Abstract
Insect mouthparts are serially homologous appendages. As such, their development and evolution are nonindependent. Arthropod appendages share similarities in their developmental origins and underlying genetics. Here, we review the development, specification, and patterning of insect mouthparts, with comparisons to the legs of Drosophila melanogaster. The expression and function of genes in the arthropod head give clues as to the homology of the labrum. The activity of Hox genes establishes appendage-specific gene expression and interactions allowing for the development of unique appendage types. Many similarities exist in the patterning of gnathal appendages and legs; however, unique variations in gene function in each appendage type provide clues to the developmental origins of mouthpart morphologies. We examine what is known about mouthpart patterning in mandibulates, as exemplified from several beetle species, as well as in the proboscis of Drosophila melanogaster and in the hemipteran rostrum of Oncopeltus fasciatus. With these findings in mind, we reflect on the evolution of serially homologous structures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
The mouthparts and other appendages of arthropods possess a versatile developmental program. The segmented body plan of these animals makes it possible for the redeployment of a conserved developmental system, which nevertheless admits variations enabling evolution and adaptation. Arthropods confront their environment with a varied array of tools for different lifestyles. Their success seems supreme in species diversity, if not also anatomical disparity. As far as we now understand it, this diversity arises from a shared set of developmental events and the genes that control them. Nevertheless, investigations of comparative developmental biology and genetics have uncovered a mixture of conservation and divergence in insect appendage development.
Here, we will attempt to contextualize the patterns of the evolution in insect mouthpart and appendage development through analogy to the musical ideas of “theme and variation. ” Compared between species and between appendage types, mouthparts and other insect appendages are both special and serial homologs , respectively. These appendages share a great deal in their developmental origins and underlying genetics. This is the common “theme.” But key differences exist and influence the generation of morphological variations. While consistent themes run throughout, individual variations enable novel life histories.
In this chapter, we will review thematic aspects of development common to arthropod species and appendage types, reflecting primarily on the mouthparts of insects. We will also explore variations that allow for unique appendage types and for the unique features of individual lineages.
Theme and Variation
In classical western music, the compositional technique of theme and variations uses a theme as the central musical idea of the piece, usually a memorable melody or chord progression. As the piece progresses, the theme is repeated again and varied in a different way. This cycle continues several times, providing the structure for the piece of music. Often the conclusion returns more closely to the theme or has a dramatic or poignant variation.
5.2 Homology: Theme and Variation
The shared developmental features of insect appendages reflect their complex evolutionary history, and it is useful to distinguish between the different ways in which these structures are related to one another. An important issue is that morphology and developmental similarities reflect both a history of common descent (homology or, formally speaking, special homology ) and the shared deployment of developmental programs at different positions in the body (serial homology ).
The first appreciation of morphological similarity in western science was closer to our current notion of serial homology and explicitly implicated development. The poet, statesman, and botanist, Johann Wolfgang von Goethe , carefully observed the development of plants and noted the similarities between leaves and floral organs (1790). Goethe described that these different structures grew from a similar meristem but diverged as development proceeded. He described the differences in their structures as arising from differences in “expansion” or “contraction” (Pfau 2010), although it seems clear he meant more than simply allometric differences. Goethe’s observation of this connection has direct historical continuity to our present idea of serial homology . Moreover, Goethe also contemplated the implications of his idea for species diversity. He considered that his model of development could, starting from the “Urpflanze” (the archetypal or primordial plant), “invent plants without limit.” This concept could also be universal: “The same law will permit itself to be applied to everything that is living” (Goethe 1814; Pfau 2010).
It is perhaps ironic that the term “homology ” was coined by Richard Owen (1843), who vocally opposed the idea of species evolution. Nevertheless, Owen clarified the ideas first expressed by Goethe , crediting him for his influential observations (1848). Owen explicitly defined what he called “serial homology ” as the repeated appearance of structures, such as vertebrae, within the body of an animal. He distinguished this from “special homology ,” which he described as “correspondency of a part or organ, determined by its relative position and connections, with a part or organ in a different animal” (1848). Without recognizing the possibility of evolution, Owen drew the distinction to what he called “general homology,” “… that in which a part or series of parts stands to the fundamental or general type, and its enunciation involves and implies a knowledge of the type on which a natural group of animals … is constructed.”
After Darwin , the concepts of special and general homology collapsed into one, as writers on the subject came to understand (special) homology as arising from shared ancestry. By the mid-twentieth century, Boyden (1943, 1947) argued that the literature had gone too far and confused serial and special homology , complicating the use of characters in taxonomy. In the 1980s, evolutionary biologists considering the implications of development (e.g., Van Valen 1982; Roth 1984) and developmental biologists considering the implications of evolution (e.g., Raff and Kaufman 1983; Wagner 1989) began to reconsider concepts of homology, arguing for a more mechanistic basis and drawing clear distinctions between special and serial homology.
In recent decades, detailed mechanistic studies of development in anatomically disparate organisms (e.g., Hinman et al. 2003; Davidson 2006) have meant that considerations of the evolution of characters often depend on consideration of their generative mechanisms. Günter Wagner (2007) has argued that the unit of homology should be considered to be the developmental genetic system responsible for the identity of a particular trait, what he terms the character identity network (ChIN).
We will return to the idea of homology in our conclusions and explore how insect appendage development reflects general principles in the evolution of homologous structures. The anatomy of insect mouthparts will be detailed elsewhere in this volume. So we will only briefly summarize their structure here, focusing on taxa relevant to studies of development.
5.3 Overview of Insect Mouthpart Anatomy
The ancestral and most common state of insect mouthparts is the mandibulate type (Grimaldi and Engel 2005; Misof et al. 2014), which is fixed in several prominent orders such as Odonata , Orthoptera , Coleoptera , and Hymenoptera (Marshall 2006). Mandibulate mouthparts are primarily used for chewing, and they appear in both generalist and specialist taxa. From anterior to posterior, the mouthpart appendages consist of the labrum , mandibles , maxillae , and labium (Snodgrass 1930, 1935). The labrum’s status as an appendage remains controversial (e.g., Popadić et al. 1998; Haas et al. 2001; Kimm and Prpic 2006; Posnien et al. 2009), and this question is considered below. Anatomically, the labrum acts as an upper lip and roof to the oral cavity. The mandibles are unjointed appendages used in chewing, and they are typically robust and well-muscled. The maxillae are paired , jointed appendages, which branch distally. The basal-most segment of the maxilla, the cardo , is jointed to the ventral head. The next segment is the stipes, which articulates with two medial endites , the lacinia and galea , which are fringed with setae in many species. Laterally, the stipes is also jointed to the maxillary palps. The palps typically consist of multiple segments, although their number may vary between different taxa. The palps typically function in the recognition of food. Chemosensory receptors on the surface of the palps aid the insect in identifying its target food (Snodgrass 1930; Chapman 1998). The posterior mouthpart appendage is the labium. The proximal labial segments fuse medially, forming the mentum and prementum . These segments may be jointed, or the joint between them may fuse, as in Tribolium (Sokoloff 1972; Angelini et al. 2012a ). Medially, the prementum articulates to a set of endites in most species. There may be as many as four labial endites, two medial glossae and two lateral paraglossae , although these are reduced or fused in some taxa (Snodgrass 1930, 1935). Lateral of the endites , the labium also articulates with a pair of palps, similar in their structure and function to the maxillary palps. The number of labial palpomeres also varies among taxa. The hypopharynx is a fleshy, non-appendicular structure that acts as a tongue or the bottom of the oral cavity in some taxa. While not prominent in many mandibulate insects, the hypopharynx is an essential component of some derived mouthpart morphologies.
Fossils and phylogenetic evidence establish mandibulate anatomy as the ancestral state for insects (Grimaldi and Engel 2005; Misof et al. 2014). Among extant orders, at least 24 of the 32 (as recognized by Misof et al. 2014) are characterized by mandibulate mouthparts . The development of mandibulate mouthparts has been examined in model species representing multiple orders, including the cricket Gryllus bimaculatus (reviewed by Liu and Popadić 2017) and the beetle species Tribolium castaneum (Angelini et al. 2012a ), Onthophagus taurus (Simonnet and Moczek 2011), and Cyclommatus metallifer (Gotoh et al. 2017).
However, some of the most successful groups of insects have exploited variations on the mandibulate theme. Among these novel morphologies is the principle insect model of development and genetics, the fruit fly Drosophila melanogaster. Diptera are characterized by the modification of mouthparts to piercing or sponging functions. In Muscomorpha, such as D. melanogaster, this involves the reduction and fusion of mouthpart appendages and surrounding head structures into a proboscis. The labial palps are absent, and the labium ends in a modified area called the labellum that is used for collection of liquid or particulate food (Snodgrass 1944). Mosquitos have evolved bladelike mandibles and maxillary laciniae, with an elongated hypopharynx used to secrete saliva (Snodgrass 1959). Emerging models of vector biology, such as Anopheles gambiae (Adolfi and Lycett 2018), have the potential to serve as comparative models of mosquito mouthpart development in the future.
The milkweed bug Oncopeltus fasciatus has also emerged as an informative system for developmental genetics (Chipman 2017; Panfilio et al. 2018), and this species represents the diverse Hemiptera . In this order, the labium is modified into a medially fused rostrum with multiple joints and no endites , while the mandibles and maxillae form thin stylets used in piercing and fluid feeding . Lepidoptera are another lineage in which existing model species, such as the silk moth Bombyx mori (Tomita and Kikuchi 2009; Ando et al. 2018), may be amenable to developmental genetic studies of mouthparts. Lepidopteran larvae retain chewing mandibulate mouthparts . Except for the early-branching lineage of Micropterigidae, adult Lepidoptera have evolved mouthparts in which the maxillary galeae form a proboscis typically used for nectar feeding (Krenn 2010). Secondarily, adults of the ghost moths (Hepialoidea) have reduced or absent maxillary palps and galeae. The mouthparts of these moths are vestigial, and the adults do not feed (Powell and Opler 2009). A fascinating novelty exists in Prodoxidae , where female Yucca moths develop an enlarged maxillary palpomere that is used independently of the proboscis to pollinate their host plant (Davis 1967; Pellmyr and Krenn 2002).
Other groups present intriguing mouthpart modifications, but few models currently lend themselves to developmental genetic investigations. For example, Thysanoptera present an interest comparison to Hemiptera, their sister taxon. The mouthparts of thrips are asymmetrical, with a single left mandible modified to form a piercing stylet . The maxillae differ in size, but each possesses a medial stylet and a small lateral palp. The thysanopteran labium is much closer in morphology to that of mandibulates. It is symmetrical, with a medial mentum and prementum , ending distally in medial endites and lateral palps (Jones 1954; Hunter and Ullman 1992). Siphonaptera (fleas) are another insect group with independently derived piercing mouthpart morphologies (Snodgrass 1946). In fleas the mandibles are absent, but bladelike mouthparts are formed by elongation of the labrum and laciniae. The maxillae and labium retain palps. Unfortunately, despite their medical importance, developmental studies of Siphonaptera have lagged behind other groups.
5.4 Development of Insect Mouthparts
5.4.1 The Embryonic Origins of Insect Mouthparts
In all hemimetabolous and many holometabolous insects, the mouthparts originate as ventral-lateral outgrowths from the embryo (Fig. 5.1; Snodgrass 1928; Butt 1949; Van Horn 1966). Limb buds appear soon after segment formation. Therefore, in species with short germ band development, the limb buds of the gnathal and thoracic segments appear before obvious external segmentation in the abdomen is completed. Initially, limb buds consist exclusively of ectoderm, but mesodermal cells from the body of each segment contribute to the appendages forming the muscles (Eastham 1931; Heming 1980). In Holometabola , muscle stem cells are associated with the imaginal discs and also give rise to the appendicular muscles at metamorphosis (Snodgrass 1935). During the germ band stage, specific gene expression establishes the components of differing character identity networks to define each appendage type.
In most insect species, appendages develop from three-dimensional embryonic limb buds , such as in the milkweed bug Oncopeltus fasciatus. (a) O. fasciatus embryos of different ages are shown stained with Sytox, a fluorescent dye that binds to DNA, highlighting nuclei. Ages are given as hours post egg-laying and as a percentage of total average developmental time. Embryos have been dissected away from yolk for clarity. (b) A 72-h embryo stained with Sytox is shown from a lateral view with the yolk intact. (c) Lateral view of an O. fasciatus first-instar nymph. Notice that appendages are visible early, before abdominal segmentation is complete. The limb buds grow rapidly, and by 96 h, regionalization within the appendages is apparent. The labial appendages are initially separate but migrate ventrally, and by 120 h, they fuse together at the midline. An antenna , Lr labrum, Mn mandible, Mx maxilla, Lb labium, T1–3 thoracic legs
5.4.2 Postembryonic Development of Appendages
Ancestrally, insects have a more-or-less direct development of the body plan. While adult structures such as wings and genitalia only appear after the adult molt (or in the subimago of Ephemeroptera ; Edmunds and McCafferty 1988), among members of the hemimetabolous orders, which lack a complete metamorphosis , juveniles hatch with appendages similar in structure to those of the adult, differing only in relative size and cuticle or sensory features. Nevertheless, the number of segments in some distal appendage structures can vary by developmental stage. For example, in Oncopeltus juveniles, the legs have two tarsomeres on each leg, while adults have three, apparently due to the formation of a novel joint within the distitarsus.
In the Holometabola , species undergo a complete metamorphosis with a non-motile pupa. During this stage, appendages undergo a more dramatic repatterning. In most holometabolous orders, legs and mouthparts are present in juveniles but have a less complex morphology compared to adults. For example, the distal segments of Tribolium juvenile legs are much smaller than in the adult, and the tibiotarsus exists as a single segment that will become two in the adult (Angelini et al. 2012b). Adult structures are produced by cells from corresponding larval structures (Švácha 1992 ). An extreme “indirect” form appendage development exists in some Holometabola . Drosophila is a familiar example, in which larval appendages are visible externally only as small sensory Keilin’s organs (Dambly-Chaudière and Ghysen 1986). In fruit flies and other Muscomorpha, most of the larval epidermis is polyploid (Smith and Orr-Weaver 1991) and must be replaced during metamorphosis . Imaginal discs give rise to the appendages and much of the surrounding body wall, while imaginal histoblasts produce to the remainder of the adult cuticle (Mandaravally Madhavan and Schneiderman 1977).
5.5 The Mystery of the Labrum
The labrum is an apical appendage-like structure on the insect head. It functions as the upper lip of insects (Snodgrass 1935); houses many sensory structures, such as setae, pressure receptors, trichoid sensilla, and coeloconic sensilla (Smith et al. 2014b); and serves a chemosensory function (Ortega-Hernández and Budd 2016). Several long-standing questions regarding the labrum have perplexed biologists (Budd 2002; Scholtz and Edgecombe 2006; Ortega-Hernández et al. 2017). Is the labrum a segmental structure, and if so, which segment is the labrum associated with? Is the labrum homologous to the paired ventral appendages that characterize insects and other arthropods? Lastly, what structure, if any, is the labrum homologous to in most distant relatives of Arthropoda? Several hypotheses have been proposed for each of these questions based on comparative studies of morphology and embryogenesis (Fig. 5.2). More recently, advances in developmental genetic techniques have provided an additional approach to testing hypotheses regarding the nature of the labrum. Here, we review the hypotheses for the nature of the insect labrum and summarize recent advances in our understanding of the labrum based on studies of developmental genes.
Gene expression and models of labrum identity in the insect head. Segments are numbered according to the different models. See main text for references. Segmental regions are shaded dark gray, and non-segmental regions are shaded light gray. (a) The protocerebral region is composed of a segmental and non-segmental region. (b) The protocerebral region is composed of two segments. (c) The protocerebral region is composed of a single segment and does not include non-segmental tissue. (d) Developmental gene expression patterns. The boundaries between wg and hh expression mark the parasegmental boundaries. The segment polarity gene wg is expressed in the labrum and in the ocular region of the developing protocerebrum . deu deutocerebral segment, lab labial segment, man mandibular segment, max maxillary segment, pro protocerebral region, tri tritocerebral segment
5.5.1 Where Is the Axial Origin of the Labrum?
The labrum has been hypothesized to be a component of the intercalary segment —the segment that gives rise to the tritocerebral brain neuromere (Butt 1960; Haas et al. 2001), the acron —an unsegmental anterior-most region of the insect head (Brusca and Brusca 2003), or the first segment of the insect head (Budd 2002). The intercalary segment hypothesis is supported by several pieces of evidence, each of which has recently come under scrutiny in the literature. The first piece of evidence is based on the position of the labrum in the insect head. The stomodeum , which the labrum is closely associated with, sits somewhere between the intercalary segment and the antennal segment in models of insect head segmentation (Rempel 1975 ; Schmidt-Ott and Technau 1992; Rogers and Kaufman 1997; Haas et al. 2001). However, during embryogenesis, the stomodeum migrates posteriorly from an apical-most region (Khila and Grbić 2007). Furthermore, expression of the gene six3 , which marks the apical-most region of the developing body axis of annelids, hemichordates, and onychophorans, also marks the labrum of insects (Fig. 5.2d; Steinmetz et al. 2010). These developmental studies suggest that the labrum originates in an apical position in the insect body axis, rather than in the intercalary segment, i.e., the ultimate position of the labrum does not reflect the position at which the labrum originates during embryogenesis. The second piece of evidence favoring an intercalary segment origin for the labrum is the fact that the labrum is innervated by the tritocerebral brain neuromere in the locusts Schistocerca gregaria and Locusta migratoria (Boyan et al. 2002). However, the labrum is innervated by the deutocerebrum in the horseshoe crab Limulus polyphemus (Mittmann and Scholtz 2003). The innervation of the labrum by either the deutocerebrum or tritocerebrum in euarthropods may represent derived conditions related to the ultimate position of the labrum, rather than its segmental origin (Scholtz and Edgecombe 2006; Bitsch and Bitsch 2010). Third, in the crustacean Porcellio scaber (Abzhanov and Kaufman 1999) and the centipede Lithobius atkinsoni (Hughes and Kaufman 2002b), the Hox gene labial (lab), which labels the intercalary/tritocerebral segment in all arthropods, is also expressed in the labrum (Haas et al. 2001). However, lab is not expressed in the labrum of other euarthropods investigated, including insects (Mlodzik et al. 1988; Rogers and Kaufman 1997; Peterson et al. 1999; Nie et al. 2001; Posnien and Bucher 2010), chelicerates (Damen et al. 1998; Sharma et al. 2012), and millipedes (Janssen and Damen 2006 ). This more comprehensive survey of lab expression suggests that its expression in the labrum of P. scaber and L. atkinsoni is likely a derived condition of the lineages leading to these species and is not indicative of the segmental origin of the labrum. In summary, most researchers now agree that the labrum originates in the insect protocerebral region. This hypothesis is supported by the expression of six3 in the labrum (Fig. 5.2d; Steinmetz et al. 2010) and the fact that the labrum migrates posteriorly from an apical-most position during insect development (Khila and Grbić 2007).
5.5.2 Is the Labrum a Segmental Structure?
While a consensus exists regarding the position of the labrum on the protocerebrum , there remains debate regarding the segmental nature of the protocerebrum. Current debates revolve around whether the protocerebrum represents a single segment, two fused segments, or a composite between a non-segmental and a segmental region. These debates have important implications for interpretations of the evolution of the labrum.
The existence of a non-segmental apical region in the insect head, and the heads of other euarthropods, originated with the Articulata hypothesis, which posits a sister-group relationship between Euarthropoda and Annelida, and a common origin of segmentation between these lineages (Scholtz 2002; Scholtz and Edgecombe 2006). The apical-most region of Annelida, referred to as the prostomium , lacks signatures of segmentation that are exhibited by body segments, such as nephridia and coelomic sacs, and unlike the body segments of Clitellata (earthworms and leaches), it does not develop from a posterior growth zone (Nielsen 2001; Ackermann et al. 2005; Scholtz and Edgecombe 2006). In polychaetes, distinct morphogenetic mechanisms underlie larval and juvenile segment development, but neither of these mechanisms is involved in development of the larval episphere, which gives rise to the prostomium (Ackermann et al. 2005; Scholtz and Edgecombe 2006). Therefore, this anterior-most region of the body axis may truly be regarded as non-segmental in nature (Scholtz and Edgecombe 2006). By extension, if segmentation is homologous between annelids and arthropods, then arthropods should exhibit an anterior-most non-segmental region.
In annelids, the prostomium is marked by six3 expression during development, while the first segment—the peristomium —is marked by expression of the insect homolog of the gene orthodenticle (otx). Likewise, six3 marks the anterior-most region of the body axis of insects and other arthropods, while otx marks a slightly more posterior region (Fig. 5.2d; Steinmetz et al. 2010). These expression domains both lie within the protocerebral region in insects and other arthropods. Therefore, in accordance with the Articulata hypothesis, the protocerebral region would represent a composite between an anterior non-segmental region and a posterior segmental region (Fig. 5.2a), much as the annelid head is composed of the prostomium and the peristomium . The labrum lies within the expression domain of six3, in insects and other euarthropods (Steinmetz et al. 2010). Since this region is predicted to be homologous to the annelid prostomium —a non-segmental region, according to the Articulata hypothesis, the labrum would represent a non-segmental structure (Fig. 5.2a).
Molecular analyses have revealed that arthropods and annelids are not closely related (Aguinaldo et al. 1997; Dunn et al. 2008). Based on these analyses, the Articulata hypothesis has been replaced by the Ecdysozoa hypothesis, which posits that insects and other arthropods are more closely related to several unsegmented phyla than they are to annelids. The Ecdysozoa hypothesis suggests that segmentation evolved independently in Euarthropoda and Annelida. While the Ecdysozoa hypothesis has now reached a consensus in the field (Giribet and Edgecombe 2017), whether an apical unsegmented region exists in the head of insects and other euarthropods remains an open question (Budd 2002; Scholtz and Edgecombe 2006; Posnien et al. 2010). This possibility might be expected, if annelids and euarthropods evolved segmentation in parallel from shared ancestral developmental mechanisms that were reiterated along an unsegmented body axis, as has been proposed (Chipman 2010). Two observations based on studies of the red flour beetle Tribolium castaneum suggest that an apical non-segmental region does exist within the protocerebrum of insects (Posnien et al. 2010). First, the V-shaped median apical-most region that gives rise to the labrum lacks the parasegment -like gene expression patterns that reliably demarcate body segments along the rest of the insect body axis (Fig. 5.2d; Posnien et al. 2009, 2010). Second, the gene regulatory network that patterns the V-shaped region is not reiterated in segmental patterns (Li et al. 1996; Schroder et al. 2000; Economou and Telford 2009; Posnien et al. 2009; Steinmetz et al. 2010). Taken together, these observations suggest that the insect protocerebrum may be composed of a median apical non-segmental region and a posterolateral segmental region.
The remaining hypotheses regarding the segmental nature of the protocerebrum region agree that this region is segmental. By extension, these hypotheses argue that the labrum is a segmental structure. However, they disagree about the number of segments that compose the protocerebrum. In one hypothesis, the protocerebrum is composed of a fusion between two ancestrally independent segments (Fig. 5.2b; Strausfeld 2012; Cong et al. 2014). In insects and other arthropods, two regions can be recognized within the protocerebrum —the anterior region is referred to as the prosocerebrum and includes the labrum and the posterior region is referred to as the archicerebrum and includes the optic lobes and mushroom bodies of the brain (Urbach and Technau 2003). According to this hypothesis, the labrum represents a fused pair of segmental appendages of a protocerebral segment, while the stalked eyes of stem group euarthropods—homologs of insect compound eyes—represent the segmental appendages of an archicerebrum segment (Strausfeld 2012; Cong et al. 2014). In both insects and other euarthropods, segment polarity genes are typically expressed in a one-stripe per segment pattern but are expressed independently in both the labrum and ocular regions of the protocerebrum (Fig. 5.2d; Damen 2002; Farzana and Brown 2008; Posnien et al. 2009; Janssen 2012), which lends some developmental support to this hypothesis (Ortega-Hernández et al. 2017).
The lack of fossil evidence for the transition between a leg and a stalked eye, a prediction of the dual segment origin of the protocerebrum, challenges this hypothesis (Ortega-Hernández et al. 2017). Additionally, it now seems clear that the insect protocerebral region is homologous to the head of tardigrades (Smith et al. 2016, 2018) and the eye-bearing segment of onychophorans (Eriksson et al. 2010). Stalked eyes evolved in the euarthropod lineage, after this lineage diverged from Tardigrada and Onychophora (Park et al. 2018). Therefore, the dual segment origin predicts that two appendage pairs should be found in the protocerebral region of tardigrades and onychophorans, but a single appendage pair—the frontal appendages—is found in this region in onychophorans, and either no appendages or a single appendage pair is found in this region in tardigrades, depending on whether the teeth-like stylets of tardigrades are derived from legs or not (Nielsen 2001).
The remaining hypothesis argues that the protocerebrum represents a single segment, with the labrum representing a fused appendage pair of this segment (Budd 2002; Budd and Telford 2009; Ortega-Hernández et al. 2017). According to this hypothesis, the independent expression domains of segment polarity genes in the insect protocerebrum are the result of co-option of these genes for novel functions in the protocerebrum, possibly in development of the ocular lobes (Ortega-Hernández et al. 2017). In this hypothesis, each segment of ancient panarthropods housed a pair of appendages, and the labrum represents the appendage pair of a single protocerebral segment (Budd 2002; Budd and Telford 2009; Ortega-Hernández and Budd 2016). This hypothesis aligns well with recent conclusions about the homology of the protocerebral region across Panarthropoda based on developmental studies (Smith et al. 2016, 2018) and fossil evidence (Park et al. 2018). Yet it remains possible that the protocerebrum evolved from a fusion of two segments. If so, based on current evidence, this fusion must have happened in the stem group of Panarthropoda (Ortega-Hernández et al. 2017), rather than in the stem group of Euarthropoda (Strausfeld 2012; Cong et al. 2014).
5.5.3 Is the Labrum Serially Homologous to the Ventral Appendages?
Studies of labrum development have clear consequences for our interpretations of the homology of this structure to the ventral appendages of insects and other euarthropods—including the gnathal appendages. One way to gauge homology is to test whether similar mechanisms control the development of the labrum and the ventral appendages. Like the ventral appendages, the labrum originates as paired bud-like structures during insect development (Scholtz and Edgecombe 2006; Posnien et al. 2009). Furthermore, the distal appendage-patterning gene Distal-less (Dll) and other components of the appendage-patterning network are active in the developing labrum of several insect species investigated (Angelini and Kaufman 2004; Ronco et al. 2008; Ohde et al. 2009; Posnien et al. 2009; Simonnet and Moczek 2011; Smith et al. 2014b; Yoshiyama et al. 2013). These results support homology between the labrum and the ventral appendages.
Although similar mechanisms control development of the labrum and the ventral appendages, there are compelling differences. The ventral appendages develop at parasegmental boundaries. The Wnt signaling protein encoded by wingless (wg) is expressed on the anterior side of parasegmental boundaries, and hedgehog (hh) is expressed on the posterior side of the boundaries (Fig. 5.2d; Hidalgo 1991; Posnien et al. 2009). In T. castaneum and other insects, wg and hh are required for activation of Dll (Morata 2001; Posnien et al. 2009). Targeting hh or wg with RNAi during T. castaneum embryogenesis leads to loss of Dll expression where ventral appendages normally develop and, in the case of hh, complete deletion of all ventral appendages (Posnien et al. 2009). By contrast, the labrum does not develop at a parasegmental boundary, and RNAi targeting hh or wg treatments does not affect Dll expression in the labrum or lead to deletions of the labrum (Posnien et al. 2009). These results suggest that there are no parasegmental boundaries in the region where the labrum develops and that different mechanisms activate Dll expression in the labrum compared to ventral appendages. These conclusions are consistent with the hypothesis that the labrum develops in a non-segmental region of the insect head and suggest that the labrum is not a serial homolog of the ventral appendages (Posnien et al. 2009). Additionally, the Notch pathway activates Dll expression in the labrum, but not in the ventral appendages (Siemanowski et al. 2015).
If the labrum is not a serial homolog of the ventral appendages, then why are there are so many similarities between labrum development and ventral appendage development? One hypothesis is that the labrum is a novel structure that evolved by co-option of the ventral appendage-patterning network (Posnien et al. 2009; Simonnet and Moczek 2011; Smith et al. 2014b). This hypothesis underpins a counterintuitive possibility. As an appendage, the labrum may not be homologous to the ventral appendages, while the developmental mechanisms that control development of the labrum and ventral appendages may be homologous.
5.5.4 How Does the Insect Labrum Relate to Structures in Other Animals?
A protocerebral appendage pair is predicted to be an ancient characteristic of Panarthropoda (Budd 2002). This ancestral appendage pair is thought to have given rise to the frontal appendages of onychophorans and possibly the teeth-like stylets of tardigrades (Nielsen 2001). This ancient appendage pair is exemplified by the “great appendages” of stem group euarthropods (Budd 2002). According to this hypothesis, the insect labrum—and the labra of other euarthropods—evolved from this ancient appendage pair. This hypothesis finds developmental support from expression of six3; six3 is expressed in the developing antenna -like frontal appendages of onychophorans and the euarthropod labrum (Steinmetz et al. 2010; Eriksson et al. 2013). More recently, several genes that are expressed in the developing euarthropod labrum were found not to be expressed in the developing onychophoran frontal appendage, casting doubt on the significance of expression patterns of a single gene, six3, for inferring homology of the euarthropod labrum and onychophoran frontal appendage (Janssen 2017b). In other words, the fact that six3 is expressed in both the labrum and frontal appendages may reflect the fact that they both develop in a homologous region of the body axis, rather than representing evidence that they share structural homology. On the other hand, differences in developmental patterning mechanisms should not be surprising, given how morphologically different the euarthropod labrum is compared to the onychophoran frontal appendages. Additional studies of labrum development and frontal appendage development need to be performed to better gauge the homology of these structures.
5.5.5 Current Outlook on Identity and Evolution of the Labrum
Although there is much to be determined regarding the origin of the labrum , the above discussion reveals three elements related to the evolution of the labrum that have reached a near consensus among zoologists. First, fossil evidence (Cong et al. 2014; Park et al. 2018) and developmental studies of Onychophora (Eriksson et al. 2010, 2013) strongly support a model in which an ancient ancestor of euarthropods had an appendage pair on the protocerebral region. Second, the labrum develops in the protocerebral region of the body axis (Steinmetz et al. 2010). Third, similar mechanisms control patterning of both the labrum and the ventral appendages (Smith et al. 2014b). However, determining whether the labrum is homologous to frontal appendages of onychophorans and ancient panarthropods and whether it is homologous to the ventral appendages requires additional studies. An important step toward addressing these questions will be to determine the segmental composition of the protocerebral region. New paleontological insights and developmental studies of a more diverse group of insects, additional euarthropods, and even onychophorans and tardigrades may be required to finally solve the mystery of the labrum.
5.6 Identity Specification of the Gnathal Appendages
The body plans of animals are established early in embryonic development. Anterior-to-posterior axial gradients activate a series of conserved transcription factors in adjacent and sometimes overlapping domains. Loss of function in these genes results in homeosis , the development of one anatomical structure in the position normally held by another. In many species these genes are linked in adjacent positions on the chromosomes. Their homeotic mutant phenotypes and linkage in a genetic complex gave them their name: Hox genes . In the 1990s and 2000s, evolutionary developmental biology (evo-devo) grew as a field in part by exploring the connections between Hox gene function and arthropod body plan variations (reviewed by Hughes and Kaufman 2002b ; Angelini and Kaufman 2005). These genes are active during embryonic development, but the specification of appendage identity is an ongoing process, as evidenced by the transformation of appendages during metamorphic or juvenile-to-adult development following knockdown by RNA interference (e.g., Tomoyasu et al. 2005; Wasik et al. 2010; Aspiras et al. 2011).
5.6.1 The Mandible
The mandible is the anterior-most head appendage that is not associated with a brain-housing segment. This appendage articulates with the head capsule but otherwise lacks joints. In zoological terms, it consists of the coxopodite (proximal) component, but not the telopodite (distal) component of the generalized insect appendicular appendage (Snodgrass 1935). In line with its coxopodite identity, the insect mandible lacks expression of the telopodite maker Distal-less during embryogenesis (Rogers et al. 2002). Additional gene expression studies suggest that the mandible is primarily composed of single endite of a single basal podomeres (Coulcher and Telford 2013).
While genetic screens of Drosophila melanogaster have laid the foundation for our understanding of how appendage identities are specified during development, fruit flies lack mandibles. For this appendage type, knowledge of mandible identity specification arose from studies of other insect species. As with other gnathal appendages, the Hox genes play important roles in regulating mandible identity. In winged insects, the only Hox gene that is strongly expressed in the mandible is Deformed (Dfd) (Fig. 5.3a; Rogers and Kaufman 1997; Brown et al. 1999a; Hughes and Kaufman 2000; Rogers et al. 2002; Angelini et al. 2005). However, the insect ortholog of Hox3, zerknüllt (zen), which is typically expressed extraembryonically during insect development (Schmidt-Ott et al. 2010), is also expressed in a more typical Hox gene pattern in the apterygote insect Thermobia domestica (Hughes et al. 2004). In this species, Hox3 is expressed in the mesoderm of the developing mandibles and maxillae. Hox3 was most likely also expressed in the developing mandibles of the last common ancestor of insects, given that it is expressed in the mandibles of crustaceans (Papillon and Telford 2007) and centipedes (Hughes and Kaufman 2002a) and given that Zygentoma —the apterygote lineage that includes T. domestica—is an out-group of all winged insects that have been investigated (Yeates et al. 2016). Like in T. domestica, Hox3 expression is restricted to the mesodermal layer of the developing mandibles of the crustacean Daphnia pulex (Papillon and Telford 2007), suggesting that this gene played a role in regulating development of mesodermal derivatives in the mandibles ancestrally in insects. Additionally, Sex combs reduced (Scr) is expressed at low levels in the mandibles of T. domestica (Passalacqua et al. 2010).
Head appendage identity specification based on studies of T. castaneum and other insects. See main text for references. (a) Expression domains of the Hox genes labial (lab), proboscipedia (pb), Deformed (Dfd), and Sex combs reduced (Scr) and the gene cap’n’collar (cnc) in the insect head. Known regulatory interactions are shown. Arrows indicate activation of expression. The horizontal bar indicates repression of expression. The thin line indicates a more restricted expression domain of Scr in the maxillary segment. (b) A model for appendage identity specification in insects. The default identity is leg (top). Expression of appendage identity selector genes in appendage anlagen (+ gene name) modifies the default leg state. Pathways leading to modified appendage identities are color-coded. deu deutocerebral segment, lab labial segment, man mandibular segment, max maxillary segment, pro protocerebral region, tri tritocerebral segment
At this juncture, the function of zen and Scr in the developing mandibles of T. domestica is unknown. However, the function of Dfd during mandible development has been investigated in insects with generalized mandibulate mouthparts—the flour beetle Tribolium castaneum—and insects with highly derived mouthparts—the milkweed bug Oncopeltus fasciatus. In T. castaneum, null Dfd mutants and RNA interference (RNAi) targeting Dfd result in nearly complete transformations of the larval mandible to antenna (Fig. 5.3a; Brown et al. 1999b). In this species, Dfd activates the transcription factor-coding genes cap’n’collar (cnc) and paired (prd) during embryogenesis (Coulcher and Telford 2012). Dfd activates expression of cnc broadly across the mandible segment, including in the developing mandibles, and prd specifically in the endites of the mandibles (Fig. 5.3a). RNAi targeting cnc during embryogenesis results in transformation of the mandible to maxilla, indicating that this gene plays an important role in specifying mandible identity (Fig. 5.3b; Coulcher and Telford 2012). Expression of cnc is restricted to the mandible segment and labrum across mandibulate euarthropods. By contrast, it is expressed broadly across the developing embryo of chelicerates (Sharma et al. 2014) and onychophorans (Janssen 2017a). These results support a model in which the mandible characteristic of Mandibulata evolved by specialization of cnc function in this lineage.
In contrast to its function during embryogenesis, Dfd does not appear to be required for establishing mandible identity during metamorphosis in T. castaneum (Smith and Jockusch 2014). Instead, targeting Dfd during this period results in minor defects in mandible morphology but does not affect the identity of this appendage type. A similar result was recovered from studies of the postembryonic function of Dfd in a hemimetabolous insect species, the termite Nasutitermes takasagoensis (Toga et al. 2013). In this species, male minor workers can molt into either presoldiers or medium workers. The mandibles regress in size between the male minor worker and presoldier molt. When Dfd is targeted with RNAi, mandible regression is inhibited, i.e., presoldiers of Dfd RNAi treatments have larger mandibles than presoldiers of control treatments (Toga et al. 2013). This result suggests that Dfd functions to determine the size of presoldier mandibles postembryonically. As with postembryonic Dfd RNAi in T. castaneum, mandible identity is not affected by postembryonic Dfd RNAi in N. takasagoensis (Toga et al. 2013).
Oncopeltus fasciatus are true bugs (Hemiptera ), and like other true bugs, they exhibit highly derived piercing-sucking mouthparts. In bugs, the mandibles and maxillae are modified into long thin stylets. The mandibles and maxillae form a piercing-sucking tube, with the mandible on the outside and the maxillae fused on the inside, with space between them for fluid to flow. The labial palps sheath and provide support to the feeding stylets . Of the Hox genes , only Dfd plays a role in establishing mandible identity in O. fasciatus (Hughes and Kaufman 2000). RNAi targeting this gene results in a transformation of the mandible to an antenna with multiple joints. The recognizable components of the ectopic antenna appear to exhibit distal antenna identity. Therefore, although bugs exhibit morphologically derived mandibles, Dfd functions to specify mandibular identity in the same manner as it does in insects with generalized mandible morphologies, by blocking antennal identity during embryogenesis.
5.6.2 The Maxilla
The Hox genes pb and Dfd are both expressed in the developing insect maxilla of most species that have been investigated (Fig. 5.3a; Brown et al. 1999a; Shippy et al. 2000; Curtis et al. 2001; Hughes and Kaufman 2002b; Angelini et al. 2005), and Scr is expressed in the maxillae of some insects that have been investigated (Passalacqua et al. 2010). Several null pb mutations cause nearly complete transformations of maxilla to leg in the homozygous state during embryogenesis in T. castaneum (Beeman et al. 1993; Shippy et al. 2000). Severely affected larvae of embryonic RNAi treatments targeting pb also exhibit nearly complete transformations of maxilla to leg (Shippy et al. 2000). Both loss-of-function pb mutations and larval RNAi targeting pb in T. castaneum also lead to transformations of the maxillae to leg during metamorphosis (Beeman et al. 1989; Smith and Jockusch 2014). In this case, only the palps are transformed, and they exhibit transformation to distal leg (femur, tibia, tarsus, pretarsus, claw). Together, these results suggest that pb played an ancient role in specifying maxilla identity in insects (Fig. 5.3b).
One might expect that the maxillae would develop into mandibles in the absence of pb function in T. castaneum. After all, in the absence of pb function, Dfd is the only Hox gene predicted to be expressed in the maxillae, and Dfd is required for specification of mandible identity (see above). Yet, the maxillae are transformed into legs when pb function is disrupted. This result can be explained by the fact that cnc is required for mandible development, and unlike Dfd, this gene is expressed in the developing mandibles, but not the maxillae (Fig. 5.3a; Coulcher and Telford 2012). However, Dfd does play an important role in maxillae development. In T. castaneum, Dfd loss-of-function embryos exhibit the telopodite component of the maxilla but lack the endite component (Fig. 5.3b; Brown et al. 2000). This suggests that Dfd is required for development of maxillary endites. When both Dfd and pb function are simultaneously disrupted, the maxilla develops into an antenna (Brown et al. 2002). Disrupting the function of Dfd and Scr simultaneously also results in maxilla to antenna transformations (Brown et al. 2002). The mechanism behind this result is unclear, but it most likely indicates that Dfd normally activates pb expression in the maxilla, but Scr can compensate for this function in the absence of Dfd function (Fig. 5.3a; Brown et al. 2002). In this model, when both Dfd and Scr function are compromised, pb is not expressed, resulting in transformation of the maxilla to antenna. There is some merit to this idea since Scr is required to activate pb expression in the labium of T. castaneum embryos (DeCamillis et al. 2001). This model of maxilla identity specification leaves open an interesting question. How does Scr affect expression of pb in the maxilla, since Scr is not expressed in the maxilla of T. castaneum embryos (Passalacqua et al. 2010)? It is possible that Scr is expressed in the maxilla when Dfd function is compromised, due to an inhibitory regulatory interaction between Dfd and Scr, but this possibility has not been tested in T. castaneum.
During T. castaneum metamorphosis , the roles that pb and Dfd play in maxilla identity specification are similar to their roles during embryogenesis (Smith and Jockusch 2014). However, as with mandible development, it appears that slightly different mechanisms are active during metamorphosis. First, disrupting Dfd function with RNAi does not delete maxillary endites (Smith and Jockusch 2014), although this result is predicted based on studies of embryogenesis (Brown et al. 2000). Second, targeting Dfd and Scr simultaneously with RNAi does not cause homeotic transformations of the maxilla (Smith and Jockusch 2014), while the embryonic model predicts that this treatment should result in transformations of the maxillae to antenna (Brown et al. 2002). The simplest explanation for this difference is that, unlike during embryogenesis, pb expression does not require activation by Dfd or Scr in the maxilla during metamorphosis (Smith and Jockusch 2014).
Functional data and expression data make it clear that pb played a primary role in specifying maxilla identity in the last common ancestor of insects (Rogers et al. 2002). Intriguingly, however, pb is not expressed in the developing maxillae of the milkweed bug O. fasciatus , nor is this gene required for specification of maxilla identity in this species (Hughes and Kaufman 2000; Rogers et al. 2002; Angelini et al. 2005). In fact, the mechanisms that specify maxilla identity in O. fasciatus resemble those that specify mandible identity (Hughes and Kaufman 2000; Rogers et al. 2002). These similarities in specification resemble morphological similarities—both the mandible and maxilla are long unjointed appendages in O. fasciatus and other true bugs. By contrast, in other insect species, the maxilla is morphologically much more similar to the labium. Therefore, the loss of pb function in the maxilla of true bugs correlates with the evolution of the maxilla in this lineage toward a mandible-like morphology (Hughes and Kaufman 2000; Rogers et al. 2002). This change in morphology coupled with the loss of gene expression recalls the loss-of-function homeotic transformation of body segments that can be produced in Hox mutations in fruit flies and other animals. This correlation has led some authors (Rogers et al. 2002) to tentatively suggest that hemipteran mouthparts represent the success of a hopeful monster (Gould 1977; West-Eberhard 2003), the rare case in which a mutation of large phenotypic effect is favored and fixed by natural selection.
5.6.3 The Labium
The Hox genes pb and Scr are both expressed in the developing insect labium (Fig. 5.3a; Hughes and Kaufman 2000; Shippy et al. 2000; Curtis et al. 2001; DeCamillis et al. 2001; Hughes and Kaufman 2002b; Rogers et al. 2002; Angelini et al. 2005; Zhang et al. 2005; Hrycaj et al. 2010; Passalacqua et al. 2010). Structurally, the labium is very similar to the maxillae—consisting of basal podomeres with endites and terminal palps. However, unlike in the maxillae, the contralateral basal podomeres and endites are fused medially in the labium. Mirroring their morphological similarities, very similar mechanisms specify the maxillary and labial identities. For instance, as with the maxillae, disrupting pb function leads to transformations of the palps of the labium to distal leg in insect species that have been investigated (Pultz et al. 1988; Beeman et al. 1993; Hughes and Kaufman 2000; Smith and Jockusch 2014). These results indicate that pb plays a primary role in insects in promoting palp morphology during development. In contrast to the typical developing insect maxilla, Scr is typically strongly expressed in the developing labium (Fig. 5.3a; Hughes and Kaufman 2000; Curtis et al. 2001; DeCamillis et al. 2001; Rogers et al. 2002; Zhang et al. 2005; Hrycaj et al. 2010; Passalacqua et al. 2010). Therefore, Scr may be playing specific roles in distinguishing the labium from the maxillae. It is difficult to test this possibility during embryogenesis because Scr function is typically required for expression of pb in the labium (Fig. 5.3b; DeCamillis et al. 2001; Angelini et al. 2005). Loss of Scr function leads to loss of pb function, and the labium develops into antennae (Curtis et al. 2001; DeCamillis et al. 2001). Therefore, discriminating between Scr specific functions and functions of Scr that are mediated through its role in regulating pb expression are difficult in studies of insect embryogenesis. However, Scr does not appear to regulate pb expression during T. castaneum metamorphosis (see above). When Scr is targeted with RNAi during metamorphosis, the labial palps and endites develop characteristics that are typically restricted to the maxillae (Smith and Jockusch 2014). This result supports a role for Scr in promoting labium specific morphologies, while pb might play a more generic role in promoting the development of palp containing appendages.
5.6.4 The Role of Homothorax and Extradenticle in Specifying Mouthpart Identities
The protein products of genes homothorax (hth) and extradenticle (exd) must come together in the cytoplasm and form a heterodimer in order to be transported to the nucleus, where they function, in tandem, as transcription factors (Abu-Shaar and Mann 1998; Abu-Shaar et al. 1999; Kurant et al. 1998; Pai et al. 1998; Rieckhof et al. 1997). Therefore, the developmental functions of these genes perfectly overlap. Disrupting the function of either hth or exd results in homeotic transformations of gnathal appendage identities in Gryllus bimaculatus (Ronco et al. 2008), O. fasciatus (Angelini and Kaufman 2004), Onthophagus taurus (Simonnet and Moczek 2011), and D. melanogaster (Rauskolb et al. 1995; Inbal et al. 2001). These transformations most likely reflect the fact that Hth and Exd act as cofactors for Hox proteins and, as such, influence the specificity of Hox proteins for DNA regulatory elements (Chang et al. 1995; Chan et al. 1996; Johnson et al. 1995). In the absence of either Hth or Exd, Hox proteins are unable to properly regulate gene expression. This explains why the resulting phenotypes when hth or exd function is disrupted phenocopy the results of experiments in which Hox gene function is disrupted. Therefore, the roles that hth and exd play in specifying gnathal appendage identities are most likely mediated through direct interactions of their corresponding proteins with Hox proteins.
5.6.5 A General Model of Gnathal Appendage Identity Specification
Based on studies that began with D. melanogaster but have since expanded across diverse insects, it appears that highly conserved mechanisms control appendage identity specification in insects. The identities of most ventral appendages, including gnathal appendages, are determined by the Hox genes that are expressed in them (Hughes and Kaufman 2002a; see above). This is true for all ventral appendages except for the antennae. Hox genes are not expressed in the antennal segment (Fig. 5.3a; Hughes and Kaufman 2002a). In the absence of Hox gene function in the developing antennae , hth and exd promote antennal identity in insects (Fig. 5.3b; Struhl 1982a; Casares and Mann 1998, 2001; Mito et al. 2008; Ronco et al. 2008; Moczek and Rose 2009; Smith et al. 2014a; Setton et al. 2017). Antennal identity is specified by these genes, at least in part, by positively regulating the expression of the bHLH-PAS family transcription factor-coding gene spineless (Struhl 1982b; Duncan et al. 1998; Dong et al. 2002; Emmons et al. 2007; Shippy et al. 2009; Angelini et al. 2009; Toegel et al. 2009; Smith et al. 2014a; Setton et al. 2017). In developing legs, Hox genes repress ss expression (Duncan et al. 2010). In the absence of Hox gene activity, all ventral appendages develop as antennae (Struhl 1982a; Casares and Mann 1998, 2001; Brown et al. 2002; Smith and Jockusch 2014). While this might suggest that antennal identity is the default state of developing appendages, this is not the case. Disruption of hth/exd results in transformations of antenna to leg, even in the absence of Hox gene activity (Casares and Mann 2001; Dong et al. 2002; Ronco et al. 2008; Smith et al. 2014a). This suggests that leg identity is the default identity for ventral appendages (Fig. 5.3b; Casares and Mann 2001). To summarize the current model of ventral appendage identity specification, leg identity is most likely the default state, hth/exd promotes antennal identity in the absence of Hox gene activity, and Hox genes promote specific gnathal and leg identities combinatorially by suppressing antennal identity and the identities of other appendage types and/or by promoting particular ventral appendage identities (Fig. 5.3b).
Several features of the insect appendage identity specification mechanism predate the origin of insects. The Hox genes that pattern the gnathal appendages exhibit remarkably conserved expression patterns across Panarthropoda (Damen et al. 1998; Telford and Thomas 1998; Jager et al. 2006; Janssen and Damen 2006; Eriksson et al. 2010; Sharma et al. 2012; Janssen et al. 2014; Smith et al. 2016). Additionally, Hox genes are not expressed in the deutocerebral segment—the segment that houses antennae in insects—in Arthropoda or Onychophora (Damen et al. 1998; Telford and Thomas 1998; Jager et al. 2006; Janssen and Damen 2006; Eriksson et al. 2010; Sharma et al. 2012; Janssen et al. 2014). This suggests that specification of the appendage type that is associated with the deutocerebral segment without input from Hox genes is an ancient feature within Panarthropoda. Furthermore, RNAi targeting hth results in homeotic transformations of chelicerae—the deutocerebral appendages of Chelicerata—to leg in the harvestman Phalangium opilio (Sharma et al. 2015). This indicates that hth was required for specification of deutocerebral appendage identity in the last common ancestor of Euarthropoda. Taken together, these results indicate that interactions among Hox genes and between Hox genes and hth were important for specifying appendage identities—including those of direct homologs of the insect gnathal appendages—in stem group Euarthropods and possibly earlier.
5.7 Developmental Genetic Patterning of Insect Appendages
While components of the core character identity network , such as Hox genes , establish the fate of different appendages, these genes activate a set of downstream genes and developmental events that direct the morphogenesis of the unique appendage types. Some of the genes involved have expression patterns and interactions that are similar across appendage types, while many are specific to the identity of the appendage. Most of our knowledge of this phase of appendage patterning comes from D. melanogaster and particularly from the leg imaginal disc . However, some studies in the fruit fly and other insects have examined patterning in diverse appendages, such as the mouthparts. Before considering the development of mouthparts, it will be useful to reflect on the thematic pattern demonstrated by development in the legs of insects. Several detailed reviews on the developmental genetics of insect appendages exist (Angelini and Kaufman 2005; Jockusch and Smith 2015; Jockusch 2017; Ruiz-Losada et al. 2018). Readers interested in an authoritative account of the developmental genetics of insect appendages should refer to Jockusch and Smith (2015).
5.7.1 Initiation of Appendage Primordia
The cells that are competent to give rise to ventral appendages are specified at the anterior-posterior parasegment boundaries (Estella et al. 2003). In D. melanogaster, cells adjacent to the posterior of the boundary express the secreted protein Hedgehog (Hh) (Ingham 1993). To the anterior, Hh induces production of secreted Wingless (Wg), in ventral cells, and Decapentaplegic (Dpp), in dorsal cells (Basler and Struhl, 1994). The areas of wg and dpp expression maintain mutually repressive interactions, reinforcing their identities (Jiang and Struhl 1996; Theisen et al. 1996). The appendage primordia ultimately inherit cells from each compartment and the expression of these segment polarity genes marking their boundaries (Diaz-Benjumea et al. 1994; Theisen et al. 1996).
Outside of Drosophila , it is unclear whether these signaling pathways also initiate the expression of appendage development genes. The expression pattern of wg is known to extend laterally into the nascent appendages in diverse species, including the mayfly Ephoron leukon (O’Donnell and Jockusch 2010), the orthopterans G. bimaculatus (Niwa et al. 2000) and Schistocerca americana (Jockusch et al. 2000), the milkweed bug O. fasciatus (Angelini and Kaufman 2004), and the flour beetle T. castaneum (Bolognesi et al. 2008). However functional tests of wg in G. bimaculatus (Miyawaki et al. 2004) and O. fasciatus (Angelini and Kaufman 2004) appendage development do not produce defects in appendage growth or patterning. In the T. castaneum embryo, wg RNAi prevents appendage initiation (Ober and Jockusch 2006), suggesting that Wnt activation of appendage development may have evolved within Holometabola .
The transcription factor Distal-less (Dll) is one of first genes to be activated in the appendage primordia. In D. melanogaster, Wg promotes the expression of Dll, and its expression is restricted to a ventral-lateral domain in each embryonic body segment by inhibition from Dpp, dorsally, and epidermal growth factor (EGF), ventrally (Goto and Hayashi 1997 ). A subset of cells at the dorsal part of the Dll-expressing embryonic leg primordia contribute to the wing and haltere imaginal discs (Requena et al. 2017). In Drosophila, once the imaginal disc has formed, the initiation and maintenance of Dll expression is regulated by two separate enhancers. The first element is activated only by high levels of Wg and Dpp. Subsequently, an autoregulatory element is activated by Dll, independent of input from Wg or Dpp (Estella et al. 2008).
Dorsal-ventral specification within the leg imaginal disc is also controlled, independently, by Dpp and Wg (Estella and Mann 2008; Svendsen et al. 2009). These signaling molecules activate expression of transcription factors encoded by optomotor blind (omb) and H15 in dorsal and ventral territories, respectively (Maves and Schubiger 2003 ; Wilder and Perrimon 1995). Orthologs of omb and H15 are expressed in similar dorsal and ventral territories in the limb buds of the pill millipede Glomeris marginata (Prpic et al. 2005), but the expression of H15 is reduced in the spider Cupiennius salei (Prpic et al. 2003) and actually appears in a dorsal area of the limb buds in the onychophoran Euperipatoides kanangrensis (Janssen et al. 2015). Moreover, patterns of wg and especially of dpp expression do not conform with the Drosophila model in most other arthropod species (Angelini and Kaufman 2005; Janssen et al. 2015). These results suggest that, while the specification of dorsal-ventral polarity may be conserved within insects, its establishment may rely on as yet unidentified factors.
5.7.2 Specification of Proximal-to-Distal Domains
By the late second instar, gene expression begins to differentiate discrete domains along the proximal-to-distal axis of the Drosophila leg imaginal disc (Lecuit and Cohen 1997), and similar patterns have been found in other insects (Fig. 5.4; Angelini and Kaufman 2005; Jockusch and Smith 2015). High levels of both Wg and Dpp occur only in the center of the D. melanogaster leg imaginal disc, where cells expressing the two signals are near each other spatially along the parasegment boundary (Lecuit and Cohen 1997; Wu and Cohen 1999). In this way, Dll expression becomes locked in at the center of the leg disc, where its activity is required for development of the telopodite , the distal region of the leg (Cohen and Jürgens 1989b).
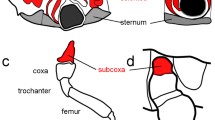
Summary of the requirement for appendage-patterning genes in the development of three insect species with different mouthpart morphologies. Distal structures are to the right in each panel, and lateral is up, except in the diagrams of legs where dorsal is up. Colored bars highlight structures affected by the manipulation of hth , dac, Dll and different components of the EGF and Notch signaling pathways. Notes: 1. While dac is expressed in an intermediate domain of the embryonic T. castaneum labrum (Prpic et al. 2001), embryonic dac RNAi has not been reported. Metamorphic-stage dac RNAi does not produce noticeable defects in the labrum (Smith et al. 2014). 2. Dll is expressed in the embryonic maxillary appendages in O. fasciatus, but Dll RNAi has no noticeable effect on their development. crd cardo , cx coxa, ds distal sclerite of the labrum, fe femur, gal galea, lac lacinia, lig ligula (single labial endite ), lp1–3 labial palp segments 1–3, ls labral sclerite, mnt mentum , mp1–4 maxillary palp segments 1–4, pmt prementum , pt pretarsus, stp stipes, t1–5 tarsomeres 1–5, Ti tibia, tr trochanter, ts tibial spurs
While the establishment of the proximal-to-distal axis by dorsal and ventral gradients of dpp and wg expression has been well described in D. melanogaster, a comparable model is lacking for insects generally. Prpic et al. (2003) have argued that this model of Dll activation, in the context of a roughly two-dimensional imaginal disc, does not generalize to the three-dimensional limb buds that are ancestral to insects and other arthropods. These authors point out that, because dpp and wg are expressed along dorsal and ventral sides of the compartment boundary, their secreted products form two hyperbola-shaped domains that intersect only at the center of the disc. However, if the same model is generalized to three dimensions, then cells along the length of the limb would experience similar concentrations of signaling proteins produced from the dorsal and ventral sides. This theoretical consideration helps to explain the diversity of dpp expression patterns that have been found (Angelini and Kaufman 2005; Janssen et al. 2015). However, it is still not clear what mechanism produces unique proximal-to-distal gene expression outside of Drosophila for genes such as Dll.
5.7.3 Proximal-Distal Domain Genes: Distal-less, Dachshund, and Homothorax
Genes such as Dll have been dubbed “limb gap genes ” because their loss-of-function phenotype eliminates structures from the limb and reduces growth of cells in those areas. This name is an analogy to the gap genes involved in Drosophila embryonic germ band patterning, where mutations in gap genes produce similar phenotypes (Nüsslein-Volhard and Wieschaus 1980; Wieschaus and Nüsslein-Volhard 2016). Distal-less is expressed in the D. melanogaster leg disc in cells that will give rise to the distal tibia and tarsus (Lecuit and Cohen 1997; Panganiban and Rubenstein 2002). A narrow ring of Dll expression also appears in the distal trochanter shortly before pupation (Wu and Cohen 1999). Strong Dll loss-of-function alleles in D. melanogaster are embryonic recessive lethal (Sunkel and Whittle 1987), but hypomorphic alleles or imaginal discs with reduced or eliminated Dll activity cause the loss of distal structures from the leg, including the femur, tibia, and tarsus (Cohen and Jürgens 1989b). The expression pattern of Dll orthologs is well conserved in the distal legs of diverse insects and other animals (Jockusch and Smith 2015). Mutations or RNA interference reducing Dll activity has also produced deletion of the legs, distal to the trochanter, in several hemi- and holometabolous insect species (Fig. 5.4; Beermann et al. 2001; Angelini and Kaufman 2004; Ohde et al. 2009; Yoshiyama et al. 2013; Angelini et al. 2012b; Moczek and Rose 2009).
The proximal domain of the insect leg is marked by expression of the homeobox transcription factor homothorax (hth). Wg and Dpp act to inhibit the expression of hth in central parts of the leg imaginal disc, restricting its expression to the periphery (Abu-Shaar and Mann 1998; Wu and Cohen 1999). This pattern of hth expression in developing legs appears conserved in many insects (Prpic et al. 2003; Angelini and Kaufman 2004; Inoue et al. 2002) and in other arthropods (Prpic and Tautz 2003). In D. melanogaster, Hth functions by binding with its cofactor encoded by extradenticle (exd; Abu-Shaar and Mann 1998; Rieckhof et al. 1997). Leg imaginal discs that lack hth develop with a fusion of proximal leg structures, aberrant joint formation, or a proximal-to-distal transformation of podomeres (Casares and Mann 1998, 2001). A similar leg phenotype is found with hth or exd RNAi in O. fasciatus (Fig. 5.4; Angelini and Kaufman 2004), G. bimaculatus (Mito et al. 2008; Ronco et al. 2008), and T. castaneum (Smith and Jockusch 2014).
A unique intermediate domain becomes established later in the second instar leg imaginal disc with the expression of dachshund (dac) (Mardon et al. 1994; Giorgianni and Mann 2011). Over time, the area of dac expression expands to encompass cells that will give rise to the femur, tibia, and basitarsus. As with the activation of Dll, Wg and Dpp promote the expression of dac in the D. melanogaster leg imaginal disc (Lecuit and Cohen 1997). Its area of expression is refined through co-activation by Brinker (Brk), which is expressed in areas of the disc outside the influence of Dpp (Estella and Mann 2008). Dll also directly binds to a dac regulatory element to initiate its expression (Giorgianni and Mann 2011). Later in the third instar, Dll and dac distinguish the distal and intermediate domains of the leg through mutually antagonistic interactions (Dong et al. 2001). Orthologs of dac are expressed in similar patterns in the developing legs of diverse insects (Abzhanov and Kaufman, 2000; Schaeper et al. 2013; Inoue et al. 2002 ; Prpic et al. 2001; Angelini and Kaufman 2004; Tanaka and Truman 2007), although some differences exist among taxa in the dynamics and precise proximal or distal limits of dac expression (Jockusch and Smith 2015). Mutations eliminating dac activity in D. melanogaster reduce the length of the leg by eliminating the tibia, giving this gene its name in reference to the short-legged dog breed. Maternal RNAi in O. fasciatus produces embryos with similar deletion of the tibia (Fig. 5.4). Surprisingly, dac RNAi in T. castaneum embryos produces only minor leg defects (Lee et al. 2013), although RNAi during metamorphosis in the species results in deletion of the tibia (Angelini et al. 2012b ), similar to the D. melanogaster dac mutant phenotype (Fig. 5.4).
Studies in diverse insects have largely supported the conservation of Dll , dac , and hth in establishing the pattern of proximal-to-distal domains in the leg. While small differences in the precise limits of expression and in timing exist (reviewed by Jockusch and Smith 2015), the homology of this network within leg development seems certain. In Drosophila , the interactions that define expression boundaries between the proximal-to-distal domain genes have been examined through elegant clonal analysis studies. Using methods for timed mosaic generation of cells with deletion alleles (Xu and Rubin 1993; Lee and Luo 1999), it is possible to see how cells lacking, for example, a distal gene change their expression of other genes or interact with neighboring wild-type cells. Using these methods, it has been found that the three principal proximal-distal domain genes, Dll, dac, and hth, interact antagonistically in a way that helps define each area (Dong et al. 2001; Wu and Cohen 1999).
The initial pattern established by Dll , dac, and hth is elaborated as other genes also become expressed in the leg, directing smaller aspects of local identity (reviewed by Angelini et al. 2012b; Jockusch and Smith 2015). The distal segmentation of the tarsus and development of the pretarsal structures are controlled by EGF signaling in D. melanogaster (Campbell 2002; Galindo et al. 2002). This terminal appendage-patterning role for EGF appears to be widely conserved. Knockdown of the EGF ligand during metamorphosis also eliminated the tarsus and tibial spurs in the legs of T. castaneum (Grossmann and Prpic 2012; Angelini et al. 2012b). Similarly, RNAi targeting the EGF receptor prevented regeneration of the distal tarsus and pretarsus in the legs of G. bimaculatus (Nakamura et al. 2008 ). Another well-conserved aspect of later appendage development is the requirement for Notch signaling in joint formation. In D. melanogaster, the Notch ligands Delta and Serrate are expressed adjacent to the locations of joint formation (de Celis et al. 1998; Bishop et al. 1999; Rauskolb and Irvine 1999; Tajiri et al. 2011), and the terminal EGF signal helps determine the position of joints in the leg by regulating the expression Notch pathway genes (Galindo et al. 2005). The role of Notch signaling in joint formation has been confirmed by RNAi in the insects G. bimaculatus (Mito et al. 2011) and T. castaneum (Angelini et al. 2012b). The spider Cupiennius salei also requires Notch signaling activity for leg growth and joint formation, leading to the suggestion that this function is an ancestral and defining feature of all euarthropods (Prpic and Damen 2009).
5.8 Developmental Genetic Patterning of Mandibulate Mouthparts
The developmental patterning of mouthparts is similar in many ways to the theme represented by legs. Unique morphologies are reflected by variations in the developmental system. Mandibulate mouthparts are the ancestral state for insects (Snodgrass 1935), but they also bear the closest resemblance to the theme established by leg development (Angelini et al. 2012a). The development of mandibulate mouthparts has been investigated through functional genetic tests in hemimetabolous and holometabolous species, including the primitively wingless insect Thermobia domestica (Schaeper et al. 2013), the cricket G. bimaculatus (Ronco et al. 2008), the beetles Onthophagus taurus (Simonnet and Moczek 2011) and Tribolium castaneum (Angelini et al. 2012a), and the stag beetle Cyclommatus metallifer (Gotoh et al. 2017).
5.8.1 The Mandible
The mandible is the most anterior gnathal appendage, and it is unique in many ways. The insect mandible is unjointed, consisting of a single heavily muscled segment. The relative simplicity of its anatomy and its resemblance to the proximal-most segments of other appendages gave rise to the suggestion that the insect mandible is homologous only to other proximal appendage segments (Snodgrass 1935; Kukalová-Peck 1998). However it has also been suggested that the mandible evolved by reduction and elimination of joints, essentially retaining homology with the full proximal-to-distal extent of other appendages (Manton 1964). The gnathobasic hypothesis has been supported by developmental genetic studies of the distal appendage gene Dll , which is not expressed in the mandibles in insects (Panganiban et al. 1994; Scholtz et al. 1998; Popadić et al. 1998), and its suppression by RNAi does not affect mandible development (Niimi et al. 2005; Moczek and Rose 2009; Beermann et al. 2001; Angelini et al. 2012a; Gotoh et al. 2017; Yoshiyama et al. 2013). In T. castaneum, the Hox gene Dfd activates expression of cnc in the mandibular body segment, which inhibits expression of Dll (Coulcher and Telford 2012).
However, studies of different beetle species have revealed diverse roles for other genes in shaping the mandible. A functional study of 13 candidate appendage-patterning genes in the tenebrionid T. castaneum identified a role for EGF signaling in the mandible (Fig. 5.4; Angelini et al. 2012a). EGF RNAi significantly reduced mandible length, reducing the medial-distal incisor area in the flour beetle. This finding was unexpected, since EGF is required for formation of distal leg structures in diverse insects, including G. bimaculatus (Nakamura et al. 2008), T. castaneum (Grossmann and Prpic 2012; Angelini et al. 2012b), and D. melanogaster (Campbell 2002; Galindo et al. 2002). RNA interference targeting other appendage-patterning genes, including dac and hth , did not produce defects in the mandible of T. castaneum. In contrast, studies in scarabaeoid species O. taurus (Simonnet and Moczek 2011) and C. metallifer (Gotoh et al. 2017) found that RNAi suppression of dac caused reduction of mandibular teeth or incisors. Male C. metallifer have enlarged mandibles, and dac RNAi also significantly reduced their growth. Both studies also identified unique aspects of mandible patterning in these species. Depletion of hth modified a ridge between the molar and incisor regions in O. taurus (Simonnet and Moczek 2011) and eliminated the development of the medial mandibular teeth in C. metallifer (Gotoh et al. 2017). Other genes have not yet been examined in O. taurus, but RNAi targeting the distal leg gene aristaless also eliminated the mandibular teeth in C. metallifer (Gotoh et al. 2017). In contrast to its prominent role in the mandible of T. castaneum, EGF RNAi in C. metallifer did not cause noticeable defects.
Fully evaluating the gnathobasic hypothesis will require additional functional studies of mandibulate insects, especially among early-branching insect lineages. One possibility is that, while the ancestral state for insects may be gnathobasic, the existing interactions among appendage-patterning genes, necessary for the development of other appendage types, may have facilitated the evolutionary co-option of these genes into mandible development for roles in patterning novel structures, such as mandibular teeth.
5.8.2 The Maxilla and Labium
Patterning of the maxillae and labium is similar, reflecting similarities in their morphology. Their development also requires the same proximal-distal patterning genes known from leg development (Fig. 5.4). Exactly how these and other developmental regulatory genes direct appendage-specific anatomy is not completely clear. However, a striking difference is that the extent of overlap in gene expression is much greater for Dll , dac , and hth in the maxilla and labium than it is in the legs (reviewed by Angelini and Kaufman 2005; Jockusch and Smith 2015). It is likely that the combination of these transcription factors, along with regulatory proteins unique to these body segments, such as specific Hox protein combinations, directs specific target genes that ultimately leads to morphogenesis of these appendage types.
Distal-less is expressed in the palps and endites of both appendage types across diverse mandibulate insects (reviewed by Angelini and Kaufman 2005; Jockusch and Smith 2015). These structures are also eliminated by Dll mutation or RNAi in T. castaneum (Beermann et al. 2001; Angelini et al. 2012a). However, some variations in the requirement of Dll may exist among species, since metamorphic-stage Dll RNAi in the dung beetle O. taurus does not affect development of the maxillary endites (Simonnet and Moczek 2011), although other appendages have phenotypes resembling similar experiments in T. castaneum (Angelini et al. 2012a). Knockdown of Dll by RNAi in the sawfly Athalia rosae (Yoshiyama et al. 2013) and in the firebrat Thermobia domestica (Ohde et al. 2009) also reduced the maxillary and labial palps, causing fusion of palp segments. These studies did not report potential effects of Dll RNAi on the medial endites.
Expression of the intermediate domain gene, dachshund , occurs in the second maxillary and labial appendage segments (the stipes and prementum ), in the maxillary and labial endites, and in a proximal to intermediate region of the palps (reviewed by Angelini and Kaufman 2005; Jockusch and Smith 2015). Tests for the functional requirement of dac in the mandibulate maxilla and labium have only been reported from the beetles T. castaneum (Angelini et al. 2012a) and O. taurus (Simonnet and Moczek 2011). In both species, dac RNAi reduces the length of and number of joints within the maxillary palps. However, in the labium, the two species have different dac RNAi phenotypes. In O. taurus, dac RNAi causes reduction of the prementum, while in T. castaneum the dac RNAi phenotype is similar in the maxilla and labium, with reductions in the length and joint number in the palps. Wider phylogenetic sampling is needed, but the serial homology among gnathal appendages suggests that a dac function in the palps may be ancestral.
Proximal appendage genes, hth and exd , are expressed across a much larger area of the maxilla and labium than they are in the legs (reviewed by Angelini and Kaufman 2005; Jockusch and Smith 2015). This creates a large degree of overlap between genes known for proximal and distal specification in the leg. While interactions among these genes have not been tested in the maxilla or labium of mandibulate insects, we predict that they should not exhibit the same antagonism seen during D. melanogaster leg development (e.g., Wu and Cohen 1999; Dong et al. 2001). RNA interference targeting hth in T. castaneum causes effects across the maxilla and labium (Angelini et al. 2012a). Shape changes occur in the proximal segments that may represent transformation toward more distal identities. Endites are present but reduced. Only the distal segments of the palps are unaffected. Depletion of hth in O. taurus produced similar phenotypes although the endites and palps of the maxilla appeared normal (Simonnet and Moczek 2011). The function of hth has also been tested in G. bimaculatus ; however, its depletion by RNAi caused the transformation of mouthparts toward a mixed antenna-leg identity (Ronco et al. 2008). As discussed above, Hth also functions as a cofactor for Hox protein function.
Other aspects of gnathal appendage development follow the theme set by the legs (Fig. 5.4). Joints express and require locally restricted components of the Notch signaling pathway (Mito et al. 2011; Angelini et al. 2012a), and terminal patterning genes, such as aristaless, are required for development of the endites and the distal tip of the palps (Miyawaki et al. 2002; Angelini et al. 2012a).
5.9 Patterning Variations in Derived Mouthpart Morphologies
A number of successful insect lineages have evolved variations on the mandibulate theme. Two representatives of such taxa have been studied at the developmental genetic level: the fruit fly Drosophila melanogaster and the milkweed bug Oncopeltus fasciatus.
5.9.1 The Labellate Proboscis of Drosophila
Muscomorpha have evolved an adult proboscis used to lap up liquid or semiliquid foods. In Drosophila , the proboscis is derived mostly from the labium , although labial palps or endites are absent. Maxillae are present on the lateral sides of the proboscis. A small maxillary base branches into a lacinia and maxillary palp. Adult mandibular structures are reduced and incorporated into the head capsule.
Signals that establish the proximal-distal axis of developing adult mouthparts are expressed in the late third instar larva, later than in the legs. Wnt and Dpp signaling is required for development of the maxillary field within the eye-antennal imaginal disc and for development of the labial imaginal discs (Joulia et al. 2005; Yasunaga et al. 2006; Doumpas et al. 2013), as they are in leg development. However, the timing of these signals is critical for the identity and patterning of both structures. If Wnt expression is activated early, the maxillary field develops as an ectopic antenna (Lebreton et al. 2008). In the labial disc, the Hox protein Pb represses hh , which results in reduced expression of wg and dpp (Joulia et al. 2005). In the absence of this repression, the labial disc develops as pair of ectopic legs.
Distal-less expression is activated in the developing adult maxillary field and labial imaginal disc by Wg and Dpp (Joulia et al. 2005; Yasunaga et al. 2006), although its expression is less intense than in the leg imaginal disc. Dll is expressed across the distal third of the labial disc (Fig. 5.4; Abzhanov et al. 2001; Joulia et al. 2005; Yasunaga et al. 2006). Mosaic mutant cells lacking Dll in the maxillary field fail to form maxillary palps, although the proximal base and lacinia remain (Cohen and Jürgens, 1989b). Distal structures of the labium are eliminated by loss of Dll mosaic clones in the labial disc (Cohen and Jürgens, 1989a; Yasunaga et al. 2006). Levels of Dll expression are also controlled by negative regulation from Scr (Abzhanov et al. 2001).
There appears to be no role for dac in the development of Drosophila mouthparts. The presence of Pb causes suppression of dac, and the maxillary field and labial disc in D. melanogaster show no expression of Dac (Abzhanov et al. 2001; Joulia et al. 2005). Abnormal phenotypes in the mouthparts have not been reported for dac loss of function.
The proximal leg patterning gene hth is expressed across the entire lateral layer of the labial imaginal disc , where it overlaps with Dll, but it is limited to the proximal two thirds as the medial layer (Yasunaga et al. 2006). Expression of hth occurs throughout the maxillary field (Pai et al. 1998). Exd, the cofactor required for Hth function, has very little expression in the maxillary field (Abzhanov et al. 2001). Nevertheless, loss of hth from the eye-antennal disc eliminates the maxillary palps (Stultz et al. 2012). The interactions among Dll and hth differ in the medial and lateral layers of the labial disc (Yasunaga et al. 2006). Dll represses hth only in the lateral layer, while the two genes are co-expressed medially. Since Dll and hth also are co-expressed in the antenna (reviewed by Angelini and Kaufman 2005; Jockusch and Smith 2015), the labium has been suggested as developing through an intermediate developmental program, rather than a completely unique one (Yasunaga et al. 2006).
5.9.2 The Rostrum of Oncopeltus
The mouthparts of Hemiptera are modified into a rostrum used for feeding by piercing and sucking liquid foods (Meek 1903; Snodgrass 1921). Different species have applied this strategy to predation and to phytophagy. One of the major differences in hemipteran mouthparts is that the difference in similarities among the gnathal appendages. While in mandibulate mouthparts the maxilla resembles the labium , in Hemiptera, it more closely resembles the mandibular appendages. However, both the mandibular and maxillary appendages of Hemiptera are modified into long, slender stylets . These interlock along their length, forming multiple channels for secretion of saliva and the uptake of liquefied food. The labial appendages are fused medially. The stylets are held in a midventral groove, and the rostrum is manipulated by the insect by means of joints between four labial segments.
The developmental genetic patterning of the hemipteran rostrum has been studied in the milkweed bug Oncopeltus fasciatus (Angelini and Kaufman 2004, 2005). The anatomical similarity in mandibular and maxillary appendages in O. fasciatus correlates with similar expression patterns in the gnathal Hox genes . In most insects, including Drosophila and Tribolium , the mandibular and maxillary body segments express the Hox gene Dfd, while pb is expressed in the maxillary and labial segments. The labial segment is also distinguished by expression of Scr (reviewed by Hughes and Kaufman 2002a ). However, in O. fasciatus, the expression of pb is limited to the labial appendages (Rogers and Kaufman 1997; Angelini et al. 2005). As a result, the mandibular and maxillary segments develop with only Dfd for Hox regulation. RNA interference targeting the gnathal Hox genes produces transformations of the appendages in segments where these genes are normally expressed (Hughes and Kaufman 2000).
Proximal-distal domain genes also demonstrate the similarity of mandibular and maxillary development in Hemiptera (Angelini and Kaufman 2004). In embryos of O. fasciatus, the mandibular and maxillary limb buds both express dac and hth throughout their length. Both of these genes are also required for proper development of the stylets . Knockdown of dac by RNAi causes failure of the embryonic appendages to differentiate into stylets. Following hth RNAi, only the distal tips of the stylets differentiate, but the proximal majority of the appendage fails to and does not invaginate and coil into the head as normal (Dorn and Hoffmann 1983; Newcomer 1948). RNAi targeting Dll has no effect on stylet development in O. fasciatus, although the antennae , labium, and legs are all truncated (Angelini and Kaufman 2004). Juvenile-stage Dll RNAi also affects development of the male and female genitalia in O. fasciatus (Aspiras et al. 2011). The absence of a functional requirement for Dll in the hemipteran mandibular and maxillary and stylets is similar to what has been found in the development of mandibles in other insects. Unexpectedly, Dll mRNA and protein are expressed strongly in the maxillary limb buds of O. fasciatus embryos, although not in the mandibular limb buds (Rogers et al. 2002; Angelini and Kaufman 2004). This suggests two implications: First, a mechanism must exist to inhibit the function of the Dll transcription factor specifically in the maxillary body segment. Second, activation of Dll in the maxillary appendages is likely independent of Hox regulation, since both the mandibular and maxillary segments share the same Hox protein milieu. One possibility is activation of Dll by the gap gene hunchback (hb), which is expressed throughout the future head region in blastoderm-stage embryos, but hb expression is markedly more intense in the maxillary and labial body segments (Liu and Kaufman 2004).
The homology of hemipteran mouthpart structures to those of mandibulates has been uncertain (Meek 1903; Snodgrass 1921). Anatomists have proposed homology of the maxillary stylets to several components of the mandibulate maxilla, including the stipes (Cobben 1979), palpigers (Muir and Kershaw 1911a), lacinia (Crampton 1923; Hamilton 1981; Muir and Kershaw 1911b, 1912; Newcomer 1948; Snodgrass 1938, 1944), and to the entire maxilla (Bourgoin 1986; Parsons 1964, 1974). Perhaps this is asking the wrong question? The shift in pb expression and the functional similarities in Dll , dac , and hth in the mandibular and maxillary appendages suggests that, rather than modification of maxillary structures, the hemipteran maxillary stylets may have evolved by redeploying the mandibular developmental program within the maxillary appendages. Viewed in this way, both hemipteran stylets are homologous to the ancestral insect mandible. Moreover, the prominent functions of dac and hth in O. fasciatus stylet development fit well with developmental and anatomical evidence suggesting that the mandible is a proximal, gnathobasic structure.
5.10 Differences in Embryonic and Postembryonic Appendage Patterning
Appendages do not reach their final state in an individual insect until adulthood. Wings and genitalia are extreme in this regard, since they are not fully functional until adulthood. The subimago of Ephemeroptera is an exception, having functional wings in the last preadult stage (Edmunds and McCafferty 1988). However, other appendages, such as the antennae , mouthparts, and legs, appear in the juveniles of most insect groups and undergo subsequent development and repatterning during nymphal or pupal molts. Juvenile legs typically lack joints in distal structures, such as the tarsus or tibiotarsus. Experiments in beetle Tenebrio molitor using amputation (Huet and Lenoir-Rousseaux 1976) suggest that the entire larval leg contributes to the adult leg with cells maintaining their approximate relative position within the limb.
Once structures are formed during development, it is unclear to what extent their identity is irreversibly determined or whether they require continuous expression of genes to maintain their identity. Such a requirement may differ by species or between hemi- and holometabolous insects. The dramatic delay in appendage development in Drosophila has meant that the fruit fly has not provided its usual insights into development regarding differences in embryonic and postembryonic appendage patterning. Instead, other model species have provided these comparisons.
In the hemimetabolous hemipteran O. fasciatus, all juvenile stages have legs that closely resemble the adult in gross anatomy but have two tarsomeres to each leg. At the imaginal molt, the distal tarsomere is divided by a new joint, producing three tarsomeres in total. While the leg distal of the trochanter is lost if Dll is suppressed by RNAi during embryogenesis (Angelini and Kaufman 2004), only the distal tarsal joints of the adult are affected by Dll RNAi during the last juvenile instar (Aspiras et al. 2011). In contrast, the holometabolous species T. castaneum requires Dll activity continuously to maintain the growth and identity of leg structures. Tribolium adult legs have four to five tarsomeres, but larvae have a fused tibiotarsus. Dll mutations in T. castaneum affect larval and adult legs distal of the trochanter, causing reduced growth and an absence of distal identity affecting the femur, tibia, tarsus, and pretarsus (Beermann et al. 2001). When Dll is targeted by RNAi during the pupal stage , even structures such as the femur, which were properly formed in the same individuals as larvae, can be affected by the loss of Dll activity (Angelini et al. 2012b). Examination of in situ gene expression in embryonic, larval, and pupal legs of Manduca sexta (Lepidoptera), another holometabolous species with robust larval legs, has found a continuity of expression of hth , dac, and Dll at their respective proximal to distal levels in the leg as individuals undergo metamorphosis (Tanaka and Truman 2007).
Similarly, in the mouthparts of T. castaneum, gene activity is required to maintain the identity of specific regions during metamorphic development. Pupal-stage RNAi targeting hth, dac, or Dll produces defects in structures that were present in the larval maxilla and labium (Angelini et al. 2012a). While the requirement for these genes is limited to the tarsus during adult development of the legs in O. fasciatus, the mandibular and maxillary stylets continue to require dac activity for proper development during the imaginal molt (Aspiras et al. 2011), similar to its role during embryonic development (Angelini and Kaufman 2004). While the gross anatomy of the adult labium (rostrum ) of O. fasciatus is not obviously altered by juvenile RNAi targeting proximal-distal domain genes, the length of the labium is reduced by Dll RNAi at this stage (Angelini and Kaufman 2005; Aspiras et al. 2011).
5.11 The Future of Research on Insect Appendage Development
Developmental genetics is still far from a detailed understanding of how genetic networks sculpt anatomy. However, we are beginning to appreciate how character identity networks initiate the development of specific structures. Mutant screens and functional analyses such as the production of mosaic discs have provided deep insights into appendage development in D. melanogaster. In contrast, studies of other species have relied heavily on a candidate-based approach, in which orthologs of genes from D. melanogaster developmental models are preferentially tested for roles in other species, highlighting instances of conservation or difference. While this path has been fruitful, it leads to a perspective of diversity that is likely biased toward conserved features of development. We often view other insects in terms of how they are “not like fruit flies” rather than how they uniquely generate their own morphologies.
Given the disparity of insect mouthparts, much remains to be learned about how genes direct this diversity of forms. Many model species are amendable to developmental and molecular genetic studies in ecologically and economically important insect groups with unique mouthparts. First among these are the Lepidoptera. A genetic model for the development of the galeate proboscis of moths or butterflies would provide important insights into this key innovation of the Lepidoptera.
Thankfully, the increasing accessibility of genomics and functional genetic manipulations is beginning to change the current situation. For example, a recent study of water striders used transcriptome comparisons among different legs to identify novel genes associated with the evolution and development of a unique, taxon-specific fan structure at the distal end of the midleg (Santos et al. 2017). RNA interference enabled tests of the gene’s requirement in fan development, as well as the fan’s function in the insect’s locomotion. Similar applications of genomic methods should enable more sophisticated approaches that are not constrained by the assumptions of conservation with traditional genetic model species.
5.12 Returning to the Theme of Homology
Focusing on the genes responsible for development of traits underscores both special homology (inheritance from a common ancestor) and serial homology (deployment at multiple locations across the body plan). Importantly, this concept can also contextualize anatomical themes and variation seen across organisms. As we have recounted, some aspects of the developmental network may be conserved among serially homologous structures, such as the specification of appendage identity by Hox genes and the requirement of Dll for development of distal appendage structures, while other aspects, such as local interactions among patterning genes, may vary.
Importantly, serial homologs are not evolutionarily independent (Wagner 2007; Angelini et al. 2012b; Jockusch and Smith 2015). Serial homologs share developmental mechanisms and the genes that comprise their components. In this way, they have a shared evolutionary history, by virtual of their common genes, and a shared developmental history, via redeployment of those genes in different locations. Nevertheless, serial homologs can experience different selection pressures and may therefore evolve independently over time. Mutations in different lineages may affect development in ways that are general or specific with regard to serial homologs (Fig. 5.5). We will term evolutionary change causing uniform, similar changes in all serial homologs homotypic change , while a change affecting a subset of serial homologs may be known as a heterotypic change.
Serial homologs may evolve in concert, via homotypic change , or independently, via heterotypic change. These differing types of evolution are depicted here for mouthparts of a generic beetle-like insect. (a) In homotypic change, all serial homologs, such as the appendages, are affected pleiotropically and exhibit similar changes compared to the ancestral state. In this example, all appendages become enlarged and green. (b) In contrast, heterotypic change is limited to one serial homolog. In this example, the maxilla increases in size and changes color to blue. We predict mutations causing heterotypic change to be qualitatively different, such that their effects are limited in scope. The most likely mechanism for this specificity is change in a gene’s regulatory elements, controlling expression in a given region
With some understanding of the developmental system, we might begin to ask, what kinds of mutations are likely to result in homotypic or heterotypic change? An intuitive hypothesis might focus on the distinction between “core” genes, which function early and upstream in the developmental network in all serial homologs, and homolog-specific genes function later and downstream to effect unique morphology (Davidson 2006). This model would predict homotypic changes would result from mutations in the “core” genes and heterotypic changes would result from mutations in the more downstream, homolog-specific genes. However, our understanding of insect appendage development does not support this hypothesis (Fig. 5.4).
From what is known of appendage development, serial homologs differ from one another in their development at all levels. A comparison of D. melanogaster labial and leg imaginal discs helps illustrate this point. In both appendages, specification of the proximal-distal axis is accomplished by Wg and Dpp gradients, but this is indirectly modulated by pb activity in the labial disc in a way that is essential for labial development (Joulia et al. 2005; Yasunaga et al. 2006). Downstream of axis specification, the proximal-distal domain genes Dll and hth are co-expressed in the medial labial disc, but not laterally, as in the leg (Yasunaga et al. 2006). Similarly, Dll, dac , and hth are all expressed in antennae , mouthparts, and legs of diverse insects, but their areas of overlap differ (Fig. 5.4).
Rather than fixing mutations at different levels of the regulatory hierarchy, heterotypic evolution of serial homologs appears to have proceeded through changes in the regulatory interactions among genes that are part of a common theme in appendage development. Therefore, we predict that the key genetic differences underlying the unique morphologies of serial homologs, as well as the sites of mutation affecting their evolution, will be found in the regulatory elements of genes required for their formation (Rebeiz and Tsiantis 2017). If it is correct that morphological evolution among species proceeds more often via regulatory changes (Stern and Orgogozo 2008; Stern 2010 ), then similarities between serial homologs are likely to be retained to some extent by pleiotropy .
Additional developmental genetic studies are necessary to fully test this hypothesis. While technically demanding, functional tests of regulatory elements will be needed in a wider diversity of insect models. Genome editing technologies are beginning to make this possible. Ultimately, our goal is to read the musical genetic notation and understand how variations in morphological diversity emerge from the theme.
References
Abu-Shaar M, Mann RS (1998) Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125:3821–3830
Abu-Shaar M, Ryoo HD, Mann RS (1999) Control of the nuclear localization of extradenticle by competing nuclear import and export signals. Genes Dev 13:935–945
Abzhanov A, Kaufman TC (1999) Homeotic genes and the arthropod head: expression patterns of the labial, proboscipedia, and Deformed genes in crustaceans and insects. Proc Natl Acad Sci U S A 96:10224–10229. https://doi.org/10.1073/pnas.96.18.10224
Abzhanov A, Kaufman TC (2000) Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol 227:673–689. https://doi.org/10.1006/dbio.2000.9904
Abzhanov A, Holtzman S, Kaufman TC (2001) The Drosophila proboscis is specified by two Hox genes, proboscipedia and Sex combs reduced, via repression of leg and antennal appendage genes. Development 128:2803–2814. https://doi.org/10.1016/S0925-4773(96)00649-1
Ackermann C, Dorresteijn A, Fischer A (2005) Clonal domains in postlarval Platynereis dumerilii (Annelida: Polychaeta). J Morphol 266:258–280
Adolfi A, Lycett GJ (2018) Opening the toolkit for genetic analysis and control of Anopheles mosquito vectors. Curr Opin Insect Sci 30:8–18. https://doi.org/10.1016/j.cois.2018.07.014
Aguinaldo AM, Turbeville JM, Linford LS et al (1997) Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387:489–493. https://doi.org/10.1038/387489a0
Ando T, Fujiwara H, Kojima T (2018) The pivotal role of aristaless in development and evolution of diverse antennal morphologies in moths and butterflies. BMC Evol Biol 18:8. https://doi.org/10.1186/s12862-018-1124-2
Angelini DR, Kaufman TC (2004) Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol:271, 306–321. https://doi.org/10.1016/j.ydbio.2004.04.005
Angelini DR, Kaufman TC (2005) Insect appendages and comparative ontogenetics. Dev Biol 286:57–77. https://doi.org/10.1016/j.ydbio.2005.07.006
Angelini DR, Liu PZ, Hughes CL, Kaufman TC (2005) Hox gene function and interaction in the milkweed bug Oncopeltus fasciatus (Hemiptera). Dev Biol 287:440–455. https://doi.org/10.1016/j.ydbio.2005.08.010
Angelini DR, Kikuchi M, Jockusch EL (2009) Genetic patterning in the adult capitate antenna of the beetle Tribolium castaneum. Dev Biol 327:240–251. https://doi.org/10.1016/j.ydbio.2008.10.047
Angelini DR, Smith FW, Aspiras AC et al (2012a) Patterning of the adult mandibulate mouthparts in the red flour beetle, Tribolium castaneum. Genetics 190:639–654. https://doi.org/10.1534/genetics.111.134296
Angelini DR, Smith FW, Jockusch EL (2012b) Extent with modification: leg patterning in the beetle Tribolium castaneum and the evolution of serial homologs. G3 Genes Genomes Genet 2:235–248. https://doi.org/10.1534/g3.111.001537
Aspiras AC, Smith FW, Angelini DR (2011) Sex-specific gene interactions in the patterning of insect genitalia. Dev Biol 360:369–380. https://doi.org/10.1016/j.ydbio.2011.09.026
Basler K, Struhl G (1994) Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368:208–214. https://doi.org/10.1038/368208a0
Beeman RW, Stuart JJ, Haas MS, Denell RE (1989) Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev Biol 133:196–209
Beeman RW, Stuart JJ, Brown SJ, Denell RE (1993) Structure and function of the homeotic gene complex (HOM-C) in the beetle, Tribolium castaneum. BioEssays 15:439–444
Beermann A, Jay DG, Beeman RW et al (2001) The short antennae gene of Tribolium is required for limb development and encodes the orthologue of the Drosophila Distal-less protein. Development 128:287–297
Bishop SA, Klein T, Arias AM, Couso JP (1999) Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development 126:2993–3003
Bitsch J, Bitsch C (2010) The tritocerebrum and the clypeolabrum in mandibulate arthropods: segmental interpretations. Acta Zool 91:249–266. https://doi.org/10.1111/j.1463-6395.2009.00402.x
Bolognesi R, Farzana L, Fischer TD, Brown SJ (2008) Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol 18:1624–1629
Bourgoin T (1986) Valeur morphologique de la lame maxillaire chez les Hemiptera; remarques phylogénétiques. Ann La Soc Entomol France 22:413–422
Boyan GS, Williams JLD, Posser S, Bräunig P (2002) Morphological and molecular data argue for the labrum being non-apical, articulated, and the appendage of the intercalary segment in the locust. Arthropod Struct Dev 31:65–76
Boyden A (1943) Homology and analogy: a century after the definitions of “homologue” and “analogue” of Richard Owen. Q Rev Biol 18:228–241
Boyden A (1947) Homology and analogy. A critical review of the meanings and implications of these concepts in biology. Am Midl Nat 37:648–669. https://doi.org/10.2307/2421470
Brown SJ, Mahaffey JP, Lorenzen MD, Denell RE, Mahaffey JW (1999a) Using RNAi to investigate orthologous homeotic gene function during development of distantly related insects. Evol Dev 1:11–15. https://doi.org/10.1046/j.1525-142x.1999.99013.x
Brown S, Holtzman S, Kaufman T, Denell R (1999b) Characterization of the Tribolium Deformed ortholog and its ability to directly regulate Deformed target genes in the rescue of a Drosophila Deformed null mutant. Dev Genes Evol 209:389–398. https://doi.org/10.1007/s004270050269
Brown S, DeCamillis M, Gonzalez-Charneco K et al (2000) Implications of the Tribolium Deformed mutant phenotype for the evolution of Hox gene function. Proc Natl Acad Sci U S A 97:4510–4514. https://doi.org/10.1073/pnas.97.9.4510
Brown SJ, Shippy TD, Beeman RW, Denell RE (2002) Tribolium Hox genes repress antennal development in the gnathos and trunk. Mol Phylogenet Evol 24:384–387. https://doi.org/10.1016/S1055-7903(02)00205-1
Brusca RC, Brusca GJ (2003) Invertebrates, 2nd edn. Sinauer Associates, Inc, Sunderland, MA
Budd GE (2002) A palaeontological solution to the arthropod head problem. Nature 417:271–275. https://doi.org/10.1038/417271a
Budd GE, Telford MJ (2009) The origin and evolution of arthropods. Nature 457:812–817. https://doi.org/10.1038/nature07890
Butt FH (1949) Embryology of the milkweed bug, Oncopeltus fasciatus (Hemiptera). Mem Cornell Univ Agric Exp Stn 283:1–43
Butt FH (1960) Head development in the arthropods. Biol Rev 35:43–91
Campbell G (2002) Distalization of the Drosophila leg by graded EGF-receptor activity. Nature 418:781–785. https://doi.org/10.1038/nature00971
Casares F, Mann RS (1998) Control of antennal versus leg development in Drosophila. Nature 392:723–726. https://doi.org/10.1038/33706
Casares F, Mann RS (2001) The ground state of the ventral appendage in Drosophila. Science 293:1477–1480. https://doi.org/10.1126/science.1062542
Chan S-K, Pöpperl H, Krumlauf R, Mann RS (1996) An Extradenticle-induced conformational change in a HOX protein overcomes an inhibitory function of the conserved hexapeptide motif. EMBO J 15:2476–2487
Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, Cleary ML (1995) Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev 9:663–674
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Chipman AD (2010) Parallel evolution of segmentation by co-option of ancestral gene regulatory networks. BioEssays 32:60–70
Chipman AD (2017) Oncopeltus fasciatus as an evo-devo research organism. Genesis:e23020. https://doi.org/10.1002/dvg.23020
Cobben RH (1979) On the original feeding habits of the Hemiptera (Insecta): a reply to Merrill Sweet. Ann Entomol Soc Am 72:711–715
Cohen SM, Jürgens G (1989a) Proximal-distal pattern formation in Drosophila: graded requirement for Distal-less gene activity during limb development. Rouxs Arch Dev Biol 198:157–169. https://doi.org/10.1007/BF02438941
Cohen SM, Jürgens G (1989b) Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J 8:2045–2055
Cong P, Ma X, Hou X, Edgecombe GD, Strausfeld NJ (2014) Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513:538–542
Coulcher JF, Telford MJ (2012) Cap‘n’collar differentiates the mandible from the maxilla in the beetle Tribolium castaneum. EvoDevo 3:25. https://doi.org/10.1186/2041-9139-3-25
Coulcher JF, Telford MJ (2013) Comparative gene expression supports the origin of the incisor and molar process from a single endite in the mandible of the red flour beetle Tribolium castaneum. EvoDevo 4:1. https://doi.org/10.1186/2041-9139-4-1
Crampton G (1923) A phylogenetic comparison of the maxillae throughout the orders of insects. J New York Entomol Soc 31:77–107
Curtis CD, Brisson JA, DeCamillis MA, Shippy TD, Brown SJ, Denell RE (2001) Molecular characterization of Cephalothorax, the Tribolium ortholog of Sex combs reduced. Genes J Genet Dev 30:12–20
Dambly-Chaudière C, Ghysen A (1986) The sense organs in the Drosophila larva and their relation to the embryonic pattern of sensory neurons. Rouxs Arch Dev Biol 195:222–228. https://doi.org/10.1007/BF02438954
Damen WGM (2002) Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development 129:1239–1250
Damen WG, Hausdorf M, Seyfarth EA, Tautz D (1998) A conserved mode of head segmentation in arthropods revealed by the expression pattern of Hox genes in a spider. Proc Natl Acad Sci U S A 95:10665–10670. https://doi.org/10.1073/pnas.95.18.10665
Davidson EH (2006) The regulatory genome: gene regulatory networks in development and evolution. Academic, Cambridge, MA
Davis DR (1967) A revision of the moths of the subfamily prodoxinae (Lepidoptera: Incurvariidae). Bull U S Natl Mus 255:1–170. https://doi.org/10.5479/si.03629236.255.1
de Celis JF, Tyler DM, de Celis J, Bray SJ (1998) Notch signalling mediates segmentation of the Drosophila leg. Development 125:4617–4626
DeCamillis MA, Lewis DL, Brown SJ, Beeman RW, Denell RE (2001) Interactions of the Tribolium Sex combs reduced and proboscipedia orthologs in embryonic labial development. Genetics 159:1643–1648
Diaz-Benjumea FJ, Cohen B, Cohen SM (1994) Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature 372:175–179. https://doi.org/10.1038/372175a0
Dong PD, Chu J, Panganiban G (2001) Proximodistal domain specification and interactions in developing Drosophila appendages. Development 128:2365–2372
Dong PDS, Dicks JS, Panganiban G (2002) Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development 129:1967–1974
Dorn A, Hoffmann P (1983) Segmentation and differentiation of appendages during embryogenesis of the milkweed bug Oncopeltus fasciatus: a scanning electron microscopical study. Zool Jahrbücher 109:277–298
Doumpas N, Jékely G, Teleman AA (2013) Wnt6 is required for maxillary palp formation in Drosophila. BMC Biol 11:104. https://doi.org/10.1186/1741-7007-11-104
Duncan DM, Burgess EA, Duncan I (1998) Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev 12:1290–1303. https://doi.org/10.1101/gad.12.9.1290
Duncan D, Kiefel P, Duncan I (2010) Control of the spineless antennal enhancer: direct repression of antennal target genes by Antennapedia. Dev Biol 347:82–91. https://doi.org/10.1016/j.ydbio.2010.08.012
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sørensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749. https://doi.org/10.1038/nature06614
Eastham LES (1931) The embryology of Pieris rapae: organogeny. Philos Trans Roy Soc B 219:1–50
Economou AD, Telford MJ (2009) Comparative gene expression in the heads of Drosophila melanogaster and Tribolium castaneum and the segmental affinity of the Drosophila hypopharyngeal lobes. Evol Dev 11:88–96
Edmunds GF, McCafferty WP (1988) The mayfly subimago. Annu Rev Entomol 33:509–538
Emmons RB, Duncan D, Duncan I (2007) Regulation of the Drosophila distal antennal determinant spineless. Dev Biol 302:412–426. https://doi.org/10.1016/j.ydbio.2006.09.044
Eriksson BJ, Tait NN, Budd GE, Janssen R, Akam M (2010) Head patterning and Hox gene expression in an onychophoran and its implications for the arthropod head problem. Dev Genes Evol 220:117–122. https://doi.org/10.1007/s00427-010-0329-1
Eriksson BJ, Samadi L, Schmid A (2013) The expression pattern of the genes engrailed, pax6, otd and six3 with special respect to head and eye development in Euperipatoides kanangrensis Reid 1996 (Onychophora: Peripatopsidae). Dev Genes Evol 223:237–246
Estella C, Mann RS (2008) Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg. Development 135:627–636. https://doi.org/10.1242/dev.014670
Estella C, Rieckhof G, Calleja M, Morata G (2003) The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development 130:5929–5941. https://doi.org/10.1242/dev.00832
Estella C, McKay DJ, Mann RS (2008) Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev Cell 14:86–96. https://doi.org/10.1016/j.devcel.2007.11.002
Farzana L, Brown SJ (2008) Hedgehog signaling pathway function conserved in Tribolium segmentation. Dev Genes Evol 218:181–192. https://doi.org/10.1007/s00427-008-0207-2
Galindo MI, Bishop SA, Greig S, Couso JP (2002) Leg patterning driven by proximal-distal interactions and EGFR signaling. Science 297:256–259. https://doi.org/10.1126/science.1072311
Galindo MI, Bishop SA, Couso JP (2005) Dynamic EGFR-Ras signalling in Drosophila leg development. Dev Dyn 233:1496–1508. https://doi.org/10.1002/dvdy.20452
Giorgianni MW, Mann RS (2011) Establishment of medial fates along the proximodistal axis of the Drosophila leg through direct activation of dachshund by distalless. Dev Cell 20:455–468. https://doi.org/10.1016/j.devcel.2011.03.017
Giribet G, Edgecombe GD (2017) Current understanding of Ecdysozoa and its internal phylogenetic relationships. Integr Comp Biol 57:455–466
Goto S, Hayashi S (1997) Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development 124:125–132
Gotoh H, Zinna RA, Ishikawa Y, Miyakawa H, Ishikawa A, Sugime Y, Emlen DJ, Lavine LC, Miura T (2017) The function of appendage patterning genes in mandible development of the sexually dimorphic stag beetle. Dev Biol 422:24–32. https://doi.org/10.1016/j.ydbio.2016.12.011
Gould SJ (1977) The return of hopeful monsters. Nat Hist 86:22–30
Grimaldi D, Engel MS (2005) Evolution of the insects. Cambridge University Press, New York
Grossmann D, Prpic NM (2012) Egfr signaling regulates distal as well as medial fate in the embryonic leg of Tribolium castaneum. Dev Biol 370:264–272. https://doi.org/10.1016/j.ydbio.2012.08.005
Haas MS, Brown SJ, Beeman RW (2001) Pondering the procephalon: the segmental origin of the labrum. Dev Genes Evol 211:89–95
Hamilton FGA (1981) Morphology and evolution of the rhychotan head (Insecta: Hemiptera, Homoptera). Can Ent 113:953–974
Heming BS (1980) Development of the mouthparts in embryos of Haplothrips verbasci (Osborn) (Insecta, Thysanoptera, Phlaeothripidae). J Morphol 164:235–263. https://doi.org/10.1002/jmor.1051640303
Hidalgo A (1991) Interactions between segment polarity genes and the generation of the segmental pattern in Drosophila. Mech Dev 35:77–87
Hinman VF, Nguyen AT, Cameron RA, Davidson EH (2003) Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc Natl Acad Sci U S A 100:13356–13361. https://doi.org/10.1073/pnas.2235868100
Hrycaj S, Chesebro J, Popadic A (2010) Functional analysis of Scr during embryonic and post-embryonic development in the cockroach, Periplaneta americana. Dev Biol 341:324–334. https://doi.org/10.1016/j.ydbio.2010.02.018
Huet C, Lenoir-Rousseaux JJ (1976) Etude de la mise en place de la patte imaginable de Tenebrio molitor. 1. Analyse expérimentale des processus de restauration au cours de la morphogenése. J Embryol Exp Morphol 35:303–321
Hughes CL, Kaufman TC (2000) RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the Hemipteran head. Development 127:3683–3694
Hughes CL, Kaufman TC (2002a) Hox genes and the evolution of the arthropod body plan. Evol Dev 499:459–499
Hughes CL, Kaufman TC (2002b) Exploring the myriapod body plan: expression patterns of the ten Hox genes in a centipede. Development 129:1225–1238. https://doi.org/10.1006/dbio.2002.0683
Hughes CL, Liu PZ, Kaufman TC (2004) Expression patterns of the rogue Hox genes Hox3/zen and fushi tarazu in the apterygote insect Thermobia domestica. Evol Dev 6:393–401. https://doi.org/10.1111/j.1525-142X.2004.04048.x
Hunter WB, Ullman DE (1992) Anatomy and ultrastructure of the piercing-sucking mouthparts and paraglossal sensilla of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Int J Insect Morphol Embryol 21:17–35. https://doi.org/10.1016/0020-7322(92)90003-6
Inbal A, Halachmi N, Dibner C, Frank D, Salzberg A (2001) Genetic evidence for the transcriptional-activating function of Homothorax during adult fly development. Development 128:3405–3413
Ingham PW (1993) Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature 366:560–562. https://doi.org/10.1038/366560a0
Inoue Y, Mito T, Miyawaki K, Matsushima K, Shinmyo Y, Heanue TA, Mardon G, Ohuchi H, Noji S (2002) Correlation of expression patterns of homothorax, dachshund, and Distal-less with the proximodistal segmentation of the cricket leg bud. Mech Dev 113:141–148
Jager M, Murienne J, Clabaut C, Deutsch J, Le Guyader H, Manuel M (2006) Homology of arthropod anterior appendages revealed by Hox gene expression in a sea spider. Nature 441:506–508
Janssen R (2012) Segment polarity gene expression in a myriapod reveals conserved and diverged aspects of early head patterning in arthropods. Dev Genes Evol 222:299–309
Janssen R (2017a) Comparative analysis of gene expression patterns in the arthropod labrum and the onychophoran frontal appendages, and its implications for the arthropod head problem. EvoDevo 8:1
Janssen R (2017b) A molecular view of onychophoran segmentation. Arthropod Struct Dev 46:341–353
Janssen R, Damen W (2006) The ten Hox genes of the millipede Glomeris marginata. Dev Genes Evol 216:451–465
Janssen R, Eriksson BJ, Tait NN, Budd GE (2014) Onychophoran Hox genes and the evolution of arthropod Hox gene expression. Front Zool 11:22
Janssen R, Jörgensen M, Prpic N-M, Budd GE (2015) Aspects of dorso-ventral and proximo-distal limb patterning in onychophorans. Evol Dev 17:21–33. https://doi.org/10.1111/ede.12107
Jiang J, Struhl G (1996) Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell 86:401–409
Jockusch EL (2017) Developmental and evolutionary perspectives on the origin and diversification of arthropod appendages. Integr Comp Biol 57:533–545. https://doi.org/10.1093/icb/icx063
Jockusch EL, Smith FW (2015) Hexapoda: comparative aspects of later embryogenesis and metamorphosis. In: Wanninger A (ed) Evolutionary developmental biology of invertebrates 5. Springer, Vienna, pp 111–208
Jockusch EL, Nulsen C, Newfeld SJ, Nagy LM (2000) Leg development in flies versus grasshoppers: differences in dpp expression do not lead to differences in the expression of downstream components of the leg patterning pathway. Development 127:1617–1626
Johnson FB, Parker E, Krasnow MA (1995) Extradenticle protein is a selective cofactor for the Drosophila homeotics: role of the homeodomain and YPWM amino acid motif in the interaction. Proc Natl Acad Sci U S A 92:739–743
Jones T (1954) The external morphology of Chirothrips hematus (Trybom) (Thysanoptera). Trans R Entomol Soc London 105:163–187
Joulia L, Bourbon H-M, Cribbs DL (2005) Homeotic proboscipedia function modulates hedgehog-mediated organizer activity to pattern adult Drosophila mouthparts. Dev Biol 278:496–510. https://doi.org/10.1016/j.ydbio.2004.11.003
Khila A, Grbić M (2007) Gene silencing in the spider mite Tetranychus urticae: dsRNA and siRNA parental silencing of the Distal-less gene. Dev Genes Evol 217:241–251
Kimm M, Prpic N-M (2006) Formation of the arthropod labrum by fusion of paired and rotated limb-bud-like primordia. Zoomorphology 125:147–155
Krenn HW (2010) Feeding mechanisms of adult Lepidoptera: structure, function, and evolution of the mouthparts. Annu Rev Entomol 55:307–327. https://doi.org/10.1146/annurev-ento-112408-085338
Kukalová-Peck J (1998) Arthropod phylogeny and “basal” morphological structures. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman Hall, London, pp 249–268
Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A (1998) Dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development 125:1037–1048
Lebreton G, Faucher C, Cribbs DL, Benassayag C (2008) Timing of wingless signalling distinguishes maxillary and antennal identities in Drosophila melanogaster. Development 135:2301–2309. https://doi.org/10.1242/dev.017053
Lecuit T, Cohen SM (1997) Proximal-distal axis formation in the Drosophila leg. Nature 388:139–145
Lee T, Luo L (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22:451–461
Lee AK, Sze CC, Kim ER, Suzuki Y (2013) Developmental coupling of larval and adult stages in a complex life cycle: insights from limb regeneration in the flour beetle, Tribolium castaneum. EvoDevo 4:20. https://doi.org/10.1186/2041-9139-4-20
Li Y, Brown SJ, Hausdorf B, Tautz D, Denell RE, Finkelstein R (1996) Two orthodenticle-related genes in the short-germ beetle Tribolium castaneum. Dev Genes Evol 206:35–45
Liu PZ, Kaufman TC (2004) hunchback is required for suppression of abdominal identity, and for proper germband growth and segmentation in the intermediate germband insect Oncopeltus fasciatus. Development 131:1515–1527
Liu J, Popadić A (2017) Early development and diversity of Gryllus appendages. In: Horch HW, Mito T, Popadić A, Ohuchi H, Noji S (eds) The cricket as a model organism. Springer, Tokyo, pp 17–30
Mandaravally Madhavan M, Schneiderman HA (1977) Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during the larval development of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol 183:269–305. https://doi.org/10.1007/BF00848459
Manton SM (1964) Mandibular mechanisms and the evolution of arthropods. Philos Trans R Soc Lond Ser B Biol Sci 247:1–183. https://doi.org/10.1098/rstb.1964.0001
Mardon G, Solomon NM, Rubin GM (1994) dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486
Marshall SA (2006) Insects: their natural history and diversity: with a photographic guide to insects of eastern North America. Firefly Books, Buffalo
Maves L, Schubiger G (2003) Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr Opin Genet Dev 13:472–479
Meek WJ (1903) On the mouth-parts of the Hemiptera. Kansas Univ Sci Bull 2:256–277, Pl. 7–11
Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, Frandsen PB, Ware J, Flouri T, Beutel RG, Niehuis O, Petersen M, Izquierdo-Carrasco F, Wappler T, Rust J, Aberer AJ, Aspöck U, Aspöck H, Bartel D, Blanke A, Berger S, Böhm A, Buckley T, Calcott B, Chen J, Friedrich F, Fukui M, Fujita M, Greve C, Grobe P, Gu S, Huang Y, Jermiin LS, Kawahara AY, Krogmann L, Kubiak M, Lanfear R, Letsch H, Li Y, Li Z, Li J, Lu H, Machida R, Mashimo Y, Kapli P, McKenna DD, Meng G, Nakagaki Y, Navarrete-Heredia JL, Ott M, Ou Y, Pass G, Podsiadlowski L, Pohl H, von Reumont BM, Schütte K, Sekiya K, Shimizu S, Slipinski A, Stamatakis A, Song W, Su X, Szucsich NU, Tan M, Tan X, Tang M, Tang J, Timelthaler G, Tomizuka S, Trautwein M, Tong X, Uchifune T, Walzl MG, Wiegmann BM, Wilbrandt J, Wipfler B, Wong TK, Wu Q, Wu G, Xie Y, Yang S, Yang Q, Yeates DK, Yoshizawa K, Zhang Q, Zhang R, Zhang W, Zhang Y, Zhao J, Zhou C, Zhou L, Ziesmann T, Zou S, Li Y, Xu X, Zhang Y, Yang H, Wang J, Wang J, Kjer KM, Zhou X (2014) Phylogenomics resolves the timing and pattern of insect evolution. Science 346:763–767. https://doi.org/10.1017/CBO9781107415324.004
Mito T, Ronco M, Uda T, Nakamura T, Ohuchi H, Noji S (2008) Divergent and conserved roles of extradenticle in body segmentation and appendage formation, respectively, in the cricket Gryllus bimaculatus. Dev Biol 313:67–79. https://doi.org/10.1016/j.ydbio.2007.09.060
Mito T, Shinmyo Y, Kurita K, Nakamura T, Ohuchi H, Noji S (2011) Ancestral functions of Delta/Notch signaling in the formation of body and leg segments in the cricket Gryllus bimaculatus. Development 138:3823–3833. https://doi.org/10.1242/dev.060681
Mittmann B, Scholtz G (2003) Development of the nervous system in the “head” of Limulus polyphemus (Chelicerata: Xiphosura): morphological evidence for a correspondence between the segments of the chelicerae and of the (first) antennae of Mandibulata. Dev Genes Evol 213:9–17. https://doi.org/10.1007/s00427-002-0285-5
Miyawaki K, Inoue Y, Mito T, Fujimoto T, Matsushima K, Shinmyo Y, Ohuchi H, Noji S (2002) Expression patterns of aristaless in developing appendages of Gryllus bimaculatus (cricket). Mech Dev 113:181–184
Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, Noji S (2004) Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev 121:119–130
Mlodzik M, Fjose A, Gehring WJ (1988) Molecular structure and spatial expression of a homeobox gene from the labial region of the Antennapedia-complex. EMBO J 7:2569–2578
Moczek AP, Rose DJ (2009) Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc Natl Acad Sci U S A 106:8992–8997. https://doi.org/10.1073/pnas.0809668106
Morata G (2001) How Drosophila appendages develop. Nat Rev Mol Cell Biol 2:89–97
Muir F, Kershaw JC (1911a) On the later embryological stages of Pristhesancus papuensis (Reduviidae). Psyche 18:75–79
Muir F, Kershaw JC (1911b) On the homologies and mechanism of the mouth-parts of Hemiptera. Psyche (Stuttg) 18:1–12
Muir F, Kershaw JC (1912) The development of the mouthparts in the Homoptera, with observations on the embryo of Siphanta. Psyche 19:77–89
Nakamura T, Mito T, Miyawaki K, Ohuchi H, Noji S (2008) EGFR signaling is required for re-establishing the proximodistal axis during distal leg regeneration in the cricket Gryllus bimaculatus nymph. Dev Biol 319:46–55. https://doi.org/10.1016/j.ydbio.2008.04.002
Newcomer WS (1948) Embryological development of the mouthparts and related structures of the milkweed bug Oncopeltus fasciatus. J Morphol 82:365–411
Nie W, Stronach B, Panganiban G, Shippy T, Brown SJ, Denell RE (2001) Molecular characterization of Tc-labial and the 3′ end of the Tribolium homeotic complex. Dev Genes Evol 211:244–251
Nielsen C (2001) Animal evolution: interrelationships of the living phyla, 2nd edn. Oxford University Press, London
Niimi T, Kuwayama H, Yaginuma T (2005) Larval RNAi applied to the analysis of postembryonic development in the ladybird beetle, Harmonia axyridis. J Insect Biotechnol Sericology 74:95–102
Niwa N, Inoue Y, Nozawa A, Saito M, Misumi Y, Ohuchi H, Yoshioka H, Noji S (2000) Correlation of diversity of leg morphology in Gryllus bimaculatus (cricket) with divergence in dpp expression pattern during leg development. Development 127:4373–4381
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
O’Donnell BC, Jockusch EL (2010) The expression of wingless and Engrailed in developing embryos of the mayfly Ephoron leukon (Ephemeroptera: Polymitarcyidae). Dev Genes Evol 220:11–24. https://doi.org/10.1007/s00427-010-0324-6
Ober KA, Jockusch EL (2006) The roles of wingless and decapentaplegic in axis and appendage development in the red flour beetle, Tribolium castaneum. Dev Biol 294:391–405
Ohde T, Masumoto M, Yaginuma T, Niimi T (2009) Embryonic RNAi analysis in the firebrat, Thermobia domestica: Distal-less is required to form caudal filament. J Insect Biotechnol Sericology 78:99–105
Ortega-Hernández J, Budd GE (2016) The nature of non-appendicular anterior paired projections in Palaeozoic total-group Euarthropoda. Arthropod Struct Dev 45:185–199. https://doi.org/10.1016/j.asd.2016.01.006
Ortega-Hernández J, Janssen R, Budd GE (2017) Origin and evolution of the panarthropod head – a palaeobiological and developmental perspective. Arthropod Struct Dev 46:354–379
Owen R (1843) Lectures on the comparative anatomy and physiology of the invertebrate animals, delivered at the Royal College of Surgeons in 1843. Longman, Brown, Green, and Longmans, London
Owen R (1848) On the archetype and homologies of the vertebrate skeleton. John Van Voorst, London
Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH (1998) The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev 12:435–446
Panfilio KA, Jentzsch IMV, Benoit JB, Erezyilmaz D, Suzuki Y, Colella S, Robertson HM, Poelchau MF, Waterhouse RM, Ioannidis P, Weirauch MT, Hughes DST, Murali SC, Werren JH, Jacobs CGC, Duncan EJ, Armisén D, Vreede BMI, Baa-Puyoulet P, Berger CS, Chang CC, Chao H, Chen MJM, Chen YT, Childers CP, Chipman AD, Cridge AG, Crumière AJJ, Dearden PK, Didion EM, Dinh H, Doddapaneni H, Dolan A, Dugan-Perez S, Extavour CG, Febvay G, Friedrich M, Ginzburg N, Han Y, Heger P, Holmes CJ, Horn T, Hsiao Y-M, Jennings EC, Johnston JS, Jones TE, Jones JW, Khila A, Koelzer S, Kovacova V, Leask M, Lee SL, Lee C-Y, Lovegrove MR, Lu H-L, Lu Y, Moore PJ, Munoz-Torres MC, Muzny DM, Palli SR, Parisot N, Pick L, Porter M, Qu J, Refki PN, Richter R, Rivera-Pomar R, Rosendale AJ, Roth S, Sachs L, Santos ME, Seibert J, Sghaier E, Shukla JN, Stancliffe RJ, Tidswell O, Traverso L, van der Zee M, Viala S, Worley KC, Zdobnov EM, Gibbs RA, Richards S (2018) Molecular evolutionary trends and feeding ecology diversification in the Hemiptera, anchored by the milkweed bug genome. bioRxiv:201731. https://doi.org/10.1101/201731
Panganiban G, Rubenstein JLR (2002) Developmental functions of the Distal-less/Dlx homeobox genes. Development 129:4371–4386
Panganiban G, Nagy L, Carroll SB (1994) The role of the Distal-less gene in the development and evolution of insect limbs. Curr Biol 4:671–675
Papillon D, Telford MJ (2007) Evolution of Hox3 and ftz in arthropods: insights from the crustacean Daphnia pulex. Dev Genes Evol 217:315–322
Park T-YS, Kihm J-H, Woo J, Park C, Lee WY, Smith MP, Harper DAT, Young F, Nielsen AT, Vinther J (2018) Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat Commun 9:1019
Parsons MC (1964) The origin and development of the hemipteran cranium. Can J Zool 42:409–432
Parsons MC (1974) The morphology and possible origin of the hemipteran loral lobes. Can J Zool 52:189–202
Passalacqua KD, Hrycaj S, Mahfooz N, Popadic A (2010) Evolving expression patterns of the homeotic gene Scr in insects. Int J Dev Biol 54:897–904. https://doi.org/10.1387/ijdb.082839kp
Pellmyr O, Krenn HW (2002) Origin of a complex key innovation in an obligate insect-plant mutualism. Proc Natl Acad Sci U S A 99:5498–5502. https://doi.org/10.1073/pnas.072588699
Peterson MD, Rogers BT, Popadic A, Kaufman TC (1999) The embryonic expression pattern of labial, posterior homeotic complex genes and the teashirt homologue in an apterygote insect. Dev Genes Evol 209:77–90
Pfau T (2010) “All is Leaf”: difference, metamorphosis, and Goethe’s phenomenology of knowledge. Stud Romanticism 49:3–41
Popadić A, Panganiban G, Rusch D, Shear WA, Kaufman TC (1998) Molecular evidence for the gnathobasic derivation of arthropod mandibles and for the appendicular origin of the labrum and other structures. Dev Genes Evol 208:142–150
Posnien N, Bucher G (2010) Formation of the insect head involves lateral contribution of the intercalary segment, which depends on Tc-labial function. Dev Biol 338:107–116. https://doi.org/10.1016/j.ydbio.2009.11.010
Posnien N, Bashasab F, Bucher G (2009) The insect upper lip (labrum) is a nonsegmental appendage-like structure. Evol Dev 11:480–488. https://doi.org/10.1111/j.1525-142X.2009.00356.x
Posnien N, Schinko JB, Kittelmann S, Bucher G (2010) Genetics, development and composition of the insect head – a beetle’s view. Arthropod Struct Dev 39:399–410. https://doi.org/10.1016/j.asd.2010.08.002
Powell JA, Opler PA (2009) Moths of Western North America. University of California Press, Berkeley, CA
Prpic NM, Damen WGM (2009) Notch-mediated segmentation of the appendages is a molecular phylotypic trait of the arthropods. Dev Biol 326:262–271. https://doi.org/10.1016/j.ydbio.2008.10.049
Prpic NM, Tautz D (2003) The expression of the proximodistal axis patterning genes Distal-less and dachshund in the appendages of Glomeris marginata (Myriapoda: Diplopoda) suggests a special role of these genes in patterning the head appendages. Dev Biol 260:97–112. https://doi.org/10.1016/S0012-1606(03)00217-3
Prpic NM, Wigand B, Damen WGM, Klingler M (2001) Expression of dachshund in wild-type and Distal-less mutant Tribolium corroborates serial homologies in insect appendages. Dev Genes Evol 211:467–477. https://doi.org/10.1007/s004270100178
Prpic NM, Janssen R, Wigand B, Klingler M, Damen WGM (2003) Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling. Dev Biol 264:119–140. https://doi.org/10.1016/j.ydbio.2003.08.002
Prpic NM, Janssen R, Damen WGM, Tautz D (2005) Evolution of dorsal-ventral axis formation in arthropod appendages: H15 and optomotor-blind/bifid-type T-box genes in the millipede Glomeris marginata (Myriapoda: Diplopoda). Evol Dev 7:51–57. https://doi.org/10.1111/j.1525-142X.2005.05006.x
Pultz MA, Diederich RJ, Cribbs DL, Kaufman TC (1988) The proboscipedia locus of the Antennapedia complex: a molecular and genetic analysis. Genes Dev 2:901–920. https://doi.org/10.1101/gad.2.7.901
Raff RA, Kaufman TC (1983) Embryos, genes, and evolution: the developmental-genetic basis of evolutionary change. Macmillan, New York City, NY
Rauskolb C, Irvine KD (1999) Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol 210:339–350. https://doi.org/10.1006/dbio.1999.9273
Rauskolb C, Smith KM, Peifer M, Wieschaus E (1995) Extradenticle determines segmental identities throughout Drosophila development. Development 121:3663–3673
Rebeiz M, Tsiantis M (2017) Enhancer evolution and the origins of morphological novelty. Curr Opin Genet Dev 45:115–123. https://doi.org/10.1016/j.gde.2017.04.006
Rempel J (1975) Evolution of the insect head: the endless dispute. Quaest Entomol 11:7–25
Requena D, Álvarez JA, Gabilondo H, Loker R, Mann RS, Estella CS (2017) Origins and specification of the Drosophila wing. Curr Biol 27:3826–3836.e5. https://doi.org/10.1016/j.cub.2017.11.023
Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS (1997) Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91:171–183. https://doi.org/10.1016/S0092-8674(00)80400-6
Rogers BT, Kaufman TC (1997) Structure of the insect head in ontogeny and phylogeny: a view from Drosophila. Int Rev Cytol 174:1–84. https://doi.org/10.1016/S0074-7696(08)62115-4
Rogers BT, Peterson MD, Kaufman TC (2002) The development and evolution of insect mouthparts as revealed by the expression patterns of gnathocephalic genes. Evol Dev 4:96–110. https://doi.org/10.1046/j.1525-142X.2002.01065.x
Ronco M, Uda T, Mito T, Minelli A, Noji S, Klingler M (2008) Antenna and all gnathal appendages are similarly transformed by homothorax knock-down in the cricket Gryllus bimaculatus. Dev Biol 313:80–92. https://doi.org/10.1016/j.ydbio.2007.09.059
Roth VL (1984) On homology. Biol J Linn Soc 22:13–29. https://doi.org/10.1111/j.1095-8312.1984.tb00796.x
Ruiz-Losada M, Blom-Dahl D, Córdoba S, Estella C (2018) Specification and patterning of Drosophila appendages. J Dev Biol 6:17. https://doi.org/10.3390/jdb6030017
Santos ME, Le Bouquin A, Crumière AJJ, Khila A (2017) Taxon-restricted genes at the origin of a novel trait allowing access to a new environment. Science 358:386–390. https://doi.org/10.1126/science.aan2748
Schaeper ND, Wimmer EA, Prpic N-M (2013) Appendage patterning in the primitively wingless hexapods Thermobia domestica (Zygentoma: Lepismatidae) and Folsomia candida (Collembola: Isotomidae). Dev Genes Evol 223:341–350. https://doi.org/10.1007/s00427-013-0449-5
Schmidt-Ott U, Technau GM (1992) Expression of en and wg in the embryonic head and brain of Drosophila indicates a refolded band of seven segment remnants. Development 116:111–125
Schmidt-Ott U, Rafiqi AM, Lemke S (2010) Hox3/zen and the evolution of extraembryonic epithelia in insects. Adv Exp Med Biol 689:133–144
Scholtz G (2002) The Articulata hypothesis–or what is a segment? Org Divers Evol 2:197–215
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216:395–415. https://doi.org/10.1007/s00427-006-0085-4
Scholtz G, Mittmann B, Gerberding M (1998) The pattern of Distal-less expression in the mouthparts of crustaceans, myriapods and insects: new evidence for a gnathobasic mandible and the common origin of Mandibulata. Int J Dev Biol 42:801–810
Schroder R, Eckert C, Wolff C, Tautz D (2000) Conserved and divergent aspects of terminal patterning in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A 97:6591–6596. https://doi.org/10.1073/pnas.100005497
Setton EVW, March LE, Nolan ED, Jones TE, Cho H, Wheeler WC, Extavour CG, Sharma PP (2017) Expression and function of spineless orthologs correlate with distal deutocerebral appendage morphology across Arthropoda. Dev Biol 430:224–236. https://doi.org/10.1016/j.ydbio.2017.07.016
Sharma PP, Schwager EE, Extavour CG, Giribet G (2012) Hox gene expression in the harvestman Phalangium opilio reveals divergent patterning of the chelicerate opisthosoma. Evol Dev 14:450–463. https://doi.org/10.1111/j.1525-142X.2012.00565.x
Sharma PP, Gupta T, Schwager EE, Wheeler WC, Extavour CG (2014) Subdivision of arthropod cap-n-collar expression domains is restricted to Mandibulata. EvoDevo 5:3. https://doi.org/10.1186/2041-9139-5-3
Sharma PP, Tarazona OA, Lopez DH, Schwager EE, Cohn MJ, Wheeler WC, Extavour CG (2015) A conserved genetic mechanism specifies deutocerebral appendage identity in insects and arachnids. ProcRSocB 282:20150698
Shippy TD, Guo J, Brown SJ, Beeman RW, Denell RE (2000) Analysis of maxillopedia expression pattern and larval cuticular phenotype in wild-type and mutant Tribolium. Genetics 155:721–731. https://doi.org/10.1002/jssc.201100117
Shippy TD, Yeager SJ, Denell RE (2009) The Tribolium spineless ortholog specifies both larval and adult antennal identity. Dev Genes Evol 219:45–51. https://doi.org/10.1007/s00427-008-0261-9
Siemanowski J, Richter T, Dao VA, Bucher G (2015) Notch signaling induces cell proliferation in the labrum in a regulatory network different from the thoracic legs. Dev Biol 408:164–177. https://doi.org/10.1016/J.YDBIO.2015.09.018
Simonnet F, Moczek AP (2011) Conservation and diversification of gene function during mouthpart development in Onthophagus beetles. Evol Dev 13:280–289. https://doi.org/10.1111/j.1525-142X.2011.00479.x
Smith FW, Jockusch EL (2014) Hox genes require homothorax and extradenticle for body wall identity specification but not for appendage identity specification during metamorphosis of Tribolium castaneum. Dev Biol 395:182–197. https://doi.org/10.1016/j.ydbio.2014.08.017
Smith AV, Orr-Weaver TL (1991) The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development 112:997–1008
Smith FW, Angelini DR, Jockusch EL (2014a) A functional genetic analysis in flour beetles (Tenebrionidae) reveals an antennal identity specification mechanism active during metamorphosis in Holometabola. Mech Dev 132:13–27. https://doi.org/10.1016/j.mod.2014.02.002
Smith FW, Angelini DR, Gaudio MS, Jockusch EL (2014b) Metamorphic labral axis patterning in the beetle Tribolium castaneum requires multiple upstream, but few downstream, genes in the appendage patterning network. Evol Dev 16:78–91
Smith FW, Boothby TC, Giovannini I, Rebecchi L, Jockusch EL, Goldstein B (2016) The compact body plan of tardigrades evolved by the loss of a large body region. Curr Biol 26:224–229
Smith FW, Cumming M, Goldstein B (2018) Analyses of nervous system patterning genes in the tardigrade Hypsibius exemplaris illuminate the evolution of panarthropod brains. EvoDevo 9:19
Snodgrass RE (1921) The mouthparts of the cicada. Proc Entomol Soc Washingt 23:1–15
Snodgrass RE (1928) Morphology and evolution of the insect head and its appendages. Smithson Misc Collect 81:1–158
Snodgrass RE (1930) Morphology and evolution of the insect head and its appendages. Smithson Misc Collect 81:1–158
Snodgrass RE (1935) Principles of insect morphology. Cornell University Press
Snodgrass RE (1938) The loral plates and the hypopharynx of Hemiptera. Proc Entomol Soc Washington 40:228–236
Snodgrass RE (1944) The feeding apperatus of biting and sucking insects affecting man and animals. Smithson Misc Collect 104:1–133
Snodgrass RE (1946) The skeletal anatomy of fleas (siphonaptera) (with 21 plates). Smithson Misc Collect 104:1–89
Snodgrass RE (1959) The anatomical life of the mosquito. Smithson Misc Collect 139:1–87
Sokoloff A (1972) The biology of Tribolium, vol 1. Clarendon Press, Oxford
Steinmetz PRH, Urbach R, Posnien N, Eriksson J, Kostyuchenko RP, Brena C, Guy K, Akam M, Bucher G, Arendt D (2010) Six3 demarcates the anterior-most developing brain region in bilaterian animals. EvoDevo 1:1–9
Stern DL (2010) Evolution, development, and the predictable genome. Greenwood Village, Colorado, Roberts and Co.
Stern DL, Orgogozo V (2008) The loci of evolution: how predictable is genetic evolution? Evolution 62:2155–2177. https://doi.org/10.1111/j.1558-5646.2008.00450.x
Strausfeld NJ (2012) Arthropod brains: evolution, functional elegance, and historical significance. Belknap Press of Harvard University Press, Cambridge
Struhl G (1982a) Genes controlling segmental specification in the Drosophila thorax. Dev Biol 79:7380–7384. https://doi.org/10.1073/pnas.79.23.7380
Struhl G (1982b) Spineless-aristapedia: a homeotic gene that does not control the development of specific compartments in Drosophila. Genetics 102:737–749
Stultz BG, Park SY, Mortin MA, Kennison JA, Hursh DA (2012) Hox proteins coordinate peripodial decapentaplegic expression to direct adult head morphogenesis in Drosophila. Dev Biol 369:362–376. https://doi.org/10.1016/J.YDBIO.2012.07.012
Sunkel CE, Whittle JRS (1987) Brista: a gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Rouxs Arch Dev Biol 196:124–132. https://doi.org/10.1007/BF00402034
Švácha P (1992) What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola). Dev Biol 154:101–117. https://doi.org/10.1016/0012-1606(92)90052-I
Svendsen PC, Formaz-Preston A, Leal SM, Brook WJ (2009) The Tbx20 homologs midline and H15 specify ventral fate in the Drosophila melanogaster leg. Development 136:2689–2693. https://doi.org/10.1242/dev.037911
Tajiri R, Misaki K, Yonemura S, Hayashi S (2011) Joint morphology in the insect leg: evolutionary history inferred from Notch loss-of-function phenotypes in Drosophila. Development 138:4621–4626. https://doi.org/10.1242/dev.067330
Tanaka K, Truman JW (2007) Molecular patterning mechanism underlying metamorphosis of the thoracic leg in Manduca sexta. Dev Biol 305:539–550
Telford MJ, Thomas RH (1998) Expression of homeobox genes shows chelicerate arthropods retain their deutocerebral segment. Proc Natl Acad Sci U S A 95:10671–10675. https://doi.org/10.1073/pnas.95.18.10671
Theisen H, Haerry TE, O’Connor MB, Marsh JL (1996) Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development 122:3939–3948
Toegel J, Wimmer E, Prpic N-M (2009) Loss of spineless function transforms the Tribolium antenna into a thoracic leg with pretarsal, tibiotarsal, and femoral identity. Dev Genes Evol 219:53–58
Toga K, Saiki R, Maekawa K (2013) Hox gene Deformed is likely involved in mandibular regression during presoldier differentiation in the nasute termite Nasutitermes takasagoensis. J Exp Zool Part B Mol Dev Evol 320:385–392
Tomita S, Kikuchi A (2009) Abd-B suppresses lepidopteran proleg development in posterior abdomen. Dev Biol 328:403–409. https://doi.org/10.1016/j.ydbio.2009.01.040
Tomoyasu Y, Wheeler SR, Denell RE (2005) Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433:643–647
Urbach R, Technau GM (2003) Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130:3621–3637
van Horn SN (1966) Studies on the embryogenesis of Aulocara elliotti (Thomas) (Orthoptera, Acrididae). I. External morphogenesis. J Morphol 120:83–113. https://doi.org/10.1002/jmor.1051200105
Van Valen LM (1982) Homology and causes. J Morphol 173:305–312. https://doi.org/10.1002/jmor.1051730307
von Goethe JW (1790) Versuch die Metamorphose der Pflanzen zu erklären. Carl Wilhelm Ettinger, Gotha
von Goethe JW (1814) Aus meinem Leben: Dichtung und Wahrheit. Cotta, Tübingen
Wagner GP (1989) The biological homology concept. Annu Rev Ecol Syst 20:51–69. https://doi.org/10.1146/annurev.es.20.110189.000411
Wagner GP (2007) The developmental genetics of homology. Nat Rev Genet 8:473–479. https://doi.org/10.1038/nrg2099
Wasik BR, Rose DJ, Moczek AP (2010) Beetle horns are regulated by the hox gene, Sex combs reduced, in a species- and sex-specific manner. Evol Dev 12:353–362. https://doi.org/10.1111/j.1525-142X.2010.00422.x
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Wieschaus E, Nüsslein-Volhard C (2016) The Heidelberg screen for pattern mutants of Drosophila: a personal account. Annu Rev Cell Dev Biol 32:1–46. https://doi.org/10.1146/annurev-cellbio-113015-023138
Wilder EL, Perrimon N (1995) Dual functions of wingless in the Drosophila leg imaginal disc. Development 121:477–488
Wu J, Cohen SM (1999) Proximodistal axis formation in the Drosophila leg: subdivision into proximal and distal domains by Homothorax and Distal-less. Development 126:109–117
Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117:1223–1237
Yasunaga K, Saigo K, Kojima T (2006) Fate map of the distal portion of Drosophila proboscis as inferred from the expression and mutations of basic patterning genes. Mech Dev 123:893–906. https://doi.org/10.1016/J.MOD.2006.08.008
Yeates DK, Meusemann K, Trautwein M, Wiegmann B, Zwick A (2016) Power, resolution and bias: recent advances in insect phylogeny driven by the genomic revolution. Curr Opin Insect Sci 13:16–23
Yoshiyama N, Tojo K, Hatakeyama M (2013) A survey of the effectiveness of non-cell autonomous RNAi throughout development in the sawfly, Athalia rosae (Hymenoptera). J Insect Physiol 59:400–407. https://doi.org/10.1016/j.jinsphys.2013.01.009
Zhang H, Shinmyo Y, Mito T, Miyawaki K, Sarashina I, Ohuchi H, Noji S (2005) Expression patterns of the homeotic genes Scr, Antp, Ubx, and abd-A during embryogenesis of the cricket Gryllus bimaculatus. Gene Expr Patterns 5:491–502. https://doi.org/10.1016/j.modgep.2004.12.006
Acknowledgments
Portions of this material are based upon work supported by US National Science Foundation grant IOS-1350207 to DRA. Special thanks to Joanna Smit Nelson for her perspective on theme and variation in musicology.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Angelini, D.R., Smith, F.W. (2019). Theme and Variation in the Development of Insect Mouthparts. In: Krenn, H. (eds) Insect Mouthparts. Zoological Monographs, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-030-29654-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-29654-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29653-7
Online ISBN: 978-3-030-29654-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)