Abstract
The present chapter describes the main anticoagulant measures required to prevent clotting of blood in the extracorporeal circuit during continuous renal replacement therapy (CRRT). Heparin is the classical choice. However, heparin confers systemic anticoagulation and thereby increases the risk of bleeding. Its efficacy and safety are further compromised by antithrombin deficiency and heparin resistance due to its binding to acute phase proteins and cells. Heparin-induced trombocytopenia is a rare but feared complication of heparin necessitating specific anticoagulant measures. Prostacyclin acts as anticoagulant by inhibiting platelet function, activation and adhesion, and could be used in patients with liver failure, who have coagulopathy and increased risk of both bleeding and thrombosis. The use of citrate provides regional anticoagulation of the circuit. It is better tolerated than heparin and provides less bleeding and longer circuit life. Citrate has therefore become first choice anticoagulant provided that accumulation is monitored, nurses and doctors are trained and the protocol is strictly maintained. CRRT without anticoagulation is an option, but is generally associated with short circuit survival. Finally, several non-anticoagulant measures can be considered to reduce the risk of filter clotting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Continuous Renal Replacement Therapy
- Activate Partial Thromboplastin Time
- Heparin Induce Thrombocytopenia Type
- Extracorporeal Circuit
- Systemic Anticoagulation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Heparin Anticoagulation for Continuous Venovenous Hemofiltration (CRRT)
Unfractionated heparin (UFH) is the anticoagulant most frequently used to prevent thrombosis in the extracorporeal circuit [1]. Its anticoagulant effect is mediated by the binding to antithrombin through a high affinity pentasaccharide sequence, thereby inactivating factors IIa, Xa, IXa, XIa and XIIa. By inactivating thrombin (IIa), UFH also inhibits thrombin-induced activation of platelets and factors V, VIII and XI [2]. After intravenous injection, UFH binds to endothelial cells, macrophages and plasma proteins such as the acute phase proteins factor VIII and fibrinogen, often elevated in critically ill patients. This explains why the response to UFH among critically ill patients is often reduced, a phenomenon known as heparin resistance. UFH is cleared from the circulation by cellular binding and renal elimination. Binding to endothelial cells and macrophages leads to rapid internalization and depolymerization, whereas renal elimination is a much slower process. Two small studies demonstrated that UFH is not cleared by continuous venovenous hemofiltration [3, 4].
The anticoagulant effect of UFH is generally monitored by means of the activated partial thromboplastin time (APTT) or the activated clotting time (ACT). The APTT tests the intrinsic and common pathways of the coagulation cascade, requiring the presence of coagulation factors I, II, V, VIII, IX, X, XI and XII to yield a normal result. The ACT is a point of care test, measuring the time to clotting of whole blood added to a surface activator such as celite, glass or kaolin. The ACT is less accurate than the APTT and is not recommended for the monitoring of treatment with heparin in the intensive care setting [5].
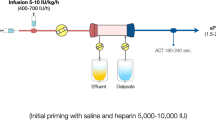
UFH can be used both for priming the circuit and keeping it open during the treatment. Priming the circuit with UFH has not been extensively studied. A small prospective randomized crossover study showed less thrombogenicity after priming the circuit with UFH, but differences in circuit survival were not mentioned [6]. To prevent thrombosis in the extracorporeal circuit during the treatment, UFH can be used systemically or regionally. No dose finding studies have been performed to establish the systemically administered UFH dose needed to prevent thrombosis in this setting. A maximum loading dose of 5,000 IU, followed by a maintenance dose of 5–10 IU/kg/h, aiming at an APTT up to 1.4 times the upper limit of normal has been recommended [7, 8]. Two small studies investigated the efficacy of regional anticoagulation with UFH, infusing UFH before the filter and reversing its action with protamine after the filter to prevent systemic anticoagulation. In the first study, circuit survival times during systemic and regional anticoagulation with UFH were similar, whereas in the second study, circuit survival was shorter during regional anticoagulation with UFH than during systemic anticoagulation with nadroparin [9, 10]. A prospective controlled study among 110 intensive care patients demonstrated that regional anticoagulation with UFH and protamine combined with intravenous prostacyclin increased circuit survival when compared with systemically administered UFH only [11]. Given the many safe citrate protocols (see below), regional anticoagulation with heparin–protamin is nowadays not recommended anymore.
Treatment with UFH carries the risks of both drug resistance and bleeding. Drug resistance is associated with antithrombin deficiency, increased heparin clearance and elevation in heparin binding proteins such as the acute phase proteins factor VIII and fibrinogen [12]. In critically ill patients, elevated levels of factor VIII may shorten the APTT without diminishing the antithrombotic effect of UFH. In this case, monitoring through an anti-Xa assay is recommended, since the result of this assay more closely mirrors the antithrombotic effect of UFH. The risk of heparin-associated bleeding increases with the dose. When doses exceeding 35,000 IU/24 h are used, monitoring by means of the anti-Xa assay is recommended [2].
The use of UFH has several advantages: Its half-life is relatively short (0.5–3 h), it is easily reversible with protamine, the experience with it is large and it is cheap. However, both drug resistance and bleeding are common and UFH carries a 1–5 % risk of heparin induced thrombocytopenia (HIT).
Low molecular weight heparins (LMWHs) are also being used to prevent thrombosis in the extracorporeal circuit, although less often than UFH [1]. The mechanism of action of LMWHs is similar to that of UFH, but because of reduced binding to plasma proteins, their pharmacokinetics are more predictable. They exhibit linear pharmacokinetics with stationary distribution volume and clearance processes, obviating the need of anti-Xa monitoring during continuous dosing. However, a small study reported resistance to LMWH in critically ill patients as well [13]. LMWHs are partially metabolized by desulfatation and depolymerization, and 5–10 % of the injected dose is eliminated by urinary excretion. Clearance by hemofiltration is insignificant [13]. The half-life is longer than that of UFH (2–4 h) and the anticoagulant action is not fully reversible with protamine. However, the incidence of HIT is much lower (0.5–1 %). Several studies have investigated the use of LMWHs during hemofiltration. The drugs most frequently investigated are dalteparin, enoxaparin and nadroparin. LMWHs differ in their ratio of anti-Xa versus anti-IIa inhibition, enoxaparin having the greatest ratio (3.8) and tinzaparin the smallest (1.9) [14]. The use of dalteparin as an anticoagulant during hemofiltration was investigated in three studies, with loading doses of 15–20 IU/kg and maintenance doses of 4–10 IU/kg/h reaching circuit survival times from 15 to 47 h [15, 16]. Enoxaparin was investigated in two studies: A dose of 0.05 – 0.06 mg/kg/h was recommended, reaching circuit survival times of 22–31 h [17]. Nadroparin has been investigated in three studies with doses of 328–475 IU/h reaching circuit survival times of 15–40 h [10, 16, 18].
In summary, when carefully dosed and monitored, both UFH and LMWHs can be used for the prevention of thrombosis in the extracorporeal circuit.
Key Messages Heparin
-
Both UFH and LMWH can be used to prevent coagulation in the extracorporeal circuit if carefully dosed and monitored.
-
Both heparin and LMWH increase the risk of bleeding.
-
Half-life of UFH is 0.5–3 h; UFH is easily reversible with protamine.
-
Half-life of LMWH is 2–4 h; anticoagulant action is only partially reversible with protamine.
-
Critically ill patients can exhibit heparin resistance due to antithrombin deficiency and increased heparin binding to acute phase proteins and cells.
-
The risk of heparin-induced thrombocytopenia (HIT) is lower with use of LMWH than with heparin.
2 Heparin Induced Thrombocytopenia
Heparins, both unfractionated heparins and low molecular weight heparins can bind to platelet factor 4 on the surface of platelets, causing activation and a reduction in the peripheral platelet count [19]. This is termed heparin induced thrombocytopenia type 1, and the fall in peripheral platelet count is typically modest, and the platelet count recovers spontaneously. On the other hand heparin induced thrombocytopenia type 2 leads to marked thrombocytopenia (typically >50 % fall in peripheral platelet count) due to autoantibody mediated platelet activation which can be life-threatening, and necessitates heparin withdrawal to aid recovery [20].
Heparins are large negatively charged proteoglycans which can nonspecifically bind to proteins. As such heparins bind to platelet factor 4 (PF4), but when heparin is in excess (heparin:PF4 27 IU:1 mg) this leads to unfolding of the PF4 molecule, exposing new epitopes to which autoantibodies form. These antibodies then bind to platelet surface FcγIIa receptors activating platelets, and also to endothelial cells increasing tissue factor expression. The ratio of heparin to PF4 is critical, and as such heparin induced thrombocytopenia type 2 is most commonly observed when patients are exposed to larger doses of heparins, for example following cardiac, major vascular and orthopaedic surgery [21]. Similarly the amount of negative charge on the heparin molecule is also important in causing changes in the shape of the PF4 molecule, so the incidence is greater with unfractionated than low molecular weight heparins, and bovine compared to porcine derived heparins. Similarly the incidence of HIT is increased in patients with solid organ malignancies and critically ill patients. Although IgA, IgG and IgM autoantibodies may be formed to the heparin-PF4 complex, it is thought that only IgG are pathological [22].
The diagnosis of heparin induced thrombocytopenia type 2 (HIT) remains a clinical diagnosis. In the critically ill patient there are often many other potential causes of peripheral thrombocytopenia, ranging from reduced platelet production to increased consumption [23]. To aid the clinician in estimating the probability of HIT, Warkentin proposed the 4 Ts scoring system (Table 15.1) [24]. Even so if the diagnosis of HIT is clinically suspected then heparin should be withdrawn, including catheter locks and line flushes whilst awaiting laboratory testing. Most laboratories now have access to ELISA assays designed to detect antibodies to heparin-PF4, but most assays are not specific for the IgGisotype, and report positive results with IgA and IgM antibodies [21, 22]. Only a few laboratories worldwide are able to perform the gold standard 5HT platelet release test for diagnosing HIT. Comparative studies between these assays have suggested that although most ELISA assays report a positive result with an optical density (OD) of >0.6, to have clinically significant disease, the OD is >1.0. As such platelet agglutination assays should also be performed to confirm the ELISA assay result. The reason that HIT remains a clinical diagnosis is that antibodies can also form from other proteins released by activated platelets.
The lower the peripheral platelet count reflects greater platelet activation and platelet adhesion to the endothelium, with greater risk of thrombosis. HIT can lead to both major venous and arterial thrombosis and platelet activation in the lung can lead to acute lung injury, so-called “pseudo-pulmonary” embolus. The management of HIT centers on both withdrawal of all heparins (including heparin flushes, catheter locks and subcutaneous administration) and also systemic anticoagulation to prevent thrombosis. The lower the platelets count the greater the risk of thrombosis and need for systemic anticoagulation. Typically thrombocytopenia starts to recover within 72 h following heparin withdrawal, and if there is no response to heparin withdrawal, then an alternative explanation for thrombocytopenia should be considered. Currently systemic anticoagulation options include the direct thrombin inhibitor argatroban, and the heparinoids, danaparoid and fondaparinux [25, 26]. Although danaparoid may cause cross reactivity with ELISA assays for HIT, this has not been reported to have adverse clinical consequences. Argatroban is a reversible thrombin inhibitor and requires a continuous infusion, with the infusion adjusted to maintain an activated partial thromboplastin ratio (aPPTr) of 2.0–2.5, and as it is hepatically metabolised then much lower dosages are required for patients with liver disease. Both danaparoid and fondaparinux are renally excreted and accumulate in patients with acute kidney injury and chronic kidney disease. As they have minimal effect on the aPPTr, monitoring requires measurement of anti-Xa activity, aiming for a therapeutic window of 0.4–0.6 IU/ml. Given the procoagulant nature of HIT, regional anticoagulants such as citrate for CRRT do not provide the systemic anticoagulation required to prevent systemic thrombosis, and as such additional systemic anticoagulation should be considered.
Once the platelet count has recovered to >150,000 × 106/l, then warfarin therapy can be considered, as there is a risk of precipitating skin gangrene if warfarin therapy is started before the platelet count has recovered. Argatroban prolongs the prothrombin time, and therefore caution is required when converting patients from intravenous argatroban to oral warfarin therapy. For the majority of patients HIT antibodies are a temporary phenomenon and disappear over time. However before considering rechallenging patients with heparin, then both ELISA and platelet agglutination assays should be negative on at least two occasions (Table 15.1).
Key Messages Heparin Induced Thrombocytopenia
-
Consider heparin induced thrombocytopenia in any patient with a 50 % fall in peripheral platelet count after starting heparin within the previous 10 days.
-
Heparin induced thrombocytopenia remains a clinical diagnosis. Use the “4 Ts” scoring system to estimate probability.
-
With a score ≥6, withdraw all heparins immediately whilst awaiting confirmation with ELISA and platelet agglutination assays, and start alternative systemic anticoagulation with argatroban as first choice and consider additional citrate anticoagulation for the circuit.
-
With a score between 3 and 6, order ELISA testing, while continuation of heparins awaiting results seems to be justified.
-
The lower the platelet count the greater the risk of thrombosis and systemic anticoagulation is required.
-
For a definite diagnosis of HIT, positive ELISA testing should be confirmed with a platelet agglutination assay.
3 Prostacyclin
Prostacyclin is an anti-hemostatic prostaglandin that inhibits platelet function, activation and adhesion by diminishing the expression of platelet fibrinogen receptors and P-selectin and reduces heterotypic platelet-leukocyte aggregation. At higher infusion doses it is also a potent smooth muscle relaxant and vasodilator. It is available for clinical therapy as a synthetic analogue, epoprostenol. It is produced primarily in the endothelial and smooth muscle cells of blood vessels. Prostacyclin has a short half-life of 2–3 min with a clinical effect on end-organs and platelets of approximately 30 min [27, 28]. It has been used in combination with low dose heparin infusions and on its own as an adjunct to prolong hemofilter life during intermittent dialysis and CRRT [29–31].
3.1 Indication
Prostacyclin has primarily been used as an anti-hemostatic to enhance hemofilter life in patients with acute kidney injury and acute liver failure and concern to avoid hemorrhage. Although many such patients have severe coagulopathy, some continually thrombosehemofilters during CRRT, even while suffering from ongoing hemorrhage, usually gastrointestinal. This is likely due to deficiencies in the synthesis of anti-thrombotic substances such as anti-thrombin III, protein C and protein S [32]. Since prostacyclin exerts its effect on platelet function, it unlikely to be of significant value in patients with severe thrombocytopenia.
Although regional citrate anticoagulation has been shown to be more effective in maintaining hemofilter patency, its use is often not possible as these patients are at risk of citrate accumulation as citrate is primarily metabolized in the liver [33]. In this difficult clinical situation, the use of prostacyclin may be valuable in prolonging hemofilter life without adding extra risk of bleeding [34].
3.2 Practical Considerations
Prostacyclin is available clinically as epoprostenol in a freeze dried powder which must be reconstituted as directed, only using the supplied diluent containing a glycine buffer to maintain a pH of 10–11. It must be infused via a separate infusion line to avoid inactivation by acidic drugs such as catecholamine vasopressor agents. It is important that only syringe infusion pumps with noncompliant intravenous tubing external to the pumps on the CRRT machine are used for infusion when using prostacyclin with CRRT. The infusion pumps on some CRRT machines, although ostensibly syringe pumps, operate by using “micro boluses” of drug rather than a smooth continuous infusion and may be associated with the development of intermittent episodes of hypotension. A similar issue may be seen using other infusion pumps that use peristaltic mechanisms. Since prostacyclin does not interfere with the coagulation systems, there is no simple clinical means of readily monitoring and titrating the infusion dose although thromboelastography could be used for this purpose [27].
3.3 Alone or Incombination with Heparin
Prostacyclin has been used both as a sole anti-hemostatic agent and in combination with unfractionated heparin. It has been shown to extend hemofilter survival, particularly when used in combination with low dose heparin [22–24].
The use of prostacyclin combined with regional anticoagulation with prefilter heparin and postfilter protamine has been studied in a prospective randomized trial and provided excellent filter survival and minimal bleeding when compared with conventional heparin [11].
3.4 Dose and Side Effects
Because of its vasodilator properties, prostacyclin can cause hypotension, although, the typical infusion doses used to enhance hemofilter life by antagonism of platelet activation and aggregation is in the range of 3–5 ng/kg/min and, generally does not impact on blood pressure significantly in most patients, although occasionally the dose of vasopressor infusions needs to be increased. (The typical dose required to achieve pulmonary vasodilatation is up to 35 ng/kg/min).
The main side effect of prostacyclin is hypotension caused by vasodilatation which may be managed by ensuring adequate fluid volume status, by reducing the rate of infusion or by titrating a vasopressor infusion. Theoretically, the risk of bleeding increases with platelet inhibition. However, in an observational study of 51 critically ill patients undergoing CRRT with prostacyclin as sole anti-hemostatic agent there was minimal bleeding (one episode per 1,000 h treatment) although 15 % required either fluids or vasopressor therapy either for the first time or an increase in dose following initiation of prostacyclin [34]. Prostacyclin is relatively expensive, but at the low doses used for CRRT the cost is similar to citrate regional anticoagulation.
4 Citrate Anticoagulation for Continuous Renal Replacement Therapy
4.1 Summary
Citrate acts as anticoagulant by chelating ionized calcium (iCa) and thereby causing hypocalcemia in the filter. At an iCa concentration of 0.25 mmol/L, anticoagulation is maximal. Part of the citrate is removed by dialysis or filtration, the remains enter the systemic circulation. Citrate is rapidly metabolized in the mitochondria, the chelated calcium is released and the lost calcium is replaced. Citrate therefore provides regional anticoagulation and does not increase the risk of bleeding.
Sodium citrate is a buffer as well provided that citrate is metabolized. The buffer strength is equivalent to 3 mmol bicarbonate per mmol citrate if all cations are sodium (trisodium citrate) and less so if part of the cations are hydrogen (citric acid).
Citrate anticoagulation is better tolerated than heparin, and is associated with less bleeding and generally longer circuit survival. Its main risk is accumulation due to decreased metabolism as a result of liver failure or systemic hypoperfusion. Accumulation is characterized by a decrease in iCa, a rise in total Ca and metabolic acidosis. It is monitored by measuring systemic iCa (to adjust calcium replacement) and acid–base balance. A rise in total/iCa is the most sensitive marker of accumulation.
4.2 Introduction
Sodium citrate has become the first choice anticoagulant for continuous renal replacement therapy (CRRT). It provides regional anticoagulation of the circuit, without increasing the patient’s risk of bleeding. Its anticoagulant properties are due to the chelation of ionized calcium (iCa) thereby causing hypocalcemia in the circuit. Calcium is a necessary cofactor in the formation of thrombin. Coagulation is inhibited as soon as ionCa falls below 0.50 mmol/L, and is maximal at an iCa concentration of 0.25 mmol/l [35].
4.3 Regional Anticoagulation
Within the CRRT circuit, sodium citrate is administered before the filter. Citrate dose is titrated to blood flow. Postfilter iCa can be monitored to fine-tune anticagulation by adjusting citrate dose to iCa targets (0.25–0.35 mmol/L),but many protocols use a fixed citrate to blood flow proportion. Part of the calcium citrate complexes are removed by dialysis or filtration. The remains enter the patient’s circulation to be metabolized in the Krebs cycle of liver, kidney and muscle. The chelated calcium is released, while the calcium lost by dialysis or filtration is replaced. Regional anticoagulation is the result, and this is the main benefit of citrate [8, 36].
4.4 Citrate Is a Buffer
Sodium citrate is a buffer base as well. According to the classical concept, each mole of trisodium citrate provides a buffer equivalent of three moles of bicarbonate, if and when citrate is metabolized. According to the Stewart concept of acid–base [30], sodium citrate increases the strong ion difference (SID = (Na+ + K+ + Ca2+ + Mg2+) – (Cl− + lactate−)) provided that citrate is metabolized. This concept explains why the buffer strength of the citrate solution depends on the accompanying cation [31]. The buffer strength is higher when using a trisodium citrate solution and lower when part of the cations are hydrogen, as is the case in the acid dextrose citrate (ACD-A) solution, as used in some protocols, in which 30 % of the cations consist of hydrogen [37–39].
4.5 Principles of the Citrate Circuit
Citrate is administered before the filter, either as a separate more or less concentrated trisodium citrate solution [18, 40–43] or as part of an isotonic balanced calcium-free predilution hemofiltration solution. In the latter, the bicarbonate is replaced by citrate and the solution is calcium-free [44–46]. When a separate sodium citrate solution is used, the associated dialysate or postdilution hemofiltration solution contains no or less bicarbonate and less sodium to compensate for the citrate buffer and the sodium content of the citrate solution. In most protocols, calcium is replaced separately. It should be noted that citrate additionally chelates magnesium and that citrate CRRT can lead to a negative magnesium balance because the magnesium content of most CRRT solutions is too low [47].
4.6 Modalities
Different modalities for citrate are in use: Hemodialysis [41],predilution hemofiltration [44–46], postdilution hemofiltration [18, 43] or hemodiafiltration [42]. Modern CRRT devices have a strict citrate protocol incorporated in the software, allowing for choices to determine the desired citrate concentration in the filter (2.5–4.5 mmol/L blood flow), to adjust acid–base derangements (more or less buffer supply) and to adjust calcium infusion rate to compensate for calcium loss (zero calcium balance). Each protocol has strict rules for citrate dosing, acid base compensation and calcium replacement. These rules depend on the composition of the fluids in use and cannot be generalized. The use of a strict protocol, adherence to the protocol and training are crucial for safety of the method.
4.7 Monitoring of Citrate Anticoagulation
Citrate anticoagulation is monitored by measuring ion- and total calcium concentration and blood gas analysis (for acid–base) in systemic blood at 6–8 h interval. Chloride and lactate can be measured to monitor anion gap and tissue perfusion, but this is not obligatory. Postfilter iCa can be measured to fine-tune anticoagulation.
4.8 Citrate Accumulation
Metabolism of citrate is conditional for its safe use. Citrate is metabolized in the mitochondria of liver, kidney and muscle and is decreased in patients with liver cirrhosis [43] and systemic hypoperfusion. Although a high lactate concentration at the start of CRRT should raise awareness of the risk of citrate accumulation, septic patients with a high lactate level and other shock patients generally tolerate citrate remarkably well if circulation improves. Citrate anticoagulation is even feasible in patients severe lactate acidosis due to metformine intoxication (personal experience). Citrate is likely to accumulate in patients with persistent severe cardiogenic shock, ischemic hepatitis and poor muscle perfusion [44], because the Krebs cycle only operates under aerobic conditions. However, most critically ill patients tolerate citrate better than heparin [18, 48]. Even in patients with liver failure, the use of citrate is feasible with intensified monitoring [49].
When citrate accumulates, iCa concentration in the patient’s blood falls, while total calcium rises due to chelation with citrate and replacement of calcium according to the protocol. A rise of total/iCa ratio is the most sensitive sign of citrate accumulation [50]. A rise above 2.25–2.5 indicates citrate accumulation. Second, if citrate is not metabolized, acidosis will ensue and anion gap will rise, because the alkalizing effect of citrate depends on its metabolism. Due to liver failure or severe hypoperfusion, citrate accumulation is associated with a rise in lactate as well. Citrate accumulation is seen in the most severely ill patients and seems a predictor of mortality [51].
Thus iCa, total calcium, total/iCa ratio, blood gas analysis (for acid base) and lactate are used to monitor citrate accumulation. In patients at risk, intensified monitoring is recommended, initially at 2-h interval. If the total/iCa ratio rises, the risks of continuing citrate should be weighed against the use of alternative anticoagulation (heparin) with risk of bleeding or CRRT without anticoagulation (early circuit clotting). In general, citrate is not toxic. If acid–base is in balance and ionized calcium can be controlled with additional calcium supplementation, the continuation of citrate seem safer than the alternatives [49]. If calcium ratio plateaus, monitoring interval can be prolonged.
4.9 Benefits of Citrate Anticoagulation
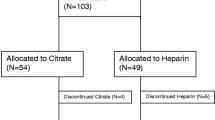
Clinical benefits of citrate are primarily related to less bleeding, a better circuit survival and lower requirement for blood products. The use of citrate does not increase the patient’s risk of bleeding. In addition, anticoagulation with citrate seems more effective than with heparin, especially when higher doses are used, and the calcium is replaced outside the CRRT circuit. Three meta-analyses, one including up to six randomized controlled trials (comparing citrate to unfractionated heparin) [52, 53], to low molecular weight heparin [18] or to heparin/protamine [54] with a total of 417 patients and one including four studies (comparing citrate to unfractionated heparin) found less bleeding and a longer circuit survival time with citrate [55–57]. After this meta-analysis, a large multicenter trial has appeared, showing that citrate was superior in terms of safety, efficacy and costs [58]. The largest randomized controlled trial (200 patients) found an unexpected survival benefit for citrate. This benefit could not be fully explained by less bleeding and not to less circuit clotting. It was especially seen in younger patient, surgical patients, and patients with sepsis or those with a high degree of organ failure, suggesting a role of citrate limiting inflammation or oxidative stress [18]. Compared to heparin, citrate confers less complement activation and neutrophil degranulation in the filter and less endothelial activation [58]. Up to now, this survival benefit had not been confirmed by other studies.
Key Messages Citrate
-
Citrate anticoagulation is first choice anticoagulant for CRRT.
-
The main benefit of citrate anticoagulation for CRRT is that it does not increase the patient’s risk of bleeding.
-
Citrate anticoagulation is associated with a less bleeding, less transfusion and longer circuit life than heparin in patients without an increased risk of bleeding.
-
The main limitation of citrate anticoagulation is accumulation, developing in case of hypoperfusion or severe liver dysfunction.
-
Citrate accumulation is associated with a decrease in iCa, a rise in total Ca and increase in total/iCa ratio, metabolic acidosis and an increase in lactate.
-
If the total/iCa ratio is higher than 2.5, continuation of citrate is only safe when acid base balance and ionized calcium concentration are under control. If not, citrate should be reduced or discontinued.
5 CRRT Without Anticoagulation
Most authors and guidelines recommend the use of some form of anticoagulation to maintain circuit patency during CRRT. This is to minimize blood loss in clotted hemofilters, maximize delivered dose of therapy and reduce nursing workload and complexity of care [55]. It has been shown that frequent hemofilter clotting is associated with more blood transfusions due to blood loss in the discarded hemofilters [59].
One of the major concerns with the use of CRRT is that, in order to maintain the extracorporeal circuit continuously, it usually requires some form of anticoagulation to prevent frequent circuit clotting. Although heparin is the most commonly used anticoagulant, it has been shown to be associated with bleeding complications, especially in high-risk patients receiving CRRT [60].
Because of this, using no anticoagulation is an option to be considered during CRRT in critically ill patients with severe coagulopathy and thrombocytopenia. Interestingly, using CRRT without anticoagulation has been reported more commonly than the use of anticoagulation in some large randomized trials of CRRT. In the NIH ATN trial, 55 % of patients treated with CVVHDF received no anticoagulation while 20 % were anticoagulated with heparin, 20 % received citrate regional anticoagulation and 5 % received other forms of anticoagulation [61]. Over 1,500 patients were treated with CVVHDF in the RENAL study. In that study, 46 % received no anticoagulation while the others received some form of anticoagulation with heparin [62]. This suggests that, despite guidelines suggesting that anticoagulation should be provided, using CRRT without anticoagulation is a default strategy for some prescribing physicians and in some critical care units.
Some studies have shown little difference in CRRT hemofilter survival between circuits with no anticoagulation compared to low dose heparin [1, 63, 64]. In general, hemofilter life is longer with more intensive heparin anticoagulation. It is noteworthy, however, that platelet levels were significantly lower in the group without anticoagulation suggesting consumption in the extracorporeal circuit. While some studies have shown a mean hemofilter life of up to 20 h without anticoagulation, this appears to be population dependent with other studies demonstrating more typical hemofilter survival times of 11–16 h. A study comparing circuit survival with citrate regional anticoagulation or no anticoagulation showed significantly longer CRRT circuit survival with citrate with a mean circuit survival time of 41 h using citrate versus 12 h with no anticoagulation [45]. Despite this, many critical care units find the relative complexity of citrate regional anticoagulation intimidating and prefer to perform CRRT without anticoagulation despite the shorter hemofilter survival.
6 Non-anticoagulant Measures to Maximize Hemofilter Survival
It is important to consider non-anticoagulant aspects of CRRT circuit management to optimize hemofilter survival, especially if no anticoagulation or low heparin doses are used [29]. The most important of these is to ensure the placement of a large bore double lumen central venous hemodialysis catheter in the right jugular or femoral position (straight course) to minimize blood flow interruptions, which promote clotting [64]. It is also important to ensure that the filtration fraction (the proportion of blood flow per minute that is removed as plasma filtrate) is maintained at 25 % or less to avoid hemoconcentration and red blood cell sludging in the hemofilter.
It has been suggested by some authors that delivering replacement fluid in a predilution mode may improve hemofilter life [1, 10, 63], while others show no significant difference [45].
Some CRRT machines incorporate deaeration chambers with a blood/air interface. This tends to encourage clotting but can be prevented by the infusion of at least some or all of the replacement fluid postfilter so that there is an air/replacement fluid/blood interface (Table 15.2).
References
Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33(9):1563–70.
Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e24S–43S.
Singer M, McNally T, Screaton G, Mackie I, Machin S, Cohen SL. Heparin clearance during continuous veno-venous haemofiltration. Intensive Care Med. 1994;20(3):212–5.
Despotis GJ, Levine V, Filos KS, Joiner-Maier D, Joist JH. Hemofiltration during cardiopulmonary bypass: the effect on anti-Xa and anti-IIa heparin activity. Anesth Analg. 1997;84(3):479–83.
De Waele JJ, Van Cauwenberghe S, Hoste E, Benoit D, Colardyn F. The use of the activated clotting time for monitoring heparin therapy in critically ill patients. Intensive Care Med. 2003;29(2):325–8.
Richtrova P, Opatrny Jr K, Vit L, Sefrna F, Perlik R. The AN69 ST haemodialysis membrane under conditions of two different extracorporeal circuit rinse protocols a comparison of thrombogenicity parameters. Nephrol Dial Transplant. 2007;22(10):2978–84.
De Pont AC, On behalf of the NVIC Committee Nephrology and Intensive Care. Guidelines for anticoagulation with unfractionated heparin and low molecular weight heparins during continuous venovenous hemofiltration in the intensive care. Neth J Crit Care. 2005;9:308–12.
Oudemans-Van Straaten HM, Wester JP, de Pont AC, Schetz MR. Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based? Intensive Care Med. 2006;32(2):188–202.
Biancofiore G, Esposito M, Bindi L, Stefanini A, Bisa M, Boldrini A, et al. Regional filter heparinization for continuous veno-venous hemofiltration in liver transplant recipients. Minerva Anestesiol. 2003;69(6):527–34; 34–8.
van der Voort PH, Gerritsen RT, Kuiper MA, Egbers PH, Kingma WP, Boerma EC. Filter run time in CVVH: pre- versus post-dilution and nadroparin versus regional heparin-protamine anticoagulation. Blood Purif. 2005;23(3):175–80.
Fabbri LP, Nucera M, Al Malyan M, Becchi C. Regional anticoagulation and antiaggregation for CVVH in critically ill patients: a prospective, randomized, controlled pilot study. Acta Anaesthesiol Scand. 2010;54(1):92–7.
Young E, Podor TJ, Venner T, Hirsh J. Induction of the acute-phase reaction increases heparin-binding proteins in plasma. Arterioscler Thromb Vasc Biol. 1997;17(8):1568–74.
Oudemans-Van Straaten HM, van Schilfgaarde M, Molenaar PJ, Wester JP, Leyte A. Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross-over trial comparing two hemofiltration rates. Crit Care. 2009;13(6):R193.
Davenport A. Review article: low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology (Carlton). 2009;14(5):455–61.
Reeves JH, Cumming AR, Gallagher L, O'Brien JL, Santamaria JD. A controlled trial of low-molecular-weight heparin (dalteparin) versus unfractionated heparin as anticoagulant during continuous venovenous hemodialysis with filtration. Crit Care Med. 1999;27(10):2224–8.
de Pont AC, Oudemans-Van Straaten HM, Roozendaal KJ, Zandstra DF. Nadroparin versus dalteparin anticoagulation in high-volume, continuous venovenous hemofiltration: a double-blind, randomized, crossover study. Crit Care Med. 2000;28(2):421–5.
Tsang DJ, Tuckfield A, Macisaac CM. Audit of safety and quality of the use of enoxaparin for anticoagulation in continuous renal replacement therapy. Crit Care Resusc. 2011;13(1):24–7.
Oudemans-Van Straaten HM, Bosman RJ, Koopmans M, van der Voort PH, Wester JP, van der Spoel JI, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37(2):545–52.
Davenport A. Antibodies to heparin-platelet factor 4 complex: pathogenesis, epidemiology, and management of heparin-induced thrombocytopenia in hemodialysis. Am J Kidney Dis. 2009;54(2):361–74.
Lee GM, Arepally GM. Diagnosis and management of heparin-induced thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):541–63.
Keeling D, Davidson S, Watson H. The management of heparin-induced thrombocytopenia. Br J Haematol. 2006;133(3):259–69.
Arnold DM, Kukaswadia S, Nazi I, Esmail A, Dewar L, Smith JW, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost. 2013;11(1):169–76.
Warkentin TE, Cook DJ. Heparin, low molecular weight heparin, and heparin-induced thrombocytopenia in the ICU. Crit Care Clin. 2005;21(3):513–29.
Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160–7.
Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med. 2013;368(8):737–44.
Eichler P, Friesen HJ, Lubenow N, Jaeger B, Greinacher A. Antihirudin antibodies in patients with heparin-induced thrombocytopenia treated with lepirudin: incidence, effects on aPTT, and clinical relevance. Blood. 2000;96(7):2373–8.
Scheeren T, Radermacher P. Prostacyclin (PGI2): new aspects of an old substance in the treatment of critically ill patients. Intensive Care Med. 1997;23(2):146–58.
Langenecker SA, Felfernig M, Werba A, Mueller CM, Chiari A, Zimpfer M. Anticoagulation with prostacyclin and heparin during continuous venovenous hemofiltration. Crit Care Med. 1994;22(11):1774–81.
Camici M, Evangelisti L. Prostacyclin and heparin during haemodialysis: comparative effects. Life Support Syst. 1986;4(3):205–9.
Zusman RM, Rubin RH, Cato AE, Cocchetto DM, Crow JW, Tolkoff-Rubin N. Hemodialysis using prostacyclin instead of heparin as the sole antithrombotic agent. N Engl J Med. 1981;304(16):934–9.
Davenport A, Will EJ, Davison AM. Comparison of the use of standard heparin and prostacyclin anticoagulation in spontaneous and pump-driven extracorporeal circuits in patients with combined acute renal and hepatic failure. Nephron. 1994;66(4):431–7.
Agarwal B, Gatt A, Riddell A, Wright G, Chowdary P, Jalan R, et al. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int. 2013;84(1):158–63.
Balik M, Waldauf P, Plasil P, Pachl J. Prostacyclin versus citrate in continuous haemodiafiltration: an observational study in patients with high risk of bleeding. Blood Purif. 2005;23(4):325–9.
Fiaccadori E, Maggiore U, Rotelli C, Minari M, Melfa L, Cappe G, et al. Continuous haemofiltration in acute renal failure with prostacyclin as the sole anti-haemostatic agent. Intensive Care Med. 2002;28(5):586–93.
Calatzis A, Toepfer M, Schramm W, Spannagl M, Schiffl H. Citrate anticoagulation for extracorporeal circuits: effects on whole blood coagulation activation and clot formation. Nephron. 2001;89(2):233–6.
Joannidis M, Oudemans-Van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11(4):218.
Swartz R, Pasko D, O'Toole J, Starmann B. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin Nephrol. 2004;61(2):134–43.
Gupta M, Wadhwa NK, Bukovsky R. Regional citrate anticoagulation for continuous venovenous hemodiafiltration using calcium-containing dialysate. Am J Kidney Dis. 2004;43(1):67–73.
Mariano F, Tedeschi L, Morselli M, Stella M, Triolo G. Normal citratemia and metabolic tolerance of citrate anticoagulation for hemodiafiltration in severe septic shock burn patients. Intensive Care Med. 2010;36(10):1735–43.
Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int. 1990;38(5):976–81.
Morgera S, Scholle C, Melzer C, Slowinski T, Liefeld L, Baumann G, et al. A simple, safe and effective citrate anticoagulation protocol for the genius dialysis system in acute renal failure. Nephron Clin Pract. 2004;98(1):c35–40.
Tolwani AJ, Prendergast MB, Speer RR, Stofan BS, Wille KM. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006;1(1):79–87.
Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med. 2004;30(2):260–5.
Palsson R, Niles JL. Regional citrate anticoagulation in continuous venovenous hemofiltration in critically ill patients with a high risk of bleeding. Kidney Int. 1999;55(5):1991–7.
Nurmohamed SA, Vervloet MG, Girbes AR, Ter Wee PM, Groeneveld AB. Continuous venovenous hemofiltration with or without predilution regional citrate anticoagulation: a prospective study. Blood Purif. 2007;25(4):316–23.
Egi M, Naka T, Bellomo R, Cole L, French C, Trethewy C, et al. A comparison of two citrate anticoagulation regimens for continuous veno-venous hemofiltration. Int J Artif Organs. 2005;28(12):1211–8.
Brain M, Anderson M, Parkes S, Fowler P. Magnesium flux during continuous venovenous haemodiafiltration with heparin and citrate anticoagulation. Crit Care Resusc. 2012;14(4):274–82.
Schilder L, Azam Nurmohamed SA, Bosch FH, Purmer IM, den Boer SS, Kleppe CG, et al. Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care. 2014;18(4):472.
Faybik P, Hetz H, Mitterer G, Krenn CG, Schiefer J, Funk GC, et al. Regional citrate anticoagulation in patients with liver failure supported by a molecular adsorbent recirculating system. Crit Care Med. 2011;39(2):273–9.
Bakker AJ, Boerma EC, Keidel H, Kingma P, van der Voort PH. Detection of citrate overdose in critically ill patients on citrate-anticoagulated venovenous haemofiltration: use of ionised and total/ionised calcium. Clin Chem Lab Med. 2006;44(8):962–6.
Link A, Klingele M, Speer T, Rbah R, Poss J, Lerner-Graber A, et al. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit Care. 2012;16(3):R97.
Hetzel GR, Schmitz M, Wissing H, Ries W, Schott G, Heering PJ, et al. Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant. 2011;26(1):232–9.
Betjes MG, van Oosterom D, van Agteren M, van de Wetering J. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol. 2007;20(5):602–8.
Fealy N, Baldwin I, Johnstone M, Egi M, Bellomo R. A pilot randomized controlled crossover study comparing regional heparinization to regional citrate anticoagulation for continuous venovenous hemofiltration. Int J Artif Organs. 2007;30(4):301–7.
Zhang Z, Hongying N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med. 2012;38(1):20–8.
Liao YJ, Zhang L, Zeng XX, Fu P. Citrate versus unfractionated heparin for anticoagulation in continuous renal replacement therapy. Chin Med J (Engl). 2013;126(7):1344–9.
Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;59(6):810–8.
Schilder L, Nurmohamed SA, Ter Wee PM, Paauw NJ, Girbes AR, Beishuizen A, et al. Citrate confers less filter-induced complement activation and neutrophil degranulation than heparin when used for anticoagulation during continuous venovenous haemofiltration in critically ill patients. BMC Nephrol. 2014;15(1):19.
Cutts MW, Thomas AN, Kishen R. Transfusion requirements during continuous veno-venous haemofiltration: the importance of filter life. Intensive Care Med. 2000;26(11):1694–7.
van de Wetering J, Westendorp RG, van der Hoeven JG, Stolk B, Feuth JD, Chang PC. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996;7(1):145–50.
Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20.
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38.
Tan HK, Baldwin I, Bellomo R. Continuous veno-venous hemofiltration without anticoagulation in high-risk patients. Intensive Care Med. 2000;26(11):1652–7.
Baldwin I, Bellomo R, Koch B. Blood flow reductions during continuous renal replacement therapy and circuit life. Intensive Care Med. 2004;30(11):2074–9.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing
About this chapter
Cite this chapter
Oudemans-van Straaten, H.M., de Pont, AC.J.M., Davenport, A., Gibney, N. (2015). Anticoagulation for Continuous Renal Replacement Therapy. In: Oudemans-van Straaten, H., Forni, L., Groeneveld, A., Bagshaw, S., Joannidis, M. (eds) Acute Nephrology for the Critical Care Physician. Springer, Cham. https://doi.org/10.1007/978-3-319-17389-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-17389-4_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17388-7
Online ISBN: 978-3-319-17389-4
eBook Packages: MedicineMedicine (R0)