Abstract
Endometriosis is a gynecological disease characterized by the presence of endometrial glandular and stromal cells existing in the extra-uterine environment. Estimates show that endometriosis affects 10–15% of all women of reproductive age. Approximately, 25–50% of infertile women may be affected by endometriosis and 30–50% patients with endometriosis may suffer from infertility. Several mechanisms have been proposed for the association of endometriosis and infertility, including distorted pelvic anatomy, impaired ovary function, altered microenvironment, affected endometrial receptivity, and reduced oocyte/embryo quality. Patients with endometriosis have inflammatory processes within the increased amount of peritoneal fluid noted. These inflammatory processes and hormonal changes have shown to alter folliculogenesis, sperm motility, tubal transport, and embryo implantation.
Oocyte quality is an important factor in the success of assisted reproductive technology treatment, and the environment of follicle growth has a significant influence on this quality. It is very difficult, however, to directly evaluate oocyte quality. Follicular fluid (FF), however, might reflect the environment during follicle and oocyte growth. Therefore, an evaluation of oxidative stress in the FF might be useful in predicting oocyte quality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Endometriosis is an inflammatory disease affecting till the 10% of reproductive-aged women, linked to infertility in almost half of the patients. Unfortunately, the pathogenesis of endometriosis and its associated infertility is unknown, even if there are some theories [1].
Literature demonstrated that patients suffering endometriosis have genetic, biochemical, or immunological dysfunction that prevents the removal of the tissue from the peritoneal cavity and rather facilitates tissue adhesion to peritoneal structures [2].
The dysfunctional immune system of patient with endometriosis generally have dysregulated a multitude of immune cell types, including neutrophils, macrophages, dendritic cells, natural killer cells, T helper cells, and B cells [3] (Fig. 17.1).
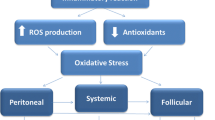
A part the oxidative stress and oocyte quality, as possible ethiopathogenic mechanisms for endometriosis (Fig. 17.2), the cytokines and the chemokines involved in inflammation process, angiogenesis, and tissue growth are increased in the plasma and peritoneal fluid (PF) of women with endometriosis. This process is suspected to stimulate symptoms commonly presented including pain and infertility [4].
Statistical reports showed that 35–50% patients affected by endometriosis experienced infertility and 25–50% of infertile women have endometriosis [5].
If in healthy couples the monthly fecundity rate, which is a couple’s probability of conceiving in 1 month, is 15–20%, on the contrary, women with endometriosis have a monthly fecundity rate of 2–10% [6].
Endometriosis is a heritable condition influenced by multiple genetic and environmental factors, with an overall heritability estimated at approximately 50%. Authors investigated whether single nucleotide polymorphisms (SNPs) rs7521902, rs10859871, and rs11031006 mapping to WNT4, VEZT, and FSHB genetic loci, respectively, are associated with risk for endometriosis in a Greek population. Genotyping of the rs7521902, rs10859871, and rs11031006 SNPs was performed with Taqman primer/probe sets. A significant association was detected with the AC genotype of rs7521902 (WNT4) in patients with stage III and IV disease only. Evidence for association with endometriosis was also found for the AC genotype of the rs10859871 of VEZT. Notably, a significant difference in the distribution of the AG genotype and the minor allele A of FSHB rs11031006 SNP was found between the patients with endometriosis and controls. They found a genetic association between rs11031006 (FSHB) SNP and endometriosis. WNT4 and VEZT genes constitute the most consistently associated genes with endometriosis. In the present study, an association of rs7521902 (WNT4) and rs10859871 (VEZT) was confirmed in women with endometriosis at the genotypic but not the allelic level [7].
17.1.1 Infertility and Endometriosis
Almost 50% of adolescents with intractable dysmenorrhea or pelvic pain are diagnosed with endometriosis, but it is not yet clear why only certain women develop the condition.
The monthly fecundity rate in normal couples of reproductive age is known to be 15–20%, whereas the rate in infertile women with endometriosis ranges from 2 to 10% [8].
A meta-analysis proposed that the chance of achieving pregnancy was lower for patients with endometriosis compared to those with tubal factor infertility (OR 0.56; 95% CI, 0.44–0.70) [9].
However, the association between infertility and early-stage disease, from minimal endometriosis [stage I] and mild endometriosis [stage II], according to the ASRM score (Fig. 17.3), in which no substantial pelvic anatomical changes are identified, remains controversial [10].
The improvement of Controlled Ovarian Hyperstimulation (COH) with GnRH-a downregulation and the application of ICSI technology may suppress some negative influences of endometriosis on pregnancy [11, 12].
Dong et al. investigated the impact of endometriosis on the IVF/ICSI outcomes, comparing ovarian stimulation parameters and IVF/ICSI outcomes. Patients with stage I-II and stage III-IV endometriosis required higher dosage and longer duration of gonadotropins, but had lower day 3 high-quality embryos rate, when compared to patients with tubal infertility. In addition, the number of oocytes retrieved, the number of obtained embryos, the number of day 3 high-quality embryos, serum E2 level on the day of hCG, and fertilization rate were lower in patients with stage III-IV endometriosis than those in tubal factors group. Except reduced implantation rate in stage III-IV endometriosis group, no differences were found in other pregnancy parameters. This study concluded that IVF/ICSI yielded similar pregnancy outcomes in patients with different stages of endometriosis and patients with tubal infertility [13].
Barbosa et al. evaluated whether the presence or severity of endometriosis affects the outcome of ART in a systematic review, investigating all studies comparing the outcome of ART in women with and those without endometriosis, or at different stages of the disease. Women with endometriosis undergoing ART have practically the same chance of achieving clinical pregnancy and live birth as do women with other causes of infertility. No relevant difference was observed in the chance of achieving clinical pregnancy and live birth following ART when comparing stage-III/IV with stage-I/II endometriosis [14].
In a recent review, Tanbo and Fedorcsak affirmed that medical or hormonal treatment alone has little or no effect and should only be used in conjunction with ART. Of the various methods of ART, intrauterine insemination, due to its simplicity, can be recommended in women with minimal or mild peritoneal endometriosis, even though insemination may yield a lower success rate than in women without endometriosis. IVF is an effective treatment option in less-advanced disease stages, and the success rates are similar to the results in other causes of infertility. However, women with more advanced stages of endometriosis have lower success rates with IVF [15].
How endometriosis affect infertility? The most widely accepted theory, which was developed by Sampson, holds that that endometrial tissue refluxed to the fallopian tubes fails to be cleared and attaches to the peritoneum. Some 70% of women who menstruate regularly exhibit bleeding reflux, but only 10% develop endometriosis [16] (Figs. 17.4, 17.5, and 17.6).
Recently, it has been suggested that abnormal immune function and dysregulation of immune mediators are responsible for the poor response to treatment, and poor clearance, of ectopic endometrium. Immune status is now considered to play an important role in the initiation and progression of endometriosis. Several studies have shown that the levels of activated macrophages, T cells, B cells, and inflammatory cytokines are increased in women with endometriosis [17, 18].
Reductions in NK cell cytotoxic function have been observed in the peritoneal fluid (PF) of patients with endometriosis implying that a defect in NK cell cytotoxic function, preventing elimination of endometrial cells from ectopic sites, may cause endometriosis [19, 20].
17.1.2 Endometriosis-Related Infertility
Endometriosis may contribute to infertility by impairing ovarian and tubal function and reducing uterine receptivity (Fig. 17.6); in fact, 35–50% of women with infertility have endometriosis [10], and about 30–50% of patients with endometriosis have impaired fertility [21].
Endometriosis is also associated with a reduced rate of pregnancy after IVF, which may be due to the poor qualities of oocytes and embryos [22].
Despite the advances in the research of endometriosis role in infertility, there are still no clearly defined treatment protocols. Low pregnancy rates after IVF are observed in patients with endometriosis, compared to those with tubal factor of infertility. The detrimental impact, if any, of endometriosis on IVF outcome would be expected to be on embryo “quality” and/or endometrial receptivity [23].
Analyzing retrospective studies on in vitro fertilization (IVF) and oocyte donation programs showed that women with endometriosis have significantly reduced pregnancy rates per cycle and per transfer as well as reduced implantation rates. Moreover, studies reported that healthy ovum donation to patients with endometriosis produces the same rate of implantation and pregnancy compared to controls [23].
Retrospective and prospective clinical trials on IVF success rate have shown decreased oocyte and embryo quality and low ovarian reserves in women with endometriosis compared to controls [24].
Nevertheless, human studies indicate poor oocyte and embryo quality and lower pregnancy rates in women with endometriosis, addressing the problem on pro-inflammatory cytokines and chemokines that negatively interact with the oocyte and embryo, with damage to the oocyte and embryo [25].
In fact, intrafollicular levels of IL-8, IL-12, and adrenomedullin are elevated in women with endometriosis undergoing IVF and are indicators of impaired embryo and oocyte quality [26].
Poor oocyte quality was observed and measured, in retrospective IVF studies, by diminished blastomere cleavage rates, increased numbers of arrested embryos, and impaired cytosolic events [27].
In addition, reports also suggest that other etiological factors, such as in utero exposure to diethylstilbestrol, environmental exposure to endocrine disrupting agents, low birth weight, and dietary choices may play significant roles in the development of endometriosis [28].
Outcomes of in vitro fertilization cycles in women with endometriosis are significantly worse than in patients without this condition. The impact of endometriosis on ovarian reserve and the quality of retrieved oocytes seems evident. Lower implantation rates, however, raise the question whether this finding is purely the consequence of lower number and poorer quality of embryos, or whether it also reflects compromised endometrial receptivity [29].
Accumulating evidence indicates that endometriosis is associated with aberrant transcriptional profiles in the eutopic endometrium of women and baboons resulting in dysregulation of critical signaling pathways [30].
Endometriosis is known to be associated with several deregulated molecules related to the pathogenesis of the disease, such as cyclooxygenase-2 (COX-2) and aromatase. The COX-2 enzyme, encoded by the PTGS2 gene (prostaglandin–endoperoxide synthase 2), is naturally induced by aromatase and is involved in the conversion of arachidonic acid into prostaglandins, which, in turn, regulate aromatase levels in endometriotic tissue [31].
In the endometrial tissue of patients with endometriosis, aberrant aromatase is induced via cyclooxygenase-2 prostaglandin-2 (COX-2–PGE2) pathway deregulation, with a positive feedback cycle. It is also related to proliferative and inflammatory properties of ectopic implants [32].
The PTGS2 gene, which encodes cyclooxygenase 2 (COX-2), is deregulated in endometriotic lesions and plays a crucial role in the acquisition of oocyte competence [33].
17.1.3 Peritoneal Fluid of Patient with Endometriosis
Endometrial fragments refluxed during menstruation induce inflammation within the peritoneal cavity [34].
Normally, neutrophils and macrophages are among the first immune cells (Fig. 17.7) to be recruited to this area and, both, are primary contributors to the elevations in pro-inflammatory and chemotactic cytokine levels found in the peritoneal fluid (PF) [35].
In addition to encouraging the growth of peritoneal implants, macrophages are a major source of angiogenic mediators, including TNF-𝛼 and IL-8 [36].
Endometriosis also involves significant disarray in the production and metabolism of nitric oxide (NO), a ubiquitous free radical in the oocyte microenvironment that plays a vital role in virtually every step of oocyte development, including meiotic maturation, fertilization, embryonic cleavage, and implantation [37].
Authors demonstrated a significant role of NO in delaying oocyte aging and maintaining the integrity of the spindle apparatus.
Decreased bioavailability of NO under certain pathologic conditions could therefore result in abnormalities in oocyte viability and developmental capacity [38].
Further, sperm, travelling through the uterus and fallopian tubes, also interact with inflammatory cytokines in the PF and similarly encounter damage.
Moreover, endometriosis is linked to the Natural Killer (NK) cells dysfunction; NK cells comprise 15% of all circulating lymphocytes, particularly those of the innate immune system, and protect against tumor development and viral infections.
Most studies have found that the numbers of cytotoxic NK cells are functionally defective and reduced in the PF and peripheral blood of patients with endometriosis and that this is accompanied by an overall decrease in NK cell activity [16] (Fig. 17.8).
In such patients, the populations of NK cells (CD32CD56+) are significantly decreased, whereas the proportions of immature NK cells (CD272CD11b2) among CD32CD56+ NK cells are increased in the PF. Functional impairment and diminished cytotoxicity of NK cells within the peritoneal cavity have also been well documented in such patients [39].
In addition, the levels of the inflammatory cytokines IL-6, IL-8, IL-1b, IFN-𝛾, and TNF-𝛼 increase in the PF of patients with endometriosis, which is consistent with the elevated levels noted in the serum [16].
Inflammatory cytokines including TNF-α and oxidative stress has been shown to directly hinder sperm motility [25, 26].
Similarly, murine embryos incubated in the PF from women with endometriosis have shown diminished growth rates of embryos, increased rates of apoptosis, DNA fragmentation, and increased number of embryos arrested in development [40].
Dexamethasone reduced the observed embryotoxic effect of the PF from women with endometriosis-associated infertility. Dexamethasone is a glucocorticoid that has been shown to reduce the expression of prostaglandins and other inflammatory mediators dysregulated in endometriosis [41].
Additionally, inhibiting TNF-α reduces embryotoxic effect on mouse embryos incubated with PF from infertile women with endometriosis [42].
Collectively, these studies link inflammation in the PF, specifically TNF-α, with embryo toxicity. Studying the toxicity of PF from women with endometriosis is limited by ethical constraints as interfering with human embryos violates moral and ethical considerations. However, this murine model provides a convincing argument to suggest the PF from women with endometriosis produces a damaging effect on the embryo [40].
17.1.4 The Oocyte Quality
Patients with endometriosis continue to pose difficulties in achieving pregnancy. Studies have shown lower implantation rates in non-endometriotic patients who received oocytes from women with endometriosis, whereas healthy donated oocytes have proven to contribute to a pregnancy with similar chances in women without the disease. The question still to be answered is whether this situation applies for natural cycles or whether it is the use of gonadotropin-releasing hormone analogs and hormonal replacement therapy used for endometrial priming in oocyte recipients that reestablishes an adequate uterine environment [29].
Endometriosis, especially at the ovarian site has been shown to have a detrimental impact on ovarian physiology. Indeed, sonographic and histologic data tend to support the idea that ovarian follicles of patients with endometriosis are decreased in number and more atretic. Moreover, the local intrafollicular environment of patients affected is characterized by alterations of the granulosa cell compartment including reduced P450 aromatase expression and increased intracellular reactive oxygen species generation [24].
Assessment of oocyte morphology is obligatory for the evaluation of oocyte quality and it has been known that quality of the oocyte has an impact on the fertilization outcomes [43].
Oocyte quality is determined by its morphological, cellular, and molecular evaluations [44].
Advances in reproductive medicine have made clear that one of the most important factors determining the outcome of embryo development is oocyte quality [45].
Many prognostic factors based on morphological characteristics of the oocyte have been devised that may allow prediction of oocyte quality, fertilization rates, and embryo development.
However, currently available techniques are not very reliable in predicting which metaphase II (MII) oocyte will lead to an embryo which will implant and result in a clinical pregnancy [46] (Fig. 17.9).
One way indirectly to assess oocyte quality is to analyze markers in cumulus cells (CCs). During follicular development, the granulosa cells differ in the mural population, limiting the follicular antrum and in the CC population, which surrounds the oocyte. Mural cells are responsible for estrogen production and rupture of the follicle, whereas CCs are intimately associated with oocyte development. CCs are regulated, in part, by factors derived from the oocyte, while contributing to oocyte maturation and development potential.
In this context, some studies have suggested that the analysis of gene expression in CCs can be used as an indirect predictor of oocyte quality and outcomes of assisted reproduction technologies, with possible clinical applications [23].
Traditionally, poor oocyte quality has been held responsible for poor ART outcome in women with endometriosis.
Barnhart et al. observed lower number of oocytes retrieved and lower fertilization rates in oocytes recovered from women with endometriosis as compared to controls of tubal factor infertility but other authors have reported conflicting results [9].
Other researches have found no difference in folliculogenesis or the number of oocytes retrieved in patients with endometriosis, as compared to other etiologies such as tubal factor infertility [47, 48].
Adverse influence on the oocyte is therefore a likely central aspect in endometriosis-related infertility. This concept is strengthened by a study reporting significant improvement in the pregnancy rate in patients with endometriosis who received donated oocytes compared with their own oocytes. Conversely, the pregnancy rates were lower in subjects without endometriosis who received donor oocytes from subjects with endometriosis [49,50,51].
Moreover, a recent meta-analysis on IVF outcomes in endometriosis indicates that live birth rates were not altered in patients with minimal/mild endometriosis, whereas patients with moderate and severe endometriosis patients had poorer outcomes including lower retrieved oocytes, implantation rates, and birth rates [52].
When retrieved oocyte number is considered as ovarian response to controlled ovarian stimulation (COS) and as a success parameter, data in the literature are more conflicting.
The acquisition of oocyte competence is known to depend on adequate cytoplasmic and nuclear maturation, the latter being dependent on the presence of a normal spindle. The meiotic spindle of human oocytes in metaphase II, a temporary and dynamic structure composed of microtubules, is associated with the oocyte cortex and its subcortical microfilaments network and is essential to ensure the fidelity of chromosome segregation during meiosis. The meiotic spindle, however, is extremely sensitive to the action of various factors such as oxidative stress, which can promote meiotic abnormalities and chromosome instability, increase apoptosis and impair the development of the preimplantation embryo [23].
17.1.5 Impact of Endometriosis on Oocyte Quality
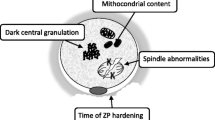
A systematic review of the literature showed that the retrieved oocytes from women affected by endometriosis are more likely to fail in vitro maturation and to show altered morphology and lower cytoplasmic mitochondrial content compared to women with other causes of infertility (Fig. 17.10). Results from meta-analyses addressing IVF outcomes in women affected would indicate that a reduction in the number of mature oocytes retrieved is associated with endometriosis while a reduction in fertilization rates is more likely to be associated with minimal/mild rather than with moderate/severe disease [24].
Women with endometriosis ovulate fewer oocytes than healthy women and those oocytes ovulated by women with endometriosis are both sometimes compromised, so that endometriosis negatively impacts embryo development [25].
Xu et al. examined the ultrastructure of oocytes from patients with minimal or mild endometriosis and control females undergoing IVF treatment by transmission electron microscopy (TEM) to investigate the physiological significance of oocyte quality for patients with minimal or mild endometriosis. The TEM results revealed that the oocytes from women with minimal or mild endometriosis exhibited abnormal mitochondrial structure and decreased mitochondria mass. Quantitative real-time PCR analysis revealed that the mitochondrial DNA copy number was significantly reduced in the oocytes from women with minimal or mild endometriosis compared with those of the control subjects. Their results suggested the decreased oocyte quality because of impaired mitochondrial structure and functions, probably an important factor affecting the fertility of patients with endometriosis [53].
A recent study has shown that women with endometriosis exhibit an increase in apoptosis of the cumulus cells surrounding the oocyte and apoptosis in ovarian cells is a good indicator of poor oocyte quality [54].
Death of cumulus cells probably leads to reduced oocyte quality and maturation attributable to the loss of the essential support that the cumulus cells give to the oocyte [55].
Aberrant nuclear and cytoplasmic events in embryos from women with endometriosis are six times more likely compared with women without endometriosis [56] (Fig. 17.11).
These events include cytoplasmic fragmentation, darkened cytoplasm, reduced cell numbers, and increased frequency of arrested embryos, leading to significantly fewer transferable blastocysts. Additionally, the quality of embryos that develop from patients with endometriosis has been shown to be reduced [57].
Treatment with a gonadotropin-releasing hormone agonist that temporarily causes regression of the endometriotic lesions and cessation of reproductive cyclicity helps to improve embryo quality in these patients [58].
Embryo quality and embryo implantation are also of particular concern in women with endometriosis. In a normal embryo, there are proteins called L-selectin that normally coat the trophoblast on the surface of the blastocyst. This protein is involved in binding of the embryo to the endometrium. Low levels of the enzyme involved in the synthesis of the endometrial ligand for L-selectin have been observed and is a possible etiology of decreased embryo receptivity in patients with endometriosis [59,60,61].
In addition, endometriosis-free patients who have oocytes donated from women with a known history of moderate to severe endometriosis have decreased implantation rates and reduced embryo quality. This decrease in implantation rate and embryo quality is in comparison with women with moderate to severe endometriosis that receive oocytes from endometriosis-free women [62].
The hormonal milieu was altered in the follicular fluid of patients with endometriosis, such as a decreased estradiol concentration [63].
A dysregulated intrafollicular hormone milieu, as well as an abnormal intrafollicular cytokine profile, might therefore be a cause of reduced fertility in endometriosis.
This suggestion is in line with studies from oocyte donation programs. Implantation rates were reduced with oocytes from women with endometriosis transferred to women without endometriosis, whereas embryos from healthy donors, transferred to women with endometriosis, did not affect implantation rates [29, 64].
Abnormal folliculogenesis, elevated oxidative stress (OS), altered immune function, changes in the hormonal milieu, or decreased endometrial receptivity may also contribute to reduced fertility [65].
OS can be considered as a process consisting in three distinct stages, with an increased production of reactive species occurring in the first stage, mobilization of antioxidant occurring in the second, and oxidative damage to the major targets (lipids, proteins, and nucleic acids) occurring in the third [66, 67].
The presence of OS markers in the follicular fluid of infertile women with endometriosis, submitted to in IVF, has been recently demonstrated (Table 17.1) [68, 69].
Donabela et al. demonstrated that infertile women with moderate and severe endometriosis showed increased expression of the superoxide dismutase 1 (SOD1) gene in cumulus cells, compared to women with minimal/mild endometriosis and controls. This evidence opens a new perspective for understanding the pathogenesis of endometriosis-related infertility, confirming that OS is involved in the worsening of oocyte quality in these women. These findings also suggest that analysis of expression of SOD1 gene in CCs might be used as a biomarker of ICSI success in women with infertility related to advanced stages of endometriosis [70].
Singh et al. evaluated the OS and trace elements in the oocytes environment in endometriosis and impact on IVF outcome. They aspirated FF at the time of oocyte retrieval from endometriosis (n = 200) and tubal infertility (n = 140) and it was analyzed. In endometriosis group, they showed an increased concentration of reactive oxygen species (ROS), nitric oxide (NO), lipid peroxidation (LPO), iron, lead, cadmium and reduced levels of total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione reductase (GR), vitamins A, C, E, copper, zinc and selenium, compared to tubal infertility. Increased ROS and NO in endometriosis and tubal infertility are associated with poor oocytes and embryo quality. Increased levels of ROS, NO, LPO, cadmium, and lead were observed in women who did not become pregnant, compared to women who did. Intrafollicular zinc levels were higher in women with endometriosis who subsequently became pregnant following IVF.
The deleterious effect of intrafollicular ROS/NO on oocytes and embryo quality and IVF pregnancy prompted authors to ascertain the ROS threshold level, beyond which it appears to be toxic, and is associated with the formation of poor quality oocytes. Further, follicular levels of lead and cadmium showed a negative association with IVF pregnancy outcome, thereby highlighting the toxicity risk of environmental pollutants [71].
An imbalance between reactive oxygen species (ROS) production and antioxidant activity causes cellular damage and dysfunction and may affect folliculogenesis. Altered folliculogenesis in patients with endometriosis may contribute to ovulatory dysfunction, poor oocyte quality, reduced fertilization, low-grade embryos, and reduced implantation [57].
Changes in the kinetics of granulosa cell cycle may also impair follicular growth and oocyte maturation in patients with endometriosis [72].
Thiols are organic sulfur derivatives, contributing to the infertility associated with endometriosis, since extracellular supply of thiols is critical for maintaining the redox state of the extracellular space or microenvironment [73].
Thus, endometriosis is associated with inflammatory changes in the intrafollicular microenvironment.
In addition, the levels of inflammatory cytokines, such as interleukin 6 (IL6), IL1b, and tumor necrosis factor alpha (TNFa), are increased in the ovarian follicular fluid (OFF) of patients with endometriosis [74].
Da Broi et al. detected a deleterious effect of FF taken from infertile women with mild endometriosis on the spindle and chromosome distribution of bovine oocytes matured in vitro, indicating the presence of harmful factors against oocyte quality in the FF of women with the disease and questioning the role of OS in the worsening of gamete quality [75].
Da Broi et al. also demonstrated both systemic and follicular oxidative stress in infertile patients with endometriosis. They demonstrate the presence of oxidative DNA damage, represented by higher 8OHdG concentrations in the follicular microenvironment of these patients, possibly related to compromised oocyte quality and associated with the pathogenesis of endometriosis-related infertility. These findings also suggest that some of the OS markers studied in serum and FF are predictive of clinical pregnancy and live birth after ICSI [76].
Baumann et al. demonstrated that endometriosis is linked with altered patterns of ovarian gene expression and demonstrated that a prominent epigenetic pathway, associated with oocyte quality and potential correct ovarian development, is severely disrupted in primate females with induced endometriosis [77].
Kasapoglu et al. showed dimorphisms significantly higher in oocytes retrieved from endometriosis group: dark cytoplasm; dark, large or thin zona pellucida; and flat or fragmented polar body (p < 0.05 for all) (Figs. 17.11, 17.12, and 17.13). When morphological parameters for oocytes of patients with endometriosis are evaluated, the oocyte defects have increased significantly in patients with endometriosis. Thus, the abnormal oocyte morphology is more common in patients with endometriosis than those with male factor infertility (Figs. 17.14, 17.15, 17.16, and 17.17).
The hemorrhagic event within an endometriotic cyst produces a slow re-adjustment of the blood with formation of a thick layer of siderotic macrophages and failure to reconstitute the endometrial mucosa. In these cases, the pathologist formulates a diagnosis of “endometriotic cyst” even without recognizing the endometrium
Thus, endometriosis may cause subfertility, and adversely affect outcomes of ART by its detrimental effects on oocyte morphology [78].
Santonastaso et al. analyzed and integrated different clinical chemistry parameters being specific of the metabolic profile, the inflammatory state, and the cell damage by H-Nuclear Magnetic Resonance (NMR) spectroscopy approach and biochemistry analysis, respectively, in follicular fluids of women with different stages endometriosis (I-II and III-IV) unrolled to IVF cycle (Figs. 17.18 and 17.19).
Their analysis evidenced that in the follicular fluids of the patients with endometriosis the levels of phospholipids, lactate, insulin, PTX3, CXCL8, CXCL10, CCL11, and VEGF were higher, whereas those of some fatty acids, lysine, choline, glucose, aspartate, alanine, leucine, valine, proline, phosphocholine, total LDH as well as its LDH-3 isoform were lower in comparison to control group. The levels of LDHB, PTX3, and insulin receptor were confirmed also by RT-PCR applied on cumulus cells surrounding oocytes retrieved from the patients. The reduced oocyte quality observed in patients with endometriosis can be certainly correlated to the different levels of these molecules [79].
Schebl et al. investigated morphological parameters of oocyte morphology in patients with endometriosis, describing oocyte morphology in patients undergoing intracytoplasmic sperm injection. Patients with endometriosis had a significantly lower rate of mature oocytes (p < 0.03) and morphologically normal oocytes (p < 0.001). In particular, brownish oocytes (p < 0.009; stage I-IV) and the presence of refractile bodies (p < 0.001; stage IV) were found to be increased. Endometriosis stage IV was associated with significantly worse-quality oocytes than stages I-III (p < 0.01). Fertilization was significantly reduced in conventional in vitro fertilization but not in intracytoplasmic sperm injection (p < 0.03). This was due to lower fertilization rates in stage III-IV endometriosis compared with stage I-II (p < 0.04). No difference was observed with respect to rates of implantation, clinical pregnancy, miscarriage, live birth, and malformation. Authors concluded that patients with endometriosis, in particular those with severe endometriosis, present lower-quality oocytes [80].
Nakagawa et al. measured the OS in the FF from a single follicle of patients with endometrioma (EM); we evaluated whether an EM might affect the environment of follicular growth. The FF was obtained during the first puncture of follicular aspiration and was evaluated. Authors showed that oxidative stress and antioxidant potential in the FF of the patients with unilateral EM showed values similar to those without an EM, and concluded that EMs do not affect the environment for follicle growth during ART treatment [81].
Nasiri et al. evaluated the levels of two OS markers including lipid peroxide (LPO) and total antioxidant capacity (TAC) in both serum and FF of women with endometriosis after puncture. They observed that women with endometriosis had significantly higher LPO and lower TAC levels in the serum and FF as compared with the control group. Therefore, authors noted that FF of women with endometriosis, regardless of disease stage, increases the proliferation power of endometrial cells in vitro, and presumed that inflammatory reactions-induced OS in ovary may be responsible for proliferation induction ability in FF obtained from women with endometriosis [82] (Figs. 17.20, 17.21, 17.22, 17.23, 17.24, 17.25, and 17.26).
The causes of follicle loss during surgery for endometriosis involve injury to blood vessels and stroma and removal of healthy cortex. (1) Endometrioma. (2) Pseudocapsule of endometriosic ovarian cyst. (3) Healthy cortex containing significant number of follicles stripped with pseudocapsule. (4) Coagulation of vascular bed. (5) Blood vessels injuries. (6) Edema/inflammation
Adenomyotic nodule, small or large, which develops in deep adenomyosis in viscera (intestine, bladder) equipped with muscular tunic. The release of cytokines with stimulatory activity in the endometriotic focus causes reactive hyperplasia in the smooth muscle cells of the host organ. These fibers have an irregular pattern and do not respect the normal functional dynamics of the organ causing dyskinesias. Macroscopically, the hypertrophic lesion has a neoplastic-like character and causes extensive resections of the organ. Histologically, Peripherical endometrial stroma may be poor or absent, causing uncertainties about the invasive character of the glands
Da Luz et al. compared the expression levels of PTGS2 in cumulus cells of infertile women, with and without endometriosis, undergoing ovarian stimulation for ICSI. A decreased expression of PTGS2 was found in cumulus cells of infertile women with endometriosis compared with controls, which might be related to reduced levels of COX-2 in the cumulus cells of women with the disease. Consequently, authors hypothesized that lower transcript levels of PTGS2 in cumulus cells may be involved in the impairment of oocyte quality, suggesting a possible mechanism involved in disease-related infertility [83].
Pauli et al. analyzed and compared peripheral plasma (PP) and FF retinoid levels, retinol (ROL) including all-trans retinoic acid (ATRA), all essential for a number of reproductive processes, in women undergoing IVF. They investigated also the relationship between retinoid levels and embryo quality.
An analysis compared the retinoid levels with day 3 embryo grades between two groups of women, patients with endometriosis and control patients. Results demonstrated distinctive levels of retinoid metabolites and isomers in FF versus PP. There was a significantly larger percentage of high-quality grade I embryos derived from the largest versus smallest follicles. An increase in follicle size also correlated with a >50% increase in FF ROL and ATRA concentrations. Independent of follicle size, FF yielding grade I versus non-grade I embryos showed higher mean levels of ATRA but not ROL. In a nested case-control analysis, control participants had 50% higher mean levels of ATRA in their FF and PP than women with endometriosis. These findings strongly supported the proposition that ATRA should play a fundamental role in oocyte development and quality, and that reduced ATRA synthesis may contribute to decreased fecundity of participants with endometriosis [84].
Du et al. evaluated the effect of endometriosis on folliculogenesis and pregnancy, and to assess the involvement of inflammatory factors (IL1b, PGE2, PGF2a, and TGFb2) in follicular fluid. Authors aspirated FF in patients with endometriosis to measure the concentrations of inflammatory factors (IL1b, PGE2, PGF2α, and TGFβ2) and steroid hormones (E2, progesterone, FSH, and LH) within follicular fluid, as well as serum E2 and LH concentrations. The oocyte retrieval, rate of metaphase II oocyte, cleavage rate, effective embryo rate, and pregnancy rates of patients with endometriosis were all significantly lower than those of the control patients. In those with endometriosis, serum E2 concentrations were lower than those observed in controls. Aromatase levels in the granulosa cells of the endometriosis group were lower while concentrations of PGE2 in follicular fluid were higher than in the control group. Concentrations of PGE2, PGF2α, TGFβ2, and IL1b were significantly correlated with each other. The study conclusion was that the outcomes of ART, in relation to serum E2 concentration, were adversely affected by the presence of endometriosis [85] (Fig. 17.27).
Furthermore, severe ovarian damage and extensive follicle loss have been reported after cystectomy for endometriomas, resulting in a shorter reproductive life span or even immediate ovarian failure as reported in few cases [86,87,88,89].
The follicle loss during surgery for endometriosis has a negative impact of reproduction (Fig. 17.4).
The amount of healthy tissue inadvertently lost during surgery is larger with excision of bilateral endometriomas and is also related to electrosurgical coagulation and the local inflammation associated with the procedure [90].
The endometrioma cyst wall contains different amounts of follicles, which are lost during surgery. The number of follicles in histological sections obtained from the cyst wall is related to patient’s age: the younger the patient, the larger the number of follicles present in the endometrioma wall [91].
Young women also have a higher recurrence rate of their endometriomas (30–50%) that often leads to repeated ovarian surgery which further compromises ovarian reserve [92].
Lastly, extensive adhesiolysis even without direct surgery to the ovaries has been associated with a significant decline in ovarian reserve. This may derive from injury to ovarian vascular bed, resulting in reduced ovarian blood supply postsurgery [93].
17.2 Transvaginal Ultrasound-Guided Oocyte Retrieval in Patients with Endometriosis
The development of transvaginal ultrasound-guided oocyte retrieval transformed IVF in the 1980s. It became possible to retrieve oocytes from the ovaries using outpatient, minimally invasive approaches, utilizing IV sedation or even just local anesthetics [94]. Previously, laparoscopy was necessary with its increased cost and need for general anesthesia. Whether oocyte retrieval is being performed laparoscopically or transvaginally, the ovary needs to be held in place in order for the follicle to be punctured and aspirated. Several consequences of advanced stages of endometriosis include scarring of the ovary and adnexa within the cul-de-sac or obscuring by bowel or omental adhesions [95, 96]. Paradoxically during transvaginal oocyte retrieval, adhesion formation may actually immobilize the ovaries facilitating the aspiration of the follicles.
Endometriosis and associated endometriomas are common in patients coming for oocyte retrieval as part of the IVF process [97]. The development of large endometriomas is unusual outside the ovary, and the pathogenesis behind their development inside the ovary is unknown; however, different hypotheses do exist. It has been shown, while the formation of superficial cysts of superficial ovarian endometriosis is similar to extra-ovarian site endometriosis, the development of large endometriomas may be the result of secondary involvement of functional (follicular or luteal) ovarian cysts by the endometriotic process. Additionally, the ovary has several unique physiologic processes, such as high concentrations of ovarian steroids and growth factors which may impact the initiation, maintenance, and growth of endometrial implants; as well as, the regular follicular rupture at time of ovulation, also known as ovulation dehiscence, causes metaplasia of the ovarian mesothelium, or stigma, potentially allowing for the progressive invagination of the ovarian cortex and the invasion of follicles or corpora lutea from endometriotic cells [98, 99]. To note, the chance of correspondence between follicular dehiscence and an endometrial implant may be higher in natural cycles due to the concomitance of molecules involved in both endometriosis-associated inflammation and ovulation dehiscence. Furthermore, such correspondence can also occur during IUI but not during IVF cycles, when the stigma is controlled by physicians [100, 101]. These two unique features of the ovary in combination may play a role in the development of large ovarian endometriomas. Different clinical types of suspected endometriomas have been observed and classifications have been developed based on size, cyst contents, ease of capsule removal, adhesions of the cyst to surrounding structures, and location of superficial endometrial implants relative to the cyst [102, 103]; see Table 17.2.
At times, the surgical approach in these cases depends on the size and location of the endometrioma (Fig. 17.5). Ordinarily, follicles will be aspirated sequentially, moving from one to the next emptying the follicular contents. The larger problem faced during transvaginal oocyte retrieval in patients with endometriosis is the presence of endometriomas in the needle path from the vagina toward the target follicle [104]. Generally, the goal is to avoid the endometrioma and “go around it” if possible (Fig. 17.28).
The reason for this approach is because spillage of the endometrioma fluid can be toxic to the oocytes, the endometrium, and the peritoneal cavity [105]. Occasionally, it is hard to avoid the endometriomas and several methods exist to combat this inconvenience. (1) The vaginal ultrasound technique may be used to traverse the cyst with the aspirating needle without aspirating the cyst itself. This is usually successful and avoids the issues mentioned above; or (2) if the endometrioma is so large as to prevent a clear path to the normal follicle, it may be drained into a separate aspiration tube and the aspiration needle thoroughly rinsed prior to using it to aspirate a normal follicle.
IVF is an outstanding treatment option with excellent success rates for couples suffering from infertility caused by endometriosis [106]. Treatment for endometriosis via laparoscopy following previous IVF failures has been shown to increase odds of conceiving naturally and/or via IVF [107]. With a little planning and attention to detail, transvaginal ultrasound-guided oocyte retrieval can be carried out safely and efficiently in women with all stages of endometriosis.
Abbreviations
- ART:
-
Assisted reproductive technique
- COH:
-
Controlled ovarian hyperstimulation
- COS:
-
Controlled ovarian stimulation
- COX-2–PGE2:
-
Cyclooxygenase-2 prostaglandin-2
- FF:
-
Follicular fluid
- GM-CSF:
-
Granulocyte macrophage-colony stimulator factor
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- ICSI:
-
Intracytoplasmatic sperm injection
- IFN-α:
-
Interferon-α
- IL-1:
-
Interleukin-1
- IL-12:
-
Interleukin-12
- IL-2:
-
Interleukin-2
- IL-4:
-
Interleukin-4
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- IVF:
-
In vitro fertilization
- LPO:
-
Lipid peroxidation
- MCP-1:
-
Monocyte chemotactic protein-1
- MCs:
-
Mast cells
- MMPs:
-
Matrix metalloproteinases
- NK:
-
Natural Killer
- NO:
-
Nitric oxide
- OFF:
-
Ovarian follicular fluid
- OS:
-
Oxidative stress
- PCR:
-
Polymerase chain reaction
- PDGF:
-
Platelet-derived growth factor
- PF:
-
Peritoneal fluid
- PG:
-
Prostaglandin
- PP:
-
Peripheral plasma
- ROL:
-
Retinol
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SOD1:
-
Superoxide dismutase 1
- TAC:
-
Total antioxidant capacity
- TEM:
-
Transmission electron microscopy
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor 3
References
Hirsch M, Begum MR, Paniz É, Barker C, Davis CJ, Duffy J. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125(5):556–64.
Cho YJ, Lee SH, Park JW, Han M, Park MJ, Han SJ. Dysfunctional signaling underlying endometriosis: current state of knowledge. J Mol Endocrinol. 2018;60(3):R97–R113.
Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J, Taylor RN. Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. 2018;50:50–60. https://doi.org/10.1016/j.bpobgyn.2018.01.006. pii: S1521-6934(18)30023-3.
Clemenza S, Sorbi F, Noci I, Capezzuoli T, Turrini I, Carriero C, Buffi N, Fambrini M, Petraglia F. From pathogenesis to clinical practice: emerging medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;51:92–101. https://doi.org/10.1016/j.bpobgyn.2018.01.021. pii: S1521-6934(18)30039-7.
Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):R63–78. https://doi.org/10.1530/REP-16-0052.
Donnez J, Donnez O, Orellana R, Binda MM, Dolmans MM. Endometriosis and infertility. Panminerva Med. 2016;58(2):143–50.
Matalliotakis M, Zervou MI, Matalliotaki C, Rahmioglu N, Koumantakis G, Kalogiannidis I, Prapas I, Zondervan K, Spandidos DA, Matalliotakis I, Goulielmos GN. The role of gene polymorphisms in endometriosis. Mol Med Rep. 2017;16(5):5881–6.
Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil Steril. 1993;59(5):963–70.
Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–55.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99.
Opoien HK, Fedorcsak P, Omland AK, Abyholm T, Bjercke S, Ertzeid G, Oldereid N, Mellembakken JR, Tanbo T. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril. 2012;97:912–8.
Lin XN, Wei ML, Tong XM, Xu WH, Zhou F, Huang QX, Wen GF, Zhang SY. Outcome of in vitro fertilization in endometriosis-associated infertility: a 5-year database cohort study. Chin Med J (Engl). 2012;125:2688–93.
Dong X, Liao X, Wang R, Zhang H. The impact of endometriosis on IVF/ICSI outcomes. Int J Clin Exp Pathol. 2013;6(9):1911–8.
Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014;44(3):261–78.
Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017;96(6):659–67.
Jeung I, Cheon K, Kim MR. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed Res Int. 2016;2016:2916070.
D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21(2):243–54.
Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60(5):383–404.
Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56(1):45–51.
Oosterlynck DJ, Meuleman C, Waer M, Vandeputte M, Koninckx PR. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil Steril. 1992;58(2):290–5.
Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–7.
Garrido N, Navarro J, Remohí J, Simón C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update. 2000;6(1):67–74.
Da Broi MG, Navarro PA. Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016;364(1):1–7.
Sanchez AM, Vanni VS, Bartiromo L, Papaleo E, Zilberberg E, Candiani M, Orvieto R, Viganò P. Is the oocyte quality affected by endometriosis? A review of the literature. J Ovarian Res. 2017;10(1):43.
Stilley JA, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349(3):849–62.
Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017;8(4):7138–47.
Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36.
Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25(6):1528–35.
Hauzman EE, Garcia-Velasco JA, Pellicer A. Oocyte donation and endometriosis: what are the lessons? Semin Reprod Med. 2013;31(2):173–7.
Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88(2):44.
Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–4.
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79.
Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, Bulun SE. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–64.
Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37.
Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35(6):696–8.
Beste MT, Pfäffle-Doyle N, Prentice EA, Morris SN, Lauffenburger DA, Isaacson KB, Griffith LG. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6(222):222ra16.
Osborn BH, Haney AF, Misukonis MA, Weinberg JB. Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis-associated infertility. Fertil Steril. 2002;77(1):46–51.
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Activation of the cGMP signaling pathway is essential in delaying oocyte aging in diabetes mellitus. Biochemistry. 2006;45(38):11366–78.
Somigliana E, Viganò P, Gaffuri B, Candiani M, Busacca M, Di Blasio AM, Vignali M. Modulation of NK cell lytic function by endometrial secretory factors: potential role in endometriosis. Am J Reprod Immunol. 1996;36(5):295–300.
Kokcu A. Possible effects of endometriosis-related immune events on reproductive function. Arch Gynecol Obstet. 2013;287(6):1225–33.
Heitmann RJ, Tobler KJ, Gillette L, Tercero J, Burney RO. Dexamethasone attenuates the embryotoxic effect of endometriotic peritoneal fluid in a murine model. J Assist Reprod Genet. 2015;32(9):1317–23.
Riccio LDGC, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. https://doi.org/10.1016/j.bpobgyn.2018.01.010. pii: S1521-6934(18)30028-2.
Harris SE, Faddy M, Levett S, et al. Analysis of donor heterogeneity as a factor affecting the clinical outcome of oocyte donation. Hum Fertil (Camb). 2002;5:193–8.
Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19:1–12.
Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–54.
De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42.
Dmowski WP, Rana N, Michalowska J, Friberg J, Papierniak C, el-Roeiy A. The effect of endometriosis, its stage and activity, and of autoantibodies on in vitro fertilisation and embryo transfer success rates. Fertil Steril. 1995;63:555–62.
Bergendal A, Naffah S, Nagy C, Bergqvist A, Sjöblom P, Hillensjö T. Outcome of IVF in patients with endometriosis in comparison with tubal factor infertility. J Assist Reprod Genet. 1998;15:530–4.
Simón C, Gutiérrez A, Vidal A, de los Santos MJ, Tarín JJ, Remohí J, Pellicer A. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–9.
Garrido N, Navarro J, Remohí J, Simón C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update. 2000;6(1):67–74.
Garrido N, Navarro J, García-Velasco J, Remoh J, Pellice A, Simón C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8(1):95–103.
Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:79–88.
Xu B, Guo N, Zhang XM, Shi W, Tong XH, Iqbal F, Liu YS. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep. 2015;5:10779.
Díaz-Fontdevila M, Pommer R, Smith R. Cumulus cell apoptosis changes with exposure to spermatozoa and pathologies involved in infertility. Fertil Steril. 2009;91(5 Suppl):2061–8.
Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–33.
Brizek CL, Schlaff S, Pellegrini VA, Frank JB, Worrilow KC. Increased incidence of aberrant morphological phenotypes in human embryogenesis—an association with endometriosis. J Assist Reprod Genet. 1995;12(2):106–12.
Garrido N, Pellicer A, Remohí J, Simón C. Uterine and ovarian function in endometriosis. Semin Reprod Med. 2003;21(2):183–92.
Kimura F, Takahashi K, Takebayashi K, Fujiwara M, Kita N, Noda Y, Harada N, et al. Fertil Steril. 2007;87(6):1468.e9–12.
Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;48:3814–26.
Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81.
Kemmann E, Ghazi D, Corsan G, et al. Does ovulation stimulation improve fertility in women with minimal/mild endometriosis after laser laparoscopy? Int J Fertil Menopausal Stud. 1993;38:16–21.
Evans MB, Decherney AH. Fertility and endometriosis. Clin Obstet Gynecol. 2017;60(3):497–502.
Wunder DM, Mueller MD, Birkhäuser MH, Bersinger NA. Steroids and protein markers in the follicular fluid as indicators of oocyte quality in patients with and without endometriosis. J Assist Reprod Genet. 2005;22(6):257–64.
von Wolff M, Kollmann Z, Flück CE, Stute P, Marti U, Weiss B, et al. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: a comparative study between natural cycle IVF and conventional IVF. Hum Reprod. 2014;29:1049–57.
Härkki P, Tiitinen A, Ylikorkala O. Endometriosis and assisted reproduction techniques. Ann N Y Acad Sci. 2010;1205:207–13.
Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13(1):126–34.
Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86(3):503–12.
Navarro PA, Liu L, Ferriani RA, Keefe DL. Arsenite induces aberrations in meiosis that can be prevented by coadministration of N-acetylcysteine in mice. Fertil Steril. 2006;85(Suppl 1):1187–94.
Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. 2012;98(1):126–30.
Donabela FC, Meola J, Padovan CC, de Paz CC, Navarro PA. Higher SOD1 gene expression in cumulus cells from infertile women with moderate and severe endometriosis. Reprod Sci. 2015;22(11):1452–60.
Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–24.
Saito H, Seino T, Kaneko T, Nakahara K, Toya M, Kurachi H. Endometriosis and oocyte quality. Gynecol Obstet Invest. 2002;53(Suppl 1):46–51.
Turkyilmaz E, Yildirim M, Cendek BD, Baran P, Alisik M, Dalgaci F, Yavuz AF. Evaluation of oxidative stress markers and intra-extracellular antioxidant activities in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:164–8.
Wunder DM, Mueller MD, Birkhäuser MH, Bersinger NA. Increased ENA-78 in the follicular fluid of patients with endometriosis. Acta Obstet Gynecol Scand. 2006;85(3):336–42.
Da Broi MG, Malvezzi H, Paz CC, Ferriani RA, Navarro PA. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum Reprod. 2014;29(2):315–23.
Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordão Junior AA, Navarro PA. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016;366(1):231–42.
Baumann C, Olson M, Wang K, Fazleabas A, De La Fuente R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction. 2015;150(4):297–310.
Kasapoglu I, Kuspinar G, Saribal S, Turk P, Avcı B, Uncu G. Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: a retrospective cohort study. Gynecol Endocrinol. 2018;34(3):206–211.11.
Santonastaso M, Pucciarelli A, Costantini S, Caprio F, Sorice A, Capone F, Natella A, Iardino P, Colacurci N, Chiosi E. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol Biosyst. 2017;13(6):1213–22.
Shebl O, Sifferlinger I, Habelsberger A, Oppelt P, Mayer RB, Petek E, Ebner T. Oocyte competence in in vitro fertilization and intracytoplasmic sperm injection patients suffering from endometriosis and its possible association with subsequent treatment outcome: a matched case-control study. Acta Obstet Gynecol Scand. 2017;96(6):736–44.
Nakagawa K, Hisano M, Sugiyama R, Yamaguchi K. Measurement of oxidative stress in the follicular fluid of infertility patients with an endometrioma. Arch Gynecol Obstet. 2016;293(1):197–202.
Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. Cell J. 2017;18(4):582–7.
da Luz CM, da Broi MG, Donabela FC, Paro de Paz CC, Meola J, Navarro PA. PTGS2 down-regulation in cumulus cells of infertile women with endometriosis. Reprod Biomed Online. 2017;35(4):379–86.
Pauli SA, Session DR, Shang W, Easley K, Wieser F, Taylor RN, Pierzchalski K, Napoli JL, Kane MA, Sidell N. Analysis of follicular fluid retinoids in women undergoing in vitro fertilization: retinoic acid influences embryo quality and is reduced in women with endometriosis. Reprod Sci. 2013;20(9):1116–24.
Du YB, Gao MZ, Shi Y, Sun ZG, Wang J. Endocrine and inflammatory factors and endometriosis-associated infertility in assisted reproduction techniques. Arch Gynecol Obstet. 2013;287(1):123–30.
Hwu YM, Wu FS-Y, Li S-H, Sun F-J, Lin M-H, Lee RK-K. The impact of endometrioma and laparoscopic cystectomy on serum anti-Mullerian hormone levels. Reprod Biol Endocrinol. 2011;9:80.
Busacca M, Riparini J, Somigliana E, Oggioni G, Izzo S, Vignali M, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421–5.
Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod. 2011;26(11):3000–7.
Roman H, Tarta O, Pura I, Opris I, Bourdel N, Marpeau L, et al. Direct proportional relationship between endometrioma size and ovarian parenchyma inadvertently removed during cystectomy, and its implication on the management of enlarged endometriomas. Hum Reprod. 2010;25:1428–32.
Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92:1428–35.
Romualdi D, Franco Zannoni G, Lanzone A, Selvaggi L, Tagliaferri V, Gaetano Vellone V, et al. Follicular loss in endoscopic surgery for ovarian endometriosis: quantitative and qualitative observations. Fertil Steril. 2011;96(2):374–8.
Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano P, Candiani M. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol. 2011;24(6):376–9.
Hirokawa W, Iwase A, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum Reprod. 2011;26(4):904–10.
Tanbo T, Henriksen T, Magnus O, Abyholm T. Oocyte retrieval in an IVF program. A comparison of laparoscopic and transvaginal ultrasound-guided follicular puncture. Acta Obstet Gynecol Scand. 1988;67(3):243–6.
Ota Y, Andou M, Ota I. Laparoscopic surgery with urinary tract reconstruction and bowel endometriosis resection for deep infiltrating endometriosis. Asian J Endosc Surg. 2018;11(1):7–14.
King CR, Lum D. Techniques in minimally invasive surgery for advanced endometriosis. Curr Opin Obstet Gynecol. 2016;28(4):316–22.
Khine YM, Taniguchi F, Harada T. Clinical management of endometriosis-associated infertility. Reprod Med Biol. 2016;15(4):217–25.
Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas: implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–6.
Vigano P, Vanni VS, Corti L, Garavaglia E, Tandoi I, Pagliardini L, et al. Unravelling the ovarian endometrioma pathogenesis: “the long and winding road across the various theories.”. J Endometr Pelvic Pain Disord. 2013;5:62–7.
Gerard N, Caillaud M, Martoriati A, Goudet G, Lalmanach AC. The interleukin-1 system and female reproduction. J Endocrinol. 2004;180:203–12.
Somigliana E, Vigano P, Benaglia L, Busnelli A, Vercellini P, Fedele L. Adhesion prevention in endometriosis: a neglected critical challenge. J Minim Invasive Gynecol. 2012;19:415–21.
Nezhat C, Nezhat F, Nezhat CH, Seidman DS. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–3.
Nezhat C, Nezhat F, Nezhat CH. Nezhat’s video-assisted and robotic-assisted laparoscopy and hysteroscopy. 4th ed. New York: Cambridge University Press; 2013.
Benaglia L, Busnelli A, Biancardi R, Vegetti W, Reschini M, Vercellini P, Somigliana E. Oocyte retrieval difficulties in women with ovarian endometriomas. Reprod Biomed Online. 2018;37(1):77–84.
Somigliana E, Vigano P, Filippi F, Papaleo E, Benaglia L, Candiani M, et al. Fertility preservation in women with endometriosis: for all, for some, for none? Hum Reprod. 2015;20:1280–6.
Cecchino GN, Garcia-Velasco JA. Endometrioma, fertility, and assisted reproductive treatments: connecting the dots. Curr Opin Obstet Gynecol. 2018;30(4):223–8.
Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tinelli, A. et al. (2020). Endometriosis, Infertility, and Oocyte Quality. In: Malvasi, A., Baldini, D. (eds) Pick Up and Oocyte Management. Springer, Cham. https://doi.org/10.1007/978-3-030-28741-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-28741-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28740-5
Online ISBN: 978-3-030-28741-2
eBook Packages: MedicineMedicine (R0)