean delivery, in which surgical site infection is more common. Unscheduled cesarean delivery is a major risk factor for wound infection.
Finally, episiotomy is the surgery with the highest risk of infection of all these commented. The area is heavily colonized by bacteria and frequently is contaminated by stool during the delivery process. However, infection is unusual because of the patient’s immune system action. Furthermore, to prevent infection, obstetricians should insist on irrigation of the treated area. If infection does occur, the risk of necrosis and dehiscence increases.
6.1 Treatment of Skin Necrosis
Treatment should be based on the debridement of wound edges, the debridement of damaged or necrotic tissue from wound bed if it is present, and healing by first or second intention. The risk of a first wound healing is that if the cause of necrosis (frequently excessive tension) is still present, the wound can suffer some tissue necrosis again. On the other hand, some disadvantages for second healing are that it is a longer process, it may worsen the esthetic result, and it increases the risk of infection.
One special case is the reconstruction of nipple-areola complex after a necrosis. Although second intention healing is possible (Fig. 12.28), especially in partial necrosis, reconstruction is often performed using more sophisticated techniques, such as grafts and flaps.
Tip
Micropigmentation is a good treatment to conceal scars and pigmentation alterations after surgeries that involve nipple-areola complex.
6.1.1 Debridement
Debridement consists of removing necrotic, damaged, or infected tissue to improve the healing potential of the remaining healthy tissue. The presence of necrotic tissue obstructs the new tissue growth and serves as a nidus for bacterial proliferation.
Debridement may be sharp, surgical, mechanical, enzymatic (Fig. 12.29), autolytic, or biological (larval). In this type of surgical acute wounds, the chosen techniques are usually sharp or surgical debridement. Nevertheless, autolytic debridement, through enzymatic debridement agents (collagenase, trypsin, …), may also be applied to remove fibrin and sloughs from the wound bed during the process of second healing [54, 55].
Tip
If autolytic debridement is indicated, a moisture-retentive dressing should be selected because it will become a high exudate wound. In addition, it is important to protect peri-wound skin to avoid maceration.
6.1.2 Principles of Second Healing
Successful wound healing is most likely to be achieved if the underlying cause of failure to heal is identified and treated. An easy way of understanding and treating wounds by second healing is using the TIME acronym. The TIME acronym, developed by a group of wound care experts, was first published in 2003 [56, 57]. It is a practical guide to wound management that relates clinical observations and interventions to the underlying wound pathology in each of the four areas:
-
T for tissue: nonviable or deficient
-
I for infection/inflammation
-
M for moisture imbalance
-
E for edge of wound, nonadvancing or undermined
Therefore, according to TIME acronym, firstly it is necessary to remove all damaged or necrotic tissue and foreign bodies on the surface of the wound. Debridement is the quickest and most efficient method of removing these materials. The second point refers to control infection and management of inappropriate inflammation unrelated to infection. Infection should be prevented or, in case it is present, treated. The best way to avoid infection is wound cleansing and shielding the wound from bacterial invasion. Thirdly, the wound should not be desiccate, neither can have excess of exudate. The relevance of moisture balance has led to the development and use of a wide range of dressings [7]. A draining wound requires a dressing with the ability to absorb moisture and protect the surrounding wound from maceration, such as hydrofibers, alginates (Figs. 12.30 and 12.31), or foam dressings (Fig. 12.32). A nondraining wound requires a dressing that provides moisture or prevents evaporative fluid loss, such as hydrogels or hydrocolloids (Fig. 12.33). Even in highly exuding wounds, negative-pressure wound therapy can be applied. NPWT provides a closed moist wound healing environment and regulates the quantity of exudate. Finally, it is advisable to use creams or dressings that facilitate the process of healing and epithelialization, but also that protect the surrounding skin. Dressings should be adapted to the wound bed, and the progress of the wound changes over time. During the phase of epithelialization, wounds require a dressing that will protect from trauma and promote a moist environment, for example films of polyurethane (Fig. 12.34).
Many studies have verified the fact that a moisturized environment promotes wound healing better than a dry one. A moist wound environment facilitates all three phases of wound healing by trapping endogenously produced enzymes to facilitate autolytic debridement, preserve endogenously produced growth factors, and reduce patient pain complaints. Furthermore, a moist wound usually results in a more cosmetically appealing scar.
Principles at second healing:
-
T (tissue) → Early excision of nonviable tissue
-
I (infection) → Microbial control
-
M (moisture) → Control exudate/avoid desiccation
-
E (edge of wound) → Advanced wound healing techniques
There are no fixed principles in choosing the dressing material. Dressings should be selected based on the information obtained from the wound examination, preferences of the patient or medical team, economic impact, frequency of dressing changes, location, etc [55]. Some characteristics of the ideal wound dressing would be the following:
-
Provide a barrier to microorganisms.
-
Create a moist wound environment.
-
Manage quantity of exudate.
-
Provide adequate gas exchange.
-
Provide thermal insulation.
-
Protect exposed nerves (decrease pain).
-
Eliminate dead space.
-
Remove debris, necrotic tissue, and foreign material.
-
Allow dressing changes painlessly and atraumatically.
-
Protect peri-wound skin.
6.2 Treatment of Dehiscence
Treatment should be based on the debridement of wound edges, and the debridement of s damaged or necrotic tissue from wound bed if it is present, and healing by first or second intention. As in skin necrosis, the chosen technique in this kind of acute wounds after surgery is usually sharp or surgical debridement and primary closure with sutures, although it depends on the preferences of the patient and the medical team. If the area of dehiscence is reduced, second healing (following the same principles as seen above) is also a good option.
6.3 Treatment of Infection
Skin is naturally colonized by many microorganisms, which are named skin microbiota. Most are nonpathogenic microorganisms; some of them are commensal (meaning they coexist without harming their host) and some are mutualistic (meaning they coexist and also offer a benefit to their host). They are found principally in the superficial layers of the epidermis and the upper parts of hair follicles.
Attention
Contamination (microorganisms are present, but do not result in signs or symptoms) and infection (there are signs or symptoms) are not the same.
When there is a wound, skin integrity is lost and microorganisms are allowed to enter the human body, where they can proliferate and grow at the expense of damaged or necrotic tissue.
Risk factors for developing a surgical wound infection are the following:
-
Patient factors: smoking, aging, obesity, diabetes, weakened immune system, chronic steroid use, malnutrition, …
-
Local factors: necrotic tissue, bad perfusion, foreign bodies, hematomas, dead space, …
-
Surgery factors: emergency surgeries, long period of surgery (>2 h), …
The basic treatment for surgical wound infection can be summarized in a three-step process [55, 58].
-
1.
Irrigation: It is important for diminishing the bacterial load and removing loose material. Open wounds should be irrigated on initial examination and with each dressing change. The most frequently used irrigation solution is normal saline. However, it has been proved that potable tap is as safe as sterile water or saline. Nonetheless, tap water must be used with caution in immunosuppressed patients, particularly if the water might be non-potable. Other cytotoxic antiseptic irrigation solutions (dilute iodine, chlorhexidine, hydrogen peroxide, …) are also frequently used, although the evidence of benefits for their use is weak and may have negative influence on tissue regeneration due to toxicity to host cells.
-
2.
Debridement: All damaged and died tissues should be removed, as well as purulent collections. Debridement plays a vital role in the management of wound infections. Debridement of necrotic tissue and exudate helps to reduce wound bioburden and may also increase the effectiveness of topical antimicrobials.
-
3.
Topical antimicrobial treatment: The term antimicrobial is used broadly to describe disinfectants, antiseptics, and antibiotics. It can be applied in the form of impregnated dressings or irrigants. Most frequently used antimicrobials are silver, iodine, and polyhexamethylene biguanide, while some antibiotics often used in infected surgical wounds are silver sulfadiazine, nitrofurazone, or mupirocin. Infected wounds should not be occluded and should be rebandaged at least once a day. Antimicrobial therapy is not indicated for all wounds and should be reserved for the following:
-
Prevention of infection in patients who are considered to be at an increased risk
-
Treatment of localized wound with clinical signs of infection
-
Local treatment of wound infection in cases of local spreading or systemic wound infection, in conjunction with systemic antibiotics
-
Systemic antimicrobial treatment would only be needed if there are systemic symptoms, for example fever.
Tip
Infection signs are similar to the cardinal signs of inflammation (redness, heat, swelling, and pain), but these signs are typically excessive or disproportionate to the size and extent of the wound. Some other signs of infection are delayed healing, presence of pus, or foul smell.
7 Conclusion
Management of scars is a therapeutic challenge. The progressive knowledge of the wound healing process and the physiological events that take place in each type of scar have allowed the development of new treatments for hypertrophic scars and keloids, as well as the development of multiple techniques for minimizing scar formation and correcting unaesthetic scars. Nevertheless, there is still much to know and to advance in this field of research, and we hope that the dream of being able to erase the scars will become true in the future.
References
Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia: Elsevier-Saunders; 2012.
Griffiths CE, Barker J, Bleier T, Chalmers R, Creamer D. Rook’s textbook of dermatology. 9th ed. Chichester: Wiley Blackwell; 2016.
Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, Wearda T, Muhonen E, McArdle A, Tevlin R, Atashroo DA, Senarath-Yapa K, Peter Lorenz H, Gurtner GC, Longaker MT. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg. 2015;135:907.
Janis J, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2014;133(2):199e–207e.
Limbert G. Skin biophysics. from experimental characterisation to advanced modelling. Switzerland: Springer; 2019.
Baranoski S, Ayello EA. Wound care essentials: practice principles. 4th ed. Philadelphia: Wolters Kluwer; 2016.
Han SK. Innovations and advances in wound healing. 2nd ed. Berlin: Springer; 2016.
Armstrong DG, Meyr AJ. Basic principles of wound healing. In: Post TW, editor. UpToDate. Waltham: UpToDate Inc.. https://www.uptodate.com. Accessed 5 Nov 2019.
Armstrong DG, Meyr AJ. Risk factors for impaired wound healing and wound complications. In: Post TW, editor. UpToDate. Waltham: UpToDate Inc.. https://www.uptodate.com. Accessed 7 Nov 2019.
Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069–79.
Reddy M. Skin and wound care: important considerations in the older adult. Adv Skin Wound Care. 2008;21(9):424–36.
Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22.
Janis JE. Essentials of plastic surgery. 2nd ed. Boca Raton: Taylor & Francis Group; 2014.
Wang J, Boerma M, Fu Q, Hauer-Jensen M. Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia. 2006;10(6):502–6.
Brown JJ, Bayat A. Genetic susceptibility to raised dermal scarring. Br J Dermatol. 2009;161(1):8–18.
Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43:S3–S18.
Hassan S, Reynolds G, Clarkson J, Brooks P. Challenging the dogma: relationship between time to healing and formation of hypertrophic scars after burn injuries. J Burn Care Res. 2014;35:e118–24.
Thorne CH. Grabb and Smith’s plastic surgery. 7th ed. Philadelphia: Wolters and Kluwers; 2014.
Tsai CH, Ogawa R. Keloid research: current status and future directions. Scars Burn Heal. 2019;5:1–8.
Rohrer TE, Cook JL, Kaufman AJ. Flaps and grafts in dermatology surgery. 2nd ed. Philadelphia: Elsevier; 2018.
Thomaidis VK. Cutaneous flaps in head and neck reconstruction: from anatomy to surgery. Berlin: Springer; 2014.
Monstrey S, Middelkoop E, Vranckx JJ, Bassetto F, Ziegler UE, Meaume S, Téot L. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67(8):1017–25.
Singh D, Lobach V, Holton T. Use of closed-incision negative-pressure therapy in aesthetic surgery. Plast Reconstr Surg. 2019;143:11S–4S.
Caviggioli F, Vinci V, Vappiani M, Lisa A, Maione L, Klinger M. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2017;139(6):1371e–3e.
Longaker MT, Rohrich RJ, Greenberg L, et al. A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg. 2014;134:536–46.
Goutos I, Owaga R. Steroid tape: a promising adjunct to scar management. Scars Burn Heal. 2017;3:1–9.
Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, Murcia C. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825–31.
Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, Murcia C. Updated international clinical recommendations on scar management: part 1—evaluating the evidence. Dermatol Surg. 2014;40(8):817–24.
Karmisholt KE, Haerskjold A, Karlsmark T, Waibel J, Paasch U, Haedersdal M. Early laser intervention to reduce scar formation—a systematic review. J Eur Acad Dermatol Venereol. 2018;32(7):1099–110.
Alster TS. Improvement of erythematous and hypertrophic scars by the 585-nm flashlamp-pumped pulsed dye laser. Ann Plast Surg. 1994;32:186.
Jin R, Huang X, Li H, Yuan Y, Li B, Cheng C, Li Q. Laser therapy for prevention and treatment of pathologic excessive scars. Plast Reconstr Surg. 2013;132:1747–58.
Vrijman C, van Drooge AM, Limpens J, Bos JD, van der Veen JP, Spuls PI, Wolkerstorfer A. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165(5):934–42.
Erol OO, Gurlek A, Agaoglu G, Topcuoglu E, Oz H. Treatment of hypertrophic scars and keloids using intense pulsed light (IPL). Aesthet Plast Surg. 2008;32(6):902–9.
Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch. 2016;83:46–53.
Ouyang HW, Li GF, Lei Y, Gold MH, Tan J. Comparison of the effectiveness of pulsed dye laser vs pulsed dye laser combined with ultrapulse fractional CO2 laser in the treatment of immature red hypertrophic scars. J Cosmet Dermatol. 2018;17(1):54–60.
Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment: a systematic review. Acta Derm Venereol. 2015;95:778–82.
Cavalié M, Sillard L, Montaudié H, Bahadoran P, Lacour JP, Passeron T. Treatment of keloids with laser-assisted topical steroid delivery: a retrospective study of 23 cases. Dermatol Ther. 2015;28:74–8.
Hædersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Rox Anderson R. Fractional CO2 laser-assisted drug delivery. Lasers Surg Med. 2010;42:113–22.
Jeong HS, Lee BH, Sung HM, Park SY, Ahn DK, Jung MS, Suh IS. Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg. 2015;136(2):171e–8e.
Hu L, Zou Y, Chang SJ, Qiu Y, Chen H, Gang M, Jin Y, Lin X. Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg. 2018;141(3):646–50.
Zhibo X, Miaobo Z. Intralesional botulinum toxin type A injection as a new treatment measure for keloids. Plast Reconstr Surg. 2009;124:275e–7e.
Siotos C, Uzosike AC, Hong H, Seal SM, Rosson GD, Cooney CM, Cooney DS. Keloid excision and adjuvant treatments. A network meta-analysis. Ann Plast Surg. 2019;83(2):154–62.
Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S–28S.
Van Leeuwen MC, Stokmans SC, Bulstra AE, Meijer OW, Heymans MW, Ket JC, Ritt MJ, van Leeuwen PA, Niessen FB. Surgical excision with adjuvant irradiation for treatment of keloid scars: a systematic review. Plast Reconstr Surg Glob Open. 2015;3(7):e440.
Ji J, Tian Y, Zhu YQ, Zhang LY, Ji SJ, Huan J, Zhou XZ, Cao JP. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol. 2015;42(1):56–63.
Kim J, Lee SH. Therapeutic results and safety of postoperative radiotherapy for keloid after repeated Cesarean section in immediate postpartum period. Radiat Oncol J. 2012;30(2):49–52.
Perez JL, Rohrich RJ. Optimizing postsurgical scars: a systematic review on best practices in preventative scar management. Plast Reconstr Surg. 2017;140(6):782e–93e.
Janis J, Harrison B. Wound healing: part II. Clinical applications. Plast Reconstr Surg. 2014;133(3):383e–92e.
Ciancio F, Parisi D, Innocenti A, Portincasa A. Effectiveness of autologous fat grafting in adherent scars: results obtained by a comprehensive scar evaluation protocol. Plast Reconstr Surg. 2017;40(2):355e–6e. Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S–120S.
Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409–22.
Glaich AS, Rahman Z, Goldberg LH, Friedman PM. Fractional resurfacing for the treatment of hypopigmented scars: a pilot study. Dermatol Surg. 2007;33:289–94.
Carter EB, Temming LA, Fowler S, Eppes C, Gross G, Srinivas SK, Macones GA, Colditz GA, Tuuli MG. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–46.
Sarsam SE, Elliott JP, Lam GK. Management of wound complications from cesarean delivery. Obstet Gynecol Surv. 2005;60(7):462–73.
Smith F, Dryburgh N, Donaldson J, Mitchell M. Debridement for surgical wounds. Cochrane Database Syst Rev. 2011;(5):CD006214.
Armstrong DG, Meyr AJ. Basic principles of wound management. In: Post TW, editor. UpToDate. Waltham: UpToDate Inc.. https://www.uptodate.com. Accessed 7 Nov 2019.
Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J. 2004;1(1):19–32.
Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J. 2012;9:1–19.
Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11:1–28.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Estiragues, M., Morillo, E., Sarrasqueta, C., Olivas-Menayo, J. (2023). Wound Care and Treatment of Scars. In: Gomes-Ferreira, M., Olivas-Menayo, J. (eds) Post-maternity Body Changes. Springer, Cham. https://doi.org/10.1007/978-3-030-43840-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-43840-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43839-5
Online ISBN: 978-3-030-43840-1
eBook Packages: MedicineMedicine (R0)