Abstract
Our own studies and those of others have shown that defects in essential fatty acid (EFA) metabolism occurs in age-related disorders such as obesity, type 2 diabetes mellitus, hypertension, atherosclerosis, coronary heart disease, immune dysfunction and cancer. It has been noted that in all these disorders there could occur a defect in the activities of desaturases, cyclo-oxygenase (COX), and lipoxygenase (LOX) enzymes leading to a decrease in the formation of their long-chain products gamma-linolenic acid (GLA), arachidonic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA). This leads to an increase in the production of pro-inflammatory prostaglandin E2 (PGE2), thromboxanes (TXs), and leukotrienes (LTs) and a decrease in anti-inflammatory lipoxin A4, resolvins, protectins and maresins. All these bioactive molecules are termed as bioactive lipids (BALs). This imbalance in the metabolites of EFAs leads to low-grade systemic inflammation and at times acute inflammatory events at specific local sites that trigger the development of various age-related disorders such as obesity, type 2 diabetes mellitus, hypertension, coronary heart disease, atherosclerosis, and immune dysfunction as seen in rheumatoid arthritis, lupus, nephritis and other localized inflammatory conditions. This evidence implies that methods designed to restore BALs to normal can prevent age-related disorders and enhance longevity and health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioactive lipids
- Aging
- Disorders
- Diseases
- Diabetes mellitus
- Hypertension
- Coronary heart disease
- Atherosclerosis
- Cancer
- Immune dysfunction
- Lupus
- Rheumatoid arthritis
- Retinopathy
- Nephritis
1 Introduction
Aging is inevitable but can be slowed. As we age, cells, tissues, organs and systems are bound to become senile and develop several health-related issues that may ultimately affect the health and lead to the onset of various diseases. But aging can be healthy so that disease occurrence and progression can be prevented, postponed or altogether avoided. Despite the fact that aging is believed to be a genetically programmed event, understanding the biochemical changes that predispose to the development of various disorders associated with aging may lead to development of strategies that keep the human organism healthy. Several studies in the field of aging have been performed with specific reference to changes in gene expression and their proteins, alterations in the activity of various enzymes and consequently alterations in the cellular functions that results in the aging process.
There are two dominant theories of aging: damage-based and programmed. The damage-based theory suggests that aging results from a continuous process of damage, presumably to DNA, which leads to alterations in several metabolic pathways. This theory implies that DNA damage occurs throughout the entire lifespan induced by byproducts released during the normal cellular process or a consequence of inefficient repair systems. In contrast to this, the programmed theory of aging argues that aging is a genetically-regulated process but not as a result of random or stochastic events. Although both the theories appear different, the underlying mechanism is in both is damage to DNA that results in altered gene(s) expression or alterations in gene expression as a programmed process. Thus, in theory, it is possible to stop, or even reverse aging provided that the altered gene(s) expressions can be identified and restored to normal. In essence both theories have a strong genetic component. It can be said that the damage-based theory of aging suggests that genetic factors such as defensive or protective genes, have a role in aging while the programmed theory does recognize that some forms of damage to DNA contribute to aging and that environmental factors have a significant role in the aging process. So the difference between these two theories lies in the underlying mechanism. Damage-based theories of aging argue that aging is predominantly a result of interactions with the environment and/or damage from chemical reactions, while the programmed theories argue that aging is predetermined and occurs on a fixed schedule triggered by genetic programs. Other theories of aging include: (i) extrinsic or intrinsic factors that cause an accumulation of damage; and (ii) changes in gene expression that are either programmed or derived from DNA structural changes. It is apparent that there is a certain amount of overlap between all of these theories of aging.
In essence, aging is unavoidable and influenced by various endogenous and exogenous factors that result in gradual cellular deterioration. Studies have revealed that certain interventions can increase life expectancy and inhibit the aging process [1]. For instance, aging can be postponed or delayed (i) in mice, worms and fruit flies by inhibiting the insulin/IGF-1 axis that results in a transcription factor (DAF-16 in C. elegans, FoXo in mice) entering the nucleus to stimulate the expression of genes encoding survival-promoting proteins such as the Klotho protein; (ii) scavenging the highly toxic reactive oxygen species (ROS) produced mainly in the mitochondria, whose accumulation leads to DNA, lipid and protein changes (that results in cell dysfunction and aging); (iii) preventing shortening of the telomeres by enhancing telomerase activity; and (iv) correcting defective autophagy that uses lysosomes to destroy altered proteins to retain cell homeostasis. Studies of genetically mediated aging disorders such as progeria have revealed the importance of laminins (intermediate nuclear filaments) which fail to mature causing accelerated aging and premature death. It is agreed that there is no single biological marker of aging but measuring a combination of markers of specific diseases associated with aging may be of help in preventing or at least prevent or postpone some disorders of aging. For example, measurement of Nt-proBNP, troponin I, C-reactive protein and cystatin that are increased in atheroma and cardiovascular diseases may help in preventing these diseases by employing suitable remedial measures. Different organs age in different ways. Vessel walls become rigid due to protein glycation and develop atheroma, the heart is invaded by fibrosis, the brain suffers from neurofibrillar degeneration and senile plaques (responsible for Alzheimer’s disease), the retina undergoes macular degeneration, renal function declines due to a gradual decrease in the nephron pool, and immune defenses become less effective due to the functional degradation of B and T lymphocytes and thymus involution, resulting in the development of cancer or autoimmune disorders . Despite all of the advances made in molecular and biochemical understanding of cell function(s), the only two measures that are known to slow the aging process are physical exercise and dietary restriction in the form of reduced calorie intake.
Recent studies by us and others showed that there are some distinct changes in the metabolism of bioactive lipids (BALs) that may predispose to the development of age-related disorders such as obesity, type 2 diabetes mellitus, hypertension, atherosclerosis, coronary heart disease (CHD), immune dysfunction and cancer. This implies that abnormalities in BAL metabolism can lead to the occurrence of these disorders at an early age and rectifying BAL metabolism could form a novel therapeutic approach for these disorders. Since most of these disorders are common with advancing age, restoring BALs to normal may have implications in preventing aging itself or may aid in healthy aging. It is possible that occurrence of some of these disorders is an indication of premature aging. This suggests that plasma levels or specific tissue levels of various BALs could be used as a measure of aging. It is interesting to note that both physical exercise and diet restriction enhance BAL metabolism such that inappropriate inflammation and imbalance in the immune system are restored to normal.
2 Essential Fatty Acid Metabolism
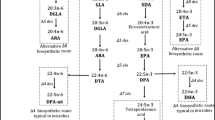
Our dietary essential fatty acids (EFAs): cis-linoleic acid (LA, 18:2 n-6) and alpha-linolenic acid (ALA, 18:3 n-3) are converted to their long-chain metabolites by the action of delta-6- desaturase and delta-5-desaturase and elongases. Thus, LA is converted to gamma-linolenic acid (GLA, 18:3 n-6), dihomo-gamma-linolenic acid (DGLA, 20:3 n-6) and arachidonic acid (AA, 20:4 n-6) whereas ALA is converted to eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3) by the same set of enzymes (Figs. 3.1 and 3.2; for EFA metabolism). It is noteworthy that several vitamins, minerals and trace elements influence EFA metabolism (Fig. 3.2) and deficiencies in these can result in the formation of higher amounts of pro-inflammatory and a decrease in the synthesis of anti-inflammatory metabolites. It is noteworthy that vitamins B1, B6, B12 and C are needed for adequate synthesis of GLA, DGLA, AA, EPA and DHA, precursors that in turn are needed for the formation of prostaglandin E1 (PGE1), prostacyclin (PGI2), lipoxins, resolvins protectins and maresins, which have potent anti-inflammatory, vasodilator, anti-platelet anti-aggregator and cytoprotective actions [2,3,4,5,6,7,8,9,10,11]. AA is the precursor of 2 series PGs, thromboxanes (TXs) and 4 series leukotrienes (LTs) whereas EPA is the precursor of 3 series PGs, TXs and 5 series LTs. PGs, TXs and LTs have pro-inflammatory actions. It is noteworthy that 3 series PGs, TXs and 5 series LTs are also pro-inflammatory in nature but are less potent compared to 2 series PGs TXs and 4 series LTs. Hence the suggestion that PGs, TXs and LTs formed from EPA have anti-inflammatory actions is not correct. AA, EPA and DHA are also metabolized by cytochrome P450 enzyme system to form various products that have been outlined in Figs. 3.3, 3.4, 3.5 and 3.6 in addition to the action of COX and LOX enzymes. In general, more detailed studies have been performed on the metabolic products formed by the action of COX and LOX enzymes compared to the cytochrome P450 enzymes system. One needs to consider P450 products formed from AA, EPA and DHA while studying the actions of various metabolites of EFAs and PUFAs.
Scheme showing metabolism of EFAs (LA and ALA) and also the metabolism of n-7 and n-9 fatty acids. Although all fatty acids are important for normal health, n-3 and n-6 seems to be more critical. It is to be noted that “n” is same as “ω”. A simplified version and other products formed from EFAs are given in Fig. 3.2
Scheme showing metabolism of AA by the cytochrome P450 enzyme system. Both EPA and DHA also undergo similar metabolism by the cytochrome P450s. AA is metabolized by cytochrome P450 mono-oxygenases to ω- and ω-1-hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acids (epoxides, EETs), and dihydroxyeicosatrienoic acids (diols, DHTs). 20-HETE and 5,6-EET can be converted by COX to analogues of PGs
In contrast to the pro-inflammatory actions of PGs, LTs and TXs, certain specific anti-inflammatory products can also be formed from AA, EPA and DHA. These include lipoxins from AA, resolvins of E series from EPA and resolvins of D series and protectins and maresins from DHA (Fig. 3.3). Hence, it is likely that under physiological conditions a balance is maintained between pro- and anti-inflammatory compounds formed from GLA, DGLA, AA, EPA, DPA and DHA. The production of various PGs, TXs, LTs, lipoxins, resolvin, protectins and maresins from their respective precursors depends on the concentrations of GLA, DGLA, AA, EPA, DPA and DHA in the cell membrane lipid pool of these fatty acids released by the action of the enzyme phospholipase A2 (PLA2). It is known that PLA2 is activated by various stimuli including injury, infection, LPS (lipopolysaccharide), IL-6, TNF-α, IL-1, IL-2, IL-4, HMGB1 and other inciting agents that are capable of perturbing the cell membrane. It is predicted that PLA2 is able to activate in a specific and coordinated fashion such that the release of GLA, DGLA, AA, EPA, DPA and DHA from the cell membrane is determined based on the context and necessity of the local events either to initiate and perpetuate inflammation and/or suppress inflammation and initiate resolution of inflammation and restore homeostasis. Thus, the release of PGs, TXs, LTs, lipoxins, resolvins, protectins and maresins appears to occur in a deliberately coordinated and smooth manner to shift the local events from pro-inflammatory status to resolution of the inflammation phase. How this occurs is not completely clear. However, it is known that local factors play a significant role in this sequence of events. Some of these local factors that may have the ability to regulate the production of BALs include pH, lactate, potassium, sodium, magnesium, and glucose and its metabolite concentrations. It is noteworthy that EFAs and PUFAs and other BAL molecules are capable of altering the function of mechanosensitive channels such as PIEZO-1 and PIEZO-2 and TRPV and thus, regulate the structure and functions of various receptors located on the cell membrane. In addition, local infiltrating leukocytes, T cells, NK cells and macrophages including endothelial cells, fibroblasts and cellular milieu are also capable of influencing the activity of PLA2, desaturases, elongases, COXs, LOXs, PG synthase, 15-PGDH (15-prostaglandin dehydrogenase) and other eicosanoid catabolic enzymes that can alter local concentrations of EFAs, PUFAs, PGs, LTs, TXs, lipoxins, resolvins, protectins and maresins. Thus, the formation of actions of BALs are complex. At the same time, in view of their large number of actions, EFAs and BALs are capable of influencing a number of cellular events and thus participate in a number of disorders that include inflammatory, immunological and degenerative disorders as discussed below. Understanding the various actions of EFAs and BALs and their role in various diseases opens a new window of opportunity to exploit these as potential drug targets for various disorders.
Note that the term EFAs is used for LA and ALA, PUFAs refer to GLA, DGLA, AA, EPA, DPA and DHA and BAL refers to EFAs, PUFAs and lipoxins, resolvins, protectins and maresins in the present discussion.
3 Actions of Bioactive Lipids
BALs have several important actions that may explain their involvement in many cellular functions and biological processes and disorders. Some of these significant actions of BALs include: (i) participation in inflammatory processes; (ii) modulation of the immune response in cancer other immunological disorders; (iii) influencing the actions of ion channels including Piezo1 and Piezo 2 and voltage gated ion channels such as the transient receptor potential cation channel subfamily V member 1 (TrpV1) in the cell mitotic process, cell signaling, cell cycle progression, and cell volume regulation; (iv) altering cell membrane fluidity, influencing the structure and composition of intermediate filaments and their multiple binding partners involved in cellular mechanics and gene regulation; (v) and regulating mitochondrial processes, telomerase activity; and G-protein–mediated signal transduction.
4 Inflammation
It is interesting to note that GLA, DGLA, AA, EPA, DPA and DHA have potent anti-inflammatory actions by themselves without the necessity of formation of their respective metabolites: PGs, TXs, LTs, lipoxins, resolvins, protectins and maresins. GLA, DGLA, AA, EPA and DHA inhibit the formation of pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), IL-1, and HMGB1 (high mobility group box-1) [12,13,14,15,16,17,18,19,20,21,22]. Since both AA and EPA form precursors to pro-inflammatory PGs, LTs, TXs and anti-inflammatory lipoxins and resolvins, it is likely that their concentrations in the cell membrane, the amount(s) of each of these fatty acids that are released in response to PLA2 activation, their conversion to the respective metabolites (PGs, LTs, TXs vs lipoxins and resolvins), and their degradation determines the final outcome of the inflammatory process. Thus, it is anticipated that there is a delicate balance between the pro- and anti-inflammatory products of AA and EPA. Hence, it is likely that inflammation is triggered and perpetuated if the pro-inflammatory PGs, LTs and TXs are produced in excess whereas inflammation is suppressed, and resolution ensues if the production of lipoxins and resolvins are formed in adequate amounts. It is noteworthy that lipoxins and resolvins are capable of suppressing the production and antagonizing the actions of PGs, LTs and TXs and thus, inhibiting inflammation and initiating inflammation resolution processes [2, 23,24,25,26,27,28,29]. DHA, the precursor of resolvins of D series, protectins and maresins have anti-inflammatory, cytoprotective and wound healing properties [1, 23, 30, 31]. It is also evident that lipoxins and maresins are capable of reducing inflammatory edema, neuropathic pain, and enhancing tissue regeneration partly by acting on the TRPV1 channels [31]. Our studies revealed that both AA and LXA4 (lipoxin A4) not only inhibit inflammation by decreasing the production of IL-6 and TNF-α but also suppress NF-kB and COX-2 expression and enhance the proliferation of pancreatic β cells [32, 33]. The beneficial actions of AA appear to be due to formation of LXA4. Surprisingly, we observed that experimental animals treated with EPA, DHA and other fatty acids also showed enhanced levels of LXA4 despite the fact that LXA4 is derived from AA. This suggests that fatty acids other than AA when administered can displace AA from the cell membrane lipid pool and this displaced AA could be converted to LXA4. The other possibility is that there is a crosstalk among lipoxins, resolvins, protectins and maresins such that they are able to augment the production of each other as the situation demands. If this is true, it is not clear why this crosstalk needs to occur. The fact that lipoxins, resolvins, protectins and maresins that have similar anti-inflammatory, inflammation resolution and wound healing properties but are produced from different precursors suggests that there are more well-designed but separate actions that are critical for wound healing and other beneficial actions. In this context, it is noteworthy that lipoxins, resolvins, protectins and maresins also show cytoprotective and cell proliferation regulatory actions in addition to their anti-inflammatory role. This suggests that lipoxins, resolvins, protectins and maresins may have more selective, specific and beneficial actions in addition to their action on inflammation. Such an assumption is supported by the observation that LXA4 has more potent anti-diabetic action compared to resolvins (unpublished data). Similarly, LXA4 is more potent that the resolvins in suppressing the production of IL-6 and TNF-α in alloxan and streptozotocin-induced type 1 and type 2 diabetes mellitus animal models. Thus, although lipoxins, resolvins, protectins and maresins show anti-inflammatory actions, their potency is variable. No studies have been performed to assess such variations in their potency but some preliminary predictions are possible as shown in Fig. 3.7. In this context, the role of PLA2 in inflammation and formation of PGs, LTs, TXs and lipoxins, resolvins, protectins and maresins needs attention.
Scheme showing the relationship among pro- and anti-inflammatory cytokines, PGs, LTs, lipoxins, resolvins, protectins and maresins and steroids. Metabolism and actions of AA are shown as a representative of various PUFAs (DGLA, EPA and DHA). For further details see the text. RSVs resolvins, PRTs protectins; MaRs maresins. During the inflammatory process it is expected that there will be activation of desaturases, COX-2 and LOX enzymes depending on the stage and duration of inflammation. The proposed levels of anti-inflammatory lipoxins (LXA4), RSVs, PRTs and MaRs, and pro-inflammatory PGE2 can be as follows (it needs to be noted that PGE2 is depicted as a representative of all pro-inflammatory lipids and the relationship among cytokines and the bioactive lipids is given in Fig. 3.8): 24 h: PGE2↑↑↑↑; LXA4↑; RSVs↔; PRTs↔; MaRs↔. 48 h: PGE2↑↑↑; LXA4↑↑; RSVs↑; PRTs↑; MaRs↑. 72 h: PGE2↑↑; LXA4↑↑↑; RSVs↑↑; PRTs↑↑↑; MaRs↑↑↑. 96 h: PGE2↑; LXA4↑↑; RSVs↑↑↑; PRTs↑↑↑; MaRs↑↑↑↑. >96 h: PGE2↑; LXA4↑; RSVs↑↑; PRTs↑↑; MaRs↑↑↑. The actions of these compounds in the inflammation and wound healing process can be as follows: LXA4 → anti-inflammatory >resolution >protection >proliferation; RSVs → resolution > anti-inflammatory >protection >proliferation; PRTs → protection > resolution > anti-inflammatory > proliferation; MaRs → proliferation > protection > resolution > anti-inflammatory. Resolution refers to resolution of inflammation. Protection refers to protection of normal cells/tissues from injurious agents. Proliferation refers to proliferation of stem cells and other cells in order to replace damaged cells/tissues. Despite the fact that all compounds have similar and overlapping actions and possess anti-inflammatory properties, each lipid may show one particular action more compared to the other actions
5 Bioactive Lipids and the Immune Response
It is likely that under normal physiological conditions, a balance is maintained between pro-inflammatory PGS, TXs, LTs and IL-6, TNF-α, IL-1β, HMGB1 and other pro-inflammatory cytokines and anti-inflammatory lipoxins, resolvins, protectins and maresins and anti-inflammatory cytokines IL-4, IL-10, TGF-β. It is noteworthy that once the inflammatory process reaches its peak, the anti-inflammatory pathway is stimulated and the formation of anti-inflammatory lipoxins, resolvins, protectins and maresins and the needed anti-inflammatory cytokines occurs. These events are likely to be accompanied by suppression of ROS (reactive oxygen species) generation and increase in the much-needed antioxidant defences. These events trigger the initiation of the inflammation resolution process and healing of the wound to restore homeostasis. Lipoxins, resolvins, protectins and maresins inhibit PMNLs (polymorphonuclear leukocytes) trans-endothelial migration, reduce leucocyte infiltration, and suppress dendritic cell (DC) migration and IL-12 production in order to suppress inflammation and enhance the anti-inflammatory process. Lipoxins, resolvins, protectins and maresins have the ability to augment the expression of antiinflammatory genes and attenuate LTB4-stimulated proinflammatory signals [2, 23].
It is known that an interaction exists among pro- and anti-inflammatory cytokines and PUFA metabolism. Proinflammatory cytokines IL-1, IL-6, TNF-α and IFN-γ are known to activate phospholipases, augment ROS generation [34,35,36,37,38], and enhance the activities of COX-2 and LOX enzymes that are needed for the production of PGE2, TXA2 and LTs to initiate and perpetuate inflammation and subsequently to suppress inflammatory process as and when the purpose of inflammation is achieved. The precursors that are common (especially AA and EPA) for the formation of both pro-inflammatory (PGs, LTs TXs from AA and EPA) and anti-inflammatory (lipoxins from AA and resolvins from EPA and DHA and protectins and maresins from DHA) lipids are derived from the cell membrane pool by the activation of phospholipase A2 (PLA2). This implies that there could be two waves of release of PUFAs especially AA, EPA and DHA from the cell membrane lipid pool. The first one to enhance the formation of pro-inflammatory PGs, LTs and TXs and the second to trigger the formation of lipoxins, resolvins, protectins and maresins by their respective and specific phospholipases (Fig. 3.8).
Scheme showing the relationship among pro- and anti-inflammatory cytokines, PGs, LTs, lipoxins, resolvins, protectins and maresins and steroids. Metabolism and actions of arachidonic acid is shown as a representative of various PUFAs (GLA, DGLA, EPA, DPA and DHA). For further details see the text. (+) Indicates increase in the synthesis/action or positive effect. (−) Indicates decrease in the synthesis/action or negative effect
The three classes of phospholipases that regulate the release of PUFAs are calcium independent PLA2 (iPLA2), secretory PLA2 (sPLA2), and cytosolic PLA2 (cPLA2). Each class of PLA2 is further divided into isoenzymes for which there are 10 for mammalian sPLA2, at least 3 for cPLA2, and 2 for iPLA2. The first wave of release of PUFAs from the cell membrane is due to the action of iPL2 that results in the formation of pro-inflammatory PGE2, TXA2 and LTB4. The second wave of release of PUFAs is due to the action of sPLA2 and cPLA2 that occurs at the time of initiation of resolution of inflammation. This results in the formation of lipoxins, resolvins, protectins and maresins that suppress inflammation, initiate resolution of the inflammatory process, cytoprotection of surrounding normal cells/tissues and regeneration of normal cells to replace the dead and damaged cells and tissue to restore homeostasis. It is noteworthy that adequate amounts of PGE2 are needed to induce optimal inflammation and also trigger initiation of resolution of inflammation. Thus, the inflammatory stimuli that induces the release of PUFAs by activating iPLA2 are utilized for the synthesis of pro-inflammatory PGs, TXs and LTs. In contrast, PUFAs released from the same cell membrane stores by the action of sPLA2 and cPLA2 at the time of initiation of resolution of inflammation are directed to form lipoxins, resolvins, protectins and maresins. This delicate yet and imperceptible and orderly switch over from pro-inflammatory to anti-inflammatory molecules seems to be determined by the type of PLAs that are activated and the activities of COX-2 and 5-, 12- and 15-LOX enzymes. Thus, a close co-operation, association and interaction(s) among PLAs, COX-2, LOX enzymes and various cytokines is needed for the appropriate inflammation to occur and to induce a gradual, smooth and orderly onset of anti-inflammatory events, resolution of inflammation and restoration of homeostasis [2]. Any defects in this process (dysfunction of cytokines, PLAs, COX, LOX enzymes, cell membrane stores of PUFAs, etc.) can lead to persistance of inflammation and damage to the target tissues as seen in autoimmune diseases, chronic infections such as tuberculosis and in aging (Figs. 3.2, 3.7 and 3.8). With advancing age, there is a decrease in the activities of desaturases, an increase in COX-2 activity and a change in the expression of 5-, 12-, and 15-LOX enzymes that can result in a decrease in the concentrations of GLA, DGLA, AA, EPA and DHA in the cell membrane pool, an increase in the formation of PGE2 and decreased synthesis and release of lipoxins, resolvins, protectins and maresins (Figs. 3.7, 3.8 and 3.9) [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. A similar relationship exists between pro- and anti-inflammatory cytokines and any imbalance in their concentrations can lead to inappropriate inflammation (Figs. 3.7 and 3.8).
Scheme showing possible relationship among PGE2, LXA4 and various PLA2 enzymes, as seen in inflammation and inflammation resolution processes.  PGE2;
PGE2;  LXA4;
LXA4;  iPLA2;
iPLA2;  sPLA2;
sPLA2;  cPLA2;
cPLA2;  COX-2. All of these concentrations and activities of enzymes are presented as expected to behave during normal inflammatory process (which finally resolve spontaneously).
COX-2. All of these concentrations and activities of enzymes are presented as expected to behave during normal inflammatory process (which finally resolve spontaneously).  PGE2 when inflammation persists;
PGE2 when inflammation persists;  COX-2 when inflammation persists;
COX-2 when inflammation persists;  LXA4 when resolution of inflammation is defective. Possible changes that may occur in the activities of various PLA2s are not shown in the figure, they are likely to behave in tune with the concentrations of PGE2 and LXA4. Despite the fact that LXA4, resolvins, protectins and maresins have anti-inflammatory actions, there could be subtle differences in their major and minor actions with some amount of overlap in their anti-inflammatory actions (Fig. 3.7). Although the role of nitrolipids is not shown, it is expected to behave similarly to LXA4. As already discussed in the text and shown in Figs. 3.7 and 3.8, there are two waves of release of AA (and other PUFAs). The first one occurs in the early period of inflammation (within the first 24 h due to activation of iPLA2) which predominantly leads to the formation of PGE2 and other pro-inflammatory molecules. Once the concentrations of PGE2 reach the optimum level (say by the end of 24–48 h), a second wave of AA release occurs (due to activation of sPLA2 and cPLA2) that results in the formation of LXA4 (resolvins, protectins and maresins from EPA and DHA), capable of inducing resolution of inflammation. The activation of cPLA2 occurs around 48–72 h to initiate and accelerate the resolution of inflammation. The activation of iPLA2 and formation of PGE2 are closely associated with the activation of COX-2. In this process of inflammation and resolution of inflammation, there is a critical role for the PGDH enzyme needed for catabolism of PGE2. It is noteworthy that LXA4, resolvins, protectins and maresins are anti-inflammatory molecules but may have slight but critically important differences in their actions to resolve the inflammation and enhance wound healing. For instance, LXA4 is needed to induce anti-inflammatory events. Fig. 3.9 (continued) It should be noted that suppression of inflammation is not equal to resolution of inflammation. To suppress inflammation, LXA4 inhibits leukocyte infiltration. While resolvins are needed for resolution of inflammation (such as removing the debris of a wound, phagocytosis of dead leukocytes, etc.); protectins may perform the important function of protecting normal cells/tissues from further damage and thus, maintain tissue integrity. Maresins may act on stem cells (to induce their differentiation) for the repair process to occur and restore tissue homeostasis. The figure also shows how this sequence of orderly activation and deactivation of PLA2, COX-2 and formation of PGE2 and LXA4 are likely to get deranged in the face of failure of resolution of inflammation processes. Patients with hypertension, diabetes mellitus and advanced age have low-grade systemic inflammation as a result of sustained activation of COX-2 and formation of PGE2 and failure of formation of adequate amounts of LXA4 and other anti-inflammatory compounds. Failure of the inflammation resolution process may lead to the onset of age-associated disorders such as hypertension, type 2 diabetes mellitus, atherosclerosis, CHD, cancer, osteoporosis and sarcopenia and when this inflammatory process is severe it can lead to the onset of sepsis and septic shock, which are common in the elderly
LXA4 when resolution of inflammation is defective. Possible changes that may occur in the activities of various PLA2s are not shown in the figure, they are likely to behave in tune with the concentrations of PGE2 and LXA4. Despite the fact that LXA4, resolvins, protectins and maresins have anti-inflammatory actions, there could be subtle differences in their major and minor actions with some amount of overlap in their anti-inflammatory actions (Fig. 3.7). Although the role of nitrolipids is not shown, it is expected to behave similarly to LXA4. As already discussed in the text and shown in Figs. 3.7 and 3.8, there are two waves of release of AA (and other PUFAs). The first one occurs in the early period of inflammation (within the first 24 h due to activation of iPLA2) which predominantly leads to the formation of PGE2 and other pro-inflammatory molecules. Once the concentrations of PGE2 reach the optimum level (say by the end of 24–48 h), a second wave of AA release occurs (due to activation of sPLA2 and cPLA2) that results in the formation of LXA4 (resolvins, protectins and maresins from EPA and DHA), capable of inducing resolution of inflammation. The activation of cPLA2 occurs around 48–72 h to initiate and accelerate the resolution of inflammation. The activation of iPLA2 and formation of PGE2 are closely associated with the activation of COX-2. In this process of inflammation and resolution of inflammation, there is a critical role for the PGDH enzyme needed for catabolism of PGE2. It is noteworthy that LXA4, resolvins, protectins and maresins are anti-inflammatory molecules but may have slight but critically important differences in their actions to resolve the inflammation and enhance wound healing. For instance, LXA4 is needed to induce anti-inflammatory events. Fig. 3.9 (continued) It should be noted that suppression of inflammation is not equal to resolution of inflammation. To suppress inflammation, LXA4 inhibits leukocyte infiltration. While resolvins are needed for resolution of inflammation (such as removing the debris of a wound, phagocytosis of dead leukocytes, etc.); protectins may perform the important function of protecting normal cells/tissues from further damage and thus, maintain tissue integrity. Maresins may act on stem cells (to induce their differentiation) for the repair process to occur and restore tissue homeostasis. The figure also shows how this sequence of orderly activation and deactivation of PLA2, COX-2 and formation of PGE2 and LXA4 are likely to get deranged in the face of failure of resolution of inflammation processes. Patients with hypertension, diabetes mellitus and advanced age have low-grade systemic inflammation as a result of sustained activation of COX-2 and formation of PGE2 and failure of formation of adequate amounts of LXA4 and other anti-inflammatory compounds. Failure of the inflammation resolution process may lead to the onset of age-associated disorders such as hypertension, type 2 diabetes mellitus, atherosclerosis, CHD, cancer, osteoporosis and sarcopenia and when this inflammatory process is severe it can lead to the onset of sepsis and septic shock, which are common in the elderly
A significant inverse correlation was noted between age and the LXA4/LTs ratio suggesting that aging is associated with a dramatic change in AA (and possibly also of GLA, DGLA, EPA and DHA) metabolism such that LXA4 (and other anti-inflammatory lipid molecules) levels are decreased whereas those of LTs (a pro-inflammatory molecule) is increased and may contribute to the development of diseases that are common in the elderly such as type 2 diabetes mellitus, hypertension, coronary heart disease (CHD), atherosclerosis, cancer, Alzheimer’s disease, depression and immune dysfunction. This may also include other inflammatory and immunological disorders such as disc prolapse, lupus and arthritis, osteoporosis and tendon tears. It is noteworthy that all these are inflammatory conditions and have an immunological component in the form of an increase in the local and/or systemic concentrations of pro-inflammatory cytokines IL-6, TNF-α, IL-1β and HMGB1 and a concomitant change in bioactive lipids seen as low plasma or tissue levels of GLA, DGLA, AA, EPA and DHA (one or more of these fatty acids or all) and an increase in pro-inflammatory molecules PGE2, LTs, TXs and a deficiency of lipoxins, resolvins, protectins and maresins [2, 6,7,8,9,10,11,12,13, 23,24,25,26,27, 30,31,32,33, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Thus, bioactive lipids seem to have a significant role in many inflammatory and immune-mediated disorders that are common in the elderly. This implies that occurrence of these inflammatory and immune-mediated diseases such as obesity, type 2 diabetes mellitus, hypertension, atherosclerosis, coronary heart disease, lupus, cancer and osteoporosis is a sign of aging. In essence, all these evidences suggest that an increase in pro-inflammatory PGs, LTs and TXs and cytokines and a decrease or deficiency of LXA4, resolvins protectins and maresins and anti-inflammatory cytokines occur in many diseases associated with aging. This implies that restoring the balance between pro- and anti-inflammatory cytokines and BALs may form a novel approach in the prevention and management of several inflammatory and immunological disorders as summarized previously (Fig. 3.10) [47].
Scheme showing aging and its associated disorders and their relationship to hypothalamus, oxidative stress, PUFA metabolism, CO (carbon monoxide), NO (nitric oxide), H2S (hydrogen sulfide) and telomere length. High calorie diet stimulates ROS generation that may overwhelm the antioxidant system in adipose and other tissues, enhance the synthesis of pro-inflammatory cytokines, and decrease the formation of anti-inflammatory cytokines, leading to the onset of low-grade systemic inflammation, induction of DNA damage and aging. These events cause aging of endothelial cells, shorten telomere length, and inhibit p53 expression. They also induce endothelial dysfunction and insulin resistance that leads to the development of hypertension, type 2 diabetes mellitus, atherosclerosis, CHD and aging. A high calorie diet, insulin resistance and lack of exercise suppress D6 and D5 desaturases leading to reduced formation of GLA, DGLA, AA, EPA and DHA, the precursors of lipoxins, resolvins, protectins and maresins and other anti-inflammatory products. Deficiency of these molecules impairs resolution of inflammation, DNA damage persists, telomere shortening occurs, p53 dysfunction sets in, and stem cell function becomes inappropriate, leading to the onset and progression of aging and age-associated disorders. These events will result in decreased CO, NO and H2S production. PUFAs and their metabolites influence stem cell biology and thus, affect the aging process and age-associated disorders including Alzheimer’s disease. Fig. 3.10 (continued) PUFAs can give rise to FAHFAs (fatty acid hydroxy fatty acids) that have anti-inflammatory properties and enhance the formation of NO, CO and H2S, and mediate exercise-induced anti-inflammatory actions. PUFAs and lipoxins, resolvins, protectins and maresins suppress IL-6, TNF-α and PG, LT and TX production. It is not yet known but possible that FAHFAs suppress tumor cell growth and inhibit inflammatory events in hypothalamus. Although the role of p53 in aging and diseases is not discussed in detail here, it may be noted that p53 is the guardian of the genome. PUFAs and their metabolites, cytokines, NO, CO, H2S, ROS, GDF-11, GnRH and NAE may modulate the action of p53. For instance, exercise reduces the incidence of cancer, possibly by augmenting the production of IL-6 and TNF-α that are cytotoxic to tumor cells either by their direct action and/or by their ability to enhance the production of ROS that are tumoricidal. Exercise enhances the expression and action of p53 that leads to apoptosis of cancer cells. PUFAs have tumoricidal action by enhancing the production of free radicals and accumulation of toxic lipid peroxides in tumor cells
6 IL-6, TNF-α and Corticosteroids Induce a Bioactive Lipid Deficiency State
It is interesting to note that IL-6 , TNF-α, HMGB1 and other pro-inflammatory cytokines and corticosteroids suppress desaturase activity that leads to decreased formation of metabolites of EFAs (LA and ALA) such as GLA, DGLA, AA, EPA and DHA. Due the presence of decreased concentrations of GLA, DGLA, AA, EPA and DHA, their metabolites such as PGE1 (from DGLA), PGI2 and LXA4 (from AA), resolvins (from EPA and DHA), protectins and maresins (from DHA) form in low amounts due to precursor deficiency but ironically excess formation of PGs, LTs and TXs occurs [2, 23, 55]. In contrast, IL-6 and TNF-α and other pro-inflammatory cytokines activate PLA2, COX-2 and LOX enzymes whereas corticosteroids suppress them. Thus, corticosteroids are potent anti-inflammatory molecules since they (i) suppress desaturases, (ii) inhibit PLA2 activity, and (iii) block COX and LOX enzymes. As a result of these actions, (i) decreased conversion of LA and ALA to their long-chain metabolites such as GLA, DGLA and AA from LA and EPA and DHA from ALA (due to suppression of desaturases) and hence, a deficiency of GLA, DGLA, AA, EPA and DHA, occurs in the cells; (ii) decreased formation and release of PGs, LTs and TXs is seen due to substrate deficiency; (iii) as a consequence of inhibition of COX and LOX enzymes reduced formation of PGs, LTs and TXs occurs; and (iv) due to the inhibitory action of corticosteroids on PLA2 activity there is a decrease in the release of GLA, DGLA, AA, EPA and DHA from the cell membrane lipid pool and so, the availability of precursors of PGs, LTs and TXs is significantly low. Thus, in the short-term corticosteroids are potent anti-inflammatory compounds. However, in the long-run they induce an EFA and PUFA deficiency state leading to continuation of the inflammatory state and failure of resolution of the injury/inflammation as a result of decreased formation of lipoxins, resolvins, protectins and maresins that are needed for wound healing and restoration of homeostasis. This deficiency of anti-inflammatory lipids is due to their precursor (GLA, DGLA, AA, EPA, DPA and DHA) deficiency. It is paradoxical to know that corticosteroids inhibit both LXA4 and LTB4 synthesis but have a much lower effect on LTB4 that results in a pro-inflammatory status [86]. This proposal is further supported by the observation that supplementation of AA during active inflammatory process when PGs, LTs and TXs are being synthesized in excess, actually results in an increase in the formation of LXA4 (and possibly, resolvins, protectins and maresins) with little change in the concentrations of PGE2, tilting the balance more towards an anti-inflammatory status that results in suppression of the inflammation [2, 55, 87, 88]. However, unlike IL-6 and TNF-α that activate PLA2 and COX-2 and thus, enhance the formation of pro-inflammatory PG2, LTs and TXs, corticosteroids block the expression of PLA2 and COX-2 and thereby block the formation of PGE2, LTs and TXs that may explain their (corticosteroids) anti-inflammatory action compared to the pro-inflammatory actions of IL-6 and TNF-α (Figs. 3.7 and 3.8). These results imply that EFAs, PUFAs , and other bioactive lipids are the mediators of the actions of corticosteroids and IL-6 and TNF-α and paradoxically both corticosteroids and IL-6 and TNF-α induce an EFA (PUFA)-deficiency state by their ability to block the activities of desaturases. These results imply that co-administration of PUFAs along with corticosteroids may sustain their anti-inflammatory actions (by enhancing the formation of lipoxins, resolvins, protectins and maresins) and, when PUFAs are administered in conjunction with IL-6 and TNF-α, may potentiate their anti-cancer action by augmenting ROS generation in tumor cells (Fig. 3.8) [2, 61, 62]. It is interesting that corticosteroids and pro-inflammatory cytokines that have physiologically opposite actions seem to mediate their actions through the same molecules, namely BALs. This speaks of the pleiotropic actions of BALs.
It is noteworthy that IL-1β that is markedly increased during inflammation is capable of inducing PG biosynthesis and also up regulating the formation of LXA4 and maresins that are necessary for the inflammation resolution process [55]. LXA4, resolvins, protectins and maresins are potent down-regulators of PGE2 production. Increased 15-prostaglandin dehydrogenase (15-PGDH) expression enhances the formation of LXA4, resolvins, protectins and maresins and augments the regeneration of tissues to reestablish tissue homeostasis [2, 23, 55, 57, 72, 89,90,91,92,93]. Thus, IL-1β and PGE2 have both pro- and anti-inflammatory actions depending on the context (Fig. 3.8). This suggests that in order to suppress both acute and chronic inflammation and inhibit the production of pro-inflammatory IL-6 and TNF-α, one needs to employ AA/EPA/DPA/DHA, LXA4, resolvins, protectins and maresins in combination with corticosteroids. Similarly, when IL-6 and TNF-α are co-administered along with GLA, DGLA, AA, EPA, DPA and DHA it could be possible to eliminate tumor cells selectively with little or no side effects of cytokines on normal cells since BALs suppress inappropriate production and action of pro-inflammatory cytokines [61, 62]. The relationship between bioactive lipids and corticosteroids suggests that Cushing’s syndrome that is due to excess production of cortisol can be considered as an EFA deficiency state since it inhibits desaturase, PLA2, COX and LOX enzymes resulting in low plasma and tissue concentrations of GLA, DGLA, AA, EPA, DPA and DHA, and altered levels of PGs, LTs, TXs, LXA4, resolvins, protectins and maresins. Since BALs have a role in obesity, hypertension, type 2 diabetes mellitus, inflammation and immune function., it is reasonable to suggest that several features seen in Cushing’s disease can be considered as a disorder of altered bioactive lipids and this offers a critical insight into the actions of BALs (Fig. 3.11). This also explains the Cushingoid-like features seen in many patients with metabolic syndrome implying that there could be a relative cortisol excess in these subjects.
The various symptoms of Cushing’s disease are shown. Most of these can occur as a result of an EFA/PUFA deficient state. The development of hypertension, type 2 diabetes mellitus features, obesity, osteoporosis, cardiac hypertrophy, depression and irritability, and erective dysfunction, may all occur due to EFA/PUFA deficiency [3, 4, 6, 7, 10, 11, 13, 32, 33, 63, 64, 66,67,68, 70, 71]
7 Bioactive Lipids Modulate Immune Response
Aging is associated with a decrease in immunity and increased susceptibility to infections that could lead to sepsis. This increased susceptibility to infections can be ascribed to increased generation of pro-inflammatory PGE2 and LTs and decreased production of LXA4 with advancing age [52,53,54]. PGE2 suppresses the proliferation of T cells, immunosuppressive in nature, and inhibits the production of IL-6 and TNF-α that are needed to induce the generation of ROS by leukocytes and macrophages to kill bacteria and other invading organisms. Furthermore, lipoxins, resolvins, protectins and maresins are capable of enhancing the anti-bacterial action of leukocytes and macrophages and possibly that of other immunocytes [94,95,96,97,98]. Hence, their deficiency due to corticosteroid therapy and in aging may lead to increased incidence of infections and sepsis. Previously, it was also shown that several PUFAs and EFAs such as LA and ALA have anti-microbial actions [99,100,101,102,103,104]. This suggests that leukocytes, macrophages, T cells, NK cells and other immunocytes including endothelial cells may release EFAs, PUFAs, lipoxins, resolvins, protectins and maresins on exposure to microorganisms and tumor cells to inactive the microbes and kill tumor cells, respectively. The ability of EFAs and PUFAs and their metabolites to selectively induce apoptosis of tumor cells is particularly interesting since the incidence of cancer increases with age.
8 Bioactive Lipids in the Immune Response and Cancer
Antigen-presenting cells (APCs) present antigen on their class II MHC molecules (MHC2s). Helper T cells recognize these, with the help of their expression of CD4 co-receptor (CD4+). The activation of the helper T cell causes it to release cytokines and other stimulatory signals that stimulate the activity of macrophages, killer T cells and B cells. The stimulation of B cells and macrophages drives the proliferation of T helper cells. The activated T cells, B cells and macrophages produce various BALs including PGE2, LTs, LXA4, resolvins, protectins and maresins, ROS, NO and cytokines that ultimately either eliminate the invading microorganisms, intracellular pathogens and/or cause autoimmune diseases depending on the regulation or inappropriate function of T suppressor cells. This is an over-simplification of the events that occur when the immunocytes are exposed to various antigens. The actual interactions are much more complex compared to what has been described in Fig. 3.12.
Scheme showing interactions of various T and B cells and macrophages and their association with various diseases. The possible role of PUFAs in these events is outlined. PUFAs and their metabolites PGE2, LXA4, resolvins (RSVs), protectins (PRTs), and maresins (MaRs) may activate/inhibit macrophages and other immunocytes depending on the type of metabolite formed, as well as the context, concentration and duration of exposure to the target
Whenever there is tissue injury due to endogenous or exogenous agents, close interactions occur among various immunocytes and macrophages and their products and growth factors (including cytokines) that is modified by BALs, as shown in Fig. 3.13. The importance of the immune system is evident when its optimal function is needed to prevent cancer and autoimmune diseases. Thus, immunosurveillance and immunoediting become important in the context of cancer and autoimmune diseases. It is noteworthy that aging is associated with decreased immunosurveillance and increased incidence of cancer. An increase in PGE2 and a decrease in LXA4 (and possibly, that of resolvins, protectins and maresins) levels, defective immunosurveillance due to an increase in exhausted CD8+ T cells that show increased expression of Tim-3 (T-cell immunoglobulin mucin domain-3, an exhaustion marker) on aged T cells, especially CD8(+) T cells, and increased expression of inhibitory receptors, such as programmed cell death protein 1 (PD-1), in the T cells of aged subjects may explain the decreased immunosurveillance seen with aging [105,106,107,108].
Scheme showing interactions among various immunocytes and macrophages and their products and growth factors (including cytokines) in response to both endogenous and exogenous stimuli and insults. Most of these events could be modified by BALs. The modulatory actions of BALs on various events depicted in the figure include their ability to influence TH1 and TH2 cells, macrophages, NF-kB and the capacity of T cells and macrophages to secrete their respective cytokines or other soluble mediators. Thus, BALs may have both positive and negative influences on various immunocytes and their actions
In this context it is noteworthy that PGE2 plays a critical role in the development of TH17 cells and impair CTL function in co-ordination with PD-1. PGE2 is a pro-inflammatory molecule but is also a potent immunosuppressor [13, 109,110,111,112,113,114,115,116,117,118,119,120,121]. The immunosuppressive action of PGE2 may be responsible for the immunosuppression seen in cancer and its ability to limit the functions of NK cells, CD4 and CTLs [122, 123]. PGE2 induces the generation of IL-10, Treg cells and myeloid-derived suppressor cells and suppresses the proliferation and cytotoxicity of CTLs and their ability to produce IFN-γ [11, 110, 124,125,126]. In view of these immunosuppressive actions of PGE2, it is likely that increased production of PGE2 and a simultaneous decrease in LXA4, resolvins, protectins and maresins generation seen with aging may be responsible for the increase in the incidence of infections, persistence of infections, inflammatory events and high incidence of cancer in aged subjects (Figs. 3.14 and 3.15). Cancer may be considered as a non-resolving/non-healing wound that could be due to increased production of PGE2 and decreased levels of LXA4 [127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145]. Hence, the increased production of PGE2 by tumor cells and infiltrating macrophages will enable tumor cells to avoid immune surveillance, enhance their proliferation, augment tumor angiogenesis and ultimately enable them to grow faster and also metastasize. Furthermore, PGE2 is an inhibitor of TNF-α and IL-6 production [146,147,148,149,150,151,152,153], and also that of IFN-γ [153], which are pro-inflammatory molecules and known to possess tumoricidal actions. This is yet another action of PGE2 that help tumor cells to avoid immune surveillance. In addition, PGE2 modulates NO generation [154] and NO, in turn, alters PGE2 synthesis [154,155,156,157,158,159,160,161,162,163]. PGE2 enhances IL-10 production [164, 165], which is an anti-inflammatory cytokine.
Factors controlling formation of different subsets of T helper cells. LXs lipoxins, RSvs resolvins, PRTs protectins, MaRs maresins. Naive CD41T cells differentiate into subsets of T helper cells: TH1, TH2 and TH17. TGF-β, converts naive T cells into FOXP3-expressing induced Treg (iTreg) cells. Each T helper cell differentiation programme needs specific transcription factors as master regulators (T-bet, GATA3 and ROR-γt). Terminally differentiated T helper cells produce specific combinations of effector cytokines that bring about specific and distinct effector functions of the adaptive immune system. TGF-β, retinoic acid or cytokines (IL-6, IL-1, IL-23 or IL-27) provided by cells of the innate immune system (immature or activated dendritic cells (DCs), respectively) dictate whether a naive T cell develops into a FOXP31 Treg cell, a TH17cell or otherwise. Prostaglandin E2 (PGE2), through its receptor EP4 on T cells and dendritic cells, facilitates TH1 cell differentiation and amplifies IL-23–mediated TH17 cell expansion and EP4-selective antagonists decrease accumulation of both TH1 and TH17 cells and suppress progression of autoimmune encephalomyelitis or contact hypersensitivity in experimental animals. Although the role of PUFAs and their various metabolites is not discussed in detail, it is known that GLA, AA, EPA, DHA, lipoxins, resolvins, protectins, maresins and prostaglandins, leukotrienes and thromboxanes can influence macrophage and other immunocytes’ phagocytosis, motility and ability to alter ROS generation and the final outcome of the inflammation and immune response
Scheme showing potential relationship and interactions among cytokines, bioactive lipids, BDNF and PD-1 and PD-L1 and their potential role in cancer and autoimmune diseases. IL-17, IL-23 and PGE2 act together to induce a pro-inflammatory status in autoimmune diseases. Cytokines IL-17, IL-23, IL-6, TNF-α and HMGB1 activate phospholipase A2 to induce the release of PUFAs (especially DGLA, AA, EPA and DHA) that form precursors to PGE1, PGE2/LXA4, resolvins, protectins and maresins as shown in the figure. DGLA, AA, EPA and DHA suppress the production of IL17, IL-23, IL-6, TNF-α and HMGB1 and thus have a negative feedback control on the formation of pro-inflammatory cytokines. IL-17 enhances resistance to PD-1 and PD-L1 blockade. LXA4, resolvins, protectins and maresins inhibit inflammatory processes and thus, are useful in protection against autoimmune diseases such as RA, lupus, inflammatory bowel disease and multiple sclerosis. In addition, LXA4, resolvins, protectins and maresins inhibit proliferation of tumor cells. Similar and more potent anti-cancer action is shown by DGLA, AA, EPA and DHA and these induce apoptosis of various types of tumor cells. PUFAs may also suppress the expression of PD-1 and PD-LI and thus, may assist in overcoming immunosuppression seen in cancer. Furthermore, these PUFAs can act on Piezo1 channel which is capable of mediating mechanoelectrical transduction that, in turn, regulates several crucial cellular processes including cell migration. This action of PUFAs on Piezo1 could be attributed to their ability to change cell membrane fluidity. Similarly, PUFAs can regulate the other ion channel, namely TRPV1. There seems to be an interaction between Piezo1 and TRPV1 channels. Thus, PUFAs by their ability to alter the properties of Piezo1 and TRPV1 channels, can regulate membrane voltage changes which can alter cell adhesion, cell volume, apoptosis and angiogenesis. Since many cancer cells over-express K+, Na+, Ca2+ and Cl- channels, it is likely that incorporation of various PUFAs into the cell membrane can effectively alter these channels leading to changes in their mitotic and other properties. This could be one of the many actions of PUFAs/BALs to result in the arrest of growth of cancer cells and their eventual apoptosis. Fig. 3.15 (continued) Not many studies have been performed on the action of PGs, LTs, TXs, lipoxin A4, resolvins, protectins, and maresins on ion channels, especially on Trpv 1 and Piezo1. However, it is likely that these bioactive lipids can also alter the behavior of various ion channels. For instance, it has been shown that PGE2 activates Ca2+ channels. It is likely that other bioactive lipids may also have similar actions on various ion channels and Trpv1 and Piezo1
Thus, PGE2 has actions on IL-17, TNF-α, IL-6, IFN-γ, Treg cells, CTL and NO, and may mediate the resistance of tumor cells to anti-VEGF therapy through its ability to enhance IL-17 secretion [164,165,166,167,168,169,170,171,172,173,174,175,176,177,178]. This may ultimately result in tumor cell proliferation, angiogenesis and metastasis (Figs. 3.14 and 3.15). Our studies have revealed that PGE1, PGE2, LTD4, LXA4, resolvins and protectins inhibit growth of IMR-32 cancer cells [179]. These and other studies have led to the suggestion that the balance between various metabolites formed from PUFAs and the cellular content and the surrounding milieu content of various PUFAs, determines the final outcome of whether tumor cells are induced to proliferate or inhibited from further growth. Consistent with this, we and others have noted that GLA, DGLA, AA, EPA and DHA have potent growth inhibitory action on several types of tumor cells both in vitro and in vivo [180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197]. Based on these findings, it is reasonable to propose that altered EFA/PUFAs metabolism can usher in a low-grade systemic inflammatory status , impair the immune surveillance system and thereby lead to higher incidence of cancer, type 2 diabetes mellitus, hypertension, osteoporosis, sarcopenia and accumulation of abdominal fat with advancing age. This implies that aging is an inflammatory condition [47].
9 Cancer and Auto-immune Diseases Are Two Sides of the Same Coin
Both cancer and autoimmune diseases are pro-inflammatory conditions although there are some distinct differences between them. Each autoimmune disease has its own distinct features despite the fact that the underlying mechanism(s) may be similar if not identical. For instance, bones and synovial membranes are predominantly involved in RA (rheumatoid arthritis), skin, blood vessels and kidney (sometimes brain) are involved in lupus and neurons in MS (e.g., multiple sclerosis). It is not known why joint deformities occur in RA but not in lupus, or why renal involvement is common in lupus but not in RA and why only brain and other neurological structures are involved in MS with no involvement of other tissues. This suggests that local inflammatory events are more important than systemic inflammatory changes despite the presence of systemic signs and symptoms such as fever, leukocytosis, loss of appetite, etc., in all of these diseases. On the other hand, in cancer both local and systemic manifestations are not uncommon and sometimes systemic events such as cachexia and immunosuppression are more dominant and can result in morbidity and mortality. But, paradoxically, in both autoimmune diseases and cancer, inflammation is present. In autoimmune diseases, the local inflammatory events are more dominant as a result of recognition of self as foreign whereas, in cancer the immune system fails to recognize cancer cells as foreign. Despite the failure of recognition of cancer cells as foreign, some amount of inflammation occurs at the site of cancer. Despite these seemingly striking differences between autoimmune diseases and cancer, it is noteworthy that cancer is not uncommon in subjects with autoimmune diseases. With the recent development of immune check point inhibitor (ICI) therapy for cancer, it has been recognized that patients treated with this can develop autoimmune diseases. Thus, both autoimmune diseases and cancer can be considered as two sides of the same coin.
IL-17, IL-6, TNF-α and PGE2, LTs and TXs seem to have a role in the autoimmune diseases RA and lupus. Similarly, there is a significant role for IL-17, IL-6, TNF-α and other pro-inflammatory cytokines and PGE2 in cancer. Thus, these same molecules seem to participate both in cancer and autoimmune diseases suggesting that similar approaches in their management can be attempted. In Table 3.1, similarities and contrasting features between cancer and autoimmune diseases are given. It is evident from this table that some overlapping features can be seen between autoimmune diseases and cancer. In both cancer and autoimmune diseases, increased levels of IL-6, TNF-α and IL-17 are seen although, in autoimmune diseases an increase in the plasma levels of these cytokines is more common whereas in cancer they are predominantly seen at the site of the malignancy. This suggests that autoimmune diseases are predominantly systemic diseases whereas cancer is a more localized disease (at least in the initial stages). However, it needs to be noted that lupus, RA and other autoimmune diseases may start locally in a specific tissue or organ and later spread to the whole organ, system or body. For instance, RA may start in one joint and later may involve several other joints. Similarly, lupus may start as non-specific skin rash, or arthralgia and later show more systemic manifestations. Thus, at the molecular/biochemical level there seem to be a role for the same cytokines in both these diseases. One would expect decreased expression of PD-1 and PD-L1 in autoimmune diseases whereas in cancer their expressions are increased to escape the immune surveillance. It is evident from the details given in Table 3.1 that there are many similarities between autoimmune diseases and cancer, implying that same therapeutic strategies could be useful in the prevention and management of both diseases.
Plasma, synovial fluid and urinary levels of IL-6, TNF-α and IL-17 are increased with low plasma concentrations of anti-inflammatory cytokine IL-10 in those with active RA and lupus [198, 199]. In addition, RA and lupus patients have increased plasma, urinary and synovial fluid levels of PGE2 and TXA2 levels and decreased plasma levels of DGLA, AA, EPA and DHA [200,201,202,203,204,205,206,207,208,209]. Recent studies have shown that patients with lupus and RA and other rheumatological (and autoimmune) conditions have low plasma and urinary levels of lipoxin A4 (LXA4) [13, 72, 210,211,212,213,214]. Restoring LXA4 levels and COX-2 activity to normal may resolve arthritis, especially in RA. Blocking COX-2 activity and consequently reducing PGE2 levels seems to perpetuate inflammation in contrast to the expectation that reducing PGE2 levels is needed for resolution of inflammation. Subsequently it was reported that repletion of PGE2 attenuated inflammation by enhancing the formation of LXA4, a lipoxygenase metabolite formed from AA, implying that PGE2 may actually trigger initiation of the inflammation resolution process. These results also indicate that there is a close relationship between COX-LOX pathways and PGE2 has a negative feedback control on the inflammation process. This is supported by the observation that inhibition of 15-PGDH that results in a two-fold increase in PGE2 levels in several tissues such as bone marrow, colon, and liver, gives a response to partial hepatectomy with a greater than two-fold increase in hepatocyte proliferation and are resistance to chemical-induced colitis. 15-PGDH inhibition also accelerated recovery of erythropoiesis after bone marrow transplantation [91] suggesting that this enzyme, and possibly PGE2, may have a regulatory role in regeneration and repair in several tissues including bone marrow, colon and liver. It is possible that 15-PGDH inhibition and the consequent increase in PGE2 levels may induce increased formation of LXA4, possibly by redirecting AA metabolism towards LXA4 formation. These results raise the interesting possibility that depending on the context, PGE2 may have both pro- and anti-inflammatory actions. Based on these findings, it is proposed that enhanced levels of PGE2 may serve as a signal for redirecting AA metabolism towards increased formation of LXA4. Thus, both PGE2 and LXA4, derived from AA, seem to be critical not only in resolving inflammation but also by enhancing tissue regeneration. In this context, it is important to note that oral supplementation of AA does not affect PGE2 levels but enhances LXA4 formation [87, 88]. We observed that oral supplementation of AA suppresses inflammation by inhibiting the formation of IL-6, TNF-α and the expression of NF-kB [32, 33]. The anti-inflammatory cytokines IL-4 and IL-10 seem to trigger the conversion of AA, EPA and DHA to lipoxins, resolvins, protectins and maresins, suggesting a mechanism by which they are able to suppress inflammation [72, 215].
Both in autoimmune diseases and cancer, an increase in IL-17 levels have been described [216,217,218,219,220,221,222,223,224]. It is noteworthy that IL-17 not only promoted lung cancer growth but also contributed to the resistance to PD-1 blockade and promoted inflammation, factors that worsen prognosis of cancer [224]. IL-17 interacts with PGE2, IL-23, IL-6, TNF-α and the immune check point inhibitors (PD-1 and PD-L1) and, thereby, may facilitate tumor cell growth.
Thus, there are many overlapping features between autoimmune diseases (especially RA and lupus) and cancer implying that both could be managed by same, if not identical, therapeutic strategies. In view of these observations, it is tempting to propose that oral or intravenous administration of AA, EPA, DHA, GLA, DGLA, vitamin C, B1, B6, B12 in conjunction with immunosuppressive drugs such as corticosteroids and cyclophosphamide, methotrexate, and cyclosporine may be effective against RA, lupus and cancer. Both PUFAs and vitamin C may serve as antioxidants with regard to autoimmune diseases and as pro-oxidants in cancer to eliminate tumor cells as shown in Fig. 3.16. The big question is why the same compounds, BALs, vitamin C and anti-cancer drugs such as cyclophosphamide, methotrexate, and cyclosporine when given together serve as pro-oxidants in tumor cells but as antioxidants in normal cells. This could be attributed to the differences in antioxidant defences of the cells. When normal cells are exposed to vitamin C and BALs, the pro-oxidant action of these compounds stimulates their antioxidant defences, whereas tumor cells fail to do so since they have a defective antioxidant system. Thus, normal cells are able to protect themselves whereas tumor cells fail to do so and undergo apoptosis. Taken together, these findings support the idea that the same regimen of administering vitamin C and BALs with or without immunosuppressive drugs would be useful in cancer and lupus and RA.
An overview of the actions of PUFAs, vitamin C and conventional anti-cancer drugs on ROS generation, GPX4 activity and accumulation of lipid peroxides and ferroptosis/apoptosis in normal and tumor cells and their possible role in rheumatological conditions. When normal cells and tumor cells are exposed to chemotherapy and radiation there will be increased generation of free radicals (ROS), accumulation of lipid peroxides and decreases in the activity of the potent endogenous antioxidant GPX4. This leads to death (apoptosis and ferroptosis) of both normal and tumor cells. However, when vitamin C and PUFAs are administered to normal cells, they function as antioxidants and so quench ROS and prevent accumulation of lipid peroxides and protect the cells. In the case of tumor cells, both vitamin C and PUFAs act in conjunction with chemotherapy and radiation to generate more ROS and enhance accumulation of toxic lipid peroxides and decrease GPX4 that ultimately leads to their apoptosis and ferroptosis. This causes the elimination of the cancer cells. Our studies have shown that these differential actions of vitamin C and PUFAs in normal and tumor cells are a result of changes in the synthesis of PGE2 and LXA4 as shown in the figure. Vitamin C, PUFAs/BALs and immunosuppressive drugs are given in rheumatological conditions, which may lead to elimination of diseased cells and protection of normal cells and increase the generation of LXA4/resolvins/protectins/maresins and decrease in PGE2/LTs/TXs. This can result in the remission of diseases such as RA and lupus
10 Ion Channels and Bioactive Lipids
Another action of BALs that is relevant to their tumoricidal activities is their ability to modulate the properties of ion channels by altering cell membrane fluidity when they are incorporated into the membrane as shown in Fig. 3.17. PUFAs can modify the properties of the TRPV group of transient receptor potential family of ion channels and Piezo1 and Piezo2 channels. There is a close interaction between Piezo channels and TRPV ion channels. It is possible that this property of PUFAs (and possibly for various PGs, LTs, TXs, LXA4, resolvins, protectins and maresins including lipid peroxides) on various ion channels may explain many of the BAL actions including their role in inflammation, resolution of inflammation, immune response, fibrosis, tissue regeneration, epithelial to mesenchymal transition, and induction of apoptosis, ferroptosis and necrosis of tumor cells.
Scheme showing possible relationship among ion channels, fatty acids and cell proliferation or apoptosis. (Modified from Accardi [227])
Depending on the type and amount of fatty acids in the cell membrane, the membrane can be fluid or rigid. The nature of the cell membrane determines the expression and function of various membrane receptors. If the cell membrane is fluid, due to the incorporation of higher amounts of PUFAs, the number of receptors, such as the insulin receptor, will be higher. In contrast, if the membrane is rigid due to higher amounts of saturated fatty acids the number of insulin receptors will decrease. The cell membrane also contains several ion channel voltage-gated ion channels (VGIC) that allow the diffusion of ions such as K+, Ca2+, Cl−, Na+. These ion channels control rapid bioelectrical signaling including action potentials and/or contraction, cell mitotic signaling, cell cycle progression, as well as cell volume regulation. Thus, they play a critical role in cancer cell proliferation. In addition to the VGIC, there are two other ion channels namely TrpV1 and Piezo1.
Phosphatidylserine (PS) is a phospholipid and an important constituent of the cell membrane. It plays a key role in cell cycle signaling including the apoptosis pathway. PS consists of two fatty acids attached via an ester linkage to the first and second carbon (C) of glycerol and serine attached through a phosphodiester linkage to the third carbon of the glycerol. Most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on the C-2 position of the glycerol backbone. The fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to its continuous degradation and remodeling. PS carries a net charge of −1 at physiological pH. PS mostly has palmitic or stearic acid on C-1 and a long chain unsaturated fatty acid (such as 18:2, 20:4 and 22:6) on C-2. However, this composition of PS is amenable to alteration depending on the diet, supplementation, state of the cell, environment and stimuli to which the cell is exposed.
TrpV1 is a member of the TRPV group of transient receptor potential family of ion channels. The function of TRPV1 is detection and regulation of body temperature and provision of a sensation of scalding heat and pain. Piezo1 and Piezo2 are nonselective Ca2+-permeable cation channels that interact with Trpv1 [225]. Changes in the cell membrane lipid composition leads to alterations in the activities of all of these channels which, in turn, can affect cell proliferation, volume and motility and, thus, metastasis in cancer. Plasma membrane depolarization can induce reorganization of PS and phosphatidylinositol 4,5-bisphosphate that can lead to amplification of K-Ras–dependent mitogen-activated protein kinase (MAPK) signaling. In contrast, plasma membrane repolarization disrupts K-Ras nanoclustering and inhibits MAPK signaling. Thus, changes in cell membrane composition can induce changes in VGIC, TrpV1 and Piezo1, which can either enhance or suppress cellular mitosis or cause apoptosis [226, 227]. It is envisaged that under normal physiological conditions, the cell membrane will contain a balanced ratio between saturated fatty acids and PUFAs (Fig. 3.17) resulting in PS appearing in small clusters that localize to K-Ras and so low activation of RAF-MAPK pathway occurs. Cancer cells contain more saturated fatty acids and lower amounts of polyunsaturated fatty acids that results in an increase in the rigidity of the cell membrane leading to clustering of PS and K-Ras such that promotion of RAF-MAPK signaling occurs. This leads to uncontrolled proliferationof cancer cells. When tumor cells are supplemented with PUFAs, the cell membrane becomes more fluid and accumulation of excess of toxic lipid peroxides occurs, which results in disruption of PS and K-Ras clustering and its inactivation which results in their mitotic arrest and apoptosis. Changes in cell membrane fluidity and composition can affect PS composition, and changes in the expression and function of various ion channels including Trpv1 and Piezo1 as shown in Fig. 3.17. In turn, this results in perturbation of ion transmission across the channels and the membrane leading to cell apoptosis. Lipid peroxides that accumulate in the cell as a result of PUFA supplementation may inactivate various ion channel receptors, block K-Ras and the MAPK pathway or suppress it. Furthermore, changes in lipid composition of the cell membrane can also alter T cell proliferation, activation, and local response of T cells to the tumor cells [228].
It is possible that K+ and other ions leak from the cancer cells into the surrounding milieu and act on infiltrating macrophages, T cells and suppress the immune response and, thus, aid in the escape of tumor cells from immune surveillance system [229]. AA and other PUFAs activate potassium channels [230, 231] and thereby enhance the T cell responses by removing excess potassium from the tumor cell milieu. In addition, KATP channels are inactivated by high glucose concentrations [232] that may explain why tumor cells have aerobic glycolysis. GABA (gamma-aminobutyric acid) inhibits KATP channels [232] and therefore neurons and local nerves may regulate tumor growth [233, 234]. Cancer cells form synaptic connections with neurons facilitated by cell adhesion proteins neurexins and neuroligins [235]. Through these synaptic connections neurotransmitters such as glutamate may be released that bind and activate AMPA and NMDA receptors that facilitates positively charged ions to enter the cells through the receptors to cause depolarization leading to a rise in intracellular positive charge. As a result, cancer cell migration and proliferation may occur [229,230,231,232,233,234]. Potassium leakage from cells activates Ca2+-independent phospholipase A2, which enhances cleavage of pro-IL-1β by the IL-1 converting enzyme capsase-1 [236, 237]. This action of potassium on the IL-1 converting enzyme can be prevented by other monovalent cations such as sodium. High intracellular concentrations of potassium suppress apoptosis [238]. Thus, higher potassium concentration seen in the tumor microenvironment may suppress the immune response [229] such that immunosuppression against tumor cells may persist for a longer time. In addition, it will also lead to apoptosis of T cells since the concentration of potassium is higher in the tumor microenvironment compared to intracellular levels of T cells. Furthermore, phospholipase A2 induces the release of PUFAs from the cell membrane lipid pool and PUFAs activate potassium channels [230, 231]. Thus, there is a close interaction of local and intracellular concentrations of Ca2+and other ions such as K+, Ca2+, Cl−, Na+; phospholipase A2 activity, IL-1 and possibly other cytokines, glutamate, GABA and other neurotransmitters , with tumor growth. Thus, the tumor milieu, including the intra- and extracellular glucose concentration, contributes to tumor cell proliferation. Furthermore, there could be a close interaction among various ions within themselves and with intracellular and extracellular glucose concentrations. Glucose can activate or suppress the activity of phospholipase A2 depending upon its local concentration and thereby influence lipid peroxide formation.
11 Connecting the Cell Membrane to the Nucleus
All of the stimuli to which the cell gets exposed need to be transmitted to the respective genes to elicit an adequate and appropriate cellular response. How this occurs is not precisely known. One possibility is that membrane fluidity can influence the structure and composition of intermediate filaments and their multiple binding partners to regulate both cellular mechanics and gene(s) expression. The intermediate filaments, actin and microtubules form distinct cytoskeletal systems, and are critical in the dynamic interplay between these networks. Intermediate filaments provide structural support for the cells, and play a major role in cellular responses to external mechanical forces. It is known that tensional force-induced reinforcement of actin stress fibers requires the interaction of the RhoA-targeting Rho-guanine nucleotide exchange factors Solo/ARHGEF40 with keratin intermediate filaments to activate RhoA signaling, which promotes stress fiber formation and keratin network organization. These results illustrate the importance of keratins to enable cells to adapt to mechanical stress [239]. The interaction of desmoplakin with keratin filaments at desmosomes supports intercellular force transmission, traction force generation, and cell stiffness that ultimately alters the expression of several genes concerned with mitosis and apoptosis [240,241,242].
2-methoxy-oestradiol (2-ME), PUFAs, thalidomide, TNF, ILs and many anti-cancer drugs, radiation and protoporphyrin derivatives (used in photodynamic therapy) enhance free radical generation and augment the lipid peroxidation process. This leads to accumulation of lipid peroxides in the cells, resulting in apoptosis of tumor cells [243,244,245,246,247,248,249,250]. PUFAs are cytotoxic to tumor cells, possess anti-angiogenic action and enhance free radical generation in the tumor cells and regress the growth of human gliomas with few side-effects [180,181,182,183,184,185,186,187,188, 245,246,247,248,249,250,251,252]. It is likely that PUFAs and lipid peroxides alter or disrupt the intermediate filaments, actin and microtubules and cytoskeletal filament systems partly by altering cell membrane fluidity and to some extent by their direct action on the cytoskeleton.
12 Polyunsaturated Fatty Acids and Bioactive Lipids Are Involved in Mitochondrial Processes
Dietary or supplementation of PUFAs are absorbed from the gut and then distributed to cells where they enrich various cellular membranes. This influences not only cell metabolic processes and survival but also modulates mitochondrial processes [253, 254]. In addition, n-3 PUFAs protect ischemic myocardium [255] especially against oxidative-induced damage due to their ability to modulate mitochondrial ROS production [256].
These results are supported by the observation that fat-1 transgenic mice which synthesize n-3 fatty acids at the cost of AA showed a decrease in ROS production from electron transport complex (ETC)-I suggesting that EPA and DHA are able to reduce oxidative stress in the mammary tissue when exposed to the carcinogen 7,12-dimethyl benz(α)anthracene (DMBA) [257].
In contrast, tumor cells exposed to PUFAs were found to produce enhanced amounts of ROS and accumulation of toxic lipid peroxides leading to apoptosis in a caspase-dependent manner, involving both the intrinsic and extrinsic pathways [179, 258,259,260,261,262].
On the other hand, we observed that GLA, AA, EPA and DHA can protect pancreatic β cells against alloxan and streptozotocin-induced cytotoxicity and prevent the development of both type 1 and type 2 diabetes mellitus in experimental animals by suppressing free radical generation, and of NF-kB, IL-6 and TNF-α [32, 33].
Based on this data, it can be suggested that PUFAs seem to be metabolized differently by normal and tumor cells such that normal cells are protected and tumor cells get exposed to increased oxidative stress [183]. This differential action and metabolism of PUFAs by normal and tumor cells implies that PUFAs can be employed to selectively eliminate tumor cells and may also be useful to prevent diabetes mellitus [5, 11, 32, 33, 183, 246,247,248,249,250, 263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285]. This cytoprotective action of PUFAs is possibly mediated by their products: PGE1, lipoxins, resolvins, protectins and maresins [11, 263, 266, 267]. Thus, the beneficial actions of PUFAs can be ascribed to their products such as PGE1, PGI2, lipoxins, resolvins, protectins and maresins and their ability to enhance NO and alter the expression of NF-kB, IkB, caspases , cytochrome C, Ras, Myc, Fos, Fas, p53, COX-2, and LOX, and by alteration of telomerase activity (Figs. 3.7, 3.10 and 3.16).
13 Bioactive Lipids Modulate G-Protein-Mediated Signals
BALs modulate G-protein–mediated signal transduction [286] and mobilize Ca2+ from intracellular stores [287]. This can induce apoptosis [288] especially of tumor cells, activate PKC and augment NADPH oxidase activity in macrophages [289], which can result in enhanced O2−. generation. GLA, AA, EPA and DHA decreased Bcl-2 and increased Bax in tumor cells [33, 290] in addition to their action on p53 [291]. DHA has been reported to enhance p27, inhibit cyclin-associated kinase, reduce pRb phosphorylation and induce apoptosis of melanoma cells [291]. BALs such as PUFAs inhibit cell division by blocking translation initiation [292]. PUFAs were found to induce free radicals in tumor cells that can directly activate heterodimeric Gi and G0 (small G proteins) [293], which are critical signaling molecules. Thus, BALs such as PUFAs and their metabolites have actions that are detrimental to the survival of tumor cells.
14 Age-Related Disorders Are Inflammatory Conditions and Can Be Modulated by Bioactive Lipids
With advancing age, there is a tendency to accumulate abdominal fat, decrease in muscle and skeletal mass (osteoporosis), with development of insulin resistance, type 2 diabetes mellitus, hypertension, and an increase in the incidence of cancer, CHD and atherosclerosis, Alzheimer’s disease and depression, and increased chances of having disc prolapse, osteoarthritis, and tendon tears. These are all inflammatory conditions. There is reasonable evidence to suggest that in all these conditions, there is a critical role for BALs (Fig. 3.2). Based on the preceding discussion it is evident that efforts directed to restore the altered BAL abnormalities to normality could be of benefit in all these disorders. The various actions of BALs as outlined above, such as the ability to alter cell membrane fluidity, influence ion channels, act on G protein coupled receptors, regulate inflammation, immune response and stem cell biology, telomerase activity, mitochondrial processes, cytoskeletal system and participate in resolution of inflammation and wound healing, are some of the crucial actions that are relevant to their involvement in these age-related disorders. Hence, analyzing the plasma and tissue concentrations of various BALs in these conditions may give clues as to the type of abnormalities that need to be corrected. In general, it is likely that the plasma tissue concentrations of GLA, DGLA, AA, EPA, DPA, DHA, lipoxins, resolvins, protectins and maresins and anti-inflammatory cytokines are likely to be low and accompanied by a deficiency of NO, H2S, CO and an increase in pro-inflammatory PGs, LTs, TXs and cytokines with a concomitant decrease in antioxidants. Although it is unlikely that all of these molecules will be abnormal in these disorders, measuring all of them together may give clues to the specific alterations in their concentrations so that the underlying pathophysiology could be deciphered to plan relevant interventions.
15 Conclusions and Future Perspectives
One of the questions that should be answered is how BALs could have a role in many conditions. It should be mentioned here that it is the local actions of BALs that make them suitable candidates for a critical role in these conditions. Thus, it is suggested that abnormalities in the BAL system in vascular endothelial cells may lead to hypertension, in the pancreatic β cells to diabetes mellitus, in adipose tissue to obesity, in the skeletal muscles to sarcopenia, in the osteoclasts and osteoblasts to osteoporosis, in the coronary vascular endothelial cells to atherosclerosis, in neuronal cells to Alzheimer’s disease and depression, in the intervertebral disc to prolapsed, herniated, or extruded intervertebral disc (PIVD) and in specific cells to relevant cancers. If this proposal is true, it implies that administration of various PUFAs and/or lipoxins, resolvins, protectins and maresins in appropriate amounts and in a timely manner will lead to relief from these age-related disorders. Since lipoxins, resolvins, protectins and maresins are highly unstable and have short half-lives, they may not be suitable for clinical use. I propose that oral or intravenous administration of GLA, DGLA, AA, EPA, DPA, DHA and various co-factors such as vitamins B1, B6, B12, and C, zinc, magnesium, and folic acid should be provided to achieve their beneficial actions (Fig. 3.2). It should be noted that it may be necessary to administer other co-factors to optimize the synthesis and action of other relevant endogenous molecules such as NO. This could include provision of L-arginine, tetrahydrobiopterin and other minerals and trace elements to obtain the much-needed beneficial actions. By providing all these precursors, it is presumed that cells and tissues will utilize these raw materials to form the much needed and relevant BALs, NO, H2S, CO and anti-inflammatory cytokines to boost the antioxidant defences. Since all these above-mentioned factors are endogenous to natural substances in our bodies, it is anticipated that their administration is unlikely to have any side effects. In some conditions such as tendon tears and PIVD, perhaps it is relevant to administer BALs locally, either using a transdermal approach or via local injections. For patients with cancer, BALs could be administered in conjunction with conventional anti-cancer drugs and immune check point inhibitors, as proposed previously [61, 62]. In our preliminary study, it was noted that administration of PUFAs along with conventional chemotherapeutic drugs can induce remission with few side effects, and reverse drug resistance to standard chemotherapy. In fact, it was noticed that co-administration of BALs and high doses of vitamin C blunted the side effects of chemotherapeutic drugs and induced full remission in one of our stage IV drug-resistant Hodgkin’s disease patients. We observed that BALs can be administered with corticosteroids and other immunosuppressive drugs in cases of lupus and RA, to induce full remission in some cases with no recurrence of the disease. Some of these patients have been on follow up for more than 10 years and are still in full remission despite stoppage of all drugs. These preliminary results are encouraging and are in support of the proposals made here. Obviously, more thorough and in-depth studies are needed to bring BAL-based therapeutics to the mainstream, but are certainly encouraging. Based on these results, it is tempting to suggest that prophylactic administration of various PUFAs and their co-factors may aid in the prevention, postponement or delay of the aging process itself. In this context, it is noteworthy that exercise and calorie restriction, the two interventions that are known to delay aging, also modulate EFA/PUFA metabolism [47].
In general, it is believed that stem cells are essential for healing and regeneration of tissues and thus have a critical role in recovery from various diseases. In this context, it is interesting to note that stem cells seem to bring about their beneficial actions by secreting LXA4 [294], which appears to regulate stem cell proliferation and differentiation [295]. Therefore, it is proposed that BALs may serve as the mediators of the beneficial actions of stem cells. Perhaps, a combination of stem cells and BALs may form a new therapeutic approach to several disorders associated with aging.
References
Le Gall JY, Ardaillou R (2009) The biology of aging. Bull Acad Natl Med 193:365–402
Poorani R, Bhatt AN, Dwarakanath BS, Das UN (2016) COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol 785:116–132
Das UN (1985) Minerals, trace elements and vitamins interact with essential fatty acids and prostaglandins to prevent hypertension, thrombosis, hypercholesterolemia and atherosclerosis and their attendant complications. IRCS J Med Sci 13:684–687
Das UN (1987) Magnesium, essential fatty acids and cardiovascular diseases. J Assoc Physicians India 35:171
Das UN, Ramadevi G, Rao KP, Rao MS (1989) Prostaglandins can modify gamma-radiation and chemical induced cytotoxicity and genetic damage in vitro and in vivo. Prostaglandins 38:689–716
Das UN (1989) Nutrients, essential fatty acids and prostaglandins interact to augment immune responses and prevent genetic damage and cancer. Nutrition 5:106–110
Das UN (2000) Interaction(s) between nutrients, essential fatty acids, eicosanoids, free radicals, nitric oxide, anti-oxidants and endothelium and their relationship to human essential hypertension. Med Sci Res 28:75–83
Das UN (2006) Essential fatty acids: biochemistry, physiology, and pathology. Biotechnol J 1:420–439
Das UN (2006) Biological significance of essential fatty acids. J Assoc Physicians India 54:309–319
Das UN (2008) Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis 7:37. https://doi.org/10.1186/1476-511X-7-37
Das UN (2011) Molecular basis of health and disease. Springer, New York. ISBN-10: 9400704941
Das UN (1991) Interaction(s) between essential fatty acids, eicosanoids, cytokines, growth factors and free radicals: relevance to new therapeutic strategies in rheumatoid arthritis and other collagen vascular diseases. Prostaglandins Leukot Essent Fatty Acids 44:201–210
Kumar GS, Das UN (1994) Effect of prostaglandins and their precursors on the proliferation of human lymphocytes and their secretion of tumor necrosis factor and various interleukins. Prostaglandins Leukot Essent Fatty Acids 50:331–334
Rotondo D, Earl CR, Laing KJ, Kaimakamis D (1994) Inhibition of cytokine-stimulated thymic lymphocyte proliferation by fatty acids: the role of eicosanoids. Biochim Biophys Acta 1223:185–194
Santoli D, Zurier RB (1989) Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol 143:1303–1309
Miles EA, Allen E, Calder PC (2002) In vitro effects of eicosanoids derived from different 20-carbon fatty acids on production of monocyte-derived cytokines in human whole blood cultures. Cytokine 20:215–223
Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y (1997) Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol 400B:589–597
Czeslick EG, Simm A, Grond S, Silber RE, Sablotzki A (2003) Inhibition of intracellular tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 production in human monocytes by iloprost. Eur J Clin Investig 33:1013–1017
Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W et al (2017) Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 14:143. https://doi.org/10.1186/s12974-017-0917-3
Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z et al (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 15:116. https://doi.org/10.1186/s12974-018-1151-3
Chang CS, Sun HL, Lii CK, Chen HW, Chen PY, Liu KL (2010) Gamma-linolenic acid inhibits inflammatory responses by regulating NF-kappaB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 33:46–57
Dooper MM, van Riel B, Graus YM, M’Rabet L (2003) Dihomo-gamma-linolenic acid inhibits tumour necrosis factor-alpha production by human leucocytes independently of cyclooxygenase activity. Immunology 110:348–357
Das UN (2010) Current and emerging strategies for the treatment and management of systemic lupus erythematosus based on molecular signatures of acute and chronic inflammation. J Inflammation Res 3:143–170
Menezes-de-Lima O Jr, Kassuya CA, Nascimento AF, Md H, Calixto JB (2006) Lipoxin A4 inhibits acute edema in mice: implications for the anti-edematogenic mechanism induced by aspirin. Prostaglandins Other Lipid Mediat 80:123–135
Benabdoun HA, Kulbay M, Rondon EP, Vallières F, Shi Q, Fernandes J et al (2019) In vitro and in vivo assessment of the proresolutive and antiresorptive actions of resolvin D1: relevance to arthritis. Arthritis Res Ther 21:72
Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M et al (2008) An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol 155:1214–1223
Wu L, Miao S, Zou LB, Wu P, Hao H, Tang K et al (2012) Lipoxin A4 inhibits 5-lipoxygenase translocation and leukotrienes biosynthesis to exert a neuroprotective effect in cerebral ischemia/reperfusion injury. J Mol Neurosci 48:185–200
Lee TH, Lympany P, Crea AE, Spur BW (1991) Inhibition of leukotriene B4-induced neutrophil migration by lipoxin A4: structure-function relationships. Biochem Biophys Res Commun 180:1416–1421
McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C (2002) Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J 16:1817–1819
Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A et al (2006) Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A 103:11276–11281
Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ et al (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26:1755–1765
Naveen KVG, Naidu VGM, Das UN (2017) Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. Biofactors 43:251–271
Naveen KVG, Naidu VGM, Das UN (2017) Arachidonic acid and lipoxin A4 attenuate streptozotocin-induced cytotoxicity to RIN5F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition 35:61–80
Das UN, Ells G, Begin ME, Horrobin DF (1986) Free radicals as possible mediators of the actions of interferon. J Free Rad Biol Med 2:183–188
Das UN, Padma M, Sangeetha P, Ramesh G, Koratkar R (1990) Stimulation of free radical generation in human leukocytes by various stimulants including tumor necrosis factor is a calmodulin dependent process. Biochem Biophys Res Commun 167:1030–1036
Tsujimoto M, Yokota S, Vilcek J, Weissman G (1986) Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun 137:1094–1100
Beton G, Zeni L, Casaatella MA, Rossi F (1986) Gamma-interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun 138:1276–1282
Das UN, Huang YS, Begin ME, Horrobin DF (1986) Interferons, phospholipid metabolism, immune responses and cancer. IRCS Med Sci 14:1069–1074
Bordoni A, Hrelia S, Lorenzini A, Bergami R, Cabrini L, Biagi PL et al (1998) Dual influence of aging and vitamin B6 deficiency on delta-6-desaturation of essential fatty acids in rat liver microsomes. Prostaglandins Leukot Essent Fatty Acids 58:417–420
Bordoni A, Biagi PL, Turchetto E, Hrelia S (1988) Aging influence on delta-6-desaturase activity and fatty acid composition of rat liver microsomes. Biochem Int 17:1001–1009
Biagi PL, Bordoni A, Hrelia S, Celadon M, Horrobin DF (1991) Gamma-linolenic acid dietary supplementation can reverse the aging influence on rat liver microsome delta 6-desaturase activity. Biochim Biophys Acta 1083:187–192
Lopez Jimenez JA, Bordoni A, Lorenzini A, Rossi CA, Biagi PL, Hrelia S (1997) Linoleic acid metabolism in primary cultures of adult rat cardiomyocytes is impaired by aging. Biochem Biophys Res Commun 237:142–145
Lorenzini A, Bordoni A, Spanò C, Turchetto E, Biagi PL, Hrelia S (1997) Age-related changes in essential fatty acid metabolism in cultured rat heart myocytes. Prostaglandins Leukot Essent Fatty Acids 57:143–147
Bourre JM, Piciotti M, Dumont O (1990) Delta 6 desaturase in brain and liver during development and aging. Lipids 25:354–356
Horrobin DF (1981) Loss of delta-6-desaturase activity as a key factor in aging. Med Hypotheses 7:1211–1220
Das UN (2007) A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot Essent Fatty Acids 76:251–268
Das UN (2018) Ageing: is there a role for arachidonic acid and other bioactive lipids? A review. J Adv Res 11:67–79
Shim JH (2019) Prostaglandin E2 induces skin aging via E-prostanoid 1 in normal human dermal fibroblasts. Int J Mol Sci 20(22). pii: E5555. https://doi.org/10.3390/ijms20225555
Young MK, Bocek RM, Herrington PT, Beatty CH (1981) Ageing: effects on the prostaglandin production by skeletal muscle of male rhesus monkeys (Macaca mulatta). Mech Ageing Dev 16:345–353
Fraifeld V, Kaplanski J, Kukulansky T, Globerson A (1995) Increased prostaglandin E2 production by concanavalin A-stimulated splenocytes of old mice. Gerontology 41:129–133
Hayek MG, Meydani SN, Meydani M, Blumberg JB (1994) Age differences in eicosanoid production of mouse splenocytes: effects on mitogen-induced T-cell proliferation. J Gerontol 49:B197–B207
Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D et al (1998) Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Phys 275:C661–C668
Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS et al (2001) Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci 56:B426–B431
Gangemi S, Pescara L, D’Urbano E, Basile G, Nicita-Mauro V, Davì G et al (2005) Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol 40:612–614
Das UN (2020) Molecular pathobiology of scleritis and its therapeutic implications. Int J Ophthalmol 13(1):163–175
Das UN (2019) Beneficial role of bioactive lipids in the pathobiology, prevention, and management of HBV, HCV and alcoholic hepatitis, NAFLD, and liver cirrhosis: a review. J Adv Res 17:17–29
Das UN (2019) Polyunsaturated fatty acids and sepsis. Nutrition 65:39–43
Das UN (2019) Bioactive lipids in intervertebral disc (IVD) degeneration and its therapeutic implications. BioSci Rep 39(10). pii: BSR20192117. https://doi.org/10.1042/BSR20192117
Das UN (2019) Bioactive lipids in shoulder tendon tears. Am J Pathol 189:2149–2153
Dakin SG, Colas RA, Wheway K, Watkins B, Appleton L, Rees J et al (2019) Proresolving mediators LXB4 and RvE1 regulate inflammation in stromal cells from patients with shoulder tendon tears. Am J Pathol 189:2258–2268
Das UN (2019) Can bioactive lipid(s) augment anti-cancer action of immunotherapy and prevent cytokine storm? Arch Med Res 50:342–349
Das UN (2020) Bioactive lipids as modulators of immune check point inhibitors. Med Hypotheses 135:109473. https://doi.org/10.1016/j.mehy.2019.109473
Das UN (2018) Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus. J Adv Res 11:43–55
Das UN (2010) Essential fatty acids and their metabolites in the context of hypertension. Hypertens Res 33:782–785
Inoue K, Kishida K, Hirata A, Funahashi T, Shimomura I (2013) Low serum eicosapentaenoic acid/arachidonic acid ratio in male subjects with visceral obesity. Nutr Metab (Lond) 10:25. https://doi.org/10.1186/1743-7075-10-25
Yagi S, Aihara K, Fukuda D, Takashima A, Bando M, Hara T et al (2015) Reduced ratio of eicosapentaenoic acid and docosahexaenoic acid to arachidonic acid is associated with early onset of acute coronary syndrome. Nutr J 14:111. https://doi.org/10.1186/s12937-015-0102-4
Yagi S, Hara T, Ueno R, Aihara K, Fukuda D, Takashima A et al (2014) Serum concentration of eicosapentaenoic acid is associated with cognitive function in patients with coronary artery disease. Nutr J 13:112. https://doi.org/10.1186/1475-2891-13-112
Das UN (2013) Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot Essent Fatty Acids 88:201–210
Das UN (2007) Vagus nerve stimulation, depression and inflammation. Neuropsychopharmacology 32:2053–2054
Das UN (2017) Is there a role for bioactive lipids in the pathobiology of diabetes mellitus? Front Endocrinol (Lausanne) 8:182. https://doi.org/10.3389/fendo.2017.00182
Börgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF et al (2012) Lipoxin A4 attenuates adipose inflammation. FASEB J 26:4287–4294
Das UN (2011) Lipoxins as biomarkers of lupus and other inflammatory conditions. Lipids Health Dis 10:76. https://doi.org/10.1186/1476-511X-10-76
Das UN (2016) Renin-angiotensin-aldosterone system in insulin resistance and metabolic syndrome. J Transl Int Med 4:66–72
Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN et al (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84:24–35
Mai J, Liu W, Fang Y, Zhang S, Qiu Q, Yang Y et al (2018) The atheroprotective role of lipoxin A4 prevents oxLDL-induced apoptotic signaling in macrophages via JNK pathway. Atherosclerosis 278:259–268
Kain V, Liu F, Kozlovskaya V, Ingle KA, Bolisetty S, Agarwal A et al (2017) Resolution agonist 15-epi-lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci Rep 7:9999. https://doi.org/10.1038/s41598-017-10441-8
Schnittert J, Heinrich MA, Kuninty PR, Storm G, Prakash J (2018) Reprogramming tumor stroma using an endogenous lipid lipoxin A4 to treat pancreatic cancer. Cancer Lett 420:247–258
Simões RL, De-Brito NM, Cunha-Costa H, Morandi V, Fierro IM, Roitt IM et al (2017) Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer 140:346–357
Wang Z, Cheng Q, Tang K, Sun Y, Zhang K, Zhang Y et al (2015) Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Lett 364:118–124
Xu F, Zhou X, Hao J, Dai H, Zhang J, He Y et al (2018) Lipoxin A4 and its analog suppress hepatocarcinoma cell epithelial-mesenchymal transition, migration and metastasis via regulating integrin-linked kinase axis. Prostaglandins Other Lipid Mediat 137:9–19
Liu C, Guan H, Cai C, Li F, Xiao J (2017) Lipoxin A4 suppresses osteoclastogenesis in RAW264.7 cells and prevents ovariectomy-induced bone loss. Exp Cell Res 352:293–303
Banu J, Bhattacharya A, Rahman M, Kang JX, Fernandes G (2010) Endogenously produced n-3 fatty acids protect against ovariectomy induced bone loss in fat-1 transgenic mice. J Bone Miner Metab 28:617–626
Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G (2003) Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res 18:1206–1216
Wei J, Chen S, Guo W, Feng B, Yang S, Huang C, Chu J (2018) Leukotriene D4 induces cellular senescence in osteoblasts. Int Immunopharmacol 58:154–159
Bhattacharya A, Rahman M, Banu J, Lawrence RA, McGuff HS, Garrett IR et al (2005) Inhibition of osteoporosis in autoimmune disease prone MRL/Mpj-Fas(lpr) mice by N-3 fatty acids. J Am Coll Nutr 24:200–209
Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E et al (2010) Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respir Res 11:71. https://doi.org/10.1186/1465-9921-11-71
Tateishi N, Kakutani S, Kawashima H, Shibata H, Morita I (2014) Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis 13:30. https://doi.org/10.1186/1476-511X-13-30
Tateishi N, Kaneda Y, Kakutani S, Kawashima H, Shibata H, Morita I (2015) Dietary supplementation with arachidonic acid increases arachidonic acid content in paw, but does not affect arthritis severity or prostaglandin E2 content in rat adjuvant-induced arthritis model. Lipids Health Dis 14:3. https://doi.org/10.1186/1476-511X-14-3
Das UN (2019) Circulating microparticles in septic shock and sepsis-related complications. Minerva Anestesiol. (in press)
Dakin SG, Ly L, Colas RA, Oppermann U, Wheway K, Watkins B et al (2017) Increased 15-PGDH expression leads to dysregulated resolution responses in stromal cells from patients with chronic tendinopathy. Sci Rep 7:11009. https://doi.org/10.1038/s41598-017-11188-y
Zhang Y, Desai A, Yang SY, Bae KB, Antczak MI, Fink SP et al (2015) Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348:aaa2340. https://doi.org/10.1126/science.aaa2340
FitzGerald GA (2015) Bringing PGE2 in from the cold. Science 348:1208–1209
Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X et al (2016) Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell–IL-22 axis. Science 351:1333–1338
Ueda T, Fukunaga K, Seki H, Miyata J, Arita M, Miyasho T et al (2014) Combination therapy of 15-epi-lipoxin A4 with antibiotics protects mice from Escherichia coli-induced sepsis. Crit Care Med 42:e288–e295
Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A et al (2011) Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 36:410–416
Wu B, Walker J, Spur B, Rodriguez A, Yin K (2015) Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fatty Acids 94:55–64
Wu B, Capilato J, Pham MP, Walker J, Spur B, Rodriguez A et al (2016) Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J 30:2400–2410
Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA et al (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461:1287–1291
Desbois AP, Lawlor KC (2013) Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar Drugs 11:4544–4557
Das UN (2018) Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res 11:57–66
Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 579:5157–5162
Le PNT, Desbois AP (2017) Antibacterial effect of eicosapentaenoic acid against Bacillus cereus and Staphylococcus aureus: killing kinetics, selection for resistance, and potential cellular target. Mar Drugs 15: pii: E334. https://doi.org/10.3390/md15110334
Giamarellos-Bourboulis EJ, Grecka P, Dionyssiou-Asteriou A, Giamarellou H (1998) In vitro activity of polyunsaturated fatty acids on Pseudomonas aeruginosa: relationship to lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids 58:283–287
Das UN (1985) Antibiotic-like action of essential fatty acids. Can Med Assoc J 132:1350
Lee KA, Shin KS, Kim GY, Song YC, Bae EA, Kim IK et al (2016) Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell 15:291–300
Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C (2010) Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell 9:785–798
Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M (2009) Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol 44:517–522
Fukushima Y, Minato N, Hattori M (2018) The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm Regen 38:24. https://doi.org/10.1186/s41232-018-0082-9
Harris SG, Padilla J, Koumas L, Ray D, Phipps RP (2002) Prostaglandins as modulators of immunity. Trends Immunol 23:144–150
Kalinski P (2012) Regulation of immune responses by prostaglandin e2. J Immunol 188:21–28
Das UN, Padma MC (1978) Prostaglandins in lymphocyte transformation. J Assoc Physicians India 26:503–506
Das UN (1980) Prostaglandins and immune response in cancer. Int J Tiss Reac 2:233–236
Das UN (1981) Inhibition of sensitized lymphocyte response to sperm antigen(s) by prostaglandins. IRCS Med Sci 9:1087
Kumar GS, Das UN, Kumar KV, Madhavi DNP, Tan BKH (1992) Effect of n-6 and n-3 fatty acids on the proliferation and secretion of TNF and IL-2 by human lymphocytes in vitro. Nutr Res 12:815–823
Das UN (2014) HLA-DR expression, cytokines and bioactive lipids in sepsis. Arch Med Sci 10:325–335
Narumiya S (2007) Physiology and pathophysiology of prostanoid receptors. Proc Jpn Acad Ser B 83:296–319
Goodwin JS, Ceuppens J (1983) Regulation of the immune response by prostaglandins. J Clin Immunol 3:295–315
Betz M, Fox BS (1991) Prostaglandin E2 inhibits production of TH1 lymphokines but not of Th2 lymphokines. J Immunol 146:108–113
Gold KN, Weyand CM, Goronzy JJ (1994) Modulation of helper T cell function by prostaglandins. Arthritis Rheum 37:925–933
Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML (1995) Differential modulation of T helper type 1 (TH1) and T helper type 2 (TH2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol 25:59–63
Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K et al (2009) Prostaglandin E2–EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med 15:633–640
Linnemeyer PA, Pollack SB (1993) Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. J Immunol 150:3747–3754
Sreeramkumar V, Fresno M, Cuesta N (2012) Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol 90:579–586
Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T (1994) Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med 180:2365–2370
Demeure CE, Yang LP, Desjardins C, Raynauld P, Delespesse G (1997) Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol 27:3526–3531
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Owen K, Gomolka D, Droller MJ (1980) Production of prostaglandin E2 by tumor cells in vitro. Cancer Res 40:3167–3171
Young MR, Knies S (1984) Prostaglandin E production by Lewis lung carcinoma: mechanism for tumor establishment in vivo. J Natl Cancer Inst 72:919–922
Balch CM, Dougherty PA, Cloud GA, Tilden AB (1984) Prostaglandin E2-mediated suppression of cellular immunity in colon cancer patients. Surgery 95:71–77
Murray JL, Kollmorgen GM (1983) Inhibition of lymphocyte response by prostaglandin-producing suppressor cells in patients with melanoma. J Clin Immunol 3:268–276
Passwell J, Levanon M, Davidsohn J, Ramot B (1983) Monocyte PGE2 secretion in Hodgkin’s disease and its relation to decreased cellular immunity. Clin Exp Immunol 51:61–68
Chiabrando C, Broggini M, Castagnoli MN, Donelli MG, Noseda A, Visintainer M et al (1985) Prostaglandin and thromboxane synthesis by Lewis lung carcinoma during growth. Cancer Res 45:3605–3608
McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA et al (1988) Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res 48:3140–3247
Fulton AM (1988) Inhibition of experimental metastasis with indomethacin: role of macrophages and natural killer cells. Prostaglandins 35:413–425
Maxwell WJ, Kelleher D, Keating JJ, Hogan FP, Bloomfield FJ, MacDonald GS et al (1990) Enhanced secretion of prostaglandin E2 by tissue-fixed macrophages in colonic carcinoma. Digestion 47:160–166
Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M (1993) Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer 72:491–501
Alleva DG, Burger CJ, Elgert KD (1994) Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol 153:1674–1686
Liu XH, Connolly JM, Rose DP (1996) Eicosanoids as mediators of linoleic acid-stimulated invasion and type IV collagenase production by a metastatic human breast cancer cell line. Clin Exp Metastasis 14:145–152
Li S, Xu X, Jiang M, Bi Y, Xu J, Han M (2015) Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway. Mol Med Rep 11:4454–4462
Kim MJ, Kim HS, Lee SH, Yang Y, Lee MS, Lim JS (2014) NDRG2 controls COX-2/PGE2-mediated breast cancer cell migration and invasion. Mol Cells 37:759–765
Zhang M, Zhang H, Cheng S, Zhang D, Xu Y, Bai X et al (2006) Prostaglandin E2 accelerates invasion by upregulating Snail in hepatocellular carcinoma cells. Tumour Biol 35:7135–7145
Han C, Michalopoulos GK, Wu T (2006) Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol 207:261–270
Han C, Wu T (2005) Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem 280:24053–24063
Xu L, Han C, Wu T (2006) A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem 281:33982–33996
Misra UK, Pizzo SV (2013) Evidence for a pro-proliferative feedback loop in prostate cancer: the role of Epac1 and COX-2-dependent pathways. PLoS One 8:e63150. https://doi.org/10.1371/journal.pone
Evans DB, Thavarajah M, Kanis JA (1990) Involvement of prostaglandin E2 in the inhibition of osteocalcin synthesis by human osteoblast-like cells in response to cytokines and systemic hormones. Biochem Biophys Res Commun 167:194–202
Hori T, Yamanaka Y, Hayakawa M, Shibamoto S, Tsujimoto M, Oku N et al (1991) Prostaglandins antagonize fibroblast proliferation stimulated by tumor necrosis factor. Biochem Biophys Res Commun 174:758–766
Kambayashi T, Alexander HR, Fong M, Strassmann G (1995) Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol 154:3383–3390
Takigawa M, Takashiba S, Takahashi K, Arai H, Kurihara H, Murayama Y (1994) Prostaglandin E2 inhibits interleukin-6 release but not its transcription in human gingival fibroblasts stimulated with interleukin-1 beta or tumor necrosis factor-alpha. J Periodontol 65:1122–1127
Fieren MW, van den Bemd GJ, Ben-Efraim S, Bonta IL (1992) Prostaglandin E2 inhibits the release of tumor necrosis factor-alpha, rather than interleukin 1 beta, from human macrophages. Immunol Lett 31:85–90
Vassiliou E, Jing H, Ganea D (2003) Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol 223:120–132
Stafford JB, Marnett LJ (2008) Prostaglandin E2 inhibits tumor necrosis factor-alpha RNA through PKA type I. Biochem Biophys Res Commun 366:104–109
Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL Jr, Albina JE (2008) Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol 180:2125–2131
Huang CN, Liu KL, Cheng CH, Lin YS, Lin MJ, Lin TH (2005) PGE2 enhances cytokine-elicited nitric oxide production in mouse cortical collecting duct cells. Nitric Oxide 12:150–158
Gaillard T, Mülsch A, Klein H, Decker K (1992) Regulation by prostaglandin E2 of cytokine-elicited nitric oxide synthesis in rat liver macrophages. Biol Chem Hoppe Seyler 373:897–902
Stadler J, Harbrecht BG, Di Silvio M, Curran RD, Jordan ML, Simmons RL et al (1993) Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol 53:165–172
Tetsuka T, Daphna-Iken D, Miller BW, Guan Z, Baier LD, Morrison AR (1996) Nitric oxide amplifies interleukin 1-induced cyclooxygenase-2 expression in rat mesangial cells. J Clin Invest 97:2051–2056
Wilson KT, Vaandrager AB, De Vente J, Musch MW, De Jonge HR, Chang EB (1996) Production and localization of cGMP and PGE2 in nitroprusside-stimulated rat colonic ion transport. Am J Phys 270(3 Pt 1):C832–C840
Sautebin L, Ialenti A, Ianaro A, Di Rosa M (1995) Endogenous nitric oxide increases prostaglandin biosynthesis in carrageenin rat paw oedema. Eur J Pharmacol 286:219–222
Biondi C, Fiorini S, Pavan B, Ferretti ME, Barion P, Vesce F (2003) Interactions between the nitric oxide and prostaglandin E2 biosynthetic pathways in human amnion-like WISH cells. J Reprod Immunol 60:35–52
Du Y, Sarthy VP, Kern TS (2004) Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol 287:R735–R741
Chien CC, Shen SC, Yang LY, Chen YC (2012) Prostaglandins as negative regulators against lipopolysaccharide, lipoteichoic acid, and peptidoglycan-induced inducible nitric oxide synthase/nitric oxide production through reactive oxygen species-dependent heme oxygenase 1 expression in macrophages. Shock 38:549–558
Stæhr M, Hansen PB, Madsen K, Vanhoutte PM, Nüsing RM, Jensen BL (2013) Deletion of cyclooxygenase-2 in the mouse increases arterial blood pressure with no impairment in renal NO production in response to chronic high salt intake. Am J Physiol Regul Integr Comp Physiol 304:R899–R907
Harizi H, Norbert G (2004) Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol 228:99–109
Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N (2002) Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 168:2255–2263
Kanda N, Koike S, Watanabe S (2005) IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J Allergy Clin Immunol 116:1144–1150
Khayrullina T, Yen JH, Jing H, Ganea D (2008) In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol 181:721–735
Chen H, Qin J, Wei P, Zhang J, Li Q, Fu L et al (2009) Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids 80:195–200
Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P (1993) Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A 90:7240–7244
Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR (1995) Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol 114:1335–1342
Mollace V, Colasanti M, Muscoli C, Lauro GM, Iannone M, Rotiroti D et al (1998) The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells. Br J Pharmacol 124:742–746
Marcinkiewicz J (1997) Regulation of cytokine production by eicosanoids and nitric oxide. Arch Immunol Ther Exp 45:163–167
Tanaka M, Ishibashi H, Hirata Y, Miki K, Kudo J, Niho Y (1996) Tumor necrosis factor production by rat Kupffer cells-regulation by lipopolysaccharide, macrophage activating factor and prostaglandin E2. J Clin Lab Immunol 48:17–31
Liu XH, Kirschenbaum A, Lu M, Yao S, Klausner A, Preston C et al (2002) Prostaglandin E(2) stimulates prostatic intraepithelial neoplasia cell growth through activation of the interleukin-6/GP130/STAT-3 signaling pathway. Biochem Biophys Res Commun 290:249–255
Reznikov LL, Kim SH, Westcott JY, Frishman J, Fantuzzi G, Novick D et al (2000) IL-18 binding protein increases spontaneous and IL-1-induced prostaglandin production via inhibition of IFN-gamma. Proc Natl Acad Sci U S A 97:2174–2179
Perkins DJ, Kniss DA (1999) Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J Leukoc Biol 65:792–799
Sakurai T, Tamura K, Kogo H (2004) Vascular endothelial growth factor increases messenger RNAs encoding cyclooxygenase-II and membrane-associated prostaglandin E synthase in rat luteal cells. J Endocrinol 183:527–533
Yao M, Kargman S, Lam EC, Kelly CR, Zheng Y, Luk P et al (2003) Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res 63:586–592
Sailaja P, Mani AM, Naveen KVG, Anasuya DH, Siresha B, Das UN (2014) Effect of polyunsaturated fatty acids and their metabolites on bleomycin-induced cytotoxic action on human neuroblastoma cells in vitro. PLoS One 9:e114766. https://doi.org/10.1371/journal.pone.0114766
Booyens J, Englebrecht P, Le Roux S, Louwrens CC, Van der Merwe CF, Katzeff IE (1984) Some effects of the essential fatty acids linoleic acid, alpha-linolenic acid, and of their metabolites gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid and of prostaglandins A and E on the proliferation of human osteogenic sarcoma cells in culture. Prostaglandins Leukot Med 15:15–33
Begin ME, Das UN, Ells G, Horrobin DF (1985) Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med 19:177–186
Begin ME, Ells G, Das UN, Horrobin DF (1986) Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst 77:1053–1062
Das UN (1991) Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett 56:235–243
Sagar PS, Das UN, Koratkar R, Ramesh G, Padma M, Kumar GS (1992) Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells: relationship to free radicals and lipid peroxidation and its modulation by calmodulin antagonists. Cancer Lett 63:189–198
Kumar GS, Das UN (1995) Free radical-dependent suppression of growth of mouse myeloma cells by α-linolenic and eicosapentaenoic acids in vitro. Cancer Lett 92:27–38
Padma M, Das UN (1996) Effect of cis-unsaturated fatty acids on cellular oxidant stress in macrophage tumor (AK-5) cells in vitro. Cancer Lett 109:63–75
Seigel I, Liu TL, Yaghoubzadeh E, Kaskey TS, Gleicher N (1987) Cytotoxic effects of free fatty acids on ascites tumor cells. J Natl Cancer Inst 78:271–277
Tolnai S, Morgan JF (1962) Studies on the in vitro anti-tumor activity of fatty acids. V. Unsaturated fatty acids. Can J Biochem Physiol 40:869–875
Monjazeb AM, High KP, Connoy A, Hart LS, Koumenis C, Chilton FH (2006) Arachidonic acid-induced gene expression in colon cancer cells. Carcinogenesis 27:1950–1960
Monjazeb AM, High KP, Koumenis C, Chilton FH (2005) Inhibitors of arachidonic acid metabolism act synergistically to signal apoptosis in neoplastic cells. Prostaglandins Leukot Essent Fatty Acids 73:463–474
Canuto RA, Muzio G, Bassi AM, Maggiora M, Leonarduzzi G, Lindahl R et al (1995) Enrichment with arachidonic acid increases the sensitivity of hepatoma cells to the cytotoxic effects of oxidative stress. Free Radic Biol Med 18:287–293
Piazzi G, D’Argenio G, Prossomariti A, Lembo V, Mazzone G, Candela M et al (2014) Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer 135:2004–2013
Sauer LA, Dauchy RT, Blask DE, Krause JA, Davidson LK, Dauchy EM (2005) Eicosapentaenoic acid suppresses cell proliferation in MCF-7 human breast cancer xenografts in nude rats via a pertussis toxin-sensitive signal transduction pathway. J Nutr 135:2124–2129
Gu Z, Wu J, Wang S, Suburu J, Chen H, Thomas MJ et al (2013) Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis 34:1968–1975
Wang S, Wu J, Suburu J, Gu Z, Cai J, Axanova LS et al (2012) Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis 33:404–412
Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chénais B (2010) Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int J Oncol 36:737–742
Collett ED, Davidson LA, Fan YY, Lupton JR, Chapkin RS (2001) n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am J Physiol Cell Physiol 280:C1066–C1075
Havemose-Poulsen A, Sørensen LK, Stoltze K, Bendtzen K, Holmstrup P (2005) Cytokine profiles in peripheral blood and whole blood cell cultures associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol 76:2276–2285
Guimarães PM, Scavuzzi BM, Stadtlober NP, Franchi Santos LFDR, Lozovoy MAB, Iriyoda TMV et al (2017) Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol 95:824–831
Yoshida T, Ichikawa Y, Tojo T, Homma M (1996) Abnormal prostanoid metabolism in lupus nephritis and the effects of a thromboxane A2 synthetase inhibitor, DP-1904. Lupus 5:129–138
Navarro E, Esteve M, Olivé A, Klaassen J, Cabré E, Tena X et al (2000) Abnormal fatty acid pattern in rheumatoid arthritis. A rationale for treatment with marine and botanical lipids. J Rheumatol 27:298–303
Suryaprabha P, Das UN, Ramesh G, Kumar KV, Kumar GS (1991) Reactive oxygen species, lipid peroxides and essential fatty acids in patients with rheumatoid arthritis and systemic lupus erythematosus. Prostaglandins Leukot Essent Fatty Acids 43:251–255
Mohan IK, Das UN (1997) Oxidant stress, anti-oxidants and essential fatty acids in systemic lupus erythematosus. Prostaglandins Leukot Essent Fatty Acids 56:193–198
Horrobin DF (1987) Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of eicosapentaenoic acid (EPA), a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med Hypotheses 22:421–428
Horrobin DF (1984) Essential fatty acid metabolism in diseases of connective tissue with special reference to scleroderma and to Sjogren’s syndrome. Med Hypotheses 14:233–247
Laitinen O, Seppalä E, Nissilä M, Vapaatalo H (1983) Plasma levels and urinary excretion of prostaglandins in patients with rheumatoid arthritis. Clin Rheumatol 2:401–406
Trang LE, Granström E, Lövgren O (1977) Levels of prostaglandins F2 alpha and E2 and thromboxane B2 in joint fluid in rheumatoid arthritis. Scand J Rheumatol 6:151–154
Egg D (1984) Concentrations of prostaglandins D2, E2, F2 alpha, 6-keto-F1 alpha and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z Rheumatol 43:89–96
Egg D, Günther R, Herold M, Kerschbaumer F (1980) Prostaglandins E2 and F2 alpha concentrations in the synovial fluid in rheumatoid and traumatic knee joint diseases. Z Rheumatol 39:170–175
Das UN (2012) Is multiple sclerosis a proresolution deficiency disorder? Nutrition 28:951–958
Conte FP, Menezes-de-Lima O Jr, Verri WA Jr, Cunha FQ, Penido C, Henriques MG (2010) Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br J Pharmacol 161:911–924
Chan MM, Moore AR (2010) Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol 184:6418–6426
Hashimoto A, Hayashi I, Murakami Y, Sato Y, Kitasato H, Matsushita R et al (2007) Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J Rheumatol 34:2144–2153
Thomas E, Leroux JL, Blotman F, Chavis C (1995) Conversion of endogenous arachidonic acid to 5,15-diHETE and lipoxins by polymorphonuclear cells from patients with rheumatoid arthritis. Inflamm Res 44:121–124
Katoh T, Lakkis FG, Makita N, Badr KF (1994) Co-regulated expression of glomerular 12/15-lipoxygenase and interleukin-4 mRNAs in rat nephrotoxic nephritis. Kidney Int 46:341–349
Jiang C, Wang H, Xue M, Lin L, Wang J, Cai G et al (2019) Reprograming of peripheral Foxp3+ regulatory T cell towards Th17-like cell in patients with active systemic lupus erythematosus. Clin Immunol 108267. https://doi.org/10.1016/j.clim.2019.108267
Mohammadi S, Sedighi S, Memarian A (2019) IL-17 is aberrantly overexpressed among under-treatment Systemic Lupus Erythematosus patients. Iran J Pathol 14:236–242
Nordin F, Shaharir SS, Abdul Wahab A, Mustafar R, Abdul Gafor AH, Mohamed Said MS et al (2019) Serum and urine interleukin-17A levels as biomarkers of disease activity in systemic lupus erythematosus. Int J Rheum Dis 22:1419–1426
Zhang Q, Liu S, Ge D, Cunningham DM, Huang F, Ma L et al (2017) Targeting Th17-IL-17 pathway in prevention of micro-invasive prostate cancer in a mouse model. Prostate 77:888–899
Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z et al (2017) Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 36:687–699
Wang B, Zhao CH, Sun G, Zhang ZW, Qian BM, Zhu YF et al (2019) IL-17 induces the proliferation and migration of glioma cells through the activation of PI3K/Akt1/NF-κB-p65. Cancer Lett 447:93–104
Zhang Y, Wang ZC, Zhang ZS, Chen F (2018) MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. Eur Rev Med Pharmacol Sci 22:3719–3726
Changchun K, Pengchao H, Ke S, Ying W, Lei W (2017) Interleukin-17 augments tumor necrosis factor α-mediated increase of hypoxia-inducible factor-1α and inhibits vasodilator-stimulated phosphoprotein expression to reduce the adhesion of breast cancer cells. Oncol Lett 13:3253–3260
Akbay EA, Koyama S, Liu Y, Dries R, Bufe LE, Silkes M et al (2017) Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol 12:1268–1279
Borbiro I, Badheka D, Rohacs T (2015) Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 8:ra15. https://doi.org/10.1126/scisignal.2005667
Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF et al (2019) Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun 10(1):1200. https://doi.org/10.1038/s41467-019-09055-7
Accardi A (2015) Lipids link ion channels and cancer. Science 349:789–790
Liu CSC, Raychaudhuri D, Paul B, Chakrabarty Y, Ghosh AR, Rahaman O et al (2018) Piezo1 mechanosensors optimize human T cell activation. J Immunol 200:1255–1260
Chandy KG, Norton RS (2016) Channelling potassium to fight cancer. Nature 537:497–498
Ordway R, Walsh JV Jr, Singer JJ (1989) Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science 244:1176–1179
Kim D, Clapham DE (1989) Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science 244:1174–1176
Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunskit M (1990) Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science 247:852–854
Isaacs JT (2013) Prostate cancer takes nerve. Science 341:134–135
Hayakawa Y, Wang TC (2017) Nerves switch on angiogenic metabolism. Science 358:305–306
Barria A (2019) Dangerous liaisons as tumours form synapses. Nature 573:1–2
Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H et al (2000) Potassium regulates IL-1β processing via calcium-independent phospholipase A2. J Immunol 164:5120–5124
Walev I, Reske K, Palmer M, Valeva A, Bhakdi S (1995) Potassium-inhibited processing of IL-1β in human monocytes. EMBO J 14:1607–1614
Hughes FM Jr, Bortner CD, Purdy GD, Cidlowski JA (1997) Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 272:30567–30576
McGowan SE, Jackson SK, Doro MM, Olson PJ (1997) Peroxisome proliferators alter lipid acquisition and elastin gene expression in neonatal rat lung fibroblasts. Am J Physiol 273:L1249–L1257
Das UN (1993) Oxy radicals and their clinical implications. Curr Sci 65:964–968
Lin HL, Liu TY, Chau GY, Lui WY, Chi CW (2000) Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer 89:983–994
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407:390–395
Das UN (2002) A radical approach to cancer. Med Sci Monit 8:RA79–RA92
Ge Y, Byun JS, De Luca P, Gueron G, Yabe IM, Sadiq-Ali SG et al (2008) Combinatorial antileukemic disruption of oxidative homeostasis and mitochondrial stability by the redox reactive thalidomide 2-(2,4-difluoro-phenyl)-4,5,6,7-tetrafluoro-1H-isoindole-1,3(2H)-dione (CPS49) and flavopiridol. Mol Pharmacol 74:872–883
Colquhoun A (2009) Mechanisms of action of eicosapentaenoic acid in bladder cancer cells in vitro: alterations in mitochondrial metabolism, reactive oxygen species generation and apoptosis induction. J Urol 181:1885–1893
Naidu MR, Das UN, Kishan A (1992) Intratumoral gamma-linoleic acid therapy of human gliomas. Prostaglandins Leukot Essent Fatty Acids 45:181–184
Das UN, Prasad VV, Reddy DR (1995) Local application of gamma-linolenic acid in the treatment of human gliomas. Cancer Lett 94:147–155
Bakshi A, Mukherjee D, Bakshi A, Banerji AK, Das UN (2003) Gamma-linolenic acid therapy of human gliomas. Nutrition 19:305–309
Das UN (2007) Gamma-linolenic acid therapy of human glioma-a review of in vitro, in vivo, and clinical studies. Med Sci Monit 13:RA119–RRA31
Reddy DR, Prasad VS, Das UN (1998) Intratumoural injection of gamma linolenic acid in malignant gliomas. J Clin Neurosci 5:36–39
Smith DL, Willis AL, Mahmud I (1984) Eicosanoid effects on cell proliferation in vitro: relevance to atherosclerosis. Prostaglandins Leukot Med 16:1–10
Sakai T, Yamaguchi N, Shiroko Y, Sekiguchi M, Fujii G, Nishino H (1984) Prostaglandin D2 inhibits the proliferation of human malignant tumor cells. Prostaglandins 27:17–26
Rohrbach S (2009) Effects of dietary polyunsaturated fatty acids on mitochondria. Curr Pharm Des 15:4103–4116
Tuo Y, Wang D, Li S, Chen C (2011) Long-term exposure of INS-1 rat insulinoma cells to linoleic acid and glucose in vitro affects cell viability and function through mitochondrial-mediated pathways. Endocrine 39:128–138
Zeghichi-Hamri S, de Lorgeril M, Salen P, Chibane M, de Leiris J, Boucher F et al (2010) Protective effect of dietary n-3 polyunsaturated fatty acids on myocardial resistance to ischemia-reperfusion injury in rats. Nutr Res 30:849–857
Hagopian K, Weber KL, Hwee DT, Van Eenennaam AL, López-Lluch G, Villalba JM et al (2010) Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS One 5:e12696. https://doi.org/10.1371/journal.pone.0012696
Kansal S, Negi AK, Kaur R, Sarotra P, Sharma G, Aggarwal R et al (2011) Evaluation of the role of oxidative stress in chemopreventive action of fish oil and celecoxib in the initiation phase of 7,12-dimethyl benz(α)anthracene-induced mammary carcinogenesis. Tumour Biol 32:167–177
Virgili F, Santini MP, Canali R, Polakowska RR, Haake A, Perozzi G (1998) Bcl-2 overexpression in the HaCaT cell line is associated with a different membrane fatty acid composition and sensitivity to oxidative stress. Free Radic Biol Med 24:93–101
Sailaja P, Dwarakanath BS, Das UN (2018) Arachidonic acid activates extrinsic apoptotic pathway to enhance tumoricidal action of bleomycin against IMR-32 cells. Prostaglandins Leukot Essen Fatty Acids 132:16–22
Dymkowska D, Wojtczak L (2009) Arachidonic acid-induced apoptosis in rat hepatoma AS-30D cells is mediated by reactive oxygen species. Acta Biochim Pol 56:711–715
Ribeiro G, Benadiba M, de Oliveira SD, Colquhoun A (2010) The novel ruthenium-gamma-linolenic complex [Ru(2)(aGLA)(4)Cl] inhibits C6 rat glioma cell proliferation and induces changes in mitochondrial membrane potential, increased reactive oxygen species generation and apoptosis in vitro. Cell Biochem Funct 28:15–23
Giros A, Grzybowski M, Sohn VR, Pons E, Fernandez-Morales J, Xicola RM et al (2009) Regulation of colorectal cancer cell apoptosis by the n-3 polyunsaturated fatty acids Docosahexaenoic and Eicosapentaenoic. Cancer Prev Res (Phila) 2:732–742
Das UN (2011) Essential fatty acids enhance free radical generation and lipid peroxidation to induce apoptosis of tumor cells. Clin Lipidol 6:463–489
Halliwell BA (2000) Superway to kill cancer cells? Nature Med 6:1105–1106
Ponnala S, Rao KP, Chaudhury JR, Ahmed J, Rama Rao B, Kanjilal S et al (2009) Effect of polyunsaturated fatty acids on diphenyl hydantoin-induced genetic damage in vitro and in vivo. Prostaglandins Leukot Essent Fatty Acids 80:43–50
Das UN, Rao KP (2006) Effect of gamma-linolenic acid and prostaglandins E1 on gamma-radiation and chemical-induced genetic damage to the bone marrow cells of mice. Prostaglandins Leukot Essent Fatty Acids 74:165–173
Das UN, Ramadevi G, Rao KP, Rao MS (1985) Prostaglandins and their precursors can modify genetic damage-induced by gamma-radiation and benzo(a)pyrene. Prostaglandins 29:911–920
Das UN (2006) Tumoricidal and anti-angiogenic actions of gamma-linolenic acid and its derivatives. Curr Pharm Biotechnol 7:457–466
Dhayal S, Morgan NG (2011) Pharmacological characterization of the cytoprotective effects of polyunsaturated fatty acids in insulin-secreting BRIN-BD11 cells. Br J Pharmacol 162:1340–1350
Suresh Y, Das UN (2003) Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: effect of omega-6 fatty acids. Nutrition 19:93–114
Suresh Y, Das UN (2003) Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus. Effect of omega-3 fatty acids. Nutrition 19:213–228
Bazan NG (2007) Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care 10:136–141
Sangeetha PS, Das UN (1993) Gamma-linolenic acid and eicosapentaenoic acid potentiate the cytotoxicity of anti-cancer drugs on human cervical carcinoma (HeLa) cells in vitro. Med Sci Res 21:457–459
Madhavi N, Das UN (1994) Reversal of KB-3-1 and KB-Ch-8-5 tumor cell drug-resistance by cis-unsaturated fatty acids in vitro. Med Sci Res 22:689–692
Madhavi N, Das UN (1994) Effect of n-6 and n-3 fatty acids on the survival of vincristine sensitive and resistant human cervical carcinoma cells in vitro. Cancer Lett 84:31–41
Das UN, Madhavi N, Sravan Kumar G, Padma M, Sangeetha P (1998) Can tumour cell drug resistance be reversed by essential fatty acids and their metabolites? Prostaglandins Leukot Essent Fatty Acids 58:39–54
Germain E, Chajès V, Cognault S, Lhuillery C, Bougnoux P (1998) Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: relationship to lipid peroxidation. Int J Cancer 75:578–583
Mahéo K, Vibet S, Steghens JP, Dartigeas C, Lehman M, Bougnoux P et al (2005) Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation. Free Radic Biol Med 39:742–751
Ilc K, Ferrero JM, Fischel JL, Formento P, Bryce R, Etienne MC et al (1999) Cytotoxic effects of two gamma linoleic salts (lithium gammalinolenate or meglumine gammalinolenate) alone or associated with a nitrosourea: an experimental study on human glioblastoma cell lines. Anticancer Drugs 10:413–417
Menendez JA, Ropero S, Lupu R, Colomer R (2004) Omega-6 polyunsaturated fatty acid gamma-linolenic acid (18:3n-6) enhances docetaxel (Taxotere) cytotoxicity in human breast carcinoma cells: Relationship to lipid peroxidation and HER-2/neu expression. Oncol Rep 11:1241–1252
Menéndez JA, Ropero S, del Barbacid MM, Montero S, Solanas M, Escrich E et al (2002) Synergistic interaction between vinorelbine and gamma-linolenic acid in breast cancer cells. Breast Cancer Res Treat 72:203–219
Menendez JA, Ropero S, Mehmi I, Atlas E, Colomer R, Lupu R (2004) Overexpression and hyperactivity of breast cancer-associated fatty acid synthase (oncogenic antigen-519) is insensitive to normal arachidonic fatty acid-induced suppression in lipogenic tissues but it is selectively inhibited by tumoricidal alpha-linolenic and gamma-linolenic fatty acids: a novel mechanism by which dietary fat can alter mammary tumorigenesis. Int J Oncol 24:1369–1383
Kong X, Ge H, Chen L, Liu Z, Yin Z, Li P et al (2009) Gamma-linolenic acid modulates the response of multidrug-resistant K562 leukemic cells to anticancer drugs. Toxicol In Vitro 23:634–639
Buckingham LE, Balasubramanian M, Safa AR, Shah H, Komarov P, Emanuele RM et al (1998) Reversal of multi-drug resistance in vitro by fatty acid-PEG-fatty acid diesters. Int J Cancer 65:74–79
Ramesh G, Das UN, Koratkar R, Padma M, Sagar PS (1992) Effect of essential fatty acids on tumor cells. Nutrition 8:343–347
Huang ZH, Hii CS, Rathjen DA, Poulos A, Murray AW, Ferrante A (1997) N-6 and N-3 polyunsaturated fatty acids stimulate translocation of protein kinase C alpha, beta I, beta II and –epsilon and enhance agonist-induced NADPH oxidase in macrophages. Biochem J 325:553–557
Peterson DA, Mehta N, Butterfield J, Husak M, Christopher MM, Jagarlapudi S et al (1988) Polyunsaturated fatty acids stimulate superoxide formation in tumor cells: a mechanism for specific cytotoxicity and a model for tumor necrosis factor? Biochem Biophys Res Commun 155:1033–1037
Chiu LC, Wan JM (1999) Induction of apoptosis in HL-60 cells by eicosapentaenoic acid (EPA) is associated with downregulation of bcl-2 expression. Cancer Lett 145:17–27
Albino AP, Juan G, Traganos F, Reinhart L, Connolly J, Rose DP et al (2000) Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: association with decreased pRb phosphorylation. Cancer Res 60:4139–4145
Chen ZY, Istfan NW (2001) Docosahexaenoic acid, a major constituent of fish oil diets, prevents activation of cyclin-dependent kinases and S-phase entry by serum stimulation in HT-29 cells. Prostaglandins Leukot Essen Fatty Acids 64:67–73
Anasuya HD, Naidu VGM, Das UN (2018) n-6 and n-3 Fatty acids and their metabolites augment inhibitory action of doxorubicin on the proliferation of human neuroblastoma (IMR-32) cells by enhancing lipid peroxidation and suppressing Ras, Myc, and Fos. Biofactors 44:387–401
Palakurthi SS, Fluckiger R, Aktas H, Changolkar AK, Shahsafaei A, Harneit S et al (2006) Inhibition of translation initiation mediates the anticancer effect of the n-3 polyunsaturated fatty acid eicosapentaenoic acid. Cancer Res 60:2919–2925
Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H (2000) Gαi and Gαo are target proteins of reactive oxygen species. Nature 408:492–495
Bai Y, Wang J, He Z, Yang M, Li L, Jiang H (2019) Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-inflammatory cytokines. Med Sci Monit 25:3069–3076
Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y et al (2006) FASEB J 2:1785–1792
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Das, U.N. (2020). Bioactive Lipids in Age-Related Disorders. In: Guest, P. (eds) Reviews on New Drug Targets in Age-Related Disorders. Advances in Experimental Medicine and Biology(), vol 1260. Springer, Cham. https://doi.org/10.1007/978-3-030-42667-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-42667-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42666-8
Online ISBN: 978-3-030-42667-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)