Abstract
An epiphyte is a nonparasitic plant that dwells on another plant and has been well studied in terrestrial plants. However, in the marine ecosystem, these epiphytes thrive on algal thallus for their support and growth, and their infestation has a prime economic impediment in commercial cultivation. They usually belong to various groups, namely, bacteria, fungi, algae, ascidians, bryozoans, sponges, protozoa, molluscs, crustaceans, and other marine sessile organisms. The seaweed farming industry is currently growing at ca. 9% per annum, with global production of 31.2 million wet tons worth US$ 11.7 billion. The first report of an epiphytic outbreak in commercial farms of Kappaphycus in the 1970s caught the attention of several researchers on this devastating epiphyte which causes retarded growth and significant loss of stocking biomass, ultimately leading to the production of inferior quality of raw material. High-density planting in commercial farms is often responsible for recurring epiphytic infestations. Nevertheless, it is almost certain that the entire crop collapses due to epiphyte outbreak in a short span of time. Therefore, the lack of reliable global statistics exerts trade deficit in commercial seaweed farming. This chapter highlights the causes of epiphytic infestations, the current status of outbreaks, methods to control epiphytes, and its economic implications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Seaweeds are marine macrophyte algae . They are exceptionally diverse in their forms and functions, are renewable in nature, and provide inimitable prospects for its utilization as a source of nutrition, food, cosmetics, fertilizer, medicinal, nutraceuticals, pharmaceutical, biofuels, personal care, and allied industries. Commercial harvesting of seaweeds has reached a new milestone with 31.2 million tonnes year−1 production (95% accounts to farming) with a market value of over US$ 11.7 billion (FAO 2018). About 221 species of seaweed are being utilized commercially. Of these, about 145 species are used for food, while nearly 110 species are exploited for phycocolloid extraction. Almost all of the seaweed production (94%) is produced through aquaculture practice, while harvesting from the wild stocks is minuscule.
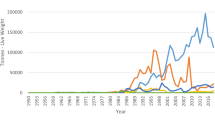
Asian countries alone account for over 99% of global seaweed production. The highest proportion of tonnage is constituted by food alga, namely, Porphyra (Nori), Laminaria (Kombu), and Undaria (Wakame), followed by seaweeds for phycocolloid extraction. As seaweed farming has been gaining impetus globally, including outside Asia, the problems faced by this industry need to be addressed; one of the several issues is an epiphytic infestation. The first report of an epiphytic outbreak in commercial farms of Kappaphycus in the 1970s caught the attention of several researchers on this devastating epiphytes which causes retarded growth and significant loss of stocking biomass ultimately leading to the production of inferior quality of raw material. It is also a major, worldwide problem in Gracilaria cultivation as well (Fig. 9.1). The epiphytic infestation has severely reduced the productivity and cost efficiency in tank cultivation systems (Fletcher 1995). Seaweed in their natural ecosystem acts as a primary producer and provides food to consumers. With this primary role in the marine ecosystem, seaweed provides shelters and habitat to many organisms including the plants and animals. The organisms which colonize the seaweeds can be called as epibiotic communities or epibionts or even referred as epiphytes (Peteiro and Freire 2013) although this word is not clearly defined (Steel and Wilson 2003) and the phenomenon is referred as epiphytism. Epiphytism is common in marine habitat (Ingle et al. 2018), and there are various types of epiphytes from microalgae, other seaweeds to invertebrates and animals from the class of gastropod, and many small crustaceans such as amphipods and isopods associated with seaweeds. With this, there are various kinds of microorganisms from bacterial, fungal, and viral species (Gachon et al. 2010) associated with seaweeds. Almost all of these microorganisms (Goecke et al. 2013, 2010) and epibiotic communities (Wahl 1989) get suitable place, habitat, food source, as well as protection from their predators and other environmental stresses.
All these epibiotic communities of seaweed can show positive as well as negative mechanism. The positive interaction however is not so well studied, but few species particularly grazers can control the epiphytic algal species. In the cultivation of seaweeds, the decrease or increase of these communities can impact on the production. The abundance and species biodiversity of epibiotic communities are dependent on crop species morphology, season, etc. There are some other important factors in defining the epifaunal assemblages apart from the algal host such as epiphytic load and height on the shore (Cacabelos et al. 2010). In many places, the seaweeds are non-native species which can show variation in acceptance of them by native epibiotic communities (Cacabelos et al. 2010), due to their metabolites as deterrents against consumers (Paul and Fenical 1986). The physiological tolerance of these animals and the variations in the habitat environment determine the distributional pattern and abundance of this epifauna related to specific marine macrophyte (Lancellotti et al. 1993). Basically, the majority of epiphytic algae species are facultative in nature and are usually found associated with more than one species (Wahl and Mark 1999), while others are known obligate epiphytes which grow on specific single host species.
Almost all epibiotic organisms are generally deposited on the thallus part of cultivated seaweeds. In case of the epiphytic seaweeds and seaweed, crops are generally competitors of each other for the resources such as sunlight and nutrient (Kersen et al. 2007) which can make host seaweed weak, resulting in bacterial infection. The abundance of epiphytic species is determined by few abiotic factors, for instance, the direction of water movement and the availability of nutrients. Their spatial distribution is dependent on their ability to dryness or removal capacity of moisture during low tides (Molina-montenegro et al. 2005). The higher level of sunlight, high temperature, and strong desiccation at the time of low tides make the intertidal zone a stressful habitat (Bertness and Leonard 1997). The species which have the capability to keep themselves alive in such adverse conditions can impact on other species (Bertness et al. 1999; Molina-montenegro et al. 2005). Few seaweed species show tolerance to such environment and can provide shelter to various organisms such as other seaweeds and small crustaceans (Bertness et al. 1999).
The upsurge in the amount of nutrient load shows a gradual increase in the number of epiphytic seaweeds and invertebrates which are dependent on the seaweeds. However, intraspecific competition is also possible in the invertebrates for light, space, and food (Lobban and Harrison 2000; Kersen et al. 2007). The complex structure of seaweed can provide larger surface results in the diverse assemblages of invertebrates associated with that seaweed (Chemello and Milazzo 2002). The spatial variability of epifauna within the same environments might vary from a few days to several months. The small spatial scale of observation shows that the seaweed is a highly appropriate habitation for an extensive variety of faunal organisms (Chemello and Milazzo 2002), but this depends on many factors such as life cycle of epibiotic organisms, the architecture, and chemical defense of host seaweeds (Duffy and Hay 1994).
9.2 Classification of Epiphytes
Linskens (Linskens 1963) classified the epiphytes in two types on the basis of their attachment to host seaweed. The holo-epiphytes attach to the outermost layer of their seaweed host, while amphi-epiphytes acutely anchor inside the host seaweed tissue. This classification is complimentary compared to the classification given by Leonardi et al. (2006), which is based on the level of host penetration and classified in five types as per their interaction with macroalgae. Leonardi classified the epiphytes in five categories as shown in Table 9.1.
These epiphytes can be other algae, bacteria, fungi, etc. which cover the parts of seaweed densely as per their requirement and possibility of spreading. Both these classifications are related with the interaction of seaweed with epiphytes particularly epiphytic algae, and no mention about microorganisms are given separately and even no discussion on the animal’s association to the seaweed. Ingle et al. (2018) defined the term pests in macroalgae cultivation, and on the basis of negative interaction of epibiotic communities with crop seaweed, pests are classified in various categories as shown in Fig. 9.2.
Classification of marine pests into three main groups, plants, animals, and microorganisms, which are further categorized into subtypes which negatively interact with seaweed and might be responsible for direct or indirect harm or injuries. (Modified from Ingle et al. 2018)
9.3 Microorganisms and Seaweed
In the marine environment , seaweed deals with all types of microorganisms including the viruses and fungal species but mostly with bacteria. Although there is a limited study on the seaweed interaction with viruses and fungi, it is found that up to half of natural seaweed is infected by viruses (Cock et al. 2010) which denotes that viruses can strongly influence the seaweed lifestyle as well (Egan et al. 2013) (Table 9.2). In case of fungal species, only a few fungal species are yet known, but majority of fungi are from Ascomycota (Loque et al. 2010; Zuccaro et al. 2008). But compared to these, the bacterial-seaweed interaction is well studied because it is one of the dominant groups, and seaweed can come under pressure due to a higher number of bacterial communities.
Many times this interaction is positive and beneficial for both. Seaweed provides favorable habitat to bacterial colonies to grow and reproduce on its surface (Englebert et al. 2008). With this, bacterial colonies get nutrients and food source from biochemical activities in the seaweed. For example, seaweed is marine photosynthetic organisms which produce oxygen is a good source of oxygen for bacteria associated to seaweed. But this interaction is not one way as for the growth and development of seaweeds, and few bacterial species are important. These symbiotic bacteria can enhance seaweed growth (Singh et al. 2011), protect seaweed from harmful bacterial and fungal species by producing certain chemicals (Lemos et al. 1985), and also help in reproduction (Joint et al. 2007). But with such positive interaction, there is negative interaction too, in which few bacterial species are responsible for many seaweed diseases (Goecke et al. 2010).
The physical association of bacteria with seaweed is generally in different ways such as tightly attached and not directly attached, and a few show syntrophic associations (Goecke et al. 2013). Bacteria can harm seaweeds by producing a chemical which is toxic (Berland et al. 1972), injuring them by degrading their cell walls (Goecke et al. 2010). Few bacterial species can produce biofilm in the seaweed crop surrounding or on the water surface which is an intricate three-dimensional cluster in nature, responsible for both direct and indirect harms by reducing the essential light penetration (Wahl et al. 2010) and gaseous exchange. Biofilm can impact negatively on the reproduction of seaweed and also in the natural environment by enhancing the attachment of spores which later results in damage.
9.4 Epiphytic Algae
The epiphytic algae on seaweed crop can be microalgae or most specifically macroalgae or other seaweed species which are not part of cultivation. It can impacts on the host seaweed as a reduction of the growth rate of the crop, a decrease of reproduction output, or even whole or partial mortality of host seaweed (Davis et al. 1989) which can further results in quality and quantity of production or yield of the crop (Table 9.3). Generally, the ephemeral epiphytes can be seen at the host tips, while large-sized epiphytes can be found attached to the basal disk (Arrontes 1990). The overall coverage of epiphytes on these seaweed crops increases as per availability and nutrients and with the age of host seaweed. The structure of epiphytic communities depends on the position of the thallus of seaweed (Longtin et al. 2009).
9.5 Epifaunal Communities
The epifaunal species assemblage undergoes frequent temporal fluctuation because of several environmental (abiotic) factors like availability of light, temperature, abundance of epiphytes, and pressure of predation (Jones and Thornber 2010). Fluctuations in these features can influence the pattern of abundance at different spatial and temporal scales for the epifaunal community. Many of these epifaunal species such as amphipods, isopods, and even smaller gastropods live in the epiphytic algae (Orav-kotta and Kotta 2004) and can use that as a food source (Leonardi et al. 2006). In this way more dense epiphytic algae host more epifaunal communities by creating a suitable environment.
In the seaweed assemblages, amphipod crustaceans are very much common which are particularly the mesograzers and impact adversely on the seaweed (Poore et al. 2012). In all ecosystems, herbivory is a key process which transfers the primary production to further consumers in ecosystem level with impact on structure and productivity of vegetated habitat (Poore et al. 2012). Similarly as herbivores control the growth of producers in the ecosystem, they are controlled by the predators, but in this natural phenomenon, the seaweed can get harm indirectly (Ingle et al. 2018). The amphipod crustaceans are important for predatory fishes and other invertebrates as a food source (Table 9.3).
9.6 Problems/Diseases Caused by Epiphytes and Their Potential Control Strategies
Due to the rapid developments in the seaweed-based farming activities, there is also an increase in the epiphytic diseases/menace worldwide. The epiphytic invasion causes significant damages for the local farmers as it drastically affects the growth of field-grown algal species and ultimately hampers the farming activity in several cases. As with any agronomical practice, several approaches are being utilized and developed to control the epiphytes growth on seaweed, although several strategies have been suggested to control the unsolicited growth of epiphytes and have been used in controlling them in Gracilaria farming (Fletcher 1995). However, some of these strategies can be used only in the cultivation tanks, and its application in an open ocean farming is highly challenging. Here we discuss about some major control strategies against seaweed epiphytes.
9.6.1 Chemical Method
The epiphytes can be removed by employing chemical procedures such as by chlorine or copper rinsing or by altering the pH. Some other preventive chemicals like sodium hypochlorite have been largely used to pre-treat the seawater, tanks, and equipment (Ugarte and Santelices 1992). However, if the contamination already starts spreading, the host material is often immersed in an appropriate toxicant and has been proven to be highly useful. Ugarte and Santelices (1992) have successfully demonstrated that immersing Ectocarpus and Enteromorpha species in 4–6% commercial hypochlorite solution for 6–10 h killed 80% of epiphytes. However, in the case of Gracilaria, it caused minor injuries until the thiosulfate was used, and the repetitive treatment led to enhanced yield of Gracilaria. Similarly, the usage of 100 g/l of copper solution significantly reduces the growth of Enteromorpha within a week without affecting the growth of Gracilaria (Haglund and Pedersén 1992). Several researchers have reported that by controlling the pH level, the epiphytes can be repressed (Friedlander 1991), particularly at the high pH level (Haglund and Pedersén 1992).
Considerable interest has been shown by the researchers to optimize the nutrient regimes in order to control the growth of epiphytes. It has been proven that incessant or higher supply of nutrients is not only extravagant but could also favor the unsolicited growth of epiphytic species (Pickering et al. 1993). By following a vigilant supervision of the nitrogen inputs, mainly by decreasing the nitrogen supply, the uncontrolled loads of epiphyte can be prohibited (Pickering et al. 1993). Contrarily, high levels of ammonia (>0.5 mmol/l) have been also proven to be lethal for several epiphytes (Friedlander et al. 1991).
9.6.2 Physical Method
Epiphytes can be physically removed by using the manual methods like mechanical brushing and rapid water movement. In certain cases, sand shifting has been implemented as an effective approach to maintain epiphyte-free Gracilaria culture (Doty 1980). Moreover, the strategy to control epiphytic growth in seaweed cultivation system includes the direct physical elimination of the host epiphytes. For the removal of diatoms, water jets are very useful and are usually used against the host material post-harvest. However, for the majority of the macroalgal species especially those affected by a special type of filament known as rhizoidal filaments can deeply penetrate and thus requires manual hand removal, which is a labor-intensive process and is unfeasible for huge culture units and may also cause damage to the host (Ugarte and Santelices 1992). Due to this problem, many farmers favor to employ some other precautionary procedures and recommend good husbandry to tackle the occurrence of epiphytism and fouling.
In tank cultivation systems, it must be confirmed that the source of seawater is devoid of impurities (Ugarte and Santelices 1992) or the seawater is routinely exchanged. For instance, filtration of seawater using diatomaceous earth or filter cartridges and sand is often utilized for blocking the entry of epiphytic organisms. Additionally, some other precautionary procedures have been also implemented to reduce the contamination, for instance, by adjusting the abiotic factors in favor of the host species. The usage of UV light has also been endorsed for tank culture system. The reduction in irradiance levels has also been successfully demonstrated to reduce the level of epiphytism in seaweed cultivation (Friedlander 1992).
9.6.3 Biological Method
The rapid prevalence of epiphytism in seaweed mariculture has enticed special attention to develop resistant strains and to utilize existing knowledge to control epiphytism in seaweed farming (e.g., Santelices and Ugarte 1990; Fletcher 1995). Acadian marine plant extract powder (AMPEP) is a commercial extract obtained from the brown alga, Ascophyllum nodosum. It harbors some major macronutrients such as total nitrogen N (0.8–1.5%), phosphoric acid P2O5 (1–2%), and soluble potash K2O (17–22%), which has been proven to augment the overall growth and development of eucheumatoids (Hurtado et al. 2013). Furthermore, promising results have been obtained in in vitro and field trials using AMPEP extract against epiphytes and pathogen. In addition, it has been linked to increase the rate of growth and carrageenan production (Loureiro et al. 2012). Interestingly, studies have also shown that soaking the algal seedlings in AMPEP, before planting, could efficiently improve the daily growth rate and productivity of both varieties of Kappaphycus. It has been also utilized to check or diminish the influence of Neosiphonia infection on commercial farming regions (Borlongan et al. 2011). However, more field trials with extracts from other algal species might lead to discovery of some new and potential anti-epiphytic compounds.
Maintaining an optimal density of host plants has been suggested by the phycologist to prevent epiphytes from colonizing. Grazers have been effectively utilized to control epiphyte growth. Gammarus lawrencianus and Idotea baltica are the two crustacean species which have been successfully demonstrated to selectively graze on Ectocarpus spp. and Enteromorpha spp. which epiphytically grow on the surface of Chondrus crispus (Shacklock and Doyle 1983). Similarly, the epiphyte growth on Fucus is controlled by Idotea which is often seen during the high nutrient load conditions (Worm et al. 2000; Orav-Kotta and Kotta 2004). Sporadic feeding by herbivores could also be beneficial for seaweeds and communities sometimes (Hay et al. 2004). The mesograzers which are the filamentous epiphytes are usually removed manually from a host. This manual removal of epiphytes allows the absorption of higher amount of light and enhances nutrient absorption by the host plant (Duffy and Hay 1990). The epiphytes associated with pond-grown seaweed species have been effectively controlled by fish such as milkfish (Chanos chanos) and Tilapia mossambica (Shang 1976).
9.7 Conclusion and Perspectives
Seaweed farming, or seagriculture, is anticipated to offer sustainable seaweed biomass, thereby enabling the rapid expansion of marine bioeconomy. But, with the increase in activities related to seaweed farming, there has been reportedly higher occurrence of epiphytic filamentous algae (EFA) disease in several parts of the world. Similar to land-based crop, the cultivation of macroalgae is also prone to diseases and infestations. This epiphytic infiltration significantly affects the algal growth and thus the local farmers and has even totally collapsed the farming activity in several parts of the globe. Moreover, because of the fragility of the marine environment, it is impractical to utilize chemical methods to control the epiphytes. In this regard, marine integrated pest management (MIPM) approach appears to be the best available option for the sustainable seagriculture. On the other hand, AMPEP (Acadian marine plant extract powder) has also been proven to be highly potential in minimizing or controlling the growth of epiphytes and the respective diseases caused by them.
References
Anandavelu I et al (2013) Epifaunal assemblage on morphologically distinct intertidal seaweeds of Kodiyaghat (South Andaman), India. Proc Int Acad Ecol Environ Sci 3(3):229–237
Anderson LM, Martone PT (2014) Biomechanical consequences of epiphytism in intertidal macroalgae. J Exp Biol:1167–1174. https://doi.org/10.1242/jeb.088955
Arrontes J (1990) Composition, distribution on host and seasonality of epiphytes on three intertidal algae. Bot Mar 33(2):205–212. https://doi.org/10.1515/botm
Berland BR, Bonin DJ, Maestrini SY (1972) Are some bacteria toxic for marine algae? Mar Biol. Springer 12(3):189–193
Bertness M, Leonard G (1997) The role of positive interactions in communities : lessons from intertidal habitats. Ecology 78(7):1976–1989
Bertness M et al (1999) Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80(8):2711–2726
Borlongan IAG, Tibubos KR, Yunque DAT, Hurtado AQ, Critchley AT (2011) Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J Appl Phycol 23:615–621. https://doi.org/10.1007/s10811-010-9649-9
Burke C et al (2011) Bacterial community assembly based on functional genes rather than species. Proc Nat Acad Sci U S A 108(34):14288–14293. https://doi.org/10.1073/pnas.1101591108
Cacabelos E et al (2010) Do grazers prefer invasive seaweeds? J Exp Mar Biol Ecol 393(1–2):182–187. https://doi.org/10.1016/j.jembe.2010.07.024.
Chemello R, Milazzo M (2002) Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar Biol 140:981–990. https://doi.org/10.1007/s00227-002-0777-x
Cock JM et al (2010) The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature, Nature Publishing Group 465(7298):617–621
Davis A et al (1989) Epibiosis of marine algae and benthic invertebrates: natural product chemistry and other mechanisms inhibiting settlement and overgrowth. In: Bioorganic marine chemistry. Springer, Berlin, pp 85–114
Doty MS (1980) Outplanting Eucheuma species and Gracilaria species in the tropics. In: Abbott IA, Foster MS, Eklund LF (eds) Pacific seaweed aquaculture, Proc. Symp. Useful algae. California Sea Grant College Program, Inst. Mar. Resources, Univ Calif, La Jolla, pp 19–22
Duffy JE, Hay ME (1990) Seaweed adaptations to herbivory. Bioscience 40(5):368–375
Duffy JE, Hay ME (1994) Herbivore resistance to seaweed chemical defense: the roles of mobility and predation risk. Ecology 75(5):1304–1319
Egan S et al (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev. The Oxford University Press 37(3):462–476
Englebert ET, McDermott C, Kleinheinz GT (2008) Effects of the nuisance algae, Cladophora, on Escherichia coli at recreational beaches in Wisconsin. Sci Total Environ 404:10–17. https://doi.org/10.1016/j.scitotenv.2008.05.025
FAO (2018) The state of world fisheries and aquaculture 2018 – meeting the sustainable development goals. Rome
Fletcher RL (1995) Epiphytism and fouling in Gracilaria cultivation: an overview. J Appl Phycol 7(3):325–333
Friedlander M (1991) Growth rate, epiphyte biomass and agar yield of Gracilaria conferta in an annual outdoor experiment. I. Irradiance and nitrogen. Bioresour Technol 38:203–208
Friedlander M (1992) Gracilaria conferta and its epiphytes. The effect of culture conditions on growth. Bot Mar 35:423–428
Friedlander M, Krom MD, Ben-Amotz A (1991) The effect of light and ammonium on growth, epiphytes and chemical constituents of Gracilaria conferta in outdoor cultures. Bot Mar 34:161–166. https://doi.org/10.1515/botm.1991.34.3.161
Gachon CMM et al (2010) Algal diseases: spotlight on a black box. Trends Plant Sci 15(11):633–640. https://doi.org/10.1016/j.tplants.2010.08.005
Ganesan M, Sahu N, Eswaran K (2011) Raft culture of Gracilaria edulis in open sea along the south-eastern coast of India. Aquaculture. Elsevier B.V 321(1–2):145–151. https://doi.org/10.1016/j.aquaculture.2011.08.040
Ganesan M et al (2014) Epiphytism differences in Gelidiella acerosa cultivated with floating rafts and concrete blocks. J Appl Phycol 27:399–412. https://doi.org/10.1007/s10811-014-0279-5
Goecke F et al (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–299. https://doi.org/10.3354/meps08607
Goecke F et al (2013) Algae as an important environment for bacteria-phylogenetic relationships among new bacterial species isolated from algae. Phycologia 52(1):14–24
Haglund K, Pedersén M (1992) Growth of the red alga Gracilaria tenuistipitata at high pH: influence of some environmental factors and correlation to an increased carbonic-anhydrase activity. Bot Mar 35:579–587
Hay ME, Parker JD, Burkepile DE, Caudill CC, Wilson AE, Hallinan ZP, Chequer AD (2004) Mutualisms and aquatic community structure: the enemy of my enemy is my friend. Annu Rev Ecol Evol Syst 35:175–197
Hurtado AQ, Montaño MNE, Martinez-Goss MR (2013) Commercial production of carrageenophytes in the Philippines: ensuring long-term sustainability for the industry. J Appl Phycol 25(3):733–742
Ingle KN et al (2018) Marine integrated pest management (MIPM) approach for sustainable seagriculture. Algal Res 29(November 2017):223–232. https://doi.org/10.1016/j.algal.2017.11.010
James PSBR, Krishnamurthy Chennubhotla VS, Rodrigo JX (1986) Studies on the fauna associated with the cultured seaweed Gracilaria edulis. The symposium of coastal aquaculture:1193–1198
Joint I, Tait K, Wheeler G (2007) Cross-kingdom signalling: exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos Trans R Soc London B Biol Sci 362(1483):1223–1233
Jones E, Thornber CS (2010) Effects of habitat-modifying invasive macroalgae on epiphytic algal communities. Mar Ecol Progress Ser 400(Rodriguez 2006):87–100. https://doi.org/10.3354/meps08391
Joseph MM (1978) Ecological studies on the fauna associated with economic seaweeds of South India-I. Species composition, feeding habits and interrelationships. Seaweed Res Util 3:2–24
Kersen P et al (2007) Epiphytes and associated fauna on the brown alga Fucus vesiculosus in the Baltic and the north seas in relation to different abiotic and biotic variables. Mar Ecol 32:87–95. https://doi.org/10.1111/j.1439-0485.2010.00418.x.
Kitayama T, Garrigue C (1998) Marine algal endophyte and epiphytes new to New Caledonia. Bull Nat Sci Mus Tokyo Ser B 24(3):93–101
Lancellotti DA et al (1993) Distribution patterns and coexistence of six species of the amphipod genus Hyale. Mar Ecol Prog Ser 93(Lancellotti 1990):131–141
Lemos ML, Toranzo AE, Barja JL (1985) Antibiotic activity of epiphytic bacteria isolated from intertidal seaweeds. Microb Ecol 11(2):149–163. https://doi.org/10.1007/BF02010487
Leonardi PI et al (2006) Diversity, phenomenology and epidemiology of epiphytism in farmed Gracilaria chilensis (Rhodophyta) in northern Chile. Eur J Phycol 41(2):247–257. https://doi.org/10.1080/09670260600645659
Lim E et al (2016) Global transcriptome analysis of Gracilaria changii (Rhodophyta) in response to agarolytic enzyme and bacterium. Mar Biotechnol Biotechnol 18:189–200. https://doi.org/10.1007/s10126-015-9680-6
Linskens HF (1963) Beitrag zur frage der beziehungen zwischen epiphyt und basiphyt bei marinen algen. Pubbl Stn Zool Napoli 33:274–293
Lobban CS, Harrison PJ (2000) Seaweed ecology and physiology. Cambridge University Press, Cambridge, 366 pp
Longtin CM et al (2009) Distribution of algal epiphytes across environmental gradients at different scales: intertidal elevation, host canopies, and host fronds. J Phycol 45:820–827. https://doi.org/10.1111/j.1529-8817.2009.00710.x.
Loque CP et al (2010) Fungal community associated with marine macroalgae from Antarctica. Polar Biol 33(5):641–648. https://doi.org/10.1007/s00300-009-0740-0
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2012) Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): a “vaccinelike” effect on Kappaphycus alvarezii (Doty) Doty ex PC Silva. J Appl Phycol 24(3):427–432
Molina-montenegro MA et al (2005) Positive associations between macroalgal species in a rocky intertidal zone and their effects on the physiological performance of Ulva lactuca. Mar Ecol Prog Ser 292:173–180
Muñoz J, Fotedar R (2010) Epiphytism of Gracilaria cliftonii (Withell, Millar & Kraft) from Western Australia. J Appl Phycol 22:371–379. https://doi.org/10.1007/s10811-009-9469-y
Norderhaug KM, Christie H, Rinde E (2002) Colonisation of kelp imitations by epiphyte and holdfast fauna; a study of mobility patterns. Mar Biol 141:965–973. https://doi.org/10.1007/s00227-002-0893-7
Norton TA, Benson MR (1983) Ecological interactions between the brown seaweed Sargassum muticum and its associated fauna. Mar Biol 75:169–177
Orav-kotta H, Kotta J (2004) Food and habitat choice of the isopod Idotea baltica in the northeastern Baltic Sea. Hydrobiologia 514:79–85
Paul VJ, Fenical W (1986) Chemical defense in tropical green algae, order Caulerpales. Mar Ecol Prog Ser 34:157–169
Peteiro C, Freire O (2013) Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. J Would Aquacult Soc 44(5):706–715
Pickering TD, Gordon ME, Tong LJ (1993) Effect of nutrient pulse concentration and frequency on growth of Gracilaria chilensis plants and levels of epiphytic algae. J Appl Phycol 5:525–533. https://doi.org/10.1007/BF02182511
Poore AGB et al (2012) Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett 15:912–922. https://doi.org/10.1111/j.1461-0248.2012.01804.x
Rindi F, Guiry MD (2004) A long-term comparison of the benthic algal flora of Clare Island, County Mayo, western Ireland. Biodivers Conserv 13:471–492
Sand-Jensen K, Borum J (1984) Epiphyte shading and its effect on photosynthesis and diel metabolism of Lobelia dortmanna l. during the spring bloom in a Danish lake. Aquat Biol 20:109–119
Santelices B, Ugarte R (1990) Ecological differences among Chilean populations of commercial Gracilaria. J Appl Phycol 2:17–26
Sarma LN, Ganapati PN (1972) Faunal association of algae in the intertidal region of Visakhapatnam. Proc Indian Natl Sci Acad Part B Biol Sci 38:380–396
Singh RPR et al (2011) Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat Biol 12(1):13–21. https://doi.org/10.3354/ab00312
Shacklock PF, Doyle RW (1983) Control of epiphytes in seaweed cultures using grazers. Aquaculture 31:141–151
Shang VC (1976) Economic aspects of Gracilaria culture in Taiwan. Aquaculture 8:1–7
Steel JB, Wilson JB (2003) Which is the phytes in epiphytes. Folia Geobot 38:97–99
Taylor RB, Cole RG (1994) Mobile epifauna on subtidal brown seaweeds in northeastern New Zealand. Mar Ecol Prog Ser 115:271–282
Totti C, Poulin ÆM, Romagnoli ÆT (2009) Epiphytic diatom communities on intertidal seaweeds from Iceland. Polar Biol 32:1681–1691. https://doi.org/10.1007/s00300-009-0668-4
Tujula NA et al (2010) Variability and abundance of the epiphytic bacterial community associated with a green marine Ulvacean alga. ISME J 4:301–311. https://doi.org/10.1038/ismej.2009.107
Ugarte R, Santelices B (1992) Experimental tank cultivation of Gracilaria in Central Chile. Aquaculture 101:7–16
Vairappan CS (2006) Seasonal occurrences of epiphytic algae on the commercially cultivated red alga Kappaphycus alvarezii (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 18:611–617. https://doi.org/10.1007/s10811-006-9062-6
Veeragurunathan V et al (2015) Feasibility of Gracilaria dura cultivation in the open sea on the Southeastern coast of India. Aquaculture. Elsevier B.V 438:68–74. https://doi.org/10.1016/j.aquaculture.2015.01.009
Viejo RM (1999) Mobile epifauna inhabiting the invasive Sargassum muticum and two local seaweeds in northern Spain. Aquat Bot 64:131–149
Wahl M (1989) Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar Ecol Progr Ser Int Res 58:175–189
Wahl M, Mark O (1999) The predominantly facultative nature of epibiosis : experimental and observational evidence. Mar Ecol Prog Ser 187:59–66
Wahl M et al (2010) Ecology of antifouling resistance in the bladder wrack Fucus vesiculosus: patterns of microfouling and antimicrobial protection. Mar Ecol Prog Ser 411:33–48
Werner FJ, Graiff A, Matthiessen B (2016) Even moderate nutrient enrichment negatively adds up to global climate change effects on a habitat-forming seaweed system. Limnol Oceanogr 61:1891–1899. https://doi.org/10.1002/lno.10342
Worm B, Lotze HK, Sommer U (2000) Coastal food web structure, carbon storage, and nitrogen retention regulated by consumer pressure and nutrient loading. Limnol Oceanogr 45(2):339–349
Zuccaro A et al (2008) Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl Environ Microbiol 74(4):931–941
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sahu, S.K., Ingle, K.N., Mantri, V.A. (2020). Epiphytism in Seaweed Farming: Causes, Status, and Implications. In: Gothandam, K., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Environmental Biotechnology Vol. 1. Environmental Chemistry for a Sustainable World, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-030-38192-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-38192-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38191-2
Online ISBN: 978-3-030-38192-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)