Abstract
The skin is the largest organ in the human body; however, it is only a few millimeters thick. Among the main functions of the skin are to serve as a barrier for protection against physical and biological insults, as a thermal regulator to control internal temperatures, and as a sensor of physical stimulus that could lead to pleasant or harmful experiences. This highly-integrated sensory and regulatory armor is also capable of self-repair in response to injury, albeit the quality and extent of healing are determined by the skin condition and the type and size of the wound. In general, the wound healing process of skin comprehends four stages, which are hemostasis, inflammation, proliferation, and remodeling. These stages can be affected by internal physiological conditions and external environmental factors compromising the healing of the wound, for example, chronic wounds and bacterial infections. This chapter opens with an overview of the physiology of skin and skin wound healing. This overview is followed by a review of nanomaterial technologies and methods that have been investigated for the treatment of skin wounds. The chapter ends with an outlook of nanotechnology strategies for improving the treatment of skin wounds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
6.1 Introduction
Skin is the largest organ in the human body. The biology of the skin involves a myriad of cell types and structures that work together to enable key organ functions [1, 2]. We interact with our environment through the skin, which also protects us from the environment. Our skin plays an essential role in how we present ourselves to society—from tanning and tattoos to scars and wrinkles. When the skin is breached or wounded, the immediate organ response is to initiate a repair process to close the gap, and while many traditional and modern standard approaches to promote wound healing have proven effective in saving countless lives, novel approaches enabling healing of complex wounds and healing by remodeling—without scarring—are still missing.
6.2 The Basic Biology of Human Skin
The skin consists of two main layers: the top layer is called the epidermis, which constitutes an epithelium (tissue that covers an inner or outer surface), and the lower layer is called the dermis, which constitutes a connective tissue (Fig. 6.1).

Adapted from Watt [1]
Back skin of mouse illustrating the epidermal and dermal layers; the type and location of different stem cell populations (LGR6 and LRIG1 are expressed in the follicle isthmus and LGR5 and CD34 in the bulge); and, the specialized mesenchymal cells of the dermal papilla and arrector pili muscle.
The epidermis is comprised mainly by a multilayered epithelium, the interfollicular epidermis, and adnexal structures. The adnexal structures, like hair follicles, sebaceous glands, and sweat glands, provide function to the skin [1]. The thickness of the epidermis and the distribution of adnexal structures vary across body sites. The main cell type in the epidermis is the keratinocyte. Among the key functions of the epidermis are the formation of a shielding interface with the outside environment, corporeal thermal regulation by hairs and sweat, sensory perception of heat, pain, pressure by nerves, and lubrication of the skin with lipids. Most of these functions stem from nondividing and terminally differentiated keratinocyte cells, which are in constant turn over. Keratinocyte cells are replenished through a variety of stem cell populations that are located in different skin sites, such as hair follicles (Fig. 6.1) [3]. Under normal physiological conditions, each stem cell cluster produces a subset of differentiated epidermal cells; however, most stem cells are able to contribute to the entire variety of differentiated epidermal lineages when the cells are relocated, or the skin is wounded.
The dermis consists of three layers: the upper layer in contact with the epidermis is the papillary dermis, the middle layer is the reticular dermis, and the deepest layer is termed the hypodermis. A basement membrane separates the dermis from the epidermis. This membrane is extracellular matrix characterized by an abundance of type IV collagen and laminin. The main cell type in the dermis is the fibroblast, and its density is higher in the papillary dermis. The reticular dermis is rich in fibrillar collagen, and the hypodermis is characterized by the presence of white adipocytes. Two mesenchymal structures important for function in the dermis are the dermal papilla—a population of cells that regulates the hair cycle and is located at the base of the follicle—and the arrector pili muscle, which a smooth muscle that upon contraction straights the hair follicles [1].
In addition to keratinocytes and fibroblasts, other important cell types, which either reside in the skin or circulate through the skin, are melanocytes, innate and adaptive immune system cells, peripheral nervous system cells, and cells of blood vessels [4,5,6].
6.3 When the Skin Is Wounded
The capacity of the skin to heal after an injury is vital for our survival, and this capacity is often limited, compromised, and disrupted in a spectrum of conditions and disorders. The process of skin wound healing is complex, it requires a highly coordinated response by a multitude of cells, like, immune cells, hematopoietic cells (immature cells that can differentiate into all types of blood cells), and resident cells of the skin (keratinocytes, fibroblasts). Skin wound healing is typically divided into four stages that overlap: hemostasis, inflammation, proliferation, and remodeling. In each stage, multiple key molecular, cellular, and physiologic events take place, and these events are coordinated primarily by signaling between immunologic, hematopoietic and resident skin cells. These stages are schematically represented in Fig. 6.2 and have been reviewed in detail in [7].

Reproduced with permission from Sun et al. [2]
Overlapping stages of skin wound healing: a hemostasis, b inflammation, c proliferation, d remodeling. A multitude of fundamental molecular and cellular processes coordinated by a myriad of secreted factors occur at each stage. The schematic illustrates representative factors from each stage.
6.3.1 Skin Wound Healing
In humans, immediately after the skin is wounded, hemostasis begins. Multiple responses are activated to stop blood loss: local vascular smooth muscle cells constrict vessels, reducing the blood flow; platelets and coagulation cascade factors produce fibrin, forming a hemostatic clot and a scaffold for the infiltration of leukocytes, keratinocytes, fibroblasts, and other cells into the wound [8]. The inflammatory stage begins within hours from the time of injury. Inflammation is driven by mediators derived from platelets, by-products from bacteria, and chemoattractants secreted in the wound. The first cell type that infiltrates the wound site is the neutrophil in order to kill bacteria and degrade matrix proteins that are damaged [9]. Within 24 h, monocytes cells infiltrate the wound and differentiate into macrophages, which kill microbes, remove tissue debris, eliminate neutrophils, and enable angiogenesis and tissue granulation [10].
During the proliferation stage, new cells populate the wound and a multitude of growth factors and chemokines that induce cell migration and proliferation, and matrix formation are released: platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), and transforming growth factor -α and -β (TGF-α and TGF-β), among others. In addition, keratinocyte cells are released from the wound edges and stem cell reservoirs located in the bulge and isthmus of hair follicles and the interfollicular epidermis. Keratinocytes proliferate and migrate to close the wound by coverage. Upon contact inhibition, keratinocytes undergo vertical stratification and differentiation to reinstate all the layers of the epidermal barrier [11, 12]. Angiogenesis, in synchrony with epidermal repair, is activated by multiple growth factors, for example, VEGF and FGF. The combination of new blood vessels, fibroblast and macrophage cells, and matrix proteins forms granulation tissue, which is the soft and pink tissue that forms at the bottom of a healing skin wound. At the end of the proliferative stage, fibroblast cells differentiate into contractile myofibroblast cells that pull together the wound edges [13].
During the remodeling stage, cells from previous stages are removed and collagen deposition in the dermis transitions from type III to type I collagen. Collagen remodeling involves matrix metalloproteinases, and its synthesis increases the tensile strength of the wounded skin, which recovers approximately 40% of its original strength in 4 weeks and about 70% in 1 year in normal healing conditions [14]. Altered collagen synthesis during this stage leads to scar formation [15]. Furthermore, poor or pathologic wound healing originates from failure to initiate, terminate, or regulate any particular wound healing stage. Examples of poor and pathologic wound healing outcomes are pyogenic granulomas (excessive formation of granulation tissue), hypertrophic scars and keloids (accumulation of excess fibrotic components), and chronic ulcers (prolonged inflammation, poor vascularization, and inability to form a new epithelium).
6.4 Nanomaterials for Engineering Skin Wound Healing
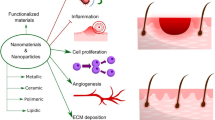
The skin endures and heals injuries throughout our lives. And healing is influenced by a wide variety of factors that affect skin wounding and the speed and quality of healing. These factors include a number of common conditions, diseases, and treatments, underscoring the far-reaching relevance of skin wound healing to medicine, public health, and the global burden of disease. Surgical incisions, thermal burns, and chronic ulcers are among the conditions that significantly burden (or delay) wound healing [2]. Current clinical approaches to promote wound healing have proven effective in saving lives; however, novel approaches enabling healing of complex wounds and healing by remodeling—without scarring—are still missing. Nanomedicine has demonstrated its potential to advance healthcare by developing nanoscale methods and technology to address a broad range of clinical limitations in drug delivery, biosensing, imaging, tissue regeneration, diagnostics, cancer, and other diseases treatments [16,17,18]. Nanomaterials have been extensively investigated for improved skin wound-healing and have demonstrated, mostly in animal models, their ability in regulating skin wound healing at different stages.
Next, we present an illustrative summary of the type of nanomaterials that have been tested in animal models of skin wounds, leaving out cell studies for succinctness and including only a few human studies because there are not many. A variety of nanomaterial frameworks—polymer nanoparticles, dendrimers, liposomes, metals, ceramics, fullerenes, nanotubes, nanoemulsions, nanopores, quantum dots—have been utilized to address specific healing limitations [19]. The unique properties of nanomaterials and their potential applications for the treatment of tissues other than skin—bone, bladder, cartilage, neural, vascular, etc.—are reviewed in [16, 20,21,22]. Excellent reviews about nanomaterials in skin wound healing can be found in [23,24,25].
6.4.1 Carbon
Carbon nanomaterials have been used for imaging, drug delivery, and gene delivery, among other applications [26, 27]. This type of nanomaterials includes fullerenes, carbon nanohorns, carbon nanotubes, and graphene. Fullerene is a powerful antioxidant capable of scavenging ROS and reactive nitrogen species and, consequently, of modulating inflammatory and proliferative processes [28]. In combination with light, fullerene has also been used to rescue mice with wounds infected with pathogenic gram-negative bacteria [28]. The biocompatibility of carbon nanomaterials remains controversial [26], and most studies in the literature are limited to in vitro cell studies [29,30,31].
6.4.2 Ceramics
Ceramic nanomaterials based on silica, calcium salts and hydroxyapatite have been investigated as nanoparticles for applications in wound healing. Intravenously administered calcium-based nanoparticles, synthesized using CaCl2 with β-glycerol-phosphate, were used to modulate local calcium levels and calcium homeostasis in open wounds in mice. These nanoparticles decreased the size of the wound by contraction, which was attributed to the release of ionized calcium into the wound bed as the pH-sensitive nanoparticles dissolved in the acidic wound microenvironment [32]. Variations in local pH levels in the wound bed are known to promote or inhibit bacteria growth, regulate enzyme prevalence, and change oxygen supply [33]. A similar approach utilized nitric NO-releasing nanoparticles, which were synthesized by means of combining chitosan, glucose, tetramethyl orthosilicate, sodium nitrite, and polyethylene glycol. In clean and infected wounds in mice, the wounds treated with NO-NPs presented reduced inflammation, increased collagen deposition, and increased blood vessel formation. NO stimulated migration and proliferation of fibroblasts and synthesis of collagen type III [34, 35].
6.4.3 Lipids
Nanoparticle treatment approaches for accelerating wound closure have been developed using liposomes [36, 37], and tested in porcine models. Lipids obtained from the cell membranes of rabbit red blood cells were combined with α-gal epitopes to make submicroscopic liposomes (Gll-Phl-Chol-α-gal). Anti-Gal is an antibody that interacts specifically with a carbohydrate antigen, the α-gal epitope (Galα1-3Galβ1-4GlcNAc-R), on glycolipids and glycoproteins in a wound and activates the complement system. Among the products generated from this complement activation, there are complement cleavage chemotactic factors, such as C5a and C3a. The increment in local concentrations of chemotactic factors within the wound stimulated rapid recruitment and migration of macrophages to the wound site [36, 37]. α-Gal nanoparticles binding to the membrane receptors of macrophages were able to activate the production of cytokines and accelerate healing and wound closure, which was demonstrated in pig wounds treated topically with nanoparticles deposited as a thin film [36].
A different approach incorporated plant-derived compounds quercetin and curcumin in liposomes. The drug and carrier efficacy were evaluated by in vitro skin distribution and in vivo ability to reduce oxidative inflammation and neutrophil infiltration in mice subjected to 12-O-tetradecanoylphorbol-13-acetate (TPA, a potent tumor promoter). These plant-based drugs present antioxidant and anti-inflammatory properties and were able to inhibit the onset of skin wounds during the application of TP [38]. A different study utilized phospholipid bilayer vesicles loaded with hemoglobin and coated with polyethylene glycol (HbVs) to improve the oxygenation of ischemic skin wounds in mice models [39]. Relative to controls, tissue survival improved by 24% and the wound-healing rate increased twofold. Immunohistochemical analysis showed a higher density of capillaries and a higher expression of endothelial NO synthase 3 in the wounds treated with HbVs. A different study investigated liposome-encapsulated hemoglobin with high O2 affinity for the treatment of full-thickness dorsal wounds in mice [40]. The treatment significantly accelerated granulation, increased epithelial thickness, suppressed early granulocyte infiltration, and increased Ki67 expression.
Another approach synthesized adenosine triphosphate (ATP)-vesicles and tested them as a topical nonionic cream for improved wound healing. ATP-vesicles constructed using phospholipids, trehalose, and a liposomal transfection reagent, were combined with a nonionic commercial cream moisturizer. For both non-ischemic and ischemic wounds, the ATP-vesicle provided a source of energy for survival of cells and improved granulation and re-epithelialization in diabetic wounds in rabbits [41].
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are two major types of lipid-based nanoparticles. SLNs advantages are good release profiles and targeted drug delivery with excellent physical stability and, consequently, have overcome the limitations of other colloidal carriers, like emulsions, liposomes, and polymeric nanoparticles. Topical administration of SLNPs and NLCs loaded with rhEGF improved re-epithelialization and restoration of the inflammatory process in diabetic wounds in mice [42]. A different approach developed silica (SiO2) nanoparticles as an alternative to conventional wound closure methods, such as sutures and adhesives like Dermabond (2-octyl cyano-acrylate). SiO2 nanoparticles synthesized by the Stöber method were applied to full-thickness dorsal skin wounds. The wound edges were maintained in contact manually for less than one minute, which is the time that took to close the wound. Macroscopic analysis shows the absence of pathological inflammation or necrosis [43]. A class of biomaterials that have been successfully implanted in millions of patients worldwide to repair bone and dental defects are bioactive glasses, primarily 45S5 glass compositions [44]. Many other bioactive glass compositions have been proposed for applications like soft tissue repair and drug delivery. A bioactive glass-based nanoformulation was applied to full-thickness wounds in a diabetic rat model. This nanoformulation promoted the proliferation of fibroblasts and deposition of granulation tissue while stimulating the production of growth factors such as VEGF and FGF2 [45].
6.4.4 Metal
A variety of metal nanoparticles has been investigated and developed for wound healing applications. Colloidal solutions of silver (Ag) nanoparticles possess a remarkably wide spectrum of antimicrobial properties, effectively reducing or preventing wound infections caused by a broad range of microbes [46, 47]. AgNPs are also effective against fungi, yeast, and viruses [48]. The origin and antimicrobial activity of these particles, including the actual contribution of ionic silver to the antimicrobial activity, is an ongoing discussion [49, 50]. However, it is well established that adequate surfaces are required for producing biologically active nanoparticles [51,52,53,54,55,56,57,58]. As a function of size and concentration, AgNPs have shown anti-inflammatory properties and the ability to minimize ROS production and improve the tensile strength of skin by modulating collagen alignment [59,60,61]. The production of stable and nontoxic nanosilver under physiological conditions constitutes a development approach for the translation of silver nanocomposites to the clinic. Cumulative data to date highlights the fundamental role of bio-inspired protecting agents to produce stable nanosilver structures that do not have toxic side effects on primary human cells and mice, i.e., protective agents like collagen and the thiol-modified LL37 antimicrobial peptide [62]. In situ preparation of fibers with AgNPs utilizing electrospinning was developed for use as a wound dressing. Nanofibrous membranes enabled the continuous release of Ag ions, which resulted in broad-spectrum antimicrobial activity against Staphylococcus aureus and Escherichia coli. These antibacterial nanofibrous membranes were able to reduce the inflammatory response and accelerate wound healing in Wistar rats [63].
In addition to silver, iron oxide, copper, and gold have been investigated for wound healing applications. Thrombin-conjugated γ-Fe2O3 nanoparticles accelerated the closure of incisional wounds in rats, improving skin tensile strength and reducing stitch-induced scarring [64]. Copper nanoparticles within a methylcellulose-based ointment were shown to induce pro-inflammatory mediators that increased blood vessel formation in full-thickness wounds in mice skin [65]. Small-sized gold nanomaterials are stable and nontoxic, which makes them an attractive platform for development of biofunctional nanomaterials. Photoluminescent gold nanodots were constructed using etching and codeposition of hybridized ligands, an antimicrobial peptide (surfactant), and 1-dodecanethiol, on gold nanoparticles. In rats, wounds infected with Methicillin-Resistant S. aureus showed faster healing, improved epithelialization, and higher collagen deposition when photoluminescent gold nanodots are used as a dressing material [66].
Metals of the lanthanide group have also been investigated in wound healing [67]. Nanoceria (cerium oxide nanoparticles) is an ROS scavenger material that stabilizes HIF-1α expression stimulating angiogenesis via upregulation of VEGF and modulating oxygen levels in the wound [24, 68]. Nanoceria/PU/cellulose acetate fibers applied to wounds exhibited anti-bacterial properties due to the release of free cerium ions [69].
6.4.5 Polymers
Polymeric nanomaterials have been used for drug delivery, imaging and sensing applications [70]. In wound healing, polymeric nanomaterials are often combined with wound dressings [71, 72]. Poly (lactide-co-glycolide) (PLGA), polycaprolactone (PCL) and polyethylene glycol (PEG) have been used to develop nanomaterials for improving wound closure outcomes in normal and infected wounds. PLGA nanoparticles loaded with curcumin reduced the inflammatory response, accelerated re-epithelialization and improved the formation of granulation tissue in mice wounds [73]. Curcumin, an organic molecule found in the spice turmeric, exhibits anti-inflammatory, antioxidant and bactericidal properties [74, 75]. PLGA nanoparticles loaded with recombinant human epidermal growth factor (rhEGF) increased the healing rate of full-thickness wounds in diabetic rats [76]. The release of rhEGH was sustained for 24 h enhancing fibroblast proliferation. The application of PCL-PEG nanoparticles loaded with hypericin (Hy) to wounds infected with methicillin-resistant S. aureus resulted in reduced expression of TNF—tumor necrosis factor, a cytokine involved in systemic inflammation—improved epithelialization and collagen synthesis in a rat model [77].
Biodegradable poly(b-amino esters) (PBAEs) and copolymers of maleic acid have been exploited to develop gene delivery and drug release systems for wound-healing applications [78, 79]. PBAEs nanoparticles carrying the sonic hedgehog gene promoted angiogenesis and tissue regeneration by activating angiogenic signaling pathways in mice wound model [80]. Chitosan, an organic polysaccharide exhibiting biocompatibility, biodegradability, mucoadhesivity and anti-infection activity, has been extensively investigated for biomedical applications [81]. Chitosan nanoparticles have shown significant bactericidal effects on different types of bacteria without cell toxicity on mouse fibroblast cells [82]. The N-acetyl glucosamine in chitosan is also present in the dermal connective tissue in elastin, which is a highly-elastic structural and cell-signaling protein [83]. Synthesized chimeric nanoparticles, formed via spontaneous self-assembling of elastin-like peptides (ELP) and loaded with keratinocytes growth factors, improved re-epithelialization and granulation in diabetic mice wounds [84].
Dendrimers, like polyamidoamine (PAMAM), have been used for cellular delivery of plasmid DNA by forming a stable complex with limited degradation. An arginine-grafted cationic dendrimer (PAM) was used to deliver minicircle plasmid DNA encoding VEGF into diabetic wounds in mice. This polycomplex PAM-RG4) consists of a highly ordered PAMAM dendrimer backbone. Relative to control wounds, PAM-RG4 improved the proliferation of basal cells and the deposition of collagen, and it also reduced the formation of immature blood vessels. Diabetic skin wounds healed within 6 days displaying a well‐ordered dermal structure [85]. The structure of dendrimers exhibits internal cavities and surface channels, making them suitable to accommodate small molecules and carry high drug loads that can be used for the treatment of complex wounds. Dendrimers have also been used in the development of nanotechnology for delivering short RNA molecules to wounds. RNA interference (RNAi) is a biological process in which small interfering RNAs (siRNAs) silence gene expression by degrading targeted mRNA molecules. Sirnaomics Inc., is developing nanoparticle formulations containing siRNA for reducing the expression of TGFβ1 and Cox-2 (cyclooxygenase-2), which are implicated in many tissue inflammation and fibrosis processes. Among other applications, Sirnaomics Inc., is testing formulations for the treatment of hypertrophic scars in phase II clinical trials.
6.5 Emerging Technologies
As illustrated in the previous section, the vast majority of the nanomaterial applications investigated for skin wound healing consist in utilizing them either to modulate different wound healing processes by direct interaction with the wound microenvironment or to deliver cargo that modulates the wound healing process. Alternative emerging technologies utilize nanomaterials in synergy with novel technologies in other fields [86].
Photosensitized crosslinking of proteins is being developed for medical treatments and many potential benefits have already been demonstrated in preclinical and clinical studies. Applications include sealing wounds, reattaching severed tissues, stiffening and strengthening tissues, decreasing inflammatory responses, and bioengineering tissues [87,88,89,90]. These treatments rely on light-initiated formation of covalent crosslinks between proteins on the surfaces of two tissues or between proteins within a tissue. Two dyes, rose Bengal (RB2−) and riboflavin-5-phosphate (R5P2−), has been used almost exclusively for medical applications of photosensitized protein crosslinking. The treatment procedure for photo-crosslinking tissue proteins is simple. In fact, the simplicity of this technique is one of its clinical advantages. An aqueous solution of the dye typically is applied to the tissue, which is then exposed for a few minutes to light wavelengths absorbed by the dye. Crosslinking occurs during the irradiation and is followed by healing processes. The light-induced effects appear to result largely from crosslinks in collagen, the major connective tissue protein, with possible crosslinking to the proteoglycans that surround collagen fibrils and to other proteins. This technique has been regarded as an effective alternative to stapling or traditional suturing. Absorption and scattering of light in tissue results from fundamental light–matter interactions and have enabled a variety of powerful optical techniques for therapy and imaging [91]. However, these interactions are also problematic as they limit the penetration of light in tissues. A poly(allylamine) (PAAm)-modified upconversion nanoparticle/hyaluronate–rose bengal (UCNP/PAAm/HA-RB) conjugate complex was developed for photochemical bonding of deep tissue with near-infrared (NIR) light illumination [92]. In a mice study, the UCNP/PAAm/HA-RB conjugate complex was efficiently delivered into deep tissue and accelerated tissue bonding upon NIR light illumination. HA in the outer layer of the complex facilitated the penetration of RB into the collagen layer of the dermis.
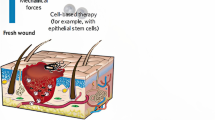
Skin grafting requires removing a layer of skin tissue from a donor site to transfer it to the wounded site. Skin grafts can be classified as split-thickness skin grafts (STSGs), consisting of the epidermis and the upper part of the dermis, or full-thickness skin grafts (FTSGs), consisting of the epidermis and the full-thickness dermis. A major limitation of this procedure is scarring at the donor site and, in STSGs, the absence of the deep dermal adnexal structures that give function to skin, such as hair follicles, sweat glands, and sebaceous glands. FTSGs require to create a full-thickness wound at the donor site and a good vascular bed for survival at the wounded site. Therefore, standard clinical outcomes are scarring at the donor and wounded site, and lack of skin function at the wounded site. Innovations in surgical grafting techniques have advanced skin grafting, but grafting methods are still limited [2, 93]. In mice and porcine models, a new approach that grafts thousands of full-thickness skin micrografts have been shown to eliminate scarring at the donor site while transferring the deep adnexal structure to the wound site [94, 95]. Again, the simplicity of this technique is one of its clinical advantages [96, 97]. This fractional grafting approach places the micrografts randomly in the wounded site, that is, micrografts are not oriented. Current development efforts are geared toward developing nanomaterials that could serve as functional scaffolds to “copy skin” by enabling controlling the spacing and orientation of micrografts, which presumably would result in faster healing and functional skin resembling the original skin, and manipulation of this hybrid construct (autologous tissue in a synthetic biomaterial) by clinicians. The concept is illustrated in Fig. 6.3. The flexibility and broad reach of nanomaterials in wound healing could enable a fractional skin grafting platform with the capability of customizing scaffolds for different wound environments; for example, in infected wounds by functionalizing the scaffold to kill bacteria while preserving the micrografts.
Full-thickness microscopic skin tissue columns (MSTCs) are harvested from the healthy donor site and placed into a scaffold matrix to assemble a hybrid skin construct for wound repair. Nanomaterials enable functionalization of the matrix for different wound environments and modifying the wound to establish favorable conditions, for example, the combination with a silver nanoparticles spray for infected wounds
6.6 Summary and Conclusions
Wound healing interventions aim to repair or promote the repair of the structure and function of organs, tissues, and cells. When the skin is wounded, repair implies restoration of all functional components, including hair follicles, sweat glands, and nerves in clean and impaired healing conditions. Although there is no perfect repair method currently available for complex wounds, rapid developments in understanding skin development and wound repair, together with advances in related fields like stem cell, tissue bioengineering, and nanomedicine, provide hope that such methods represent a tractable goal in the future. Nanomaterials have a high surface area to mass ratio that enables favorable interactions with wound environment and constituents. Cells naturally interact with the extracellular matrix which can be better modulated by nanoscale materials. Nanomaterials will have a critical role to play in the engineering of novel clinical strategies that enable healing of large and complex wounds by remodeling without scarring.
References
Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science. 2014;346(6212):937–40.
Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941–5.
Schepeler T, Page ME, Jensen KB. Heterogeneity and plasticity of epidermal stem cells. Development. 2014;141(13):2559–67.
Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346(6212):954–9.
Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945–9.
Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346(6212):950–4.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21.
Clark RAF. Fibrin and wound healing. In: Nieuwenhuizen W, Mosesson MW, DeMaat MPM, editors. Fibrinogen, vol. 936. Annals of the New York Academy of Sciences; 2001. p. 355–67.
Ross R, Odland G. Human wound repair: II. Inflammatory cells epithelial-mesenchymal interrelations and fibrogenesis. J Cell Biol. 1968;39(1):152–68.
Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23.
Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344(6189):1243.
Ito M, Liu YP, Yang ZX, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–4.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63.
Levenson SM, Geever EF, Crowley LV, Oates JF, Berard CW, Rosen H. Healing of rat skin wounds. Ann Surg. 1965;161(2):293.
Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–47.
Tocco I, Zavan B, Bassetto F, Vindigni V. Nanotechnology-based therapies for skin wound regeneration. J Nanomater. 2012;11.
Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9(1):1–14.
Ryan SM, Brayden DJ. Progress in the delivery of nanoparticle constructs: towards clinical translation. Curr Opin Pharmacol. 2014;18:120–8.
Athar M, Das AJ. Therapeutic nanoparticles: State-of-the-art of nanomedicine. Adv Mater Rev. 2014;1(1):25–37.
Cortivo R, Vindigni V, Iacobellis L, Abatangelo G, Pinton P, Zavan B. Nanoscale particle therapies for wounds and ulcers. Nanomedicine. 2010;5(4):641–56.
Obregon R, Ramon-Azcon J, Ahadian S, Shiku H, Bae H, Ramalingam M, Matsue T. The use of microtechnology and nanotechnology in fabricating vascularized tissues. J Nanosci Nanotechnol. 2014;14(1):487–500.
Zhang LJ, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4(1):66–80.
Chakrabarti S, Chattopadhyay P, Islam J, Ray S, Raju PS, Mazumder B. Aspects of nanomaterials in wound healing. Curr Drug Deliv. 2019;16(1):26–41.
Kalashnikova I, Das S, Seal S. Nanomaterials for wound healing: scope and advancement. Nanomedicine. 2015;10(16):2593–612.
Mordorski B, Prow T. Nanomaterials for wound healing. Curr Dermatol Rep. 2016;5(4):278–86.
Zhang YB, Petibone D, Xu Y, Mahmood M, Karmakar A, Casciano D, Ali S, Biris AS. Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine. Drug Metab Rev. 2014;46(2):232–46.
Zhou Z. Liposome formulation of fullerene-based molecular diagnostic and therapeutic agents. Pharmaceutics. 2013;5(4):525–41.
Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine. 2010;5(10):1525–33.
Gao J, Wang HL, Iyer R. Suppression of proinflammatory cytokines in functionalized fullerene-exposed dermal keratinocytes. J Nanomater. 2010.
Ryoo SR, Kim YK, Kim MH, Min DH. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies. ACS Nano. 2010;4(11):6587–98.
Zhang YY, Wang B, Meng XA, Sun GQ, Gao CY. Influences of acid-treated multiwalled carbon nanotubes on fibroblasts: proliferation, adhesion, migration, and wound healing. Ann Biomed Eng. 2011;39(1):414–26.
Kawai K, Larson BJ, Ishise H, Carre AL, Nishimoto S, Longaker M, Lorenz HP. Calcium-based nanoparticles accelerate skin wound healing. PLOS One. 2011;6(11).
Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298(9):413–20.
Blecher K, Martinez LR, Tuckman-Vernon C, Nacharaju P, Schairer D, Chouake J, Friedman JM, Alfieri A, Guha C, Nosanchuk JD and others. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine. 2012;8(8):1364–71.
Han G, Nguyen LN, Macherla C, Chi YL, Friedman JM, Nosanchuk JD, Martinez LR. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol. 2012;180(4):1465–73.
Hurwitz ZM, Ignotz R, Lalikos JF, Galili U. Accelerated porcine wound healing after treatment with alpha-gal nanoparticles. Plast Reconstr Surg. 2012;129(2):242E–51E.
Wigglesworth KM, Racki WJ, Mishra R, Szomolanyi-Tsuda E, Greiner DL, Galili U. Rapid recruitment and activation of macrophages by anti-gal/alpha-gal liposome interaction accelerates wound healing. J Immunol. 2011;186(7):4422–32.
Castangia I, Nacher A, Caddeo C, Valenti D, Fadda AM, Diez-Sales O, Ruiz-Sauri A, Manconi M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014;10(3):1292–300.
Plock JA, Rafatmehr N, Sinovcic D, Schnider J, Sakai H, Tsuchida E, Banic A, Erni D. Hemoglobin vesicles improve wound healing and tissue survival in critically ischemic skin in mice. Am J Physiol Heart Circ Physiol. 2009;297(3):H905–10.
Fukui T, Kawaguchi AT, Takekoshi S, Miyasaka M, Tanaka R. Liposome-encapsulated hemoglobin accelerates skin wound healing in mice. Artif Organs. 2012;36(2):161–9.
Wang JP, Wan R, Mo YQ, Li M, Zhang QW, Chien SF. Intracellular delivery of adenosine triphosphate enhanced healing process in full-thickness skin wounds in diabetic rabbits. Am J Surg. 2010;199(6):823–32.
Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, Igartua M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51–61.
Meddahi-Pelle A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem. 2014;53(25):6369–73.
Baino F, Hamzehlou S, Kargozar S. Bioactive glasses: where are we and where are we going? J Funct Biomat. 2018;9(1).
Lin C, Mao C, Zhang JJ, Li YL, Chen XF. Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed Mat. 2012;7(4).
Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem Rev. 2013;113(7):4708–54.
Alarcon EI, Griffith M, Udekwu KI. Silver nanoparticle applications. New York: Springer; 2015.
Lara HH, Garza-Trevino EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9.
Xiu ZM, Ma J, Alvarez PJJ. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol. 2011;45(20):9003–8.
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271–5.
Ahumada M, McLaughlin S, Pacioni NL, Alarcon EI. Spherical silver nanoparticles in the detection of thermally denatured collagens. Anal Bioanal Chem. 2016;408(8):1993–6.
Alarcon EI, Udekwu K, Skog M, Pacioni NL, Stamplecoskie KG, Gonzalez-Bejar M, Polisetti N, Wickham A, Richter-Dahlfors A, Griffith M and others. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials. 2012;33(19):4947–56.
Alarcon EI, Udekwu KI, Noel CW, Gagnon LBP, Taylor PK, Vulesevic B, Simpson MJ, Gkotzis S, Islam MM, Lee CJ and others. Safety and efficacy of composite collagen-silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale. 2015;7(44):18789–98.
Poblete H, Agarwal A, Thomas SS, Bohne C, Ravichandran R, Phospase J, Comer J, Alarcon EI. New insights into peptide-silver nanoparticle interaction: deciphering the role of cysteine and lysine in the peptide sequence. Langmuir. 2016;32(1):265–73.
Pokhrel LR, Dubey B, Scheuerman PR. Impacts of select organic ligands on the colloidal stability, dissolution dynamics, and toxicity of silver nanoparticles. Environ Sci Technol. 2013;47(22):12877–85.
Seitz F, Rosenfeldt RR, Storm K, Metreveli G, Schaumann GE, Schulz R, Bundschuh M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to daphnia magna. Ecotoxicol Environ Saf. 2015;111:263–70.
Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL. Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interface Sci. 2014;204:15–34.
Vignoni M, Weerasekera HDA, Simpson MJ, Phopase J, Mah TF, Griffith M, Alarcon EI, Scaiano JC. LL37 peptide@silver nanoparticles: combining the best of the two worlds for skin infection control. Nanoscale. 2014;6(11):5725–8.
Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–19.
Kwan KHL, Liu XL, To MKT, Yeung KWK, Ho CM, Wong KKY. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomedicine. 2011;7(4):497–504.
Mishra M, Kumar H, Tripathi K. Diabetic delayed wound healing and the role of silver nanoparticles. Dig J Nanomater Bios. 2008;3(2):49–54.
Alarcon EI, Vulesevic B, Argawal A, Ross A, Bejjani P, Podrebarac J, Ravichandran R, Phopase J, Suuronen EJ, Griffith M. Coloured cornea replacements with anti-infective properties: expanding the safe use of silver nanoparticles in regenerative medicine. Nanoscale. 2016;8(12):6484–9.
Dong RH, Jia YX, Qin CC, Zhan L, Yan X, Cui L, Zhou Y, Jiang XY, Long YZ. In situ deposition of a personalized nanofibrous dressing via a handy electrospinning device for skin wound care. Nanoscale. 2016;8(6):3482–8.
Ziv-Polat O, Topaz M, Brosh T, Margel S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials. 2010;31(4):741–7.
Trickler WJ, Lantz SM, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Paule MG, Slikker W, Biris AS, Hussain SM and others. Effects of copper nanoparticles on rat cerebral microvessel endothelial cells. Nanomedicine. 2012;7(6):835–46.
Chen WY, Chang HY, Lu JK, Huang YC, Harroun SG, Tseng YT, Li YJ, Huang CC, Chang HT. Self-assembly of antimicrobial peptides on gold nanodots: against multidrug-resistant bacteria and wound-healing application. Adv Funct Mater. 2015;25(46):7189–99.
Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, Seal S, Mattson MP. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194–201.
Das S, Baker AB. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol. 2016;4.
Unnithan AR, Sasikala ARK, Sathishkumar Y, Lee YS, Park CH, Kim CS. Nanoceria doped electrospun antibacterial composite mats for potential biomedical applications. Cer Int. 2014;40(8):12003–12.
Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7):2545–61.
Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4(14):413–37.
Mogosanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127–36.
Chereddy KK, Coco R, Memvanga PB, Ucakar B, des Rieux A, Vandermeulen G, Preat V. Combined effect of PLGA and curcumin on wound healing activity. J Control Release. 2013;171(2):208–15.
Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, Kim YC, Lee DS, Shin DW, Kweon KT and others. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20(8–9):714–18.
Durgaprasad S, Reetesh R, Hareesh K, Rajput R. Effect of a topical curcumin preparation (BIOCURCUMAX) on burn wound healing in rats. J Pharm Biomed Sci. 2011;8(08).
Chu YJ, Yu DM, Wang PH, Xu J, Li DQ, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18(5):499–505.
Nafee N, Youssef A, El-Gowelli H, Asem H, Kandil S. Antibiotic-free nanotherapeutics: hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int J Pharm. 2013;454(1):249–58.
Angelova N, Yordanov G. Nanoparticles of poly(styrene-co-maleic acid) as colloidal carriers for the anticancer drug epirubicin. Colloids Surf A. 2014;452:73–81.
Keeney M, Ong SG, Padilla A, Yao ZY, Goodman S, Wu JC, Yang F. Development of poly(beta-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNA. ACS Nano. 2013;7(8):7241–50.
Park HJ, Lee J, Kim MJ, Kang TJ, Jeong Y, Um SH, Cho SW. Sonic hedgehog intradermal gene therapy using a biodegradable poly (beta-amino esters) nanoparticle to enhance wound healing. Biomaterials. 2012;33(35):9148–56.
Archana D, Dutta J, Dutta PK. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol. 2013;57:193–203.
Gao WJ, Lai JCK, Leung SW. Functional enhancement of chitosan and nanoparticles in cell culture, tissue engineering, and pharmaceutical applications. Front Physiol. 2012;3.
Rnjak-Kovacina J, Weiss AS. The role of elastin in wound healing and dermal substitute design. In: Dermal replacements in general, burn, and plastic surgery. New York: Springer; 2013. p. 57–66.
Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, Yarmush ML. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. PNAS. 2011;108(3):1034–9.
Kwon MJ, An S, Choi S, Nam K, Jung HS, Yoon CS, Ko JH, Jun HJ, Kim TK, Jung SJ and others. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J Gene Med. 2012;14(4):272–8.
Zarrintaj P, Moghaddam AS, Manouchehri S, Atoufi Z, Amiri A, Amirkhani MA, Nilforoushzadeh MA, Saeb MR, Hamblin MR, Mozafari M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine. 2017;12(19):2403–22.
Bhagat V, Becker ML. Degradable adhesives for surgery and tissue engineering. Biomacromol. 2017;18(10):3009–39.
Pupkaite J, Ahumada M, McLaughlin S, Temkit M, Alaziz S, Seymour R, Ruel M, Kochevar I, Griffith M, Suuronen EJ and others. Collagen-based photoactive agent for tissue bonding. ACS Appl Mater Interfaces. 2017;9(11):9265–70.
Xu N, Yao M, Farinelli W, Hajjarian Z, Wang Y, Redmond RW, Kochevar IE. Light-activated sealing of skin wounds. Lasers Surg Med. 2015;47(1):17–29.
Zhao X, Sun X, Yildirimer L, Lang Q, Lin ZYW, Zheng R, Zhang Y, Cui W, Annabi N, Khademhosseini A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017;49:66–77.
Nizamoglu S, Gather MC, Humar M, Choi M, Kim S, Kim KS, Hahn SK, Scarcelli G, Randolph M, Redmond RW and others. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat Comm. 2016;7.
Han S, Hwang BW, Jeon EY, Jung D, Lee GH, Keum DH, Kim KS, Yun SH, Cha HJ, Hahn SK. Upconversion nanoparticles/hyaluronate-rose bengal conjugate complex for noninvasive photochemical tissue bonding. ACS Nano. 2017;11(10):9979–88.
Singh M, Nuutila K, Kruse C, Robson MC, Caterson E, Eriksson E. Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast Reconstr Surg. 2015;136(4):524E–30E.
Tam J, Wang Y, Farinelli WA, Jimenez-Lozano J, Franco W, Sakamoto FH, Cheung EJ, Purschke M, Doukas AG, Anderson RR. Fractional skin harvesting: autologous skin grafting without donor-site morbidity. Plast Reconstr Surg Glob Open. 2013;1(6):e47.
Tam J, Wang Y, Vuong LN, Fisher JM, Farinellil WA, Anderson RR. Reconstitution of full-thickness skin by microcolumn grafting. J Tissue Eng Regen Med. 2017;11(10):2796–805.
Franco W, Jimenez-Lozano JN, Tam J, Purschke M, Wang Y, Sakamoto FH, Farinelli WA, Doukas AG, Anderson RR. Fractional skin harvesting: device operational principles and deployment evaluation. J Med Dev. 2014;8(4).
Tam J, Farinelli W, Franco W, Anderson RR. Apparatus for harvesting tissue microcolumns. J Vis Exp. 2018;(140).
Acknowledgements
We would like to thank Dr. Rox R. Anderson for his support. All authors have read and approved this final version. Dr. Ahumada acknowledges the support of CONICYT-FONDECYT Iniciación (grant #11180616). Illustrating support was provided by Yanjie Jack Guo.
Disclosure
All authors have read and approved this final version.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ahumada, M., Wang, Y., Franco, W. (2019). Nanomaterials for Engineering the Treatment of Skin Wounds. In: Alarcon, E., Ahumada, M. (eds) Nanoengineering Materials for Biomedical Uses. Springer, Cham. https://doi.org/10.1007/978-3-030-31261-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-31261-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31260-2
Online ISBN: 978-3-030-31261-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)