Abstract
Achalasia is a primary disorder of esophageal motility and is the most common motor neuron disorder of the esophagus. Classic presentation involves dysphagia to solids and liquids with regurgitation and chest pain. The gold standard test is using high-resolution manometry to evaluate the motility of the esophagus when an endoscopy has ruled out causes of pseudo-achalasia, such as a cancer at the gastroesophageal junction. Endoscopic appearance of the esophagus at the lower esophageal sphincter often shows a “puckered appearance” with retained food or saliva proximal to this narrowing. Barium esophagram may reveal distal tapering of the lower esophageal sphincter giving the classic “bird beak’s appearance.” Several therapeutic options for achalasia are available including both endoscopic and surgical options for those able to tolerate the risks of these procedures. Increased attention has been placed on management based on subtype of achalasia to direct a personalized approach. Limitations of the current state of achalasia include the lack of etiological risk factor and lack of curative approaches. The goals of therapy are to improve symptoms and prevent esophageal stasis. Future work guided at understanding fundamental causal factors of achalasia, which may provide insight to more durable therapies is needed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Esophageal peristalsis
- EPT
- EGJOO
- Esophagogastroduodenoscopy
- Functional lumen imaging probe
- Botulinum toxin injection
- Pneumatic dilation
- POEM
- Laparoscopic Heller myotomy
Introduction
Achalasia is a primary esophageal motility disorder that results from loss of intrinsic inhibitory innervation of the lower esophageal sphincter (LES) and the smooth muscle segment of the esophageal body. Classic symptoms include dysphagia to solids and liquids associated with regurgitation of undigested food. The etiology of achalasia is unclear with several proposed theories including immune-mediated response of neuronal degeneration. Histologically, there has been evidence of inflammation of the myenteric inhibitory ganglion cells with some studies showing loss of inhibitory neurons via inflammation with subsequent neuronal destruction and fibrosis [1, 2]. Improvement in diagnostic modalities with esophageal pressure topography (EPT) has identified subgroups of achalasia patients based on carefully validated metrics to quantify LES relaxation and esophageal peristaltic function. Currently, the Chicago Classification is used to determine the subtype of achalasia (type I, II, or III) based on high-resolution manometry (HRM) . Along with the improvement in diagnostic tools, treatment options including endoscopic and surgical options have advanced management for achalasia. As the etiology of achalasia is still undefined, our treatment options are aimed at mechanical disruption of the LES, but a cure for achalasia is still not available.
Epidemiology
Incidence and Prevalence
The incidence of achalasia is 1/100,000, and due to the chronicity of symptoms, the prevalence is around 10/100,000 [3,4,5,6]. Achalasia has no age nor gender preference, and its chronicity affects patient’s health-related quality of life, work productivity, and functional status [7]. In Iceland, 62 cases of achalasia were diagnosed over a 51-year surveillance (overall incidence 0.6/100,000 per year) [4]. In the United States, hospitalization for achalasia ranged from 0.25/100,000 (<18 years old) to 37/100,000 (>85 years old) [8, 9].
Age
The peak incidence is between 30 and 60 years old [10, 11].
Gender and Race
Achalasia occurs equally among women and men and is without racial predilection.
Genetics
Utilizing research from twin and sibling studies, genetic underpinnings of achalasia show an association with other diseases, such as Parkinson’s, Allgrove syndrome, and Down syndrome [12,13,14]. The most well-known genetic syndrome is Allgrove syndrome , also known as “triple A” syndrome , which included achalasia, alacrima, and adrenal insufficiency due to a defect in the AAAS gene (chromosome 12q13) with defective tryptophan-aspartic acid repeat protein [15,16,17]. Familial cases of achalasia combined with abnormal polymorphisms of nitric oxide or interleukin expression (IL-23 and IL-10) have added support for a genetic etiology [18,19,20].
Case-control studies and a genetic association study have shown the contribution of human leukocyte antigen (HLA) class II genes in to susceptibility to achalasia [21,22,23]. A genetic association study in achalasia and controls mapped a strong major histocompatibility complex association signal by imputing classical HLA haplotypes and amino acid polymorphism. To date, the only known achalasia risk factor is an eight-residue insertion located in the cytoplasmic tail of HLA-DQβ1 receptor [24]. Data are otherwise sparse on genetic and/or phenomic association in achalasia. Studies of molecular pathology have also suggested the consideration of achalasia as an autoimmune inflammatory disorder [25, 26]. This is supported by the presence of anti-myenteric antibodies in the circulation and inflammatory T-cell infiltrates in the myenteric plexus in achalasia. Patients with achalasia are 3.6× more likely to have other autoimmune diseases including uveitis, Type I diabetes, rheumatoid arthritis, systemic lupus erythematous, and Sjögren’s syndrome [27]. However, at this time, there is no role for genetic testing in routine clinical practice.
Pathogenesis
Esophageal peristalsis is a result of complicated contractile and relaxation forces. One of the keys to understanding the pathogenesis of achalasia is to better characterize the role of autonomic ganglia in controlling distal esophageal and LES contractility. Esophageal contraction is predominately orchestrated by the postganglionic neurons which are the neurons targeted in achalasia (Fig. 7.1) [28].
Neuronal injury that secretes VIP and NO leads to unopposed excitatory activity and failure of LES relaxation. (Adapted from: Patel et al. [28])
Precise balance of the contractions and inhibitions is responsible for the manometric observation of a normal esophageal peristalsis post deglutition [29,30,31,32]. In achalasia, the selective destruction of the neuroinhibitory fibers lead to loss of peristalsis and inability of the LES to relax leading to the classic manometry findings of achalasia (Fig. 7.2). The causes of an initial reduction of inhibitory neurons is unknown with theories including a possible autoimmune etiology (herpes, measles) which may trigger neuronal degeneration in a genetically predisposed host [33]. Achalasia patients are more likely to have concomitant autoimmune diseases and higher prevalence of serum neural antibodies [27, 34]. However, infectious etiologies should also be kept in the differential as seen in Chagas disease by the parasite Trypanosoma cruzi, which can cause achalasia [35].
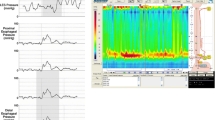
High-resolution manometry showing the three subtypes of achalasia. Type I is characterized by absent contractility; type II shows pan-esophageal pressurization; and type III shows simultaneous contractions. (Adapted from: Patel et al. [28])
The exact cause of the alterations in the myenteric plexus, including progressive degeneration and destruction of myenteric neurons, in patients with achalasia remains to be determined. However, studies have suggested a significant decrease or absent NO innervation in the myenteric plexus of patients with achalasia [29, 36]. The current hypothesis is that an initiating event, probably an environmental insult such as a viral infection, creates a cascade of inflammatory changes and damage to the myenteric plexus [33, 37, 38]. This inflammation triggers an autoimmune response, leading to chronic inflammation with subsequent complete destruction of the inhibitory neurons in the myenteric plexus (Fig. 7.3) [1]. A recent study evaluated 26 specimens in patients with achalasia and found inflammatory changes including capillaritis (51%), plexitis (23%), nerve hypertrophy (16%), venulitis (7%), and fibrosis (3%) [26].
Possible mechanisms for the development of achalasia ranging from viral triggers, genotype susceptibility, and genetic changes interacting with immune changes which can lead to esophageal neuronal changes. (Adapted from: Patel et al. [28])
Opioids
Detrimental effects of opioids on esophageal motility has been known since the 1980s where repeated dosing of morphine in healthy individuals led to an increase in LES pressure and decreased sphincter relaxation [39]. However, recently multiple studies have shown increased rate of esophagogastric junction outflow obstruction (EGJOO) , type III achalasia, and esophageal spasm in patients on chronic opiates suggesting possible opioid-induced esophageal dysfunction [40,41,42,43]. The largest retrospective cohort included 2342 patients (224 on chronic daily opioids) and found that patients on opioids were more likely to report dysphagia (62% vs. 43%, P < 0.01) and were more likely to have type III achalasia (13% vs 1%, P < 0.01), EGJOO (13% vs. 3%, P < 0.01), and esophageal spasm (3% vs. 0.5%, P < 0.01) [44].
Management of patients with narcotics is difficult, but in the case of achalasia-like symptoms, reduction of narcotics to the lowest dose tolerated or transitioning to non-opioid analgesia is preferred. Manometric abnormalities in patients with opioid-induced esophageal dysfunction can normalize when patients are studied off opiates [45]. In one small case series, three out of five patients using pneumatic dilation for opioid-mediated esophageal dysfunction had little improvement in symptoms [43]. If the opioid cannot be stopped, injection with botulinum toxin can be considered. More invasive procedures, such as pneumatic dilation, surgical myotomy, and peroral endoscopic myotomy (POEM), should be approached with significant caution and reserved for refractory cases after discussion about the risks, benefits, and potential failure given the lower than average response rate in patients on chronic opioids [46, 47].
Diagnosis
Clinical Manifestations
The diagnosis of achalasia starts with symptom presentation of dysphagia, typically to solid and liquids. Patients can also present with associated regurgitation of undigested food or chest pain. Occasionally, patients report having reflux symptoms and are nonresponsive to acid suppression. A high index of suspicion for achalasia should be present for patients with reflux symptoms and regurgitation without symptom improvement despite acid suppression. Younger patients are more likely to report heartburn and chest pain compared to older patients [48]. Obese patients (body mass index >30) present frequently with choking and vomiting [49]. Despite their symptoms of dysphagia, the degree of weight loss varies with a recent study showing the correlation of achalasia with phenotype, where type II achalasia patients were most likely and type III achalasia least likely to have weight loss compared to type I achalasia [50].
Respiratory symptoms are also common due to the increased risk of aspiration secondary to retained food and saliva in the esophagus. Of 110 patients with achalasia, 40% of patients reported at least 1 respiratory symptom daily, which improved after therapy directed at the LES [51, 52]. In a retrospective study, the symptoms of dysphagia preceded respiratory symptoms by an average of 24 months, supporting the retention hypothesis as the etiology for aspiration and respiratory complaints [53]. However, there are several other etiologies of respiratory causes and dysphagia, including oropharyngeal dysphagia, connective tissues diseases (i.e., scleroderma), or extraesophageal gastroesophageal reflux disease (GERD) which should be on the differential.
Subtypes
EPT is a major advancement in the field of esophageal physiology [54]. With the innovative advent of EPT and HRM, achalasia is now recognized to present with three distinct manometric subtypes (Fig. 7.2) [55].All three phenotypes have impaired EGJ relaxation and absent esophageal peristalsis, but the distinguishing features are in the pattern of esophageal pressurization. Type I achalasia is characterized by absence of esophageal pressurization to more than 30 mmHg and has 100% failed peristalsis (aperistalsis), type II is associated with panesophageal pressurization to greater than 30 mm Hg, and type III has spastic contractions due to abnormal lumen obliterating contractions with or without periods of panesophageal pressurization [56].
Manometric subtypes have been shown to have prognostic and treatment implications. Success rates for both pneumatic dilation (PD) and Heller myotomy (HM) are significantly higher in subtypes I and II than type III. The latter subtype (type III) responds the least to reducing the LES pressure, as the segment affected by the spastic motility extends well above the LES [57]. This subtype of achalasia is characterized by chest pain due to lumen obliterating spastic contractions in the distal esophagus. It is proposed that type III achalasia may represent early disease with progression to type II followed by type I over time. However, pathophysiologic basis of this proposed progression is lacking. Recent studies also suggest that type I achalasia may represent decompensated esophagus to outflow obstructions caused by a dysfunctional LES accompanied with a complete aganglionosis [58].
Esophagogastric Junction Outflow Obstruction (EGJOO)
When the IRP is greater than 15 mmHg but there is peristalsis that does not meet criteria for type I, II, or III achalasia, the Chicago Classification labels this as EGJOO. This potential phenotype of achalasia is an important but heterogenous group [59]. There are multiple etiologies of EGJOO including incompletely expressed or early achalasia, esophageal wall stiffness, infiltrative cancer, hiatal hernia, obesity, or opiate-induced [45]. Further evaluation with endoscopic ultrasound, CT, or functional luminal imaging probe (FLIP) can be done to better elucidate the etiology of EGJOO. In some studies, patients with EGJOO were monitored conservatively, and their “disorder” resolved spontaneously [60, 61]. To increase the yield of EGJOO, provocative maneuvers during HRM can help, including rapid drink challenge or solid meal challenge [62, 63]. The mechanism of these maneuvers is that increasing the volume or viscosity of the bolus increases esophageal pressurization and thus IRP increases [47].
Esophagogastroduodenoscopy
Symptoms of dysphagia should prompt an esophagogastroduodenoscopy (EGD) with mucosal biopsies. These findings can help rule out GERD, eosinophilic esophagitis (EoE), or structural causes, such as rings or webs. On EGD, a “puckered” gastroesophageal (GE) junction with retention of solid or liquid material proximal to GE junction is commonly seen (Fig. 7.4). In more advanced cases, the esophagus can be dilated or tortuous due to chronic stasis. There can be resistance with passage of the endoscope through the GE junction due to failure of the LES to relax. When achalasia is suspected, a thorough retroflexion should be completed to fully evaluate the GE junction and cardia to rule out malignancy, which can cause pseudo-achalasia. Due to the stasis from the failure of the LES to relax, there can be esophageal candidiasis, which in the context of an intact immune function should prompt concern for esophageal dysmotility. Endoscopy can be helpful for its ability to rule out other causes of dysphagia and help support a diagnosis of achalasia, but other testing is often needed to confirm the diagnosis of achalasia.
Endoscopic evaluation of achalasia showing (a) puckered GE junction and (b) retained saliva. (Adapted from: Patel et al. [28])
Histological Features
Though biopsies are more helpful to rule out other causes of dysphagia, such as EoE, histopathological analysis has been performed on achalasia patients. Prior studies have shown predominantly capillaritis with varying amounts of plexitis, nerve hypertrophy, venulitis, and fibrosis with identified presence of HSV-1 antibodies supporting a possible neurotropic viral infection leading to an autoimmune inflammatory cascade [25]. In a concentrated histopathological examination, subtypes of achalasia showed greater degree of myenteric ganglion cell loss in type I achalasia compared to type II proposing that type I achalasia may represent disease progression from type II achalasia [58]. In all types of achalasia, there was a spectrum from complete neuronal loss to lymphocytic inflammation to apparently normal tissue suggesting a pathogenically heterogeneous patient group with a common esophagogastric junction outflow obstruction.
Barium Esophagram
A noninvasive method to help with the diagnosis of achalasia is to perform a barium esophagram, which can show the classic “bird beak’s” appearance due to the narrowing of the GE junction. Other findings include aperistalsis, dilated esophagus, or a “cork appearance” of the esophagus (Fig. 7.5). However, this method is not sensitive for diagnosis of achalasia, and other modalities such as manometry are essential to confirm the diagnosis.
Barium esophagram showing retained contents in the proximal esophagus and “bird beak’s” appearance due to incomplete relaxation of the lower esophageal sphincter. (Adapted from: Patel et al. [28])
Esophageal Manometry
The gold standard for diagnosis of achalasia is esophageal manometry, which involves transnasal placement of a flexible catheter into the esophagus to measure esophageal pressures and contractions along the length of the esophagus. Prior line tracings from water-perfused or strain gauge systems have now been replacement with high-resolution manometry systems that present pressure tracings in EPT plots [64, 65]. Building on the work of Clouse and plots of contractile activity, the Chicago Classification was created to define and diagnose motility disorders (Fig. 7.6) [55].
Manometric diagnosis of achalasia and EGJOO . The Chicago Classification v3.0, hierarchical classification. (Modified from Kahrilas et al. [55])
By using EPT , achalasia has been further characterized into three clinically important subtypes that have shown differences in response to therapy (Fig. 7.2). The diagnosis of achalasia is made by demonstrating impaired relaxation of the lower esophageal sphincter and absent peristalsis in the absence of esophageal obstruction due to a secondary cause (i.e., pseudo-achalasia from a GE junction tumor). Manometric analysis showing an elevated integrated relaxation pressure and 100% failed peristalsis or spasm meets criteria for achalasia. The phenotype depends if there is no contractility (type I), greater than 20% pan-esophageal pressurization (type II), or greater than 20% spasm [a distal latency less than 4.5 seconds] (type III). These three subtypes of achalasia have prognostic and therapeutic implications [56].
Functional Lumen Imaging Probe (FLIP)
Per Chicago Classification version 3.0, the IRP must be greater than 15 mmHg which means the EGJ pressure is greater than 15 mmHg, which is not always the case, particularly in type I achalasia. The etiology for this may be due to in part for those with advanced disease having very low LES pressures. Prior attempts to decrease the IRP cutoff have been rejected as there are some diagnosis of achalasia with IRP values of 3 mmHg and 5 mmHg, which were seen with the use of functional luminal imaging probe (FLIP) technology and stasis on esophagram [66, 67]. FLIP has aimed to better assess this group of patients with being able to measure a distensibility index, which is a metric relating EGJ opening diameter to intraluminal distensible pressure. Using this index, a threshold of 2.8 mm2 per mmHg has been the most helpful in diagnosing abnormal EGJ function [68]. Alternatively, one can use minimal bolus flow time during HRM, a timed barium esophagram, or rapid drink challenge to also obtain this diagnosis [63, 69,70,71]. Intraoperative use of FLIP during laparoscopic HM or POEM might also offer the ability to assess the efficacy of LES myotomy in real-time and predict postoperative symptomatic outcomes [72,73,74].
Treatment Options
There is no curative option for achalasia; all treatment options are directed toward improving quality of life and attempting to preserve esophageal function and preventing esophageal stasis. Current treatment options aim to reduce the hypertonicity of the LES to improve esophageal emptying by gravity.
Therapeutic options are divided into oral pharmacological, endoscopic (pharmacological, pneumatic dilation, myotomy), and surgical (myotomy or esophagectomy) (Fig. 7.7). The choice of treatment is based on surgical candidacy, age, comorbidities, dilation of esophagus, patient preference, local expertise, and manometric subtype. The most effective therapies to help preserve esophageal function include pneumatic dilation, surgical myotomy, and POEM. Pharmacological therapy, whether oral or endoscopically injected, has decreased efficacy as compared to the three aforementioned techniques. In patients who have end-stage achalasia with severely dilated “sigmoid”-shaped esophagus and nonresponsive to other options, esophagectomy can be considered.
Proposed mechanism of treatment for achalasia based on low and high surgical risk. (Adapted from: Patel et al. [28])
Pharmacological Therapies
Pharmacological therapy is the least effective treatment modality for achalasia. The response to these agents is short lived, and their side-effect profile often limits compliance or dose escalation. Oral therapies are reserved for those patients who are not candidate for more definitive endoscopic or surgical options due to comorbidities. Options for therapy are varied, but the most common include calcium channel blockers (i.e., nifedipine , 10–30 mg given 30–45 minutes prior to meals) or long-acting nitrates (isosorbide dinitrate, 5–10 mg given 15 minutes prior to meals) [75,76,77,78,79,80,81]. Both calcium channel blockers and long-acting nitrates cause a rapid reduction in lower esophageal sphincter of up to 47–64%, but unfortunately this translates poorly to symptom improvement with modest dysphagia improvement [76]. An alternative option includes the use of off-label phosphodiesterase-5 inhibitors (i.e., sildenafil ) which lowers esophagogastric junction pressure, but symptom improvement is also modest, and long-term studies are lacking [80, 82]. Given the limited efficacy of oral pharmacological therapy, this option is reserved for patients who cannot undergo a more definitive therapeutic approach (Table 7.1).
Endoscopic Options
Botulinum Toxin
For patients who cannot tolerate a more invasive approach, botulinum toxin injection (BTI), a potent inhibitor of acetylcholine release from the presynaptic terminals, is a useful treatment strategy. BTI blocks unopposed cholinergic stimulation caused by the selective loss of inhibitory interneurons. This is injected during endoscopy where under direct visualization, 100 units of toxin are injected in 25 units aliquots in 4 quadrants via a sclero-needle just proximal to the squamo-columnar junction. Issues with the use of BTI are the transitory effect of the injection which often needs repeat procedures typically every 6–12 months. Complications with the procedure include chest pain (16–25%) and rarely more serious complications of mediastinitis and allergy to an egg-based protein.
The immediate response to BTI is as high as 80–90%, but over half of patients are symptomatic at 1 year (Table 7.2) [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Predictive factors for response to BTI include older age (over 40 years old), type II phenotype, and decreased basal LES pressure following treatment [84]. Repeated BTI can make surgical myotomy more difficult due to the creation of fibrosis; hence BTI should not be first line for patients who are eligible for more definite endoscopic or surgical options [98]. Though more effective than oral pharmacological therapy, BTI is not as effective as PD, POEM, or surgical myotomy. As discussed previously, for patients with achalasia-like phenotype from opioids who cannot stop their opioid, BTI might be a better alternative.
Pneumatic Dilation (PD)
PD is performed using an noncompliant balloon that employs air pressures to disrupt or fracture the LES circular muscle fibers and is an effective nonsurgical option in the treatment of achalasia [10, 99]. Currently, the most widely used balloon dilator for PD is the Rigiflex, a nonradiopaque graded size polyethylene balloon. The Rigiflex dilators can be performed under direct visualization or under radiological guidance (fluoroscopy) [100, 101]. The dilators are available in three diameters (3.0, 3.5, and 4.0 cm), which allow a graded dilated approach. When employing this graded approach, relief of symptoms is possible in 50–93% of patients (Table 7.3) [100, 102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127].
Graded PD is performed by an initial dilation at 3.0 cm, then 3.5 cm, and finishing at 4.0 cm with 4–6 weeks in between dilations. Reassessment of symptoms and LES pressure can be performed between each session to determine the necessity of subsequent treatments. It is estimated that a third of patients treated with PD will experience symptom relapse within 4–6 years.
Predictive factors of a poor clinical response to treatment include age less than 40 years, male sex, LES pressure after dilation greater than 10–15 mmHg, and continued symptoms after one or two treatments [128,129,130,131]. Additionally, males younger than 45 years of age may not be as responsive to the serial approach possibly due to thicker LES musculature. In these patients, it is recommended to either start with PD at 3.5 cm or proceed straight to surgical myotomy as the initial step in management.
Of the manometric subtypes, type II achalasia has better outcomes with PD [132]. Surgical myotomy has a greater response than a single pneumatic dilation, but a graded approach with PD is a reasonable alternative to surgery as it has similar efficacy. Given the risk of perforation, which is around 2%, all patients who undergo PD must be surgical candidates in case a perforation were to occur [133]. Depending on the length and extent of the perforation, the complication can be managed conservatively with stent placement, antibiotics, and parenteral nutrition; however, larger perforations with mediastinal contamination will need a surgical repair via thoracostomy. Post-dilation, there is an increased risk of GERD, seen in 15–35% of patients post PD due to the disruption of the LES. Hence, initiation of acid suppression is recommended for patients with pre-existing GERD or new symptoms of heartburn or reflux [134]. It is important to note that dysphagia after PD can be due to the underlying achalasia or could be due to a reflux stricture; endoscopy can help separate these two etiologies.
Peroral Endoscopic Myotomy (POEM)
Peroral endoscopic myotomy (POEM) is a minimally invasive endoscopic technique and is one of the most recent advances in the treatment of achalasia (Table 7.4). The procedure is performed endoscopically with a small mucosal incision in the mid-esophagus and creating a submucosal tunnel to the gastric cardia. This technique allows careful and selective myotomy of the circular muscle.
In 2010, Inoue and investigators published a prospective trial of 17 patients undergoing endoscopic myotomy that revealed significant reduction in the index of dysphagia symptoms (10 to 1.3, P = 0.01) as well as resting LES pressure (52.4 to 19.9 mmHg, P = 0.01) [135]. Given the safety profile of this procedure, POEM entered into clinical practice and has been studied since its inception. In 2014, Bhayani conducted a prospective observational study that compared 64 patients treated by LHM and 37 by POEM, which showed that mean operative time and length of stay were significantly higher in the LHM cohort, but complication rates were similar [136]. Moreover, patient symptoms, manometry, and postoperative esophageal acid exposure revealed similar outcomes among the two groups.
The preparation for POEM begins with a liquid diet 1–5 days prior to the procedure to minimize residual food in the esophagus [137]. The first step in the procedure involves injection of 10 mL of saline solution with contrast (methylene blue or indigo carmine) to the central esophagus 10–16 cm proximal to the squamo-columnar junction [138]. Following this, a 2 cm incision is made to gain access into the submucosal space. Then, a submucosal tunnel is dissected through the EGJ and 2–3 cm into the gastric cardia [139]. Once access is made to the circular muscle layer of the LES, the myotomy is usually extended to 6 cm into the esophagus and 2 cm below the EGJ. Since its inception, there have been multiple studies showing its efficacy in improvement of dysphagia scores and manometric or imaging modalities, with ranges of 87.5–100% efficacy [135, 136, 140,141,142,143,144,145,146,147,148,149,150,151,152,153,154]. Patients with type III achalasia have a greater than 90% response rate to POEM, possibly owing to the longer myotomy length [155].
Serious adverse events are rare with POEM. They occur at a rate of less than 0.1% with the most common serious event being perforation [156]. Another, albeit less serious, complication following POEM is GERD. Although initial studies showed significantly higher prevalence of GERD post-POEM up to 58%, recent studies in carefully selected patients have shown short-term postoperative clinical symptoms of GERD following POEM is 10.9% and might be comparable to that of LHM [157, 158]. However, given the high potential risk of reflux post-POEM, a recent clinical practice update from expert review and best practice advice from the American Gastroenterological Association recommended that this should be discussed with patients undergoing POEM including potential ramifications of indefinite need for proton pump inhibitor therapy and/or surveillance endoscopy after POEM [159].
Surgical Options
Laparoscopic Heller Myotomy
Surgical myotomy, a technique involving the division of the circular muscle fibers of the LES, was initially performed via an open thoracotomy and laparotomy approach. Studies at the time revealed good response with 60–94% of patients achieving symptomatic improvement when followed over 1–36 years, and this approach has since been replaced with laparoscopic Heller myotomy (LHM) which resulted in less morbidity and faster recovery time (Table 7.5) [75, 102, 121, 160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186]. A systematic review analyzing surgical techniques in 4871 patients reported patient symptom improvement after all surgical myotomies, which included 84.5% of those who underwent the open transabdominal approach, 83.3% of those with the open transthoracic approach, 77.6% of those with the thoracoscopic approach, and 89.3% of those who had a LHM [103]. A subset of the analysis comparing studies with LHM (3086), and the thoracoscopic approach (211) showed better symptomatic improvement with the laparoscopic approach compared to the thoracoscopic approach (89.3 vs 77.6%, P = 0.048) [103].
A complication of any myotomy is GERD, and given the surgical approach, a fundoplication at time of myotomy has helped to decrease postoperative GERD. Reflux may be less if fundoplication is added to myotomy (41.5% without fundoplication vs 14.5% with fundoplication, P = 001) [103]. A randomized controlled trial comparing myotomy with or without fundoplication reported that performing intraoperative fundoplication was associated with a lower incidence of postoperative reflux [187]. Rawlings and investigators demonstrated in a randomized control trial comparing anterior Dor with posterior Toupet fundoplication that both provide similar outcomes in terms of postoperative reflux following LHM [188].
Furthermore, LHM and POEM have been shown to have similar efficacy with a recent systematic review and meta-analysis comparing the two interventions noting improvement in dysphagia at 24 months were 92.7% for POEM and 90.0% for LHM, but patients undergoing POEM were more likely to develop GERD symptoms (OR 1.69, 95% CI 1.33–2.14) and erosive esophagitis (9.31, 95% CI 4.71–18.85) [189].
Prognosis
Despite no curative therapies for achalasia, current management allows an improved quality of life. With the advent of HRM, achalasia phenotypes have also shown prognostic implications with type II achalasia having the best prognosis after myotomy or pneumatic dilation (96% success rate) compared to type I which has 81% success rate and type III which has a 66% success rate [57]. However, success rate for type I and type III are also now >90% with the advent of POEM. Post-intervention, a timed barium esophagram by taking radiographs at 1, 2, and 5 minutes post-barium to evaluate esophageal emptying can also be considered to predict the effectiveness of treatment [190].
Achalasia is a lifelong disease and these patients need continued follow-up. These evaluations are based on determining esophageal symptoms, nutritional status, and imaging when indicated, including a timed barium esophagram [99]. For the patient who is willing to repeat a manometry, HRM can be completed to evaluate for return of esophageal contractile activity [191]. The decision for repeat treatment is based on a combination of symptoms, fitness for repeat treatment, and signs of retention on either a timed barium esophagram, EGD, or continued absence peristalsis on manometry.
Long-term complications of achalasia include an increased risk of squamous cell carcinoma (SCC) with a prevalence of 26 cases in 1000 achalasia patients [192]. The etiology of SCC is felt to be due to persistent esophageal stasis [193]. There is insufficient evidence to support routine screening for SCC; however, this decision for surveillance should be discussed with the patient and provider on a personalized approach [194]. In addition to SCC, patients with achalasia have an increased incidence of aspiration pneumonia, lower respiratory tract infections, and higher mortality [195].
Treatment Failures
Currently there is no curative treatment for achalasia. Up to 20% of patients will need additional treatment within 5 years [196,197,198,199]. Achalasia can progress to mega-esophagus or end-stage disease in around 6–20% of patients [200]. Options for these patients include botulinum toxin injection, repeat pneumatic dilation, or repeat myotomy. A recent multicenter retrospective cohort study assessing both technical and clinical efficacy of POEM in treating achalasia after a failed HM showed technical success of 98% with clinical response up to 81% in patients who had previously failed HM with median follow-up of 9 months [201]. Similarly, in an intention-to-treat analysis at 12 months, clinical success of PD after HM was also comparable to POEM at 89% [158, 202]. Lastly, redo HM also has similar clinical success rate in this group at 73–89% with median follow-up of 2–3.6 years [203, 204]. Thus, all these options can be considered in patients who have lack of response to initial therapy [205]. For patients with severe esophageal dilation with symptoms not responsive to repeat endoscopic options or myotomy, a surgical esophagectomy can be considered (Fig. 7.7).
Conclusion
Achalasia is characterized by impairment in nitrergic inhibitory neurotransmission resulting in non-relaxing LES and aperistalsis of the esophageal body. Patients often present with dysphagia to solids and/or liquid with varying degree of weight loss. Endoscopy is essential to rule out causes of pseudo-achalasia, and high-resolution manometry is the gold standard test for diagnosis. Current treatment options provide excellent palliation of symptoms in patients with achalasia (Table 7.6).
References
Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–54.
Frieling T, et al. Family occurrence of achalasia and diffuse spasm of the oesophagus. Gut. 1988;29:1595–602. https://doi.org/10.1136/gut.29.11.1595.
Farrukh A, DeCaestecker J, Mayberry JF. An epidemiological study of achalasia among the South Asian population of Leicester, 1986–2005. Dysphagia. 2008;23:161–4. https://doi.org/10.1007/s00455-007-9116-1.
Birgisson S, Richter JE. Achalasia in Iceland, 1952–2002: an epidemiologic study. Dig Dis Sci. 2007;52:1855–60. https://doi.org/10.1007/s10620-006-9286-y.
Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256–61. https://doi.org/10.1111/j.1365-2982.2010.01511.x.
Enestvedt BK, Williams JL, Sonnenberg A. Epidemiology and practice patterns of achalasia in a large multi-centre database. Aliment Pharmacol Ther. 2011;33:1209–14. https://doi.org/10.1111/j.1365-2036.2011.04655.x.
Nenshi R, et al. The cost of achalasia: quantifying the effect of symptomatic disease on patient cost burden, treatment time, and work productivity. Surg Innov. 2010;17:291–4. https://doi.org/10.1177/1553350610376392.
Sonnenberg A. Hospitalization for achalasia in the United States 1997–2006. Dig Dis Sci. 2009;54:1680–5. https://doi.org/10.1007/s10620-009-0863-8.
Sonnenberg A, Massey BT, McCarty DJ, Jacobsen SJ. Epidemiology of hospitalization for achalasia in the United States. Dig Dis Sci. 1993;38:233–44.
Vaezi MF, Richter JE. Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am J Gastroenterol. 1999;94:3406–12. https://doi.org/10.1111/j.1572-0241.1999.01639.x.
Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–74. https://doi.org/10.1053/j.gastro.2010.06.024.
Johnston BT, et al. Repetitive proximal esophageal contractions: a new manometric finding and a possible further link between Parkinson’s disease and achalasia. Dysphagia. 2001;16:186–9.
Viegelmann G, Low Y, Sriram B, Chu HP. Achalasia and Down syndrome: a unique association not to be missed. Singapore Med J. 2014;55:e107–8. https://doi.org/10.11622/smedj.2013260.
Jung KW, et al. Genetic evaluation of ALADIN gene in early-onset achalasia and alacrima patients. J Neurogastroenterol Motil. 2011;17:169–73. https://doi.org/10.5056/jnm.2011.17.2.169.
Stuckey BG, Mastaglia FL, Reed WD, Pullan PT. Glucocorticoid insufficiency, achalasia, alacrima with autonomic motor neuropathy. Ann Intern Med. 1987;106:61–3.
Sarathi V, Shah NS. Triple-A syndrome. Adv Exp Med Biol. 2010;685:1–8.
Gordillo-Gonzalez G, et al. Achalasia familiar: report of a family with an autosomal dominant pattern of inherence. Dis Esophagus. 2011;24:E1–4. https://doi.org/10.1111/j.1442-2050.2010.01124.x.
Vigo AG, Martinez A, de la Concha EG, Urcelay E, Ruiz de Leon A. Suggested association of NOS2A polymorphism in idiopathic achalasia: no evidence in a large case-control study. Am J Gastroenterol. 2009;104:1326–7. https://doi.org/10.1038/ajg.2009.72.
de Leon AR, et al. Association between idiopathic achalasia and IL23R gene. Neurogastroenterol Motil. 2010;22:734–738, e218. https://doi.org/10.1111/j.1365-2982.2010.01497.x.
Nunez C, et al. Association of IL10 promoter polymorphisms with idiopathic achalasia. Hum Immunol. 2011;72:749–52. https://doi.org/10.1016/j.humimm.2011.05.017.
Wong RK, Maydonovitch CL, Metz SJ, Baker JR Jr. Significant DQw1 association in achalasia. Dig Dis Sci. 1989;34:349–52.
De la Concha EG, et al. Contribution of HLA class II genes to susceptibility in achalasia. Tissue Antigens. 1998;52:381–4.
Verne GN, et al. Association of HLA-DR and -DQ alleles with idiopathic achalasia. Gastroenterology. 1999;117:26–31.
Gockel I, et al. Common variants in the HLA-DQ region confer susceptibility to idiopathic achalasia. Nat Genet. 2014;46:901–4. https://doi.org/10.1038/ng.3029.
Furuzawa-Carballeda J, et al. Achalasia--an autoimmune inflammatory disease: a cross-sectional study. J Immunol Res. 2015;2015:729217. https://doi.org/10.1155/2015/729217.
Furuzawa-Carballeda J, et al. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroenterol: WJG. 2016;22:7892–907. https://doi.org/10.3748/wjg.v22.i35.7892.
Booy JD, Takata J, Tomlinson G, Urbach DR. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis Esophagus. 2012;25:209–13. https://doi.org/10.1111/j.1442-2050.2011.01249.x.
Patel DA, Lappas BM, Vaezi MF. An overview of achalasia and its subtypes. Gastroenterol Hepatol (NY). 2017;13:411–21.
Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol. 2005;100:1404–14. https://doi.org/10.1111/j.1572-0241.2005.41775.x.
Kuramoto H, Kadowaki M, Yoshida N. Morphological demonstration of a vagal inhibitory pathway to the lower esophageal sphincter via nitrergic neurons in the rat esophagus. Neurogastroenterol Motil. 2013;25:e485–94. https://doi.org/10.1111/nmo.12146.
Murray J, Du C, Ledlow A, Bates JN, Conklin JL. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991;261:G401–6.
Guelrud M, et al. The effect of vasoactive intestinal polypeptide on the lower esophageal sphincter in achalasia. Gastroenterology. 1992;103:377–82.
Boeckxstaens GE. Achalasia: virus-induced euthanasia of neurons? Am J Gastroenterol. 2008;103:1610–2. https://doi.org/10.1111/j.1572-0241.2008.01967.x.
Kraichely RE, Farrugia G, Pittock SJ, Castell DO, Lennon VA. Neural autoantibody profile of primary achalasia. Dig Dis Sci. 2010;55:307–11. https://doi.org/10.1007/s10620-009-0838-9.
de Oliveira RB, Rezende Filho J, Dantas RO, Iazigi N. The spectrum of esophageal motor disorders in Chagas’ disease. Am J Gastroenterol. 1995;90:1119–24.
Mearin F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993;23:724–8.
Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013;145:954–65. https://doi.org/10.1053/j.gastro.2013.08.038.
Ates F, Vaezi MF. The pathogenesis and management of achalasia: current status and future directions. Gut Liver. 2015;9:449–63. https://doi.org/10.5009/gnl14446.
Dowlatshahi K, Evander A, Walther B, Skinner DB. Influence of morphine on the distal oesophagus and the lower oesophageal sphincter--a manometric study. Gut. 1985;26:802–6. https://doi.org/10.1136/gut.26.8.802.
Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113:409–14.
Penagini R, Picone A, Bianchi PA. Effect of morphine and naloxone on motor response of the human esophagus to swallowing and distension. Am J Physiol. 1996;271:G675–80. https://doi.org/10.1152/ajpgi.1996.271.4.G675.
Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31:601–6. https://doi.org/10.1111/j.1365-2036.2009.04212.x.
Gonzalez ES, Bellver VO, Jaime FC, Cortes JA, Gil VG. Opioid-induced lower esophageal sphincter dysfunction. J Neurogastroenterol Motil. 2015;21:618–20. https://doi.org/10.5056/jnm15108.
Babaei A, Szabo A, Shad S, Massey BT. Chronic daily opioid exposure is associated with dysphagia, esophageal outflow obstruction, and disordered peristalsis. Neurogastroenterol Motil. 2019;31:e13601. https://doi.org/10.1111/nmo.13601.
Ratuapli SK, et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol. 2015;110:979–84. https://doi.org/10.1038/ajg.2015.154.
Ortiz V, Garcia-Campos M, Saez-Gonzalez E, del Pozo P, Garrigues V. A concise review of opioid-induced esophageal dysfunction: is this a new clinical entity? Dis Esophagus. 2018;31:doy003. https://doi.org/10.1093/dote/doy003.
Kahrilas PJ, Bredenoord AJ, Carlson DA, Pandolfino JE. Advances in management of esophageal motility disorders. Clin Gastroenterol Hepatol. 2018;16:1692–700. https://doi.org/10.1016/j.cgh.2018.04.026.
Schechter RB, Lemme EM, Novais P, Biccas B. Achalasia in the elderly patient: a comparative study. Arq Gastroenterol. 2011;48:19–23.
Rakita SS, Villadolid D, Kalipersad C, Thometz D, Rosemurgy A. BMI affects presenting symptoms of achalasia and outcome after Heller myotomy. Surg Endosc. 2007;21:258–64. https://doi.org/10.1007/s00464-006-0113-5.
Patel DA, et al. Weight loss in achalasia is determined by its phenotype. Dis Esophagus. 2018;31:doy046. https://doi.org/10.1093/dote/doy046.
Khandelwal S, et al. Improvement of respiratory symptoms following Heller myotomy for achalasia. J Gastrointest Surg. 2011;15:235–9. https://doi.org/10.1007/s11605-010-1397-2.
Sinan H, et al. Prevalence of respiratory symptoms in patients with achalasia. Dis Esophagus. 2011;24:224–8. https://doi.org/10.1111/j.1442-2050.2010.01126.x.
Gupta M, et al. Respiratory dysfunction is common in patients with achalasia and improves after pneumatic dilation. Dig Dis Sci. 2014;59:744–52. https://doi.org/10.1007/s10620-013-2971-8.
Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–84.
Kahrilas PJ, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74. https://doi.org/10.1111/nmo.12477.
Pandolfino JE, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–33. https://doi.org/10.1053/j.gastro.2008.07.022.
Rohof WO, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144:718–25; quiz e713–714. https://doi.org/10.1053/j.gastro.2012.12.027.
Sodikoff JB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28:139–45. https://doi.org/10.1111/nmo.12711.
Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–25. https://doi.org/10.1007/s11605-009-0975-7.
van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2015;27:1310–6. https://doi.org/10.1111/nmo.12625.
Perez-Fernandez MT, Santander C, Marinero A, Burgos-Santamaria D, Chavarria-Herbozo C. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil. 2016;28:116–26. https://doi.org/10.1111/nmo.12708.
Sweis R, Anggiansah A, Wong T, Brady G, Fox M. Assessment of esophageal dysfunction and symptoms during and after a standardized test meal: development and clinical validation of a new methodology utilizing high-resolution manometry. Neurogastroenterol Motil. 2014;26:215–28. https://doi.org/10.1111/nmo.12252.
Ang D, et al. Rapid Drink Challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil. 2017;29:e12902. https://doi.org/10.1111/nmo.12902.
Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Reproducibility of esophageal high-resolution manometry. Neurogastroenterol Motil. 2011;23:e271–6. https://doi.org/10.1111/j.1365-2982.2011.01713.x.
Fox M, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–42. https://doi.org/10.1111/j.1365-2982.2004.00539.x.
Lin Z, et al. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil. 2012;24:e356–63. https://doi.org/10.1111/j.1365-2982.2012.01952.x.
Ponds FA, Bredenoord AJ, Kessing BF, Smout AJ. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29:e12908. https://doi.org/10.1111/nmo.12908.
Pandolfino JE, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25:496–501. https://doi.org/10.1111/nmo.12097.
Lin Z, et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol. 2014;307:G158–63. https://doi.org/10.1152/ajpgi.00119.2014.
Lin Z, et al. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27:1232–8. https://doi.org/10.1111/nmo.12613.
Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil. 2009;21:718–e741. https://doi.org/10.1111/j.1365-2982.2009.01273.x.
Teitelbaum EN, et al. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc. 2014;28:2840–7. https://doi.org/10.1007/s00464-014-3563-1.
Teitelbaum EN, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522–8. https://doi.org/10.1007/s00464-014-3733-1.
Ngamruengphong S, et al. Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surg Endosc. 2016;30:2886–94. https://doi.org/10.1007/s00464-015-4574-2.
Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21–35.
Gelfond M, Rozen P, Gilat T. Isosorbide dinitrate and nifedipine treatment of achalasia: a clinical, manometric and radionuclide evaluation. Gastroenterology. 1982;83:963–9.
Rozen P, Gelfond M, Salzman S, Baron J, Gilat T. Radionuclide confirmation of the therapeutic value of isosorbide dinitrate in relieving the dysphagia in achalasia. J Clin Gastroenterol. 1982;4:17–22.
Gelfond M, Rozen P, Keren S, Gilat T. Effect of nitrates on LOS pressure in achalasia: a potential therapeutic aid. Gut. 1981;22:312–8. https://doi.org/10.1136/gut.22.4.312.
Traube M, Dubovik S, Lange RC, McCallum RW. The role of nifedipine therapy in achalasia: results of a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 1989;84:1259–62.
Bortolotti M, Labo G. Clinical and manometric effects of nifedipine in patients with esophageal achalasia. Gastroenterology. 1981;80:39–44.
Coccia G, Bortolotti M, Michetti P, Dodero M. Prospective clinical and manometric study comparing pneumatic dilatation and sublingual nifedipine in the treatment of oesophageal achalasia. Gut. 1991;32:604–6. https://doi.org/10.1136/gut.32.6.604.
Eherer AJ, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut. 2002;50:758–64. https://doi.org/10.1136/gut.50.6.758.
Vaezi MF, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44:231–9. https://doi.org/10.1136/gut.44.2.231.
Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110:1410–5.
Pasricha PJ, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–8. https://doi.org/10.1056/NEJM199503233321203.
Pasricha PJ, Ravich WJ, Kalloo AN. Botulinum toxin for achalasia. Lancet. 1993;341:244–5.
Annese V, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597–600. https://doi.org/10.1136/gut.46.5.597.
Cuilliere C, et al. Achalasia: outcome of patients treated with intrasphincteric injection of botulinum toxin. Gut. 1997;41:87–92. https://doi.org/10.1136/gut.41.1.87.
Rollan A, Gonzalez R, Carvajal S, Chianale J. Endoscopic intrasphincteric injection of botulinum toxin for the treatment of achalasia. J Clin Gastroenterol. 1995;20:189–91.
Fishman VM, et al. Symptomatic improvement in achalasia after botulinum toxin injection of the lower esophageal sphincter. Am J Gastroenterol. 1996;91:1724–30.
Annese V, et al. Controlled trial of botulinum toxin injection versus placebo and pneumatic dilation in achalasia. Gastroenterology. 1996;111:1418–24.
Gordon JM, Eaker EY. Prospective study of esophageal botulinum toxin injection in high-risk achalasia patients. Am J Gastroenterol. 1997;92:1812–7.
Muehldorfer SM, et al. Esophageal achalasia: intrasphincteric injection of botulinum toxin A versus balloon dilation. Endoscopy. 1999;31:517–21. https://doi.org/10.1055/s-1999-56.
Kolbasnik J, Waterfall WE, Fachnie B, Chen Y, Tougas G. Long-term efficacy of Botulinum toxin in classical achalasia: a prospective study. Am J Gastroenterol. 1999;94:3434–9. https://doi.org/10.1111/j.1572-0241.1999.01605.x.
Mikaeli J, Fazel A, Montazeri G, Yaghoobi M, Malekzadeh R. Randomized controlled trial comparing botulinum toxin injection to pneumatic dilatation for the treatment of achalasia. Aliment Pharmacol Ther. 2001;15:1389–96.
Allescher HD, et al. Treatment of achalasia: botulinum toxin injection vs. pneumatic balloon dilation. A prospective study with long-term follow-up. Endoscopy. 2001;33:1007–17. https://doi.org/10.1055/s-2001-18935.
Neubrand M, Scheurlen C, Schepke M, Sauerbruch T. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519–23. https://doi.org/10.1055/s-2002-33225.
Smith CD, Stival A, Howell DL, Swafford V. Endoscopic therapy for achalasia before Heller myotomy results in worse outcomes than heller myotomy alone. Ann Surg. 2006;243:579–84; discussion 584–576. https://doi.org/10.1097/01.sla.0000217524.75529.2d.
Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238–49; quiz 1250. https://doi.org/10.1038/ajg.2013.196.
Lambroza A, Schuman RW. Pneumatic dilation for achalasia without fluoroscopic guidance: safety and efficacy. Am J Gastroenterol. 1995;90:1226–9.
Thomas V, Harish K, Sunilkumar K. Pneumatic dilation of achalasia cardia under direct endoscopy: the debate continues. Gastrointest Endosc. 2006;63:734. https://doi.org/10.1016/j.gie.2005.11.023.
Vela MF, et al. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–7.
Campos GM, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. https://doi.org/10.1097/SLA.0b013e31818e43ab.
Cox J, Buckton GK, Bennett JR. Balloon dilatation in achalasia: a new dilator. Gut. 1986;27:986–9. https://doi.org/10.1136/gut.27.8.986.
Gelfand MD, Kozarek RA. An experience with polyethylene balloons for pneumatic dilation in achalasia. Am J Gastroenterol. 1989;84:924–7.
Barkin JS, Guelrud M, Reiner DK, Goldberg RI, Phillips RS. Forceful balloon dilation: an outpatient procedure for achalasia. Gastrointest Endosc. 1990;36:123–6.
Stark GA, Castell DO, Richter JE, Wu WC. Prospective randomized comparison of Brown-McHardy and microvasive balloon dilators in treatment of achalasia. Am J Gastroenterol. 1990;85:1322–6.
Makela J, Kiviniemi H, Laitinen S. Heller’s cardiomyotomy compared with pneumatic dilatation for treatment of oesophageal achalasia. Eur J Surg. 1991;157:411–4.
Levine ML, Moskowitz GW, Dorf BS, Bank S. Pneumatic dilation in patients with achalasia with a modified Gruntzig dilator (Levine) under direct endoscopic control: results after 5 years. Am J Gastroenterol. 1991;86:1581–4.
Kim CH, et al. Achalasia: prospective evaluation of relationship between lower esophageal sphincter pressure, esophageal transit, and esophageal diameter and symptoms in response to pneumatic dilation. Mayo Clin Proc. 1993;68:1067–73. https://doi.org/10.1016/s0025-6196(12)60900-8.
Lee JD, Cecil BD, Brown PE, Wright RA. The Cohen test does not predict outcome in achalasia after pneumatic dilation. Gastrointest Endosc. 1993;39:157–60.
Abid S, et al. Treatment of achalasia: the best of both worlds. Am J Gastroenterol. 1994;89:979–85.
Wehrmann T, Jacobi V, Jung M, Lembcke B, Caspary WF. Pneumatic dilation in achalasia with a low-compliance balloon: results of a 5-year prospective evaluation. Gastrointest Endosc. 1995;42:31–6.
Muehldorfer SM, Hahn EG, Ell C. High- and low-compliance balloon dilators in patients with achalasia: a randomized prospective comparative trial. Gastrointest Endosc. 1996;44:398–403.
Bhatnagar MS, Nanivadekar SA, Sawant P, Rathi PM. Achalasia cardia dilatation using polyethylene balloon (Rigiflex) dilators. Indian J Gastroenterol. 1996;15:49–51.
Gideon RM, Castell DO, Yarze J. Prospective randomized comparison of pneumatic dilatation technique in patients with idiopathic achalasia. Dig Dis Sci. 1999;44:1853–7.
Khan AA, et al. Massively dilated esophagus in achalasia: response to pneumatic balloon dilation. Am J Gastroenterol. 1999;94:2363–6. https://doi.org/10.1111/j.1572-0241.1999.01358.x.
Kadakia SC, Wong RK. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary esophageal achalasia. Am J Gastroenterol. 1993;88:34–8.
Chan KC, et al. Short-term and long-term results of endoscopic balloon dilation for achalasia: 12 years’ experience. Endoscopy. 2004;36:690–4. https://doi.org/10.1055/s-2004-825659.
Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long-term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10:3322–7. https://doi.org/10.3748/wjg.v10.i22.3322.
Kostic S, et al. Pneumatic dilatation or laparoscopic cardiomyotomy in the management of newly diagnosed idiopathic achalasia. Results of a randomized controlled trial. World J Surg. 2007;31:470–8. https://doi.org/10.1007/s00268-006-0600-9.
Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20:431–6. https://doi.org/10.1111/j.1365-2036.2004.02080.x.
Ghoshal UC, et al. Long-term follow-up after pneumatic dilation for achalasia cardia: factors associated with treatment failure and recurrence. Am J Gastroenterol. 2004;99:2304–10. https://doi.org/10.1111/j.1572-0241.2004.40099.x.
Guardino JM, Vela MF, Connor JT, Richter JE. Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol. 2004;38:855–60.
Boztas G, et al. Pneumatic balloon dilatation in primary achalasia: the long-term follow-up results. Hepatogastroenterology. 2005;52:475–80.
Karamanolis G, et al. Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol. 2005;100:270–4. https://doi.org/10.1111/j.1572-0241.2005.40093.x.
Katsinelos P, et al. Long-term results of pneumatic dilation for achalasia: a 15 years’ experience. World J Gastroenterol. 2005;11:5701–5. https://doi.org/10.3748/wjg.v11.i36.5701.
Tuset JA, Lujan M, Huguet JM, Canelles P, Medina E. Endoscopic pneumatic balloon dilation in primary achalasia: predictive factors, complications, and long-term follow-up. Dis Esophagus. 2009;22:74–9. https://doi.org/10.1111/j.1442-2050.2008.00874.x.
Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–8.
Gockel I, Junginger T, Bernhard G, Eckardt VF. Heller myotomy for failed pneumatic dilation in achalasia: how effective is it? Ann Surg. 2004;239:371–7. https://doi.org/10.1097/01.sla.0000114228.34809.01.
Farhoomand K, Connor JT, Richter JE, Achkar E, Vaezi MF. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol. 2004;2:389–94.
Salvador R, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg. 2010;14:1635–45. https://doi.org/10.1007/s11605-010-1318-4.
Eckardt VF, Kanzler G, Westermeier T. Complications and their impact after pneumatic dilation for achalasia: prospective long-term follow-up study. Gastrointest Endosc. 1997;45:349–53.
Vanuytsel T, et al. Conservative management of esophageal perforations during pneumatic dilation for idiopathic esophageal achalasia. Clin Gastroenterol Hepatol. 2012;10:142–9. https://doi.org/10.1016/j.cgh.2011.10.032.
Inoue H, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–71. https://doi.org/10.1055/s-0029-1244080.
Bhayani NH, et al. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg. 2014;259:1098–103. https://doi.org/10.1097/SLA.0000000000000268.
Stavropoulos SN, Modayil RJ, Friedel D, Savides T. The international per oral endoscopic myotomy survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc. 2013;27:3322–38. https://doi.org/10.1007/s00464-013-2913-8.
Lujan-Sanchis M, et al. Management of primary achalasia: the role of endoscopy. World J Gastrointest Endosc. 2015;7:593–605. https://doi.org/10.4253/wjge.v7.i6.593.
Bechara R, Ikeda H, Inoue H. Peroral endoscopic myotomy: an evolving treatment for achalasia. Nat Rev Gastroenterol Hepatol. 2015;12:410–26. https://doi.org/10.1038/nrgastro.2015.87.
Tan Y, et al. Efficacy of anterior versus posterior per-oral endoscopic myotomy for treating achalasia: a randomized, prospective study. Gastrointest Endosc. 2018;88:46–54. https://doi.org/10.1016/j.gie.2018.03.009.
Tyberg A, et al. Peroral endoscopic myotomy as salvation technique post-Heller: international experience. Dig Endosc. 2018;30:52–6. https://doi.org/10.1111/den.12918.
Yao S, Linghu E. Peroral endoscopic myotomy can improve esophageal motility in patients with achalasia from a large sample self-control research (66 patients). PLoS One. 2015;10:e0125942. https://doi.org/10.1371/journal.pone.0125942.
Hu JW, et al. Peroral endoscopic myotomy for advanced achalasia with sigmoid-shaped esophagus: long-term outcomes from a prospective, single-center study. Surg Endosc. 2015;29:2841–50. https://doi.org/10.1007/s00464-014-4013-9.
Teitelbaum EN, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surg Endosc. 2014;28:3359–65. https://doi.org/10.1007/s00464-014-3628-1.
Zhou PH, et al. Peroral endoscopic remyotomy for failed Heller myotomy: a prospective single-center study. Endoscopy. 2013;45:161–6. https://doi.org/10.1055/s-0032-1326203.
Swanstrom LL, et al. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg. 2012;256:659–67. https://doi.org/10.1097/SLA.0b013e31826b5212.
Shiwaku H. et al. Multicenter collaborative retrospective evaluation of peroral endoscopic myotomy for esophageal achalasia: analysis of data from more than 1300 patients at eight facilities in Japan. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-06833-8.
Grimes KL, et al. Double-scope per oral endoscopic myotomy (POEM): a prospective randomized controlled trial. Surg Endosc. 2016;30:1344–51. https://doi.org/10.1007/s00464-015-4396-2.
Liu W. et al. Open peroral endoscopic myotomy for the treatment of achalasia: a case series of 82 cases. Dis Esophagus. 2019. https://doi.org/10.1093/dote/doz052.
Chandan S. et al. Clinical efficacy of per-oral endoscopic myotomy (POEM) for spastic esophageal disorders: a systematic review and meta-analysis. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-06819-6.
Kim WH, et al. Comparison of the outcomes of peroral endoscopic myotomy for achalasia according to manometric subtype. Gut Liver. 2017;11:642–7. https://doi.org/10.5009/gnl16545.
Kane ED, Budhraja V, Desilets DJ, Romanelli JR. Myotomy length informed by high-resolution esophageal manometry (HREM) results in improved per-oral endoscopic myotomy (POEM) outcomes for type III achalasia. Surg Endosc. 2019;33:886–94. https://doi.org/10.1007/s00464-018-6356-0.
Zhang W, Linghu EQ. Peroral endoscopic myotomy for type III achalasia of Chicago classification: outcomes with a minimum follow-up of 24 months. J Gastrointest Surg. 2017;21:785–91. https://doi.org/10.1007/s11605-017-3398-x.
Chen X, et al. Two-year follow-up for 45 patients with achalasia who underwent peroral endoscopic myotomy. Eur J Cardiothorac Surg. 2015;47:890–6. https://doi.org/10.1093/ejcts/ezu320.
Khashab MA, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc. 2015;81:1170–7. https://doi.org/10.1016/j.gie.2014.10.011.
Stavropoulos SN, et al. Per-oral endoscopic myotomy white paper summary. Surg Endosc. 2014;28:2005–19. https://doi.org/10.1007/s00464-014-3630-7.
Talukdar R, Inoue H, Nageshwar Reddy D. Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis. Surg Endosc. 2015;29:3030–46. https://doi.org/10.1007/s00464-014-4040-6.
Kumbhari V, et al. Gastroesophageal reflux after peroral endoscopic myotomy: a multicenter case-control study. Endoscopy. 2017;49:634–42. https://doi.org/10.1055/s-0043-105485.
Kahrilas PJ, Katzka D, Richter JE. Clinical practice update: the use of per-oral endoscopic myotomy in achalasia: expert review and best practice advice from the AGA Institute. Gastroenterology. 2017;153:1205–11. https://doi.org/10.1053/j.gastro.2017.10.001.
Rosati R, et al. Laparoscopic approach to esophageal achalasia. Am J Surg. 1995;169:424–7.
Ancona E, et al. Esophageal achalasia: laparoscopic versus conventional open Heller-Dor operation. Am J Surg. 1995;170:265–70.
Mitchell PC, et al. Laparoscopic cardiomyotomy with a Dor patch for achalasia. Can J Surg. 1995;38:445–8.
Swanstrom LL, Pennings J. Laparoscopic esophagomyotomy for achalasia. Surg Endosc. 1995;9:286–90; discussion 290–282.
Raiser F, et al. Heller myotomy via minimal-access surgery. An evaluation of antireflux procedures. Arch Surg. 1996;131:593–7; discussion 597–598.
Morino M, Rebecchi F, Festa V, Garrone C. Laparoscopic Heller cardiomyotomy with intraoperative manometry in the management of oesophageal achalasia. Int Surg. 1995;80:332–5.
Robertson GS, Lloyd DM, Wicks AC, de Caestecker J, Veitch PS. Laparoscopic Heller’s cardiomyotomy without an antireflux procedure. Br J Surg. 1995;82:957–9.
Bonavina L, Rosati R, Segalin A, Peracchia A. Laparoscopic Heller-Dor operation for the treatment of oesophageal achalasia: technique and early results. Ann Chir Gynaecol. 1995;84:165–8.
Delgado F, et al. Laparoscopic treatment of esophageal achalasia. Surg Laparosc Endosc. 1996;6:83–90.
Hunter JG, Trus TL, Branum GD, Waring JP. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg. 1997;225:655–64; discussion 664–655. https://doi.org/10.1097/00000658-199706000-00003.
Kjellin AP, Granqvist S, Ramel S, Thor KB. Laparoscopic myotomy without fundoplication in patients with achalasia. Eur J Surg. 1999;165:1162–6. https://doi.org/10.1080/110241599750007702.
Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Laparoscopic cardiomyotomy and anterior partial fundoplication for achalasia. Surg Endosc. 2001;15:683–6. https://doi.org/10.1007/s004640080037.
Yamamura MS, Gilster JC, Myers BS, Deveney CW, Sheppard BC. Laparoscopic heller myotomy and anterior fundoplication for achalasia results in a high degree of patient satisfaction. Arch Surg. 2000;135:902–6.
Patti MG, et al. Laparoscopic Heller myotomy and Dor fundoplication for achalasia: analysis of successes and failures. Arch Surg. 2001;136:870–7.
Pechlivanides G, et al. Laparoscopic Heller cardiomyotomy and Dor fundoplication for esophageal achalasia: possible factors predicting outcome. Arch Surg. 2001;136:1240–3.
Sharp KW, Khaitan L, Scholz S, Holzman MD, Richards WO. 100 consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg. 2002;235:631–8; discussion 638–639. https://doi.org/10.1097/00000658-200205000-00004.
Donahue PE, Horgan S, Liu KJ, Madura JA. Floppy Dor fundoplication after esophagocardiomyotomy for achalasia. Surgery. 2002;132:716–22; discussion 722–713.
Zaninotto G, et al. Etiology, diagnosis, and treatment of failures after laparoscopic Heller myotomy for achalasia. Ann Surg. 2002;235:186–92. https://doi.org/10.1097/00000658-200202000-00005.
Luketich JD, et al. Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg. 2001;72:1909–12; discussion 1912–1903.
Decker G, et al. Gastrointestinal quality of life before and after laparoscopic heller myotomy with partial posterior fundoplication. Ann Surg. 2002;236:750–8; discussion 758. https://doi.org/10.1097/00000658-200212000-00007.
Mineo TC, Ambrogi V. Long-term results and quality of life after surgery for oesophageal achalasia: one surgeon’s experience. Eur J Cardiothorac Surg. 2004;25:1089–96. https://doi.org/10.1016/j.ejcts.2004.01.043.
Gockel I, Junginger T, Eckardt VF. Long-term results of conventional myotomy in patients with achalasia: a prospective 20-year analysis. J Gastrointest Surg. 2006;10:1400–8. https://doi.org/10.1016/j.gassur.2006.07.006.
Wright AS, Williams CW, Pellegrini CA, Oelschlager BK. Long-term outcomes confirm the superior efficacy of extended Heller myotomy with Toupet fundoplication for achalasia. Surg Endosc. 2007;21:713–8. https://doi.org/10.1007/s00464-006-9165-9.
Khajanchee YS, Kanneganti S, Leatherwood AE, Hansen PD, Swanstrom LL. Laparoscopic Heller myotomy with Toupet fundoplication: outcomes predictors in 121 consecutive patients. Arch Surg. 2005;140:827–33; discussion 833–824. https://doi.org/10.1001/archsurg.140.9.827.
Zaninotto G, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239:364–70. https://doi.org/10.1097/01.sla.0000114217.52941.c5.
Csendes A, et al. Very late results of esophagomyotomy for patients with achalasia: clinical, endoscopic, histologic, manometric, and acid reflux studies in 67 patients for a mean follow-up of 190 months. Ann Surg. 2006;243:196–203. https://doi.org/10.1097/01.sla.0000197469.12632.e0.
Ramacciato G, et al. The laparoscopic approach with antireflux surgery is superior to the thoracoscopic approach for the treatment of esophageal achalasia. Experience of a single surgical unit. Surg Endosc. 2002;16:1431–7. https://doi.org/10.1007/s00464-001-9215-2.
Richards WO, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405–12; discussion 412–405. https://doi.org/10.1097/01.sla.0000136940.32255.51.
Rawlings A, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc. 2012;26:18–26. https://doi.org/10.1007/s00464-011-1822-y.
Schlottmann F, Luckett DJ, Fine J, Shaheen NJ, Patti MG. Laparoscopic Heller myotomy versus peroral endoscopic myotomy (POEM) for achalasia: a systematic review and meta-analysis. Ann Surg. 2018;267:451–60. https://doi.org/10.1097/SLA.0000000000002311.
Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765–70. https://doi.org/10.1136/gut.50.6.765.
Roman S, et al. Partial recovery of peristalsis after myotomy for achalasia: more the rule than the exception. JAMA Surg. 2013;148:157–64. https://doi.org/10.1001/2013.jamasurg.38.
Tustumi F, et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis Esophagus. 2017;30:1–8. https://doi.org/10.1093/dote/dox072.
Leeuwenburgh I, et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 2010;105:2144–9. https://doi.org/10.1038/ajg.2010.263.
Ravi K, Geno DM, Katzka DA. Esophageal cancer screening in achalasia: is there a consensus? Dis Esophagus. 2015;28:299–304. https://doi.org/10.1111/dote.12196.
Harvey PR, et al. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut. 2019;68:790–5. https://doi.org/10.1136/gutjnl-2018-316089.
Zaninotto G, et al. Long-term results (6–10 years) of laparoscopic fundoplication. J Gastrointest Surg. 2007;11:1138–45. https://doi.org/10.1007/s11605-007-0195-y.
Zaninotto G, et al. Four hundred laparoscopic myotomies for esophageal achalasia: a single centre experience. Ann Surg. 2008;248:986–93. https://doi.org/10.1097/SLA.0b013e3181907bdd.
Bonatti H, et al. Long-term results of laparoscopic Heller myotomy with partial fundoplication for the treatment of achalasia. Am J Surg. 2005;190:874–8. https://doi.org/10.1016/j.amjsurg.2005.08.012.
Costantini M, et al. The laparoscopic Heller-Dor operation remains an effective treatment for esophageal achalasia at a minimum 6-year follow-up. Surg Endosc. 2005;19:345–51. https://doi.org/10.1007/s00464-004-8941-7.
Eckardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol. 2008;20:956–60. https://doi.org/10.1097/MEG.0b013e3282fbf5e5.
Ngamruengphong S, et al. Efficacy and safety of peroral endoscopic myotomy for treatment of achalasia after failed Heller myotomy. Clin Gastroenterol Hepatol. 2017;15:1531–1537.e1533. https://doi.org/10.1016/j.cgh.2017.01.031.
Legros L, et al. Long-term results of pneumatic dilatation for relapsing symptoms of achalasia after Heller myotomy. Neurogastroenterol Motil. 2014;26:1248–55. https://doi.org/10.1111/nmo.12380.
Rakita S, Villadolid D, Kalipersad C, Thometz D, Rosemurgy A. Outcomes promote reoperative Heller myotomy for symptoms of achalasia. Surg Endosc. 2007;21:1709–14. https://doi.org/10.1007/s00464-007-9226-8.
Grotenhuis BA, et al. Reoperation for dysphagia after cardiomyotomy for achalasia. Am J Surg. 2007;194:678–82. https://doi.org/10.1016/j.amjsurg.2007.01.035.
Patel DA, Vaezi MF. Refractory achalasia: is POEM changing the paradigm? Clin Gastroenterol Hepatol. 2017;15:1504–6. https://doi.org/10.1016/j.cgh.2017.04.032.
Hoogerwerf WA, et al. Pharmacologic therapy in treating achalasia. Gastrointest Endosc Clin N Am. 2001;11:311–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Naik, R.D., Patel, D.A. (2020). Achalasia. In: Patel, D., Kavitt, R., Vaezi, M. (eds) Evaluation and Management of Dysphagia . Springer, Cham. https://doi.org/10.1007/978-3-030-26554-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-26554-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26553-3
Online ISBN: 978-3-030-26554-0
eBook Packages: MedicineMedicine (R0)