Abstract
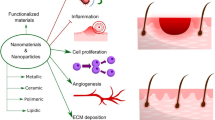
Being the largest organ and the protective shield of the human body, the skin is highly vulnerable to potential injuries. A cascade of biological events initiates after the injury to regenerate and repair the damaged tissue and this process is referred to as wound healing. Wound dressings have been introduced as a temporary protective physical barrier to prevent the invasion of pathogenic microorganisms and to keep the wound from dehydration while facilitating the healing process. In recent times, nanomaterials have emerged as a source for developing highly effective and innovative wound dressings. Particularly, several polymeric nanofibres have shown promising results as scaffolds for skin regeneration while some metal nanoparticles possess intrinsic antibacterial properties thus making them potential candidates for integration into wound dressings. Moreover, encapsulating drugs, biomolecules, and growth factors, within nanocarriers is also offering new treatment modalities, especially for chronic wounds. Therefore, this chapter provides an overview of the recent advances in nanotechnology-assisted wound healing and tissue regeneration and the applicability of nanomaterials in the treatment of chronic and acute wounds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 The Wound Healing Process
Skin is a complex organ and it is composed of the epidermis, dermis, and skin appendages like hair follicles, sebaceous glands, etc. It acts as a barrier and protects the internal organs from harmful ultraviolet rays, mechanical damage, and harmful pathogens. In addition, skin prevents the evaporation of water from the body and maintains the fluid imbalance and is involved in thermal dysregulation. Therefore, the integrity of healthy skin is of great importance in maintaining the physiological homeostasis of the human body. However, the skin is functioning as the protective shield of the body and becomes vulnerable to potential injury. Hence, wound healing is an essential process for the survival of organisms (Bentley 2004; Takeo et al. 2015; Sorg et al. 2017).
A wound is defined as a “disruption of normal anatomic structure and function of the skin”. Generally, wounds can be categorized as acute wounds and chronic wounds. Acute wounds are caused by irradiation, exposure to heat, mechanical damages, etc. and are healable within 8–12 weeks. On the other hand, chronic wounds are caused due to diseases like diabetes, tumours, etc. and the healing would take more than 12 weeks (Zahedi et al. 2010).
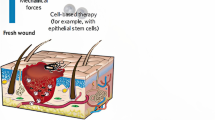
Wound healing is a cascade of biological events which initiates after the injury to regenerate and repair the damaged tissue. The wound healing process can be described under four main phases; haemostasis, Inflammation, proliferation, and maturation (Dalisson and Barralet 2019). The haemostasis phase starts soon after wounding (within the first few minutes) and it involves vasoconstriction, platelet plug formation, activation of the coagulation cascade, and finally the formation of a fibrin plug (final clot). Vasoconstriction helps to reduce the blood flow while platelet adhesion, activation, and aggregation lead to platelet plug formation. Due to the activation of the extrinsic pathway (tissue factor pathway) and intrinsic pathway of blood coagulation, a cascade of enzymatic reactions takes place and ultimately causes the conversion of fibrinogen to fibrin. The fibrin monomers get polymerized to form a fibrin polymer mesh and this results in a cross-linked fibrin clot around the platelet plug (LaPelusa and Dave 2022). Within an hour after clotting, the second phase of the wound healing process, i.e. inflammation starts. Polymorphonuclear neutrophils (PMNs) are predominately found in the wound bed up to about two days after the injury. These PMNs perform several functions such as the release of free radicals to kill bacteria in the wound, phagocytosis of debris, and breakdown of damaged tissues through the action of proteases. After two days, these PMNs either undergo apoptosis (cell death) or get degraded by macrophages. Thereafter, monocytes enter the wound bed and get converted into macrophages. Matured macrophages secrete several growth factors and cytokines that promote angiogenesis, granulation tissue formation as well as re-epithelialization. Before the end of the inflammatory phase of wound healing, the factors which are released by the macrophages activate fibroblasts, endothelial cells, and keratinocytes from surrounding tissues and initiate the migration and proliferation processes. These will be followed by events like the laying down of a new extracellular matrix, the formation of granulation tissue to form a barrier between the wound and the environment, and the closing of the wound. Usually, the final phase of the wound healing process, i.e. maturation starts three days after an injury. In this phase, the collagen matrix which has been previously laid down is remodelled slowly (Dalisson and Barralet 2019).
5.2 Wound Dressings
According to the clay tablets of Mesopotamian origin that date back to 2500 BCE, Mesopotamians used milk or water to clean the wounds before dressing them with honey or resin (Daunton et al. 2012). In the early clay tablets, three healing gestures were described. Those were washing the wounds, making the “plasters”, and bandaging the wound. These ancient “plasters” (the present-day equivalent of wound dressings) were mixtures of clay/mud, herbs, oils, etc. (Shah 2011). Similarly in ancient Greece (460–370 BCE), wine and vinegar were employed to clean the wounds while honey, wine, and oil were used as further treatments. Wool boiled in water or wine was used as bandages. In traditional Chinese medicine, green tea, liquorice, soaked mushrooms, and many herbal powders have been employed to promote granulation tissue, help in debridement and prevent infection (Daunton et al. 2012).
5.2.1 Traditional Wound Dressings
Over the years wound dressings have evolved from the crude application of herbal materials and animal fat to tissue-engineered scaffolds. Gauze, lint, plasters, cotton wool, as well as synthetic or natural bandages, can be considered traditional wound dressings. These are used as primary dressings (i.e. the dressing that makes physical contact with the wound surface) or secondary dressings (i.e. dressings that are used to cover the primary dressing) and help to prevent contaminations and thereby protect the wound.
Gauze is made using cotton fibres, rayon, and polyesters which can act against some bacterial infections. Mostly, sterile gauze pads are used to absorb fluids and exudates in open wounds. Although gauzes are widely used as wound dressings, there are several disadvantages associated. For example, gauzes should be changed from time to time and are not cost-effective. Moreover, excessive wound drainage causes it to become adherent to the wound and as a result, the patient would experience pain at the time it is being removed. Bandages are made using cotton wool, cellulose, or polyamide materials. Cotton bandages are used to keep light dressings in place, whereas high compression bandages and short-stretch compression bandages are used to provide sustained compression. Tulle dressings like Bactigras, Jelonet, and Paratulle (some commercially available tulle dressings) are impregnated with paraffin and these are appropriate for superficial clean wounds. Because of the inability to provide a moist atmosphere for wound healing, nowadays dry traditional wound dressings comprised of topical liquid and semi-solid formulations have been replaced by new dressings with more sophisticated formulations (Boateng et al. 2008; Dhivya et al. 2015).
5.2.2 Modern Wound Dressings
These kinds of dressings have been produced to keep the wound from dehydration and facilitate the healing process. Modern dressings are usually made up of synthetic polymers. Some examples are given below.
-
(a)
Semi-permeable film dressings
These dressings are made of transparent and adherent polyurethane and are impermeable to bacteria but permit the transfer of water vapour, O2, and CO2 from the wound while providing autolytic debridement eschar. In the early days, the films were produced from nylon derivatives with adhesive polyethylene frames and the adhesive layer permits the dressing to adhere to intact skin. However, these nylon-derived film dressings were not used for highly exudating wounds because of their limited absorption capacity. Moreover, maceration of the wound and the surrounding healthy tissues were also observed with the use of these materials. These dressings are recommended as a primary dressing for dry and superficial wounds. Yet, these can also be used in more exuding wounds, as a secondary dressing on top of foam dressings or dressing pads. When applying to intact skin in vulnerable areas, these dressings can also help to reduce friction, though, the removal should be done with caution to prevent any damage to the epidermal layer of the skin by the adhesive (Abdelrahman and Newton 2011; Shi et al. 2020).
-
(b)
Semi-permeable foam dressings
Semi-permeable foam dressings are made up of hydrophobic and hydrophilic foam, sometimes with adhesive borders (Morgan 2002). These dressings are usually produced from polyurethane and silicone and are available as adhesive or non-adhesive foam dressings (Abdelrahman and Newton 2011). The outer layer of the dressing protects the wound from a liquid due to its hydrophobic properties while allowing gaseous exchange and water vapour. Depending on the thickness of the wound, foam can absorb varying amounts of wound drainage. These dressings are designed mainly for lower leg ulcers and can be used for moderate to highly exudating wounds or granulating wounds (Ramos-e-Silva and Ribeiro de Castro 2002). Some foam dressings are demonstrated to be effective cavity fillers. The foam absorbs exudates preventing pooling and skin leakage while maintaining a low adherence to allow for easy, painless removal (Abdelrahman and Newton 2011).
-
(c)
Alginate dressings
Alginate dressings are made out of calcium or sodium alginate derived from seaweeds. These dressings are usually recommended for moderately to heavily exuding wounds but are not suitable for dry wounds. Calcium component of the dressing functions as a haemostat thus useful in bleeding wounds (Abdelrahman and Newton 2011). Alginate dressings have achieved a good absorption capability through the formation of a strong hydrophilic gel which can limit the wound exudates and reduce bacterial contamination (Dhivya et al. 2015). Despite there were some concerns that alginate can inhibit keratinocyte migration, Thomas et al. revealed that alginate speed up the healing process. This has occurred by activating macrophages to synthesize TNF-α that initiates inflammatory signals. After applying alginate dressings to the wound, the ions in the alginate exchange with the blood and form a protective film. Furthermore, these dressings require secondary dressings as they could dehydrate the wound causing a delay in healing (Thomas et al. 2000; Boateng et al. 2008).
-
(d)
Hydrogel dressings
Hydrogels are a form of insoluble hydrophilic material made up of synthetic polymers like poly (methacrylates) and polyvinyl pyrrolidine. A high water content (70–90%) in hydrogels assists granulation of tissues and epithelium in a moist environment. In addition, the soft elastic properties of these dressings make them easy to apply and also to remove once the wound is healed without causing any damage. Furthermore, hydrogels reduce the temperature of cutaneous wounds, providing those with a cooling effect. These dressings are designed for chronic wounds, necrotic wounds, burn wounds, and also for pressure ulcers (Dhivya et al. 2015). These hydrogel dressings are produced as tubes or as flat sheets and by considering the depth and location of the wound the most suitable one should be selected (Abdelrahman and Newton 2011).
-
(e)
Hydrocolloid dressings
Hydrocolloid dressings are amongst the most commonly used interactive dressings which have two layers named inner colloidal layer and the outer water-impermeable layer. These dressings are formed with the help of gel-forming agents such as carboxymethyl cellulose, gelatin, and pectin and are combined with other materials like elastomers and adhesives (Boateng et al. 2008; Dhivya et al. 2015; Vowden and Vowden 2017). Hydrocolloids have the properties of debridement and absorb wound exudates while being permeable to water vapour but impermeable to bacteria (Thomas 1992). These kinds of dressings can be used for pressure sores, superficial burns, and traumatic wounds. In addition to that, hydrocolloid dressings do not cause any pain during the removal. Therefore, such dressings can also be recommended for paediatric wound care management. Nevertheless, these are usually not recommended for neuropathic ulcers or wounds with a lot of exudates and are often used as a secondary dressing (Boateng et al. 2008; Dhivya et al. 2015).
-
(f)
Antimicrobial dressings
Topical antimicrobial dressings are designed to minimize the growth of microorganisms in wounds and can be used in both chronic and acute wounds. These dressings are designed using silver and iodine. In addition, polyhexamethylene biguanide-based antimicrobial dressings that affect bacterial cell metabolism have recently been introduced. All these dressings are found to be effective against a broad range of microorganisms commonly associated with chronic wounds (Flores and Kingsely 2007; Abdelrahman and Newton 2011).
5.2.3 Significance of Nanotechnological Approaches in Wound Healing and Tissue Regeneration
Recent advances in nanotechnology have opened up new avenues for drug delivery applications, allowing the delivery of biomolecules or growth factors, which can be used in chronic wound healing. The small size and physicochemical properties of the nanomaterials facilitate the intracellular delivery of biomolecules or drugs by protecting them from degradation and improving the drug penetration into the wound. This enables the topical administration of drugs and increment of the half-life thus reducing the number of applications and costs. Furthermore, encapsulating drugs and biomolecules within nanocarriers would allow different drug release profiles that may match the requirements of the wound healing process (Blanco-Fernandez et al. 2021).
Therefore, this chapter summarizes the recent advances in nanotechnology-assisted wound healing and tissue regeneration and the applicability of nanomaterials like nanoparticles, nanofibers, self-assembled nanocarriers, etc. in the treatment of chronic and acute wounds.
5.3 Application of Nanomaterials in Wound Healing and Tissue Regeneration
Nanoparticles are very small particles typically with a diameter of 1–100 nm. They have been extensively used in biomedicine and tissue engineering applications. Nanoparticles play two roles in wound healing; i.e. with their intrinsic properties positive for wound healing, and as drug delivery systems (Blanco-Fernandez et al. 2021).
Nanoparticles are employed in wound dressing materials mainly due to their intrinsic antimicrobial properties. Moreover, nanoparticles are capable of penetrating cell membranes whereas conventional antimicrobial agents often have a limited ability to cross certain cell membranes. Nanoparticles exert antimicrobial properties by degrading cell membranes, blocking and altering enzymatic pathways, etc. (Yah and Simate 2015).
Metal nanoparticles such as silver, gold, copper, copper oxide, iron oxide, zinc oxide, and titanium dioxide possess antibacterial properties which are useful in wound healing. The antibacterial activity of the above-mentioned metal nanoparticles triggers by the formation of reactive oxygen species (ROS) as well as interaction with biomolecules such as DNA, proteins, or inhibition of enzymes (Chatterjee et al. 2014; Shaikh et al. 2019). For example, silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) exert antibacterial activity via several modes of action. These nanoparticles can disrupt the bacterial cell membrane, leach into the cytosol, and destabilize/disrupt membrane proteins, cytoplasmic proteins, and enzymes. This would ultimately lead to metabolic impairment and ultimately bacterial cell death. Further, AuNPs and AgNPs can generate ROS creating oxidative stress. The presence of ROS can damage proteins and nucleic acids in bacterial cells and also inhibit the electron transport chain of bacterial cells leading to bacterial cell death (Joshi et al. 2020).
Silver has been widely used in the field of medicine for over centuries in the form of metallic silver, AgNO3, and silver sulphadiazine, to treat open wounds, burns, and also to treat chronically infected wounds. With the introduction of antibiotics, the usage of silver compounds had drastically reduced. However, with the development of nanotechnology, silver metal in its nano form re-emerged as a prospective antimicrobial agent (Rai et al. 2009). The extremely small size and large surface area to volume ratios have made AgNPs an effective antimicrobial agent. The formation of “pits” was observed in the cell wall of Escherichia coli cells treated with AgNPs, while AgNPs got accumulated in the bacterial membrane increasing the permeability and ultimately resulting in cell death (Sondi and Salopek-Sondi 2004). A more recent study conducted using Staphylococcus aureus and E. coli revealed that AgNPs can completely inhibit the growth of bacterial cells by destroying the permeability of the cell membrane and decreasing the activity of some enzymes (Gomaa 2017). Xiu et al. suggested that the antimicrobial activity of AgNPs depends on the release of Ag+, thus it can be modulated by manipulating oxygen availability, size and shape of the particles, and the type of coating (Xiu et al. 2012). Bhattacharya et al. studied the antibacterial activity of AgNPs coated with a functionalizing agent. Here, polyethylene glycol, tween 80, and sodium dodecyl sulphate were added separately to coat AgNPs. Amongst those, polyethylene glycol-coated AgNPs were found to be most effective against both normal and multi-drug resistant strains of bacteria. It was observed that intracellular ROS production in bacteria was high in polyethylene glycol-coated nanoparticles (Bhattacharya et al. 2012).
AgNPs can increase the rate of wound closure by promoting the proliferation and migration of keratinocytes. Further, AgNPs can facilitate the differentiation of fibroblasts into myofibroblasts and thereby promoting wound contraction (Liu et al. 2010). Tian et al. demonstrated that AgNPs can contribute to the wound healing process through their antimicrobial action, by reducing wound inflammation and modulating fibrogenic cytokines (Tian et al. 2007). Tannic acid (TA)-modified AgNPs were also found to have effective antibacterial activity against Pseudomonas aeruginosa, S. aureus, and E. coli. Furthermore, in a mouse splint wound model, TA-modified AgNPs enhanced wound closure, epithelialization, angiogenesis, and granulation tissue formation. TA-AgNPs also induced the expression of vascular endothelial growth factor-α (VEGF-α), platelet-derived growth factor-β (PDGF-β), and transforming growth factor-β1(TGF-β1) cytokines which are involved in the efficient wound healing process (Orlowski et al. 2018).
Gold nanoparticles (AuNPs) are widely used in tissue regeneration, wound healing, and also in drug delivery. On the surface of AuNPs, there is a possibility to conjugate different ligands like polypeptide sequences, antibodies, and proteins (Fathi-Achachelouei et al. 2019). For example, Gu et al. reported that AuNPs can be conjugated with existing antimicrobial drugs by preparing vancomycin-conjugated gold nanoparticles (Au@Van) (Fig. 5.1). These nanoparticles have displayed increased activity against vancomycin-resistant Enterococci (VRE) and a notable activity against E. coli which is a Gram-negative bacterium normally unaffected by vancomycin (Gu et al. 2003).

(Adapted with permission from Gu et al. 2003)
Transmission electron micrographs of a Au@Cys and b Au@Van nanoparticles in the aggregated state after cryodrying at concentrations of 6.7 and 50 µg/mL (The insets show TEM images taken at the MIC concentration for Au@Cys and b Au@Van) and TEM images of E. coli after being treated by c Au@Cys and d Au@van nanoparticles at minimum inhibition concentrations (In this study Au nanoparticles conjugated with cysteine; Au@Cys were employed as the control)
Recently, Korani et al. evaluated the antibacterial, antioxidant, cytotoxic, and cutaneous wound healing potential in AuNPs synthesized using Abelmoschus esculentus extract. Transmission electron microscopy (TEM) images indicated that the prepared nanoparticles were spherical and the size was around 75 nm. These nanoparticles exhibited significant antioxidant and antibacterial activities while being non-toxic. Interestingly, these AuNPs could accelerate wound closure, thus indicating the prospective application as a wound healing therapeutic (Korani et al. 2021).
AuNPs can be adjusted to strongly absorb near-infrared radiation and eventually transfer this energy in the form of heat into the surrounding environment. When nanoparticles are attached to bacterial cells, this localized heating during irradiation of near-infrared radiation can lead to irreversible cellular damage. On this basis, Norman et al. experimented with gold nanorods that have been covalently linked to pathogen-specific antibodies to selectively destroy one of the multi-drug resistance Gram-negative bacterium P. aeruginosa (Norman et al. 2008). Similarly, Gil-Tomas et al. attempted to covalently couple toluidine blue O–tiopronin to AuNPs and thereby enhance the antimicrobial activity against S. aureus. This conjugate entity showed activity under both 632.8 nm laser light and white light and appeared as a rapidly acting photosensitiser in the photodynamic therapy of wound and burn infections (Gil-Tomás et al. 2007). Moreover, Sherwani et al. demonstrated that photosensitizer conjugated-AuNPs can be used in the treatment of cutaneous Candida albicans infections. These nanoparticles have significantly reduced the fungal burden in topical skin wounds and tongues infected with C. albicans in mice (Sherwani et al. 2015).
Further, Naraginti et al. demonstrated that the application of green synthesized AgNPs and AuNPs formulations on open skin wounds in rats has accelerated the wound healing process. This has occurred via increased granulation tissue formation, collagen deposition, and re-epithelialization and thereby shortening the overall healing time. The topical application of AuNP formulation was found to be more effective than the application of AgNP formulation and the conspicuous wound healing activity observed with AuNPs was linked to its high anti-inflammatory and antioxidant activities (Naraginti et al. 2016).
Laser-tissue welding and laser-tissue soldering techniques have been introduced as an alternative approach to traditional suturing to repair skin. Although laser-tissue welding results in faster healing, it inherits several disadvantages like thermal damage and low penetration depths. In order to overcome these issues, exogenous chromophores have been employed to absorb the laser energy within the near-infrared window. In this respect, gold nanoshells have displayed great promise as exogenous near-infrared absorbers in laser-tissue welding. The in vivo experiments with rat skin wound healing model indicated that the aforementioned approach can result in a good wound healing response while minimizing the destruction of surrounding tissues and permitting the welding of thicker tissues (Gobin et al. 2005).
Zinc oxide nanoparticles (ZnONPs) possess antibacterial, anti-inflammatory, and skin regeneration properties and thus can be considered an ideal material for wound dressings and to speed up the healing of both acute and chronic wounds. Hu et al. employed the electrospinning technique to fabricate zinc oxide/silver/polyvinylpyrrolidone/polycaprolactone (ZnO/Ag/PVP/PCL) nanofibres. In this approach, ZnONPs and AgNPs were mixed with PVP and PCL to obtain nanofibers. The ZnO/Ag/PVP/PCL bimetallic nanofibers displayed potent antibacterial activity and low cytotoxicity than the single metal nanomaterial-loaded nanofibres (Hu et al. 2018).
Copper plays many roles during the wound healing process. The antibacterial activity in copper is well-known while its involvement in skin re-modulation, stimulation of angiogenesis, and enhancement of anti-inflammatory power have also been documented. As copper in its nano form displays better bioavailability than the native bulk form, Thiwari et al. conducted experiments to synthesize copper nanoparticles (CuNPs) and to evaluate the wound healing activity. In this study, CuNPs were prepared using P. aeruginosa. Synthesized CuNPs have exhibited better antimicrobial efficacy and a higher margin of safety on HaCaT normal cell line in comparison to native copper. Further treatment with CuNP-gel showed an increased rate of wound healing in rats compared to native copper (Tiwari et al. 2014). Similarly, Xiao et al. hypothesized that copper metal–organic framework nanoparticles can be modified for the slow release of Cu2+ions and thereby to enhance the rate of wound healing. Folic acid was added during the synthesis of nanoparticles as a stabilizer and the resulting folic acid-modified copper metal–organic framework nanoparticles slowly released copper ions and promoted cell migration in vitro. Further, the synthesized nanoparticles were capable of promoting angiogenesis, deposition of collagen, re-epithelialization, and accelerating wound closure rates (Xiao et al. 2018). Sankar et al. have synthesized copper oxide nanoparticles (CuONPs) using Ficus religiosa leaf extract and evaluated antibacterial, and wound healing activity. Interestingly, CuONPs have inhibited the growth of some pathogenic bacterial strains that can delay the wound healing process (Fig. 5.2). It increased the rate of wound healing in Wistar Albino rats (Sankar et al. 2015).

(Adapted with permission from Sankar et al. 2015)
Photographic illustration on different days of control and copper oxide nanoparticles treated animals
Similarly, CuNPs were synthesized from aqueous extract of the leaves of Falcaria vulgaris and the antioxidant, antimicrobial, and wound healing properties of the nanoparticles were evaluated. Notable antioxidant and antimicrobial activities were observed while the nanoparticles did not affect the viability of the human umbilical vein endothelial cell line. The in vivo experiments revealed that the treatment with CuNPs ointment remarkably increased the cutaneous wound healing process in rats induced with cutaneous wounds (Zangeneh et al. 2019).
Recently, Ahmed et al. reported an ecofriendly approach for the synthesis of CeO2 nanoparticles and the wound healing potential of the synthesized nanoparticles was evaluated after incorporating them into a chitosan hydrogel membrane as a wound dressing. Here, the synthesis of CeO2 nanoparticles was mediated by Abelmoschus esculentus extract which functioned as a reducing and stabilizing agent. The green synthesized nanoparticles exhibited bactericidal effects against both Gram-positive and Gram-negative bacterial species while the in vivo studies revealed that these nanoparticles were highly effective in treating wounds. CeO2 nanoparticles induced collagen deposition and enhanced the tensile strength of skin and thereby promoted wound healing (Ahmed et al. 2021).
Different growth factors like epidermal growth factor (EGF), basic fibroblast growth factor, granulocyte–macrophage colony-stimulating factor, vascular endothelial growth factor (VEGF) are involved in the wound healing process. For example, EGF stimulates epithelial cell proliferation and the synthesis of the extracellular matrix while VEGF stimulates new vessel growth and increases vascular permeability. Similarly, basic fibroblast growth factors (bFGFs) promote fibroblast proliferation and neovascularization. Ribeiro et al. attempted to encapsulate VEGF and EGF in chitosan microparticles which were then embedded inside a dextran-based hydrogel. The in vitro and in vivo assays revealed the applicability of the above system as a biocompatible approach to improve mechanical, chemical, and biological protection of the damaged skin and thereby to re-establish the skin architecture (Ribeiro et al. 2013). In an early study, Elçin et al. used calcium alginate microspheres as VEGF carriers and examined the in vivo wound healing potency on the Wistar rat model. The results indicated that VEGF-encapsulated alginate microspheres were capable of promoting vigorous angiogenesis, thus showing great promise in tissue engineering (Elçin et al. 2001).
AuNPs have recently emerged as gene delivery vectors. Wang et al. attempted to functionalize ultra-small gold nanoparticles (AuNPs@LL37) with the antimicrobial peptide LL37 which exerts various immunomodulatory functions. Thereafter, the suitability of this system for the topical treatment of diabetic wounds was investigated. Here AuNPs@LL37 system was combined with pro-angiogenic (VEGF) plasmids (AuNPs@LL37/pDNAs) and this has significantly improved the gene transfection efficiency in keratinocytes. It promoted angiogenesis and inhibited bacterial infection in diabetic wounds ultimately resulting in accelerated wound closure rates (Wang et al. 2018).
Lipid nanoparticles have been widely investigated over recent years as a safe and cost-effective drug delivery system. Moreover, these lipid nanoparticles also help to protect the drug from biological degradation. Solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid drug conjugates are some examples of lipid nanoparticles widely employed in pharmaceutical research (Attama et al. 2012).
Impaired wound healing and the development of non-healing diabetic foot ulcers are common amongst patients suffering from diabetes mellitus. The overproduction of tumour necrosis factor α (TNFα) causes further damage to the wound due to the up-regulation of cellular apoptosis, formation of reactive oxygen species, and degradation of the matrix. Therefore, Kasiewicz and Whitehead developed lipid nanoparticles (LNPs) loaded with siRNA-specific for TNFα. The topical application of the nanopreparation decreased TNFα mRNA expression in the wound in both diabetic and non-diabetic mice. Further, TNF α knockdown has increased the wound healing process in diabetic mice compared to untreated controls (Fig. 5.3). These findings demonstrated that LNP-based RNA interference therapy can decrease the severity and duration of chronic diabetic wounds (Kasiewicz and Whitehead 2018).

(Adapted with permission from Kasiewicz and Whitehead 2018)
LNP-mediated TNFα gene silencing accelerated wound healing in diabetic mice. a Mice were double-wounded on day 1 and treated on days 2 and 3 with either PBS (control, left wounds) or LNPs containing siTNFα (250 nM, right wounds). Mice were sacrificed on day 4. b The wound area is represented as a percent of its original size. By day 4, nanoparticle‐treated wounds are statistically significantly smaller than PBS‐treated wounds. c Wounds treated with lipid nanoparticles containing siRNA specific against TNFα (siTNFα) experienced a 43% reduction in TNFα expression compared to PBS‐treated control wounds. In each panel, error bars represent s. d (n = 4–5, *p < 0.05, **p < 0.01)
The topical administration of nanoparticle-based delivery systems is often facilitated by incorporating into either semi-solid hydrogels or solid scaffolds like fibrin-based biomaterials. Human recombinant EGF-loaded solid lipid nanoparticles (rhEGF-SLN) and human recombinant EGF-loaded nanostructured lipid carriers (rhEGF-NLC) were embedded in either semi-solid hydrogels or fibrin-based solid scaffolds and the performance of each wound dressing-the delivery system was investigated. The results indicated that both rhEGF-SLN and rhEGF-NLC nanocarrier systems were equally effective with respect to their in vitro performance. However, the long-term stability of fibrin-based scaffolds suggested that fibrin-based scaffolds might be the ideal approach to formulate rhEGF-loaded lipid nanoparticles (Gainza et al. 2015).
Saporito et al. attempted to load eucalyptus and rosemary essential oils into solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) prepared from natural lipids. Thereafter the antimicrobial activity of these lipid nanoparticles was determined against S. aureus and Streptococcus pyogenes. The in vitro proliferation enhancement, as well as wound healing potential on normal human dermal fibroblasts, was also tested while the rat burn model was used to determine the wound healing activity in vivo. The nanostructured lipid carriers made up of olive oil and loaded with eucalyptus oil demonstrated promising activity in both in vitro and in vivo assays. The synergic effect of eucalyptus oil with olive oil which is rich in oleic acid was proved to be responsible for antimicrobial activity and wound repair promotion (Saporito et al. 2017). Similarly, Rosseto et al. developed solid lipid nanoparticles and nanostructured lipid carriers as topical propolis delivery systems for wound healing purposes. Good cell viability was observed in human immortalized keratinocytes treated with propolis and by-product extracts entrapped systems. Moreover, in vivo wound healing process was accelerated by SLN modified with propolis (SLN-2 and SLN-4) as depicted in Fig. 5.4 (Rosseto et al. 2017).

(Adapted with permission from Rosseto et al. 2017)
Results of wound closure test—representative photographs showing the wounds and the different treatments in SKH1-mice after treatment (d0) and 15 days after (d15)
Various electrospinning nanofiber scaffolds have been evaluated as wound dressings by many researchers. The electrospinning method is a quick, cost-effective, and repeatable method that can be used to obtain nanomaterials formed by the deposition of polymer nanofibers. Natural polymers (e.g. collagen, gelatin, chitosan, silk fibroin), as well as synthetic biodegradable polymers like poly(vinyl alcohol) (PVA), polyacrylonitrile (PAN), poly(vinyl acetate) (PVAc), polyurethane (PU), polylactic acid (PLA), poly(ε-caprolactone) (PCL), poly(lactide-co-glycolide) (PLGA), are employed in the development of these nanofibrous mats. Electrospinning results in an interconnected three-dimensional network with features like the large surface area to volume ratio and high porosity that can facilitate wound homeostasis while also allowing gas and nutrient exchange. Further, loading drugs or other biomolecules into these fibres is also possible (Abrigo et al. 2014). Particularly, in the treatment of chronic non-healing wounds, much attention is given to dressings that are capable of preventing microbial infiltration while maintaining a balanced moisture and gas exchange environment. In this respect, electrospun wound dressings with the ability to simultaneously impair or treat infection and encourage cell proliferation/wound healing are receiving much attention (Abrigo et al. 2014; Coelho et al. 2018).
Gonis et al. also conducted experiments to develop bioactive wound dressing materials capable of promoting wound healing as well as being involved in the regeneration of healthy tissue. These experiments led to the development of poly (1,8 octanediol-co-citrate) and poly (acrylic acid) nanofibrous scaffolds. Antibacterial potential, hydrogel-like water uptake capability, and the capacity to deliver growth factor at physiologically relevant concentrations are some important features of this nanofibrous scaffold. Further, it demonstrated low toxicity and cellular adhesion while enhancing the proliferation of skin fibroblasts (Gonis et al. 2017).
Fibrin is a coagulation protein that plays an important role in the wound healing process. It is derived from its precursor fibrinogen, a soluble protein synthesized by the liver and found in blood plasma. Fibrin is often utilized as a scaffold for wound healing and also as a drug carrier. For example, Alphonsa et al. developed ciprofloxacin-loaded and fluconazole-loaded fibrin nanoparticles in order to achieve sustained delivery of encapsulated antimicrobial drugs to treat microbial infected wounds. A good antimicrobial activity was observed in the above drug-loaded systems while maintaining adequate cell viability of human dermal fibroblast cell lines (Alphonsa et al. 2014). Similarly, Losi et al. developed a fibrin-coated poly(ether)urethane-polydimethylsiloxane scaffold containing poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with recombinant human VEGF and bFGF (Losi et al. 2013).
Nanofibrous scaffolds fabricated with natural polymers such as collagen in the presence of bioactive metabolites have great promise in tissue engineering. Kandhasamy et al. conducted experiments to develop collagen-coated ostholamide (OSA) electrospun nanofiber scaffold and thereafter to evaluate the wound healing potential. Ostholamide (OSA) was derivatised from osthole, a natural coumarin with antimicrobial, anti-inflammatory, and antioxidant activities. Here, OSA was added to polyhydroxybutyrate (PHB) and gelatin (GEL), which were used as templates for electrospun nanofibers. Thereafter, the electrospun PHB-GEL-OSA scaffolds were coated with collagen and this resulted in PHB-GEL-OSA-COL nanofibrous scaffold that mimics the extracellular matrix (Fig. 5.5).

(Adapted with permission from Kandhasamy et al. 2017)
SEM micrographs of nanofibers; a and a1 PHB-GEL-OSA (overlay view at 6000X and 24000X) and b and b1 PHB-GEL-OSA-COL (Collagen coated PHB-GEL-OSA fibres at 6000X and 24000X magnification) scaffold
The PHB-GEL-OSA-COL nanofibers have displayed exceptional mechanical stability, which is important in wound healing. Moreover, the presence of OSA provided antibacterial properties to this nanofiber scaffold. Further, the nanofibrous scaffold was able to release OSA in a controlled manner and it was found to be stable against enzymatic degradation, a property that is important for the repair and remodelling of tissues during wound healing. The in vivo experiments conducted using Wistar rats revealed enhanced wound healing in rats treated with PHB-GEL-OSA-COL nanofibrous scaffold in comparison to untreated rats (Fig. 5.6) (Kandhasamy et al. 2017).

(Adapted with permission from Kandhasamy et al. 2017)
Photographs showing wound closure efficiency in the control (untreated wound), collagen scaffold (COLF), PHB-GEL-COL fibres, and PHB-GEL-OSA-COL electrospun nanofibrous scaffold
Hyaluronic acid (HA) is a component of the extracellular matrix involved in the wound healing process. It is widely used as a hydrogel scaffold for wound healing applications. Pereira et al. developed polymeric films containing hyaluronate, sodium alginate, and polyvinyl alcohol (PVA) and loaded with Aloe vera extract and vitamin E. Although this formulation was found to be more effective than the conventional vitamin E cream, it showed a burst release of the vitamin and was not occlusive (Pereira et al. 2014). Therefore, a new form of hyaluronic acid-coated lipid nanoparticles was formulated for topical administration on burns. Here, vitamin E-loaded nanoparticles based on hyaluronate and lecithin were developed with the use of the cationic lipid dioctadecyldimethylammonium bromide (DODMA). The prepared nanoparticles were subsequently embedded in a polymeric film containing Aloe vera extracts. Interestingly, a slow release of the vitamin was observed along with more efficient occlusion properties in comparison to the formulation containing the vitamin but in the absence of nanoparticles. Further, this nanoparticle-loaded polymer film reduced the water loss through damaged skin while protecting the vitamin against degradation from light and oxygen (Pereira et al. 2016).
Recently Buntum et al. formulated semi-solid poly(vinyl alcohol) (PVA) hydrogels containing essential oil-loaded chitosan nanoparticles for wound dressing applications. Essential oils of clove and turmeric were used in this study. The essential oil-loaded chitosan nanoparticles in semi-solid PVA hydrogels helped to sustain and prolong the release rate of essential oil from the hydrogels. Moreover, the investigated semi-solid PVA hydrogels were found to be non-toxic to the cells as those were able to retain the viability of NCTC clone 929 and NHDF cells above 70% (Buntum et al. 2021).
Oxidative stress adversely affects tissue repair and regeneration, thus the removal of excessive ROS may result in an accelerated healing process. In this respect, antioxidant biomolecules play a significant role with their ability to neutralize free radicals. Therefore, Kandhasamy et al. designed a quinone-based chromenopyrazole (QCP) antioxidant-laden silk fibroin nanofiber scaffold (QCP-SF) for skin tissue engineering applications. For this, 3-methyl-4-(4-oxo4H-chromen-3-yl)-1-phenylbenzo[6,7]chromeno[2,3-c]pyrazole-5,10 (1H,4H)-dione (QCP) derivatives inherited with potent antioxidant activity were synthesized and incorporated into silk fibroin nanofiber scaffold. Silk fibroin (SF) is a natural protein derived from cocoons of silkworm Bombyx mori and it has various tissue engineering applications mainly due to its ability to enhance re-epithelization, adhesion, and massive proliferation of keratinocytes, fibroblasts, and endothelial cells. Upon the incorporation of QCP, the nanofiber scaffold displayed an increased cell attachment and proliferation in NIH 3T3 fibroblasts and rat bone marrow stem cells (rBMSCs). Moreover, QCP-SF scaffold displayed a rapid cell migration in the scratch assay and hence an excellent wound healing activity (Kandhasamy et al. 2019).
Vitamin E-loaded silk fibroin nanofibrous scaffolds have also been developed for wound healing applications. Vitamin E (α-tocopherol) functions as an antioxidant as well as a stabilizing agent in biological membranes. The water-soluble derivative of vitamin E, RRR-α-tocopherol polyethylene glycol 1000 succinate (VE TPGS) was loaded into silk fibroin nanofibrous mat and the wound healing activity was evaluated by Sheng et al. Sustained release behaviour of VE TPGS from the nanofibrous mat was observed in the in vitro study. Moreover, this novel scaffold encouraged the proliferation of skin fibroblasts and enhanced the survival of the cells against oxidative stress (Sheng et al. 2013). In another study, vitamin E-loaded starch nanoparticles were incorporated into silk fibroin/poly(vinyl alcohol)/Aloe vera nanofibers system prepared by the electrospinning method. The incorporation of Aloe vera and vitamin E silk fibroin/poly(vinyl alcohol) nanomatrix enhanced the fibroblast attachment, proliferation, and collagen secretion while the presence of vitamin E has exerted an antioxidant effect facilitating the wound healing process (Kheradvar et al. 2018).
In another study, soy protein isolate/silk fibroin (SPI/SF) nanofibrous scaffolds were developed and the wound healing potential was evaluated. These scaffolds were found to be non-toxic to normal mammalian cells and were capable of healing full-thickness wounds in rats within 14 days (Varshney et al. 2020). Similarly, nanofibrous scaffolds comprised of gum tragacanth/poly (ε-caprolactone) have also been developed for tissue engineering/wound dressing applications. Gum tragacanth is a mixture of water-soluble polysaccharides of plant origin reported with wound healing potency. Poly (ε-caprolactone) is an aliphatic polyester with good mechanical properties which is widely employed in biomedical applications due to its biocompatibility, slow biodegradability, and non-toxicity. The fabricated scaffold was capable of enhancing fibroblast adhesion and proliferation and also displayed antibacterial activity against Gram-positive and Gram-negative bacterial species (Ranjbar-Mohammadi and Bahrami 2015).
Rathinavel et al. fabricated a nanoscaffold comprised of functionalized SBA-15 (Santa Barbara Amorphous) polycaprolactone (PCL) and curcumin for wound healing applications. The high biocompatibility and cell adhesion property of amine-functionalized SBA-15 as well as antimicrobial potential in curcumin have made this nanoscaffold an effective wound healing therapeutic. The in vivo experiments with Wister rats indicated that the treatment with this nanoscaffold has led to re-epithelization, collagen deposition, and formation of granulation tissue resulting in 99% scar-less wound healing within 21 days (Rathinavel et al. 2021).
The combination of different nanotechnological approaches can result in the development of more sophisticated and improved wound healing materials. For example, Chen et al. synthesized a novel wound dressing with the use of AgNP-loaded Konjac Glucomannan composite sponges (KGM/AgNP). In this approach, AgNPs were prepared with egg whites and then KGM powder was added while vigorously stirring. Thereafter this mixture was freeze-dried to obtain a nanocomposite sponge. This sponge possessed properties like antibacterial activity against E. coli and S. aureus, good cytocompatibility, higher capacity of water absorption and retention as well as significant mechanical properties. In addition, animal experiments demonstrated that the fabricated nanocomposite was capable of enhancing wound healing and promoting fibroblast growth and epithelialization (Chen et al. 2018).
Furthermore, Ding et al. prepared a chemically cross-linked spongy bilayer composite as a wound dressing. The upper layer was formed from chitosan-Ag nanoparticles cross-linked with genipin. Genipin is a compound isolated from the fruit of Gardenia jasminoides and it is being widely used to cross-link the free amino groups on chitosan. The lower layer of the bilayer composite was made of chitosan cross-linked with genipin and partially oxidized Bletilla striata polysaccharide. B. striata is a plant employed in traditional Chinese medicine and several in vitro assays have reported that polysaccharides obtained from this plant can enhance the proliferation of human vascular endothelial cells and the expression of vascular endothelial growth factor. The newly developed wound dressing material exhibited good water retention, mechanical strength, L929 cell proliferation, and antimicrobial activity. The in vivo studies revealed that the new wound dressing material has accelerated the healing rate of cutaneous wounds in mice (Ding et al. 2017).
Similarly, Yang et al. employed some of the structural components of β-lactam antibiotics; i.e. 6-aminopenicillanic acid (6-APA), 7-aminocephalosporanic acid (7-ACA), and 7-amino-desacetoxy-cephalosporanic acid (7-ADCA) to modify the surfaces of AuNPs. Thereafter these modified AuNPs were incorporated into electrospun poly(ε-caprolactone) (PCL)/gelatin fibres to fabricate a wound dressing material. The antibacterial activity studies indicated that APA-coated AuNPS were more effective than AuNPs coated with 7-ACA and 7-ADCA to inhibit the growth of P. aeruginosa and Klebsiella pneumonia as well as clinically isolated MDR (multi-drug resistant) strains. Furthermore, the in vivo bacteria-infected wound healing study showed that it has a remarkable ability to treat MDR bacteria-infected wounds (Yang et al. 2017).
Thanusha et al. developed a hydrogel composed of the triterpenoid asiatic acid, zinc oxide nanoparticles, and copper oxide nanoparticles which were incorporated into the gelatin and glycosaminoglycans (hyaluronic acid and chondroitin sulphate). Thereafter the efficacy of this hydrogel composite in healing second-degree burn wounds was determined using Wistar rats. The presence of either ZnONPs or CuONPs in the hydrogel composite resulted in a higher antibacterial activity compared to the hydrogel composite scaffold. Moreover, a significant wound healing activity was seen in Wistar rats treated with hydrogel composite in comparison to the control (NeuSkin™ and cotton gauze). The histopathology study demonstrated that re-epithelialization, arrangement of collagen fibres, and angiogenesis were facilitated by the above treatment (Fig. 5.7). Further, a decrease in TNF-α and an increase in MMP-2 expression were observed with the treatment and supposed to be contributed to the healing process (Thanusha et al. 2018).

(Adapted with permission from Thanusha et al. 2018)
Photographic images of (i) procedure followed for applying second-degree burn wound: a removal of hairs, b applying burn punch (2 cm2) of 95 ℃ for 20 s, c formation of burn, d after 24 h, removed epidermal burnt position-0th day. (ii) a–c hydrogel composite applied wound on 7d, 14d and 28d, d–f NeuSkinTM applied wound on 7d, 14d, and 28d, g–i Cotton gauze (Control) applied wound on 7d, 14d, and 28 day
The wound healing potential of phytochemical-decorated AuNPs was evaluated by Lee et al. The skin regeneration study revealed that the treatment of gallic acid-isoflavone-covered AuNPs/protocatechuic acid-isoflavone-covered AuNPs on the dorsal skin of rats has resulted in a thicker epidermis, a decrease in the metalloproteinase-1 level and an increased activity in superoxide dismutase. Moreover, phytochemical-decorated AuNPs treatment on surgical and burn wounds in Sprague–Dawley (SD) rats has improved the expression of VEGF and angiopoietin-2 and induced the proliferation of granulation tissue and keratinocytes. Further, the study revealed that nanoparticles exert an anti-inflammatory function during the wounding healing process while gallic acid-isoflavone-covered AuNPs can activate the transforming growth factor β (TGF-β) function for skin regeneration (Lee et al. 2015).
Tissue-engineered skin substitutes are emerging as an effective therapeutic option to treat patients with skin damage caused by congenital defects, burns, etc. Although collagen-based dermal substitutes (e.g. Dermagraft, Apligraf) are used as skin substitutes, the issues like antigenicity could lead to unsatisfactory outcomes. As a result, several synthetic or natural polymers have been investigated and silk fibroin has shown great promise as an alternative material for the construction of dermal substitutes (Park et al. 2016). Although nanofibers fabricated by electrospinning silk fibroin possess desirable biocompatibility, the small pore size makes it difficult for cells to infiltrate these electrospun silk fibroin. Therefore, Lee et al. developed a method using NaCl crystals to control the pore size of the electrospun silk fibroin and thereby allowing cells to easily infiltrate into the nanofibers and proliferate internally. The newly developed three-dimensional electrospun silk fibroin nanofiber did not show any cytotoxicity while displaying increased cell infiltration in NIH 3T3 fibroblast cells. The in vivo experiments revealed that it could accelerate re-epithelialization and wound closure and was equally effective as Matriderm. However, it almost completely degraded without inducing wound contracture like with Matriderm. Therefore, this electrospun silk fibroin nanofiber displayed a high potential to be used in human patients with full-thickness skin defects (Lee et al. 2014). In the follow-up study, a skin substitute was developed by using the 3-D electrospun SF nanofiber scaffold. The high interconnectivity between pores and the porosity as well as the water uptake abilities have facilitated cell infiltration and proliferation leading to increased infiltration of fibroblasts and the stratification of keratinocytes. These observations further confirmed the suitability of the 3-D electrospun silk fibroin nanofiber scaffold for skin tissue engineering (Park et al. 2016).
An important concern during tissue engineering is to mimic the fibrous structure of the extracellular matrix. The development of scaffold materials with good biodegradability, surface properties, mechanical strength as well as controlled drug release potential is important in tissue engineering. Li et al. fabricated core-sheath nanofibers comprised of poly (ɛ-caprolactone) and silk fibroin blends. This scaffold resulted in an improved cell attachment and proliferation and sustained drug release (Li et al. 2011). In another study, the natural antioxidant fenugreek was incorporated into silk fibroin nanofibers and the wound healing potential of this nanofiber scaffold was evaluated using full-thickness excisional wounds in the rat model. The developed material has displayed excellent mechanical, thermal, and antioxidant properties and resulted in complete re-epithelialization and enhanced collagen deposition accelerating the wound healing process (Selvaraj and Fathima 2017).
Bioactive glasses (BGs) are normally employed for hard tissue regeneration. However, these can also be used to prepare wound dressings because of their ability to stimulate rapid haemostasis, fibroblasts proliferation, and angiogenesis. Particularly the nano-scale bioactive glasses were found to be more effective than conventional bioactive glasses due to the larger specific surface area. Wang et al. developed an easy-to-use hydrogel by mixing bioactive glasses with gelatin. This hydrogel caused a rapid cutaneous-tissue regeneration and tissue-structure formation in rats (Wang et al. 2017).
Based on the aforementioned examples, it is clear that nanomaterials have a great potential to be employed as wound healing therapeutics and tissue engineering scaffolds in near future.
5.4 Conclusion
Skin is the largest external organ in the human body and it can be damaged due to various reasons. Therefore, wounds may occur on the skin very often. Wound healing is a complex process and it may get delayed due to several factors. Nowadays traditional wound dressings have been replaced by more sophisticated modern wound dressings. Nanomaterials are playing an important role in the development of highly effective wound dressings. Tissue engineering scaffolds nanoparticles, nanofibers, as well as nanocomposites, appear as excellent wound healing agents and tissue engineering materials due to the intrinsic antimicrobial activity, cytocompatibility, low toxicity, etc. Hence, nanomaterials have an inevitable future as wound dresses and skin substitutes.
References
Abdelrahman, T., Newton, H.: Wound dressings: principles and practice. Surg. Infect. (Larchmt.) 29(10), 491–495 (2011)
Abrigo, M., McArthur, S.L., Kingshott, P.: Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol. Biosci. 14(6), 772–792 (2014)
Ahmed, H.E., Iqbal, Y., Aziz, M.H., Atif, M., Batool, Z., Hanif, A., Yaqub, N., Farooq, W.A., Ahmad, S., Fatehmulla, A., Ahmad, H.: Green synthesis of CeO2 nanoparticles from the Abelmoschus esculentus extract: evaluation of antioxidant, anticancer, antibacterial, and wound-healing activities. Molecules 26(15), 4659 (2021). https://doi.org/10.3390/molecules26154659
Alphonsa, B.M., Sudheesh Kumar, P.T., Praveen, G., Biswas, R., Chennazhi, K.P., Jayakumar, R.: Antimicrobial drugs encapsulated in fibrin nanoparticles for treating microbial infested wounds. Pharm. Res. 31(5), 1338–1351 (2014)
Attama, A.A., Mamoh, M.A., Builders, P.F.: Lipid nanoparticulate drug delivery systems: a revolution in dosage form design and development. In: Sezer, A.D. (ed.) Recent Advances in Novel Drug Carrier Systems, pp. 107–140. IntechOpen (2012). https://www.intechopen.com/chapters/40253
Bentley, J.: Understanding the wound healing process. Pract. Nurs. 15(4), 181–188 (2004)
Buntum, T., Kongprayoon, A., Mungyoi, W., Charoenram, P., Kiti, K., Thanomsilp, C., Supaphol, P., Suwantong, O.: Wound-aided semi-solid poly(vinyl alcohol) hydrogels incorporating essential oil-loaded chitosan nanoparticles. Int. J. Biol. Macromol. 189, 135–141 (2021)
Bhattacharya, D., Samanta, S., Mukherjee, A., Santra, C.R., Ghosh, A.N., Niyogi, S.K., Karmakar, P.: Antibacterial activities of polyethylene glycol, tween 80 and sodium dodecyl sulphate coated silver nanoparticles in normal and multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 12(3), 2513–2521 (2012)
Blanco-Fernandez, B., Castaño, O., Mateos-Timoneda, M.Á., Engel, E., Pérez-Amodio, S.: Nanotechnology approaches in chronic wound healing. Adv. Wound Care 10(5), 234–256 (2021)
Boateng, J.S., Matthews, K.H., Stevens, H.N., Eccleston, G.M.: Wound healing dressings and drug delivery systems: a review. J. Pharm. Sci. 97(8), 2892–2923 (2008)
Chatterjee, A.K., Chakraborty, R., Basu, T.: Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25(13), 135101 (2014). https://doi.org/10.1088/0957-4484/25/13/135101
Chen, H., Lan, G., Ran, L., Xiao, Y., Yu, K., Lu, B., Dai, F., Wu, D., Lu, F.: A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 183, 70–80 (2018)
Coelho, D.S., Veleirinho, B., Alberti, T., Maestri, A., Yunes, R., Dias, P.F., Maraschin, M.: Electrospinning technology: designing nanofibers toward wound healing application. In: Clichici, S., Filip, A., do Nascimento, G.M. (eds.) Nanomaterials—Toxicity, Human Health and Environment. IntechOpen (2018). https://doi.org/10.5772/intechopen.81530
Dalisson, B., Barralet, J.: Bioinorganics and wound healing. Adv. Healthc. Mater. 9, 1900764 (2019). https://doi.org/10.1002/adhm.201900764
Daunton, C., Kothari, S., Smith, L., Steele, D.: A history of materials and practices for wound management. Wound Pract. Res. 20(4), 174–186 (2012)
Dhivya, S., Padma, V.V., Santhini, E.: Wound dressings—a review. Biomedicine 5(4), 22 (2015). https://doi.org/10.7603/s40681-015-0022-9
Ding, L., Shan, X., Zhao, X., Zha, H., Chen, X., Wang, J., Cai, C., Wang, X., Li, G., Hao, J., Yu, G.: Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 157, 1538–1547 (2017)
Elçin, Y.M., Dixit, V., Gitnick, G.: Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif. Organs 25(7), 558–565 (2001)
Fathi-Achachelouei, M., Knopf-Marques, H., Ribeiro da Silva, C.E., Barthès, J., Bat, E., Tezcaner, A., Vrana, N.E.: Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 7, 113 (2019). https://doi.org/10.3389/fbioe.2019.00113
Flores, A., Kingsley, A.: Topical antimicrobial dressings: an overview. Wound Essentials 2, 182–185 (2007)
Gainza, G., Chu, W.S., Guy, R.H., Pedraz, J.L., Hernandez, R.M., Delgado-Charro, B., Igartua, M.: Development and in vitro evaluation of lipid nanoparticle-based dressings for topical treatment of chronic wounds. Int. J. Pharm. 490(1–2), 404–411 (2015)
Gil-Tomás, J., Tubby, S., Parkin, I.P., Narband, N., Dekker, L., Nair, S.P., Wilson, M., Street, C.: Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O–tiopronin–gold nanoparticle conjugate. J. Mater. Chem. 17(35), 3739–3746 (2007)
Gobin, A.M., O’Neal, D.P., Watkins, D.M., Halas, N.J., Drezek, R.A., West, J.L.: Near infrared laser-tissue welding using nanoshells as an exogenous absorber. Lasers Surg. Med. 37(2), 123–129 (2005)
Goins, A., Ramaswamy, V., Dirr, E., Dulany, K., Irby, S., Webb, A., Allen, J.: Development of poly (1,8 octanediol-co-citrate) and poly (acrylic acid) nanofibrous scaffolds for wound healing applications. Biomed. Mater. 13(1), 015002 (2017). https://doi.org/10.1088/1748-605X/aa8439
Gomaa, E.Z.: Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 63(1), 36–43 (2017)
Gu, H., Ho, P.L., Tong, E., Wang, L., Xu, B.: Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 3(9), 1261–1263 (2003)
Hu, M., Li, C., Li, X., Zhou, M., Sun, J., Sheng, F., Shi, S., Lu, L.: Zinc oxide/silver bimetallic nanoencapsulated in PVP/PCL nanofibres for improved antibacterial activity. Artif. Cells Nanomed. Biotechnol. 46(6), 1248–1257 (2018)
Joshi, A.S., Singh, P., Mijakovic, I.: Interactions of gold and silver nanoparticles with bacterial biofilms: molecular interactions behind inhibition and resistance. Int. J. Mol. Sci. 21(20), 7658 (2020). https://doi.org/10.3390/ijms21207658
Kandhasamy, S., Perumal, S., Madhan, B., Umamaheswari, N., Banday, J.A., Perumal, P.T., Santhanakrishnan, V.P.: Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl. Mater. Interfaces 9(10), 8556–8568 (2017)
Kandhasamy, S., Arthi, N., Arun, R.P., Verma, R.S.: Synthesis and fabrication of novel quinone-based chromenopyrazole antioxidant-laden silk fibroin nanofibers scaffold for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 102, 773–787 (2019)
Kasiewicz, L.N., Whitehead, K.A.: Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng. Transl. Med. 4(1), 75–82 (2018)
Kheradvar, S.A., Nourmohammadi, J., Tabesh, H., Bagheri, B.: Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly(vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surf. B Biointerfaces 166, 9–16 (2018)
Korani, S., Rashidi, K., Hamelian, M., Jalalvand, A. R., Tajehmiri, A., Korani, M., Sathyapalan, T., Sahebkar, A.: Evaluation of antimicrobial and wound healing effects of gold nanoparticles containing Abelmoschus esculentus (L.) aqueous extract. Bioinorg. Chem. Appl. 2021, 7019130 (2021). https://doi.org/10.1155/2021/7019130
LaPelusa, A., Dave, H.D.: Physiology, hemostasis. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL) (2022). PMID: 31424847
Lee, O.J., Ju, H.W., Kim, J.H., Lee, J.M., Ki, C.S., Kim, J.H., Moon, B.M., Park, H.J., Sheikh, F.A., Park, C.H.: Development of artificial dermis using 3D electrospun silk fibroin nanofiber matrix. J. Biomed. Nanotech. 10(7), 1294–1303 (2014)
Lee, J., Kim, J., Go, J., Lee, J.H., Han, D.W., Hwang, D., Lee, J.: Transdermal treatment of the surgical and burned wound skin via phytochemical-capped gold nanoparticles. Colloids Surf. B Biointerfaces 135, 166–174 (2015)
Li, L., Li, H., Qian, Y., Li, X., Singh, G.K., Zhong, L., Liu, W., Lv, Y., Cai, K., Yang, L.: Electrospun poly(ɛ-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int. J. Biol. Macromol. 49(2), 223–232 (2011)
Liu, X., Lee, P.-Y., Ho, C.-M., Lui, V., Chen, Y., Che, C.-M., Tam, P., Wong, K.: Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem 5, 468–475 (2010). https://doi.org/10.1002/cmdc.200900502
Losi, P., Briganti, E., Errico, C., Lisella, A., Sanguinetti, E., Chiellini, F., Soldani, G.: Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 9(8), 7814–7821 (2013)
Morgan, D.A.: Wounds-what should a dressing formulary include? Hosp. Pharm. 9, 261–266 (2002)
Naraginti, S., Kumari, P.L., Das, R.K., Sivakumar, A., Patil, S.H., Andhalkar, V.V.: Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 62, 293–300 (2016)
Norman, R.S., Stone, J.W., Gole, A., Murphy, C.J., Sabo-Attwood, T.L.: Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 8(1), 302–306 (2008)
Orlowski, P., Zmigrodzka, M., Tomaszewska, E., Ranoszek-Soliwoda, K., Czupryn, M., Antos-Bielska, M., Szemraj, J., Celichowski, G., Grobelny, J., Krzyzowska, M.: Tannic acid-modified silver nanoparticles for wound healing: the importance of size. Int. J. Nanomed. 13, 991–1007 (2018)
Park, Y.R., Ju, H.W., Lee, J.M., Kim, D.K., Lee, O.J., Moon, B.M., Park, H.J., Jeong, J.Y., Yeon, Y.K., Park, C.H.: Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 93(Pt B), 1567–1574 (2016)
Pereira, G.G., Guterres, S.S., Balducci, A.G., Colombo, P., Sonvico, F.: Polymeric films loaded with vitamin E and Aloe vera for topical application in the treatment of burn wounds. Biomed Res. Int. 2014, 641590 (2014). https://doi.org/10.1155/2014/641590
Pereira, G.G., Detoni, C.B., Balducci, A.G., Rondelli, V., Colombo, P., Guterres, S.S., Sonvico, F.: Hyaluronate nanoparticles included in polymer films for the prolonged release of vitamin E for the management of skin wounds. Eur. J. Pharm. Sci. 83, 203–211 (2016)
Rai, M., Yadav, A., Gade, A.: Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27(1), 76–83 (2009)
Ramos-e-Silva, M., Ribeiro de Castro, M.C.: New dressings, including tissue-engineered living skin. Clin. Dermatol. 20(6), 715–723 (2002)
Ranjbar-Mohammadi, M., Bahrami, S.H.: Development of nanofibrous scaffolds containing gum tragacanth/poly (ε-caprolactone) for application as skin scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 48, 71–79 (2015)
Rathinavel, S., Korrapati, P.S., Kalaiselvi, P., Dharmalingam, S.: Mesoporous silica incorporated PCL/curcumin nanofiber for wound healing application. Eur. J. Pharm. Sci. 167, 106021 (2021). https://doi.org/10.1016/j.ejps.2021.106021
Ribeiro, M.P., Morgado, P.I., Miguel, S.P., Coutinho, P., Correia, I.J.: Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 33(5), 2958–2966 (2013)
Rosseto, H.C., Toledo, L., Francisco, L., Esposito, E., Lim, Y., Valacchi, G., Cortesi, R., Bruschi, M.L.: Nanostructured lipid systems modified with waste material of propolis for wound healing: design, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 158, 441–452 (2017)
Sankar, R., Baskaran, A., Shivashangari, K.S., Ravikumar, V.: Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J. Mater. Sci. Mater. Med. 26(7), 214 (2015). https://doi.org/10.1007/s10856-015-5543-y
Saporito, F., Sandri, G., Bonferoni, M.C., Rossi, S., Boselli, C., Icaro Cornaglia, A., Mannucci, B., Grisoli, P., Vigani, B., Ferrari, F.: Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 13, 175–186 (2017)
Selvaraj, S., Fathima, N.N.: Fenugreek incorporated silk fibroin nanofibers—a potential antioxidant scaffold for enhanced wound healing. ACS Appl. Mater. Interfaces 9(7), 5916–5926 (2017)
Shah, J.B.: The history of wound care. J. Am. Col. Certif. Wound Spec. 3(3), 65–66 (2011). https://doi.org/10.1016/j.jcws.2012.04.002
Shaikh, S., Nazam, N., Rizvi, S., Ahmad, K., Baig, M.H., Lee, E.J., Choi, I.: Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 20(10), 2468 (2019). https://doi.org/10.3390/ijms20102468
Sheng, X., Fan, L., He, C., Zhang, K., Mo, X., Wang, H.: Vitamin E-loaded silk fibroin nanofibrous mats fabricated by green process for skin care application. Int. J. Biol. Macromol. 56, 49–56 (2013)
Sherwani, M.A., Tufail, S., Khan, A.A., Owais, M.: Gold nanoparticle-photosensitizer conjugate based photodynamic inactivation of biofilm producing cells: Potential for treatment of C. albicans infection in BALB/c mice. PloS One 10(7), e0131684 (2015). https://doi.org/10.1371/journal.pone.0131684
Shi, C., Wang, C., Liu, H., Li, Q., Li, R., Zhang, Y., Liu, Y., Shao, Y., Wang, J.: Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 8, 182 (2020). https://doi.org/10.3389/fbioe.2020.00182
Sondi, I., Salopek-Sondi, B.: Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275(1), 177–182 (2004)
Sorg, H., Tilkorn, D.J., Hager, S., Hauser, J., Mirastschijski, U.: Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. 58(1–2), 81–94 (2017)
Takeo, M., Lee, W., Ito, M.: Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 5(1), a023267 (2015). https://doi.org/10.1101/cshperspect.a023267
Thanusha, A.V., Dinda, A.K., Koul, V.: Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater. Sci. Eng. C 89(378–386), 2018 (2018)
Tian, J., Wong, K.K., Ho, C.M., Lok, C.N., Yu, W.Y., Che, C.M., Chiu, J.F., Tam, P.K.: Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2(1), 129–136 (2007)
Thomas, S.: Hydrocolloids. J. Wound Care 1(2), 27–30 (1992). https://doi.org/10.12968/jowc.1992.1.2.27
Thomas, A., Harding, K.G., Moore, K.: Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-alpha. Biomaterials 21(17), 1797–1802 (2000)
Tiwari, M., Narayanan, K., Thakar, M.B., Jagani, H.V., Venkata Rao, J.: Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 8(4), 230–237 (2014)
Varshney, N., Sahi, A.K., Poddar, S., Mahto, S.K.: Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 160, 112–127 (2020)
Vowden, K., Vowden, P.: Wound dressings: Principles and practice. Surg. Infect. (Larchmt.) 35(9), 489–494 (2017)
Wang, C., Zhu, F., Cui, Y., Ren, H., Xie, Y., Li, A., Ji, L., Qu, X., Qiu, D., Yang, Z.: An easy-to-use wound dressing gelatin-bioactive nanoparticle gel and its preliminary in vivo study. J. Mater. Sci. Mater. Med. 28(1), 10 (2017). https://doi.org/10.1007/s10856-016-5823-1
Wang, S., Yan, C., Zhang, X., Shi, D., Chi, L., Luo, G., Deng, J.: Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 6(10), 2757–2772 (2018)
Xiao, J., Zhu, Y., Huddleston, S., Li, P., Xiao, B., Farha, O.K., Ameer, G.A.: Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 12(2), 1023–1032 (2018)
Xiu, Z.M., Zhang, Q.B., Puppala, H.L., Colvin, V.L., Alvarez, P.J.: Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 12(8), 4271–4275 (2012)
Yah, C.S., Simate, G.S.: Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 23, 43 (2015). https://doi.org/10.1186/s40199-015-0125-6
Yang, X., Yang, J., Wang, L., Ran, B., Jia, Y., Zhang, L., Yang, G., Shao, H., Jiang, X.: Pharmaceutical intermediate-modified gold nanoparticles against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11, 5737–5745 (2017)
Zahedi, P., Rezaeian, I., Ranaei-Siadat, S.-O., Jafari, S.-H., Supaphol, P.: A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 21, 77–95 (2010)
Zangeneh, M.M., Ghaneialvar, H., Akbaribazm, M., Ghanimatdan, M., Abbasi, N., Goorani, S., Pirabbasi, E., Zangeneh, A.: Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem. Photobiol. B, Biol. 197, 111556 (2019). https://doi.org/10.1016/j.jphotobiol.2019.111556
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Napagoda, M., Madhushanthi, P., Witharana, S. (2023). Nanomaterials for Wound Healing and Tissue Regeneration. In: Witharana, S., Napagoda, M.T. (eds) Nanotechnology in Modern Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-19-8050-3_5
Download citation
DOI: https://doi.org/10.1007/978-981-19-8050-3_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8049-7
Online ISBN: 978-981-19-8050-3
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)