Abstract
Achalasia is a motility disorder of the esophagus, most commonly presenting as dysphagia to solids and liquids, with gradual symptom progression. Pathophysiology arises from degeneration of inhibitory neurons of the lower esophageal sphincter (LES), resulting in unopposed tonicity and poor relaxation ability of the LES. The etiology of primary achalasia is unknown, but may involve viral, genetic, autoimmune, or neurodegenerative causes. Secondary achalasia, or pseudoachalasia, may arise from Chagas disease, malignancy associated with invasive or paraneoplastic disease, or other rare syndromes. Diagnosis is made using esophagogastroduodenoscopy, barium swallow radiography, and esophageal manometry, in order to demonstrate the effects of the underlying pathophysiologic changes and to allow for manometric subtyping which predicts treatment response. Treatment includes mechanical and biochemical methods to disrupt the pathophysiologic process. Complications of untreated disease may include overlying infectious esophagitis, esophageal diverticulosis, megaesophagus, and a slight increase in esophageal cancer risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Primary achalasia
- Lower esophageal sphincter
- Neuronal degeneration
- Nitric oxide
- Vasoactive inhibitory peptide
- Pseudoachalasia
- Megaesophagus
- Aperistalsis
Achalasia is a motility disorder of the esophagus with a prevalence of 1:100,000 [1]. The most common primary presenting symptom is dysphagia to both solids and liquids, with gradual symptom progression. Other non-specific symptoms may include regurgitation, chest pain (predominantly in younger patients), heartburn, and halitosis. In advanced cases, patients may also report weight loss, nocturnal cough, and finding regurgitated food or mucous on the pillow upon waking from sleep.
Normally, the lower esophageal sphincter (LES) has myogenic tone, i.e., remains intrinsically contracted in the absence of neural input or hormones, to prevent reflux of gastric contents. It relaxes in response to swallowing and esophageal or gastric distention. This muscle is also under neurogenic control involving the myenteric plexus, which contain both excitatory (acetylcholine-producing) and inhibitory (nitric oxide- and vasoactive intestinal peptide-producing) motor neurons. In contrast, the smooth muscle of the esophageal body lacks demonstrable tone, likely owing to differences in contractile proteins and isoforms compared to smooth muscle of the LES [2]. Unlike contraction in the skeletal muscles controlled by central sequential activation of motor neurons, primary peristalsis along the smooth muscle portion (approximate distal two-thirds) of the esophageal body is initiated by non-sequential simultaneous central activation, and is believed to be propagated largely by peripheral mechanisms to produce a deglutitive inhibition followed by excitation. There is an intrinsic gradient of decreasing cholinergic and increasing nitrergic innervation distally in the esophagus [3, 4].
Pathophysiology involves the selective degeneration of inhibitory neurons in the esophagus, which are needed for peristalsis of the smooth muscle of the esophageal body, as well as relaxation of the tonic LES [5]. The etiology of primary achalasia remains largely unknown. Based on viral antigen reactivity in some patients with achalasia, such viruses as varicella-zoster, human papilloma and herpes have been implicated in initiating an inflammatory reaction [6, 7]. The preference of herpes virus for squamous rather than columnar epithelium could explain predominant esophageal involvement in achalasia while largely sparing the rest of the gastrointestinal tract, and increased risk for esophageal squamous carcinoma. However, polymerase chain reaction amplification failed to detect such viruses in myotomy specimens from achalasic patients [8]. Nonetheless, this negative finding does not rule out the role of other viruses, or an earlier viral assault that is cleared by the time symptoms arise. There are also known familial cases of achalasia, including a case report of siblings with coexistent Hirschsprung’s disease [9]. Albeit extremely rare, such cases raise the possibility of a genetic basis of the disease [10]. An autoimmune etiology has been suggested, with evidence of circulating autoantibodies [11], and antibodies against myenteric neurons in the serum of approximately a third of achalasic patients [12], as well as association with Class II histocompatibility antigen [13]; however, antibody detection had low specificity for the disease, suggesting the likelihood of epiphenomenon rather than true causation [14]. Neurodegeneration may be a primary etiology given the detection in one study of Lewy bodies, as found in Parkinson’s disease [15], or secondary to the aforementioned viral or autoimmune processes, but no central neurologic lesion has ever been implicated [16].
Secondary achalasia, or pseudoachalasia, is considered when achalasia arises secondary to other known causes. For example, Chagas’ disease is a tropical parasitic disease found in South America, in which infection by the protozoan Trypanosoma cruzi results in systemic invasion of internal organs, thereby disrupting normal functions of structures including the heart, brain, and gastrointestinal system [17]. Malignancy is also an important cause of secondary achalasia, and must be excluded before proceeding with treatment for primary achalasia [18]. Invasive disease, such as esophageal cancer, or extrinsic compression from lung or gastric cancer, can result in achalasia-like symptoms with suggestive findings on testing modalities. Additionally, several malignancies, including breast and small cell lung cancer, have been associated with a paraneoplastic phenomenon of dysmotility based on elaboration of humoral factors, neuronal degeneration, and possibly abnormal neurotransmission [5, 19]. Type 1 antineuronal nuclear autoantibodies (ANNA-1, also called anti-Hu) react with both small cell lung cancer cells and with various nerve cells, and has been found in patients with achalasia, gastroparesis, and pseudobstruction, even before overt diagnosis of cancer [20]. Allgrove’s syndrome, consisting of achalasia, alacrima, and adrenal insufficiency, is another secondary cause of achalasia with autosomal recessive inheritance that has been linked to 12q13 chromosome with features also of mental retardation and peripheral and autonomic neuropathy [21].
Whether primary or secondary, the resulting esophageal aperistalsis and incomplete relaxation of the LES impede passage of the swallowed food bolus into the stomach, leading to accumulation of undigested material in the esophagus. Over time, this may result in permanent dilation of the body of the esophagus. In most cases, histologic examination confirms evidence of decreased neurons in the myenteric plexi, with significant inflammatory infiltration including lymphocytosis [22]. The nitric oxide-producing, inhibitory neurons are preferentially affected [23], while cholinergic neurons are largely preserved [24]. As such, the acetylcholinesterase inhibitor edrophonium choline produces enhanced contraction in achalasia. Specific targeted deletion of the neuronal nitric oxide synthase gene in an animal model produces the phenotype of achalasia [25]. Exceptions to this pathological finding include secondary achalasia from multiple endocrine neoplasia (MEN) type 2B and von Recklinghausen’s disease (neurofibromatosis), which are characterized not by dropout, but by hyperganglionosis or dysplasia of the myenteric plexus. A mutation in the RET protooncogene, associated with Hirschsprung’s disease, was also identified in 90 % of patients with MEN type2, which may explain improper neural crest migration and differentiation [26]. However, other hereditary forms of achalasia require further genetic characterization. Achalasia is also described in patients with autoimmune polyglandular syndrome [27].
Many of the treatments applied for achalasia address and add clarity to these pathophysiologic pathways. The goal of treatment is symptom improvement by decreasing the LES resting pressure to enhance esophageal clearance, and to minimize the effects of esophageal stasis leading to progressive esophageal dilation. However, no treatments to date have shown restoration of peristalsis in the esophageal body. The non-relaxing LES can be treated by mechanical methods (pneumatic dilation or surgical myotomy), or biochemical means (endoscopic botulinum toxin injection (EBTI) and oral medications). While mechanical methods treat the anatomic obstruction resulting from incomplete LES relaxation, biochemical methods are targeted at specific portions of the proposed pathway. In EBTI, botulinum neurotoxin type A is endoscopically injected into the LES. Botulinum toxin inhibits acetylcholine release to reduce the unopposed excitation of the LES seen in achalasia, thereby allowing the LES to function as normal [28].
Oral medications such as calcium channel blockers (nifedipine 10–30 mg SL, 30–45 min before meals) [29] and nitrates (isosorbide dinitrate 5 mg SL, 10–15 min before meals) [30] can also induce relaxation of the smooth muscle of the LES to enhance esophageal transit in achalasia. The efficacy of these medications, though limited, suggest that the underlying function of the LES remains preserved. More interesting, sildenafil has also been investigated for treatment of achalasia in a smaller study with some success [31]. Sildenafil is a phosphodiesterase inhibitor used in functional impotence, and results in enhancement of inhibitory pathway induced by nitric oxide. Its application in achalasic patients results in improved LES relaxation, further supporting the importance of the above pathophysiologic pathway in achalasia.
Complications of achalasia may include esophageal candidiasis or frank esophagitis, due to retention of food matter in the esophagus. This can contribute to symptoms of dysphagia or odynophagia. There have also been reports of esophageal diverticula, developing as a result of slowed esophageal transit with alteration in bolus flow [32].
The diagnosis of achalasia is usually made with a combination of three testing modalities, which demonstrate evidence of the pathophysiologic process. Esophagogastroduodenoscopy (EGD) may often reveal esophageal dilation with retained foodstuff, as well as complications of esophagitis or candidiasis (Fig. 2.1). Endoscopy is also helpful to exclude other findings such as esophageal or gastric malignancy that can result in secondary achalasia. Barium swallow radiography will often reveal the characteristic finding of smooth tapering or “bird-beaking” in the distal esophagus, which suggests lack of overt mucosal pathology but represents poor LES relaxation (Fig. 2.2). Finally, esophageal manometry is key to the diagnosis of achalasia by revealing evidence of aperistalsis, poor LES relaxation, and often an elevation in baseline LES pressure.
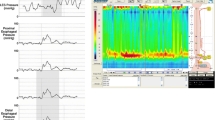
As a result of advances in high resolution esophageal manometry (HREM), the diagnosis of achalasia can be further divided into manometric subtypes, with impact on treatment response [33, 34]. Although esophageal aperistalsis, poor LES relaxation, and elevation in basal LES pressure are seen commonly across subtypes, distinguishing manometric characteristics allows for further sub-classification. Type 1 is the classic subtype, with absent esophageal pressurization (Fig. 2.3). Type 2 is the esophageal compression subtype, with pan-esophageal pressurization of the esophagus in greater than 20 % of swallows (Fig. 2.4). Type 3 is the spastic subtype, with high amplitude spastic contractions of the esophagus in greater than 20 % of swallows (Fig. 2.5). Distal esophageal peristalsis may be preserved in this subtype, but proximal peristalsis remains absent. A normal esophageal manometric swallow is included for reference (Fig. 2.6).
In candidates with suggestive history or risk factors, chest imaging such as x-ray or CT scan may assist in excluding etiologies of secondary achalasia, including lung cancer, which cannot be identified on the aforementioned testing modalities.
The natural disease course of patients with achalasia that do not receive treatment includes progressive esophageal dilation and tortuosity. In late-stage achalasia, megaesophagus is irreversible and may require esophagectomy [35]. Additionally, an increased risk of squamous cell esophageal cancer has been identified in patients with achalasia, but as the absolute risk is low (with annual incidence of 0.34 %) [36], endoscopic surveillance is not routinely recommended. An association with esophageal adenocarcinoma has also been reported [37]. The pathway has not been elucidated, though it has been proposed that chronic stasis may result in bacterial overgrowth and mucosal dysplasia, leading to the increased cancer risk [38].
References
Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscope. JAMA. 1998;280(7):638–42.
Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol. 1998;275:C684–92.
Mashimo H, Goyal RK. Physiology of esophageal motility. GI Motility Online. 2006. www.GIMotilityonline.com. doi:10.1038/gimo3.
Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610–9.
Goyal RK, Chaudhury A. Pathogenesis of achalasia: lessons from mutant mice. Gastroenterology. 2010;139(4):1086–90.
Castagliuolo I, Brun P, Costantini M, Rizzetto C, Palu G, Costantino M, Baldan N, Zaninotto G. Esophageal achalasia: is the herpes simplex virus really innocent? J Gastrointest Surg. 2004;8(1):24–30.
Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut. 1993;34(3):299–302.
Birgisson S, Galinski MS, Goldblum JR, et al. Achalasia is not associated with measles or known herpes and human papilloma viruses. Dig Dis Sci. 1997;42:300–6.
Kelly JL, Mulcahy TM, O’Riordain DS, et al. Coexistent Hirschsprung’s disease and esophageal achalasia in male siblings. J Pediatr Surg. 1997;32(12):1809–11.
Evsyutina YV, Trukhmanov AS, Ivashkin VT. Family case of achalasia cardia: case report and review of literature. World J Gastroenterol. 2014;20(4):1114–8.
Kallel-Sellami M, Karoui S, Romdhane H, Laddhar L, Serghini M, Boubaker J, Lahmar H, Filali A, Makni S. Circulating antimyenteric autoantibodies in Tunisian patients with idiopathic achalasia. Dis Esophagus. 2013;26(8):782–7.
Verne GN, Sallustio JE, Eaker EY. Anti-myenteric neuronal antibodies in patients with achalasia: a prospective study. Dig Dis Sci. 1997;42:307–13.
Wong RK, Maydonovitch CL, Metz SJ, Baker Jr JR. Significant DQw1 association in achalasia. Dig Dis Sci. 1989;34:349–52.
Moses PL, Ellis LM, Anees MR, Ho W, Rothstein RI, Meddings JB, Sharkey KA, Mawe GM. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut. 2003;52(5):629–36.
Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87(4):848–56.
Atkinson M, Ogilvie AL, Robertson CS, Smart HL. Vagal function in achalasia of the cardia. Q J Med. 1987;63(240):297–303.
De Oliveira RB, Rezende Filho J, Dantas RO, Iazigi N. The spectrum of esophageal motor disorders in Chagas’ disease. Am J Gastroenterol. 1995;90(7):1119–24.
Kahrilas PJ, Kishk SM, Helm JF, Dodds WJ, Harig JM, Hogan WJ. Comparison of pseudoachalasia and achalasia. Am J Med. 1987;82(3):439–46.
Katzka DA, Farrugia G, Arora AS. Achalasia secondary to neoplasia: a disease with a changing differential diagnosis. Dis Esophagus. 2012;25(4):331–6.
Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50:652–7.
Weber A, Wienker TF, Jung M, et al. Linkage of the gene for the triple A syndrome to chromosome 12q13 near the type II keratin gene cluster. Hum Mol Genet. 1996;5:2061–6.
Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111(3):648–54.
Dodds WJ, Dent J, Hogan WJ, Patel GK, Toouli J, Arndorfer RC. Paradoxical lower esophageal sphincter contraction induced by cholecystokinin-octapeptide in patients with achalasia. Gastroenterology. 1981;80(2):327–33.
Holloway RH, Dodds WJ, Helm JF, et al. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology. 1986;90:924–9.
Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS−/− and hypotensive in W/Wv mutant mice. Gastroenterology. 2001;121:34–42.
Eng C. Seminars in medicine of the Beth Israel Hospital, Boston: the RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med. 1996;335:943–51.
Fritzen R, Bornstein SR, Scherbaum WA. Megaoesophagus in a patient with autoimmune polyglandular syndrome type II. Clin Endocrinol (Oxf). 1996;45:493–8.
Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110(5):1410–5.
Traube M, Dubovik S, Lange RC, McCallum RW. The role of nifedipine therapy in achalasia: results of a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 1989;84(10):1259–62.
Wen ZH, Gardener E, Wang YP. Nitrates for achalasia. Cochrane Database Syst Rev. 2004;1, CD002299.
Bortolotti M, Mari C, Lopilato C, Porrazzo G, Miglioli M. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118(2):253–7.
Ott DJ, Hodge RG, Chen MY, Wu WC, Gelfand DW. Achalasia associated with esophageal diverticula. Prevalence and potential implications. J Clin Gastroenterol. 1994;18(4):343–6.
Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135(5):1526–33.
Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144(4):718–25.
Eckardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol. 2008;20(10):956–60.
Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 2010;105(10):2144–9.
Zendehdel K, Nyren O, Edberg A, Ye W. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol. 2011;106(1):57–61.
Brucher BL, Stein HL, Bartels H, Feussner H, Siewert JR. Achalasia and esophageal cancer: incidence, prevalence, and prognosis. World J Surg. 2001;25(6):745–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lo, WK., Mashimo, H. (2016). Pathophysiology of Achalasia. In: Fisichella, P., Herbella, F., Patti, M. (eds) Achalasia. Springer, Cham. https://doi.org/10.1007/978-3-319-13569-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-13569-4_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13568-7
Online ISBN: 978-3-319-13569-4
eBook Packages: MedicineMedicine (R0)