Abstract
Peripheral nerve electrodes, with the goal of restoring muscle functions or recording neural signals, have been extensively studied. Recently, not only have existing designs been re-scrutinized, but several new electrode designs have been developed. Despite several excellent comprehensive review articles on peripheral nerve electrodes, most of them focus on different types of devices but lack a systematic emphasis on the challenges and design motivations. In addition, more recent developments involve redesigning conventional electrodes, but these new advances have not been covered in any review article yet. Here, we review recent advances on peripheral nerve electrodes, by analyzing three major challenges and corresponding strategies, which may guide the community to a problem-driven path and inspire further innovations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
With the goal of restoring muscle functions or recording neural signals, peripheral nerve electrodes have been extensively studied for decades. For restoration of lost motor function, in most cases, multiple groups of muscle fibers need to be electrically activated. The straightforward approach is to use muscle-targeted electrodes (either epimysial or intramuscular electrodes (Navarro et al. 2005)), by implanting at least one electrode on/in each muscle (Veraart et al. 1993). Apparently, due to the sparse distribution of implanted electrodes, the wiring scheme is not only complicated for surgical implantation procedure and maintenance but also fragile to limb movements. Therefore, within the options of muscular or neural interfaces, targeting peripheral nerves has the advantage of much lower activation thresholds, a smaller number of required electrodes, and higher recruiting accuracy.
In contrast to the complexity in the central nervous system, the relatively simple anatomical structure of peripheral nerves makes them easier to be accessed by electrodes. Typically, neurons in the peripheral nervous system have their somas located in or around the spinal cord while extending their axons all the way to target organs. While eventually ending with branches and forming neuromuscular junctions with skeletal muscle fibers, the axons, for the most part of their lengths, are grouped together in fascicles that are wrapped by three protective layers: epineurium, perineurium, and endoneurium (Fig. 5.1). Such a compact and clearly mapped structure not only minimizes electrode size and quantity but also leaves adequate space for high-resolution interfaces such as intrafascicular electrodes.
Cross-sectional view of a typical peripheral nerve where nerve fibers are bundled into perineurium-ensheathed fascicles. (Adapted with permission from Yoshida et al. 2010)
The advances of peripheral nerve electrodes have been summarized in multiple excellent comprehensive reviews (Navarro et al. 2005; Yoshida et al. 2010; Ortiz-Catalan et al. 2012; Patil and Thakor 2016) which organize their discussions based on different types of devices. Instead, by focusing on challenges and corresponding design motivations, this chapter covers the efforts made in the field to improve issues of selectivity and noise reduction, electrode-neural interfaces, and surgical implantations. To provide an overview on the working principles of peripheral nerve electrodes, Sect. 5.2 will briefly introduce three major types of electrodes. The three major challenges and corresponding strategies will then be discussed in detail in Sect. 5.3.
5.2 Peripheral Nerve Electrodes
In this section, we discuss three types of conventional peripheral nerve electrodes used to stimulate peripheral nerves or record neural activity: cuff electrodes, intrafascicular electrodes, and regenerative electrodes.
5.2.1 Cuff Electrodes
Among peripheral electrodes, cuff electrodes are perhaps the most studied and investigated toward clinical applications. They work by completely encircling the nerve with an insulated tubular sheath and using two or more electrode contacts on the inner surface to either stimulate the nerve or record neural activity. These electrodes can either completely encircle the nerve (circumferential) or come in contact with just a section of it (differential). Circumferential contacts have mainly been used for recording purposes, while differential contacts have been found to provide better stimulation (McNeal et al. 1989; Sweeney et al. 1990; Grill and Mortimer 1998). Furthermore, differential contacts offer a variety of designs as they can be placed in a bipolar, tripolar, or short-circuit tripolar configuration to reduce noise or current leaks. However, the use of differential contacts for recording has been rarely reported in the current literature.
The flexibility of cuff electrodes allows them to avoid the problems of mechanical stress and displacement that are common in muscle-based electrodes, thereby reducing the possibility of electrode or lead failure. They also have a higher accuracy when recording data because they increase the resistance of the extracellular return path, which increases the amplitude of the recorded signals (Sahin et al. 1997; Struijk et al. 1999).
Although several issues with cuff electrodes have been observed, they were often remedied in subsequent investigations. For example, it was found that cuff electrodes caused a loss of myelinated fibers, but these fibers regenerated to a smaller diameter over time without any signs of control or strength loss (Larsen et al. 1998). It was also found that the self-sizing spiral cuff electrode could cause demyelination and axon losses, perineurium thickening, increased intraneural tissue, or axonal swelling (Naples et al. 1988). However, a different study found that these effects could be circumvented by placing the cuff farther away from a joint (Romero et al. 2001).
5.2.2 Intrafascicular Electrodes
An intrafascicular electrode is directly inserted into the peripheral nerve for direct contact with the nerve tissue that they are intended to activate or record. This direct contact enhances the selectivity and increases the signal-to-noise ratio (SNR) of the recording. Stimulation on a certain nerve fascicle has less effect on adjacent ones. More than one intrafascicular electrode can be implanted to stimulate multiple areas along the nerve.
Longitudinally implanted intrafascicular electrodes (LIFEs) are implanted parallel to the nerve fibers and are inserted a few millimeters into the endoneurium before exiting. The active section inside the nerve can have multiple contacts placed in different orientations to improve selectivity comparing to the transverse intrafascicular multichannel electrodes (TIME).
Studies of metal and polymer LIFE show no damage caused by the electrode or biocompatibility issues and good selectivity for stimulation and multiunit extracellular recording (Nannini and Horch 1991; Goodall et al. 1993). They also have long-term reliability, being used for studies for over 6 months (Goodall et al. 1991).
Furthermore, the electrical stimulation produced by LIFE is able to elicit sensations of touch, joint movement, and position, potentially allowing amputees to have prosthetic limbs with a more natural feel and control. However, the selectivity is challenging to achieve, as it is difficult to implant multiple LIFEs in different fascicles to stimulate the appropriate muscle groups (Dhillon et al. 2004a, b).
5.2.3 Regenerative Electrodes
Unlike cuff and intrafascicular electrodes that passively access intact nerves, regenerative electrodes employ a completely different strategy in which nerve fibers of interest are severed first and then regenerate through micro via-holes or channels to reconstruct neural connections. Probes or stimulation electrodes are integrated in the channels to directly interface with regenerated fascicles, giving rise to highly intimate electrode–tissue interface as well as excellent selectivity to individual fibers.
Due to its highly aggressive nature, however, many factors should be considered during design to ensure successful nerve regeneration. For example, appropriate channel dimensions should be customized so that regenerated nerve fibers will not be damaged by compression force. Additionally, it may also require trophic factors to facilitate regeneration .
5.3 Challenges and Strategies on Electrode Design
5.3.1 Toward Better Selectivity and SNR
Selectivity and SNR are the two most important properties of peripheral neural interfaces regarding to functionality. For both stimulation and recording purposes, it is always desired to recruit or record a smaller group of nerve fibers with less interference from others. Higher selectivity in stimulation enables finer and more coordinated control over many muscle fibers, yielding a more effective motor prosthesis process.
Although recording peripheral nerve activities cannot improve neural modulation directly, it provides important information on the functionality and mechanism of peripheral nervous system and can be used for controlling an external prosthesis. In addition, neural recording is an indivisible part of feedback control that enhances the neural modulation effectiveness. Therefore, the goal of recovering action potentials while suppressing noise from muscle activities and stimulation artifacts has been pursued for any type of electrodes.
5.3.1.1 Selectivity
Steering electric fields with cuff electrodes Selectively activating nerve fascicles can be realized in two ways: (1) manipulating the electric field inside a nerve trunk and (2) directly accessing individual fascicles by electrodes. The first method, also referred as electric field steering, was developed for cuff electrodes which have no direct access to axons inside a nerve. And it can be considered as a remote control of electric field distribution by electrodes wrapping around the nerve trunk. This technique is based on two findings: (1) the excitability of a myelinated axon is determined by longitudinal electric field rather than transverse field (Rushton 1927; Coburn 1980; Rattay 1986); and (2) a transverse current could be used to steer the longitudinal current, restricting the region of excitation (Sweeney et al. 1990). As shown in the longitudinal tripolar configuration (Fig. 5.2a), by passing a transverse current flow at a subthreshold level from a “steering” electrode (anode) 180° opposite to the central cathode, the longitudinal current can be repelled away from the steering electrode and into the region near the cathode. In this way, only a portion of the nerve trunk is stimulated rather than the entire region. Based on this longitudinal tripolar configuration, further modifications have been proposed and investigated (Fig. 5.2b) (Veraart et al. 1993), in which double tripolar configuration shows the best selectivity to activate fascicles that could not be activated selectively by a single tripole, at the cost of more electrodes and lead wires that complicate the system. To achieve the best selectivity for a given muscle, it is necessary to modulate the amplitude of both longitudinal and transverse current, which means the stimulation conditions are not predictable and highly sensitive to electrodes’ relative position to nerve fascicles. In clinical practices, however, such a trial-and-error process may result in unstable implantation because changes in electrodes’ position may invalidate the preset stimulation parameters .
Electric field-steering techniques for cuff electrodes . (a) Schematic view of longitudinal tripolar cuff with field steering where the transverse current serves as steering current. (Adapted with permission from Sweeney et al. 1990). (b) Four longitudinal configurations based on field steering. Adapted with permission from Veraart et al. (1993). (c) The configuration of four-contact transversal cuff electrode. (Adapted with permission from Tarler and Mortimer 2004)
As an alternative approach, arranging electrodes transversely can achieve the same selectivity without compromising on robustness and simplicity. Such concept originates from studies of longitudinal configurations where spatial selectivity is maximized when the transverse current constitutes a high proportion of the total current (Goodall et al. 1996; Deurloo et al. 1998). Accordingly, this suggests that 100% transverse current would result in maximum spatial selectivity. In that case, the two anodes from the longitudinal tripole are effectively eliminated (Goodall et al. 1996). This has been tested and confirmed in modeling works (Parrini 1997; Deurloo et al. 1998) and cat sciatic nerve implantations (Tarler and Mortimer 2003; Tarler and Mortimer 2004). A representative device is the four-contact cuff electrode (Fig. 5.2c) (Tarler and Mortimer 2004). By modulating anodic and cathodic steering current to hyperpolarize undesired fascicles and excite the target fascicle, respectively, each of the four motor fascicles in the cat sciatic nerve can be recruited selectively and independently. However, since nerve fibers are more sensitive to longitudinal electric fields, the activation threshold is significantly higher for transverse configurations than for longitudinal ones (Goodall et al. 1996).
Reshaping Nerve Trunk with Flat Interface Nerve Electrode (FINE)
While selective recruitment of cat nerve fascicles can be achieved by a field-steering cuff interface, the circular distribution of electrode sites reduces their ability to selectively access central axon populations due to a significant distance to inner fascicles (Veltink et al. 1988, 1989a, b; Altman and Plonsey 1990). Different from the simple structure of cat sciatic nerve, human peripheral nerves typically contain more fascicles and may not have a cylindrical cross-section. For example, a human femoral nerve (12 mm wide, 4 mm thick) is composed of at least 20 fascicles (Schiefer et al. 2008), making it difficult for field-steering cuffs to work effectively. As an evolution, a noninvasive FINE was proposed to form a rectangular cross-section. By slowly squeezing the nerve trunk into an elongated and flattened oval, fascicles are lined up on a two-dimensional flat surface instead of a three-dimensional cylinder (Fig. 5.3a, b), resulting in more exposure of smaller and inner fascicles under stimulating electrodes. The nerve reshaping process may take from hours to days, depending on the force applied on nerve trunk.
FINE. (a) Schematic cross-section of a FINE on a nerve. (b) Relative position of the electrodes and the nerve fascicles. Electrical contacts giving functionally equivalent torque outputs are circled. (c) Example of selective recruitment of four fascicles within the sciatic nerve. The lines with symbols show recruitment curves of individual branches having electrodes distal to the FINE. The recruitments from the FINE contacts are shown with the thin lines. The contacts are listed next to their respective recruitment trajectories. (Adapted with permission from Tyler and Durand 2002)
In comparison with conventional cuff electrodes , this design exploits the oblong cross-section of the nerve and takes advantage of the nerve’s ability to reshape (Tyler and Durand 2002). Since electrodes are more intimate to individual fascicles, they are theoretically more effective to recruit a certain fascicle without causing cross-talks to other fascicles (Choi et al. 2001; Schiefer et al. 2005). Using simple monopolar stimulation without field steering, acute experiments on cat and beagle have shown high degree of selectivity for major fascicles (Fig. 5.3c) (Tyler and Durand 2002; Leventhal and Durand 2004; Yoo et al. 2004), and even subfascicular recruitment can be achieved with optimized electrode design (Leventhal and Durand 2003). Moreover, studies on human peripheral nerves also proved the effectiveness of FINE design. Modeling and simulation predicted that eight-contact FINE is enough to selectively stimulate each of the six muscles innervated by the proximal femoral nerve (Schiefer et al. 2008), which was further confirmed for femoral, tibial, and peroneal nerves in human subject implantation carried by the same group (Schiefer et al. 2010; Schiefer et al. 2013).
However, with a flat interface to nerve trunk, the high selectivity and the simple monopolar stimulation without complicated field steering are actually achieved at the cost of greater possibility of nerve damage by pressure. Although chronic implantation on cat sciatic nerves up to 3 months suggested that moderate reshaping caused no axonal damage (Daniel et al. 2006), the chronical biocompatibility of FINE still needs to be confirmed with human subject test .
Subfascicular Selectivity with Intrafascicular Electrodes
To further enhance the selectivity, either field-steering cuff or FINE has seemed to be reaching their limits due to the inherent disadvantage of remote stimulation. The need for finer neural modulation interfaces promotes intrafascicular electrodes that are placed within individual fascicles to directly access the nerve fibers, instead of manipulating electric fields outside the nerve fascicles. These electrodes are usually composed of an insulated conducting wire with small openings at stimulation sites. Essentially, it is the proximity to nerve fibers and restricted current flow in space that give rise to their excellent selectivity and low-activation threshold.
A variety of designs have been developed for intrafascicular recording and modulation. Based on the implanting orientation, they can be categorized into LIFEs and TIMEs. As indicated by the name, LIFEs are designed to be implanted in parallel to the nerve fibers (Fig. 5.4a) (Malagodi et al. 1989). Basically, it is a conductive wire wrapped by an insulation layer, with several stimulation sites where insulation is removed to expose the conductive core. During the surgical implantation, an insertion of micro-needle will penetrate the nerve tissue and lead the wire through the nerve trunk (Fig. 5.4b). Since stimulating sites are physically in contact with individual nerve fibers within a certain fascicle, this interface has shown high selectivity with low-activation threshold (Yoshida and Horch 1993; Micera et al. 2008; Jordi et al. 2011) and little cross-talk to adjacent fascicles (Navarro et al. 2005) in acute animal studies, as opposed to field-steering cuff electrodes.
Longitudinal and transversal intrafascicular electrodes . (a) Schematic diagrams and sciatic nerve implantations of TIME, LIFE, and cuff electrodes. (Adapted with permission from Jordi et al. 2011). (b) Structure of a longitudinal intrafascicular electrode. (Adapted with permission from Malagodi et al. 1989). (c) Electron microscopic picture of the Utah electrode array with 25 probes on a 2 mm by 2 mm base. (Adapted with permission from Branner and Normann 2000). (d) Schematic comparison of planar UEA and USEA. The varying length of electrodes allows more nerve fibers to be recruited. (Adapted with permission from Branner et al. 2001). (e) Scanning electron microscope image of the USEA. (Adapted with permission from Branner et al. 2001)
The major disadvantage of LIFEs , however, is the challenging task to access multiple fascicles with just one device. Based on the guiding-needle method of implantation, it is difficult to implant a few wires in different fascicles without causing substantial tissue damage or device malfunction (Navarro et al. 2005). Therefore, TIMEs are designed to address this issue and further enhance the selectivity. By inserting the wire transversally to the nerve trunk, a single TIME is able to interface with several fascicles (Fig. 5.4a), thus reducing the number of implanted electrodes required by LIFEs (Boretius et al. 2010). Aside from positive confirmation from computational modeling (Raspopovic et al. 2011), acute studies on rat sciatic nerve and human amputee have also shown remarkable selectivity of TIME, despite the small distance between active sites and the small size of the nerve (Boretius et al. 2010; Jensen et al. 2010; Badia et al. 2011; Boretius et al. 2012; Kundu et al. 2014; Harreby et al. 2015).
With similar principles of TIMEs but much more recording/stimulating channels, the Utah electrode array (UEA) can be customized for peripheral nerve interfacing, though originally developed for use in the cerebral cortex (Branner and Normann 2000). Benefiting from microfabrication technologies, 25 probes can be fabricated on a single device (Fig. 5.4c), significantly improving the accessibility to nerve fibers comparing to LIFEs and TIMEs. Furthermore, the planar array was improved by the Utah slanted electrode array (USEA) to avoid a nerve fiber being recruited by many electrodes along the axis (Fig. 5.4d, e) (Branner et al. 2001). The three dimensional structure of USEA with electrodes of varying length can provide access to more individual fibers in each fascicle and enhance graded recruitment of force in muscle groups in a highly selective fashion (Branner et al. 2001).
Axonal Selectivity with Regenerative Sieve Electrodes
Regenerative electrodes are designed to precisely interface with each axon in a nerve fascicle, which reaches the highest resolution a peripheral nerve electrode can get. Ideally, as mentioned in Sect. 5.2, by guiding transected axons to grow through an array of microscale via-holes, individual axons can be selectively stimulated or recorded by the conductive layer deposited around the holes (Fig. 5.5). Practically, since the nerve trunk has to be severed before implantation, this method actually reorganizes nerve structure rather than simply accessing target axons . Therefore, successful application of regenerative electrodes depends on robust axonal regeneration that is remarkably affected by the dimensions of via-holes. Excluding the feasibility of the ideal one-axon-one-hole design (Navarro et al. 2005), most chronic tests on animals found the optimal diameter to be 30–60 μm (Navarro et al. 1996; Wallman et al. 2000) for rat sciatic nerve. However, 5 μm designs with higher selectivity have also been realized and proved successful in recording afferent signals from a rat glossopharyngeal nerve (Bradley et al. 1992, 1997).
Although regenerative electrodes offer the highest selectivity among peripheral nerve devices, their traumatic implantation procedure brings more risks than others. Such design requires high regenerative ability of peripheral nerves, and it takes a long period of time for severed nerve fibers to regenerate and recover. Moreover, it is challenging to perfectly and coherently match the number, size, and layout of via-holes with the intact nerve structure, resulting in functional loss and instability from case to case. For example, thermal and taste responses were lost (Bradley et al. 1997) using the nearly same device that successfully recorded tactile, thermal, and taste signals (Bradley et al. 1992). Also, due to the variety in neuron types, each nerve fiber does not have the same or even similar regenerating capability. Implantations in rat sciatic nerve reveal that regeneration is much less successful for myelinated axons than unmyelinated ones, that motor axons regenerate more poorly than sensory axons, and that some subclasses of sympathetic fibers regenerate better than others (Negredo et al. 2004; Castro et al. 2008).
5.3.1.2 SNR
For cuff electrodes, noise rejection can be realized by optimizing electrode configuration and dimensions or an external shielding layer. Based on the simple principle of Ohm’s law , the innovative design of tripole configuration has significantly improved the quality of recorded signals (Stein et al. 1975; Hoffer and Marks 1976). Instead of detecting the voltage between two electrodes, three equally spaced electrodes are placed along the nerve trunk (Fig. 5.6a) (Stein et al. 1975), and the two terminal ones are electrically shorted. In principle, such a design eliminates the potential gradient for external sources outside the cuff, allowing noises to bypass the device. In the meanwhile, action potentials that propagate inside the nerve trunk are still able to be detected using the central contact. However, this tripolar configuration is based on the assumption that the potential gradient of noise is linearly distributed along the cuff, which is often compromised in clinical applications due to cuff imbalance and connective tissue (Rahal et al. 2000a; Triantis and Demosthenous 2008; Sadeghlo and Yoo 2013). Therefore, several variations on wiring scheme with amplifiers have been reported to achieve a robust recording performance (Fig. 5.6b) (Pflaum et al. 1996; Rahal et al. 2000b; Demosthenous et al. 2004; Jun-Uk et al. 2012). In addition to focusing on electrode configurations, studies on electrode dimensions found that splitting the ring-shaped central contact into discrete electrode pads (Fig. 5.6c) leads to considerable enhancement on SNR (Ortiz-Catalan et al. 2013). Moreover, inspired by the principle of electromagnetic shielding , a conductive shielding layer has shown its effectiveness on external noise rejection (Fig. 5.6d) (Perez-Orive and Durund 2000; Sadeghlo and Yoo 2013) and can simplify the circuit complexity by working with bipolar cuffs (Parisa et al. 2017). Conductive shielding has also been integrated with thin-film LIFE (tf-LIFE) to reject electromyogram (EMG) artifacts (Djilas et al. 2007).
Noise rejection for cuff electrodes . (a) Original tripolar configuration where two terminal electrodes are shorted. (Adapted with permission from Stein et al. 1975). (b) Variations based on the original tripolar configuration. (Adapted with permission from Ortiz-Catalan et al. 2013). (c) Schematic diagram of split-contact design . (Adapted with permission from Ortiz-Catalan et al. 2013). (d) Noise amplitude comparison between conventional cuff and a shielded cuff. (Adapted with permission from Sadeghlo and Yoo 2013)
Compared to cuff electrodes, intrafascicular electrodes typically have a higher SNR due to the insulating effect from the perineurium. The currents of EMG from nearby muscles tend to go around the fascicle rather than into it (Clark and Plonsey 1968). However, conventional monopolar scheme that records potential difference between intrafascicular space and extrafascicular space can still result in substantial EMG pickup. Based on the concept of differential recording, bipolar recording was proposed on LIFEs where axonal signals were detected between two intrafascicular electrodes (Yoshida and Stein 1999), eliminating the interference from extrafascicular sources. Moreover, the interelectrode spacing of 2 mm was found to provide an optimal SNR (Fig. 5.7) (Yoshida and Stein 1999).
The recording of sieve electrodes relies on close proximity of the ring electrode to the axons and the corresponding nodes of Ranvier; therefore, not all electrodes record equally well and are subject to cross-talk (Lago et al. 2007a). Microchannel regenerative interface combines the electrode–axon proximity of sieve electrodes and the snug enclosure of cuff electrodes. Instead of going through via-holes, the regenerated nerve fibers are enclosed by electrically insulated microchannels (Fig. 5.8) (Ivan et al. 2012) that restrict the amount of extracellular fluids. Such an encircle of axons works as a natural amplifier due to the restriction of extracellular current return path (Ivan et al. 2012). In addition, an axon is intimately interfaced by multiple recording sites. Consequently, it is easy to pick up weak axonal signals and obtain a high SNR up to four (Ivan et al. 2012; Musick et al. 2015).
5.3.2 Toward a Better Tissue–Device Interface
Clinical applications require not only high selectivity and noise suppression but also a good tissue–device interface that maintains the stability over long-term implantation. The implanted neural interface should be able to adapt to local motions and minimize neural damage without significant degradation on the stimulation/recording capability. Taking advantages on microfabrication techniques and material advances, the quality of tissue–device interface can be improved through structural optimization, flexible base materials, and high-performance electrode materials.
5.3.2.1 Innovations on Structural Design
tf-LIFE and ACTIN tf-LIFE (thin-film longitudinal intrafascicular electrode) was proposed to improve not only selectivity but also mechanical matching of the neural interface (Yoshida et al. 2000). Using micromachining process, electrode sites can be fabricated on a flat ribbon of polyimide substrate (Fig. 5.9a, b). Comparing to metal wire LIFEs, such a 2D structure allows for more recording sites and a much smaller interfacial area, which significantly enhances the selectivity without applying intensive spike-sorting algorithms (Mirfakhraei and Horch 1994). More importantly, the flexibility of the polymer substrate relieves the mechanical stress between the implanted electrodes and endoneural tissue (Yoshida et al. 2010). While a fibrous response is inevitable, tf-LIFEs produced less axonal damage than conventional LIFEs or polyLIFEs during a 3-month implantation (Lago et al. 2007b).
tf-LIFE , ACTIN , and SELINE designs. (a, b) tf-LIFE and its implantation on a rat sciatic nerve. (Adapted with permission from Lago et al. 2007b). (c, d) Corrugated structure of ACTIN and its thermal responses under applied voltages. (Adapted with permission from Bossi et al. 2009). (e) The wing-shaped anchoring structure of SELINE. (Adapted with permission from Cutrone et al. 2015)
To address the issue of formation of encapsulation layer in chronic implantation, the concept of “movable interface” has been proposed and tested by embedding microactuators in the core of a polyLIFE device, referred to as actuated intraneural (ACTIN) electrodes (Bossi et al. 2009) (Fig. 5.9c, d). The ACTIN is actuated by the deforming of a TiNi thin film sandwiched by top and bottom polyimide layers. When applying voltage to the TiNi actuator, the bending force of the TiNi core (due to heating) causes the entire interface to turn from flat to corrugated. In addition, the memorized shape of TiNi microactuator is optimally designed so that its peaks coincide with the active sites of the interface, maximizing the displacement of active sites without undesired movements of the rest of the structure. With displacement of up to 60 μm, such micro-adjustment after implantation is very helpful to recover the lost connection between electrodes and nerve tissue. However, in vivo test of this design has not been reported yet, and due to the cytotoxicity of pure Ni, the biocompatibility of TiNi microactuator still remains unknown .
SELINE
The issues with LIFEs and TIMEs inspire the development of SELINE (self-opening intrafascicular neural interface), an evolution of LIFEs and TIMEs. The electrode has a main shaft with two lateral wings on each side (Fig. 5.9e). After insertion into a nerve and gently being pulled back, the two wings open transversely and remain anchored to the nerve tissue, making the implanted device more stable over long-term implantation (Cutrone et al. 2015). In addition, since there are active stimulation sites on both the main shaft and opening wings, axons from different sub-fascicles can be accessed in a three-dimensional way. Comparing to LIFEs and TIMEs, this design significantly improves the mechanical stability of the tissue–device interface, while inheriting the outstanding selectivity from TIMEs .
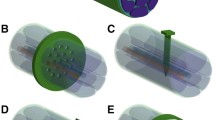
For regenerative electrodes that have more intimate contact with nerve fibers, an implantable interface must be able to facilitate healthy axonal regeneration and maturation while maintaining the close contact with axons (Srinivasan et al. 2015). Moreover, the mixed motor and sensory fibers after transection make it even harder to separately record motor signals and stimulate sensory nerves. Rigid sieve electrodes fall short on these requirements due to the difficulty of designing a suitable layout for the complicated axon populations (Thompson et al. 2016). Even with the flexible polyimide polymer, the thin-plate structure can generate substantial compressive force on a small area to cause damage to nerve fibers. Likewise, the low-channel density also limits chronic applications of the flat microchannel electrode. Therefore, several three-dimensional microchannel interfaces have been proposed to promote the chronic performance by combining sieve and microchannel structures. Rolling a flat microchannel device for implantation not only increases channel density significantly (Fig. 5.10a) (Srinivasan et al. 2015), but also forms a more friendly interface with the nerve fibers, as its tubing structure provides more support to relieve localized compressive force and mimics the natural cross-sectional architecture of a nerve. With modifications , such tubing microchannel structure can also realize guided growth of motor and sensory fibers, separately. As shown in Fig. 5.10b, the Y-shape tubing together with nerve growth factor (NGF) and neurotrophin-3 (NT-3) has preferentially enticed specific axonal populations into separate compartments (Lotfi et al. 2011). Alternatively, multiple nerve fibers have been guided into wider channels first and then grow into bifurcated channels with diminished sizes (Fig. 5.10c) (Irina et al. 2013), which facilitates the separation of the regenerating axonal bundles in a more gradual and effective way than sieve electrodes.
Microchannel electrodes as an improved neural interface. (a) Schematic view of microchannel structure consisting of PDMS substrate, SU8 walls, and gold electrodes (up); cross-sectional view of the device rolled for implantation (down). (Adapted with permission from Srinivasan et al. 2015). (b) Mixed nature of regenerative nerve in the absence of any molecular cues (up); specific growth factors attract a subtype of neurons to the modality-specific compartment (down). (Adapted with permission from Lotfi et al. 2011). (c) Three stages of bifurcated microchannels with diminished sizes for axons to grow through. (Adapted with permission from Irina et al. 2013)
Expanding the principle of in situ amplification of microchannel electrodes, a biohybrid regenerative peripheral nerve interface (RPNI) has been proposed by inclusion of living muscle grafts (Fig. 5.11) (Stieglitz et al. 2002; Urbanchek et al. 2011; Kung 2014; Langhals et al. 2014a; b; Urbanchek et al. 2016). This design integrates muscle grafts and recording electrodes together and then interfaces with transected nerves. Consequently, nerve fibers will grow toward these muscle grafts and regenerate neuromuscular junctions to form new innervations. In this way, action potentials from regenerated nerves give rise to contraction of muscle cells whose membrane potentials are picked up by recording probes. Comparing to sieve and microchannel devices, RPNI innovatively exploits the tendency of neurons to form neuromuscular junctions, which serve as biological amplifiers as muscular ionic currents are much larger in amplitude than those of neurons. In addition, since axons are interfaced by the RPNI in a natural way rather than going through a passive structure, foreign body reactions and traumas are significantly alleviated. However, nerve fibers innervating artificial muscle grafts cannot engage again with natural muscles, thus this strategy only applies to amputees whose original muscles are already beyond repair.
Biohybrid muscle graft regenerative electrode. (Adapted with permission from Urbanchek et al. 2016). (a) Schematic view of RPNI in which the peripheral nerve is wrapped by acellular muscle with PEDOT and the device is populated with cultured myoblasts (b) RPNI after 4 months of implantation on rat peroneal nerve
5.3.2.2 Innovations on Materials
The development of more stable and biocompatible materials is crucial to improving tissue–device interface and often serves as the basis of novel design on electrode structures. For peripheral nerve interfaces, material innovations are often used for either the skeleton of devices or recording/stimulation sites, in which the former focuses on mechanical and biological properties while the latter on electrical characteristics.
For chronic implantations, the high stiffness of conventional Pt-Ir LIFEs compared to surrounding neural tissue often leads to relative motions of electrodes, causing nerve damage and fibrous encapsulation (Lefurge et al. 1991). To minimize the mechanical mismatch, flexible polymers are used as core structures to replace pure metal wires (Yoshida et al. 2010). Based on the metalization techniques (McNaughton and Horch 1996), metalizing a 12-μm Kevlar fiber and insulating it with silicone (Fig. 5.12a) can make a polyLIFE device 60 times more flexible than Pt-Ir electrodes, without a significant loss in the recording capability (Lawrence et al. 2003). Similarly, the tf-LIFE mentioned before uses flexible polyimide as the substrate material to reduce the structural rigidity. These efforts have demonstrated reduced nerve damage and high degree of biocompatibility in chronic implantations (Malmstrom et al. 1998; Lawrence et al. 2002; Dhillon et al. 2004a, b; Dhillon and Horch 2005; Dhillon et al. 2005). In addition, flexible polyimide has shown better long-term stability in sieve electrodes and promotes faster axonal regeneration than traditional silicon-based devices (Navarro et al. 1998; Lago et al. 2005). To further increase the flexibility, liquid GaIn alloy as electrode material has been proposed to fabricate stretchable peripheral nerve interfaces (Rui and Jing 2017) similar to cuff electrodes (Fig. 5.12b). While liquid metal cannot be surpassed by solid materials in terms of mechanical flexibility, whether it is suitable for intrafascicular applications is still unclear.
Innovative neural interfaces based on advanced materials. (a) General design of polymer-based intrafascicular electrode. (Adapted with permission from Lawrence et al. 2003). (b) Flexible cuff electrode using liquid alloy as the electrode core. (Adapted with permission from Rui and Jing 2017). (c) Neural modulation using ion-selective membrane. (Adapted with permission from Song et al. 2011)
The critical stimulation/recording sites that electrically interact with nerve fibers also benefit from the advances of biomaterials. Among four popular electrode materials, Pt, IrOx, poly(3,4-ethylenedioxythiophene) (PEDOT), and platinum black (Pt black), acute and long-term stimulations with cuff electrodes have demonstrated Pt black as the best candidate for chronically implantable electrodes, due to its excellent performance on charge delivery capacity, charge injection capacity, interfacial impedance, and most importantly the stable electrochemical properties (Lee et al. 2016). Although tested on cuff electrodes, the application of Pt black can also be extended to smaller and more invasive interfaces that demand higher charge delivery capability for stimulating sites.
In contrast, the application of ion-selective membrane (ISM) stands out of the traditional electron–ion interfaces by directly modulating ion concentrations along the nerve (Fig. 5.12c) (Song et al. 2011). Since the essence of action potential is the motion of different ions into or out of plasma membrane, such innovative design actually works the same way as neurons and eliminates the electron–ion conversion process at the neural interface, resulting in 40% reduction of stimulation threshold on a frog sciatic nerve.
5.3.3 Toward Easier Surgical Operation and Implantation
Surgical operation is not only the very first step of peripheral nerve electrode implantation but also determines the performance and stability of implanted devices. Since this process is very likely to introduce neural trauma and displacement of electrodes, it is critical to make surgical protocols as simple and robust as possible when designing a neural interface. Therefore, this section will discuss the strategies for easier surgical implantation, from the improvement on electrodes and device wirings.
5.3.3.1 Modification of Electrodes
Since cuff, intrafascicular, and regenerative electrodes are significantly distinguished from each other in both structure and working principles, the corresponding surgical procedures should also be discussed individually. As discussed before, a conventional split-cylinder cuff interface is made of silicon rubber with metal contacts on its inner side, which requires sutures to secure the device around the target nerve during implantation. Not only does the suturing complicate the surgical installation but causes a non-intimate interface as well (Naples et al. 1988). Spiral cuff and shape memory alloy (SMA) cuff are therefore proposed to eliminate the suturing process using self-closed scheme. By bonding two pieces of silicone sheet with different resting lengths, stress is stored in the spiral cuff, and the device can spontaneously coil into a spiral tube once released (Fig. 5.13a) (Naples et al. 1988). With a similar strategy, the SMA cuff utilizes the shape memory effect and super elasticity of NiTi alloy to make the device self-closed at 37 °C while self-opening at 10 °C (Fig. 5.13b) (Crampon et al. 1998; Crampon et al. 1999). Benefitted from advances on microfabrication and flexible materials, various new designs have been reported under the concept of self-closed cuff (see the table in Kang et al. (2015)). A typical example is the microfabricated parylene-based cuff electrode (Fig. 5.13c), which further reduces mechanical mismatch and increases the number of channels to 16 (Kang et al. 2015). Compared to the original design, these modifications help the cuff wrap snugly around nerve trunk and adapt to size changes, significantly enhancing the quality of the neural interface and its recording/stimulating efficiency.
Self-closed cuff electrodes . (a) Spiral cuff electrode is fully closed when stress is released, forming snug and adaptive wrapping on a nerve trunk. (Adapted with permission from Naples et al. 1988). (b) Structure of the shape memory alloy electrode. (Adapted with permission from Crampon et al. 1998). (c) Fabrication procedure of self-closed parylene cuff electrode. (Adapted with permission from Kang et al. 2015)
Compared to cuff electrodes that only need to wrap the entire nerve trunk, the surgical installation of intrafascicular electrodes is much more complicated and easier to cause tissue damage. According to the basic design of intrafascicular electrodes (Malagodi et al. 1989), the device can be introduced into a nerve fascicle by an electrosharpened tungsten needle that is cut off after implantation (Fig. 5.14a). However, this apparently leaves the electrode unsecured and subject to longitudinal movements. This was addressed by anchoring the device on a silicone tube (Lefurge et al. 1991). Specifically, medical-grade Silastic adhesive was injected into the tubing to anchor the electrode wires and suture (Fig. 5.14b). Furthermore, since electrodes are glued with the introducing needle, the entry terminal of a wire LIFE has to be cut off when removing the needle, and this may cause further longitudinal and rotatory motion of already positioned electrode, as well as the exposure of the metal core to body fluids. The creative dual electrode from a single insulated wire can solve this issue by eliminating the need of gluing (Fig. 5.14c, d). By breaking the metal contact between two interfacial sites and stretching the insulation layer, the device can be folded into a loop and linked with a sacrificial polyaramid loop glued to the needle. In this way, the needle can be released after implantation by simply cutting off the sacrificial loop rather than the electrode body.
Surgical implantation of intrafascicular electrodes . (a) Schematic representation of implantation process for LIFE and TIME. (Adapted with permission from Yoshida et al. 2010). (b) Anchoring of LIFE using a tube filled with Silastic adhesive. (Adapted with permission from Lefurge et al. 1991). (c, d) Implantation of LIFE (Lago et al. 2007b) and TIME (Boretius et al. 2010) without gluing to the introducer needle (Lago et al. 2007b). (Adapted with permission from Lago et al. (2007b) and Boretius et al. (2010))
5.3.3.2 Modification of Device Wirings
In addition to the implantation of the device itself, wiring and accessories of a peripheral nerve interface, especially those with multiple channels, also constitute substantial liability to surgical operation as well as chronic implantation after surgery. On the one hand, it is highly desired to minimize the overall dimensions of the implanted device using integrated circuit technology. For FINE, despite plenty of leads required for electrode contacts, the wiring scheme could be significantly simplified by integrating a multiplexer (Lertmanorat et al. 2009) with the electrodes. Similar integration design can also be implemented in sieve electrodes to enable chronic recordings (Bradley et al. 1997).
On the other hand , wireless transmission has been proposed to completely eliminate the need for tethered communication as well as powering of implanted devices. Based on electromagnetic energy coupling and communication, many systems have been developed using radiofrequency (RF) electromagnetic transmission. Wireless powering for in vivo stimulation can be realized by direct antenna harvesting (Park et al. 2015), resonant cavity (Montgomery et al. 2015), or midfield regime transfer (Tanabe et al. 2017). Through capacitive coupling between an adjacent serpentine antenna, RF power can be harvested to provide electricity for LEDs that are implanted to optogenetically stimulate rat sciatic nerves (Fig. 5.15a). Alternatively, electromagnetic energy can be uniformly localized on an aluminum resonant cavity to power LEDs implanted on mouse peripheral nerve endings (Fig. 5.15b). In addition to optogenetic probes, RF transmission has also been applied to cuff electrodes on the vagus nerve using midfield regime that significantly enhanced transmission efficiency (Fig. 5.15c).
Electromagnetic wireless powering for peripheral nerve electrodes. (a) Soft and wireless optogenetic stimulation device based on capacitive coupling through serpentine antenna. (Adapted with permission from Park et al. 2015). (b) Resonant cavity-based light delivery system and implanted devices (subfigure). (Adapted with permission from Montgomery et al. 2015). (c) Schematic diagram (left) and photos (right) of the wireless cuff, which consist of electrode sites, a meandered antenna, and integrated circuits. (Adapted with permission from Tanabe et al. 2017). (d) Neural Dust mote implanted on rat sciatic nerve. A piezoelectric crystal, a transistor, and two recording electrodes are assembled on a flexible PCB. (Adapted with permission from Seo et al. 2016)
However, further miniaturization of electromagnetic wireless electronics is bottlenecked by their poor efficiency at dimensions lower than 5 mm due to the inefficient RF coupling within tissue (Rabaey et al. 2011). To overcome this inherent limitation, ultrasonic transmission has been proposed to replace the electromagnetic strategy. With much smaller wavelength and less attenuation within tissue, ultrasonic wave is able to achieve higher spatial resolution and penetration depth, yielding excellent power efficiency at smaller dimensions compared to its electromagnetic counterparts (Seo et al. 2015). By integrating a piezocrystal to convert ultrasonic energy into electricity, the recording transistor can be electrically powered, and neural potentials can be detected by the transistor’s gate, modulating the current flowing through the piezocrystal (Fig. 5.15d) (Seo et al. 2016). In turn, this current modulation affects the vibration of the crystal and the reflected ultrasonic wave that is then reconstructed externally. This technology, named Neural Dust, is well known for its sub-mm size and high efficiency, which makes the surgical operation easier and reduces trauma caused by micromotion of the electrodes during chronic implantation .
5.4 Conclusion
Directly acting on nerve fibers, peripheral nerve interfaces can modulate muscular activity with less activation energy and more compact structure. Decades of extensive studies on electrode-based interfaces have witnessed significant progress on selectivity, noise rejection, tissue–device interface, and surgical implantation. Although the challenges are discussed individually here, they are inherently correlated to each other, particularly between the functionality and tissue–device interface. Both higher resolution of recruitment and lower noise level require more intimate interface between the implanted probes and nerve fibers, which inevitably brings issues of scar encapsulation, neural trauma, and instability of recording/stimulation during chronic implantation. While difficult to be perfectly eliminated, these side effects have been much alleviated through more stabilized device structures, the application of flexible materials, and robust implantation procedures. Moreover, the emergence of muscle-graft interface and ion-selective membrane has opened up the possibility of accessing peripheral nerves in a more natural way than conventional electrodes.
References
Altman, K. W., & Plonsey, R. (1990). Point source nerve bundle stimulation: Effects of fiber diameter and depth on simulated excitation. IEEE Transactions on Biomedical Engineering, 37(7), 688–698.
Badia, J., Boretius, T., et al. (2011). Biocompatibility of chronically implanted transverse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE Transactions on Biomedical Engineering, 58(8), 2324–2332.
Boretius, T., Badia, J., et al. (2010). A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosensors and Bioelectronics, 26(1), 62–69.
Boretius, T., Yoshida, K., et al. (2012). A transverse intrafascicular multichannel electrode (TIME) to treat phantom limb pain — Towards human clinical trials. 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob).
Bossi, S., Kammer, S., et al. (2009). An implantable microactuated intrafascicular electrode for peripheral nerves. IEEE Transactions on Biomedical Engineering, 56(11), 2701–2706.
Bradley, R. M., Smoke, R. H., et al. (1992). Functional regeneration of glossopharyngeal nerve through micromachined sieve electrode arrays. Brain Research, 594(1), 84–90.
Bradley, R. M., Cao, X., et al. (1997). Long term chronic recordings from peripheral sensory fibers using a sieve electrode array. Journal of Neuroscience Methods, 73(2), 177–186.
Branner, A., & Normann, R. A. (2000). A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Research Bulletin, 51(4), 293–306.
Branner, A., Stein, R. B., et al. (2001). Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. Journal of Neurophysiology, 85(4), 1585–1594.
Castro, J., Negredo, P., et al. (2008). Fiber composition of the rat sciatic nerve and its modification during regeneration through a sieve electrode. Brain Research, 1190(Supplement C), 65–77.
Choi, A. Q., Cavanaugh, J. K., et al. (2001). Selectivity of multiple-contact nerve cuff electrodes: A simulation analysis. IEEE Transactions on Biomedical Engineering, 48(2), 165–172.
Clark, J., & Plonsey, R. (1968). The extracellular potential field of the single active nerve fiber in a volume conductor. Biophysical Journal, 8(7), 842–864.
Coburn, B. (1980). Electrical stimulation of the spinal cord: Two-dimensional finite element analysis with particular reference to epidural electrodes. Medical and Biological Engineering and Computing, 18(5), 573–584.
Crampon, M. A., Sawan, M., et al. (1998). New nerve cuff electrode based on a shape memory alloy armature. Proceedings of the 20th annual international conference of the IEEE Engineering in Medicine and Biology Society. Vol.20 Biomedical Engineering Towards the Year 2000 and Beyond (Cat. No.98CH36286).
Crampon, M.-A., Sawan, M., et al. (1999). New easy to install nerve cuff electrode using shape memory alloy armature. Artificial Organs, 23(5), 392–395.
Cutrone, A., Valle, J. D., et al. (2015). A three-dimensional self-opening intraneural peripheral interface (SELINE). Journal of Neural Engineering, 12(1), 016016.
Daniel, K. L., Mark, C., et al. (2006). Chronic histological effects of the flat interface nerve electrode. Journal of Neural Engineering, 3(2), 102.
Demosthenous, A., Taylor, J., et al. (2004). Design of an adaptive interference reduction system for nerve-cuff electrode recording. IEEE Transactions on Circuits and Systems I: Regular Papers, 51(4), 629–639.
Deurloo, K. E. I., Holsheimer, J., et al. (1998). Transverse tripolar stimulation of peripheral nerve: A modelling study of spatial selectivity. Medical and Biological Engineering and Computing, 36(1), 66–74.
Dhillon, G. S., & Horch, K. W. (2005). Direct neural sensory feedback and control of a prosthetic arm. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 13(4), 468–472.
Dhillon, G. S., Lawrence, S. M., et al. (2004a). Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs. The Journal of Hand Surgery, 29(4), 605–615. discussion 616-608.
Dhillon, G. S., Lawrence, S. M., et al. (2004b). Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs1 1No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The Journal of Hand Surgery, 29(4), 605–615.
Dhillon, G. S., Krüger, T. B., et al. (2005). Effects of short-term training on sensory and motor function in severed nerves of long-term human amputees. Journal of Neurophysiology, 93(5), 2625–2633.
Djilas, M., Yoshida, K., et al. (2007). Improving the signal-to-noise ratio in recordings with thin-film longitudinal intra-fascicular electrodes using shielding cuffs. 2007 3rd international IEEE/EMBS conference on neural engineering.
Goodall, E. V., Lefurge, T. M., et al. (1991). Information contained in sensory nerve recordings made with intrafascicular electrodes. IEEE Transactions on Biomedical Engineering, 38(9), 846–850.
Goodall, E. V., Horch, K. W., et al. (1993). Analysis of single-unit firing patterns in multi-unit intrafascicular recordings. Medical and Biological Engineering and Computing, 31(3), 257–267.
Goodall, E. V., de Breij, J. F., et al. (1996). Position-selective activation of peripheral nerve fibers with a cuff electrode. IEEE Transactions on Biomedical Engineering, 43(8), 851–856.
Grill, W. M., & Mortimer, J. T. (1998). Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Transactions on Rehabilitation Engineering, 6(4), 364–373.
Harreby, K. R., Kundu, A., et al. (2015). Subchronic stimulation performance of transverse intrafascicular multichannel electrodes in the median nerve of the Göttingen Minipig. Artificial Organs, 39(2), E36–E48.
Hoffer, J. A., & Marks, W. B. (1976). Long-term peripheral nerve activity during behavior in the rabbit. In R. M. Herman, S. Grillner, P. S. G. Stein, & D. G. Stuart (Eds.), Neural control of locomotion (pp. 767–768). Boston: Springer.
Irina, I. S., van Wezel, R. J. A., et al. (2013). In vivo testing of a 3D bifurcating microchannel scaffold inducing separation of regenerating axon bundles in peripheral nerves. Journal of Neural Engineering, 10(6), 066018.
Ivan, R. M., Daniel, J. C., et al. (2012). High sensitivity recording of afferent nerve activity using ultra-compliant microchannel electrodes: An acute in vivo validation. Journal of Neural Engineering, 9(2), 026005.
Jensen, W., Micera, S., et al. (2010). Development of an implantable transverse intrafascicular multichannel electrode (TIME) system for relieving phantom limb pain. 2010 annual international conference of the IEEE engineering in medicine and biology.
Jordi, B., Tim, B., et al. (2011). Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. Journal of Neural Engineering, 8(3), 036023.
Jun-Uk, C., Kang-Il, S., et al. (2012). Improvement of signal-to-interference ratio and signal-to-noise ratio in nerve cuff electrode systems. Physiological Measurement, 33(6), 943.
Kang, X., Liu, J. Q., et al. (2015). Self-closed parylene cuff electrode for peripheral nerve recording. Journal of Microelectromechanical Systems, 24(2), 319–332.
Kundu, A., Harreby, K. R., et al. (2014). Stimulation selectivity of the “thin-film longitudinal intrafascicular electrode” (tfLIFE) and the “transverse intrafascicular multi-channel electrode”; (TIME) in the large nerve animal model. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 22(2), 400–410.
Kung, T. A. (2014). Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plastic and Reconstructive Surgery (1963), 133(6), 1380–1394.
Lago, N., Ceballos, D., et al. (2005). Long term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomaterials, 26(14), 2021–2031.
Lago, N., Udina, E., et al. (2007a). Neurobiological assessment of regenerative electrodes for bidirectional interfacing injured peripheral nerves. IEEE Transactions on Biomedical Engineering, 54(6), 1129–1137.
Lago, N., Yoshida, K., et al. (2007b). Assessment of biocompatibility of chronically implanted polyimide and platinum intrafascicular electrodes. IEEE Transactions on Biomedical Engineering, 54(2), 281–290.
Langhals, N. B., Urbanchek, M. G., et al. (2014a). Update in facial nerve paralysis: Tissue engineering and new technologies. Current Opinion in Otolaryngology & Head and Neck Surgery, 22(4), 291–299.
Langhals, N. B., Woo, S. L., et al. (2014b). Electrically stimulated signals from a long-term regenerative peripheral nerve interface. 2014 36th annual international conference of the IEEE engineering in medicine and biology society.
Larsen, J. O., Thomsen, M., et al. (1998). Degeneration and regeneration in rabbit peripheral nerve with long-term nerve cuff electrode implant: A stereological study of myelinated and unmyelinated axons. Acta Neuropathologica, 96(4), 365–378.
Lawrence, S. M., Larsen, J. O., et al. (2002). Long-term biocompatibility of implanted polymer-based. Journal of Biomedical Materials Research, 63(5), 501–506.
Lawrence, S. M., Dhillon, G. S., et al. (2003). Fabrication and characteristics of an implantable, polymer-based, intrafascicular electrode. Journal of Neuroscience Methods, 131(1), 9–26.
Lee, Y. J., Kim, H.-J., et al. (2016). Characterization of nerve-cuff electrode interface for biocompatible and chronic stimulating application. Sensors and Actuators B: Chemical, 237(Supplement C), 924–934.
Lefurge, T., Goodall, E., et al. (1991). Chronically implanted intrafascicular recording electrodes. Annals of Biomedical Engineering, 19(2), 197–207.
Lertmanorat, Z., Montague, F. W., et al. (2009). A flat Interface nerve electrode with integrated multiplexer. IEEE transactions on neural systems and rehabilitation engineering: A publication of the IEEE Engineering in Medicine and Biology Society, 17(2), 176–182.
Leventhal, D. K., & Durand, D. M. (2003). Subfascicle stimulation selectivity with the flat interface nerve electrode. Annals of Biomedical Engineering, 31(6), 643–652.
Leventhal, D. K., & Durand, D. M. (2004). Chronic measurement of the stimulation selectivity of the flat interface nerve electrode. IEEE Transactions on Biomedical Engineering, 51(9), 1649–1658.
Lotfi, P., Garde, K., et al. (2011). Modality-specific axonal regeneration: Toward selective regenerative neural interfaces. Front Neuroeng, 4, 11.
Malagodi, M. S., Horch, K. W., et al. (1989). An intrafascicular electrode for recording of action potentials in peripheral nerves. Annals of Biomedical Engineering, 17(4), 397–410.
Malmstrom, J. A., McNaughton, T. G., et al. (1998). Recording properties and biocompatibility of chronically implanted polymer-based intrafascicular electrodes. Annals of Biomedical Engineering, 26(6), 1055–1064.
McNaughton, T. G., & Horch, K. W. (1996). Metallized polymer fibers as leadwires and intrafascicular microelectrodes. Journal of Neuroscience Methods, 70(1), 103–107.
McNeal, D. R., Baker, L. L., et al. (1989). Recruitment data for nerve cuff electrodes: Implications for design of implantable stimulators. IEEE Transactions on Biomedical Engineering, 36(3), 301–308.
Micera, S., Navarro, X., et al. (2008). On the use of longitudinal intrafascicular peripheral interfaces for the control of cybernetic hand prostheses in amputees. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 16(5), 453–472.
Mirfakhraei, K., & Horch, K. (1994). Classification of action potentials in multi-unit intrafascicular recordings using neural network pattern-recognition techniques. IEEE Transactions on Biomedical Engineering, 41(1), 89–91.
Montgomery, K. L., Yeh, A. J., et al. (2015). Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature Methods, 12(10), 969–974.
Musick, K. M., Rigosa, J., et al. (2015). Chronic multichannel neural recordings from soft regenerative microchannel electrodes during gait. Scientific Reports, 5, 14363.
Nannini, N., & Horch, K. (1991). Muscle recruitment with intrafascicular electrodes. IEEE Transactions on Biomedical Engineering, 38(8), 769–776.
Naples, G. G., Mortimer, J. T., et al. (1988). A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Transactions on Biomedical Engineering, 35(11), 905–916.
Navarro, X., Calvet, S., et al. (1996). Peripheral nerve regeneration through microelectrode arrays based on silicon technology. Restorative Neurology and Neuroscience, 9(3), 151–160.
Navarro, X., Calvet, S., et al. (1998). Stimulation and recording from regenerated peripheral nerves through polyimide sieve electrodes. Journal of the Peripheral Nervous System, 3(2), 91–101.
Navarro, X., Krueger, T. B., et al. (2005). A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. Journal of the Peripheral Nervous System, 10(3), 229–258.
Negredo, P., Castro, J., et al. (2004). Differential growth of axons from sensory and motor neurons through a regenerative electrode: A stereological, retrograde tracer, and functional study in the rat. Neuroscience, 128(3), 605–615.
Ortiz-Catalan, M., Brånemark, R., et al. (2012). On the viability of implantable electrodes for the natural control of artificial limbs: Review and discussion. Biomedical Engineering Online, 11, 33–33.
Ortiz-Catalan, M., Marin-Millan, J., et al. (2013). Effect on signal-to-noise ratio of splitting the continuous contacts of cuff electrodes into smaller recording areas. Journal of Neuroengineering and Rehabilitation, 10, 22–22.
Parisa, S., Milos, R. P., et al. (2017). Optimizing the design of bipolar nerve cuff electrodes for improved recording of peripheral nerve activity. Journal of Neural Engineering, 14(3), 036015.
Park, S. I., Brenner, D. S., et al. (2015). Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature Biotechnology, 33(12), 1280–1286.
Parrini, S. (1997). A modeling study to compare tripolar and monopolar cuff electrodes for selective activation of nerve fibers. Conf. Neural Prosthesis: Motor Systems.
Patil, A. C., & Thakor, N. V. (2016). Implantable neurotechnologies: A review of micro- and nanoelectrodes for neural recording. Medical & Biological Engineering & Computing, 54(1), 23–44.
Perez-Orive, J., & Durund, D. M. (2000). Modeling study of peripheral nerve recording selectivity. IEEE Transactions on Rehabilitation Engineering, 8(3), 320–329.
Pflaum, C., Riso, R. R., et al. (1996). Performance of alternative amplifier configurations for tripolar nerve cuff recorded ENG. Proceedings of 18th annual international conference of the IEEE engineering in medicine and biology society.
Rabaey, J. M., M. Mark, et al. (2011). Powering and communicating with mm-size implants. 2011 design, automation & test in Europe.
Rahal, M., Taylor, J., et al. (2000a). The effect of nerve cuff geometry on interference reduction: A study by computer modeling. IEEE Transactions on Biomedical Engineering, 47(1), 136–138.
Rahal, M., Winter, J., et al. (2000b). An improved configuration for the reduction of EMG in electrode cuff recordings: A theoretical approach. IEEE Transactions on Biomedical Engineering, 47(9), 1281–1284.
Raspopovic, S., Capogrosso, M., et al. (2011). A computational model for the stimulation of rat sciatic nerve using a transverse intrafascicular multichannel electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 19(4), 333–344.
Rattay, F. (1986). Analysis of models for external stimulation of axons. IEEE Transactions on Biomedical Engineering, BME-33(10), 974–977.
Romero, E., Denef, J. F., et al. (2001). Neural morphological effects of long-term implantation of the self-sizing spiral cuff nerve electrode. Medical and Biological Engineering and Computing, 39(1), 90–100.
Rui, G., & Jing, L. (2017). Implantable liquid metal-based flexible neural microelectrode array and its application in recovering animal locomotion functions. Journal of Micromechanics and Microengineering, 27(10), 104002.
Rushton, W. A. H. (1927). The effect upon the threshold for nervous excitation of the length of nerve exposed, and the angle between current and nerve. The Journal of Physiology, 63(4), 357–377.
Sadeghlo, B. & Yoo, P. B. (2013). Enhanced electrode design for peripheral nerve recording. 2013 6th international IEEE/EMBS conference on neural engineering (NER).
Sahin, M., Haxhiu, M. A., et al. (1997). Spiral nerve cuff electrode for recordings of respiratory output. Journal of Applied Physiology, 83(1), 317–322.
Schiefer, M. A., Triolo, R. J., et al. (2005). Modeling selective stimulation with A flat Interface nerve electrode for standing neuroprosthetic systems. Conference proceedings. 2nd international IEEE EMBS conference on neural engineering, 2005.
Schiefer, M. A., Triolo, R. J., et al. (2008). A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 16(2), 195–204.
Schiefer, M. A., Polasek, K. H., et al. (2010). Selective stimulation of the human femoral nerve with a flat interface nerve electrode. Journal of Neural Engineering, 7(2), 26006–26006.
Schiefer, M. A., Freeberg, M., et al. (2013). Selective activation of the human tibial and common peroneal nerves with a flat interface nerve electrode. Journal of Neural Engineering, 10(5), 056006.
Seo, D., Carmena, J. M., et al. (2015). Model validation of untethered, ultrasonic neural dust motes for cortical recording. Journal of Neuroscience Methods, 244(Supplement C), 114–122.
Seo, D., Neely, R. M., et al. (2016). Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron, 91(3), 529–539.
Song, Y.-A., Melik, R., et al. (2011). Electrochemical activation and inhibition of neuromuscular systems through modulation of ion concentrations with ion-selective membranes. Nature Materials, 10, 980.
Srinivasan, A., Tahilramani, M., et al. (2015). Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees. Biomaterials, 41(Supplement C), 151–165.
Stein, R. B., Charles, D., et al. (1975). Principles underlying new methods for chronic neural recording. The Canadian Journal of Neurological Sciences, 2(3), 235–244.
Stieglitz, T., Ruf, H. H., et al. (2002). A biohybrid system to interface peripheral nerves after traumatic lesions: Design of a high channel sieve electrod. Biosensors and Bioelectronics, 17(8), 685–696.
Struijk, J. J., Thomsen, M., et al. (1999). Cuff electrodes for long-term recording of natural sensory information. IEEE Engineering in Medicine and Biology Magazine, 18(3), 91–98.
Sweeney, J. D., Ksienski, D. A., et al. (1990). A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Transactions on Biomedical Engineering, 37(7), 706–715.
Tanabe, Y., Ho, J. S., et al. (2017). High-performance wireless powering for peripheral nerve neuromodulation systems. PLoS One, 12(10), e0186698.
Tarler, M. D., & Mortimer, J. T. (2003). Comparison of joint torque evoked with monopolar and tripolar-cuff electrodes. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 11(3), 227–235.
Tarler, M. D., & Mortimer, J. T. (2004). Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 12(2), 251–257.
Thompson, C. H., Zoratti, M. J., et al. (2016). Regenerative electrode interfaces for neural prostheses. Tissue Engineering. Part B, Reviews, 22(2), 125–135.
Triantis, I. F., & Demosthenous, A. (2008). Tripolar-cuff deviation from ideal model: Assessment by bioelectric field simulations and saline-bath experiments. Medical Engineering & Physics, 30(5), 550–562.
Tyler, D. J., & Durand, D. M. (2002). Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 10(4), 294–303.
Urbanchek, M. G., Wei, B., et al. (2011). Long-term stability of regenerative peripheral nerve interfaces (RPNI). Plastic and Reconstructive Surgery Plastic and Reconstructive Surgery, 128, 88–89.
Urbanchek, M. G., Kung, T. A., et al. (2016). Development of a regenerative peripheral nerve interface for control of a neuroprosthetic limb. BioMed Research International, 2016, 5726730.
Veltink, P. H., van Alste, J. A., et al. (1988). Influences of stimulation conditions on recruitment of myelinated nerve fibres: A model study. IEEE Transactions on Biomedical Engineering, 35(11), 917–924.
Veltink, P. H., van Alsté, J. A., et al. (1989a). Multielectrode intrafascicular and extraneural stimulation. Medical and Biological Engineering and Computing, 27(1), 19–24.
Veltink, P. H., Veen, B. K. v., et al. (1989b). A modeling study of nerve fascicle stimulation. IEEE Transactions on Biomedical Engineering, 36(7), 683–692.
Veraart, C., Grill, W. M., et al. (1993). Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Transactions on Biomedical Engineering, 40(7), 640–653.
Wallman, L., Rosengren, A., et al. (2000). Geometric design and surface morphology of sieve electrodes-nerve regeneration and biocompatibility studies. 1st annual international IEEE-EMBS special topic conference on microtechnologies in medicine and biology. Proceedings (Cat. No.00EX451).
Yoo, P. B., Sahin, M., et al. (2004). Selective stimulation of the canine hypoglossal nerve using a multi-contact cuff electrode. Annals of Biomedical Engineering, 32(4), 511–519.
Yoshida, K., & Horch, K. (1993). Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Transactions on Biomedical Engineering, 40(5), 492–494.
Yoshida, K., & Stein, R. B. (1999). Characterization of signals and noise rejection with bipolar longitudinal intrafascicular electrodes. IEEE Transactions on Biomedical Engineering, 46(2), 226–234.
Yoshida, K., Pellinen, D., et al. (2000). Development of the thin-film longitudinal intra-fascicular electrode. 5th annual conference of the International Functional Electrical Stimulation Society, Aalborg, Denmark, Center for Sensory-Motor Interaction (SMI), Department of Health Science and Technology, Aalborg University.
Yoshida, K., Farina, D., et al. (2010). Multichannel intraneural and intramuscular techniques for multiunit recording and use in active prostheses. Proceedings of the IEEE, 98(3), 432–449.
Acknowledgments
This work was supported by the Defense Advanced Research Projects Agency through Grant # D17AP00031 of the USA and the Chronic Brain Injury Program of The Ohio State University through a Pilot Award. The views, opinions, and/or findings contained in this article are those of the author and should not be interpreted as representing the official views or policies, either expressed or implied, of the Defense Advanced Research Projects Agency or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wu, Y., Guo, L. (2020). Peripheral Nerve Electrodes. In: Guo, L. (eds) Neural Interface Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-41854-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-41854-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41853-3
Online ISBN: 978-3-030-41854-0
eBook Packages: EngineeringEngineering (R0)