Abstract
Despite the advancements in the treatment of squamous head and neck cancer in the past decade, clinical outcomes still remain poor. The use of multimodality treatment and the advent of targeted therapies have improved outcomes. This chapter will provide a brief overview of the current management for squamous head and neck cancers in both the locally advanced and metastatic settings. We will then outline targeted therapies currently in use or under investigation for the treatment of both locally advanced and metastatic head and neck cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Head and neck cancers are a heterogeneous group of malignancies, and their management requires a multidisciplinary approach including input from medical oncologists, radiation oncologists, surgeons, dentists, specialized nursing care, speech and language pathologists, physiotherapists, nutritionists, as well as psychologists [1]. Overall survival (OS) is improved when patients are treated at high-volume centers [2].
Patients with early stage disease (stage I or II) are treated with surgical resection or definitive radiation therapy (RT) to the primary site. Locoregionally advanced disease (stage III or IV) is treated with a combined modality approach such as surgery and RT with or without chemotherapy given the increased risk of local recurrence and distant metastasis in this patient population. Patients with metastatic disease require systemic therapy as well as best supportive care. Patient prognosis is often poor with median survival between 6 and 12 months. Therapeutic options for head and neck cancer patients with metastatic disease include cytotoxic chemotherapeutic agents or molecularly targeted agents.
This chapter will describe the novel and emerging chemotherapeutic agents in head and neck cancer. The role for immunotherapy will be outlined in a later chapter.
9.2 Systemic Therapy for Locoregionally Advanced Disease
Locoregionally advanced squamous head and neck cancer is associated with high rates of local recurrence of up to 50% [3,4,5] and rates of distant metastases between 4% and 26% [6,7,8]. Chemotherapy has therefore been integrated into the multimodality treatment plans in an effort to improve the rates of both locoregional and distant recurrence, as well as to reduce patient morbidity related to surgery and radiation using a functional organ preservation approach. These approaches can be classified into induction chemotherapy (neoadjuvant chemotherapy), concurrent chemoradiotherapy, and sequential chemoradiotherapy (combined induction chemotherapy followed by concurrent chemoradiotherapy). Prior to initiation of a multimodality treatment regimen, individual patient characteristics such as age, comorbidities, performance status, and support system should be assessed.
Various prospective studies have validated the role for chemotherapy in this patient population. Although there was no survival benefit for single-agent induction chemotherapy in comparison to surgery or RT alone, it was found that there was an OS benefit in patients receiving cisplatin plus fluorouracil [9]. Concurrent chemotherapy significantly improved OS in comparison to surgery or RT alone [9]. In contrast, the benefit for sequential chemoradiotherapy is still unclear [10,11,12,13] with suggested benefit for high-risk patients with bulky N2b, N3 nodal status, or those with T3 or T4 disease [12].
9.3 Chemotherapy Regimens for Locoregionally Advanced Disease
For induction chemotherapy, a three-drug combination of cisplatin, fluorouracil, plus a taxane is most commonly used and is the approach of choice [3, 14, 15]. Important toxicities include myelosuppression, febrile neutropenia, stomatitis, dysphagia, nausea, and anorexia [16].
For concurrent therapy in patients with good performance status, high-dose bolus cisplatin (100 mg/m2 on days 1, 22, 43) can be administrated concurrently with RT [17]. Given the frequent onset of both acute and late-onset adverse events, other dosing regimens are sometimes used. The most commonly associated toxicities included hematological toxicities, stomatitis, dysphagia, as well as nausea and vomiting, neurotoxicity, and nephrotoxicity [18]. Although not as effective as cisplatin in the treatment of locally advanced squamous head and neck cancer [19], weekly carboplatin (AUC of 1.5–2) is an appropriate choice for patients with renal disease or poor performance status [20]. Myelosuppression is an important limitation; however, there is less neurotoxicity associated with this treatment. Carboplatin in combination with fluorouracil given concurrently with RT is another approach [21].
9.4 Treatment Regimens for Recurrent Metastatic Disease in Previously Untreated Patients
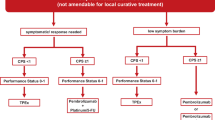
The median survival in patients with metastatic head and neck cancer is poor and approaches 6–12 months depending on disease and individual patient factors, such as performance status, presence of comorbidities, and disease-related factors. Systemic treatment options are chosen based on whether the patient has already received systemic treatment as part of organ preservation strategy or if they have already received a first-line agent for presence of systemic or recurrent disease. The role of traditional cytotoxic agents, targeted molecular agents, and checkpoint inhibitor therapy in the treatment of metastatic or recurrent head and neck cancer will be discussed in detail herein. A small subgroup of patients with good performance status who recur may be candidates for “salvage” therapy with curative intent, but most patients require a palliative approach using the regimens discussed in this chapter. It is important to note that best supportive care is also an important component to the management plan in all of these patients.
In otherwise healthy patients with a good performance status and those with advanced disease who are not appropriate candidates for curative therapy, combinations of platinum-based chemotherapy with fluorouracil or a taxane is the preferred approach [22,23,24,25]. The data supporting this recommendation are discussed in detail below.
Cisplatin (100 mg/m2 intravenous on day 1) and fluorouracil (1000 mg/m2/day continuous infusion over 4 days), and in comparison to single-agent cisplatin or methotrexate, this doublet regimen was associated with higher response rates across all studies, albeit no survival benefit was shown. For example, the EORTC Head and Neck Cancer Cooperative Group conducted a randomized controlled trial three arms (1) cisplatin, methotrexate, bleomycin, and vincristine (CABO), (2) combination cisplatin and fluorouracil (CF), and (3) cisplatin alone in previously untreated head and neck metastatic squamous cell cancer. Both CABO and CF were superior in terms of overall response rates with no difference in progression-free survival or overall survival. The Southwest Oncology Group (SWOG) performed a randomized controlled trial of (1) CF, (2) carboplatin plus fluorouracil, and (3) single-agent methotrexate. Once again, both the combination regimens (CF and carboplatin plus fluorouracil) were associated with improved response rates in comparison to methotrexate alone with similar median survival times across all three groups. There was however increased incidence of adverse events in the combination treatment groups. Further adding to the data supporting a doublet treatment regimen approach, a study by Jacobs et al. randomized patients to receive either cisplatin alone, fluorouracil alone, or their combination and once again found improved overall response rates with no significant difference in survival outcomes. Toxicities were more important in the combination treatment arm, with alopecia and myelosuppression being the most important. One study by Gibson et al. failed to show any statistically significant difference in response rate or survival between the single-agent and combination arms, and toxicities were similar in both groups.
We have seen above the data for combination therapy for fluorouracil and cisplatin or carboplatin; however, both cisplatin and carboplatin have been combined with a taxane regimen, either paclitaxel or docetaxel. No statistically significant benefit in response rate or overall survival exists with this regimen; however, common gastrointestinal adverse events and lack of need for prolonged infusion time make the taxane regimen more convenient.
Cisplatin is sometimes replaced for carboplatin in the taxane combination for more frail individuals as the side effect profile is more favorable with less ototoxicity, kidney failure, vomiting, and neuropathy; however, this has not been validated in phase III trials.
Single-agent therapy is reserved for patients with poor performance status and options include single-agent taxane, cisplatin, carboplatin, or methotrexate. Cetuximab (discussed later in this chapter) can be added to these regimens, and as seen in the EXTREME trial, when added to platin-fluorouracil, confers an OS and PFS improvement when compared to cisplatin-fluorouracil alone [26]. Best supportive care is also an important component to the management plan.
The role of immune checkpoint inhibition with pembrolizumab in the first-line setting is discussed in the final section of this chapter.
9.5 Epidermal Growth Factor Receptor (EGFR)-Targeted Therapy
EGFR is a member of the ErbB/Her group of ligand-activated receptor tyrosine kinases (RTKs) [27]. Through ligand-binding and activation of various downstream pathways, these receptors promote cancer cell proliferation, migration, angiogenesis, and tumor resistance to chemotherapy [28,29,30,31,32]. EGFR expression occurs in over 90% of squamous head and neck cancers, and overexpression is associated with decreased survival, resistance to radiotherapy, locoregional recurrence, and increased rate of distant metastases [27, 33].
9.5.1 Monoclonal Antibodies (mAb) Against EGFR
Monoclonal antibodies targeting EFGR and used in the treatment of locoregionally advanced squamous head and neck cancer include cetuximab, panitumumab, zalatumumab, and nimotuzumab. Their mechanism of action is through direct inhibition of ligand-receptor binding [27].
9.5.1.1 Cetuximab
Cetuximab is a highly specific, human-murine chimeric immunoglobulin G (IgG) mAb targeting EGFR [27]. As demonstrated in this landmark randomized controlled trial by Bonner et al., when administered at a dose of 400 mg/m2 1 week prior to RT followed by 250 mg/m2 weekly during high-dose RT in patients with locally advanced squamous head and neck cancer, cetuximab was associated with improved OS (49.0 months compared to 29.3 months HR0.74; p = 0.03) and locoregional control (24.4 months compared to 14.9 months (HE 0.68; p = 0.05) [34]), in comparison to high-dose radiation therapy alone. Progression-free survival was also improved in the combination treatment arm. This improvement in outcome was particularly important in patients 65 years of age or less with good performance status, albeit the study was not powered to detect differences in subgroups. In this study, there were no statistically significant difference in the incidence of grade 3 or higher adverse events; however, patients treated with cetuximab may have higher incidence of serious radiation dermatitis and another rare, but important side effect is the occurrence of cetuximab-induced infusion reaction, particularly in the first cycle. Interstitial lung disease was also an important side effect [35]. Current available data did not show any benefit to the use of concurrent cetuximab plus cisplatin with RT and is therefore not currently indicated in the treatment of locally advanced squamous head and neck cancer [36]. In the metastatic setting, a randomized, phase III clinical trial in patients with recurrent or metastatic squamous head and neck cancer the addition of cetuximab was compared with cisplatin/carboplatin plus fluorouracil. Chemotherapy plus cetuximab was associated with prolonged OS, PFS, and response rates. The main toxicities associated with the addition of cetuximab in this trial were severe hypomagnesemia, rash, and sepsis [26].
9.5.1.2 Panitumumab
Panitumumab is a fully humanized IgG anti-EGFR mAb, and like cetuximab, it inhibits EGFR ligand-dependant activation. Multiple prospective and randomized studies have failed to show an overall survival benefit in adding panitumumab to concurrent regimens in head and neck cancer, and its use is also associated with increased toxicity such as grade 3 rash or mucosal inflammation [37,38,39,40]. There was however a survival benefit p16-negative patients in the metastatic setting [41].
9.5.1.3 Zalutumumab
Zalutumumab, another fully humanized IgG anti-EGFR, works in a similar fashion as cetuximab and zalutumumab. Similar to panitumumab, zalutumumab has failed to show any benefit in the treatment of patients with squamous cell head and neck cancer in multiple phase III randomized controlled trials [42,43,44].
9.5.1.4 Nimotuzumab
Nimotuzumab, another fully humanized IgG anti-EGFR is now being compared to the administration of cisplatin in phase III trials in the management of locally and regionally advanced nasopharyngeal carcinoma when administered during radiotherapy following preoperative chemotherapy.
9.5.2 Tyrosine Kinase Inhibitors (TKI) Against EGFR
The intracellular domain of EGFR has important tyrosine kinase activity. TKIs serve to inhibit the activation and subsequent phosphorylation of EGFR [27]. In contrast to EGFR mAb, the small nature of these molecules allow for good GI absorption and therefore are prescribed orally in a daily fashion [27]. At the time of writing of this text book, TKIs are under review in several randomized, controlled trials, and none of the TKIs have been approved in the treatment for squamous head and neck cancer.
9.5.2.1 Gefitinib
In a randomized phase III trial, the addition of gefitinib to docetaxel did not improve survival for patients with recurrent or metastatic head and neck cancer [45] despite a phase II trial showing an overall response rate of 10.6% [46].
9.5.2.2 Erlotinib
Erlotinib, the second most common TKI was combined with cisplatin and compared to cisplatin alone in a phase II trial in which the cisplatin was given concurrently with definitive RT. In this study, there was no improvement in the response rate or survival [47].
9.5.2.3 Lapatinib
Lapatinib, a dual TKI, selectively inhibits the activation of EGFR as well as HER-2 [27]. A phase II trial compared the addition of lapatinib to standard chemoradiotherapy and showed promising results for the complete response rate in patients with locally advanced squamous head and neck cancer [48]. At this time, no benefit was shown in survival. In the metastatic setting, no objective response rate was observed in a phase II trial [49].
9.5.2.4 Afatinib
Afatinib is an irreversible TKI and, similarly to lapatinib, binds to the Erb2 receptor to inhibit EGFR [27]. Preliminary results from a phase II trial in the metastatic setting showed that there is significant disease activity for afatinib, and that it may be comparable to cetuximab [50]. It is currently being studied in the locally advanced setting.
9.5.2.5 Dacomitinib
Dacomitinib is an irreversible tyrosine kinase inhibitor for both EGFR and HER2. Two phase II clinical trials in recurrent or metastatic head and neck cancer demonstrated important clinical activity with the most common grade 3 adverse event being diarrhea [51, 52]. Exploratory analyses suggest that certain subgroups of patients with specific biomarkers may have improved responses to dacomitinib, but these findings need to be validated in phase III randomized control trials before their implementation into clinical practice.
9.6 Vascular Endothelial Growth Factor Receptor (VEGFR)-Directed Therapies
Vascular endothelial growth factor is an important cytokine for tumor angiogenesis, which is essential for tumor growth and metastatic dissemination [27]. Overexpression of VEGFR in patients with squamous head and neck cancer is associated with worse OS [53], making the VEGFR pathway an appealing therapeutic target. The VEGFR-directed therapies currently being studied in clinical models in squamous head and neck cancer include bevacizumab, sorafenib, sunitinib, and vandetanib. Other VEGF inhibitors that are currently under investigation for head and neck cancers include pazopanib, axitinib, nilotinib, and linifanib [54,55,56,57].
9.6.1 Monoclonal Antibodies (mAb) Against the VEGFR
9.6.1.1 Bevacizumab
Bevacizumab is an antiangiogenic mAb against VEGFR. A phase II study in patients with locally advanced squamous head and neck cancer compared the addition of bevacizumab to concurrent radiation therapy with cetuximab and pemetrexed. The addition of bevacizumab increased toxicity without improvement in efficacy or clinical outcomes [58]. Another phase II trial in squamous head and neck patients with locally advanced disease studying the addition of bevacizumab to concurrent intensity-modulated RT with cetuximab and cisplatin was associated with favorable clinical outcomes with the most common grade 3 adverse events being lymphopenia, mucositis, and dysphagia [59]. In the metastatic setting, a phase II trial showed an overall response rate of 30% with the addition of bevacizumab to pemetrexed with frequent (15%) bleeding adverse events [60].
9.6.2 Tyrosine Kinase Inhibitors (TKIs) Against VEGFR
9.6.2.1 Sorafenib
Sorafenib is a multiple kinase inhibitor targeting VEGFR, RAF, and platelet-derived growth factor receptor (PDGFR) [27]. To date, evidence has been conflicting with two phase II trials in the recurrent or metastatic setting showing little clinical activity [61, 62] and a more recent phase II trial showing an overall response rate of 55% with the combination of sorafenib with paclitaxel and carboplatin [63].
9.6.2.2 Sunitinib
Sunitinib, a second multiple kinase inhibitor targeting VEGFR, PDGFR, RET, and c-kit was evaluated as palliative monotherapy in patients with metastatic head and neck cancer [27]. Outcomes were poor with a significant amount of grade 3–5 hemorrhage [64]. A second study was closed after interim analysis due to only one out of the 19 patients in the study showing partial response [65].
9.6.2.3 Vandetanib
Vandetanib has activity against EGFR, VEGFR, and RET [27]. Currently its use has only been shown to be feasible in the phase I setting [66] with preclinical data showing it may overcome resistance to EGFR as well as RT [67].
9.7 P13K/AKT/mTOR Pathway Inhibitors
An important therapeutic hurdle to the use of EGFR and VEGFR inhibition is resistance to these molecules, either primarily or by prolonged use [68]. Prolonged treatment with EGFR can induce initiation of feedback loops thereby activating the P13/AKT pathway which promotes protein synthesis, cell survival, and tumor growth [27]. The mTOR pathway is another important pathway promoting tumor growth through regulation of cell proliferation, cell motility, and protein synthesis and has shown to be stimulated in 57–81% of patients with squamous head and neck cancer [27]. Temsirolimus is an mTOR inhibitor that was studied in a phase II trial in patients with cetuximab-resistant metastatic squamous head and neck cancer. This study showed a nonstatistically significant improvement in response rate. Two other studies evaluating the combination of temsirolimus with erlotinib and everolimus (a second mTOR inhibitor) with cetuximab and cisplatin were terminated early due to toxicity [69,70,71].
9.8 Palbociclib
Palbociclib, a selective cyclin-dependant kinase (CDK) 4/6 inhibitor was evaluated in a phase II trial of patients with platinum-resistant recurrent or metastatic head and neck squamous cell carcinoma. This study showed encouraging response rates of 35% with improved PFS and OS in comparison with similar patient cohorts [72].
9.9 Immune Checkpoint Inhibitors
The advent of immune checkpoint inhibitors has revolutionized the therapeutic landscape in many solid tumors and is now emerging as an important therapeutic option in the treatment of metastatic head and neck cancer.
At the time of writing of this text, the data on the use of immune checkpoint inhibitors in the first-line setting have been presented in abstract form only and therefore should be interpreted with caution until regulatory authorities approve these agents in this setting. Nevertheless, the preliminary results are promising and merit discussion.
In an open-label, phase III, randomized controlled study (NCT02358031), patients were randomly assigned to single-agent pembrolizumab, a PD-L1 inhibitor, versus pembrolizumab plus flourouracil/platinum combination, versus cetuximab and a fluorouracil/platinum combination. Patients were stratified based on PD-L1 score which was evaluated using the combined positive score (CPS). Single-agent pembrolizumab improved OS in comparison to the cetuximab and fluorouracil/platinum combination in patients with a high CPS score (above 20). Overall survival in the pembrolizumab arm was 14.9 months compared to 10.7 months. Strangely, this OS benefit did not translate in an improvement in PFS or response rate. As expected, toxicity was less in the single-agent pembrolizumab arm. A similar benefit in OS was seen in the pembrolizumab and fluorouracil/cisplatin arm (13.0 months compared to 10.7 months), and once again no significant differences were seen in response rates or PFS.
In the second-line setting, pembrolizumab was recently approved in the second-line setting for patients with metastatic head and neck squamous carcinoma. The KEYNOTE-040 randomized controlled, phase III trial of patients who had failed standard platinum-based chemotherapy was randomized to either pembrolizumab or standard of care with either methotrexate, docetaxel, or cetuximab [73]. Crossover was allowed at progression. There was a small but non-negligible improvement in overall survival in the pembrolizumab group of 8.4 months versus 6.9 months, and this benefit was most important in those with PDL-1 expression greater than 50%. There were less grade 3 or higher adverse events in the chemotherapy arm, but as expected a higher incidence of grade 1–2 immune-related adverse events, hypothyroidism being the most common. As a result of these studies and two other studies showing favorable response rate, pembrolizumab was approved by the FDA for the treatment of recurrent or metastatic head and neck squamous cell carcinoma at a dose of 200 mg intravenous every 3 weeks.
In a phase III randomized controlled study in the second- and later-line settings in patients with recurrent or metastatic head and neck squamous cell carcinoma, nivolumab, a PD-1 inhibitor demonstrated an overall survival benefit (7.7 months versus 5.1 months), and this was most important for patients with PDL-1 status more than 1% [74]. It is important to note that crossover was not allowed in this study. Based on the results of this study, the FDA approved nivolumab in this setting, at a dose of 240 mg intravenous every 2 weeks.
Darvalumab, another PD-1 inhibitor, has shown clinical activity in a phase II study of patients with recurrent or metastatic and previously treated squamous cell head and neck cancer [75]. The role for ipilimumab, a CTLA-4 inhibitor, is currently undergoing investigation (NCT02369874).
9.10 Oligometastatic Disease
In carefully selected patients with oligometastatic disease (limited metastatic disease) good performance status and who are good candidates for aggressive management, it may be reasonable to consider metastasectomy. As seen above, one of the most common sites of metastasis for head and neck squamous cell carcinoma is the lung. Around 30% of patients with metastatic head and neck squamous cell carcinoma who undergo pulmonary metastatecomy experience long-term survival. Poor prognostic factors in this approach include male sex, oral cavity lesions, lymph node involvement, and incomplete resection [76,77,78].
9.11 Drug Resistance
Despite significant improvements in the survival rates and organ preservation seen in the treatment of head and neck cancer care, significant challenges still exist as many patients experience drug resistance. Sensitivity to chemotherapeutic agents is associated with tumor heterogeneity, which is a result of patient factors (ethnic differences, age, weight, gender), and genetic differences in clonal tumor cells [79]. Mechanisms of resistance will be discussed in this section of this chapter.
Firstly, decreased concentration of antineoplastic agent within the tumor cells is an important mechanism of resistance and is thought to occur through a ATB-Binding Cassette (ABC)-mediated mechanism [80]. The ABC plays an important role in the transportation of antineoplastic treatments outside of the cell and also transports nutrients within the tumor cells thus allowing for drug resistance.
Secondly, head and neck squamous carcinoma cells are able to perform DNA repair, mediated by base-excision repair (BER). For example, polymorphisms in genes encoding BERs have been described, such as ERCC1 (C8092A), which plays a role in mRNA stability and DNA reparation capability, and ERCC1 expression may be associated with improved chemoradiation sensitivity perhaps clinical outcome as well [81].
Thirdly, there is an increased capability of tumor dissemination through a variety of mechanisms. For example, tumors that show an overexpression of p53 are resistant to both chemo and radiation therapy, and this has been associated with increased tumor progression and decreased survival rates [82]. There may also be increased chemoresistance through matrix metalloproteinase (MMP) through a Fas/FasL-mediated fashion, as some studies have indicated that polymorphisms in MMPs are independently associated with increased chemotherapy resistance [83].
Lastly, inactivation of antineoplastic drugs within the tumor cells can occur, also contributing to resistance. This may be particularly important for EGFR-mediated resistance, as EGF expression may be critical for maintaining tumor cell proliferation, and thus perhaps resistance to cetuximab [84]. However, it is still not been determined which of the EGFR ligands predict response to anti-EGFR treatment in patients with squamous cell carcinoma head and neck cancer. Resistance may also be due to autocrine growth factor production [85]. More data are required in order to fully elucidate the mechanisms of resistance to single-agent cetuximab, but this may be related to the capability of EGF to inhibit epithelial differentiation, and this may be in a cancer stem cell-related fashion [86, 87].
These findings prompt the need for continued search for biomarkers for resistance, with the goal of a personalized approach when prescribing therapy for head and neck squamous cell carcinoma patients.
9.12 Conclusions and Future Directions
The treatment of head and neck cancer continues to be challenging due to its heterogeneous nature as well as its increased incidence of resistance to conventional chemoradiation as well as targeted therapy. The distinct responses of HPV-positive and HPV-negative patients require further study. Advancements in elucidation of cancer cell biology have allowed for the development of several targeted therapies; however, more phase III trials need to be undertaken in order to implement these targeted therapies in daily practice.
References
Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg. 2010;143(5):650–4.
Wuthrick EJ, Zhang Q, Machtay M, Rosenthal DI, Nguyen-Tan PF, Fortin A, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33(2):156–64.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–15.
Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179–86.
Bourhis J, Le Maitre A, Baujat B, Audry H, Pignon JP. Meta-analysis of chemotherapy in head NCCG, et al. Individual patients’ data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19(3):188–94.
Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck. 1997;19(6):500–5.
Bhatia R, Bahadur S. Distant metastasis in malignancies of the head and neck. J Laryngol Otol. 1987;101(9):925–8.
Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(7):762–6.
Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–64.
Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25(1):216–25.
Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–43.
Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codeca C, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017;28(9):2206–12.
Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12(2):153–9.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–704.
Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Cruz Hernandez JJ, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31(23):2854–60.
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–8.
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8.
Fountzilas G, Ciuleanu E, Dafni U, Plataniotis G, Kalogera-Fountzila A, Samantas E, et al. Concomitant radiochemotherapy vs radiotherapy alone in patients with head and neck cancer: a Hellenic Cooperative Oncology Group Phase III Study. Med Oncol. 2004;21(2):95–107.
Posner MR, Norris CM, Wirth LJ, Shin DM, Cullen KJ, Winquist EW, et al. Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol. 2009;20(5):921–7.
Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(2):145–53.
Clavel M, Vermorken JB, Cognetti F, Cappelaere P, de Mulder PH, Schornagel JH, et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1994;5(6):521–6.
Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1245–51.
Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10(2):257–63.
Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(15):3562–7.
Rivera F, Garcia-Castano A, Vega N, Vega-Villegas ME, Gutierrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther. 2009;9(10):1421–8.
Dorsey K, Agulnik M. Promising new molecular targeted therapies in head and neck cancer. Drugs. 2013;73(4):315–25.
Hynes NE, Horsch K, Olayioye MA, Badache A. The ErbB receptor tyrosine family as signal integrators. Endocr Relat Cancer. 2001;8(3):151–9.
Kruger JS, Reddy KB. Distinct mechanisms mediate the initial and sustained phases of cell migration in epidermal growth factor receptor-overexpressing cells. Mol Cancer Res. 2003;1(11):801–9.
Masuda M, Toh S, Koike K, Kuratomi Y, Suzui M, Deguchi A, et al. The roles of JNK1 and Stat3 in the response of head and neck cancer cell lines to combined treatment with all-trans-retinoic acid and 5-fluorouracil. Jpn J Cancer Res. 2002;93(3):329–39.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, et al. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001;61(5):1855–61.
Agulnik M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). Med Oncol. 2012;29(4):2481–91.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8.
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–50.
Saloura V, Vokes EE. EGFR-based bioradiotherapy in SCCHN. Lancet Oncol. 2015;16(2):129–30.
Mesia R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):208–20.
Siu LL, Waldron JN, Chen BE, Winquist E, Wright JR, Nabid A, et al. Effect of standard radiotherapy with cisplatin vs accelerated radiotherapy with panitumumab in locoregionally advanced squamous cell head and neck carcinoma: a randomized clinical trial. JAMA Oncol. 2017;3(2):220–6.
Giralt J, Trigo J, Nuyts S, Ozsahin M, Skladowski K, Hatoum G, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):221–32.
Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697–710.
Bastholt L, Specht L, Jensen K, Brun E, Loft A, Petersen J, et al. Phase I/II clinical and pharmacokinetic study evaluating a fully human monoclonal antibody against EGFr (HuMax-EGFr) in patients with advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 2007;85(1):24–8.
Machiels JP, Subramanian S, Ruzsa A, Repassy G, Lifirenko I, Flygare A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12(4):333–43.
Schick U, Gujral DM, Richards TM, Harrington KJ, Nutting CM. Zalutumumab in head and neck cancer. Expert Opin Biol Ther. 2012;12(1):119–25.
Argiris A, Ghebremichael M, Gilbert J, Lee JW, Sachidanandam K, Kolesar JM, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2013;31(11):1405–14.
Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21(10):1980–7.
Martins RG, Parvathaneni U, Bauman JE, Sharma AK, Raez LE, Papagikos MA, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31(11):1415–21.
Harrington KJ, Berrier A, Robinson M, Remenar E, Housset M, Mendoza FH, et al. Phase II study of oral lapatinib, a dual-tyrosine kinase inhibitor, combined with chemoradiotherapy (CRT) in patients (pts) with locally advanced, unresected squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2010;28(15 Suppl):5505.
Abidoye OO, Cohen EE, Wong SJ, Kozloff MF, Nattam SR, Stenson KM, et al. A phase II study of lapatinib (GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2006;24(18 Suppl):5568.
Seiwert TY, Fayette J, Cupissol D, del Campo JM, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck†. Ann Oncol. 2014;25(9):1813–20.
Abdul Razak AR, Soulières D, Laurie SA, Hotte SJ, Singh S, Winquist E, et al. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck†. Ann Oncol. 2013;24(3):761–9.
Kim HS, Kwon HJ, Jung I, Yun MR, Ahn M-J, Kang BW, et al. Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin Cancer Res. 2015;21(3):544–52.
Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131(9):624–30.
Brands RC, Knierim LM, De Donno F, Steinacker V, Hartmann S, Seher A, et al. Targeting VEGFR and FGFR in head and neck squamous cell carcinoma in vitro. Oncol Rep. 2017;38(3):1877–85.
Swiecicki PL, Zhao L, Belile E, Sacco AG, Chepeha DB, Dobrosotskaya I, et al. A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Investig New Drugs. 2015;33(6):1248–56.
Hsu HW, de Necochea-Campion R, Williams V, Duerksen-Hughes PJ, Simental AA Jr, Ferris RL, et al. Linifanib (ABT-869), enhances cytotoxicity with poly (ADP-ribose) polymerase inhibitor, veliparib (ABT-888), in head and neck carcinoma cells. Oral Oncol. 2014;50(7):662–9.
Kramer B, Hock C, Birk R, Sauter A, Stuck BA, Hormann K, et al. Targeted therapies in HPV-positive and -negative HNSCC – alteration of EGFR and VEGFR-2 expression in vitro. Anticancer Res. 2016;36(6):2799–807.
Argiris A, Bauman JE, Ohr J, Gooding WE, Heron DE, Duvvuri U, et al. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann Oncol. 2016;27(8):1594–600.
Fury MG, Xiao H, Sherman EJ, Baxi S, Smith-Marrone S, Schupak K, et al. Phase II trial of bevacizumab + cetuximab + cisplatin with concurrent intensity-modulated radiation therapy for patients with stage III/IVB head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E566–70.
Argiris A, Karamouzis MV, Gooding WE, Branstetter BF, Zhong S, Raez LE, et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2011;29(9):1140–5.
Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28(20):3330–5.
Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–73.
Blumenschein GR, Glisson BS, Lu C, Sabichi AL, Ginsberg LE, Bartos CI, et al. Final results of a phase II study of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic or recurrent squamous cell cancer of the head and neck (SCCHN). J Clin Oncol. 2012;30(15 Suppl):5592.
Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28(1):21–8.
Choong NW, Kozloff M, Taber D, Hu HS, Wade J 3rd, Ivy P, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Investig New Drugs. 2010;28(5):677–83.
Papadimitrakopoulou VA, Frank SJ, Cohen EW, Hirsch FR, Myers JN, Heymach JV, et al. Phase I study of vandetanib with radiation therapy with or without cisplatin in locally advanced head and neck squamous cell carcinoma. Head Neck. 2016;38(3):439–47.
Sano D, Matsumoto F, Valdecanas DR, Zhao M, Molkentine DP, Takahashi Y, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. 2011;17(7):1815–27.
Zibelman M, Mehra R. Overview of current treatment options and investigational targeted therapies for locally advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39(4):396–406.
Chawla A, Adkins D, Worden FP, Rao KA, Hu HS, Price KAR, et al. Effect of the addition of temsirolimus to cetuximab in cetuximab-resistant head and neck cancers: results of the randomized PII MAESTRO study. J Clin Oncol. 2014;32(15 Suppl):6089.
Bauman JE, Arias-Pulido H, Lee SJ, Fekrazad MH, Ozawa H, Fertig E, et al. A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol. 2013;49(5):461–7.
Chung CH, Wang H, Tsottles N, Gourin CG, Agrawal N, Molinolo A, et al. A phase I study of everolimus in combination with cetuximab and cisplatin as first-line therapy in recurrent and metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2012;30(15 Suppl):e16061.
Adkins D, Oppelt PJ, Ley JC, Trinkaus K, Neupane PC, Sacco AG. Multicenter phase II trial of palbociclib, a selective cyclin dependent kinase (CDK) 4/6 inhibitor, and cetuximab in platinum-resistant HPV unrelated (−) recurrent/metastatic head and neck squamous cell carcinoma (RM HNSCC). J Clin Oncol. 2018;36:6008.
Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–67.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with >/=25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–52.
Shiono S, Kawamura M, Sato T, Okumura S, Nakajima J, Yoshino I, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg. 2009;88(3):856–60.
Liu D, Labow DM, Dang N, Martini N, Bains M, Burt M, et al. Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol. 1999;6(6):572–8.
Mochizuki T, Okumura S, Ishii G, Ishikawa Y, Hayashi R, Kawabata K, et al. Surgical resection for oral tongue cancer pulmonary metastases. Interact Cardiovasc Thorac Surg. 2010;11(1):56–9.
Lopez-Verdin S, Lavalle-Carrasco J, Carreon-Burciaga RG, Serafin-Higuera N, Molina-Frechero N, Gonzalez-Gonzalez R, et al. Molecular markers of anticancer drug resistance in head and neck squamous cell carcinoma: a literature review. Cancers. 2018;10(10):pii: E376.
Becker M, Levy D. Modeling the transfer of drug resistance in solid tumors. Bull Math Biol. 2017;79(10):2394–412.
Quintela-Fandino M, Hitt R, Medina PP, Gamarra S, Manso L, Cortes-Funes H, et al. DNA-repair gene polymorphisms predict favorable clinical outcome among patients with advanced squamous cell carcinoma of the head and neck treated with cisplatin-based induction chemotherapy. J Clin Oncol. 2006;24(26):4333–9.
Cutilli T, Leocata P, Dolo V, Altobelli E. Evaluation of p53 protein as a prognostic factor for oral cancer surgery. Br J Oral Maxillofac Surg. 2013;51(8):922–7.
Blons H, Gad S, Zinzindohoue F, Maniere I, Beauregard J, Tregouet D, et al. Matrix metalloproteinase 3 polymorphism: a predictive factor of response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10(8):2594–9.
Ansell A, Jedlinski A, Johansson AC, Roberg K. Epidermal growth factor is a potential biomarker for poor cetuximab response in tongue cancer cells. J Oral Pathol Med. 2016;45(1):9–16.
Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10(8):1115–27.
Setubal Destro Rodrigues MF, Gammon L, Rahman MM, Biddle A, Nunes FD, Mackenzie IC. Effects of cetuximab and erlotinib on the behaviour of cancer stem cells in head and neck squamous cell carcinoma. Oncotarget. 2018;9(17):13488–500.
Gemenetzidis E, Gammon L, Biddle A, Emich H, Mackenzie IC. Invasive oral cancer stem cells display resistance to ionising radiation. Oncotarget. 2015;6(41):43964–77.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Elkrief, A., Makhoul, N., Bouganim, N. (2020). Novel and Emerging Chemotherapeutic Agents in Head and Neck Cancer. In: Kademani, D. (eds) Improving Outcomes in Oral Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-30094-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-30094-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30093-7
Online ISBN: 978-3-030-30094-4
eBook Packages: MedicineMedicine (R0)