Summary
Background Sunitinib is an orally administered multitargeted tyrosine kinase inhibitor of RET, VEGFR, PDGFR, and c-KIT. We conducted a phase II trial to evaluate the tolerability and efficacy of sunitinib in metastatic and/or recurrent SCCHN patients. Methods Patients who had received no more than two prior chemotherapy regimens were eligible and, depending on ECOG performance status (PS), were entered into either Cohort A (PS 0-1) or Cohort B (PS 2). Sunitinib was administered in 6-week cycles at 50 mg daily for 4 weeks followed by 2 weeks off. Primary endpoint for Cohort A was objective tumor response. A Simon two-stage design required twelve patients to be enrolled in the first stage and if 1 or fewer responses were observed, further study of this cohort would be terminated due to lack of treatment efficacy. Primary endpoint of Cohort B was to determine the feasibility of sunitinib in patients with ECOG performance status 2. Results Twenty-two patients were accrued (Cohort A — 15 patients, Cohort B — 7 patients). Median age in cohort A and B was 56 and 61 years, respectively. Grade 3 hematologic toxicities encountered were lymphopenia (18%), neutropenia (14%) and thrombocytopenia (5%). There was only one incidence of grade 4 hematologic toxicity which was thrombocytopenia. Fatigue and anorexia were the most common non-hematologic toxicities. Grade 3 fatigue occurred in 23% of patients. The only grade 4 non-hematologic toxicity was one incidence of gastrointestinal hemorrhage. Non-fatal hemorrhagic complications occurred in 8 patients: epistaxis (3 patients), pulmonary hemorrhage (2 patients), gastrointestinal hemorrhage (2 patients) and tumor hemorrhage (1 patient). Four patients were not evaluable for tumor response (Cohort A — 3patients, Cohort B — 1 pt). One partial response was observed in the entire study. Dose reduction was required in 5 patients (Cohort A — 3 patients for grd 3 fatigue, grd 3 mucositis and recurrent grd 3 neutropenia; Cohort B — 2 patients for grd 3 fatigue and grd 3 nausea). Median time to progression for cohort A and B were 8.4 and 10.5 weeks, respectively. Median overall survival for cohort A and B was 21 and 19 weeks, respectively. Conclusions Sunitinib had low single agent activity in SCCHN necessitating early closure of cohort A at interim analysis. Sunitinib was well tolerated in PS 2 patients. Further evaluation of single agent sunitinib in head and neck is not supported by the results of this trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Approximately 70% of patients with squamous cell carcinoma of the head and neck (SCCHN) present with locoregional disease and despite aggressive locoregional therapy, more than half will succumb to recurrent and/or metastatic disease [1]. If untreated, the median survival of patients with metastatic disease is less than 4 months [2]. Systemic chemotherapy with palliative intent is often the only therapeutic option for these patients. The response rate of single-agent and combination chemotherapy ranges between 10-30% [3]. Despite the use of more aggressive chemotherapy combinations and achieving higher response rates, this has not translated into significant improvement in survival [1] until recently when cetuximab in combination with platinum and fluorouracil was shown to significantly improved survival [4].
Sunitinib malate (sunitinib; SU11248; SU011248; Sutent®) is a novel, multi-targeted, small molecule inhibitor of receptor tyrosine kinases (RTKs). It inhibits RTKs involved in tumor proliferation and angiogenesis, including vascular endothelial growth factor receptor-1 (VEGFR-1), -2, and -3, platelet-derived growth factor receptor (PDGFR) -α and -β, stem cell factor receptor (KIT), the tyrosine kinase (TK) receptor encoded by the ret proto-oncogene, and fms-like tyrosine kinase 3 (Flt3) [5].
VEGFR and PDGFR as well as their ligands are highly expressed in SCCHN tumor tissues [6–10]. The expression of VEGFR and various isoforms of VEGF have been correlated with lymph node metastasis [7, 9–11], local tumor invasion [11, 12], tumor recurrences [13] and prognosis [10, 14, 15]. Inhibition VEGF signaling has successfully resulted in tumor growth suppression in SCCHN xenografts [16, 17].
In mouse models, overexpression of PDGF enhances tumor formation by stimulating vascular endothelial growth factor (VEGF) expression in neovessels and by attracting vessel-associated pericytes [18]. Pericyte recruitment results in increased vasculogenesis in the central regions of these tumors leading to improved tumor perfusion [18]. Dual inhibition of PDGFR and VEGFR results in a significantly reduced neovascularization by inducing endothelial cell apoptosis through interference of pericyte-endothelium interaction [19]. Based on these preclinical data and the documented activity, safety and tolerability of sunitinib in clinical trials [20, 21], we performed this phase II study of sunitinib in recurrent and/or metastatic SCCHN.
Methods
Objective
The primary objective of this trial was to determine the overall response rate and toxicity of sunitinib in Eastern Cooperative Group (ECOG) performance status (PS) 0–1 patients with recurrent and/or metastatic SCCHN who have never been treated with anti-VEGFR therapy. The secondary objective was to determine the feasibility of sunitinib in recurrent and/or metastatic SCCHN patients with ECOG PS 2.
Patient selection
Eligibility criteria included previously treated (no more than two prior regimens for recurrent or metastatic disease), histologically or cytologically confirmed recurrent or metastatic SCCHN in patients older than 18 years. Patients were not permitted to have undergone previous anti-angiogenic therapy for SCCHN. Additional eligibility requirements included ECOG PS of 0, 1, or 2 and measurable or evaluable disease by RECIST criteria. Patients must have had normal renal (creatinine clearance ≥ 60 mL/min/1.73 m2, serum calcium ≤ 12.0 mg/dL), hepatic (total serum bilirubin within normal institutional limits AST/ALT ≤ 2.5 X institutional upper limit of normal), cardiac (QTc < 500 msec, New York Heart Association Class II or better) and marrow (leukocytes ≥ 3,000/mcL, absolute neutrophil count ≥ 1,500/mcL, platelets ≥ 100,000/mcL, hemoglobin ≥ 9 g/dL) function. Patients were excluded if they had prior anthracycline exposure, received central thoracic radiation that included the heart in the radiotherapy port, a serious medical or psychiatric illness which might interfere with protocol compliance, on therapeutic doses of coumarin-derivative anticoagulants, pregnant or lactating, HIV positive or with brain metastasis. The use of proarrhythmic drugs and potent cytochrome P3A4 liver enzyme inducers or inhibitors was not allowed. Furthermore, patients who were unable to take sunitinib orally were excluded. All patients gave written, witnessed, and informed consent before study entry.
Treatment and response assessment
All subjects were entered into either Cohort A or Cohort B defined by ECOG PS: Cohort A — ECOG PS 0–1 and Cohort B — ECOG PS 2. Sunitinib was administered orally at 50 mg once daily for 4 consecutive weeks followed by 2 weeks off sunitinib. A 6-week period constituted one treatment cycle. Treatment was continued until disease progression, intercurrent illness that prevented further administration of treatment, unacceptable adverse event(s), or patient withdrawal. Response and progression were evaluated every 12 weeks (2 cycles) using the Response Evaluation Criteria in Solid Tumors (RECIST). All adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v3.0).
Dose reduction of sunitinib (to 37.5 mg and then to 25 mg) was allowed depending on the type and severity of adverse effects. Patients who developed hypertension were managed with anti-hypertensives. Dose reduction or discontinuation of sunitinib for hypertension occurred only if the patient was persistently hypertensive after maximal antihypertensive therapy (four antihypertensives for 2 weeks without dose modification of antihypertensive medications).
Statistical analysis
The primary endpoint for Cohort A was objective tumor response based on the RECIST criteria. A Simon two-stage design was used to test the null hypothesis that the response rate (CR + PR) is 10% against the alternative that it is 30%. Twelve patients were to be enrolled in the first stage and if 1 or fewer responses were observed, further study of this cohort would be terminated due to lack of treatment efficacy. Otherwise an additional 23 patients were to be enrolled for a total of 35. At the end of the second stage if 5 or fewer responses were observed, the treatment would be rejected whereas if 6 or more responses were observed (> 17%) the regimen would be considered sufficiently active to warrant further study. This design had an alpha level of 10% and a power of 90%. The probability of stopping after the first stage if the true response rate was only 10% was 0.65.
The objective of Cohort B was to determine the feasibility of sunitinib in patients with ECOG performance status 2. Eight patients were to be enrolled in Cohort B. All observed toxicities were recorded and summarized using descriptive statistics. Time to progression (defined as the interval between the first day of therapy and disease progression) and overall survival (defined as the interval between the first day of therapy and death from any cause) curves were calculated using the Kaplan-Meier method. Patients were followed until March 1, 2009.
Results
Patient characteristics
Twenty-two patients were accrued (Cohort A — 15patients, Cohort B — 7patients) and their characteristics are summarized in Table 1. In Cohort A, 2 patients had locoregional recurrence while the other 13 patient had distant metastatic disease with or without locoregional recurrence. Thirteen patients in Cohort A had previously received definitive curative-intent therapy and 2 patients had metastatic disease at presentation. Every patient in Cohort B had metastatic disease. Only 2 patients had metastatic disease at presentation, while the rest had received prior definitive curative-intent therapy.
Toxicity
Hematologic toxicities encountered were anemia (Cohort A — 20%, Cohort B — 14%), leucopenia (40%, 14%), neutropenia (20%, 14%), and thrombocytopenia (34%, 43%) (Table 2). Grade 3/4 anemia was not observed in either cohort. Grade 3 leucopenia (Cohort A — 13%, Cohort B — 0%), neutropenia (13%, 14%) and thrombocytopenia (0%, 14%) was observed in both cohorts. The only grade 4 hematologic toxicity was thrombocytopenia, which was observed in one Cohort A patient (7%).
Fatigue was the predominant non-hematologic toxicity occurring in 73% in Cohort A and 43% in Cohort B (Table 3). Grade 3 fatigue was observed in 13% of patients in Cohort A and 43% in Cohort B. Hypertension occurred in 20% and 28% of patients in Cohort A and B, respectively. Grade 3 hypertension was observed in 1 patient in Cohort B. Other non-hematologic adverse effects include anorexia, nausea, vomiting and mucositis. The only grade 4 non-hematologic toxicity was gastrointestinal bleeding, which was observed in one Cohort B patient (7%).
Dose reduction was required in 5 patients. In Cohort A, 3 patients required dose reduction for grade 3 fatigue, grade 3 mucositis and recurrent grade 3 neutropenia. In Cohort B, 2 patients required dose reduction for grade 3 fatigue and grade 3 nausea.
Eight patients experienced hemorrhagic events associated with sunitinib: epistaxis (3 patients — all grade 1), pulmonary hemorrhage (1 patient — grade 2, 1 patient — grade 3), gastrointestinal bleeding (1 patient — grade 3, 1 patient — grade 4) and superficial tumor hemorrhage (1 patient — grade 3).
Response evaluation and survival
Twelve patients in Cohort A and 6 patients in Cohort B were evaluable for disease response. Three patients in Cohort A were not evaluable because of premature withdrawal from the study (2 patients) and one due to death from sepsis. In Cohort B, one patient died from a sudden cardiac death after developing a gastrointestinal bleed and prior to disease evaluation.
In Cohort A, partial response was observed in one patient. Three patients (25%) experienced disease stabilization for 8, 19 and 26 weeks, respectively. No responses were observed in cohort B, but two patients (29%) experienced disease stabilization for 15 and 30 weeks, respectively.
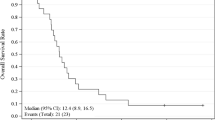
At the time of database lock, one patient was still alive. The median overall survival for all patients in Cohort A and B were 21.1 and 19.1 weeks, respectively (Fig. 1). The actual 1-year overall survival in Cohort A and B were 22% and 14%, respectively.
Every patient had experienced disease progression on sunitinib. The median time to progression for all patients in Cohort A and B were 8.4 and 10.5 weeks, respectively (Fig. 2).
Discussion
The objectives of this study were to evaluate the response rate and tolerability of sunitinib in metastatic and/or recurrent head and neck cancer given the strong scientific rationale underlying inhibition of sunitinib’s putative targets that are critical in SCCHN angiogenesis and neovascularization [19]. Furthermore the adverse effect profile of sunitinib was acceptable in previously reported studies [20, 21].
Sunitinib was well tolerated in this study but efficacy parameters in cohort A were not met at interim analysis and the study was closed prior to completing planned accrual. The response rate observed with sunitinib was low and stable disease as best response occurred only in 25% of patients. We observed one patient who did not respond to sunitinib by standard RECIST criteria but developed central necrosis of the tumor (Fig. 3). Tumor cavitation, a class effect of anti-angiogenic agents, has only a minor impact on response assessment in lung tumors treated with anti-angiogenic agents [22]. This observation suggests that sunitinib may have some anti-tumor activity in head and neck cancer despite low response rates. However, PFS and OS rates do not suggest that, single agent sunitinib has equal or greater efficacy than currently available drugs.
Sorafenib, a multikinase inhibitor of C-Raf, B-Raf, VEGFR and PDGFR has been evaluated for patients with metastatic and/or recurrent head and neck cancer [23, 24]. Although the response rate observed was similar to our study (3–4%), the rate of stable disease was higher at 40–45%. Nonetheless, the reported median time to progression and median overall survival by Elser et al of 1.8 months and 4.2 months, respectively, approximated the current study [23]. The study reported by Williamson et al had an encouraging median survival of 9 months [24]. This study excluded patients who had received prior chemotherapy for recurrent or metastatic disease, which could explain the longer median survival despite a median progression-free survival of 4 months. Another anti-angiogenic small molecule tyrosine kinase inhibitor, SU5416, has demonstrated similar efficacy outcomes as sorafenib and sunitinib in head and neck cancer but with a higher toxicity profile [25].
Fatigue was the most common adverse effect noted and tended to be more severe in patients with PS2. Compared to sorafenib and to other sunitinib trials, cutaneous reactions were uncommon in our study. The incidence of hypertension was comparable to other trials of single-agent sunitinib [20, 21].
Vascular events observed in our study include hypertension, epistaxis, pulmonary hemorrhage, gastrointestinal bleeding and superficial tumor hemorrhage. In the two patients who developed pulmonary hemorrhage, one patient developed hemoptysis in the setting of lobar pneumonia while the other had lung metastases and probably bled from the tumor. In the two patients who developed gastrointestinal bleed, one patient was anticoagulated for an upper extremity deep venous thrombosis from an intravenous catheter and developed a colonic bleed while the other patient had a base of tongue tumor recurrence, developed an upper gastrointestinal bleed 10 days into therapy leading to sudden cardiac death soon after. The exact cause of death was unclear but may be a result of tumor hemorrhage. Therefore apart from the one patient who we directly witnessed tumor hemorrhage, two other patients described above may have also had tumor hemorrhage. Minor bleeding complications have been noted to occur in up to 25% of patients treated with sunitinib [26]. Tumor related hemorrhage has been observed in head and neck cancer studies involving sorafenib (1 fatal nasopharyngeal hemorrhage) and SU5416 (1 fatal carotid hemorrhage) [23, 25]. It is unclear if tumor hemorrhage represents disease response despite its fatal consequences.
The low activity in this study may be because advanced head and neck cancers do not depend entirely on the pathways that are inhibited by sunitinib. Although our data suggest that further investigation of single agent sunitinib should not be performed in recurrent and/or metastatic head and neck cancer, there is evidence to support that anti-angiogenic agents may potentiate other agents when used in combination [27]. Further investigation into sunitinib combination therapy should be considered.
References
Choong N, Vokes E (2008) Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin 58:32–53
Kowalski LP, Carvalho AL (2000) Natural history of untreated head and neck cancer. Eur J Cancer 36:1032–7
Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK et al (1992) Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol 10:1245–51
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–27
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9:327–37
Lalla RV, Boisoneau DS, Spiro JD, Kreutzer DL (2003) Expression of vascular endothelial growth factor receptors on tumor cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 129:882–8
O-charoenrat P, Rhys-Evans P, Eccles SA (2001) Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer 92:556–68
Ongkeko WM, Altuna X, Weisman RA, Wang-Rodriguez J (2005) Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol 124:71–6
Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H (2004) Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol 40:13–20
Tanigaki Y, Nagashima Y, Kitamura Y, Matsuda H, Mikami Y, Tsukuda M (2004) The expression of vascular endothelial growth factor-A and -C, and receptors 1 and 3: correlation with lymph node metastasis and prognosis in tongue squamous cell carcinoma. Int J Mol Med 14:389–95
Sauter ER, Nesbit M, Watson JC, Klein-Szanto A, Litwin S, Herlyn M (1999) Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res 5:775–82
Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ (2005) Potential autocrine function of vascular endothelial growth factor in head and neck cancer via vascular endothelial growth factor receptor-2. Mod Pathol 18:485–94
Neuchrist C, Erovic BM, Handisurya A, Fischer MB, Steiner GE, Hollemann D, Gedlicka C, Saaristo A, Burian M (2003) Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck 25:464–74
Kyzas PA, Stefanou D, Agnantis NJ (2004) Immunohistochemical expression of vascular endothelial growth factor correlates with positive surgical margins and recurrence in T1 and T2 squamous cell carcinoma (SCC) of the lower lip. Oral Oncol 40:941–7
Teknos TN, Cox C, Yoo S, Chepeha DB, Wolf GT, Bradford CR, Carey TE, Fisher SG (2002) Elevated serum vascular endothelial growth factor and decreased survival in advanced laryngeal carcinoma. Head Neck 24:1004–11
Riedel F, Gotte K, Hormann K, Grandis JR (2005) Antiangiogenic therapy of head and neck squamous cell carcinoma by vascular endothelial growth factor antisense therapy. Adv Otorhinolaryngol 62:103–20
Riedel F, Gotte K, Li M, Hormann K, Grandis JR (2003) Abrogation of VEGF expression in human head and neck squamous cell carcinoma decreases angiogenic activity in vitro and in vivo. Int J Oncol 23:577–83
Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY (2003) Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol 162:1083–93
Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18:338–40
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–24
Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, Leighl NB, Shepherd FA, Powers J, Seymour L, Laurie SA (2009) Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 27:404–10
Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, Francis P, Cheiken R, Elting J, McNabola A, Wilkie D, Petrenciuc O, Chen EX (2007) Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol 25:3766–73
Williamson, S., Moon, J., Huang, C., Guaglianone, P., Wolf, G. and Urba, S. (2007) A phase II trial of sorafenib in patients with recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC): A Southwest Oncology Group (SWOG) trial. Proc Am Soc Clin Oncol 25:Abstract 6044
Fury MG, Zahalsky A, Wong R, Venkatraman E, Lis E, Hann L, Aliff T, Gerald W, Fleisher M, Pfister DG (2007) A Phase II study of SU5416 in patients with advanced or recurrent head and neck cancers. Invest New Drugs 25:165–72
Verheul HM, Pinedo HM (2007) Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 7:475–85
Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, Kozloff MF, Clark JI, Yan DH, Liu W, Pierce C, Dancey JE, Stenson K, Blair E, Dekker A, Vokes EE (2009) Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol 10:247–57
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant support
This study was supported by NIH Cooperative Contract NO1 CM-07003-74 and UCCRC Cancer Center Grant.
Rights and permissions
About this article
Cite this article
Choong, N.W., Kozloff, M., Taber, D. et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs 28, 677–683 (2010). https://doi.org/10.1007/s10637-009-9296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9296-7