Abstract
Ovarian endometriomas may have a direct detrimental impact on ovarian follicles. In this chapter, we review the literature specific to the biological relationship between ovarian endometriomas and oocyte quality and quantity. Anatomically, endometriomas are associated with tubo-ovarian adhesions and deep endometriosis that may negatively affect ovulation and trapping of the oocyte by the tubal fimbriae. Within the ovary, there is iron-mediated oxidative stress and inflammation in endometriosis cyst fluid, peritoneal fluid, and follicular fluid. Abnormalities in granulosa cells, such as increased apoptosis, may result in decreased aromatase activity and estradiol. Stretch of tissues caused by the endometrioma may also trigger signaling cascades that result in the hyperactivation of ovarian follicles, and thus a decrease in ovarian reserve. Together, these biological mechanisms may impair oocyte quantity and quality, and thus fertility, in patients with ovarian endometriomas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
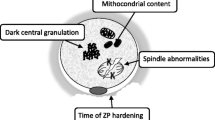
This chapter will focus on the biological impact of ovarian endometriomas on ovarian structure and function, which may lead to infertility. We will begin with a brief overview of the etiology of ovarian endometriomas, and then review potential biological mechanisms including (a) anatomical distortion and other non-ovarian mechanisms; (b) endometrioma fluid and cyst wall; (c) iron metabolism, oxidative stress, and local inflammation, and their relation to abnormalities in granulosa cells and follicular fluid; and (d) pathways leading to a reduction in oocyte quantity. There will be a focus on the published literature specific to ovarian endometriomas, rather than endometriosis in general. These mechanisms are illustrated in Fig. 1.
Biological effects of ovarian endometriomas on ovarian follicles. Ovarian endometriomas are associated with increased iron, oxidative stress, and inflammation, which can diffuse to surrounding follicles and reduce oocyte quality. Granulosa cells demonstrate higher rates of senescence, apoptosis, and autophagy, which can lead to decreased estradiol production. Endometriomas also induce surrounding ovarian fibrosis and decreased vascularization, as well as stretch-induced hyperactivation of primordial follicles, which reduces oocyte quantity. Around endometrioma-affected ovaries, the presence of non-ovarian endometriosis, tubo-ovarian adhesions, and peritoneal inflammation also reduce fertility. It should be noted that this is a simplified diagram only; for example, the ovarian endometrioma would consist of chocolate fluid, endometrial epithelium/stroma cyst wall, as well as fibrosis. Created with BioRender
2 Etiology of Ovarian Endometriomas
There are several hypotheses for the genes of the endometrioma cyst wall [1]. One hypothesis is metaplasia of invaginated mesothelial inclusions, where mesothelium covering the ovary invaginates into the cortex and subsequently undergoes coelomic metaplasia. A second hypothesis is that superficial implants invaginate into the ovarian cortex, for example, where the ovary becomes attached to adjacent non-ovarian endometriosis, followed by invagination into the ovarian cortex. A third hypothesis is that adjacent non-ovarian endometriosis invades a corpus luteum. Regardless, the resulting endometrioma has a mean cyst wall thickness of 1.4 mm, with the endometriosis epithelium/stroma penetrating the cyst wall only 0.6 mm on average [2].
While a full account of the biological studies of ovarian endometriomas is beyond the scope of this chapter, a brief review of recent novel methodological approaches will be provided. In a review of epigenetic studies of endometriomas [3], epigenetic alterations were noted in histones H3 and H4, and notably hypomethylation of steroidogenic factor-1 (SF-1) that binds promoters of steroidogenic acute regulatory protein (STAR) and aromatase. The latter was replicated in a genome-wide methylation study of endometrial stromal cells from endometriomas [4]. Other genes have been found to be hypomethylated or hypermethylated in ovarian endometriomas in another genome-wide analysis by Borghese et al. [5], although only a specific subset of epigenetic events were correlated to nearby gene expression.
Furthermore, somatic cancer driver mutations and other somatic genomic events in the epithelium of endometriosis (without cancer), including endometriomas, were recently reviewed [6]. In ovarian endometriomas, a variety of abnormalities have been noted such as chromosome abnormalities (e.g. chromosome 17 aneuploidy, as well as a variety of chromosome arm gains or losses using comparative genomic hybridization), areas of loss of heterozygosity (e.g. 10q23.3), loss of BAF250 (ARID1A) immunohistochemistry expression in a proportion (8–19%) of endometriomas, and recurrent somatic cancer driver mutations in endometriosis epithelium in work done by Suda et al. (e.g. in KRAS and PIK3CA) [7, 8]. The biological implications of these somatic genomic events remain unclear, but as they are characteristic of malignancies, they may promote invasion or invagination of endometriosis cells into the ovary.
Sanchez et al. [3] reviewed the literature for microarray gene expression studies on ovarian endometriomas specifically in comparison to the eutopic uterine endometrium. They found that endometriomas had comparatively higher expression of hydroxysteroid 11beta-dehydrogenase that converts cortisone to cortisol; phospholipase A2 group II and group V that produce arachidonic acid precursor for prostaglandins; apolipoprotein E expressed by macrophages; peroxisome proliferator-activated receptor gamma that regulates cytokine transcription; as well as complement proteins (C1R, C3, and C7), cytoskeletal components actin alpha2 and myosin 11, and various major histocompatibility complex molecules.
Finally, Hayashi et al. [9] generated a mouse model of ovarian endometriomas, where uterine tissue was implanted in the ovaries of syngeneic mice. They found that the endometrioma-affected ovaries had elevated iron levels and more oxidative stress in follicles, accompanied by a reduction in FSH expression. The role of iron and oxidative stress in endometriomas and surrounding follicles will be explained in more detail below.
3 Anatomic Distortion and Other Non-ovarian Mechanisms
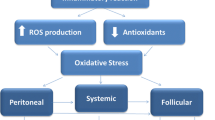
Endometriomas may be associated with tubo-ovarian adhesions and non-ovarian endometriosis (particularly, deep endometriosis), resulting in anatomic distortion that negatively affects the ability of the tubal fimbriae to capture the ovulated oocyte. Endometriomas and endometriosis, in general, are also associated with peritoneal inflammation (e.g. elevated IL-1beta, IL-6, and tumor necrosis factor) that may affect tubo-ovarian function and also hinder sperm motility and oocyte–sperm interaction [10]. The increase in peritoneal inflammation may also potentially impair oocyte quality [11]. Moreover, the peritoneal fluid has evidence of oxidative stress due to iron from shed blood from endometriosis lesions and from retrograde menstruation, which contributes to the inflammation in the peritoneal fluid that surrounds the ovary [10]. If macrophages take up the iron, then the iron not be accessible to ferritin, which further increases oxidative stress [12]. In addition, it is also plausible that endometriosis (and endometriomas) may affect endometrial receptivity and implantation, if there is an increase in eutopic endometrial inflammation in endometriosis (e.g. related to increased aromatase producing higher estradiol) [13], with perhaps another mechanism being anterograde flow of endometriosis-associated inflammatory peritoneal fluid into the endometrial cavity.
4 Endometrioma Fluid and Cyst Wall
Cellular and molecular features of endometriomas were extensively reviewed by Sanchez et al. [1], who divided their review into the endometriosis fluid, the cyst wall and other cellular elements lining the inside of the endometrioma, and the local environment around the endometrioma. One hypothesis is that the endometrioma fluid itself, which arises from repeated bleeding into the cyst from the endometrioma cyst wall, is toxic to surrounding ovarian tissue. Similar to peritoneal fluid, the endometrioma fluid may have an increase in iron that can mediate an increase in oxidative stress and subsequent inflammation (e.g. IL-8). There may also be an imbalance among activins, inhibins, and follistatin, as well as changes in soluble adhesion molecules, in endometrioma cyst fluid. Unlike other cysts, endometriomas are not surrounded by a true capsule such that there is less of the barrier of diffusion from the endometrioma to surrounding ovarian tissue and follicles [14]. This local diffusion of molecules from the endometrioma is supported by the observation of an increase in total iron and ferritin in the follicular fluid of follicles proximal to the endometrioma compared to follicles distal to the endometrioma and from the contralateral ovary [15].
For the cyst wall, there are regions of endometriosis epithelium/stroma, but there can also be the presence of metaplasia and regions of the cyst wall being replaced with fibrotic tissue, as well as surrounding hemosiderin macrophages (particularly M2 macrophages) that may support endometriosis lesion growth [1]. It has been postulated that iron-mediated oxidative stress, such as in the endometrioma fluid, is one mechanism that can predispose to the somatic cancer driver mutations seen in ovarian endometrioma epithelium [1].
5 Iron, Oxidative Stress, and Inflammation
Before moving on to a discussion of changes in granulosa cells and follicular fluid, the relationship among iron metabolism, oxidative stress, and inflammation will be reviewed. Gupta et al. reviewed proteomic studies of the role of oxidative stress in infertility, including in endometriosis [12]. Reactive oxygen species arise from mitochondrial respiration (electron transport chain), and when antioxidants cannot clear these reactive oxygen species, the result is oxidative stress. Reactive oxygen species lack electrons which makes them reactive with surrounding molecules, with examples being hydrogen peroxide, hydroxyl radicals, and superoxide anion. Iron can be a cause of reactive oxygen species, due to its ability to shift between Fe2+ and Fe3+ forms [16], and is important in endometriosis due to shed blood in endometrioma fluid, in peritoneal fluid and via retrograde menstruation.
Anti-oxidants can be enzymatic (e.g. superoxide dismutase and glutathione oxidase) and non-enzymatic (e.g. Vitamins A and E, zinc, and selenium) [12]. There is a balance between reactive oxygen species and anti-oxidants: a homeostatic level of reactive oxygen species being important for physiological processes during ovulation such as resumption of meiosis I and formation of the dominant follicle, while anti-oxidants promote resumption of meiosis II. Thus, either excessive or inadequate reactive oxygen species may negatively affect reproduction. Specifically, oxidative stress results when reactive oxygen species exceed anti-oxidant activities, with the oxidative stress in endometriomas then resulting in an increase in pro-inflammatory cytokines [1].
6 Granulosa Cell Abnormalities
Huo studied granulosa cells with associated endometriomas for evidence of mitochondrial abnormalities [17]. They found evidence that endometrioma-associated granulosa cells had fewer mitochondria, more abnormal morphology, and lower ATPase and proteins involved in oxidative phosphorylation. There was also a higher level of cell-free mitochondrial DNA in follicular fluid in endometriosis cases compared to controls that were in turn inversely associated with cell-free mitochondrial DNA in granulosa cells. The authors interpreted these findings as suggesting a negative impact on oocyte quality, particularly as mitochondrial DNA has been correlated with embryo quality. Urs et al. [18] found that endometrioma-affected ovarian granulosa cells had less mitochondrial mass and membrane potential and less expression of STAR and 3beta-hydroxysteroid dehydrogenase (which together were correlated with decreased follicular estradiol), in comparison to different control groups. There was also an increase in apoptosis of cumulus cells in the endometrioma group.
Another study examined granulosa cells from patients with endometrioma and studied the role of endoplasmic reticulum stress [19]. There was evidence of endoplasmic reticulum stress (e.g. increased expression of unfolded protein response and phosphorylated endoplasmic reticulum stress sensor proteins). In functional culture studies, hydrogen peroxide (a feature of oxidative stress) promoted the expression of unfolded protein response in cultured granulosa cells, as well as apoptosis-associated caspase 8 and caspase 3. Therefore, oxidative stress in the ovary due to endometrioma may lead to endoplasmic reticulum stress and apoptosis in granulosa cells. Similarly, lipidomic profiling showed an increase in sphingolipids and phosphatidylcholines in endometrioma-affected follicular fluid, which could also be involved in apoptosis [20].
Recently the role of autophagy (catabolic process to recycle cell components) in granulosa cells with endometrioma was investigated [21]. They found that these granulosa cells had increased autophagy and expression of Beclin-1 (a mediator of autophagy) and that these patients had an increase in serum progesterone in the late follicular phase that may be a marker of poorer oocyte quality. In functional studies, they showed that Beclin-1 promoted progesterone expression through the degradation of low-density lipoprotein.
Li et al. [22] examined the nuclear factor-ĸB (NF-ĸB) pathway and found that granulosa cells in patients with endometriomas had higher NF-ĸB binding activity. They also examined telomerase activity, which was inversely related to NF-ĸB binding levels. In cultured granulosa cells, tumor necrosis factor-alpha (TNF-alpha) reduced human telomerase reverse transcriptase (hTERT) and telomerase. The authors hypothesized that in the presence of ovarian endometriomas, there may be higher TNF-alpha that increases NF-ĸB pathway activation and reduces telomerase activity in granulosa cells, resulting in increased granulosa cell senescence. Given the importance of granulosa cells in promoting aromatase, this granulosa cell senescence, apoptosis, and autophagy may together account in part for the observation of decreased estradiol concentrations in endometriosis [11].
Recent studies have utilized innovative technologies to study granulosa cells in the presence of endometriomas. Notarstefano et al. [23] used infrared and Raman microspectroscopy on luteinized granulosa cells and found indirect evidence for oxidative stress and lipid/carbohydrate metabolism abnormalities, both in the endometrioma-affected ovary and in the normal contralateral ovary, in comparison to control ovaries. Da Luz et al. examined the transcriptome of cumulus cells from endometriosis patients with or without endometrioma, compared to controls, using RNA sequencing [24]. There were 461 differentially expressed genes between endometrioma cases and control, and 66 between endometriosis (non-endometrioma) cases and controls. These differentially expressed genes were involved in oocyte competence including oxidative phosphorylation, mitochondrial functioning, and steroid metabolism. Interestingly, there were no differentially expressed genes comparing endometriosis cases with or without endometrioma. Another study [25] involved microRNA profiling in cumulus cells and found that miR-532-3p was significantly lower in stage III/IV endometriosis compared to stage I/II and to the infertile control group (only five cases per group). The authors noted that this microRNA-regulated pathway is involved in oocyte competence and oocyte meiosis.
7 Follicular Fluid Abnormalities
In general, there is evidence that the follicular fluid in ovaries affected by endometriomas may be associated with increased oxidative stress (e.g. mediated by iron) and inflammation (e.g. IL-8 and IL-12) that lead to decreased oocyte quality [11]. It should be noted that one study did not find a difference in oxidative stress in endometriomas [26], while another did find evidence for an increase in ferritin and reactive oxygen species pathways using a proteomic tandem mass spectrometry approach in endometriomas [27]. Li et al. [28] also sampled follicular fluid in patients with stage III and IV endometriosis (anatomic subtypes not specified) and found the endometriosis group to have decreased transferrin and iron overload and, using a mouse model, demonstrated that this may contribute to abnormal oocyte maturation. Another study found increased ferritin in the affected ovary compared to the contralateral normal ovary, but no difference in iron [29].
This iron overload and subsequent oxidative stress leads to local inflammation. Mao et al. [30] found that the follicular fluid cytokine profile in patients with a history of endometriosis compared to controls showed some that were elevated (e.g. IL-14, IL-13, IL-3, and IL-1alpha) and some were decreased (e.g. IFN-gamma). Yland et al. [31] recently profiled cytokines in follicular fluid in patients with endometriomas compared to controls. They found that a set of cytokines that were hypothesized to be abnormal in endometriosis (e.g. IL-6, IL-8, and IL-1beta) were generally elevated in endometrioma-affected ovaries (and, in some cases, the contralateral normal ovary in the same patient) compared to control ovaries. Toll-like receptors (TLRs) and associated inflammation have also been investigated in ovarian endometriosis [32]. In follicular fluid of endometrioma-affected ovaries, there was an increase in cytokines such as IL-6 and IL-8, and, in cell pellets from the follicular fluid, there was an increase in TLR1, 5, 6, 7, 8, 10, as well as NF-ĸB, IL-10 and transforming growth factor-beta (TGF-β).
It should be noted that mitochondrial superoxide dismutase (SOD2) is an anti-oxidant that converts superoxide to hydrogen peroxide that is subsequently detoxified [33]. Imbalances between enzymes may result in imbalances in reactive oxygen species, and, in fact, the accumulation of hydrogen peroxide may promote cell proliferation. Thus, while SOD2 has an anti-oxidant effect, there is some evidence that it can promote tumor cell proliferation and progression perhaps via hydrogen peroxide. In this study [33], endometriomas had increased expression of SOD2 (in response to increased oxidative stress), and, in endometrial primary cell cultures, there was evidence of SOD2-promoting cell proliferation and migration.
Finally, a microRNA profiling study was done on follicular fluid from 30 patients with ovarian endometriomas compared to controls [34]. The authors found that miR-451 was decreased in endometriosis, and, in functional studies, inhibiting miR-451 in human and mouse oocytes negatively affected oocyte and embryonic development with possible involvement of the Wnt pathway.
8 Reduction in Oocyte Quantity
The above mechanisms can reduce oocyte quality, as evidenced by changes in morphology, the spindle apparatus, and the mitochondrial content of the cytoplasm [11]. For example, Ferrero et al. [35] examined metaphase II oocytes from patients with ovarian endometriomas compared to healthy egg donors. Single-cell RNA sequencing was performed. They found numerous differentially expressed genes, typically overexpression, for oocytes from both the affected ovary and the normal contralateral ovary, in comparison to the egg donors. These genes were involved in a variety of processes such as cell growth, oxidative stress, and steroid metabolism, with particular enrichment for the mitochondria.
However, endometriomas may also reduce oocyte quantity [36]; for example, a prospective longitudinal study found that a larger reduction in markers of ovarian reserve in women with endometrioma-affected ovaries compared to controls [37]. As well, follicle density is lower in ovaries with endometriomas compared to the unaffected contralateral ovary [38], and, more so, in comparison to other non-endometriosis benign cysts [39].
Both oxidative stress and fibrosis induced by the associated local inflammation in endometriomas may lead to follicular depletion and decreased oocyte quantity [10]. A reduction in ovarian cortical stromal vascularization may also contribute [10]. In the presence of endometriomas, there may also be an increase in early follicular development and subsequent atresia [10]. Di Nisio et al. found that the ovarian cortex adjacent to an ovarian endometrioma had higher expression of apoptosis-associated caspase 8, and also of p53 that is involved in the regulation of oxidative stress response and apoptosis [40]. Altogether these mechanisms may lead to a “burnout” of follicles and decreased ovarian reserve [10].
Notably, Takeuchi et al. utilized a mouse model of endometriosis and oocytes from ovaries with endometriomas [41]. In the mouse model, there was a decrease in primordial follicles and an increase in primary, secondary, and antral follicles, suggesting elevated primordial follicle activation. In human oocytes from ovaries with endometriomas, there was an activation of the phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) pathway that when inhibited in a mouse model, increased the primordial follicles. Therefore, endometriomas may be associated with over-activation of primordial follicles mediated via the PI3K-Akt pathway, leading to “burnout” and a decrease in ovarian reserve.
The decrease in primordial follicles in endometrioma-affected ovaries may involve the Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) pathway known to be involved in primordial follicle activation [42]. In particular, YAP/TAZ are regulated by tissue stiffness and stretching. Thus, the stretching caused by an ovarian endometrioma may mechanotransduce YAP/TAZ that leads to the hyperactivation of primordial follicles, although the authors note that there are likely multiple pathways involved than just simple stretching of ovarian tissue. For example, they hypothesize that endometriomas may release reactive oxygen species and inflammatory factors that can promote the PI3K/Akt pathway, which can lead to hyperactivation of primordial follicles that further promote a reduction in ovarian reserve.
Regarding the environment around the endometrioma, reactive oxygen species may promote local tissue fibrosis, a change in follicular pattern, and vascular alterations [1]. Fibrosis results in a reduction in follicles and cortex-specific stroma and may also negatively affect follicular development. The loss of stroma is also important due to its role in providing blood supply to primordial follicles. This fibrosis and reduction in vascularization further compound the decrease in oocyte quantity.
9 Conclusion
In conclusion, endometrioma-affected ovaries are characterized by anatomic distortion and several pathophysiological changes including increased iron-mediated oxidative stress and inflammation. Together, these pathways may impair oocyte quality and quantity (Fig. 1). These biological observations have potential implications for clinical management, in terms of the potential long-term ongoing effects of an un-operated endometrioma on ovarian structure and function (due to oxidative stress and inflammation), and whether these effects can be attenuated by hormonal therapy or are in any way altered by surgical removal.
References
Sanchez AM, Vigano P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2014;20(2):217–30.
Muzii L, Bianchi A, Bellati F, Cristi E, Pernice M, Zullo MA, et al. Histologic analysis of endometriomas: what the surgeon needs to know. Fertil Steril. 2007;87(2):362–6.
Sanchez AM, Vigano P, Somigliana E, Cioffi R, Panina-Bordignon P, Candiani M. The endometriotic tissue lining the internal surface of endometrioma: hormonal, genetic, epigenetic status, and gene expression profile. Reprod Sci. 2015;22(4):391–401.
Yamagata Y, Nishino K, Takaki E, Sato S, Maekawa R, Nakai A, et al. Genome-wide DNA methylation profiling in cultured eutopic and ectopic endometrial stromal cells. PLoS One. 2014;9(1):e83612.
Borghese B, Barbaux S, Mondon F, Santulli P, Pierre G, Vinci G, et al. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24(9):1872–85.
Yong PJ, Talhouk A, Anglesio MS. Somatic genomic events in endometriosis: review of the literature and approach to phenotyping. Reprod Sci. 2021; https://doi.org/10.1007/s43032-020-00451-9.
Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24(7):1777–89.
Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Adachi S, Kase H, et al. Different mutation profiles between epithelium and stroma in endometriosis and normal endometrium. Hum Reprod. 2019;34(10):1899–905.
Hayashi S, Nakamura T, Motooka Y, Ito F, Jiang L, Akatsuka S, et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020;37:101726.
Donnez J, Donnez O, Orellana R, Binda MM, Dolmans MM. Endometriosis and infertility. Panminerva Med. 2016;58(2):143–50.
Sanchez AM, Vanni VS, Bartiromo L, Papaleo E, Zilberberg E, Candiani M, et al. Is the oocyte quality affected by endometriosis? A review of the literature. J Ovarian Res. 2017;10(1):43.
Gupta S, Ghulmiyyah J, Sharma R, Halabi J, Agarwal A. Power of proteomics in linking oxidative stress and female infertility. Biomed Res Int. 2014;2014:916212.
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79.
Giacomini E, Sanchez AM, Sarais V, Beitawi SA, Candiani M, Vigano P. Characteristics of follicular fluid in ovaries with endometriomas. Eur J Obstet Gynecol Reprod Biol. 2017;209:34–8.
Sanchez AM, Papaleo E, Corti L, Santambrogio P, Levi S, Vigano P, et al. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum Reprod. 2014;29(3):577–83.
Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta, Mol Cell Res. 2019;1866(12):118535.
Huo P, Zhang N, Zhang P, Wu X. The levels of follicular fluid cell-free mitochondrial DNA could serve as a biomarker for pregnancy success in patients with small ovarian endometriosis cysts: a case-control study. Medicine (Baltimore). 2020;99(48):e23348.
Sreerangaraja Urs DB, Wu WH, Komrskova K, Postlerova P, Lin YF, Tzeng CR, et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int J Mol Sci. 2020;21:10.
Kunitomi C, Harada M, Takahashi N, Azhary JMK, Kusamoto A, Nose E, et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol Hum Reprod. 2020;26(1):40–52.
Cordeiro FB, Cataldi TR, Perkel KJ, do Vale Teixeira da Costa L, Rochetti RC, Stevanato J, et al. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J Assist Reprod Genet. 2015;32(12):1817–25.
Ding Y, Zhu Q, He Y, Lu Y, Wang Y, Qi J, et al. Induction of autophagy by Beclin-1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl Res. 2021;227:15–29.
Li Y, Li R, Ouyang N, Dai K, Yuan P, Zheng L, et al. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil Steril. 2019;112(5):882–91. e1
Notarstefano V, Gioacchini G, Byrne HJ, Zaca C, Sereni E, Vaccari L, et al. Vibrational characterization of granulosa cells from patients affected by unilateral ovarian endometriosis: new insights from infrared and Raman microspectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2019;212:206–14.
Da Luz CM, Da Broi MG, Placa JR, Silva WA Jr, Meola J, Navarro PA. Altered transcriptome in cumulus cells of infertile women with advanced endometriosis with and without endometrioma. Reprod Biomed Online. 2021;42(5):952–62.
da Silva LFI, Da Broi MG, da Luz CM, da Silva L, Ferriani RA, Meola J, et al. miR-532-3p: a possible altered miRNA in cumulus cells of infertile women with advanced endometriosis. Reprod Biomed Online. 2021;42(3):579–88.
Nakagawa K, Hisano M, Sugiyama R, Yamaguchi K. Measurement of oxidative stress in the follicular fluid of infertility patients with an endometrioma. Arch Gynecol Obstet. 2016;293(1):197–202.
Regiani T, Cordeiro FB, da Costa LV, Salgueiro J, Cardozo K, Carvalho VM, et al. Follicular fluid alterations in endometriosis: label-free proteomics by MS(E) as a functional tool for endometriosis. Syst Biol Reprod Med. 2015;61(5):263–76.
Li A, Ni Z, Zhang J, Cai Z, Kuang Y, Yu C. Transferrin insufficiency and iron overload in follicular fluid contribute to oocyte dysmaturity in infertile women with advanced endometriosis. Front Endocrinol (Lausanne). 2020;11:391.
Benaglia L, Paffoni A, Mangiarini A, Restelli L, Bettinardi N, Somigliana E, et al. Intrafollicular iron and ferritin in women with ovarian endometriomas. Acta Obstet Gynecol Scand. 2015;94(6):646–53.
Mao XD, Hu CY, Zhu MC, Ou HL, Qian YL. Immunological microenvironment alterations in follicles of women with proven severe endometriosis undergoing in vitro fertilization. Mol Biol Rep. 2019;46(5):4675–84.
Yland J, Carvalho LFP, Beste M, Bailey A, Thomas C, Abrao MS, et al. Endometrioma, the follicular fluid inflammatory network and its association with oocyte and embryo characteristics. Reprod Biomed Online. 2020;40(3):399–408.
Jafari R, Taghavi SA, Amirchaghmaghi E, Yazdi RS, Karimian L, Ashrafi M, et al. Detailed investigation of downstream TLR signaling in the follicular cells of women with endometriosis. J Reprod Infertil. 2020;21(4):231–9.
Chen C, Zhou Y, Hu C, Wang Y, Yan Z, Li Z, et al. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic Biol Med. 2019;136:22–34.
Li X, Zhang W, Fu J, Xu Y, Gu R, Qu R, et al. MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod Biol Endocrinol. 2019;17(1):96.
Ferrero H, Corachan A, Aguilar A, Quinonero A, Carbajo-Garcia MC, Alama P, et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum Reprod. 2019;34(7):1302–12.
Broi MGD, Ferriani RA, Navarro PA. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist Reprod. 2019;23(3):273–80.
Kasapoglu I, Ata B, Uyaniklar O, Seyhan A, Orhan A, Yildiz Oguz S, et al. Endometrioma-related reduction in ovarian reserve (ERROR): a prospective longitudinal study. Fertil Steril. 2018;110(1):122–7.
Kitajima M, Defrere S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96(3):685–91.
Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Dechelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20(7):1786–92.
Di Nisio V, Rossi G, Di Luigi G, Palumbo P, D'Alfonso A, Iorio R, et al. Increased levels of proapoptotic markers in normal ovarian cortex surrounding small endometriotic cysts. Reprod Biol. 2019;19(3):225–9.
Takeuchi A, Koga K, Satake E, Makabe T, Taguchi A, Miyashita M, et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-Akt-Foxo3 pathway. J Clin Endocinol Metab. 2019;104(11):5547–54.
Matsuzaki S, Pankhurst MW. Hyperactivation of dormant primordial follicles in ovarian endometrioma patients. Reproduction. 2020;160(6):R145–R53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yong, P.J., Bedaiwy, M.A. (2024). Impact of the Endometriomas on the Ovarian Follicles. In: Ferrero, S. (eds) Endometriosis-related Infertility. Springer, Cham. https://doi.org/10.1007/978-3-031-50662-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-50662-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50661-1
Online ISBN: 978-3-031-50662-8
eBook Packages: MedicineMedicine (R0)