Abstract
An appropriate anticoagulation strategy is crucial to ensure safe and uninterrupted continuous renal replacement therapy (CCRT) sessions. In fact, filter clotting remains the principal reason for unplanned circuit replacement and unintentional interruption of the treatment. Different modalities of anticoagulation are now available, essentially divided into systemic strategies (unfractioned heparin, low-molecular-weight heparins, direct factor IIa inhibitors) with which the patient’s blood is entirely anticoagulated, and regional ones (citrate, heparin–protamine) with which only the extracorporeal blood coagulation is affected. Non-pharmacological strategies should be also considered in specific cases. The currently available guidelines are very clear, and a personalized approach, focused on clinical condition, is strongly recommended. The aim of this chapter is to present a global overview of the currently available strategies (systemic, regional, and non-pharmacologic) aimed at prolonging circuit and filter patency as much as possible always giving priority to specific patient needs. The time for precision anticoagulation strategy has come.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Acute kidney injury (AKI) occurs in about 50% of all critically ill patients admitted to the intensive care unit (ICU), and 10–20% of them require renal replacement therapy (RRT) [1]. In patients with hemodynamic instability and shock, continuous RRT (CRRT) is preferred over intermittent hemodialysis [2]. In order to deliver the prescribed therapy minimizing the downtime, extracorporeal circuit and filter patency has to be effectively obtained. Early extracorporeal circuit and filter clotting is a frustrating experience that reduces treatment efficacy and increases bedside workload and costs. Until recently, the most common approach to extracorporeal circuit and filter anticoagulation was based on the infusion of unfractionated heparin (UFH) [2]. Although generally infused into the circuit, UFH infusion generally leads to systemic anticoagulation, with an increased risk of bleeding, especially in critically ill patients and surgical patients who may have impaired hemostasis independently of heparin administration. In view of this and other potential complications (e.g., heparin-induced thrombocytopenia [HIT]), alternative modalities of anticoagulation during CRRT have gained popularity in recent years and have somehow changed the way CRRT is prescribed and delivered.
2 Systemic Strategies
2.1 Unfractionated and Low-Molecular-Weight Heparin
Heparin increases antithrombin (AT) activity on factors Xa, IIa, IXa, Xia, and XIIa. During CRRT, heparin can (theoretically) be infused directly to the patient (dedicated external line) or into the extracorporeal circuit via a dedicated internal line. The latter approach is recommended since, logically, the highest heparin concentration is reached at the prefilter site and thus at the location where the coagulation system is activated. In many centers, systemic heparin anticoagulation is the standard modality for CRRT. Heparin administration implies low costs, good drug availability, easy monitoring with activated prothrombin time (aPTT), and the possibility of administering an antagonist (protamine). However, systemic heparin anticoagulation has some adverse effects that are contributing to its replacement with alternative techniques: increased bleeding risks (e.g., secondary to sepsis-associated thrombocytopenia and/or coagulopathy; major surgery, hepatectomy; trauma), HIT, and ineffective anticoagulation due to heparin resistance [3]. Bleeding occurs in 5–40% of patients undergoing CRRT with systemic heparin anticoagulation [3,4,5,6,7,8], and this risk appears higher than with regional citrate anticoagulation [3, 9] (Fig. 28.1).
The complex of heparin and platelet factor 4 leads to the production of auto-antibodies that are at the basis of HIT. The antibody-platelet binding leads to platelet activation, causing potentially life-threatening thrombosis or thromboembolism. HIT is not a rare phenomenon and patients who receive unfractionated heparin for 7–10 days are at the highest risk with an incidence of 1–3% after cardiac surgery [10]. Logically, many studies have demonstrated a higher incidence of HIT during systemic heparin anticoagulation than with regional citrate anticoagulation [4, 5, 11].
In some patients, UFH infusion is not able to maintain sufficient anticoagulation and or the dose of UFH has to be increased in order to achieve the same aPTT level. This form of “resistance” can be related to different phenomena: insufficient AT concentration (AT should be measured in patients undergoing systemic heparin anticoagulation-CRRT) due to congenital deficit (rare) [12], decrease due to clinical conditions (e.g., chronic, acute or acute-on-chronic liver failure, bleeding, consumption). Heparin resistance may occur independently from AT concentration: heparin can be bound by a number of molecules including platelet factor 4, collagen, growth factors, elastase, and factor VIII [12, 13].
A potential alternative to UFH is low-molecular-weight heparin (LMWH) that can be suggested because of a lower incidence of HIT, less platelet activation, less inactivation by platelet factor-4, greater and more consistent bioavailability, and no metabolic adverse effects. Many disadvantages limit the widespread use of LMWH as an anticoagulation strategy for CRRT: (1) LMWH is eliminated via the kidneys and in case of renal failure its biological half-life is prolonged causing a risk of accumulation [14]; (2) CRRT only partially removes LMWH [15]; monitoring of the effect of LMWH requires the measurement of anti-Xa-activity (expensive and not available in all centers) [16]; (3) due to the difficulty in evaluating its concentration, LMWH cannot be completely antagonized by protamine. Few data can be found in the literature comparing LMWH and UFH. One randomized controlled study dates back more than 10 years [17]: enoxaparin showed difference in bleeding events compared to UFH, but anti-Xa-activity was tested every day and enoxaparin adjusted accordingly. Interestingly, the filter lifespan was longer in the LMWH group versus UFH (31 vs. 22 h, respectively; p < 0.017) [17]. Due to the paucity of data available on LMWH as an anticoagulant during CRRT, a final recommendation for or against its use cannot be made. Probably LMWH could be considered as a second-line anticoagulant after systemic heparin anticoagulation, when adverse effects, such as resistance or inadequate anticoagulation, are observed with UFH.
2.2 Direct Thrombin Inhibitors
Two techniques are available as potential alternatives to UFH or LMWH: hirudine and argatroban. Recombinant hirudin, a direct inhibitor of factor IIa (thrombin), can be used in cases of HIT. Since hirudin is eliminated by the kidneys, its half-life can be prolonged from 1 to 2 h to over 50 h in case of renal insufficiency [18]. The molecule cannot be eliminated via hemofiltration (molecular weight is about 7000 Da) and no antidote exists. Its effect can be measured using the ecarin clotting time, which is not available in all the hospitals. Filter lives are shorter in comparison with other techniques and bleeding complications more frequent [18]. Argatroban, another factor IIa inhibitor, is a 500 Da molecule derived from L-arginine and metabolized in the liver [19]. With a half-life of 45 min, its anticoagulant effect decreases 2–4 h after cessation of continuous infusion [20]. Argatroban is licensed for use in HIT. There are few data available in the literature. In a prospective study of 30 patients with HIT, argatroban was used as anticoagulant during continuous veno-venous hemodialysis (CVVHD) . Only two patients developed minor bleeding and no patient developed severe bleeding . Ninety-eight percent of the extracorporeal filters ran for at least 24 h [21]. In conclusion, in case of HIT and/or extracorporeal circuit and filter thrombosis, when regional citrate anticoagulation is not available or not deemed to prevent clotting [22], argatroban could be the anticoagulant of choice. Interestingly, repeated and unexplained filter clotting during CRRT under regional citrate anticoagulation should encourage clinicians to exclude HIT [22].
3 Regional Strategies
3.1 Regional Citrate Anticoagulation
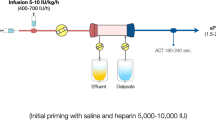
Techniques aimed to manage the coagulation in the extracorporeal circuit without affecting systemic coagulation (regional anticoagulation), although theoretically more complex to deliver, have been considered and significantly developed in recent years. Indeed, regional citrate anticoagulation has been demonstrated to prolong filter life and decrease the rate of complications, downtime, and costs compared with heparin [23] and is now recommended as the first-line anticoagulation strategy for CRRT in patients without contraindications [2]. This regional technique is based on the reversible chelation of ionized calcium, a cofactor of many steps in the clotting cascade, in the extracorporeal circuit and filter. In order to optimize the anticoagulant effect, citrate is infused proximally to the vascular access by means of the pre-dilution line (Fig. 28.2). The application of regional citrate anticoagulation requires particularly dedicated protocols, knowledge of the biochemical mechanisms underpinning this particular technique of anticoagulation and specific training of both medical and nursing staff.
In this section, some “advanced” aspects of citrate anticoagulation strategy will be summarized. Clinicians aware of the basic concepts of regional citrate anticoagulation could take advantage of specific aspects of regional citrate anticoagulation to better personalize the strategy to individual patients.
3.1.1 Citrate Infusion Rate and Citrate Load
The infusion rate of citrate and the patient’s resultant citrate load depend on the following:
-
The prescribed citrate/blood flow (QB) ratio: the citrate infusion depends on the dose of citrate (in mmol) the operator decides to infuse per liter of blood flow (QB)—mmol (citrate) per l (blood). Companies suggest starting with an initial dose of 3 or 4 mmol of citrate per liter of blood. The target range of ionized calcium in the circuit is 0.25–0.35 mmol/l (slightly variable in the literature) [24, 25]. An ionized calcium of <0.2 mmol/l prevents activation of coagulation cascades and platelets. The infusion rate of the citrate solution is modified according to the ionized calcium concentration sampled from the outflow line (Fig. 28.3). In modern CRRT machines, the citrate administration rate is electronically coupled with blood flow.
-
The blood flow rate—citrate administration is coupled with the blood pump. Since a citrate dose/QB ratio is set by the operator and modifiable at any time, depending on the ionized calcium concentration in the outflow line, the higher the QB, the higher the citrate infusion into the extracorporeal circuit.
-
Concentration of the citrate solution used: two main solutions are commonly used. A diluted solution (e.g., 18 mmol of citrate per l of solution; bags of 5 l) or a concentrated solution (e.g., 136 mmol of citrate per l of solution; bags of 2 l). Once the dose is prescribed and the QB set, the citrate solution used will depend on the concentration. By changing the concentration, the total amount of citrate infused into the extracorporeal circuit does not change; what will change will be the flow of the solution infused into the inflow line via the pre-dilution line.
Importantly, the citrate–calcium complex has a molecular weight of 298 Da, high hydrosolubility (due to the negative charge of a free carboxylate radical) and a sieving coefficient of 1 [25]. Thus, up to 50% or 30–60% of the infused citrate–calcium complexes are removed via the hemofilter during the first passage (Table 28.1). Consequently, depending on the prescription, the citrate–calcium complexes can be actively removed in the effluent flow (QEFF). By increasing the CRRT dose, dialysate flow (QD), and/or an increase in replacement flow (QR POST), more citrate–calcium complexes will be filtered into the QEFF leaving the extracorporeal circuit and eventually reducing the citrate load .
Depending on the removal rate of citrate–calcium complexes, some degree of hypocalcemia (and hypomagnesemia) will occur and to avoid a negative calcium balance an infusion of calcium is recommended and a mandatory step in any protocol specifically dedicated to regional citrate anticoagulation (calcium chloride must be infused either in the outflow line—via a dedicated line and pump—or directly through a separate central line). Clinical signs of hypocalcemia in humans appear below a level of 0.8 mmol/l of plasma ionized calcium [26]. The citrate–calcium complexes that are not filtered into the QEFF enter the systemic circulation (citrate load ). In conditions of normal perfusion and oxygenation, citrate–calcium complexes dissociate and under physiological conditions, citrate’s half-life is approximately 5 min [25]. The citrate is metabolized via the Krebs’s cycle (a mitochondrial metabolic pathway involved in the chemical conversion of carbohydrates, fats, and proteins to generate adenosine triphosphate [ATP]), being an intermediate in aerobic organisms, mostly in liver cells and also in the skeletal muscle and in the renal cortex releasing sodium as well as calcium ions [25,26,27] (Fig. 28.4). Even though solutes containing citrate vary in concentration, the actual citrate delivery rate to the CRRT extracorporeal circuit ranges from 17 to 45 mmol/h [28].
3.1.2 Acid Base Disorders and Citrate Load Management
Within its metabolic pathway, one molecule of citrate yields energy (2.48 kJ [593 cal]/mmol citrate) [29] and three molecules of bicarbonate represent a consistent source of bases. Metabolic alkalosis can result during regional citrate anticoagulation from two different pathways: (a) citrate solutions have a high sodium content (three Na+ for one citrate molecule); trisodium citrate [Na3C3H5O(COO)3] is a rich source of sodium that may increase the plasma strong ion difference leading to increase in pH; (b) bicarbonate results from the metabolism of citrate as trisodium citrate can react with carbonic acid to form sodium bicarbonate (Table 28.1). Therefore, when citrate is regularly metabolized, regional citrate anticoagulation may be associated with metabolic alkalosis. Nevertheless, AKI and, more generally, critical illness are frequently associated with metabolic acidosis, and the buffer supplementation provided by citrate in terms of bicarbonates could be desirable.
In case of metabolic alkalosis, the operator may modify the CRRT prescription in different ways choosing one or a combination of the following possibilities:
-
By increasing the CRRT dose (QD and/or QR POST), more citrate–calcium complexes will be eliminated in the QEFF reducing the citrate load to the patient.
-
By reducing the citrate dose/QB ratio, less citrate will be infused into the extracorporeal circuit.
-
By reducing QB, less citrate will be infused into the extracorporeal circuit.
In clinical practice, the choice that the operator applies will depend on patient condition: ionized calcium concentration in the outflow line (post-filter), QB, prescribed dose, etc.
Limited QB, aimed at a minimum citrate load administration , is usually recommended during regional citrate anticoagulation, and most protocols using diffusive modes (CVVHD) would recommend QB between 80 and 150 ml/min [25].
In case of metabolic acidosis , it is very important to distinguish citrate accumulation (acidosis due to impaired citrate metabolism) from AKI-related metabolic acidosis. In fact, citrate (C6H5O7) is an organic weak acid and circulating citrate–calcium complexes (C6H5O7 = Ca++) might lead to (potential) plasma acidification that in normal conditions is negligible due to their rapid clearance from the blood (about 5 min). Nevertheless, when citrate catabolism is markedly impaired, citrate–calcium complexes accumulate leading to citrate accumulation that may further worsen any previously existing metabolic acidosis. When a validated protocol is correctly applied, citrate accumulation is unlikely to occur [30]. Citrate accumulation must be promptly diagnosed and in the absence of a specific assay, it can only be suspected by an increased total calcium/ionized calcium ratio > 2.5 when both total and ionized calcium are measured in mmol/l (or > 10 if total calcium is measured in mg/dl) [25, 28] (Fig. 28.5). The accumulation of citrate in a patient’s blood leads to a decrease in the systemic ionized calcium concentration, whereas the bound fraction of calcium rises because the calcium infused to correct the low ionized calcium binds to citrate. Consequently, there is a disproportional increase in total Ca, but ionized calcium remains low. The calcium gap (total calcium—ionized calcium) and the total calcium/ionized calcium ratio increases. Worsening metabolic acidosis and hypocalcemia leading to systolic myocardial dysfunction and vasodilatation could be additional findings.
It is hard to identify those patients who are unable to tolerate the citrate load a priori, but some categories of patients should be considered at risk: acute liver failure or acute-on-chronic liver failure (not an absolute contraindication for regional citrate anticoagulation), circulatory shock with impairment of the Krebs cycle, intoxications causing mitochondrial blockage. Serum lactate concentration may help to appraise this risk since hyperlactatemia is a common finding in these conditions. Nevertheless, it has to be noted that hyperlactatemia per se is not a contraindication for regional citrate anticoagulation.
In general, to minimize citrate accumulation, a few rules can help:
-
Identify high-risk patients for reduced citrate clearance (liver failure, shock, intoxications) and decide whether a different anticoagulation strategy should be provided or a modified regional citrate anticoagulation protocol (e.g., accepting higher intra-filter ionized calcium levels by delivering a reduced citrate load).
-
Use limited QB (to limit citrate administration): since during convective modalities (continuous veno-venous hemofiltration [CVVH]) a low QB is associated with high filtration rate (to increase citrate–calcium complex clearance), and early membrane clogging, using diluted citrate solutions delivered in pre-dilution (QR PRE), may help to minimize this issue. On the other hand, in diffusive modes (CVVHD), low blood flow (>80 ml/min) may still provide adequate blood purification since QD is not restricted by filtration fraction and high flux membranes allow high clearance of citrate–calcium complexes.
-
Increase QD and/or QR POST, depending on the prescribed modality, to increase citrate removal.
In case of metabolic acidosis and a total calcium/ionized calcium ratio < 2.5, an increase in QB should be sufficient to compensate the clinical picture. In fact, an increase in QB will be followed by an increase in citrate infusion and, therefore, bicarbonate production and release into the circulation. Alternatively, or in association, a decrease in QD and/or a decrease in QR POST should reduce the filtration of citrate–calcium complexes in the QEFF increasing the amount of citrate metabolized to bicarbonate. It is important to consider that in case of reduction of a filter’s clearance capacity (e.g., progressive clogging), a decrease in citrate–calcium complex elimination may occur. In such situations, it is important to promptly replace the filter to avoid excessive citrate administration and underdialysis.
3.1.3 Regional Citrate Anticoagulation and Outcomes
Until now, evidence of a reduction in mortality with regional citrate anticoagulation compared to systemic anticoagulation is still lacking, but a prospective randomized controlled trial (RCT) comparing regional citrate anticoagulation with systemic heparin anticoagulation and targeting >1000 patients is currently being executed (Clinicaltrials.gov Identifier: NCT02669589). In the meantime, a prolonged filter lifetime is the most evident positive outcome related to the use of regional citrate anticoagulation as shown by multiple studies [23]: (1) with regional citrate anticoagulation, 17% of all circuits run up to 72 h, but none of those with systemic heparin anticoagulation; (2) clotting is the cause for discontinuation of therapy in 80% of systems using heparin and in 30% of those using regional citrate anticoagulation; (3) the mean hemofilter lifespan/benchmark is about 15–20 h during systemic heparin anticoagulation versus 60 h with regional citrate anticoagulation.
The protocol published by Morgera and collaborators in 2009 gives clear recommendations to adapt the citrate dose following measurement of ionized calcium in the circuit [31]. The same group, in an observational prospective study analyzing 100 filters in 75 patients treated with a CRRT dose of 45 ml/kg/h, showed a mean filter running time of 78 h [32]. Interestingly, 51 circuits had to be replaced because of extended filter running time (96 h), 33 for reasons not related to RRT (62 h), and only 13 due to filter clotting (58 h) [32]. Additional interesting results were as follows: (1) the mean dose during the first 72 h was 49 ml/kg/h; (2) acid–base status after 72 h was well controlled in 62% of patients, metabolic alkalosis occurred in 29%, and metabolic acidosis in 9% and in only 1 patient treatment was stopped because of citrate accumulation; (3) no bleeding complications occurred even if the selected population was deemed at high bleeding risk [32].
3.1.4 Patients at High Risk of Bleeding
Recently, results from an RCT designed to compare CVVH with regional citrate anticoagulation and with no anticoagulation in patients with a high risk of bleeding (admitted to the ICU after major surgery) were published [33]. Fifty-six patients were equally allocated into the regional citrate anticoagulation or no anticoagulation group. Compared to the no anticoagulation group, the regional citrate anticoagulation group had fewer transfusions of packed red blood cells (RBCs) and platelets and a longer filter lifespan. The authors concluded that regional citrate anticoagulation used in CVVH is a safe and effective modality to deliver RRT to patients with an elevated risk profile for bleeding complications. Among the first studies exploring bleeding as a complication of regional citrate anticoagulation, one crossover RCT was published in 2004 [34]. Patients received systemic heparin or regional citrate anticoagulation and those who needed a second CVVH run received the other study medication in a cross-over design until the fourth circuit. Forty-nine circuits were analyzed and major bleeding only occurred during heparin anticoagulation [34]. Morabito and collaborators evaluated 33 cardiac surgery patients who were switched from hemofiltration with no anticoagulation or systemic heparin to regional citrate anticoagulation (using a 12 mmol/l citrate solution). Interestingly, the transition to regional citrate anticoagulation significantly reduced transfusion requirements by more than 50% compared to both systemic heparin and no anticoagulation [35]. Moreover, regional citrate anticoagulation-CVVH filter life (about 50 h) was significantly longer (p < 0.0001) when compared with heparin (30 h) or no anticoagulation (25 h) [35].
3.1.5 Patients with Liver Failure
Patients with liver failure are one of the categories at higher risk for citrate accumulation, and liver dysfunction or failure was originally considered a contraindication for regional citrate anticoagulation because early clinical observations had raised concerns about the safety and efficacy of regional citrate anticoagulation in these patients [3]. However, in patients with liver dysfunction, coagulation is often impaired and even if the bleeding risk is high (e.g., major liver surgery; major surgery in patients with cirrhosis; shock in trauma), the extracorporeal circuit and filter undergo frequent clotting due to a tendency of these patients to have increased clotting [36]. Thus, patients with impaired liver function might particularly benefit from regional citrate anticoagulation versus systemic heparin anticoagulation.
In 2015, Slowinski and collaborators published a multicenter, prospective, observational study, which included 133 patients (48 with normal liver function—bilirubin <2 mg/dl, 43 with mild liver dysfunction—bilirubin 2–7 mg/dl, and 42 with severe liver dysfunction—bilirubin >7 mg/dl) who were treated with regional citrate anticoagulation during CVVHD [37]. Metabolic imbalance was the main focus of the trial. The frequency of safety endpoints [acidosis or alkalosis (pH ≤7.2 or ≥7.55, respectively)] in the three patient strata did not differ and severe acidosis, the most feared complication, was found in 13, 16, and 14% in normal, mild, and severe liver dysfunction groups, respectively (p = 0.95). Only 3 patients showed signs of impaired citrate metabolism. Overall filter patency was 49% at 72 h and after eliminating for interruption of the treatment due to non-clotting causes, estimated 72-h filter survival was 96%. Recently, a systematic review [38], which included 10 studies and 1241 patients with liver failure , concluded that regional citrate anticoagulation can be considered safe in liver failure patients undergoing CRRT, yielding a favorable filter lifespan (55 h). Specifically, the pooled rate of citrate accumulation was 12% and the bleeding rate was 5%. No significant increase in serum citrate was observed at the end of CRRT. Compared with non-liver failure patients, the liver failure patients showed no significant difference in the pH, serum lactate level, or total calcium/ionized calcium ratio during CRRT.
Since liver failure patients represent a category at risk for metabolic derangements, a close monitoring for citrate accumulation is mandatory (but this is also true for all patients undergoing regional citrate anticoagulation).
3.1.6 Hypoxemic Patients
A vast majority of patients are admitted to the ICU with cellular hypoxia due to circulatory and or respiratory failure. The metabolic pathway of citrate is oxygen dependent, and severe hypoxemia or inability to bring oxygen to the cells might impair this cycle and citrate metabolism. Nonetheless, there are very few data in the literature regarding regional citrate anticoagulation in patients with cellular hypoxia. A small study including 10 severely hypoxemic patients (PaO2 < 60 mmHg) concluded that regional citrate anticoagulation can be safely used in patients with hepatic function impairment but may induce acidosis and a decline in serum ionized calcium when used with hypoxemic patients [39]. Hence, hypoxemia should be acknowledged as an important risk factor for citrate accumulation and possibly alternative anticoagulation strategies should be considered. Larger trials are currently awaited to confirm this biologically plausible observation.
In conclusion, understanding of citrate “kinetics” may help the clinician correctly manage regional citrate anticoagulation in any clinical condition. In case of acid–base disorder, clinicians should be able to distinguish citrate overload (metabolic alkalosis) from accumulation (elevated total calcium/ionized calcium ratio, increase need for calcium replacement , and worsening of acidosis). If an initial regional citrate anticoagulation strategy is delivered and eventually stopped due to an emerging contraindication or strategy failure, a switch to an alternative modality should be promptly considered, even within the context of the same CRRT session (e.g., stop regional citrate anticoagulation and start systemic heparin anticoagulation, a possibility that is commonly allowed by third- and fourth-generation machines).
3.2 Regional Heparin–Protamine Anticoagulation
In this strategy, UFH is infused into the inflow line of the extracorporeal circuit, while protamine is infused into the outflow line to neutralize the anticoagulant effect of AT. aPTT must be measured in the circuit and in the systemic circulation [40]. This strategy is not recommended by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines: “It is cumbersome and difficult to titrate because heparin has a much longer half-life than protamine, inducing a risk of rebound. In addition, it exposes the patient to the side-effects of both heparin (mainly the risk of HIT) and protamine (mainly anaphylaxis, platelet dysfunction, hypotension, and pulmonary vasoconstriction with right ventricular failure) and is therefore not recommended” [2]. Even if regional heparin–protamine anticoagulation, in comparison with the other techniques, is more complex and associated with a high risk of adverse effects [13], it can be considered when heparin dosage is increased due to repeated filter clotting and excessively short extracorporeal filter life, regional citrate anticoagulation is unavailable or contraindicated, and UFH in excess may expose the patient to unacceptable bleeding risk. Clinicians applying regional heparin–protamine anticoagulation should be aware that this technique has to be limited to skilled centers and continued for short periods.
4 No Anticoagulation Strategies
The KDIGO guidelines recommend that regional citrate anticoagulation should be the first choice for CRRT in a patient without contraindications for citrate and in patients with a high bleeding risk rather than no anticoagulation. In the ICU, some patients should avoid heparin because of bleeding risk and citrate for contraindications. RRT can be done without anticoagulation, but some aspects should be considered in order to avoid very early clotting of the extracorporeal circuit and filter.
4.1 Determinants of Clotting Risk: Vascular Access, Circuit, Modality
The most frequent clotting sites are the venous access (vascular catheter), the hemofilter, and the venous air trap [41]. In particular, the vascular access, sometimes neglected, should be considered a sort of “Achille’s heel” for CRRT performance and coagulation since a well-functioning vascular access is a key factor to avoid premature failure of the extracorporeal circuit. In fact, catheter malfunction eventually leads to intermittent stasis of blood flow, which promotes clotting and subsequent circuit failure. Site of insertion, catheter length, and size and shape all represent key aspects to carefully consider as soon as the physician has decided that RRT is needed. Inadequate QB has been demonstrated to contribute to circuit failure [42]. Recommended sites of vascular access placement are the right internal jugular vein (with the tip in the right atrium) or femoral vein (with a length > 24 cm). A catheter size around 11.5–12 Fr is also strongly suggested [2, 43, 44]. The subclavian position should be avoided given the high risk of kinking, the potential for subclavian stenosis [45], and inherent risks (pneumothorax, bleeding) [2]. Intrathoracic sites should be avoided in case of high intrathoracic pressures and, similarly, intra-abdominal sites should be avoided in case of intra-abdominal hypertension. Catheters with side holes are discouraged because turbulent flow initiates clotting and contact of the holes with the vessel wall can arrest flow, thereby activating clotting. Short-term catheters for CRRT are made largely of polyurethane or silicone. The first are stiffer, more traumatic for the vessel wall, easier to place, and with a larger inner lumen. The second are more flexible, less traumatic, less easy to place and with a narrower inner lumen (thicker wall).
The bio-incompatibility of the membrane surface sustains a complex activation of tissue factor, leukocytes, and platelets, favoring clotting [46]. To reduce the thrombogenicity of the membrane, surface coating with substances such as heparin or polyethyleneimine has been applied (e.g., Oxiris membrane by Baxter, Cleveland, MS, USA). However, the use of polyethyleneimine-coated membranes has not been demonstrated to prolong circuit lifespan during CVVH without anticoagulation in the critically ill population [47]. Similarly, the use of heparin in the priming solution (a common procedure applied while setting up the machine) did not reduce thrombogenicity of the membrane in continuous veno-venous hemodiafiltration (CVVHDF) [48].
In air trap chambers, the contact of blood with air may favor clotting. The addition of a continuous flow of water may significantly reduce the risk. For example, giving post-dilution fluids into the chamber can create a fluid layer on top of the blood level, possibly reducing clot development.
Hemodialysis is associated with a longer circuit life than hemofiltration [49]. During CVVH (basic solute transport mechanism is convection), hemoconcentration eventually occurs, promoting clotting because of higher concentrations of cells and coagulation factors in the filter. To reduce hemoconcentration, a blood filtration fraction (filtrate/QB) <0.15–0.20 is recommended and since higher blood flows are crucial to keep filtration fraction low, vascular access is key [42]. In order to reduce hemoconcentration, pre-dilution (the fluid lost by ultrafiltration is replaced before the filter) clearly represents a non-pharmacologic measure for clotting prevention. Some studies have demonstrated a longer filter lifespan with pre-dilution [50]. A recent RCT was designed to determine whether QB influences circuit life in CRRT: 96 patients were randomized at 150 or 250 ml/min in CVVH or CVVHD (50% pre-dilution in CVVH and 100% post-dilution in CVVHD; vascular catheter 13.5 Fr). The authors found no difference in extracorporeal circuit and filter lifespan: 462 circuits showed a median life for the first circuit (clotted) of 9 h (150 ml/min) vs 10 h (250 ml/min); p = 0.37. It should be underlined that patients at risk of bleeding received no anticoagulation, and regional heparin–protamine anticoagulation was delivered in the others. Although the external validity of this study can be questioned due to extremely low extracorporeal circuit lives, the important message here is that QB could be inadequate both with excessively low rates (presumably favoring hemoconcentration and coagulation processes) and with excessively high rates (likely due to shear stress at resistance points). Not only should anticoagulation be tailored to patients during CRRT but also many other aspects such as vascular access performance and an optimally coupled QB.
Finally, training and education for staff has a direct relationship to success and therefore circuit life. Machine “troubleshooting” alarms, recognizing access failure and correct use of anticoagulation (non-pharmacologic and pharmacologic), are the key areas for education and training.
5 Conclusion
When a clinician has decided that CRRT is indicated, the choice of the anticoagulation strategy is crucial to guarantee the optimal delivery of dialysis therapy. In patients without absolute contraindications, regional citrate anticoagulation is strongly recommended as it is safe, and effective for both extracorporeal circuit patency and bleeding complications. Regional citrate anticoagulation must be safely managed by an adequately trained staff according to precise protocols, including any deviation for specific patients. When impaired citrate metabolism and accumulation risks are significant (severe liver failure, hypoxemia, shock), UFH may represent a second-line approach. In case of HIT, argatroban could be considered if regional citrate anticoagulation is not efficient. Alternative techniques include LMWH, hirudin, and regional heparin–protamine anticoagulation, which are probably not recommended as routine practice but could be considered in very specific situations.
References
Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.
Kidney Disease Improving Global Outcomes. Kidney disease improving global outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Brandenburger T, Dimski T, Slowinski T, et al. Renal replacement therapy and anticoagulation. Best Pract Res Clin Anaesthesiol. 2017;31:387–401.
Schilder L, Nurmohamed SA, Bosch FH, et al. Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care. 2014;18:1–9.
Stucker F, Ponte B, Tataw J, et al. Efficacy and safety of citrate-based anticoagulation compared to heparin in patients with acute kidney injury requiring continuous renal replacement therapy: a randomized controlled trial. Crit Care. 2015;19:1–9.
Betjes MGH, van Oosterom D, van Agteren M, et al. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol. 2007;20:602–8.
Hetzel GR, Schmitz M, Wissing H, et al. Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant. 2011;26:232–9.
Oudemans-Van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37:545–52.
Liu C, Mao Z, Kang H, et al. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20:1–13.
Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129:2864–72.
Gattas DJ, Rajbhandari D, Bradford C, et al. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med. 2015;43:1622–9.
Tait RC, Walker ID, Reitsma PH, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106–12.
Thota R, Ganti AK, Subbiah S. Apparent heparin resistance in a patient with infective endocarditis secondary to elevated factor VIII levels. J Thromb Thrombolysis. 2012;34:132–4.
Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl):24–38.
Straaten O, Wester JPJ, Leyte A. Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross-over trial comparing two hemofiltration rates. Crit Care. 2009;13:R139.
Oudemans-van Straaten HM, Wester JPJ, De Pont ACJM, et al. Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based? Intensive Care Med. 2006;32:188–202.
Joannidis M, Kountchev J, Rauchenzauner M, et al. Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med. 2007;33:1571–9.
Hein OV, Von Heymann C, Diehl T, et al. Intermittent hirudin versus continuous heparin for anticoagulation in continuous renal replacement therapy. Ren Fail. 2004;26:297–303.
Koster A, Fischer KG, Harder S, et al. The direct thrombin inhibitor argatroban: a review of its use in patients with and without HIT. Biol Targets Ther. 2007;1:105–12.
Di Nisio M, Middeldorp S, Büller H. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–40.
Link A, Girndt M, Selejan S, et al. Argatroban for anticoagulation in continuous renal replacement therapy. Crit Care Med. 2009;37:105–10.
Lehner GF, Schöpf M, Harler U, et al. Repeated premature hemofilter clotting during regional citrate anticoagulation as indicator of heparin induced thrombocytopenia. Blood Purif. 2014;38:127–30.
Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41:2098–110.
Ataullakhanov F, Pohilko A, Sinauridze E, et al. Calcium threshold in human plasma clotting kinetics. Thromb Res. 1994;75:383–94.
Schneider AG, Journois D, Rimmelé T. Complications of regional citrate anticoagulation: accumulation or overload? Crit Care. 2017;21:1–7.
Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2017;56:28–30.
Ricci D, Panicali L, Facchini MG, Mancini E. Citrate anticoagulation during continuous renal replacement therapy. Contrib Nephrol. 2017;190:19–30.
Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2:439–47.
New AM, Nystrom EM, Frazee E, et al. Continuous renal replacement therapy: a potential source of calories in the critically ill. Am J Clin Nutr. 2017;105:1559–63.
Khadzhynov D, Schelter C, Lieker I, et al. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J Crit Care. 2014;29:265–71.
Morgera S, Schneider M, Slowinski T, et al. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37:2018–24.
Kalb R, Kram R, Morgera S, et al. Regional citrate anticoagulation for high volume continuous venovenous hemodialysis in surgical patients with high bleeding risk. Ther Apher Dial. 2013;17:202–12.
Gao J, Wang F, Wang Y, et al. A mode of CVVH with regional citrate anticoagulation compared to no anticoagulation for acute kidney injury patients at high risk of bleeding. Sci Rep. 2019;9:1–10.
Monchi M, Berghmans D, Ledoux D, et al. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med. 2004;30:260–5.
Morabito S, Pistolesi V, Tritapepe L, et al. Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding: a continuous veno-venous hemofiltration protocol with a low concentration citrate solution. Crit Care. 2012;16:R111.
Habib M, Roberts LN, Patel RK, et al. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int. 2014;34:672–8.
Slowinski T, Morgera S, Joannidis M, et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: The Liver Citrate Anticoagulation Threshold (L-CAT) observational study. Crit Care. 2015;19:1–11.
Zhang W, Bai M, Yu Y, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23:1–11.
Gong D, Ji D, Xu B, et al. Regional citrate anticoagulation in critically ill patients during continuous blood purification. Chin Med J. 2003;116:360–3.
Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial. 2009;22:141–5.
Oudemans-Van Straaten HM. Hemostasis and thrombosis in continuous renal replacement treatment. Semin Thromb Hemost. 2015;41:91–8.
Baldwin I, Bellomo R, Koch B. Blood flow reductions during continuous renal replacement therapy and circuit life. Intensive Care Med. 2004;30:2074–9.
Huriaux L, Costille P, Quintard H, et al. Haemodialysis catheters in the intensive care unit. Anaesth Crit Care Pain Med. 2017;36:313–9.
Parienti J-J, Mégarbane B, Fischer MO, et al. Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: a randomized controlled study. Crit Care Med. 2010;38:1118–25.
Schillinger F, Schillinger D, Montagnac R, et al. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–4.
Joannidis M, Oudemans-van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11:1–10.
Schetz M, Van Cromphaut S, Dubois J, et al. Does the surface-treated AN69 membrane prolong filter survival in CRRT without anticoagulation? Intensive Care Med. 2012;38:1818–25.
Opatrný K, Polanská K, Kroužecký A, Vít L, Novák I, Kasal E. The effect of heparin rinse on the biocompatibility of continuous veno-venous hemodiafiltration. Int J Artif Organs. 2002;25:520–8.
Ricci Z, Romagnoli S, Ronco C. Acute kidney injury: to dialyse or to filter? Nephrol Dial Transplant. 2019; Feb 18 https://doi.org/10.1093/ndt/gfz022. [Epub ahead of print].
Uchino S, Bellomo R, Morimatsu H, et al. Continuous renal replacement therapy: a worldwide practice survey: The Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators. Intensive Care Med. 2007;33:1563–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Romagnoli, S., Ricci, Z., Ronco, C. (2020). Extracorporeal Filter and Circuit Patency: A Personalized Approach to Anticoagulation. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2020. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-37323-8_28
Download citation
DOI: https://doi.org/10.1007/978-3-030-37323-8_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37322-1
Online ISBN: 978-3-030-37323-8
eBook Packages: MedicineMedicine (R0)