Abstract
Enterococci are Gram-positive facultative anaerobes that have changed over epoch as highly modified representer of the gastrointestinal (GI) consortia of an extensive array of organisms like insects, birds, reptiles, mammals, and human. These commensal microorganisms have grossed resistance to all the antimicrobial drugs that currently exist. Multidrug-resistant (MDR) enterococci shows an extensive repertoire of mechanisms of drug resistance including drug target modification, overexpression of efflux pumps, inactivation of antibacterial agents, and cell membrane adaptive response that helps to persist in the body of the host and nosocomial atmosphere. MDR enterococci are renewed to persist in the GI environment and predisposing to invasive infections in those patients who are severely ill and immunocompromised. This chapter mainly focuses the resistance mechanisms of antimicrobial drugs and also role of certain new antimicrobial genes like optrA and cfr in enterococci. Moreover different strategies to control and therapeutic approaches for controlling MDR enterococci especially using nanotechnology are also highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Enterococci are a primitive genus of microorganisms that are highly adapted to surviving in heterogeneous and harsh environmental conditions. In the ending of nineteenth century, a saprophytic and infectious cocci found in intestine was described as “Enterococcus” (Thiercelin 1899). MacCallum and Hastings also characterized an enterococcal organism, Enterococcus faecalis, from a fatal endocarditis case, and provide first comprehensive picture of its pathogenesis (MacCallum and Hastings 1899). Early report attested that enterococcal pathogens are basically commensal opportunist (Lebreton et al. 2014). With the development of genomic technologies, an array of enterococcal species has explored. Enterococci are the principal causes of healthcare-associated infections (HAIs) all around the globe. The last few decades have witnessed the development of multidrug-resistant (MDR) enterococci which extensively complicates this issue and also enhances the chance of treatment failure and sometimes leads to death. In the last decade, antibiotic-resistant enterococci have become familiar as the prime cause of nosocomial bacteremia, postsurgical wound infection, urinary tract infections, and device-associated infections (García-Solache and Rice 2019; Prabaker and Weinstein 2011; Emori and Gaynes 1993; McDonald et al. 1997).
In this section, we will explain the overall characteristics of the genus Enterococcus species, diseases induced by it, and the historical viewpoint behind the creation of MDR enterococci as pathogens and current knowledge of the molecular foundation of drug resistance in Enterococcus. Finally we addressed briefly the necessity to advance new drug targets, development of new approaches of nanobiotechnological methods against these dangerous and insubordinate organisms as well as difficulties and opportunities for the future.
18.1.1 Features of Enterococcus Genus
Enterococcus species are catalase negative Gram-positive bacteria, are natural inhabitants, and can be isolated easily from their habitats. They are also an important intestinal microfloral component of humans and animals (Van and Willems 2010). The basic physiological and morphological characteristics of all enterococcal strain include Gram-positive, ovoid/spherical cells organized in pairs/chains; among them a few strains exhibit pathogenic potential (Thiercelin 1899). Different salient feature of genus streptococci is represented in Fig. 18.1. They are obligatory fermentative chemoorganotrophs and non-spore-forming facultative anaerobes. They usually grow at an optimal temperature of 35 °C and can growth in the range of 10–45 °C. They normally have an optimal growth in medium having 6.5% sodium chloride (Facklam 1973). They are generally unable to produce catalase and all cytochromes. A few species are able to produce nominal catalase with weak effervescence. Usually they are homofermentative and produce only lactic acid as end product by fermenting glucose (Klein 2003).
18.1.2 Phylogenetic Diversity of Enterococcus Genus
In recent decades, knowledge regarding the biology, ecology, virulence, and genetics of the genus Enterococcus has sharply increased. The enterococci’s taxonomy has changed considerably since the end of the twentieth century when the genus had only 20 species. As a consequence of improvements in differentiation techniques coupled with enhanced interest in enterococci, many fresh species have been well documented. Being ubiquitous, three Enterococcal species, namely, E. durans, E. faecalis, and E. faecium, were documented before 1950. E. faecium and E. faecalis account for most of the enterococcal diseases (Malani et al. 2002). Other species like E. avium, E. casseliflavus, E. hirae, E. gallinarum, E. mundtii, and E. raffinosus have also been isolated from human infection (Devriese et al. 1994; Hammerum 2012; Murray 1990; Lebreton et al. 2014). In the era of 1992–2012, about 30 species of Enterococcus were documented, and only four of them were associated with human infection and pathogenesis (E. sanguinicola, E. pallens, E. gilvus, and E. canintestini). Till date, there are 52 species available that belong to Enterococcus genus.
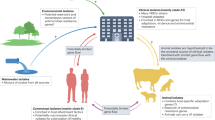
18.1.3 Enterococci-Associated Infections
Over the last couple of eras, enterococci emerged as significant pathogens (Arias and Murray 2012). The variety of diseases caused by streptococci becomes devastating which is attributed to their tendency to become increased antibiotic resistance. Although other microorganisms are often isolated from the source site with enterococci, it is often not well understood and remains a paradox that the enterococci are directly associated with the manifestations of the diseases or whether they are avirulent and opportunistic one and suppose to play an insignificant role in the manifestation of diseases (Higuita and Huycke 2014). Among the several types of enterococcal infection, endocarditis and bacteremia are the leading life-threatening disease.
18.1.3.1 Urinary Tract Infections (UTI)
Urinary tract is the most susceptible area of enterococci infection. Lower urinary tract portions, especially cystitis, prostatitis, and epididymitis, are the frequent sites of UTI caused by enterococci in older man. Young women are also affected by uncomplicated cystitis, infected by enterococci. Occurrences of bacteremia in upper UTI are most often reported in older men. Enterococci-induced UTIs are more likely to be acquired in hospitals or in long-term settings, making them more resistant to antibiotics. Moreover, ICU setting also contributes to 15% of healthcare-associated UTI. Among the ICU patients, enterococci resistant to vancomycin have become the major urinary tract pathogens associated with healthcare (Hidron et al. 2008).
18.1.3.2 Intra-abdominal Infection with Pelvic and Soft Tissue
Intra-abdominal infection with pelvic and soft tissue is also the site of enterococcal infections. Enterococci are isolated from these samples often associated with other microbial flora and infrequently cause mono-microbial infection at the above sites. Bacteremia caused by enterococci is mainly associated with abscesses and wounds in the intra-abdominal and pelvic regions (Graninger and Ragette 1992; Maki and Agger 1988). Though most of the physician routinely follows antibiotic regimens to treat such type of infections, drainage of abscesses and debridement of wounds are also essential adjuncts to antibiotic therapy. Moreover, conjunction of liver cirrhosis or patients receiving chronic peritoneal dialysis most often suffered from an infection called peritonitis. Peritonitis mainly occurs in the abdominal lining. Moreover, abdominal or pelvic mixed aerobic-anaerobic infections should be considered separately. Though, in this type of cases, enterococci show monomicrobial infection, Escherichia coli, coagulase-negative Staphylococci , and Staphylococcus aureus are also responsible for bacterial peritonitis and dialysis-associated peritonitis. Over and above, enterococci are also often isolated in cultures from decubitus and foot ulcers. However, their roles in causing such site-specific infections are not clearly understood till date.
18.1.3.3 Bacteremia
Enterococci are presently one of the major causes of bacteremia associated with healthcare. Over the last couples of years, bacteremia is usually associated with gastrointestinal tract, although sources of bacteremia also reported from biliary and intra-abdominal regions, indwelling central lines, or infections in soft tissues. Polymicrobial bacteremia is associated mainly with enterococci, though there are also other microorganisms partially involved in the occurrence of such type of infection. Enterococci-associated bacteremia causes metastatic abscesses. The rate of overall mortality in enterococci associated bacteremia is varied (Maki and Agger 1988; Patterson et al. 1995; Higuita and Huycke 2014). Several scientific reviews regarding bloodstream infections clearly reported that enterococci is the only Gram-positive bacteria associated with high risks of death. Moreover, higher mortality rate was reported in the case of E. faecium-associated bacteremia than E. faecalis (Noskin et al. 1995a, b; Higuita and Huycke 2014). The chances of occurrence of enterococci-associated bacteremia are higher in the case of elderly people with multiple underlying diseases like malignancy, diabetes mellitus, cardiovascular diseases, transplantation, and postsurgery infection.
18.1.3.4 Endocarditis
Among different types of infection caused by enterococci, endocarditis is the most fatal enterococcal infections. The alimentary or urinogenital tract is the primary bacteremia source which leads to endocarditis. In reality, left-sided participation is much more prevalent than right-sided participation. Prosthetic valve enterococcal endocarditis has been increasingly marked. This is mainly associated with increasing application of prostheses in aged persons who have higher risks for bacteremia caused by enterococci (Anderson et al. 2004; Rice et al. 1991). Enterococcal endocarditis is more common in men compared to women (McDonald et al. 2005). Several retrospective analysis reported that between 15 and 39% of enterococcal endocarditis are healthcare associated (Anderson et al. 2004; McDonald et al. 2005). Endocarditis associated with enterococci is a subacute infection followed by cardiac failure, rather than an embolic effect (McDonald et al. 2005). Though death rates are low (9–15%) in enterococcal endocarditis in comparison to other infective endocarditis (McDonald et al. 2005; Rice et al. 1991; Wilson et al. 1984; Higuita and Huycke 2014), selection of effective therapy against the multidrug-resistant enterococci is definitely a challenging task.
18.1.3.5 Uncommon Infections
Meningitis, septic arthritis, hematogenous osteomyelitis, and pneumonia are the less common or rarely seen infections caused by enterococci. Pneumonia is quite rare even in the presence of ventilators, and it is reported in significantly weakened or in immunocompromised patients who have received antibiotic drugs of a broad spectrum. Antibiotic-resistant enterococci (VRE) are likely to be responsible for such types of infection than antibiotic-susceptible enterococcal isolates.
18.2 Expansion of Antibiotic Resistance
With the innovation of antimicrobial drugs discovery and understanding the microbiological foundation of diseases, infection became remediable with remarkable recovery. Clinicians, however, quickly understood that certain microbes appear to be less effective in responding to specific antimicrobials and that’s why generations of antibiotics had come. It was also documented that penicillin and aminoglycoside are less effective against many enterococcal species, and conjugation of aminoglycosides with penicillin was prescribed which showed synergistic response that improved enterococcal endocarditis cure rates from 40 to 88% (Robbins and Tompsett 1951). Thus the particular combination of a cell-wall-active agent (i.e., penicillin/ampicillin) along with an aminoglycoside will be the solution for the treatment of deep-rooted Enterococcus-associated diseases, and this combination remains effective (Baddour et al. 2005).
Unknowingly the seeds of the resistance of enterococci against an array of drug were already being sown and propagated. By the help of comparative genomics, it was documented that the modern MDR Enterococcus faecium is a part of a genetic class that seems to have divergent root of ancestry from animal-adapted E. faecium strains in clinical practice approximately 75 years ago, corresponding to the introduction of antibiotics (Lebreton et al. 2013). This was achieved by various means which include an upsurge in horizontal gene transfer, metabolic bypass, and hyper-mutability in the enterococcal strains. The acquisition of genes for vancomycin resistance is one of the utmost examples of this adaptability. Vancomycin-resistance enterococci (VRE) was first time documented in 1988, and within two decades, more than 80% of E. faecium acquired the said property in the USA (Arias and Murray 2012). Of particular concern, E. faecium is also increasingly reported to cause nosocomial infection, which now occurs as often frequently as E. faecalis (Hidron et al. 2008). Recently, enterococci have also reported to share the vancomycin-resistant gene clusters with potential pathogens (such as methicillin-resistant Staphylococcus aureus) through horizontal gene transfer, which is matter of a great health risk (Chang et al. 2003; Ray et al. 2003). Enterococci have adapted rapidly despite the abundance of anti-Gram-positive antimicrobials, and the emergence of resistance against these agents has been theorized. This becomes a clinical challenge to treat enterococcal MDR infections. The following sections give a picture of the mechanisms and prevalence of antimicrobial resistance in enterococci which is summarized in Table 18.1.
18.3 Biofilm Formation in Enterococcal Infections
Biofilm is multicellular community of microbes attached on abiotic and biotic surfaces or interfaces, enclosed in a hydrated self-produced extracellular polymeric matrix (Costerton 2001). Development of biofilm is a multistep phenomenon which includes surface attachment, immobilization, cell-cell interaction, microcolony formation, confluent biofilm formation, and subsequently three-dimensional biofilm formation (O’Toole et al. 2000). Biofilms are the reservoir of many chronic infections and extremely difficult to eliminate (Mohamed and Huang 2007). As per the National Institutes of Health, about 4/5 share of all bacterial infection in the body associated with biofilm formation (Lewis 2001). Biofilm containing bacteria are phagocytosis resistant, and therefore it is an extremely challenging task to eliminate from the host or infected individual (Lewis 2001). Biofilms are reported to form in/on a broad range of medically used devices like pacemakers, catheters, orthopedic appliances, and prosthetic heart valves, which is correlated with multiple pathogenic consequences (Costerton et al. 1999).
Biofilm producing enterococci are extremely antibiotic resistant and therefore the impact of biofilm development is very crucial. Perusal of literature attested that enterococci were found to form biofilm in an array of infection like UTI, wounds, GI dysbiosis, endocarditis, etc. Though exopolymeric matrix and antibiotic resistance are the two major hurdles to eradicate enterococci, the foremost problem is the dissemination of the genetic trait of antibiotic resistance to other microbes (Ch’ng et al. 2019). As like as other biofilm-forming bacteria, adherence and biofilm formation by E. faecalis and E. faecium on diverse biomaterials and numerous medical apparatus (biliary stents, intravascular catheters, silicone gastrostomy devices, ureteral stents, etc.) have been documented (Joyanes et al. 2000; Distel et al. 2002; Dowidar et al. 1991; Sandoe et al. 2003; Dautle et al. 2003; Keane et al. 1994). Formation of enterococcal biofilm of on ocular lens has also been demonstrated (Kobayakawa et al. 2005).
18.3.1 Factors Contributing Formation of Biofilm in Enterococci
18.3.1.1 Biofilm Formation in E. faecalis
Development of biofilm generally consists of four phases: initial attachment, formation of microcolony, maturation of biofilm, and, finally, dispersal. There are multiple factors that influence formation of biofilm in enterococci within or outside their host condition (Dunny et al. 2014); however, the dispersion mediators have yet to be identified (Table 18.2).
Adherence to the surface is the early stage for the establishment of biofilm. Various factors like surface adhesins, glycolipids, and proteases perform significant tasks in the first step of biofilm formation. The multi-subunit (viz., A, B, and C) endocarditis and biofilm-associated pilus (Ebp) encoded by ebpABC facilitates surface adherence both in vivo and in vitro (Nielsen et al. 2012; Nallapareddy et al. 2011a; Singh et al. 2007). The role of Ebp in the early development of biofilm was showed by in vivo models of UTIs, catheter-associated UTI, and infective endocarditis (Nallapareddy et al. 2006, 2011a, b; Nielsen et al. 2013). Several in vivo experiments in cultured human cell also described the significance of surface adhesins in formation of biofilm (Mohamed et al. 2006; Rozdzinski et al. 2001; Sussmuth et al. 2000; Sillanpaa et al. 2010). It was also demonstrated that biofilm-associated glycolipid synthesis A influences in vitro surface adherence and subsequent biofilm development (Theilacker et al. 2009).
Initial attachment followed by formation of microcolony in which bacteria divided repeatedly and produce minute sizes of biofilm which subsequently get aggregated (Monds and O’Toole 2009). In vitro findings have clearly showed that microcolony formation is the mature stage of biofilm development, and this is significant for gut colonization. An enterococcal polysaccharide antigen gene cluster (epaOX) encodes a glycosyltransferase which is associated with the production of rhamnopolysaccharide associated with cell wall, and mutant E. faecalis for the particular trait showed a reduction in biofilm reduction (Ch’ng et al. 2019; Xu et al. 2000).
Maturation of E. faecalis biofilm is associated with the vigorous growth and development of extracellular matrix materials like extracellular DNA, polysaccharide, glycoprotein, modified lipid, lipoteichoic acid, etc. (Ch’ng et al. 2019; Fabretti et al. 2006). Deletion of atlA reduces the release of extracellular DNA, thus decreasing biofilm formation (Guiton et al. 2009). In vitro deletion of dltABCD operon causes inhibition of biofilm development by Gram-positive bacteria by reducing the production of D-alanine esters of lipoteichoic acid.
Biofilm formation is also contributed by population density-dependent signaling mechanism like quorum sensing and peptide pheromone signaling which upgrade expression of genes towards biofilm formation by enterococci (Krasteva et al. 2012; Camilli and Bassler 2006; Cook and Federle 2014; Li and Tian 2012; Cook et al. 2011). Recently, transfer of plasmid DNA between E. faecalis cells in GI tract has been documented which encourages biofilm formation (Chen et al. 2017; Hirt et al. 2018). Eep (Chandler and Dunny 2008), fsrABC (Ali et al. 2017), bopABCD, gelE, sprE (Dunny et al. 2014), and AI-2 (Shao et al. 2012) are also involved in quorum sensing system of enterococcal biofilm formation.
18.3.1.2 Biofilm Formation in E. faecium
Multiple genes are responsible for the development of biofilm in E. faecium like atlA, ebpABC, esp, fsrB, luxS, spx, acm, scm, sgrA, pilA, pilB, ecbA, and asrR (Dunny et al. 2014; Lim et al. 2017; Sava et al. 2010; Hendrickx et al. 2009; Sillanpaa et al. 2008). Among these genes, atlA, ebpABC, esp, acm, and asrR are responsible to cause biofilm-associated infection in in vivo condition (Dunny et al. 2014; Sava et al. 2010). The cell surface adhesin, Esp, and EbpABC perform a crucial task in the initial attachment of E. faecium, followed by biofilm development in the case of UTI and infective endocarditis model (Montealegre et al. 2016a, b; Almohamad et al. 2014). Deletion of the gene esp and ebpABC operon reduced the chances of biofilm formation by the organism (Heikens et al. 2011). There are similarities in the occurrence of biofilm formation in the case of E. faecium and E. faecalis (Ch’ng et al. 2019). AtlA-dependent release of extracellular DNA plays a crucial role in biofilm formation in vitro in both the species (Paganelli et al. 2013). Several reports suggest that upregulation of gene like ebpABC and downregulation of genes like fsrB, luxS, and spx might regulate biofilm-forming potential of E. faecium (Lim et al. 2017). Moreover, deletion of asrR gene involves in growth and maturation of biofilm and also influences biofilm-associated infections (Lebreton et al. 2012).
18.4 Mechanism of Antimicrobial Drug Resistance in Enterococci
18.4.1 Mechanism of Resistance of β-Lactam Derivatives (Cell-Wall-Active Agents)
18.4.1.1 Resistance to β-Lactams
Penicillin and ampicillin are the foremost pronounced β-lactams which competitively block peptidoglycan (PPG) biosynthesis which is basic and the most common component of the bacterial cell wall. However, the lack of analogous structural component in eukaryotes excludes the lethality of these agents and makes them an ideal against bacterial infection as therapeutics. Penicillin-binding proteins (PBPs) are the flagship of the cell wall biosynthesis machinery which is broadly subdivided into two classes: class A, which exhibits bipartite enzymatic activity, namely D,D-transpeptidase and transglycosylase, and class B, which exhibits transpeptidase activity towards other enzymes.
Enterococci are inherently resistant to most β-lactams and hence less prone to restricted by the antibiotics. This is due to the expression of one kind of PBPs which have low affinity towards β-lactam antibiotics. Consequently, the minimum inhibitory concentration (MIC) of penicillin is higher in enterococci in contrast with streptococci or other Gram-positive bacteria, which do not produce chromosomally encoded low affinity PBPs. Lower MIC values of penicillin were documented for E. faecalis strains than E. faecium.
Every enterococci have at least 5 PBPs, and 6 putative PBP genes were recognized by studying the genome of E. faecalis and E. faecium (class A, ponA, pbp F, pbpZ; class B, pbp5, pbp A, pbpB) (Miller et al. 2014). Inherent tolerance against the β-lactam antibiotics is linked with the expression of species-specific pbp5 gene (class B PBP) that minimizes binding affinity cell wall with the antibiotics. In E. faecium, the pbp5 gene is a part of operon which has three genes (including pbp5) that take part in cell wall synthesis (psr and ftsW) (Miller et al. 2014). Enhanced resistance against β-lactam antibiotics has frequently been noticed among clinically isolated E. faecium but rarely noticed in the case of E. faecalis. High-level ampicillin resistance of E. faecium (MIC>128 μg/ml) has been correlated with concomitant production of Pbp5 or with specific amino acid modifications in its sequence, which minimizes affinity of the same with penicillins resulting in less vulnerable to be inhibited. The substitutions of amino acid at or near the active-site cavity (Ser-Thr-Phe-Lys, Ser-Asp-Ala, and Lys-Thr-Gly motifs) seem to be the utmost significant ones (Rybkine et al. 1998; Zorzi et al. 1996). Combinations of specific amino acid alterations in the carboxyl-terminal transpeptidase domain of PBP5 (substitution Met-485-Ala/Thr, Ala-499-Ile/Thr, Glu-629-Val and Pro-667-Ser) and the insertion of serine or aspartate after position 466 have been related to ampicillin resistance of E. faecium isolates (Montealegre et al. 2016a, b; Jureen et al. 2003; Poeta et al. 2007; Klibi et al. 2008; Arbeloa et al. 2004; Rice et al. 2004).
Alongside, β-lactam antibiotic resistance is also facilitated by a β-lactamase enzyme which restricts the antibiotic action by cleaving the β-lactam ring. The phenomenon was documented in both E. faecalis and E. faecium (Rice and Murray 1995; Murray 1992; Coudron et al. 1992). Selected strains of E. faecalis produce a plasmid-mediated β-lactamase that is similar to the enzyme produced by Staphylococcus aureus, encoded by the blaZgene, although some polymorphisms in this gene have also been detected in some isolates (Hollenbeck and Rice 2012; Murray et al. 1992).
18.4.1.2 Resistance to Cephalosporin
As like as β-lactam antibiotic resistance , the intrinsic resistance of enterococci is correlated with a decline in the affinity of binding of cephalosporin with enterococcal PBPs, especially Pbp5 (Rice et al. 2009; Arbeloa et al. 2004). It was documented that expression of either ponA or pbpF gene in E. faecalis and E. faecium is required to exhibit cephalosporin resistance, and PbpZ alone is incapable of offering the transglycosylation property.
An array of regulatory pathways manifested by two-component system is responsible for showing cephalosporin resistance by enterococci. Downstream effector like CroRS was publicized to be imperative for the same. Besides, two-component system implicated a role in resistance also relayed by a serine/threonine kinase, namely, IreK and IreP (phosphorylated). IreB was proven as target of both the aforementioned proteins and in turn upgrade the expression of cephalosporin resistance (Comenge et al. 2003; Muller et al. 2006; Kristich et al. 2007; Hall et al. 2013). MurAA protein involved at the downstream of the IreK signaling pathway and catalyzes the first committed step in PPG biosynthesis (Miller et al. 2014).
18.4.1.3 Resistance to Glycopeptide
Vancomycin and teicoplanin belongs to glycopeptide family employed for the treatment of severe human diseases. Glycopeptides actually bind with the terminal D-alanyl-D-alanine of the pentapeptide of PPG precursors that subsequently inhibit cross-linking of PPG chains and thus restrict the bacterial cell wall synthesis. The mechanism underlying the glycopeptide resistance of enterococcal strains is the alteration of the PPG synthesis pathway. The terminus D-alanyl-D-alanine with which vancomycin binds is modified to D-alanyl-D-lactate (high resistance, MIC >64 μg/ml) or to D-alanyl-D-serine (low resistance, MIC >4–32 μg/ml). This kind of alteration in the cell wall precursors leads to reduced binding affinity of the glycopeptide with the former (Miller et al. 2014; Ahmed and Baptiste 2018; Shlaes et al. 1989; Arthur et al. 1993).
Vancomycin-resistant enterococci are formed by van operons, which encode the modified PPG precursors. Nine van operons have been recognized so far in enterococci-mediating vancomycin resistance (for D-alanyl-D-lactate modification, vanA, vanB, vanD, vanM, and for D-alanyl-D-serine modification, vanC, vanE, vanG, vanL, and vanN) (Miller et al. 2014; Courvalin 2006; Depardieu et al. 2015). The vanA and vanB are the most common genotypes among VRE with acquired resistance mechanisms of humans and animals, mostly among E. faecalis and E. faecium (Ahmed and Baptiste 2018). VanC operon is the fundamental component of E. gallinarum and E. casseliflavus that helps to produce PPG precursor with terminal D-alanyl-D-serine residue reported first time (Leclercq et al. 1992; Reid et al. 2001). Apart from VanC (which is a D-alanine-D-serine ligase), the enterococcal cells encode a serine racemase (VanT), combined dipeptidase-carboxypeptidase (VanXY) and regulators encoded by vanR and vanS genes which encode (cytoplasmic) transcriptional regulator and membrane-bound histidine kinase, respectively (Depardieu et al. 2015; Sassi et al. 2018).
The vanA operon is associated with the transposon Tn1546 and includes seven open reading frames (ORFs) transcribed under two different promoters. Regulation is mediated by vanS-vanR (sensor-kinase-response regulator) two-component system, transcribed with a common promoter. The vanH- and vanA-encoded protein modifies the PPG precursors, whereas vanY interrupt the creation of the D-alanyl-D-alanine termini of the pentapeptide by its D,D-carboxypeptidase activity. Moreover, vanZ gene is associated with teicoplanin resistance in enterococci.
Tn1547, Tn1549, and Tn5382 are the transposons associated with vanB operon. Among the transposons, Tn1549 is widely predominant among vanB-type enterococci located in chromosome. vanB has two promoters and seven ORFs. vanB enterococci represent vancomycin resistance but susceptibility towards teicoplanin (Ahmed and Baptiste 2018; Arthur and Courvalin 1993). It was well documented that a few of van operons belong to transposable genetic element which triggers the spreading of the antibiotic resistance trait.
18.4.2 Mechanism of Resistance to Protein Synthesis Interfering Antibiotics
18.4.2.1 Resistance to Aminoglycosides
Aminoglycosides are effective bactericidal chemotherapeutic agents that interfere with the protein synthesis of the bacterial cell by binding with 30S ribosomal subunit followed by misread of genetic code. The intrinsic resistance of enterococci against aminoglycosides is imparted by inactivating the aminoglycoside through covalent modification of amino or hydroxyl groups which is carried out by enterococcal enzymes.
E. faecium express 6′-acetyltransferase enzymes [AAC (6′)-Ii] which was reported to modify tobramycin, kanamycin, sisomicin, and netilmicin. Moreover, numerous isolates from clinical samples also possess the enzyme APH(3′)-IIIa which triggers the resistance against amikacin and kanamycin owing to its phosphotransferase activity (Costa et al. 1993). Alongside, in E. faecium, the bypassing of the aminoglycoside action was carried out by modifying the ribosomal target through the action of rRNA methyltransferase which methylates cytidine residue at 1404 position (Galimand et al. 2011).
Gentamycin and streptomycin are the aminoglycosides that are used in clinical practice reliably because these two are not readily degraded by enterococci-produced intrinsic enzymes. APH(2′)-Ic is another gene encoding phosphotransferases reported in E. gallinarum, E. faecium, and E. faecalis which counteracts against gentamycin (Chow et al. 1997) and tobramycin but not in against of amikacin, whereas APH(2′)-Id, isolated from E. casseliflavus and E. faecium, confers gentamycin resistance but not against amikacin. Moreover the presence of another gene, aph (2')-Ib, in E. faecium causes amino-glycoside resistance except for amikacin and streptomycin (Eliopoulos et al. 1984; Courvalin et al. 1980).
18.4.2.2 Resistance to Oxazolidinones and Linezolid
Bacteriostatic agent linezolid binds to the 23S rRNA of Gram-positive bacteria and causes disruption in the docking of charged tRNA in ribosomal A site, followed by inhibition in the peptide delivery and elongation of the polypeptide chain subsequently (Shinabarger et al. 1997; Leach et al. 2007; Locke et al. 2009; Mendes et al. 2008). The mechanism of linezolid resistance is the gene mutation which generally encodes 23S rRNA, an important ribosomal drug-binding site (Marshall et al. 2002; Chen et al. 2013; Diaz et al. 2012, 2013). Moreover, linezolid resistance develops in enterococci through acquirement of methyltransferase gene followed by modification of A2503 in the 23S rRNA (Kehrenberg et al. 2005; Vester 2018; Wang et al. 2015). Many copy of the 23S rRNA gene present in enterococci, and as much as the gene becomes mutated, the resistance property is increased concomitantly (Boumghar-Bourtchaï et al. 2009; Bourgeois-Nicolaos et al. 2007; Toh et al. 2007).
18.4.2.3 Resistance to Streptogramins, Macrolides, and Lincosamides
Unlike E. faecium, E. faecalis is resistant to pristinamycin derivatives, streptomycin A and B.
In E. faecalis genome, Isa gene encodes an ATP-binding cassette (ABC) transporter protein necessary for efflux pump which eliminates the action of lincosamide and streptogramin A (Singh et al. 2002). Similar type of pumps coded by msrC has also been reported to act in removing the streptomycin A and B (Portillo et al. 2000). An intrinsic resistance mechanism of chromosome towards macrolides by msr(A) and to linosamides by linB in E. faecium has been documented (Portillo et al. 2000; Bozdogan et al. 1999). Several other genes in Enterococcus genus are also responsible for conferring resistance like gene mef(A), causing resistance to macrolides; vgb(A), causing resistance to lincosamide; and vat(D) and vat(E), causing resistance to streptogramins.
18.4.2.4 Resistance to Daptomycin
Daptomycin binds with cellular membrane facilitated by calcium that causes alterations in its characteristics and function. It is a cyclic lipopeptide that primarily interacts with phosphatidylglycerol and, in the presence of calcium ions, aggregates and enters into the cell membrane and reaches to the inner leaflet. This causes leakage of ions, and also formation of pores occurs on the cell membrane. It also causes lipid aggregation on the membrane surface by “lipid extraction effect.” Daptomycin-resistant enterococci are reported and it is achieved by means of mutations. Report suggests that E. faecium repulses daptomycin from its cell surface by changing membrane phospholipids which is commonly associated with mutation in liaFSR operon (García-Solache and Rice 2019; Miller et al. 2016). Mutation in liaFSR system causes synergism between ampicillin and daptomycin in daptomycin-resistant E. faecium (Mishra et al. 2012).
18.4.2.5 Resistance to Tetracyclines and Glycylcyclines
Tetracyclines exhibit bacteriostatic effect by interfering with the aminoacyl-tRNA docking in the ribosome. Enterococci-acquired tetracycline resistance by ribosome shielding mechanism is facilitated by tet(M), and antibiotic efflux mechanism is facilitated by tet(L) genes (García-Solache and Rice 2019). Several other genes like tetO and tetS confer resistance to doxycyclines, minocyclines, and tetracyclines and are transferred via the Tn916 transposon. The encoded proteins of the above-mentioned genes hydrolyze GTP in the presence of ribosome and cause alteration of ribosomal conformation and finally displace bound tetracyclines (Rice 1998; Speer et al. 1992).
Tigecycline belongs to glycylcycline which is a broad-spectrum antibiotic used as therapeutics in severe infections in skin, soft tissues, and abdomen. It binds with the 16S rRNA and causes inhibition in the association of aminoacyl-tRNA. In tigecycline-resistant E. faecium, increased expressions of tet(M) and tet(L) genes were reported to confer tigecycline resistance (Fiedler et al. 2016).
18.4.3 Mechanism of Resistance to Antibiotics That Interfere in Central Dogma
18.4.3.1 Resistance to Quinolones
For the onset of cell division, starting of replication and transcription of DNA is important. Quinolones generally target two enzymes like DNA gyrase and topoisomerase IV. Those enzymes are responsible for the replication and transcription process. Administration of quinolones causes disruption of strand continuity, stopping replication process (Hawkey 2003). This antibacterial compound shows broad-spectrum effect on numerous bacteria by targeting the two said enzymes. Reduction of antibacterial activity of fluoroquinolones against Enterococci has also been reported (Oyamada et al. 2006). Though enterococci acquire low levels of quinolone resistance, sometimes it can also confer high-level resistance by several mechanisms (López et al. 2011; Werner et al. 2010; Yasufuku et al. 2011). Mutations in the gyrA and parC genes are responsible for the acquisition of resistance (in the case of levofloxacin and moxifloxacin) in E. faecium and E. faecalis (Tankovic et al. 1999; Kanematsu et al. 1998). EmeA and NorA like efflux pumps have also been reported for conferring the resistance of E. faecalis and E. faecium against quinolones, respectively (Hooper 2000). Another gene, qnr-encoded protein, is also responsible for the formation of quinolone-gyrase complex, protecting DNA gyrase, and in this way it confers resistance in Enterobacteriaceae (Arsène and Leclercq 2007; Tran et al. 2005).
18.4.3.2 Resistance to Rifampicin
Rifampicin binds with the β-subunit of DNA-dependent RNA polymerase and thus inhibits the process of transcription. Rifampicin-resistant E. faecium is developed due to substituted mutation in rpoB gene (H486Y) which encodes the said enzyme (Kristich and Little 2012). Moreover, rpoB-mutated E. feecium and E. faecalis show elevated resistance to cephalosporin (Enne et al. 2004; Rand et al. 2007).
18.4.3.3 Resistance to Trimethoprim and Sulfamethoxazole
Trimethoprim and sulfamethoxazole are the two notable antibacterial compounds that mainly target the enzymes associated with folate biosynthesis. Folate is synthesized from the p-amino benzoic acid and essential for synthesis of nucleic acids. The aforementioned compounds decrease the production of dihydrofolate and also blocked the conversion of tetrahydrofolate by inhibiting several enzymes in folate biosynthesis pathway. Though in vitro susceptibility is present, in vivo reports showed that these two antibiotics are ineffective against enterococci as they have gained the ability to utilize exogenous folate (Chenoweth et al. 1990; Grayson et al. 1990).
18.5 Alternative Strategies for Combating Multidrug-Resistant Enterococcus
The evolution of MDR enterococci has boosted interest towards alternative therapies to alleviate the disease causing potentiality of enterococci. Though virulence factors do not directly confer resistance, it will help bacteria to withstand in an unfavorable environmental condition and resist host defense mechanisms. Host bio-macromolecules associated with the cell surface of Enterococcus and release of these molecules into the extracellular matrix inhibit the antimicrobial drugs from reaching their targeted sites (Otto 2006). Cyclic-AMP (cAMP) as an important mediator of innate immune system imparts antimicrobial activity by disturbing PPG biosynthesis and cytoplasmic membrane structure (bacterial) as well as promotes autolysins which collectively help to keep microbial populations within threshold level. However, coevolution of cAMPs and their bacterial targets is well documented (Kandaswamy et al. 2013; Gilmore et al. 2013). Exploitation of host adaptive immunity is also targeted through vaccination for the production of antibodies against enterococci. In this context, the lipoteichoic acids and diheteroglycans present over the cell walls of enterococci are marked as an epitope as they will help to induce an antibody response. This will protect the host (mouse bacteremia model) against E. faecalis (Theilacker et al. 2011). Application of antibodies against those specific enterococcal antigenic motifs could be a possible therapeutic to combat MDR strains in the future.
18.6 Application of Nanotechnology Against Enterococcal Infections
Development of multidrug-resistant enterococci becomes a most pressing concern in community health worldwide. The WHO (World Health Organization) and CDC (Center for Disease Control) have already expressed major concern about the gradual increase in the formation of multidrug-resistant bacteria (Baptista et al. 2018). This has boosted researchers to develop potent strategies for drug delivery and, finally, targeting bacteria. Nanostructured materials (e.g., organic, inorganic, metallic, carbon nanotubes, etc.) are being synthesized to circumvent such types of drug resistance as they easily convey antimicrobials, assist novel drug delivery, exert antimicrobial activities, and inhibit biofilm development (Baptista et al. 2018).
Several attempts were made for the synthesis of potent nanoparticles and subsequent effective delivery of the same against multidrug-resistant enterococci (Katva et al. 2018). Silver is a nontoxic, safe antimicrobial inorganic agent, and silver nanoparticles (AgNPs) have obtained much more attention as compared to other metal-based nanoparticles due to its strong antimicrobial activity. AgNPs are the utmost encouraging inorganic nanoparticles that can be applied for the alleviation of enterococcal infections. It was demonstrated that AgNPs in combination with vancomycin exhibited excellent antibacterial potential against vancomycin-resistant E. faecalis. Likewise, a mixture of gentamycin, chloramphenicol, and AgNPs could be promising to treat MDR E. faecalis infection than both the above-mentioned antibiotics separately (Katva et al. 2018). The antibacterial efficiency of AgNPs was also evaluated by Wu et al. (2014) against E. faecalis biofilm. Otari et al. (2013) also showed the effect of AgNPs on the erythromycin-resistant E. faecalis. It was suggested that AgNPs inhibit bacterial growth and proliferation by adhering on the cell wall of bacteria, leading to cell wall modification followed by penetration of AgNPs into the bacterial cell, which consequently damages the DNA leading to cell death (Aziz et al. 2015, 2016; Kumar et al. 2016; Saini et al. 2019).
Khiralla and El-Deeb (2015) developed biogenic spherical selenium nanoparticles using cell-free supernatant of Bacillus licheniformis which imparted paramount antimicrobial and antibiofilm potential against E. faecalis. Likewise, biogenic palladium nanoparticles were prepared by using flower extract of Moringa oleifera which showed significant antibacterial effect against the same bacteria (Anand et al. 2016).
Graphene oxide (GO) has unique physicochemical characteristics and has therefore attracted attention for antibacterial use (Hu 2010). The GO nanosheets exhibit antibacterial activity through direct interaction with bacteria and increased the reactive oxygen species (ROS) level within the cell (Akhavan and Ghaderi 2010). Govindaraju et al. (2016) demonstrated that UV-irradiated form of glucosamine-gold nanoparticle-graphene oxide composite exhibited paramount antimicrobial activity against E. faecalis which is better than kanamycin, and several functional groups (like carboxyl, hydroxyl, and epoxy) present in the GO-based nanomaterial are responsible for the activity. Nanocomposite of indocyanine green and GO was also reported to exhibit potential antibacterial effect against E. faecalis during photodynamic therapy (Akbari et al. 2017).
In order to treat vancomycin-resistant Enterococcus(VRE) , Zhou et al. (2018) prepared Au/Ag bimetallic NPs and demonstrated that it has immense potential to be a good anti-enterococcal agent. Both in vitro (bacterial surface-enhanced Raman scattering imaging) and in vivo (mouse infection assays) results clearly revealed the effectiveness of this newly developed nanocomposite against VRE.
Chifiriuc et al. (2013) also investigated the capability of magnetic nanoparticle for a sustained and controlled release of drug which subsequently increases the effectiveness of antibiotics against resistant opportunistic pathogen, E. faecalis. They also suggested that magnetic nanoparticles might be a potent carrier for delivery of amino-glucoside antibiotics.
The antibacterial efficacy of calcium hydroxide nanoparticle (NCH) showed better result against E. faecalis in dentin block model. The MIC determination and agar diffusion test revealed that low concentration of the NCH inhibited E. faecalis than the native form of calcium hydroxide which is due to the enhancement of surface area due to smaller size which encourages the penetration of the NPs into the deeper layers of dentin which subsequently inhibits E. faecalis growth (Dianat et al. 2015).
Despite the expected potential of newly reported nanoparticles against multidrug-resistant Enterococcus, there are still few shortfall related to their safety when they are used in long-term basis in human. Therefore, in-depth assessment of the physical, chemical, and biological compatibility must be addressed. Experimental proof is also desirable for establishment of mechanism of action against the targeted enterococci in vivo. Moreover, the fruitful translation of the R&D work into real-life large-scale production of the newly discovered nanoparticles needs comprehensive guidelines, and effort is needed.
18.7 Conclusions and Future Challenges
Enterococcal species can colonize and survive in different biological and environmental niches. Owing to their biofilm-forming ability, multiple-drug resistance, and tendency of transfer of resistant trait to other enterococci, it became a great burden in healthcare sectors. Among the several species of enterococci, E. faecalis and E. faecium are associated with most clinical cases and hence they are marked as important nosocomial pathogens. Continuous exposure to prophylactic or metaphylactic and random application of antimicrobial agents by clinicians in human and animal hosts against enterococci contributed its ability to acquire and develop unique profiles of virulence and antimicrobial drug resistance. Moreover, expression of a wide variety of virulence characteristics promotes enterococci to colonize and also causes infections in the host body. Extensive tolerance to the antibacterial agents as well as their wondrous capacity to acquire resistance to marketed antibiotics becomes a great challenge to clinicians throughout the globe to combat with enterococcal pathogenesis. In the recent future, MDR enterococci will be immense clinical challenges to treat infections in hospitalized patients. Current trends in the epidemiology and population structure of antibiotic-resistant Enterococcus species clearly suggest that MDR enterococci may become the most common species isolated from patients in the upcoming eons. Nanotechnology is an emerging branch of science which could restrict the propagation of enterococci. Various attempts were already made worldwide to develop versatile nanomaterials that exhibited immense potentiality to limit enterococcal growth in in vitro and in vivo. However, with the advent and advancement of nanotechnology, more studies are extremely necessary to develop comprehensive strategies to limit the Enterococcus-associated infections and their large-scale implementation in upcoming eons.
References
Ahmed MO, Baptiste KE (2018) Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb Drug Resist 24:590–606. https://doi.org/10.1089/mdr.2017.0147
Akbari T, Pourhajibagher M, Hosseini F et al (2017) The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn Photodyn Ther 20:148–153. https://doi.org/10.1016/j.pdpdt.2017.08.017
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4:5731–5736. https://doi.org/10.1021/nn101390x
Ali L, Goraya M, Arafat Y et al (2017) Molecular mechanism of quorum-sensing in Enterococcus faecalis: its role in virulence and therapeutic approaches. Int J Mol Sci 18:960. https://doi.org/10.3390/ijms18050960
Almohamad S, Somarajan SR, Singh KV et al (2014) Influence of isolate origin and presence of various genes on biofilm formation by Enterococcus faecium. FEMS Microbiol Lett 353:151–156. https://doi.org/10.1111/1574-6968.12418
Anand K, Tiloke C, Phulukdaree A et al (2016) Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. J Photochem Photobiol B Biol 165:87–95. https://doi.org/10.1016/j.jphotobiol.2016.09.039
Anderson DJ, Murdoch DR, Sexton DJ, Reller LB (2004) Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 32:72–77. https://doi.org/10.1007/s15010-004-2036-1
Arbeloa A, Segal H, Hugonnet J-E et al (2004) Role of class A penicillin-binding proteins in PBP5-mediated-lactam resistance in Enterococcus faecalis. J Bacteriol 186:1221–1228. https://doi.org/10.1128/JB.186.5.1221-1228.2004
Arias CA, Murray BE (2012) The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. https://doi.org/10.1038/nrmicro2761
Arsène S, Leclercq R (2007) Role of a qnr-like gene in the intrinsic resistance of Enterococcus faecalis to fluoroquinolones. Antimicrob Agents Chemother 51:3254–3258. https://doi.org/10.1128/AAC.00274-07
Arthur M, Courvalin P (1993) Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother 37:1563–1571. https://doi.org/10.1128/AAC.37.8.1563
Arthur M, Molinas C, Depardieu F, Courvalin P (1993) Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 175:117–127. https://doi.org/10.1128/jb.175.1.117-127.1993
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605−11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Baddour LM, Wilson WR, Bayer AS et al (2005) Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. https://doi.org/10.1161/CIRCULATIONAHA.105.165564
Baptista PV, McCusker MP, Carvalho A et al (2018) Nano-strategies to fight multidrug resistant bacteria—“A battle of the titans”. Front Microbiol 9:1441. https://doi.org/10.3389/fmicb.2018.01441
Boumghar-Bourtchaï L, Dhalluin A, Malbruny B et al (2009) Influence of recombination on development of mutational resistance to linezolid in Enterococcus faecalis JH2-2. Antimicrob Agents Chemother 53:4007–4009. https://doi.org/10.1128/AAC.01633-08
Bourgeois-Nicolaos N, Massias L, Couson B et al (2007) Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J Infect Dis 195:1480–1488. https://doi.org/10.1086/513876
Bozdogan B, Berrezouga L, Kuo MS et al (1999) A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob Agents Chemother 43:925–929. https://doi.org/10.1128/AAC.43.4.925
Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311:1113–1116. https://doi.org/10.1126/science.1121357
Cavassin ED, de Figueiredo LF, Otoch JP et al (2015) Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J Nanobiotechnol 13(1): 64. https://doi.org/10.1186/s12951-015-0120-6
Ch’ng JH, Chong KK, Lam LN et al (2019) Biofilm-associated infection by enterococci. Nat Rev Microbiol 17:124–124. https://doi.org/10.1038/s41579-018-0128-7
Chandler JR, Dunny GM (2008) Characterization of the sequence specificity determinants required for processing and control of sex pheromone by the intramembrane protease Eep and the plasmid-encoded protein PrgY. J Bacteriol 190:1172–1183. https://doi.org/10.1128/JB.01327-07
Chang S, Sievert D, Hageman J et al (2003) Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 348:1342–1347. https://doi.org/10.1056/NEJMoa025025
Chen H, Wu W, Ni M et al (2013) Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents 42:317–321. https://doi.org/10.1016/j.ijantimicag.2013.06.008
Chen Y, Bandyopadhyay A, Kozlowicz BK et al (2017) Mechanisms of peptide sex pheromone regulation of conjugation in Enterococcus faecalis. Microbiology 6:e00492. https://doi.org/10.1002/mbo3.492
Chenoweth C, Robinson K, Schaberg D (1990) Efficacy of ampicillin versus trimethoprim sulfamethoxazole in a mouse model of lethal enterococcal peritonitis. Antimicrob Agents Chemother 34:1800–1802. https://doi.org/10.1128/AAC.34.9.1800
Chifiriuc MC, Grumezescu AM, Andronescu E et al (2013) Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 22:14–19. https://doi.org/10.1016/j.anaerobe.2013.04.013
Chow J, Zervos M, Lerner S et al (1997) A novel gentamicin resistance gene in Enterococcus. Antimicrob Agents Chemother 41:511–514
Comenge Y, Quintiliani R Jr, Li L et al (2003) The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J Bacteriol 185:7184–7192. https://doi.org/10.1128/jb.185.24.7184-7192.2003
Cook LC, Federle MJ (2014) Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol Rev 38(3):473–492. https://doi.org/10.1111/1574-6976.12046
Cook L, Chatterjee A, Barnes A et al (2011) Biofilm growth alters regulation of conjugation by a bacterial pheromone. Mol Microbiol 81:1499–1510. https://doi.org/10.1111/j.1365-2958.2011.07786.x
Costa Y, Galimand M, Leclercq R et al (1993) Characterization of the chromosomal aac (6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother 37:1896–1903. https://doi.org/10.1128/aac.37.9.1896
Costerton JW (2001) Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol 9:50–52. https://doi.org/10.1016/S0966-842X(00)01918-1
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. https://doi.org/10.1126/science.284.5418.1318
Coudron PE, Markowitz SM, Wong ES (1992) Isolation of a betalactamase-producing, aminoglycoside-resistant strain of Enterococcus faecium. Antimicrob Agents Chemother 36:1125–1126. https://doi.org/10.1128/AAC.36.5.1125
Courvalin P (2006) Vancomycin resistance in Gram-positive cocci. Clin Infect Dis 42(Supplement_1):S25–S34. https://doi.org/10.1086/491711
Courvalin P, Carlier C, Collatz E (1980) Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol 143:541–551. https://doi.org/10.1128/aac.13.5.716
Dautle MP, Wilkinson TR, Gauderer MW (2003) Isolation and identification of biofilm microorganisms from silicone gastrostomy devices. J Pediatr Surg 38:216–220. https://doi.org/10.1053/jpsu.2003.50046
Depardieu F, Mejean V, Courvalin P (2015) Competition between VanU(G) repressor and VanR(G) activator leads to rheostatic control of vanG vancomycin resistance operon expression. PLoS Genet 11:e1005170. https://doi.org/10.1371/journal.pgen.1005170
Devriese LA, Pot B, Van Damme L, Kersters K, Haesebrouck F (1994) Identification of Enterococcus species isolated from foods of animal origin. Int J Food Microbiol 26:187–197. https://doi.org/10.1016/0168-1605(94)00119-Q
Dianat O, Saedi S, Kazem M, Alam M (2015) Antimicrobial activity of nanoparticle calcium hydroxide against Enterococcus faecalis: an in vitro study. Iran Endod J 10:39
Diaz L, Kiratisin P, Mendes R et al (2012) Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 56:3917–3922. https://doi.org/10.1128/AAC.00419-12
Diaz L, Kontoyiannis DP, Panesso D et al (2013) Dissecting the mechanisms of linezolid resistance in a Drosophila melanogaster infection model of Staphylococcus aureus. J Infect Dis 208:83–91. https://doi.org/10.1093/infdis/jit138
Distel JW, Hatton JF, Gillespie MJ (2002) Biofilm formation in medicated root canals. J Endod 28:689–693. https://doi.org/10.1097/00004770-200210000-00003
Dowidar N, Moesgaard F, Matzen P (1991) Clogging and other complications of endoscopic biliary endoprostheses. Scand J Gastroenterol 26:1132–1136. https://doi.org/10.3109/00365529108998604
Dunny GM, Hancock LE, Shankar N (2014) In: Gilmore MS (ed) Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston
Eliopoulos GM, Farber BF, Murray BE et al (1984) Ribosomal resistance of clinical enterococcal to streptomycin isolates. Antimicrob Agents Chemother 25:398–399. https://doi.org/10.1128/AAC.25.3.398
Emori TG, Gaynes RP (1993) An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 6:428–442
Enne V, Delsol A, Roe J et al (2004) Rifampicin resistance and its fitness cost in Enterococcus faecium. J Antimicrob Chemother 53:203–207. https://doi.org/10.1093/jac/dkh044
Fabretti F, Theilacker C, Baldassarri L et al (2006) Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect Immun 74:4164–4171. https://doi.org/10.1128/IAI.00111-06
Facklam RR (1973) Comparison of several laboratory media for presumptive identification of enterococci and group D streptococci. J App Microbiol 26(2):138–145
Fiedler S, Bender JK, Klare I et al (2016) Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet(L) and tet(M). J Antimicrob Chemother 71:871–881. https://doi.org/10.1093/jac/dkv420
Galimand M, Schmitt E, Panvert M et al (2011) Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase Efm M. RNA 17:251–262. https://doi.org/10.1261/rna.2233511
García-Solache M, Rice LB (2019) The enterococcus: a model of adaptability to its environment. Clin Microbiol Rev 32(2):e00058–e00018. https://doi.org/10.1128/CMR.00058-18
Gilmore M, Lebreton F, Van Tyne D (2013) Dual defensin strategy for targeting Enterococcus faecalis. Proc Natl Acad Sci U S A 110:19980–19981. https://doi.org/10.1073/pnas.1319939110
Govindaraju S, Samal M, Yun K (2016) Superior antibacterial activity of GlcN-AuNP-GO by ultraviolet irradiation. Mater Sci Eng C 69:366–372. https://doi.org/10.1016/j.msec.2016.06.052
Graninger W, Ragette R (1992) Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis 15:49–57. https://doi.org/10.1093/clinids/15.1.49
Grayson M, Thauvin-Eliopoulos C, Eliopoulos G et al (1990) Failure of trimethoprim-sulfamethoxazole therapy in experimental enterococcal endocarditis. Antimicrob Agents Chemother 34:1792–1794. https://doi.org/10.1128/AAC.34.9.1792
Guiton PS, Hung CS, Kline KA et al (2009) Contribution of autolysin and sortase a during Enterococcus faecalis DNA dependent biofilm development. Infect Immun 77:3626–3638. https://doi.org/10.1128/IAI.00219-09
Hall CL, Tschannen M, Worthey EA et al (2013) IreB, a Ser/Thr kinase substrate, influences antimicrobial resistance in Enterococcus faecalis. Antimicrob Agents Chemother 57:6179–6186. https://doi.org/10.1128/AAC.01472-13
Hammerum AM (2012) Enterococci of animal origin and their significance for public health. Clin Microbiol Infect 18:619–625. https://doi.org/10.1111/j.1469-0691.2012.03829.x
Hawkey P (2003) Mechanisms of quinolone action and microbial response. J Antimicrob Chemother 51:29–35. https://doi.org/10.1093/jac/dkg207
Heikens E, Singh KV, Jacques-Palaz KD et al (2011) Contribution of the enterococcal surface protein Esp to pathogenesis of Enterococcus faecium endocarditis. Microbes Infect 13:1185–1190. https://doi.org/10.1016/j.micinf.2011.08.006
Hendrickx AP, van Luit-Asbroek M, Schapendonk CM et al (2009) SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect Immun 77:5097–5106. https://doi.org/10.1128/IAI.00275-09
Hidron AI, Edwards JR, Patel J et al (2008) NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. https://doi.org/10.1086/591861
Higuita NIA, Huycke MM (2014) Enterococcal disease, epidemiology, and implications for treatment. In: Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]. Massachusetts Eye and Ear Infirmary, Boston
Hirt H, Greenwood-Quaintance KE, Karau MJ et al (2018) Enterococcus faecalis sex pheromone cCF10 enhances conjugative plasmid transfer in vivo. MBio 9:e00037–e00018. https://doi.org/10.1128/mBio.00037-18
Hollenbeck BL, Rice LB (2012) Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 3:421–433. https://doi.org/10.4161/viru.21282
Hooper D (2000) Mechanisms of action and resistance of older and newer fluoroquinolones. Clin Infect Dis 31:S24–S28. https://doi.org/10.1086/314056
Hu W (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323. https://doi.org/10.1021/nn101097v
Joyanes P, Pascual A, Martinez-Martinez L et al (2000) In vitro adherence of Enterococcus faecalis and Enterococcus faecium to urinary catheters. Eur J Clin Microbiol Infect Dis 19:124–127. https://doi.org/10.1111/j.1469-0691.1999.tb00160.x
Jureen R, Top J, Mohn SC et al (2003) Molecular characterization of ampicillin-resistant Enterococcus faecium isolates from hospitalized patients in Norway. J Clin Microbiol 41:2330–2336. https://doi.org/10.1128/JCM.41.6.2330-2336.2003
Kandaswamy K, Liew T, Wang C et al (2013) Focal targeting by human β-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. Proc Natl Acad Sci U S A 110:20230–20235. https://doi.org/10.1073/pnas.1319066110
Kanematsu E, Deguchi T, Yasuda M et al (1998) Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of DNA topoisomerase IV associated with quinolone resistance in Enterococcus faecalis. Antimicrob Agents Chemother 42:433–435. https://doi.org/10.1128/AAC.42.2.433
Katva S, Das S, Moti HS et al (2018) Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Pharmacogn Mag 13:S828–S833. https://doi.org/10.4103/pm.pm_120_17
Keane PF, Bonner MC, Johnston SR et al (1994) Characterization of biofilm and encrustation on ureteric stents in vivo. Br J Urol 73:687–691. https://doi.org/10.1111/j.1464-410X.1994.tb07557.x
Kehrenberg C, Schwarz S, Jacobsen L et al (2005) A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol 57:1064–1073. https://doi.org/10.1111/j.1365-2958.2005.04754.x
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci Technol 63:1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
Klein G (2003) Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro intestinal tract. Int J Food Microbiol 88:123–131. https://doi.org/10.1016/S0168-1605(03)00175-2
Klibi N, Sáenz Y, Zarazaga M et al (2008) Polymorphism in pbp5 gene detected in clinical Enterococcus faecium strains with different ampicillin MICs from a Tunisian hospital. J Chemother 20:436–440. https://doi.org/10.1179/joc.2008.20.4.436
Kobayakawa S, Jett BD, Gilmore MS (2005) Biofilm formation by Enterococcus faecalis on intraocular lens material. Curr Eye Res 30:741–745. https://doi.org/10.1080/02713680591005959
Krasteva PV, Giglio KM, Sondermann H (2012) Sensing the messenger: the diverse ways that bacteria signal through c-di-GMP. Protein Sci 21:929–948. https://doi.org/10.1002/pro.2093
Kristich C, Little J (2012) Mutations in the β subunit of RNA polymerase alter intrinsic cephalosporin resistance in Enterococci. Antimicrob Agents Chemother 56:2022–2027. https://doi.org/10.1128/AAC.06077-11
Kristich CJ, Wells CL, Dunny GM (2007) A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci U S A 10:3508–3508. https://doi.org/10.1073/pnas.0608742104
Kumar NI, Das SA, Jyoti AN, Kaushik SA (2016) Synergistic effect of silver nanoparticles with doxycycline against Klebsiella pneumoniae. Int J Pharm Pharm Sci 8:183–186
Leach K, Swaney S, Colca J et al (2007) The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol Cell 26:393–402. https://doi.org/10.1016/j.molcel.2007.04.005
Lebreton F, Van Schaik W, Sanguinetti M et al (2012) AsrR is an oxidative stress sensing regulator modulating Enterococcus faecium opportunistic traits, antimicrobial resistance, and pathogenicity. PLoS Pathog 8:e1002834. https://doi.org/10.1371/journal.ppat.1002834
Lebreton F, van Schaik W, McGuire AM et al (2013) Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio 4:e00534–e00513. https://doi.org/10.1128/mBio.00534-13
Lebreton F, Willems RJ, Gilmore MS (2014) Enterococcus diversity, origins in nature, and gut colonization. In: Enterococci: from commensals to leading causes of drug resistant infection [Internet]. Massachusetts Eye and Ear Infirmary, Boston
Leclercq R, Dutka-Malen S, Duval J, Courvalin P (1992) Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother 36:2005–2008. https://doi.org/10.1128/aac.36.9.2005
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. https://doi.org/10.1128/AAC.45.4.999-1007.2001
Li YH, Tian X (2012) Quorum sensing and bacterial social interactions in biofilms. Sensors 12:2519–2538. https://doi.org/10.3390/s120302519
Lim SY, Teh CSJ, Thong KL (2017) Biofilm-related diseases and omics: global transcriptional profiling of Enterococcus faecium reveals different gene expression patterns in the biofilm and planktonic cells. OMICS 21:592–602. https://doi.org/10.1089/omi.2017.0119
Locke JB, Hilgers M, Shaw KJ (2009) Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob Agents Chemother 53:5275–5278. https://doi.org/10.1128/AAC.01032-09
López M, Tenorio C, Del Campo R et al (2011) Characterization of the mechanisms of fluoroquinolone resistance in vancomycin-resistant enterococci of different origins. J Chemother 23:87–91. https://doi.org/10.1179/joc.2011.23.2.87
MacCallum WG, Hastings TW (1899) A case of acute endocarditis caused by Micrococcus zymogenes (nov. spec.), with a description of the microorganism. J Exp Med 4:521–534. https://doi.org/10.1084/jem.4.5-6.521
Maki DG, Agger WA (1988) Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine 67:248–269
Malani PN, Kauffman CA, Zervos MJ (2002) Enterococcal disease, epidemiology, and treatment. In: Gilmore MS, Clewell DB, Courvalin P, Dunny GM, Murray BE, Rice LB (eds) The Enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC, pp 385–408
Marshall S, Donskey C, Hutton-Thomas R et al (2002) Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother 46:3334–3336. https://doi.org/10.1128/AAC.46.10.3334-3336.2002
McDonald LC, Kuehnert MJ, Tenover FC, Jarvis WR (1997) Vancomycin-resistant enterococci outside the health-care setting: prevalence, sources, and public health implications. Emerg Infect Dis 3:311. https://doi.org/10.3201/eid0303.970307
McDonald JR, Olaison L, Anderson DJ, Hoen B, Miro JM, Eykyn S et al (2005) Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am J Med 118:759–766. https://doi.org/10.1016/j.amjmed.2005.02.020
Mendes R, Deshpande L, Castanheira M et al (2008) First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother 52:2244–2246. https://doi.org/10.1128/AAC.00231-08
Miller WR, Munita JM, Arias CA (2014) Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti-Infect Ther 12:1221–1236. https://doi.org/10.1586/14787210.2014.956092
Miller WR, Bayer AS, Arias CA (2016) Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb Perspect Med 6:a026997. https://doi.org/10.1101/cshperspect.a026997
Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, Guan Z, Arias CA (2012) Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS One 7:e43958. https://doi.org/10.1371/journal.pone.0043958
Mohamed JA, Huang DB (2007) Biofilm formation by enterococci. J Med Microbiol 56:1581–1588. https://doi.org/10.1099/jmm.0.47331-0
Mohamed JA, Teng F, Nallapareddy SR, Murray BE (2006) Pleiotrophic effects of 2 Enterococcus faecalis sagA-like genes, salA and salB, which encode proteins that are antigenic during human infection, on biofilm formation and binding to collagen type I and fibronectin. J Infect Dis 193:231–240. https://doi.org/10.1086/498871
Monds RD, O’Toole GA (2009) The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol 17:73–87. https://doi.org/10.1016/j.tim.2008.11.001
Montealegre MC, Singh KV, Somarajan SR et al (2016a) Role of the Emp pilus subunits of Enterococcus faecium in biofilm formation, adherence to host extracellular matrix components, and experimental infection. Infect Immun 84:1491–1500. https://doi.org/10.1128/IAI.01396-15
Montealegre MC, Roh JH, Rae M et al (2016b) Differential penicillin-binding protein 5 (PBP5) levels in the Enterococcus faecium clades with different levels of ampicillin resistance. Antimicrob Agents Chemother 61:e02034–e02016. https://doi.org/10.1128/AAC.02034-16
Muller C, Le Breton Y, Morin T et al (2006) The response regulator CroR modulates expression of the secreted stress-induced SalB protein in Enterococcus faecalis. J Bacteriol 188:2636–2645. https://doi.org/10.1128/JB.188.7.2636-2645.2006
Murray BE (1990) The life and times of the Enterococcus. Clin Microbiol Rev 3:46–65. https://doi.org/10.1128/cmr.3.1.46
Murray BE (1992) Beta-lactamase-producing enterococci. Antimicrob Agents Chemother 36:2355–2359. https://doi.org/10.1128/aac.36.11.2355
Murray BE, Lopardo HA, Rubeglio EA et al (1992) Intrahospital spread of a single gentamicin-resistant, beta-lactamase producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother 36:230–232. https://doi.org/10.1128/AAC.36.1.230
Nallapareddy SR, Singh KV, Sillanpää J et al (2006) Endocarditis and biofilm associated pili of Enterococcus faecalis. J Clin Invest 116:2799–2807. https://doi.org/10.1172/JCI29021
Nallapareddy SR, Singh KV, Sillanpaa J et al (2011a) Relative contributions of Ebp Pili and the collagen adhesin ace to host extracellular matrix protein adherence and experimental urinary tract infection by Enterococcus faecalis OG1RF. Infect Immun 79:2901–2910. https://doi.org/10.1128/IAI.00038-11
Nallapareddy SR, Sillanpää J, Mitchell J et al (2011b) Conservation of Ebp-type pilus genes among Enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets. Infect Immun 79:2911–2920. https://doi.org/10.1128/IAI.00039-11
Nielsen HV, Guiton PS, Kline KA et al (2012) The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. MBio 3:e00177–e00112. https://doi.org/10.1128/mBio.00177-12
Nielsen HV, Flores-Mireles AL, Kau AL et al (2013) Pilin and sortase residues critical for endocarditis- and biofilm-associated pilus biogenesis in Enterococcus faecalis. J Bacteriol 195:4484–4495. https://doi.org/10.1128/JB.00451-13
Noskin GA, Cooper I, Peterson LR (1995a) Vancomycin-resistant Enterococcus faecium sepsis following persistent colonization. JAMA Intern Med 155:1445–1447. https://doi.org/10.1001/archinte.1995.00430130139015
Noskin GA, Stosor V, Cooper I, Peterson LR (1995b) Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 16:577–581. https://doi.org/10.2307/30141097
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Otari SV, Patil RM, Waghmare SR et al (2013) A novel microbial synthesis of catalytically active Ag–alginate biohydrogel and its antimicrobial activity. Dalt Trans 42:9966–9975. https://doi.org/10.1039/C3DT51093J
Otto M (2006) Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol 306:251–258
Oyamada Y, Ito H, Fujimoto K et al (2006) Combination of known and unknown mechanisms confers high-level resistance to fluoroquinolones in Enterococcus faecium. J Med Microbiol 55:729–736. https://doi.org/10.1099/jmm.0.46303-0
Paganelli FL, Willems RJ, Jansen P et al (2013) Enterococcus faecium biofilm formation: identification of major autolysin AtlAEfm, associated Acm surface localization, and AtlAEfm independent extracellular DNA Release. MBio 4:e00154. https://doi.org/10.1128/mBio.00154-13
Patterson JE, Sweeney AH, Simms M et al (1995) An analysis of 100 serious enterococcal infections: epidemiology, antibiotic susceptibility, and outcome. Medicine 74:191–200. https://doi.org/10.1097/00005792-199507000-00003
Poeta P, Costa D, Igrejas G et al (2007) Polymorphisms of the pbp5 gene and correlation with ampicillin resistance in Enterococcus faecium isolates of animal origin. J Med Microbiol 56:236–240. https://doi.org/10.1099/jmm.0.46778-0
Portillo A, Ruiz-Larrea F, Zarazaga M et al (2000) Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother 44:967–971. https://doi.org/10.1128/aac.44.4.967-971.2000
Prabaker K, Weinstein RA (2011) Trends in antimicrobial resistance in intensive care units in the United States. Curr Opin Crit Care 17:472–479. https://doi.org/10.1097/MCC.0b013e32834a4b03
Rand K, Houck H, Silverman J et al (2007) Daptomycin-reversible rifampicin resistance in vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother 59:1017–1020. https://doi.org/10.1093/jac/dkm045
Ray A, Pultz N, Bhalla A et al (2003) Coexistence of vancomycin-resistant enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin Infect Dis 37:875–881. https://doi.org/10.1086/377451
Reid KC, Cockerill FR III, Patel R (2001) Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteremia: a report of 20 cases. Clin Infect Dis 32:1540–1546. https://doi.org/10.1086/320542
Rice LB (1998) Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob Agents Chemother 42:1871–1877. https://doi.org/10.1128/AAC.42.8.1871
Rice LB, Murray BE (1995) β-lactamase-producing enterococci. In: Brown F, Ferretti JJ (eds) Genetics of streptococci, enterococci and lactococci, Developmental and biological standards, vol 85. Karger, Basel, Switzerland
Rice LB, Calderwood SB, Eliopoulos GM et al (1991) Enterococcal endocarditis: a comparison of prosthetic and native valve disease. Rev Infect Dis 13:1–7. https://doi.org/10.1093/clinids/13.1.1
Rice LB, Bellais S, Carias LL et al (2004) Impact of specific pbp5 mutations on expression of β-lactam resistance in Enterococcus faecium. Antimicrob Agents Chemother 48:3028–3032. https://doi.org/10.1128/AAC.48.8.3028-3032.2004
Rice LB, Carias LL, Rudin S et al (2009) Role of class A penicillin-binding proteins in the expression of beta-lactam resistance in Enterococcus faecium. J Bacteriol 191:3649–3656. https://doi.org/10.1128/JB.01834-08
Robbins WC, Tompsett R (1951) Treatment of enterococcal endocarditis and bacteremia: results of combined therapy with penicillin and streptomycin. Am J Med 10:278–299. https://doi.org/10.1016/0002-9343(51)90273-2
Rozdzinski E, Marre R, Susa M et al (2001) Aggregation substance mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins. Microb Pathog 30:211–220. https://doi.org/10.1006/mpat.2000.0429
Rybkine T, Mainardi J-L, Sougakoff W et al (1998) Penicillin-binding protein 5 sequence alterations in clinical isolates of Enterococcus faecium with different levels of β-lactam resistance. J Infect Dis 178:159–163. https://doi.org/10.1086/515605
Saeb ATM, Alshammari AS, Al-brahim H, Al-rubeaan KA (2014) Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci World J 2:704708. https://doi.org/10.1155/2014/704708
Saini MK, Das S, Moti HS et al (2019) Biogenic synthesis and characterization of silver nanoparticles from bacteria isolated from garden soil and its antibacterial activity against Enterococcus faecalis. Int J Res Pharm Sci 10:21–26. https://doi.org/10.26452/ijrps.v10i1.1774
Sandoe JA, Witherden IR, Cove JH et al (2003) Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J Med Microbiol 52:547–550. https://doi.org/10.1099/jmm.0.05201-0
Sassi M, Guerin F, Lesec L et al (2018) Genetic characterization of a VanG-type vancomycin-resistant Enterococcus faecium clinical isolate. J Antimicrob Chemother 73:852–855. https://doi.org/10.1093/jac/dkx510
Sava IG, Heikens E, Huebner J (2010) Pathogenesis and immunity in enterococcal infections. Clin Microbiol Infect 16:533–540. https://doi.org/10.1111/j.1469-0691.2010.03213.x
Shao C, Shang W, Yang Z et al (2012) LuxS-dependent AI-2 regulates versatile functions in Enterococcus faecalis V583. J Proteome Res 11:4465–4475. https://doi.org/10.1021/pr3002244
Shinabarger D, Marotti K, Murray R et al (1997) Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother 41:2132–2136. https://doi.org/10.1128/AAC.41.10.2132
Shlaes DM, Bouvet A, Devine C et al (1989) Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother 33:198–203. https://doi.org/10.1128/AAC.33.2.198
Sillanpaa J, Nallapareddy SR, Prakash VP et al (2008) Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology 154:3199–3211. https://doi.org/10.1099/mic.0.2008/017319-0
Sillanpaa J, Nallapareddy SR, Singh KV et al (2010) Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence 1:236–246. https://doi.org/10.4161/viru.1.4.11966
Singh K, Weinstock G, Murray B (2002) An Enterococcus faecalis ABC homologue (Lsa) is required for the resistance of this species to clindamycin and quinupristin-dalfopristin. Antimicrob Agents Chemother 46:1845–1850. https://doi.org/10.1128/AAC.46.6.1845-1850.2002
Singh KV, Nallapareddy SR, Murray BE (2007) Importance of the ebp (endocarditis- and biofilm associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis 195:1671–1677. https://doi.org/10.1086/517524
Speer BS, Shoemaker NB, Salyers AA (1992) Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev 5:387–399. https://doi.org/10.1128/cmr.5.4.387
Sussmuth SD, Muscholl-Silberhorn A, Wirth R et al (2000) Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect Immun 68:4900–4906. https://doi.org/10.1128/iai.68.9.4900-4906.2000
Tankovic J, Bachoual R, Ouabdesselam S et al (1999) In-vitro activity of moxifloxacin against fluoroquinolone-resistant strains of aerobic Gram-negative bacilli and Enterococcus faecalis. J Antimicrob Chemother 43:19–23. https://doi.org/10.1093/jac/43.suppl_2.19
Theilacker C, Sanchez-Carballo P, Toma I et al (2009) Glycolipids are involved in biofilm accumulation and prolonged bacteraemia in Enterococcus faecalis. Mol Microbiol 71:1055–1069. https://doi.org/10.1111/j.1365-2958.2009.06587.x
Theilacker C, Kaczyński Z, Kropec A et al (2011) Serodiversity of opsonic antibodies against Enterococcus faecalis – glycans of the cell wall revisited. PLoS One 6:e17839. https://doi.org/10.1371/journal.pone.0017839
Thiercelin ME (1899) Morphology and modes of reproduction of the enterococcus. Min Proc Soc Biol Its Affiliates 11:551–553
Toh S, Xiong L, Arias C et al (2007) Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 64:1506–1514. https://doi.org/10.1111/j.1365-2958.2007.05744.x
Tran J, Jacoby G, Hooper D (2005) Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob Agents Chemother 49:118–125. https://doi.org/10.1128/AAC.49.1.118-125.2005
van SW, Willems RJ (2010) Genome-based insights into the evolution of enterococci. Clin Microbiol Infect 16:527–532. https://doi.org/10.1111/j.1469-0691.2010.03201.x
Vester B (2018) The cfr and cfr-like multiple resistance genes. Res Microbiol 169:61–66. https://doi.org/10.1016/j.resmic.2017.12.003
Wang Y, Lv Y, Cai J et al (2015) A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. https://doi.org/10.1093/jac/dkv116
Werner G, Fleige C, Ewert B et al (2010) High-level ciprofloxacin resistance among hospital-adapted Enterococcus faecium (CC17). Int J Antimicrob Agents 35:119–125. https://doi.org/10.1016/j.ijantimicag.2009.10.012
Wilson WR, Wikowske CJ, Wright AJ et al (1984) Treatment of streptomycin-susceptible and streptomycin-resistant enterococcal endocarditis. Ann Intern Med 100:816–823. https://doi.org/10.7326/0003-4819-100-6-816
Wu D, Fan W, Kishen A et al (2014) Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod 40:285–290. https://doi.org/10.1016/j.joen.2013.08.022
Xu Y, Singh KV, Qin X et al (2000) Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect Immun 68:815–823. https://doi.org/10.1128/iai.68.2.815-823.2000
Yasufuku T, Shigemura K, Shirakawa T et al (2011) Mechanisms of and risk factors for fluoroquinolone resistance in clinical Enterococcus faecalis isolates from patients with urinary tract infections. J Clin Microbiol 49:3912–3916. https://doi.org/10.1128/JCM.05549-11
Zhou Z, Peng S, Sui M et al (2018) Multifunctional nanocomplex for surface-enhanced Raman scattering imaging and near-infrared photodynamic antimicrobial therapy of vancomycin-resistant bacteria. Colloids Surf B Biointerfaces 161:394–402. https://doi.org/10.1016/j.colsurfb.2017.11.005
Zorzi W, Zhou XY, Dardenne O et al (1996) Structure of the low-affinity penicillin-binding protein5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J Bacteriol 178:4948–4957. https://doi.org/10.1128/jb.178.16.4948-4957.1996
Acknowledgments
The authors are grateful to the Department of Science and Technology and Biotechnology, Govt. of West Bengal, India, for the financial assistance (Memo No: 532/(Sanc.)\ST/P/S&T/2G-48/2018 dated: 27/03/2019). The first author is also thankful to the Department of Physiology, Midnapore College (Autonomous), West Bengal, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Banik, A., Halder, S.K., Ghosh, C., Mondal, K.C. (2020). Enterococcal Infections, Drug Resistance, and Application of Nanotechnology. In: Prasad, R., Siddhardha, B., Dyavaiah, M. (eds) Nanostructures for Antimicrobial and Antibiofilm Applications. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-40337-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-40337-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40336-2
Online ISBN: 978-3-030-40337-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)