Abstract
Hypertrophic scars and keloids can have significant detrimental effects on patients both psychosocially and functionally. A careful identification of patient risk factors and a comprehensive management plan are necessary to optimize outcomes. Patients with a history of dystrophic scarring should avoid unnecessary procedures and enhance the wound-healing process using various preventive strategies. As there is no single, fully efficacious treatment modality, prevention remains the best approach in reducing aberrant scar formation. When prevention therapies fail, keloids have been shown to be respond to a variety of therapies including topical and injectable corticosteroids, 5-fluorouracil, radiotherapy, lasers, and surgical excision, all with varying efficacies. As such, management should be tailored to the individual patient’s risk factors with the use of combination therapies to reduce recurrence rates. Still, keloid and hypertrophic scar therapies are widely diverse with novel treatment modalities providing alternatives for recurring lesions. Laser-assisted drug delivery, skin priming, and novel topical therapies may provide alternative options for the management of hypertrophic scars and keloids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Identification of the risk factors for the development of hypertrophic scars and keloids is essential to implement the most appropriate preventive and therapeutic strategies. |
Optimization of wound healing and prevention remain the most effective methods to avoid aberrant scar formation. |
Established treatment modalities generally combine the use of excision, laser, or injectables with topical products to optimize wound healing and decrease the recurrence of keloid formation. |
1 Introduction
Pathologic scars, classified as either hypertrophic or keloid, can have significant impacts on patient quality of life through both cosmetic and functional impairments. Surgery, trauma, burns, or inflammatory wounds are typical causes of deep cutaneous injury that can result in the formation of pathologic scars. The molecular mechanism of pathologic scar formation is still not well established but involves dysregulation of myofibroblasts and formation of a thick, highly vascularized dermis marked by an abundance of immature collagen [1, 2].
Despite the wide array of available treatment options, treatment of hypertrophic scars and keloids continues to challenge physicians as no single treatment is currently recommended for all lesions and no therapy is fully efficacious [3]. Treatment therefore often involves a combination of multiple treatment modalities with consideration needed for many features, including patient risk factors, scar properties, and anatomical location.
The goal of care is to provide patients optimal function and appearance, with a decreased risk of recurrence. In this review, we provide a comprehensive overview of the established treatments for hypertrophic scars and keloids as well as recent developments in therapeutic options, including new laser technologies, intralesional therapies, and novel prophylactic methods such as priming the skin prior to surgery.
1.1 Literature Search
A broad literature review was performed in the PubMed and Web of Science databases without a language and publishing time restriction in the areas of keloid and hypertrophic scar management. Two reviewers (FSF and RU) determined the eligibility of the studies and performed a quality assessment based on individual study content. Both reviewers agreed with the study quality and content before inclusion within this article. The following inclusion criteria were applied: (1) studies must be available in English and (2) studies must discuss keloid or hypertrophic scar management. We included retrospective studies, systematic reviews, meta-analyses, randomized controlled trials, quality-improvement studies, and guideline reviews.
2 Understanding Hypertrophic Scars and Keloids
2.1 Scar Formation
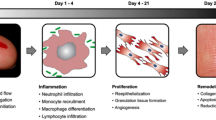
The classic model of wound healing is defined by three inter-related phases: inflammatory, proliferative, and remodeling. The initial inflammatory phase begins immediately following tissue injury and typically lasts 2–3 days with the formation of platelet plugs and the fibrin matrix to prevent further blood loss [4]. The immune system is also activated in this initial phase along with the inflammatory response to start the removal of dead tissue.
The proliferative phase begins with angiogenesis, the creation of new blood vessels and capillaries, to deliver oxygen and nutrients essential for the proliferation of fibroblasts and other cells [4]. Activated fibroblasts and macrophages aid in the replacement of the fibrin matrix with granulation tissue, the key marker of this phase. Proliferation may last up to 6 weeks and later involves the differentiation of fibroblasts into myofibroblasts that produce the extracellular matrix [5], mainly collagen, that will form the eventual scar [6].
The final phase, remodeling, is responsible for scar formation and is essential to understanding the formation of hypertrophic and keloid scars and their treatments. Remodeling involves interactions of proteolytic enzymes, mainly matrix metalloproteinases and their inhibitors. This phase typically lasts more than a year [6]. The goal is to degrade excess extracellular matrix and convert immature collagen type III to mature collagen type I. An imbalance between collagen production and degradation, specifically excess inflammatory mediators leading to enhanced fibroblast proliferation and differentiation into myofibroblasts, is proposed as the mechanism for pathologic scar formation [7].
Further studies are still needed to fully elucidate the molecular mechanisms of pathologic scars and represent a limitation to current treatment methods. Proteins such as decorin, a proteoglycan found in dermal connective tissue that neutralizes the stimulatory effects of transforming growth factor-β (TGF-β) on collagen and fibronectin synthesis, have been found to be decreased in keloids and hypertrophic scars and may be of therapeutic significance [8,9,10]. Excessive angiogenesis is another component of the pathophysiology of these scar types that furthers inflammation and is targeted in many available treatment options [11].
2.2 Diagnosis of Hypertrophic Scars and Keloids
There are several established differences between hypertrophic scars and keloids. Keloids are characterized by horizontal growth extending beyond the lateral boundaries of the initial wound and may grow for many years and spread aggressively, with a higher chance of recurrence [11, 12]. Meanwhile, hypertrophic scars tend to regress within 1 year and remain confined to the initial wound margins [1]. Keloids may appear years after the initial trauma or even spontaneously [13], whereas hypertrophic scars typically present within 4–8 weeks of wounding [14]. Histological differences have also been established. Mainly, the presence of thick, disorganized, hyalinized type I and III collagen bundles in keloids versus fine, well-organized, wavy type III collagen bundles accompanied by myofibroblast nodules in hypertrophic scars [14].
Despite the established differences, it can be difficult to distinguish between hypertrophic scars and keloids both clinically and histologically as many scars can bear overlapping features [11]. This has sparked continued debate over whether these are in fact separate entities [11, 15].
2.3 Risk Factors
Certain factors have been identified that may lead to a higher likelihood of developing pathologic scarring. Areas of cutaneous tension such as the anterior chest, shoulder, and across joints are considered high risk for the development of both hypertrophic scars and keloids. A study on the distribution of 1500 keloids in Japanese patients found 48.9% and 26.9% located on the anterior chest and scapular regions, respectively [16]. Meanwhile, none was reported on the scalp or anterior lower leg, which are areas of low stretching tension [16]. Stretching is believed to increase the likelihood of pathologic scar formation via worsening inflammation and prolonging wound healing [17]. As such, patients undergoing surgery on these areas should be monitored for 3–12 months [11]. Other anatomical locations with a higher propensity for keloid formation include the ear lobes and pelvic area. Patient-specific risk factors include age and genetics. Ages 11–30 years are at a higher risk for the formation of pathologic scars owing to the greater inflammatory response in the skin of younger adults compared with their older counterparts [3]. The increase of hormones associated with puberty is another potential influence for the increased risk of keloids in younger individuals, which will begin to wane in the 30s and 40s [18]. Pregnant women are also shown to be at an increased risk because of the similar hormonal influence of pregnancy estrogens [19, 20]. Genetics has been established as a risk factor for keloids only, with predisposition inherited in an autosomal dominant transmission and an estimation of 5–10% being familial cases [3]. It is recommended when considering keloid treatment to first assess scar number and size, as multiple or larger scars should lead to consideration of genetic and systemic factors [11]. Darker phototypes can be considered a risk factor for the formation of hypertrophic scars, and individuals of African and Asian ancestry are known to be particularly susceptible to keloid scarring, with genetic studies having identified chromosome variants associated with keloid formation in these populations [3, 21,22,23,24]. A three to seven times increase in keloid incidence has been seen in black patients versus white patients post-surgery [25, 26]. More recently, atopic dermatitis (AD) has been indicated as an independent risk factor for keloid development, along with a rising debate over dupilumab as an emerging therapy [27,28,29].
2.4 Epidemiology
The prevalence of hypertrophic scars is much higher than that of keloids, with estimated rates that range from 40 to 70% post-surgery and up to 91% following burn injuries [14]. Incidence rates of keloids vary across limited studies, with the highest incidence reported for patients of African descent (16% reported in Zaire) and the lowest rates seen in white individuals (0.09% in England) [30,31,32]. Both hypertrophic scars and keloids are more common in the second and third decades of life, hypothesized not only due to a higher risk from a stronger inflammatory response but also due to the role of cutaneous tension in younger patients [31, 33]. Equal sex distribution has been reported for hypertrophic scars but may warrant further analysis as female sex is generally considered a risk factor for hypertrophic scar formation [14, 34, 35]. Keloid prevalence is reported as slightly higher in young female individuals, possibly owing to higher rates of ear piercing as a confounding variable, but equal among both sexes in other age groups.[7].
3 Goals of Care
Hypertrophic scars and keloids can have dramatic effects on patient quality of life through pain, pruritus, and functional limitations such as contractures. Keloid scars are reported to cause pain or pruritus in 20–40% of cases [33]. Additionally, patients are reported to experience psychological consequences owing to a perception of disfigurement [36]. The goal of care should be focused on improving both function and appearance, while minimizing recurrence rates. It is important to note that the first goal in the prevention of hypertrophic scars and keloids is proper wound care, given that it has been shown that wound healing and scar treatment should always be considered together [7, 37].
Challenges to physicians involve the lack of a “one-size-fits-all” approach and the multitude of available therapies that may be required in different combinations based on individual scar presentation and the complicated pathophysiologic mechanisms. Given the ethnic differences in scar propensity, specific healing characteristics of each patient should be accounted for through algorithms optimized to each race and achieved through international collaboration [11].
4 Non-Invasive Strategies
4.1 Preventive Measures
When addressing the sequelae of wound healing in patients, it is essential to determine the likelihood of hypertrophic scarring or keloid formation based on a patient’s prior history, genetics, wound type, and anatomic location. Preventive measures are most effective when undertaken early in the wound-healing process by optimizing and encouraging epithelialization to occur as early as possible. Regardless of the wound type, repair must be prioritized, whether it be through primary closure, secondary intention healing, flaps, skin grafts, or other non-surgical methods, in order to prevent and limit greater inflammation and potential infection to ensue [38]. Additionally, after repair of the lesion is achieved, significant care must be taken in order to reduce mechanical forces on the wound type as dermal repair can take up to 3 months [39]. Excessive tensile strength that is placed on a wound has been associated with the induction of scar formation and aberrant healing through induction of myofibroblasts and fibroblasts resulting in impaired granulation tissue remodeling [40]. As such, several non-invasive preventive therapies have been developed that may decrease the chances of hypertrophic scarring or keloid formation.
4.1.1 Occlusive Dressings and Silicone Gel
Occlusive dressings have long been proposed and used as prophylactic therapy in the acute wound-healing period to prevent dystrophic scar formation. Some of the proposed mechanisms through which it promotes appropriate wound healing and prevention of abnormal scarring include enhanced epidermal migration leading to faster healing times, increased angiogenesis, decreased infections rates, and enhanced leukocyte migration and connective tissue synthesis [41,42,43]. There are several types of occlusive dressings that are currently widely available for different wound types such as polymer films and foams, hydrogel dressings, hydrocolloid dressings, and alginates. Silicone dressings are currently first line and have been used in acute wound healing since 1983 to ameliorate and optimize this process [44, 45]. Some of the proposed mechanisms through which silicone dressings specifically achieve their anti-keloidal effects include the maintenance of hydration, which is lacking when no epidermal barrier exists, reduction in reactive epidermal hyperplasia, and decreasing interleukin (IL)-1 signaling [42, 46].
Silicone gels were developed after the rise of silicone dressings for the treatment of areas where fixation of silicone sheets was more difficult or undesirable, such as on the face, scalp, or joints. Similar to silicone dressings, many studies have proved the effectiveness of silicone gels in the treatment of hypertrophic scars and keloids [47,48,49,50,51,52]. More recently, there has been a rise in the number of silicone gel products available. A modified silicone gel combined with hypochlorous acid (Celacyn; Sonoma Pharmaceuticals, Woodstock, GA, USA) has been utilized post-procedure on recent scars and found to be effective, well tolerated, and safe [53, 54]. Hypochlorous acid is reported to act on all phases of wound healing as a potent antimicrobial, antipruritic, and anti-inflammatory agent [53]. Other formulations of silicone gels have incorporated herbal or onion extracts. A silicone gel formulation containing onion extract and aloe vera was found to be as effective as silicone gel sheeting in preventing postoperative pathologic scars [55]. Another study of 10% onion extract in a silicon derivative gel observed a significantly decreased incidence of hypertrophic scars following median sternotomy in pediatric patients [56]. Further large-scale studies are needed on the efficacy of silicone gel formulations as recent systematic reviews have identified the low quality of the current trials and their high susceptibility to bias [57, 58].
Tan et al. and Shi et al. summarize the different types of occlusion dressings with their specific characteristics and their appropriateness for various wound types [41, 59]. Interestingly, a randomized controlled trial showed that both silicone gel and non-silicone gel dressings are equally effective in treating hypertrophic scars and keloids, suggesting that the type of occlusive therapy may not significantly affect outcomes [60]. The choice of occlusive dressing for appropriate wound healing should be guided by the specific characteristics of the lesions in order to encourage the most optimal environment for that specific lesion.
4.1.2 Pressure Therapy
Pressure therapy has been widely used in combination with other more invasive treatment modalities in the acute wound-healing period since the 1960s [61]. Specifically, pressure garments have been used prophylactically in lesions showing delayed wound healing and those requiring grafts, as well as a therapeutic modality in certain hypertrophic scars [62,63,64]. There are many proposed mechanisms through which pressure therapy achieves the desired wound-healing outcomes including the stimulation of mechanoreceptors to induce apoptosis of extracellular matrix components, pressure-induced ischemia resulting in collagen degradation and alteration of fibroblast activity, and induction of metalloproteinase-9 and prostaglandin E2 release [65,66,67,68]. Nonetheless, the full mechanism is not completely understood. There are different types of pressure garments available for use in different locations. Some of these include elastic wraps, common wraps, elastane bandages, magnets, pressure ear molds, and earrings. Several studies suggest that pressure garments should be maintained at a pressure of 15–30 mm Hg and worn continuously (at least 23 h daily) until the scar is mature, which normally takes at least 6 months [69,70,71,72]. As such, patient compliance is highly variable because of the extensive amount of time needed for a clinically significant benefit. Additionally, this can be complicated by the location of lesions in areas of excessive movement such as joints and extremities. Although pressure garment therapy is widely used, its efficacy in preventing or treating hypertrophic scars and keloids has been questioned. A meta-analysis of six trials involving 316 patients with burns showed minor benefits in scar height only, with all studies included in the analysis deemed as high quality [73]. A recent meta-analysis of 12 randomized controlled trials involving 710 patients with hypertrophic scars due to burn injuries showed that there was improvement in thickness, brightness, pigmentation, and hardness with 15–25 mm Hg [74]. It was noted however that the analysis was limited in the inclusion of lower quality studies owing to a lack of specifications on blinding methods [74].
4.1.3 Onion Extracts
Onion extract has been commonly found in over-the-counter products for its potential in scar prevention and treatment. Flavonoids are the active compounds that are attributed to be responsible for the amelioration of excess scarring. It is hypothesized that these compounds act by the induction of matrix metalloproteinase-1 and a decrease in fibroblast proliferation [75, 76]. Nonetheless, the exact mechanism has yet to be fully elucidated. The effectiveness of onion extracts as prophylaxis and the treatment of dystrophic scarring have been reported in the literature [77, 78]; however, other studies question its effectiveness in achieving a clinically significant effect when compared to other topical therapies [79, 80]. A recent systematic review and meta-analysis on 13 randomized controlled trials found that only five studies showed onion extract to be superior to no treatment, with the remaining studies suggesting mixed results in the benefit of onion extracts over other topical therapies [81]. As such, the current evidence in the ability of onion extracts in preventing or treating hypertrophic scars and keloids is inconclusive. Other therapies should be considered for their use in combination with onion extract to potentially optimize wound healing in the perioperative period.
4.1.4 Skin Priming
Early intervention in the wound-healing process has been posited as a possible means to prevent poor scarring outcomes. Specifically, the treatment of the skin prior to a surgical procedure can be “primed” with other topical or intralesional therapies to optimize wound healing and aberrant scar formation. An in vivo study involving the pre-treatment of diabetic mice having full-thickness incisional wounds with proangiogenic growth factors and endothelial progenitor cells showed reduced wound-healing time and improved tensile strengths compared with the control [82]. This suggests that pretreatment of tissue prior to surgery might significantly improve wound healing. Similarly, other studies have suggested that the use of prophylactic negative-pressure therapy may decrease surgical-site infection and optimize wound healing [83, 84]. Skin priming has also been reported to be successful with the use of fractional carbon dioxide (CO2) lasers used immediately after scar revision surgeries [85]. Ozog showed in a randomized split-scar study that the intraoperative treatment of wound edges with a CO2 laser improves the appearance and texture of scars [86].
Topical therapies such as epigallocatechin-3-gallate have been shown to be beneficial pre-operatively in optimizing future scarring. A double-blind, randomized, placebo-controlled study evaluating the pre-emptive use of epigallocatechin-3-gallate prior to surgery showed reduced mast cell components, blood flow, and increased elastin compared with the control [87]. Priming the skin prior to surgical intervention may provide a way to improve wound healing and avoid aberrant scarring, especially in high-risk patients. More studies are needed to fully elucidate the role of skin priming in wound healing and establish standardized guidelines.
4.2 Available Treatments
4.2.1 Corticosteroids and 5-Fluorouracil
Corticosteroids have been extensively utilized in the treatment of hypertrophic scars and keloids. Currently, they are considered first line in the treatment of keloid scars [88]. Although they are generally used as first-line treatment for the regression of these scars, they are also commonly used in conjunction with other more invasive modalities such as surgery and laser therapies. Their effectiveness has been attributed to their ability to inhibit fibroblast growth, through apoptosis and inhibition of TGF-β1 expression, as well as inhibition of angiogenesis through disruption of vascular-endothelial growth factor and alpha-globulin signaling [89,90,91,92,93]. Triamcinolone acetonide (TAC) remains the most commonly used injectable form at a concentration range of 2.5–40 mg/mL depending on the size and location of the lesion. Intralesional formulations have been associated with a response rate of 50–100% and recurrence rates of 9–50% [65, 94,95,96]. Additionally, intralesional TAC monotherapy has also been shown to be effective in reducing keloid recurrence rates [97, 98]. Nonetheless, injectable corticosteroids are often combined with other therapies such as intralesional 5-fluorouracil (5-FU), laser, and surgery owing to their improved efficacy compared with monotherapy [99]. In the Japanese guidelines for the treatment of hypertrophic scars and keloids, corticosteroid tapes and plasters are now considered first-line therapy with injectables used as adjuncts when lesions are unremitting [100]. Tapes and plasters have been associated with decreased coloration and flattening of the lesions [101]. Corticosteroid ointments and creams in combination with injections have also shown some efficacy in both treating and preventing keloid formation [38, 102]. If used, ointments and creams should be utilized as adjunctive treatments rather than monotherapy because of the lack of randomized controlled trials assessing their effectiveness.
5-Fluorouracil is an antineoplastic agent involved in the inhibition of thymidylate synthase [103]. In vitro studies have shown that 5-FU has the capacity to inhibit fibroblast proliferation and TFG-β expression [104]. 5-Fluorouracil emerged in the treatment of keloids and hypertrophic scars as a therapy to avoid the potential adverse effects of corticosteroid injections. Combination therapy of 5-FU and TAC has been shown to increase the effectiveness of keloid management while avoiding the side effects associated with corticosteroid therapy such as atrophy, hypopigmentation, and telangiectasia [105]. A randomized controlled trial assessing combination therapy in keloid treatment showed that the skin atrophy rate and telangiectasia formation were 44% and 50%, respectively, with TAC monotherapy compared with 8% and 21%, respectively, with combination therapy [106]. It was noted that there was no clinical or statistical difference in the remission rate after 6 months of follow-up [106]. 5-Fluorouracil alone or in combination with TAC has been shown to reduce the keloid recurrence rate. Interestingly, there is conflicting evidence with some studies finding no recurrence with the use of 5-FU for keloid treatment [107,108,109], while other studies report higher recurrence rates with longer follow-up times [104, 110].
4.2.2 Imiquimod
A toll-like receptor agonist, imiquimod, has been posited as a potential therapy for keloid treatment owing to its immune-modulating activities by the induction of tumor necrosis factor‐α, interferon‐α, and IL-1, IL-6, and IL-8 [111,112,113]. Notably, imiquimod is generally used as adjunct therapy with other treatment modalities in order to prevent keloid recurrence. In combination with surgical excision of keloids, imiquimod has been shown to have a recurrence rate of 24.7% [114]. While some studies suggest some improvement, others have shown no benefit in the postsurgical use of imiquimod for keloids [115, 116]. A recent meta-analysis of seven studies and 82 keloids showed that the recurrence rate after application of topical 5% imiquimod was 39%, with no alteration in the recurrence with surgical excision [117]. More clinical trials are needed to concretely ascertain the clinical efficacy of topical imiquimod.
4.2.3 Verapamil
Verapamil is a non-dihydropyridine calcium channel blocker that is currently approved by the US Food and Drug Administration for the management of hypertension, chronic stable and unstable angina, and supraventricular tachycardia [118]. Alterations in intracellular calcium can induce collagenase activity as well as inhibit signaling factors associated with keloid formation, such as vascular-endothelial growth factor, TGF-β1, and IL-6 [119]. A recent parallel-group study comparing intralesional verapamil, TAC, and, fractional CO2 therapy for the treatment of keloids showed that verapamil was as effective as TAC [120]. However, another randomized controlled trial comparing TAC and verapamil found that 2.5 mg/mL of intralesional verapamil every 3 weeks showed no therapeutic benefit in treating keloids [95]. Similarly, another study showed that verapamil is not as effective as TAC for keloid management [96]. A meta-analysis of six studies and 331 patients showed that verapamil therapy was not associated with improvements in hypertrophic scars and keloids compared to other treatment modalities, suggesting that verapamil may serve as a potential alternative only when other intralesional therapies may not be available [121].
5 Invasive Therapies
5.1 Surgery
The goals of surgical excision are to reduce tensile forces affecting the area, debulk large lesions, and improve a limited range of motion as a result of aberrant scarring [7]. Surgical excision has proven to be inferior as a monotherapy because of recurrence rates that range from 45 to 100% [122]. As such, surgical excision should be carried out with other adjunct therapies including occlusive dressings, compression therapy, intralesional triamcinolone, radiotherapy, and lasers. Specific surgical techniques such as Z-plasties, W-plasties, and flaps may provide a benefit in decreasing the likelihood of keloid or hypertrophic scar recurrence in areas of high tension [94].
A case-series study of 141 patients with keloids who were treated with subcutaneous or fascial tensile reduction sutures and z-plasties in combination with postoperative radiotherapy over 3 consecutive days showed that only 10.6% of lesions recurred [123]. Similarly, a meta-analysis of surgical excision of auricular keloids in combination with 5–10 mg/mL of intralesional triamcinolone postoperatively has shown a recurrence of 15.4% after a 12-month follow-up [124]. Combination therapy with other topicals such as mitomycin-C (MMC) and imiquimod has also been reported to be successful with recurrence rates of 16.5% and 24.7%, respectively [114]. Thus, surgical excision should generally be reserved when lesions are not amenable to other less invasive modalities and combination therapy should be pursued in order to minimize the risk of recurrence.
5.2 Lasers
Argon and CO2 laser therapy for the treatment of hypertrophic scars and keloids was first proposed in the 1980s with little success [125]. Fractional ablative lasers, namely erbium-doped yttrium aluminum garnet and CO2 lasers, have since been evaluated for the treatment of hypertrophic scars and keloids. These lasers exert their effects by targeting water molecules within the cutaneous tissue and promoting destruction of the local environment [126]. Fractional CO2 lasers are found to replace irregular collagen bands with new organized collagen fibrils in the upper dermis [127, 128]. Significantly decreased TGF-β1expression is also seen following CO2 laser therapy [127]. Clinically, fractional CO2 lasers have demonstrated improved scar appearance, thickness, pliability, and surface relief [129,130,131,132]. Pulsed dye laser (PDL) emits light selectively absorbed by oxyhemoglobin, resulting in selective photothermolysis of vascular tissue with intact surrounding tissue [133]. Pulsed dye laser is thus used as a first-line therapy for many cutaneous vascular disorders; however, recent reports have demonstrated its effectiveness in conditions of other etiologies. The 585-nm and 595-nm PDLs are found to be a safe and effective option for the treatment of hypertrophic scars and keloids [134,135,136]. One comparison study between the two wavelengths found that 585 nm was preferred, having a demonstrated ability to reduce height substantially in a significant number of scars [136]. In addition to PDL, other vascular lasers such as neodymium-doped yttrium garnet lasers have been proposed for the treatment of pathologic scars and shown to effectively reduce the Vancouver Scar Scale (VSS) score, with a notably greater effect on hypertrophic scars compared with keloids [137, 138]. Another recent study investigating the use of a 1470-nm bare-fiber diode laser found an overall VSS score improvement of 42% for hypertrophic scars and 37.9% for keloids, with no reported adverse events or recurrence [139]. Non-ablative fractional lasers are currently being studied as monotherapy and in combination with topical therapies for hypertrophic scars; however, large clinical trials to characterize its efficacy are still lacking [140,141,142,143].
International and national consensus statements have recommended the use of fractional ablative lasers as first-line therapy especially in thicker and hypopigmented hypertrophic scars, as well as in combination with other therapies such as topical agents or intralesional therapy [144, 145]. Nonetheless, the laser choice should be guided with specifics on the lesion encountered, given that certain characteristics or the type of scarring may respond better with other lasers. For instance, erythematous hypertrophic scars may respond better with PDL therapy initially with the possibility of using it in combination with ablative lasers in thicker scars [144].
5.2.1 Combination Laser Therapy
Recent evolutions in laser treatments point to more favorable outcomes and higher patient satisfaction for combination laser therapy over monotherapy for pathologic scars (Table 1). Improved efficacy of CO2 lasers in treating keloids has been shown in combination with other local therapies such as IFN-α, 5-FU, verapamil, topical corticosteroids, and even in combination with PDL [146,147,148,149,150]. In vivo studies on hypertrophic scars in rabbit models found that a combination of PDL and CO2 lasers significantly improved thickness, size, and hardness as well as suppressed the levels of TGF-β1 and the proliferating cell nuclear antigen on a histologic analysis when compared with their respective laser monotherapy [151]. One study comparing outcomes on 25 patients randomized to four treatment groups (PDL alone, CO2 laser alone, a combined treatment of these two, and another combination with CO2 ablative fractional resurfacing on the day of surgery) found that the best outcomes in scar appearance were seen for scars that underwent a combination treatment [150]. A retrospective study involving 35 Korean patients with keloids treated with a non-ablative fractional erbium-glass laser followed by an ablative fractional CO2 laser, superficial cryotherapy, and an intralesional triamcinolone injection showed significant improvements in both total and subcategory VSS scores [152]. No patient experienced significant adverse effects, and most reported improvements to pain, itch, and range of motion [152]. Combinations of laser therapy and an intralesional steroid injection have also been studied for hypertrophic scar and keloid management [141, 153, 154]. A study of 38 patients with pathologic scars found that overall there were fewer treatment sessions, higher patient satisfaction, and longer remission periods for combination therapy of a non-ablative fractional laser and an intralesional triamcinolone injection versus the steroid injection alone [141]. Better results have also been reported for the ablative CO2 laser when given in addition to intralesional triamcinolone acetonide 40 mg/mL [153, 155]. It should be noted that the efficacy of laser treatments is not widely studied in skin of color because of the increased risk of adverse events from laser therapy, such as hyperpigmentation, in higher Fitzpatrick skin types [156].
5.2.2 Laser-Assisted Drug Delivery
Laser-assisted drug delivery (LADD) was proposed in 2002 for improving the delivery of topical anesthetics [157]. Generally, LADD is achieved through the use of fractional ablative lasers owing to their ability to create cylindrical micro-ablation zones that may facilitate drug absorption deeper into the affected tissue, thus improving the bioavailability of the drug [158]. Therefore, the use of LADD for the treatment of hypertrophic scarring has been evaluated in multiple studies. Waibel et al. [159] conducted a prospective case series of 15 patients with hypertrophic scars treated with CO2 laser and immediate application of topical TAC showing significant improvements in texture, hypertrophy, and dyschromia. Similarly, a retrospective study of 23 patients with 70 keloids treated with ablative fraction lasers and topical betamethasone showed a median improvement of 50%, with recurrence in 22% of the lesions [147]. Recently, a split-face study of intralesional corticosteroids versus 2940 nm erbium-doped yttrium aluminum garnet laser therapy in conjunction with a topical corticosteroid application for the treatment of keloids showed improved VSS scores in both therapies [160]. Neither therapy was deemed to be superior in keloid management; however, patients reported significantly less pain with the laser and topical corticosteroid combination [160]. Other studies have shown improved efficacy of fractional ablative therapies in a combination of topical botulinum toxin, 5-FU, and verapamil [148, 161,162,163]. Although LADD is a promising emerging technology for the treatment of hypertrophic scars and keloids, more studies and consensus are needed for delineating parameters for laser settings and specific drug preparations to standardize its use in wound healing. Larger randomized controlled trials are also necessary to fully characterize its efficacy because of the large heterogeneity of current studies [145, 164]. Laser-assisted drug delivery remains an investigational therapy that shows significant promise in the treatment of hypertrophic scars and keloids.
5.3 Radiation Therapy
Radiation therapy has been widely used as an adjunctive therapy to prevent the regrowth of keloids after excision. Monotherapy is relatively limited because of higher reported recurrence rates (37%) when compared with the use after surgical excision of keloids (22%), with some studies reporting recurrence rates as low as 9.59% [165,166,167]. Proposed mechanisms through which radiotherapy decreases keloid formation include the inhibition of angiogenesis and the reduction in fibroblast activity [168]. Currently, three primary modalities of radiation are used as an adjunctive therapy in the treatment of pathologic scars: electron beam therapy, brachytherapy, and X-ray therapy [169]. The optimal biological effective dose (BED) that maximizes the prevention of keloid recurrence while minimizing adverse reactions is 20–30 Gy [170]. A BED of 30 Gy can be achieved by delivering four fractions of 5Gy, three fractions of 6 Gy, two fractions of 8 Gy, one fraction of 13 Gy, or one dose of 27 Gy at a low dose rate [171].
Electron beam therapy uses a linear accelerator to deliver high-energy electrons to surface structures while minimizing the dose to underlying tissue. Advantages of this modality include the treatment of large surface areas and homogeneous dose delivery to surface structures [169]. In a study of 45 patients with chest keloids who underwent an excision followed by fractionated electron-beam radiation therapy, wounds were almost completely flattened after 12 months, with only one relapse (2.2%) within 2 years of follow-up [172]. Challenges associated with electron beam therapy include the complicated dosimetry, high cost, and inability to treat curved surfaces [169].
Brachytherapy delivers gamma rays to target tissues using radioactive sources and catheters. Brachytherapy is classified as either interstitial or surface, depending on whether the radioactive source is placed within or on top of the wound [173]. Advantages to brachytherapy include the ability to target uneven surfaces and potentially spare more surrounding tissue. In the treatment of keloids, brachytherapy has been reported to have lower recurrence rates (15%) after surgical excision when compared with electron beam and X-ray therapy (23%) [165]. However, this modality requires high-dose rates in order to deliver the appropriate dose in short time frames [169]. A retrospective multicenter comparison of the recurrence and complications of brachytherapy for the treatment of 238 keloids at different fractionation schemes concluded that a BED of 20 Gy was effective and safe in preventing the recurrence of keloids [174]. The fractionation schemes of 2 × 9 Gy and 3 × 6 Gy were associated with more adverse reactions without being more effective in preventing keloid recurrence than a 2 × 6 Gy fractionation scheme [174].
Superficial radiation therapy (SRT) [175] delivers low-energy X-rays, generally in the 50–150 kV range, and has long been used for the treatment of superficial lesions including keloid scars [176, 177]. Past studies on its efficacy were limited, but many recent trials have led to growing evidence for the role of SRT as a practical and efficient treatment for hypertrophic and keloid scars [178,179,180]. One retrospective study of 96 keloid scars treated with SRT with a BED of 30 Gy found a recurrence rate of 12.7% at 18 months [181]. Another prospective study of 48 keloids treated with 18 Gy of SRT for 3 consecutive days post-resection found 81% successful remission over 12 months [180]. Prior to these studies, a consensus in 2019 had determined that SRT following keloid excision significantly reduces keloid recurrence rates with no evidence that exposing surrounding healthy skin will cause skin cancer [182]. Many studies report no adverse reactions; however, transient hyperpigmentation was reported by some as the most frequent adverse effect, the incidence and severity of which can be predicted by the location and incision length of the scar [181, 183]. Some guidelines recommend the optimal BED for SRT is 30 Gy delivered over 3 fractions with a total dose fraction of 6 Gy given on the first 3 days after surgical excision [182].
Although radiotherapy is a viable option for the treatment of keloids after surgery, there have been reports of radiation-induced carcinogenesis [184]. However, this risk is considered to be low, if any, when appropriate protective measures are taken. Radiotherapy after surgical excision is an appropriate and effective option to prevent the recurrence of keloids and hypertrophic scars.
5.4 Cryotherapy
Cryotherapy was first introduced in the early 1990s as a potential safe therapeutic modality for hypertrophic scars and keloids [185, 186]. There are various methods of delivery that can be used including spray, contact probes, and an intralesional cryoprobe. Generally, cryotherapy is carried out under local anesthesia, wherein a cryoprobe connected to a canister containing nitrogen is inserted into the core of the keloid allowing freezing to occur from the center outwards up to a 5- to 10-mm margin of normal skin. [187] Success in the treatment of keloids with cryotherapy has been highly variable with some studies suggesting full remission of the lesions, while the efficacy reported in others ranges from 32% to 74%. [186, 188] A meta-analysis of eight studies found that intralesional cryotherapy was able to decrease scar volume with a range of 51–61%, with a recurrence range of 0–24%. [189] Intralesional cryotherapy may be useful in patients with darker skin, as it can minimize the destruction of melanocytes within the epithelium. [190] Although cryotherapy may be efficacious in treating keloids, several adverse reactions have been reported including blistering, pain, infection, hypopigmentation, and depigmentation. [187]
6 Emerging Therapies (Table 2)
6.1 Mitomycin-C
Mitomycin-C is an antibiotic derived from Streptomyces caespitosus that has been used as a chemotherapeutic agent and for ophthalmologic purposes [191, 192]. In vitro studies have shown that MMC is able to decrease the fibroblast concentration in keloids [193]. Earlier studies suggested that topical MMC after surgical excision of therapies showed no significant therapeutic benefit in prevention [194]. However, a recent study comparing topical and intralesional MMC showed that both preparations were able to significantly improve auricular keloids in 40 patients. [195] Similarly, other studies have shown success with topical and intralesional MMC in preventing keloid recurrence after surgical removal [196, 197]. Mitomycin-C may serve as an alternative preventive therapy for keloid recurrence; however, larger randomized clinical trials are needed to appropriately characterize its efficacy.
6.2 Botulinum Toxin
Botulinum toxin type-A (BoNT-A) has been used since the 1980s for cosmetic purposes. It is a neurotoxin derived from Clostridium botulinum bacteria that acts on the neuromuscular plate to cause muscular paralysis lasting up to 6 months [198]. Botulinum toxin type-A was first introduced for keloid treatment in the 2000s. By inhibiting the release of Ach, BoNT-A is thought to paralyze both muscle tissue and scar fibroblasts, reducing muscle tension and elastic fiber contracture at the site of the wound to prevent further hyperplasia of the scar [199]. The full extent of the mechanism of BoNT-A in keloids is still being studied, and has recently been suggested to involve the promotion of keloid myofibroblasts into adipocytes, leading to activation of the BMP4/Smad signaling pathway [200].
In recent years, a large number of relevant studies have examined the safety and efficacy of intralesional BoNT-A in the treatment of pathologic scars. A study of 50 patients with keloids treated with either intralesional BoNT-A or 5-FU demonstrated a significantly better therapeutic response to BoNT-A with regard to flattening of lesions [201]. The same study also found BoNT-A was significantly better at treating larger lesions, and was associated with fewer side effects than 5-FU, including less pain, pruritus, hyperpigmentation, and recurrence [201]. A systematic review and meta-analysis of 18 randomized controlled trials saw similar results, with better improvement in terms of scar width, VSS score, Visual Analogue Scale [202] score, and patient satisfaction with BoNT-A compared with the control group for 915 patients [199].
Botulinum toxin type-A has also been studied for its use in combination with intralesional corticosteroids for the treatment of keloids and hypertrophic scars. Multiple studies have suggested improved efficacy of corticosteroids combined with BoNT-A compared with corticosteroids alone [203, 204]. One meta-analysis found that corticosteroid and BoNT-A combination therapy provided faster, more effective results in terms of VSS score, VAS score, scar thickness itching, and patient satisfaction than corticosteroids alone [203]. This study also noted that combination therapy was associated with fewer adverse reactions than corticosteroid monotherapy [203]. Another meta-analysis comparing the effects of four drugs used in the treatment of pathologic scars found the greatest efficacy with BoNT-A combined with corticosteroids, followed by 5-FU combined with corticosteroids, then by BoNT-A alone [204]. A systematic review and meta-analysis of studies comparing BoNT-A with intralesional TAC for the treatment of keloids and hypertrophic scars found BoNT-A to be more effective than both TAC and placebo, and associated with decreased pain after an injection [205]. Botulinum toxin type-A has also been evaluated in combination with surgical excision of keloids and intralesional 5-FU [206]. One study on 80 patients with keloids found that combination therapy resulted in a recurrence rate of 3.75% in follow-ups that ranged from 17 to 24 months [206]. Botulinum toxin type-A has shown promise in the treatment of hypertrophic scars and keloids; however, further large-scale controlled studies are needed to determine its efficacy in monotherapy and combination therapy, as well as the most appropriate injection method, dose, spacing, and timing [199]
6.3 Hyaluronic Acid and Hyaluronidase
Hyaluronic acid is a high-molecular-weight mucopolysaccharide that is usually found in high quantities in the extracellular matrix of connective tissues and synovial fluids [207]. Hyaluronic acid is generally used in dermatology for correction and improvement in injectable filler volume and contour. Case reports have shown that hyaluronic acid injections may be useful in the treatment of keloids in combination with corticosteroids [208]. The mechanism through which this occurs is not clear. An ex vivo histological analysis of keloid samples showed that keloidal tissue contained more hyaluronic acid compared with normal dermal tissue. Additionally, ex vivo treatment of keloid fibroblasts with TAC reduced hyaluronic acid synthesis [209]. This suggests that a decrease in hyaluronic acid may affect keloid formation. Other more recent in vitro studies have shown that hyaluronic acid is decreased in keloidal tissue [210]. Additionally, hyaluronan synthase and hyaluronidase messenger RNA levels were found to be reduced in keloidal tissue [210]. A study using a combination of TAC with hyaluronidase showed 68.75% effectiveness in treating keloids with better tolerability than other regimens [211]. The role of hyaluronic acid in wound healing has not been fully elucidated. The current literature shows conflicting evidence in regard to the impact of hyaluronic acid in the treatment of keloids. Some data suggest hyaluronic acid plays a role in the downregulation of fibroblastic signaling factors, while clinical evidence suggests that hyaluronidase therapy is efficacious in keloid treatment [5, 211]. Further research is needed to characterize the role of hyaluronidase and hyaluronic acid therapy in the wound-healing process.
6.4 Picosecond Lasers
Picosecond lasers have been used for a wide variety of dermatologic conditions including tattoo removal, Nevus of Ota and Hori’s macules, solar lentigines, and freckles, among others [212]. Picosecond lasers offer several advantages in that they are associated with reduced discomfort and downtime [212]. Guida et al. recently showed that picosecond lasers can be efficacious in treating atrophic and hypertrophic scars [175]. Their reflectance confocal microscopy analysis showed a significant reduction in the thickness of the scars [175]. A retrospective study of 24 patients with hypertrophic scars treated with a 1064-nm-picosecond neodymium-doped yttrium aluminum garnet laser showed improved cosmetic outcomes and patient satisfaction [213]. Taken together, these studies provide some evidence that picosecond laser treatment of hypertrophic scars may become a viable option in the future. Larger split-face studies are needed in order to characterize their efficacy more clearly.
6.5 Dupilumab
Dupilumab, an IL-4 receptor-α monoclonal antibody that blocks type 2-driven inflammation via inhibition of IL-4/IL-13 signaling, is the first biological agent approved for treatment of moderate-to-severe AD [214]. Recently, there have been inconsistent reports over the ability of dupilumab to treat keloid scaring. In 2020, Diaz et al. reported the case report of a 53-year-old African American man with severe AD who experienced shrinkage of a large keloid and complete disappearance of a smaller keloid after 7 months of treatment with dupilumab [28]. The following year, another case study of a 37-year-old South Asian woman found no physical reduction in keloid size, but markedly improved symptoms after 3 months of dupilumab treatment that allowed for better tolerance of intralesional corticosteroid injections [215]. Improvement of a symptomatic hypertrophic scar in an 80-year-old woman was also reported after 10 months of dupilumab therapy [216]. Meanwhile, further studies of two 17-year-old patients found no improvement after 3 months of dupilumab treatment, with one patient experiencing an increase in both the size of existing lesions and the number of new keloids [217]. Another study of eight patients treated with dupilumab 300 mg subcutaneously every 2 weeks saw disease progression as the most common response, with only one patient willing to continue the treatment [29]. All eight patients in this study were advised to stop treatment. In their paper, Diaz et al. also reported on how the efficacy of dupilumab against keloid scars they observed may suggest an underlying type 2-centered pathogenesis shared by AD and keloids, and found increased IL-4/IL-13 signaling in nodules to confirm this rationale [28]. However, Tirgan et al. pointed out that an established diagnosis of AD would increase IL-4/IL-13 signaling independently [29]. Despite these conflicting reports, future studies on dupilumab will help determine its role in the treatment of pathologic scars and may enhance the current pathophysiological understanding. A phase IV clinical trial investigating the use of dupilumab for keloids is currently underway (NCT04988022).
6.6 Pentoxifylline
Pentoxifylline is a methylated xanthine derivative and phosphodiesterase inhibitor used in the treatment of the peripheral artery as a vasodilator, anti-inflammatory, and antifibrotic agent [218]. It has been shown that pentoxifylline can inhibit the rate of collagen synthesis in fibroblasts of keloids, thereby halting growth [219]. In severely symptomatic keloids, pentoxifylline has also been shown to decrease pain and itch [220]. The use of pentoxifylline for the treatment of keloids is not fully established but has recently been studied as both an adjunctive systemic agent and intralesional injection [218, 221]. Tan et al. [218] published a retrospective study in 2020 where they found a statistically significant decrease in keloid recurrence rates after surgical excision for groups treated with pentoxifylline, supporting the use of pentoxifylline as an adjunctive systemic agent to decrease keloid recurrence. Another recent study comparing the efficacy and safety of intralesional pentoxifylline, TAC, and their combination for the treatment of keloids found pentoxifylline to be helpful, safe, and well tolerated [221]. The efficacy of pentoxifylline alone was lower than that of TAC alone; however, more satisfactory results with less TAC-induced side effects were seen with combination therapy [221]. Pentoxifylline is a safe option that appears potentially helpful for both keloid management and the prevention of scar recurrence, warranting future studies to explore its role as both an adjunctive and stand-alone treatment.
6.7 Oral Tranilast
Oral tranilast, an analog of a tryptophan metabolite, is an anti-allergic agent known to improve inflammatory disease with few adverse effects [222]. Tranilast was first suggested for the treatment of pathologic scars in the late 1980s after investigators discovered its ability to inhibit fibroblast proliferation in vitro and suppress collagen deposition in vivo [223]. Since then, this drug has been used for many years in Japan to treat keloids and hypertrophic scars and shown in randomized controlled trials to reduce the redness of postsurgical hypertrophic scars [224, 225]. However, clinical studies on the efficacy and safety of oral tranilast for the treatment of hypertrophic and keloid scars remain limited. One recent retrospective study found a significant improvement of total VSS scores with no specific side effect in 42 patients with keloids who were treated with oral tranilast for more than 3 months [226].
7 Conclusions
Hypertrophic scar and keloid management remains a multi-step endeavor involving careful identification of patient risks factors as well as a comprehensive perioperative approach to optimize patient outcome and satisfaction. Newer treatment modalities may offer promising alternatives in the use as monotherapy or in combination with more established treatment modalities such as surgical excision, lasers, radiotherapy, and injectables. Regardless of the treatment modality, patient expectation and education are of utmost importance to establish understanding of the therapeutic options, their adverse reactions, and efficacy rates. Additionally, patient education cannot be understated especially when pursuing a prophylactic approach in scar management. Preventive strategies such as priming of the skin prior to surgery with lasers and topical agents show promise, but further studies are needed to characterize its efficacy. Future efforts should focus on delineating guidelines and parameters for laser-assisted drug delivery specifically in regard to drug formulations and duration of treatment.
References
Ghazawi FM, Zargham R, Gilardino MS, Sasseville D, Jafarian F. Insights into the pathophysiology of hypertrophic scars and keloids: how do they differ? Adv Skin Wound Care. 2018;31(1):582–95.
Feng Y, Wu JJ, Sun ZL, Liu SY, Zou ML, Yuan ZD, et al. Targeted apoptosis of myofibroblasts by elesclomol inhibits hypertrophic scar formation. EBioMedicine. 2020;54: 102715.
Elsaie ML. Update on management of keloid and hypertrophic scars: a systemic review. J Cosmet Dermatol. 2021;20(9):2729–38.
Darby IA, Desmoulière A. Scar formation: cellular mechanisms. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on scar management: state of the art management and emerging technologies. Cham: Springer International Publishing; 2020. p. 19–26.
Hoffmann A, Hoing JL, Newman M, Simman R. Role of hyaluronic acid treatment in the prevention of keloid scarring. 2013.
Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19:3.
Gianatasio C, Abrouk M, Waibel JS. Treatment approaches for treating hypertrophic scars and keloids. Dermatol Rev. 2021;2(1):11–22.
Armour A, Scott PG, Tredget EE. Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen. 2007;159(1):S6–17.
Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Chemical characterization and quantification of proteoglycans in human post-burn hypertrophic and mature scars. Clin Sci (Lond). 1996;90(5):417–25.
Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns. 2007;33(5):634–41.
Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: a 2020 update of the algorithms published 10 years ago. Plast Reconstr Surg. 2022;149(1):79e–94e.
Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80(3):253–60.
Jfri A, Alajmi A. Spontaneous keloids: a literature review. Dermatology. 2018;234(3–4):127–30.
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–25.
Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. Hypertrophic scars and keloids: overview of the evidence and practical guide for differentiating between these abnormal scars. Exp Dermatol. 2021;30(1):146–61.
Ogawa R, Okai K, Tokumura F, Mori K, Ohmori Y, Huang C, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20(2):149–57.
Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg. 2005;116(6):1648–56.
Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40(7):1255–66.
Ibrahim NE, Shaharan S, Dheansa B. Adverse effects of pregnancy on keloids and hypertrophic scars. Cureus. 2020;12(12): e12154.
Moustafa MF, Abdel-Fattah MA, Abdel-Fattah DC. Presumptive evidence of the effect of pregnancy estrogens on keloid growth: case report. Plast Reconstr Surg. 1975;56(4):450–3.
Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. 2021;30(1):132–45.
Chike-Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg. 2009;23(3):178–84.
Hellwege JN, Russell SB, Williams SM, Edwards TL, Velez Edwards DR. Gene-based evaluation of low-frequency variation and genetically-predicted gene expression impacting risk of keloid formation. Ann Hum Genet. 2018;82(4):206–15.
Nakashima M, Chung S, Takahashi A, Kamatani N, Kawaguchi T, Tsunoda T, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42(9):768–71.
Tulandi T, Al-Sannan B, Akbar G, Ziegler C, Miner L. Prospective study of intraabdominal adhesions among women of different races with or without keloids. Am J Obstet Gynecol. 2011;204(2):132.e1-4.
Young WG, Worsham MJ, Joseph CL, Divine GW, Jones LR. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surg. 2014;16(5):379–80.
Lu YY, Lu CC, Yu WW, Zhang L, Wang QR, Zhang CL, et al. Keloid risk in patients with atopic dermatitis: a nationwide retrospective cohort study in Taiwan. BMJ Open. 2018;8(7): e022865.
Diaz A, Tan K, He H, Xu H, Cueto I, Pavel AB, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34(4):e161–4.
Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36(2):e120–2.
Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review). Int J Mol Med. 2009;24(3):283–93.
El Kinani M, Duteille F. Scar epidemiology and consequences. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on scar management: state of the art management and emerging technologies. Cham: Springer International Publishing; 2020. p. 45–9.
Taylor SC. Epidemiology of skin diseases in ethnic populations. Dermatol Clin. 2003;21(4):601–7.
Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84(5):827–37.
Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg. 2008;10(2):93–102.
Kruglikov IL, Scherer PE. Caveolin-1 as a target in prevention and treatment of hypertrophic scarring. NPJ Regen Med. 2019;4:9.
Rumsey N, Clarke A, White P. Exploring the psychosocial concerns of outpatients with disfiguring conditions. J Wound Care. 2003;12(7):247–52.
Tripathi S, Soni K, Agrawal P, Gour V, Mondal R, Soni V. Hypertrophic scars and keloids: a review and current treatment modalities. Biomed Dermatol. 2020;4(1):1–11.
Ogawa R, Dohi T, Tosa M, Aoki M, Akaishi S. The latest strategy for keloid and hypertrophic scar prevention and treatment: the Nippon Medical School (NMS) Protocol. J Nippon Med School. 2021;88(1):2–9.
Levenson SM, Geever R EF, Crowley LV, Oates JF 3rd, Berard CW, Rosen H. The Healing Of Rat Skin Wounds. Ann Surg. 1965;161(2):293-308.
Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011;19:10–5.
Tan ST, Winarto N, Dosan R, Aisyah PB. The benefits of occlusive dressings in wound healing. Open Dermatol J. 2019;13(1):27–33.
Mustoe TA, Gurjala A. The role of the epidermis and the mechanism of action of occlusive dressings in scarring. Wound Repair Regen. 2011;19 Suppl 1(0 1):s16-21.
Hutchinson JJ, McGuckin M. Occlusive dressings: a microbiologic and clinical review. 1990.
de Oliveira GV, Gold MH. Silicone sheets and new gels to treat hypertrophic scars and keloids: A short review. Dermatol Ther. 2020;33(4):e13705.
Perkins K, Davey RB, Wallis KA. Silicone gel: a new treatment for burn scars and contractures. Burns Incl Therm Inj. 1983;9(3):201–4.
O'Shaughnessy KD, De La Garza M, Roy NK, Mustoe TA. Homeostasis of the epidermal barrier layer: a theory of how occlusion reduces hypertrophic scarring. Wound Repair Regen. 2009;17(5):700-8.
De Decker I, Hoeksema H, Verbelen J, Vanlerberghe E, De Coninck P, Speeckaert MM, et al. The use of fluid silicone gels in the prevention and treatment of hypertrophic scars: a systematic review and meta-analysis. Burns. 2022;48(3):491–509.
Wiseman J, Ware RS, Simons M, McPhail S, Kimble R, Dotta A, et al. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: a randomized controlled trial. Clin Rehabil. 2020;34(1):120–31.
Jenwitheesuk K, Surakunprapha P, Kuptarnond C, Prathanee S, Intanoo W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int Wound J. 2012;9(4):397–402.
de Giorgi V, Sestini S, Mannone F, Papi F, Alfaioli B, Gori A, et al. The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clin Exp Dermatol. 2009;34(6):688–93.
Shirazi M, Mohammadi AA, Shamohammadi I, Mahboubi A, Makarem A. Efficacy of silicone gel in reducing scar formation after hypospadias repair: a randomized placebo-controlled trial. Res Rep Urol. 2019;11:291–8.
van der Wal MBA, van Zuijlen PP, van de Ven P, Middelkoop E. Topical silicone gel versus placebo in promoting the maturation of burn scars: a randomized controlled trial. Plast Reconstr Surg. 2010;126(2):524–31.
Gold MH, Andriessen A, Dayan SH, Fabi SG, Lorenc ZP, Henderson Berg MH. Hypochlorous acid gel technology: its impact on postprocedure treatment and scar prevention. J Cosmet Dermatol. 2017;16(2):162–7.
Bucko AD DZ, Dubois JC, Jones TM. A double-blind, randomized study to compare microcyn scar management hydrogel, K103163, and Kelo-cote scar gel for hypertrophic or keloid scars. In: Poster presented at: 14th Annual Caribbean Dermatology Symposium, 20–24; 2020.
Pangkanon W, Yenbutra P, Kamanamool N, Tannirandorn A, Udompataikul M. A comparison of the efficacy of silicone gel containing onion extract and aloe vera to silicone gel sheets to prevent postoperative hypertrophic scars and keloids. J Cosmet Dermatol. 2021;20(4):1146–53.
Wananukul S, Chatpreodprai S, Peongsujarit D, Lertsapcharoen P. A prospective placebo-controlled study on the efficacy of onion extract in silicone derivative gel for the prevention of hypertrophic scar and keloid in median sternotomy wound in pediatric patients. J Med Assoc Thai. 2013;96(11):1428–33.
O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;2013(9):CD003826.
Jiang Q, Chen J, Tian F, Liu Z. Silicone gel sheeting for treating hypertrophic scars. Cochrane Database Syst Rev. 2021;9(9):CD013357.
Shi C, Wang C, Liu H, Li Q, Li R, Zhang Y, et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020;8:182.
de Oliveira GV, Nunes TA, Magna LA, Cintra ML, Kitten GT, Zarpellon S, Raposo Do Amaral CM. Silicone versus nonsilicone gel dressings: a controlled trial. Dermatol Surg. 2001;27(8):721-6.
Cronin TD. The use of a molded splint to prevent contracture after split skin grafting on the neck. Probl Sovrem Neirokhirurgii. 1961;27:7-18.
Kischer CW. Alteration of hypertrophic scars induced by mechanical pressure. Arch Dermatol. 1975;111(1):60.
Van den Kerchhove E, Boeckx W, Kochuyt A. Silicone patches as a supplement for pressure therapy to control hypertrophic scarring. J Burn Care Rehabil. 1991;12(4):361-9.
Ward RS. Pressure therapy for the control of hypertrophic scar formation after burn injury. A history and review. J Burn Care Rehabil. 1991;12(3):257-62.
Berman B, Maderal A, Raphael B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol Surg. 2017;43 Suppl 1:S3-S18.
Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-β and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–56.
Renò F. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol. 2002;138(4):475.
Renò F, Grazianetti P, Cannas M. Effects of mechanical compression on hypertrophic scars: prostaglandin E2 release. Burns. 2001;27(3):215-8.
Macintyre L, Baird M. Pressure garments for use in the treatment of hypertrophic scars--a review of the problems associated with their use. Burns. 2006;32(1):10-5.
Poetschke J, Gauglitz GG. Current options for the treatment of pathological scarring. JDDG. 2016;14(5):467–77.
Williams F, Knapp D, Wallen M. Comparison of the characteristics and features of pressure garments used in the management of burn scars. Burns. 1998;24(4):329-35.
Yildiz N. A novel technique to determine pressure in pressure garments for hypertrophic burn scars and comfort properties. Burns. 2007;33(1):59-64.
Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62(1):77-84.
Ai J-W, Liu J-T, Pei S-D, Liu Y, Li D-S, Lin H-M, et al. The effectiveness of pressure therapy (15–25 mmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Sci Rep. 2017;7(1):40185.
Lee K. Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int J Mol Med. 2010;25:3.
Phan TT, Lim IJ, Sun L, Chan SY, Bay BH, Tan EK, Lee ST. Quercetin inhibits fibronectin production by keloid-derived fibroblasts. Implication for the treatment of excessive scars. J Dermatol Sci. 2003;33(3):192-4.
Ho WS, Ying SY, Chan PC, Chan HH. Use of onion extract, heparin, allantoin gel in prevention of scarring in chinese patients having laser removal of tattoos: a prospective randomized controlled trial. Dermatol Surg. 2006;32(7):891-6.
Sidgwick GP, Mcgeorge D, Bayat A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res. 2015;307(6):461–77.
Chung VQ, Kelley L, Marra D, Jiang SB. Onion extract gel versus petrolatum emollient on new surgical scars: prospective double-blinded study. Dermatol Surg. 2006;32(2):193-7.
Jackson BA, Shelton AJ. Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg. 1999;25(4):267-9.
Yuan X, Shen J, Chen L, Wang L, Yan Q, Zhang J. Onion extract gel is not better than other topical treatments in scar management: a meta-analysis from randomised controlled trails. Int Wound J. 2021;18(3):396–409.
Ackermann M, Pabst AM, Houdek JP, Ziebart T, Konerding MA. Priming with proangiogenic growth factors and endothelial progenitor cells improves revascularization in linear diabetic wounds. Int J Mol Med. 2014;33(4):833–9.
Huang H-P, Zhao W-J, Pu J, He F. Prophylactic negative pressure wound therapy for surgical site infection in obese women undergoing cesarean section: an evidence synthesis with trial sequential analysis. J Matern Fetal Neonat Med. 2021;34(15):2498–505.
Antoniou GA, Onwuka CC, Antoniou SA, Russell D. Meta-analysis and trial sequential analysis of prophylactic negative pressure therapy for groin wounds in vascular surgery. J Vasc Surg. 2019;70(5):1700-10.e6.
Du F, Yu Y, Zhou Z, Wang L, Zheng S. Early treatment using fractional CO2 laser before skin suture during scar revision surgery in Asians. J Cosmet Laser Ther. 2018;20(2):102-105.
Ozog DM. A randomized split-scar study of intraoperative treatment of surgical wound edges to minimize scarring. Arch Dermatol. 2011;147(9):1108.
Ud-Din S, Wilgus TA, Mcgeorge DD, Bayat A. Pre-emptive priming of human skin improves cutaneous scarring and is superior to immediate and delayed topical anti-scarring treatment post-wounding: a double-blind randomised placebo-controlled clinical trial. Pharmaceutics. 2021;13(4):510.
Ogawa R. Effectiveness of corticosteroid tapes and plasters for keloids and hypertrophic scars. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on scar management: state of the art management and emerging technologies. Cham: Springer International Publishing; 2020. p. 491–6.
Roques C, Téot L. The use of corticosteroids to treat keloids: a review. Int J Lower Extremity Wounds. 2008;7(3):137–45.
Diegelmann RF, Bryant CP, Cohen IK. Tissue alpha-globulins in keloid formation. Plast Reconstr Surg. 1977;59(3):418-23.
Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional triamcinolone acetonide for keloid treatment: a systematic review. Aesthetic Plastic Surg. 2008;32(4):705–9.
Wu W-S, Wang F-S, Yang KD, Huang C-C, Kuo Y-R. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006;126(6):1264–71.
Salem A, Assaf M, Helmy A, Nofal A, Ibrahim S, Eldeeb F, et al. Role of vascular endothelial growth factor in keloids: a clinicopathologic study. Int J Dermatol. 2009;48(10):1071–7.
Ogawa R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast Reconstr Surg. 2022;149(1):79e-94e.
Abedini R, Sasani P, Mahmoudi HR, Nasimi M, Teymourpour A, Shadlou Z. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: A randomized controlled trial. Burns. 2018;44(6):1482-1488.
Saki N, Mokhtari R, Nozari F. Comparing the efficacy of intralesional triamcinolone acetonide with verapamil in treatment of keloids: a randomized controlled trial. Dermatol Pract Concept. 2019;9(1):4–9.
Muneuchi G, Suzuki S, Onodera M, Ito O, Hata Y, Igawa HH. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconst Surg Hand Surg. 2006;40(2):111–6.
Trisliana Perdanasari A, Lazzeri D, Su W, Xi W, Zheng Z, Ke L, et al. Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg. 2014;41(06):620–9.
Kafka M, Collins V, Kamolz L-P, Rappl T, Branski LK, Wurzer P. Evidence of invasive and noninvasive treatment modalities for hypertrophic scars: a systematic review. Wound Repair Regen. 2017;25(1):139–44.
Ogawa R, Akita S, Akaishi S, Aramaki-Hattori N, Dohi T, Hayashi T, et al. Diagnosis and treatment of keloids and hypertrophic scars: Japan Scar Workshop Consensus Document 2018. Burns Trauma. 2019;7:1.
Goutos I, Ogawa R. Steroid tape: a promising adjunct to scar management. Scars Burns Healing. 2017;3:205951311769093.
te Hayashi T, Furukawa H, Oyama A, Funayama E, Saito A, Murao N, Yamamoto Y. A new uniform protocol of combined corticosteroid injections and ointment application reduces recurrence rates after surgical keloid/hypertrophic scar excision. Dermatol Surg. 2012;38(6):893-7.
Wigmore PM, Mustafa S, El-Beltagy M, Lyons L, Umka J, Bennett G. Effects of 5-FU. Adv Exp Med Biol. 2010;678:157-64.
Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, Stefanaki K, Argyrakos T, Petridis A, Katsambas A. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathologic study. J Am Acad Dermatol. 2005;52(3 Pt 1):474-9.
Huang L, Cai YJ, Lung I, Leung BC, Burd A. A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J Plast Reconstr Aesthet Surg. 2013;66(9):e251-9.
Hietanen K, Järvinen T, Huhtala H, Tolonen T, Kuokkanen H, Kaartinen I. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections: a randomized controlled trial. J Plast Reconstruct Aesthet Surg. 2019;72(1):4–11.
Sharma S, Vinay K, Bassi R. Treatment of Small Keloids Using Intralesional 5-fluorouracil and Triamcinolone Acetonide versus Intralesional Bleomycin and Triamcinolone Acetonide. J Clin Aesthet Dermatol. 2021;14(3):17-21.
Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204(2):130-2.
Nanda S, Reddy BS. Intralesional 5-fluorouracil as a treatment modality of keloids. Dermatol Surg. 2004;30(1):54-6; discussion 56-7.
Saha AK, Mukhopadhyay M. A comparative clinical study on role of 5-flurouracil versus triamcinolone in the treatment of keloids. Ind J Surg. 2012;74(4):326–9.
Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47(4 Suppl):S209-11.
Berman B, Villa A. Imiquimod 5% cream for keloid management. Dermatol Surg. 2003;29(10):1050–1.
Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157:8–13.
Shin JY, Yun SK, Roh SG, Lee NH, Yang KM. Efficacy of 2 Representative Topical Agents to Prevent Keloid Recurrence After Surgical Excision. J Oral Maxillofac Surg. 2017;75(2):401.e1-401.e6.
Berman B, Harrison-Balestra C, Perez OA, Viera M, Villa A, Zell D, Ramirez C. Treatment of keloid scars post-shave excision with imiquimod 5% cream: A prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol. 2009;8(5):455-8.
Cação FM, Tanaka V, Messina MC. Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg. 2009;35(4):629-33.
Klotz T, Munn Z, Aromataris EC, Greenwood JE. Imiquimod to prevent keloid recurrence postexcision: A systematic review and meta-analysis. Wound Repair Regen. 2020;28(1):145–56.
Fahie S, Cassagnol M. Verapamil. London: BTI-StatPearls: StatPearls Publishing; 2022.
Doong H, Dissanayake S, Gowrishankar TR, LaBarbera MC, Lee RC. The 1996 Lindberg Award. Calcium antagonists alter cell shape and induce procollagenase synthesis in keloid and normal human dermal fibroblasts. J Burn Care Rehabil. 1996;17(6 Pt 1):497-514.
Srivastava S, Kumari H, Singh A. Comparison of fractional CO2 laser, verapamil, and triamcinolone for the treatment of keloid. Adv Wound Care. 2019;8(1):7–13.
Li Z, Jin Z. Comparative effect and safety of verapamil in keloid and hypertrophic scar treatment: a meta-analysis. Ther Clin Risk Manage. 2016;12:1635–41.
McGinty S, Siddiqui WJ. Keloid. London: BTI-StatPearls: StatPearls Publishing; 2021.
Arima J, Dohi T, Kuribayashi S, Akaishi S, Ogawa R. Z-plasty and Postoperative Radiotherapy for Anterior Chest Wall Keloids: An Analysis of 141 Patients. Plast Reconstr Surg Glob Open. 2019;7(3):e2177.
Shin JY, Lee JW, Roh SG, Lee NH, Yang KM. A Comparison of the Effectiveness of Triamcinolone and Radiation Therapy for Ear Keloids after Surgical Excision: A Systematic Review and Meta-Analysis. Plast Reconstr Surg. 2016;137(6):1718-1725.
Apfelberg DB, Maser MR, Dds HL, White D, Weston J. Preliminary results of argon and carbon dioxide laser treatment of keloid scars. Lasers Surg Med. 1984;4(3):283–90.
Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26(03):109–16.
Makboul M, Makboul R, Abdelhafez AH, Hassan SS, Youssif SM. Evaluation of the effect of fractional CO 2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J Cosmet Dermatol. 2014;13(3):169–79.
Lee SJ, Suh DH, Lee JM, Song KY, Ryu HJ. Dermal remodeling of burn scar by fractional CO2 laser. Aesthetic Plast Surg. 2016;40(5):761–8.
Patel SP, Nguyen HV, Mannschreck D, Redett RJ, Puttgen KB, Stewart FD. Fractional CO2 laser treatment outcomes for pediatric hypertrophic burn scars. J Burn Care Res. 2019;40(4):386–91.
Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31(1):9–18.
Poetschke J, Dornseifer U, Clementoni MT, Reinholz M, Schwaiger H, Steckmeier S, et al. Ultrapulsed fractional ablative carbon dioxide laser treatment of hypertrophic burn scars: evaluation of an in-patient controlled, standardized treatment approach. Lasers Med Sci. 2017;32(5):1031–40.
Levi B, Ibrahim A, Mathews K, Wojcik B, Gomez J, Fagan S, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37(2):106–14.
Liu A, Moy RL, Victor Ross E, Hamzavi I, Ozog DM. Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38(3):351–66.
Artzi O, Friedman O, Al-Niaimi F, Wolf Y, Mehrabi JN. Mitigation of postsurgical scars using lasers: a review. Plast Reconstr Surg Glob Open. 2020;8(4): e2746.
Pongcharoen P, Pongcharoen B, Disphanurat W. The effectiveness of a 595 nm pulsed-dye-laser in the treatment of surgical scars following a knee arthroplasty. J Cosmet Laser Ther. 2019;21(6):352–6.
Nouri K, Rivas MP, Stevens M, Ballard CJ, Singer L, Ma F, et al. Comparison of the effectiveness of the pulsed dye laser 585 nm versus 595 nm in the treatment of new surgical scars. Lasers Med Sci. 2009;24(5):801–10.
Koike S, Akaishi S, Nagashima Y, Dohi T, Hyakusoku H, Ogawa R. Nd:YAG laser treatment for keloids and hypertrophic scars: an analysis of 102 cases. Plast Reconstr Surg Glob Open. 2014;2(12): e272.
Pan L, Qin H, Li C, Zhang G, Yang L, Zhang L. Efficacy of the neodymium-doped yttrium aluminum garnet laser in the treatment of keloid and hypertrophic scars: a systematic review and meta-analysis. Aesthetic Plast Surg. 2022.
Li K, Nicoli F, Cui C, Xi WJ, Al-Mousawi A, Zhang Z, et al. Treatment of hypertrophic scars and keloids using an intralesional 1470 nm bare-fibre diode laser: a novel efficient minimally-invasive technique. Sci Rep. 2020;10(1):21694.
Lin JY, Warger WC, Izikson L, Anderson RR, Tannous Z. A prospective, randomized controlled trial on the efficacy of fractional photothermolysis on scar remodeling. Lasers Surg Med. 2011;43(4):265–72.
Shin J, Cho JT, Park SI, Jung SN. Combination therapy using non-ablative fractional laser and intralesional triamcinolone injection for hypertrophic scars and keloids treatment. Int Wound J. 2019;16(6):1450–6.
Verhaeghe E, Ongenae K Fau - Bostoen J, Bostoen J Fau - Lambert J, Lambert J. Nonablative fractional laser resurfacing for the treatment of hypertrophic scars: a randomized controlled trial. 2013.
Tierney E, Mahmoud BH, Srivastava D, Ozog D, Kouba DJ. Treatment of surgical scars with nonablative fractional laser versus pulsed dye laser: a randomized controlled trial. Dermatol Surg. 2009;35(8):1172-80.
Anderson RR, Donelan MB, Hivnor C, Greeson E, Ross EV, Shumaker PR, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing. JAMA Dermatol. 2014;150(2):187.
Seago M, Shumaker PR, Spring LK, Alam M, Al-Niaimi F, Rox Anderson R, et al. Laser treatment of traumatic scars and contractures: 2020 International Consensus recommendations. Lasers Surg Med. 2020;52(2):96–116.
Conejo-Mir JS, Corbi R, Linares M. Carbon dioxide laser ablation associated with interferon alfa-2b injections reduces the recurrence of keloids. J Am Acad Dermatol. 1998;39(6):1039-40.
Cavalié M, Sillard L, Montaudié H, Bahadoran P, Lacour J-P, Passeron T. Treatment of keloids with laser-assisted topical steroid delivery: a retrospective study of 23 cases. Dermatol Ther. 2015;28(2):74–8.
Sabry HH, Abdel Rahman SH, Hussein MS, Sanad RR, Abd El Azez TA. The Efficacy of Combining Fractional Carbon Dioxide Laser With Verapamil Hydrochloride or 5-Fluorouracil in the Treatment of Hypertrophic Scars and Keloids: A Clinical and Immunohistochemical Study. Dermatol Surg. 2019;45(4):536-546.
Ouyang H-W, Li G-F, Lei Y, Gold MH, Tan J. Comparison of the effectiveness of pulsed dye laser vs pulsed dye laser combined with ultrapulse fractional CO2 laser in the treatment of immature red hypertrophic scars. J Cosmet Dermatol. 2018;17(1):54–60.
Cohen JL, Geronemus R. Safety and efficacy evaluation of pulsed dye laser treatment, CO2 ablative fractional resurfacing, and combined treatment for surgical scar clearance. J Drugs Dermatol. 2016;15(11):1315–9.
Zhang J, Zhou S, Xia Z, Peng Z, Cheng X, Yang X, et al. 595-nm pulsed dye laser combined with fractional CO2 laser reduces hypertrophic scar through down-regulating TGFβ1 and PCNA. Lasers Med Sci. 2021;36(8):1625–32.
Lee YI, Kim J, Yang CE, Hong JW, Lee WJ, Lee JH. Combined therapeutic strategies for keloid treatment. Dermatol Surg. 2019;45(6):802–10.
Verma KK, Garg T, Raj T. Carbon dioxide leaser for the treatment of keloids. Indian J Dermatol. 2002;47(2):91.
Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387–96.
Wang J, Wu J, Xu M, Gao Q, Chen B, Wang F, et al. Combination therapy of refractory keloid with ultrapulse fractional carbon dioxide (CO2). Dermatol Ther. 2020;33(6):14359.
Forbat E, Al-Niaimi F. Treatment of striae distensae: an evidence-based approach. J Cosmet Laser Ther. 2019;21(1):49–57.
Yun PL, Tachihara R, Anderson RR. Efficacy of erbium:yttrium-aluminum-garnet laser-assisted delivery of topical anesthetic. J Am Acad Dermatol. 2002;47(4):542-7.
Braun SA, Schrumpf H, Buhren BA, Homey B, Gerber PA. Laser-assisted drug delivery: mode of action and use in daily clinical practice. JDDG. 2016;14(5):480–8.
Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45(3):135–40.
Abd El-Dayem DH, Nada HA, Hanafy NS, Elsaie ML. Laser-assisted topical steroid application versus steroid injection for treating keloids: a split side study. J Cosmet Dermatol. 2021;20(1):138–42.
Sabry HH, Ibrahim EA, Hamed AM. Assessment of laser-assisted delivery vs intralesional injection of botulinum toxin A in treatment of hypertrophic scars and keloids. Dermatol Ther. 2020;33:6.
Park JH, Chun JY, Lee JH. Laser-assisted topical corticosteroid delivery for the treatment of keloids. Lasers Med Sci. 2017;32(3):601–8.
Waibel JS, Wulkan AJ, Rudnick A, Daoud A. Treatment of Hypertrophic Scars Using Laser-Assisted Corticosteroid Versus Laser-Assisted 5-Fluorouracil Delivery. Dermatol Surg. 2019;45(3):423-430.
Truong K, Prasidha I, Wain T. A systematic review of randomised controlled trials investigating laser assisted drug delivery for the treatment of keloid and hypertrophic scars. Lasers Med Sci. 2022;37(1):47–59.
Mankowski P, Kanevsky J, Tomlinson J, Dyachenko A, Luc M. Optimizing Radiotherapy for Keloids: A Meta-Analysis Systematic Review Comparing Recurrence Rates Between Different Radiation Modalities. Ann Plast Surg. 2017;78(4):403-411.
Miles OJ, Zhou J, Paleri S, Fua T, Ramakrishnan A. Chest keloids: effect of surgical excision and adjuvant radiotherapy on recurrence, a systematic review and meta-analysis. ANZ J Surg. 2021;91(6):1104–9.
Shen J, Lian X, Sun Y, Wang X, Hu K, Hou X, et al. Hypofractionated electron-beam radiation therapy for keloids: retrospective study of 568 cases with 834 lesions. J Radiat Res. 2015;56(5):811–7.
Ji J, Tian Y, Zhu Y-Q, Zhang L-Y, Ji S-J, Huan J, et al. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol. 2015;42(1):56–63.
Liu EK, Cohen RF, Chiu ES. Radiation therapy modalities for keloid management: a critical review. J Plast Reconstr Aesthet Surg. 2022.
Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med School. 2016;83(2):46–53.
Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol. 2005;181(11):717–23.
Wang LZ, Ding JP, Yang MY, Chen B. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. Dermatol Surg. 2014;40(12):1378–84.
Pashazadeh A, Boese A, Friebe M. Radiation therapy techniques in the treatment of skin cancer: an overview of the current status and outlook. J Dermatolog Treat. 2019;30(8):831–9.
Bijlard E, Verduijn GM, Harmeling JX, Dehnad H, Niessen FB, Meijer OWM, et al. Optimal high-dose-rate brachytherapy fractionation scheme after keloid excision: a retrospective multicenter comparison of recurrence rates and complications. Int J Radiat Oncol Biol Phys. 2018;100(3):679–86.
Guida S, Pellacani G, Bencini PL. Picosecond laser treatment of atrophic and hypertrophic surgical scars: In vivo monitoring of results by means of 3D imaging and reflectance confocal microscopy. Skin Res Technol. 2019.
Gold MH, Nestor MS, Berman B, Goldberg D. Assessing keloid recurrence following surgical excision and radiation. Burns Trauma. 2020;8:tkaa01.
Cognetta AB, Wolfe CM, Goldberg DJ, Hong HG. Practice and educational gaps in radiation therapy in dermatology. Dermatol Clin. 2016;34(3):319–33.
Song C, Wu HG, Chang H, Kim IH, Ha SW. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55(5):912–6.
Emad M, Omidvari S, Dastgheib L, Mortazavi A, Ghaem H. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19(5):402–5.
Jones ME, Ganzer CA, Bennett D, Finizio A. Surgical excision of keloids followed by in-office superficial radiation therapy: prospective study examining clinicaloOutcomes. Plast Reconstr Surg Glob Open. 2019;7(5): e2212.
Berman B, Nestor MS, Gold MH, Goldberg DJ, Weiss ET, Raymond I. A retrospective registry study evaluating the long-term efficacy and safety of superficial radiation therapy following excision of keloid scars. J Clin Aesthet Dermatol. 2020;13(10):12–6.
Nestor MS, Berman B, Goldberg D, Cognetta AB, Gold M, Roth W, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12(2):12–8.
Li L, Yuan C, Zhang X, Wang B, Yan Y. Superficial X-ray-induced hyperpigmentation in postoperative keloid radiotherapy: a study of 70 keloids to identify clinical features and risk factors. J Cosmet Dermatol. 2021;20(9):2880–6.
Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124(4):1196-1201.
Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111(6):1841-52.
Zouboulis CC. Outcomes of cryosurgery in keloids and hypertrophic scars. Arch Dermatol. 1993;129(9):1146.
O’Boyle CP, Shayan-Arani H, Hamada MW. Intralesional cryotherapy for hypertrophic scars and keloids: a review. Scars Burns Healing. 2017;3:205951311770216.
Rusciani L, Rossi G, Bono R. Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol. 1993 Jun;19(6):529-34.
van Leeuwen MC, Bulstra AE, Ket JC, Ritt MJ, van Leeuwen PA, Niessen FB. Intralesional cryotherapy for the treatment of keloid scars: evaluating effectiveness. 2015.
Har-Shai Y, Sabo E Fau - Rohde E, Rohde E Fau - Hyams M, Hyams M Fau - Assaf C, Assaf C Fau - Zouboulis CC, Zouboulis CC. Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies. 2006.
Zhang X, Wu Q, Wei M, Deng X, Gu C, Wang Z. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: a systematic review and meta-analysis of comparative studies. Int J Colorect Dis. 2020;35(10):1831–9.
Mearza AA, Aslanides IM. Uses and complications of mitomycin C in ophthalmology. Expert Opin Drug Saf. 2007;6(1):27-32.
Simman R, Alani H, Williams F. Effect of mitomycin C on keloid fibroblasts: an in vitro study. Ann Plast Surg. 2003;50(1):71-6.
Sanders KW, Gage-White L, Stucker FJ. Topical mitomycin C in the prevention of keloid scar recurrence. Arch Facial Plast Surg. 2005;7(3):172-5.
Mandour Y, Bake H, Mofty E, Ramadan E, Gomaa M, Akl E, Elrefae A. Topical versus interlesional mitomycin C in auricular keloids. Acta Otorrinolaringol Esp (Engl Ed). 2021;72(5):280-287.
Seo S-H, Sung H-W. Treatment of keloids and hypertrophic scars using topical and intralesional mitomycin C. J Eur Acad Dermatol Venereol. 2012;26(5):634–8.
Stewart Iv CE, Kim JY. Application of mitomycin-C for head and neck keloids. Otolaryngol Head Neck Surg. 2006;135(6):946–50.
Frampton JE, Easthope SE. Botulinum toxin A (Botox Cosmetic): a review of its use in the treatment of glabellar frown lines. Am J Clin Dermatol. 2003;4(10):709-25.
Yang W, Li G. The Safety and efficacy of botulinum toxin type A injection for postoperative scar prevention: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19(4):799–808.
Dai X, Lei TC. Botulinum toxin A promotes the transdifferentiation of primary keloid myofibroblasts into adipocyte-like cells. Basic Clin Pharmacol Toxicol. 2021;129(6):462–9.
Ismail SA, Mohammed NHK, Sotohy M, Abou-Taleb DAE. Botulinum toxin type A versus 5-fluorouracil in treatment of keloid. Arch Dermatol Res. 2021;313(7):549–56.
Cho SB, Lee SJ, Cho S, Oh SH, Chung WS, Kang JM, et al. Non-ablative 1550-nm erbium-glass and ablative 10 600-nm carbon dioxide fractional lasers for acne scars: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24(8):921–5.
Liu XG, Zhang D. Evaluation of efficacy of corticosteroid and corticosteroid combined with botulinum toxin type A in the treatment of keloid and hypertrophic scars: a meta-analysis. Aesthetic Plast Surg. 2021;45(6):3037–44.
Sun P, Lu X, Zhang H, Hu Z. The efficacy of drug injection in the treatment of pathological scar: a network meta-analysis. Aesthetic Plast Surg. 2021;45(2):791–805.
Bi M, Sun P, Li D, Dong Z, Chen Z. Intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: a systematic review and meta-analysis. Med Sci Monit. 2019;25:2950–8.
Wilson AM. Eradication of keloids: Surgical excision followed by a single injection of intralesional 5-fluorouracil and botulinum toxin. Can J Plast Surg. 2013;21(2):87–91.
Laurent TC, Fraser JR. Hyaluronan. 1992.
A DIS. Ear Keloid Treated with Infiltrated non-cross-linked hyaluronic acid and cortisone therapy. 2016;1791–7549.
Alaish SM, Yager DR, Diegelmann RF, Cohen IK. Hyaluronic acid metabolism in keloid fibroblasts. J Pediatr Surg. 1995;30(7):949-52.
Sidgwick GP, Iqbal SA, Bayat A. Altered expression of hyaluronan synthase and hyaluronidase mRNA may affect hyaluronic acid distribution in keloid disease compared with normal skin. Exp Dermatol. 2013;22(5):377–9.
Aggarwal A, Ravikumar BC, Vinay KN, Raghukumar S, Yashovardhana DP. A comparative study of various modalities in the treatment of keloids. Int J Dermatol. 2018;57(10):1192–200.
Wu DC, Goldman MP, Wat H, Chan HHL. A systematic review of picosecond laser in dermatology: evidence and recommendations. Lasers Surg Med. 2021;53(1):9–49.
Choi YJ, Kim JY, Nam JH, Lee GY, Kim WS. Clinical outcome of 1064-nm picosecond neodymium-doped yttrium aluminium garnet laser for the treatment of hypertrophic scars. 2018;2018:1476–80.
Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–9.
Wong AJS, Song EJ. Dupilumab as an adjuvant treatment for keloid-associated symptoms. JAAD Case Rep. 2021;13:73–4.
Peterson DM, Damsky WE, Vesely MD. Treatment of lichen sclerosus and hypertrophic scars with dupilumab. JAAD Case Rep. 2022;23:76–8.
Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21(2):197–9.
Tan A, Martinez Luna O, Glass DA. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46(10):1353–6.
Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123(3):339–46.
Wong TW, Lee JY, Sheu HM, Chao SC. Relief of pain and itch associated with keloids on treatment with oxpentifylline. Br J Dermatol. 1999;140(4):771–2.
Serag-Eldin YMA, Mahmoud WH, Gamea MM, Hegab DS. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20(10):3330–40.
Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res. 2015;91:15–28.
Isaji M, Nakajoh M, Naito J. Selective inhibition of collagen accumulation by N-(3,4-dimethoxycinnamoyl)anthranilic acid (N-5’) in granulation tissue. Biochem Pharmacol. 1987;36(4):469–74.
Nanba K, Oura T, Soeda S, Sioya N, Tsukada S, Hanaoka K. Clinical evaluation of tranilast for keloid and hypertrophic scarring: optimal dose finding study in a double blind study. Nessho. 1992;18:30–45.
Nakamura K, Irie H, Inoue M, Mitani H, Sunami H, Sano S. Factors affecting hypertrophic scar development in median sternotomy incisions for congenital cardiac surgery. J Am Coll Surg. 1997;185(3):218–23.
Kim J, Lee YI, Lee JH. Efficacy and safety of oral tranilast for keloid scar: a single-center, retrospective observational study.2020;72(1):416.
El-Hamid El-Azhary EA, Abd Al-Salam FM, El-Hafiz HSA, Maghraby HM. Fractional carbon dioxide (CO). Dermatol Pract Concept. 2022;12(2): e2022072.
Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399–408.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no funding for the completion of this review.
Conflicts of interest/competing interests
The authors have no conflict of interests to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
F.S.F., R.U., L.H., and K.N. developed the main conceptual ideas and proof outline. F.S.F. and R.U. worked out almost all the technical details within the manuscript. F.S.F., R.U., and L.H. wrote the manuscript with the support of G.A.Z. and I.D. Tables were devised and created by R.U. and F.S.F. K.N. supervised the manuscript preparation. All authors approved the final version of the submitted manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frech, F.S., Hernandez, L., Urbonas, R. et al. Hypertrophic Scars and Keloids: Advances in Treatment and Review of Established Therapies. Am J Clin Dermatol 24, 225–245 (2023). https://doi.org/10.1007/s40257-022-00744-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00744-6