Abstract
Background Axitinib is an oral, potent, small molecule tyrosine kinase inhibitor with selective inhibition of VEGFR 1,2, 3, as well as inhibition of potential downstream effectors of the EGFR pathway. Given the upregulation of EGFR and VEGFR in head and neck squamous cell carcinoma, treatment with axitinib holds promise as a rational targeted therapy. Patients and Methods Patients with unresectable, recurrent or metastatic head and neck squamous cell carcinoma were included in this open label, single arm, phase II trial. Primary endpoint was 6 month progression free survival. All patients received single agent axitinib with planned dose escalation based on tolerability. A planned interim efficacy analysis was performed after enrollment of 30 patients. Results Forty-two patients were registered, 30 were evaluable. While treatment was well-tolerated with no severe bleeding events, only 19 patients were able to achieve full planned dose. The best overall response rate was 6.7 % (two partial responses) with a disease control rate of 76.7 %. Median progression free survival was 3.7 months (95 % Confidence Interval (CI): 3.5–5.7) and overall survival was 10.9 months (95 % CI: 6.4–17.8). Exploratory analysis demonstrated that patients with a smaller sum of diameter of target lesions experienced improved response rates, and better progression-free and overall survival. Conclusion Treatment with single agent axitinib should be considered due to acceptable toxicity profile and favorable median overall survival compared to standard therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unresectable recurrent (R) or distant metastatic (M) head and neck squamous cell carcinoma (HNSCC) has a median survival of less than one year, and novel treatment options have been relatively disappointing to date. Documented response rates to cytotoxic chemotherapy in the palliative or treatment-refractory setting typically range between 10 and 30 % with single agent regimens and 20–40 % for multi-drug regimens.
Axitinib is an oral, potent, multi-receptor, small molecule tyrosine kinase inhibitor, with clinical activity in multiple cancer types, including renal cell carcinoma and differentiated thyroid cancers [1–4]. Axitinib inhibits several receptors including VEGFR 1, 2, and 3, PDGFR, and c-kit [5]. Given it is a multi-tyrosine kinase inhibitor, axitinib may also inhibit downstream effectors in the EGFR pathways. Additionally, the toxicity profile of axitinib is quite manageable, allowing for patients to remain on treatment for a longer duration of time as compared to historical controls of standard cytotoxic agents [6].
Based on the known mechanism of axitinib and corresponding dysregulated pathways in metastatic HNSCC, we conducted a single institution phase II trial to investigate the clinical activity of this agent in patients with unresectable recurrent or metastatic HNSCC.
Methods
Patient eligibility
This was a phase 2, single-arm, non-randomized, open label trial approved by the Institutional Review Board (IRBMED) of the University of Michigan Comprehensive Cancer Center. Informed consent was obtained from all individual participants included in the study. Patients ≥18 years old with unresectable R/M HNSCC were eligible. All patients had histologically documented HNSCC, the presence of measurable disease by CT scan, an ECOG performance status of 0–2, and a life expectancy of ≥12 weeks. Patients had to have adequate hematopoietic, hepatic, and renal function defined as: prothrombin time < 1.5, white blood cell count ≥3x109 cells/ml, absolute neutrophil count ≥1.5x109 cell/ml, platelets ≥75,000 cells/mm3, hemoglobin ≥9.0 g/dL, concentrations of total serum bilirubin within 1.5 × upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) within 2.5× institutional upper limits of normal unless there were liver metastases in which case AST and ALT within 5.0 × ULN, serum creatinine clearance ≥60 ml/min), urinary protein <2+ by urine dipstick (if dipstick is >2+ then a 24- h urine collection was done and patients entered only if urinary protein was <2 g per 24 h). Eligible patients were required to have no evidence of preexisting, uncontrolled hypertension as documented by two baseline blood pressure readings taken at least 1 h apart. The baseline systolic blood pressure readings had to be ≤140 mmHg and diastolic blood pressure of ≤90 mmHg. Women of childbearing potential must have had a negative serum or urine pregnancy test within 3 days prior to treatment initiation.
Patients were excluded if they had central lung lesions involving major blood vessels or a tumor encasing major blood vessels, history of hemoptysis, previous treatment with select therapies (antiangiogenesis agents including thalidomide, inhibitors of epidermal growth factor (EGF), platelet derived growth factor (PDGF), or fibroblast growth factor (FGF)), brain metastases, history of bleeding diatheses or venous thromboembolism. Patients were also excluded if they were unable to ingest the drug orally.
Treatment plan
Axitinib (Inlyta™) was supplied by Pfizer Inc. Patients were initiated on a dose of 5 mg by mouth twice daily. If there were no grade 2 or greater toxicities, there was a planned dose escalation to 7 mg twice daily after two weeks and to 10 mg twice daily after three weeks following treatment initiation. If toxicities were encountered, escalation could be resumed at the next visit if adverse events resolved to grade 1 or less and if the blood pressure was adequately controlled (defined as systolic ≤150 mmHg and diastolic ≤100 mmHg). The cycle length was 28 days. All patients were continued on therapy until evidence of disease progression, unacceptable toxicity, patient withdrawal of informed consent, or investigator discretion. There was a pre-planned interim efficacy analysis after enrollment of 25 patients to determine 6 month progression free survival.
Pretreatment assessment of patients enrolled in the trial included a complete history and physical examination, baseline laboratory studies (CBC with differential, comprehensive metabolic profile, thyroid stimulating hormone (TSH), urinalysis, serum or urine pregnancy test as indicated), and radiographic staging studies (CT Neck, Chest, Abdomen, and Pelvis); all screening assessments were completed within 28 days prior to the start of treatment. A biopsy of the primary lesion or a suspected metastatic lesion, when feasible, was obtained within 28 days prior to initiation of treatment.
Evaluation of response
Imaging studies for evaluation of response of target radiologic lesions were performed starting at 8 weeks following treatment initiation and continued at 8 week intervals. Target lesions were followed on each imaging study and analyzed primarily by following the sum of the largest diameter of all target lesions. Secondary radiologic evaluation data points included number of lesions, size of largest lesions, and location of target lesions. Radiologic response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST v1.0). If there was gross evidence of clinical disease progression based on physical examination, patients were taken off study. However, if the physical examination raised suspicion for clinical progression, the patient was continued on study for another 4 weeks and reassessed clinically. When feasible, a repeat biopsy was obtained 4 weeks after treatment initiation for the purposes of correlative analyses.
Statistical considerations
Treatment-related adverse events were graded according to the Common Terminology for Adverse Events version 3.0 (CTCAE v3). Disease control rate (DCR) was defined as the sum of patients with complete response (CR), partial response (PR) and stable disease (SD) per RECIST v1.0. Overall survival (OS) was the defined as the time from study enrollment to death from any cause. Progression-free survival (PFS) was defined as the time from study enrollment until disease progression or death. Data were censored at the last follow-up for patients who were progression-free or alive at the time of analysis. The posterior probability of P (defined as PFS at 6-month < 25 %) was calculated at the interim to determine if the trial needed to be halted. If this probability was larger than 80 %, the trial was planned to be stopped due to the lack of evidence that axitinib was at least equal to the standard regimen. Furthermore, we used a simulation study at the interim. This simulation study was to determine if there was sufficient evidence to demonstrate axitinib was more efficacious than the standard if we completed the planned trial. A lower confidence interval larger than 30 % would support that axitinib was better. Median survival times were computed using Kaplan-Meier methods with standard error computed using Greenwood’s formula. Differences in survival functions between human papillomavirus (HPV) negative and positive patients were assessed using the log-rank test. A Cox proportional hazards model was used to evaluate continuous radiologic factors to survival outcomes and the importance of each factor was determined from the algorithm of Furnival and Wilson [7]. Two-sample t-tests were used to associate changes of (log transformed) biomarkers before and after therapy to patient best overall response. All analyses were done using SAS 9.4 software. P < 0.05 considered as significant.
Results
Patient characteristics
Thirty evaluable patients were enrolled. The patient characteristics are summarized in Table 1. The mean age was 63.3 years (range: 40.0–84.0). HPV status was available for 26 patients. (14 vs. 12 patients, 47 % vs. 40 %, respectively).
The patients included in this study were heavily pretreated (Table 1). All patients had previously undergone radiation therapy and 63 % had previous oncologic extirpative surgery at the time of initial diagnosis. Ninety percent of patients received prior chemotherapy, with 50 % receiving more than 2 lines of palliative chemotherapy.
The study protocol had a planned interim efficacy analysis to evaluate 6-month PFS after enrollment of 20 patients. A second planned interim analysis was performed after the enrollment of 30 patients, at which time it was determined that 6-month PFS was 27 %. We predicted that all future patients on study would have to respond, but due to futility, the study was stopped.
Toxicity
Among all 42 enrolled patients, axitinib was reasonably well tolerated (Table 2). Per protocol, there was a planned dose escalation from 5 mg twice daily to 10 mg twice daily as previously noted. Only 19 patients were able to receive the 10 mg dose, with mean duration of treatment of 1.7 months (range: 0–10). A change in the plan for dose administration (i.e. a lack of planned dose escalation or dose reduction due to toxicity) was encountered in 36 patients (76 %). The most common toxicity encountered was fatigue, which was seen in 26 patients (7 [27 %] with grade 3–4 severity) (Table 3). Hypertension was encountered in 10 patients (23.8 %), all of which were grade 1 or 2 in severity. Two patients had bleeding events: one patient had epistaxis and one patient experienced bleeding from the feeding tube insertion site. Both of these grade 2 bleeding events resolved with conservative measures and axitinib was resumed without reoccurrence of bleeding.
Efficacy
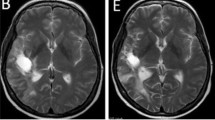
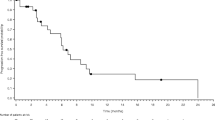
The best overall response rate was 7 % (Table 4). No patients achieved CR however there were two patients with PR. One patient received 12 cycles of axitinib prior to developing gross clinical disease progression at 22 months. The second patient with PR received a total of 5 cycles of axitinib and similarly was noted to have disease progression at 10 months (Fig. 1). The DCR was 76.7 % (n = 23) with 70 % of patients noted to SD on axitinib. The mean number of months of axitinib treatment in those with disease control was 6.6 months (range: 3–19). The median PFS was 3.7 months (95 % CI: 3.5–5.7) with a median OS of 10.9 months (95 % CI: 6.4–17.8) (Fig. 2). Neither OS (p = 0.59) nor DCR (p = 0.91) was found to be statistically different in those presenting with locoregional recurrence versus distant metastatic disease. Although not statistically significant, a trend towards improved overall survival was seen in the HPV positive population (Fig. 3).
We conducted an exploratory analysis to assess radiologic factors associated with response. Patients with a smaller sum of the diameter of target lesions experienced the best response rate, progression free survival (p = 0.02), and overall survival (p = 0.04). A higher number of lesions seemed to be associated with a worse overall response; however, this did not achieve statistical significance (p = 0.09). Regression modelling was performed to analyze the relative impact of contributors to response. The longest diameter was the most significant contributor to response (Chi2 = 7.67), followed by number of lesions (Chi2 = 4.40), and finally maximum target size (Chi2 = 1.59).
Correlative studies
Correlative studies were performed on patient serum obtained prior to enrollment and at various time points during trial involvement of which samples were available for 28 patients. Cytokines analyzed included EGF, PDGF-alpha, PDGF-alpha, interleukin (IL)-6, IL-8, hepatocyte growth factor (HGF), FGF-2, and VEGF. Although there was limited data available, it was noted that the change in IL-8 after the first dose of axitinib was associated with response to therapy (p = 0.037). Specifically, persistent increase in IL-8 was seen in all patients with no response to axitinib. Amongst patients experiencing clinical benefit, a mixed picture was seen with patients however there was a trend towards decreased IL-8 levels after initiation of therapy. Analysis of the remaining biomarkers did not reveal significant findings.
Discussion
This is the first trial to evaluate the role of axitinib in R/M HNSCC. This single arm phase II trial demonstrates that axitinib is not only is well-tolerated in heavily pre-treated R/M HNSCC patients, but also achieves good disease control. Although PFS was unremarkable compared to historical controls, median OS was noted to be markedly increased (10.9 months) compared to both historical controls in first line cytotoxic chemotherapeutic regimens. In exploratory analyses, patients with a smaller sum of the longest diameter of metastatic lesions were noted to have better responses as well as outcomes.
Tyrosine kinase inhibitors have the potential to not only target EGFR but also other associated pathways. Multiple tyrosine kinase inhibitors have been studied including Gefitinib [8–10], Erlotinib [11], Sorafenib [12, 13], and Dacomitinib [14] (Table 5). Of the prior tyrosine kinase inhibitors studied, the only one with anti-VEGFR activity is sorafenib, targeting anti-VEGFR-2, PDGFR, and Raf kinase. Axitinib varies significantly compared to sorafenib in that it targets multiple isoforms of VEGFR (−1, −2, −3) and c-kit. The greater targeting of VEGFR especially leads to the possibility of having greater anti-angiogenic activity and hence clinical efficacy. In this study, we found that although response rates were low, axitinib demonstrated both an encouraging DCR (77 %) and OS of 10.9 months. Despite these findings, the study was closed at interim analysis as it failed to meet its primary endpoint.
Treatment with axitinib was relatively well-tolerated with a low incidence of grade 3–4 toxicities, although the majority of patients could not tolerate the planned dose of 10 mg BID. Our trial demonstrates relative improved tolerability with the use of single agent axitinib, as only 40 % of patients had grade 3–4 toxicities with only six patients discontinuing therapy due to toxicities. Although bleeding is a significant concern with VEGFR targeted therapies, our study only noted two bleeding events which were mild in nature, neither necessitating cessation of the drug. However, we excluded patients with carotid artery encasement due to concern of significant life-threatening bleeding. This is a limitation to its use as many unresectable patients have disease surrounding the carotid artery, hence necessitating systemic therapy.
Assessment of tumor response to tyrosine kinase inhibitors can be difficult in patients with solid tumors. Tyrosine kinase inhibitors act to inhibit further cell growth (“cytostatic”) by a variety of mechanisms including anti-angiogenesis. As such, if a response to therapy is elicited, the tumor is expected to remain stable or even mildly increase in tumor size. This phenomenon has been extensively described in the base of advanced gastrointestinal stromal tumors (GISTs) being treated with Imatinib. Furthermore, cystic attenuation and increased tumor size on CT imaging has been shown histopathologically to correspond to treatment effect via intratumoral hemorrhage and myxoid degeneration rather than tumor progression [15, 16]. Based on RECIST v1.0, this pattern of treatment response has not been adequately captured, and as a result, responding patients have been labelled as stable disease or, in some cases, progressing on therapy. An alternate set of response criteria known as Choi Criteria have been proposed for evaluating the treatment effect of tyrosine kinase inhibitor therapy [17, 18]. In one study, GIST tumor patients treated with imatinib had a 48 % response rate per RECIST compared to an 84 % response rate by Choi Criteria [19]. Additionally, only changes as defined by Choi criteria correlated with patient-directed endpoints, including PFS and disease specific survival [17]. Subsequent studies have demonstrated the utility of Choi Criteria in the evaluation of other malignancies treated with tyrosine kinase inhibitors such as metastatic renal cell carcinoma [20]. Hence, we hypothesize that the use of Choi Criteria in the evaluation of response might yield more accurate data.
During our exploratory analyses, we noted that patients with an overall low disease burden (demonstrated by lower sums of the longest diameter of target lesions as well as number of target lesions) tended to have better responses to axitinib as well as improved clinical outcomes. This is consistent with the predominantly anti-angiogenic mechanism of axitinib. As a VEGFR inhibitor, we would expect that this would inhibit the growth of neovascularization to small metastases much more efficiently than large masses. This is due to the fact that not only is there a greater degree of established vasculature supplying the larger tumors but also larger tumors often have a degree of internal necrosis hence limiting effective targeting [21].
We noted that HPV tumors treated with axitinib had a trend, albeit not statistically significant, towards improved overall survival (Fig. 3). This is the first report of activity with a tyrosine kinase inhibitor in HPV positive patients in patients with metastatic SCCHN [22–25].
On an exploratory correlative analysis of serum cytokines, we demonstrated that a lack of clinical response to axitinib was associated with progressive increase in serum IL-8 levels following the initiation of therapy. Although fluctuations were seen in several other cytokines, no significant results were noted. IL-8 is a proinflammatory cytokine that acts to activate several pathways, and in doing so, IL-8 may modulate the tumor microenvironment. Through activation of the MAPK signaling cascade and Akt, tumor production of IL-8 results in promotion of endothelial angiogenesis, cell survival, and increased cell proliferation [26]. Interestingly, IL-8 signaling has been demonstrated to induce phosphorylation of VEGFR-2 [27] and has been demonstrated to mediate resistance to bevacizumab, an anti-VEGF-A monoclonal antibody in preclinical models [28]. Amongst patients with HNSCC, elevated IL-8 levels were demonstrated to be a prognostic factor [29, 30]. Hence, we postulate that in tumors that did not respond to treatment with axitinib, increased serum levels of IL-8 following treatment may have reflected the ability of some tumors to continue to activate angiogenesis through alternate cytokine driven pathways. Although our findings are interesting, caution is warranted given the lack of a similar pattern in similar markers (ie IL-6).
In conclusion, further evaluation with axitinib should be considered due to its tolerability and favorable median overall survival. Future studies are needed to define whether other radiologic definitions of response are more appropriate in defining response to tyrosine kinase inhibitor anti-VEGF therapy in head and neck cancer and whether improvements in outcomes may be gained with the addition of cytotoxic chemotherapy or checkpoint inhibitors in combination with axitinib.
References
Hutson TE, Lesovoy V, Al-Shukri S, et al (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14:1287–1294
Motzer RJ, Escudier B, Tomczak P, et al (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14:552–562
Locati LD, Licitra L, Agate L, et al (2014) Treatment of advanced thyroid cancer with axitinib: phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer 120:2694–2703
Cohen EE, Tortorici M, Kim S, et al (2014) A phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother Pharmacol 74:1261–1270
Hu-Lowe DD, Zou HY, Grazzini ML, et al (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14:7272–7283
Schiller JH, Larson T, Ou SH, et al (2009) Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol 27:3836–3841
Furnival GM, Wilson RW (1974) Regressions by leaps and bounds. Technometrics 16:499–511
Cohen EE, Kane MA, List MA, et al (2005) Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 11:8418–8424
Cohen MH, Williams GA, Sridhara R, et al (2003) FDA drug approval summary: gefitinib (ZD1839) (iressa) tablets. Oncologist 8:303–306
Stewart JS, Cohen EE, Licitra L, et al (2009) Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 27:1864–1871
Soulieres D, Senzer NN, Vokes EE, et al (2004) Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22:77–85
Williamson SK, Moon J, Huang CH, et al (2010) Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: southwest oncology group study S0420. J Clin Oncol 28:3330–3335
Gilbert J, Schell MJ, Zhao X, et al (2015) A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol 51:376–382
Kim HS, Kwon HJ, Jung I, et al (2015) Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin Cancer Res 21:544–552
Bechtold RE, Chen MY, Stanton CA, et al (2003) Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with gleevec. Abdom Imaging 28:808–814
Linton KM, Taylor MB, Radford JA (2006) Response evaluation in gastrointestinal stromal tumours treated with imatinib: misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol 79:e40–e44
Benjamin RS, Choi H, Macapinlac HA, et al (2007) We should desist using RECIST, at least in GIST. J Clin Oncol 25:1760–1764
Choi H, Charnsangavej C, Faria SC, et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759
Therasse P, Arbuck SG, Eisenhauer EA, et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92:205–216
Goh V, Ganeshan B, Nathan P, et al (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261:165–171
Kambadakone A, Yoon SS, Kim TM, et al (2015) CT perfusion as an imaging biomarker in monitoring response to neoadjuvant bevacizumab and radiation in soft-tissue sarcomas: comparison with tumor morphology, circulating and tumor biomarkers, and gene expression. AJR Am J Roentgenol 204:W11–W18
Fakhry C, Westra WH, Li S, et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269
Chaturvedi AK, Engels EA, Pfeiffer RM, et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301
Ang KK, Harris J, Wheeler R, et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Huang H, Zhang B, Chen W, et al (2012) Human papillomavirus infection and prognostic predictors in patients with oropharyngeal squamous cell carcinoma. Asian Pac J Cancer Prev 13:891–896
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14:6735–6741
Petreaca ML, Yao M, Liu Y, et al (2007) Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell 18:5014–5023
Gyanchandani R, Sano D, Ortega Alves MV, et al (2013) Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol 49:761–770
Le QT, Fisher R, Oliner KS, et al (2012) Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clin Cancer Res 18:1798–1807
Rischin D, Peters LJ, O'Sullivan B, et al (2010) Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the trans-Tasman radiation oncology group. J Clin Oncol 28:2989–2995
Acknowledgments
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swiecicki, P.L., Zhao, L., Belile, E. et al. A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Invest New Drugs 33, 1248–1256 (2015). https://doi.org/10.1007/s10637-015-0293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0293-8