Abstract
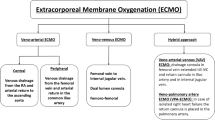
Extracorporeal membrane oxygenation (ECMO) provides mechanical support for either (1) the heart and lungs, termed venoarterial (VA) ECMO or (2) the lungs alone, termed venovenous (VV) ECMO. This chapter will focus primarily on the application of VV ECMO in the trauma patient, but will also briefly touch upon the relatively new use of VA ECMO in this population. Basic tenets of circuitry, cannulation strategies, and management will be reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
15.1 VV and VA ECMO Circuit Overview
An extracorporeal membrane oxygenation (ECMO) circuit consists of drainage and return cannulae, a pump and a membrane oxygenator with heat exchanger (Fig. 15.1a) [1, 2]. Venoarterial (VA) ECMO drains deoxygenated blood through a venous cannula to a centrifugal pump, arranged in series with a membrane oxygenator and returns oxygenated blood via an arterial cannula [2, 3]. In contrast, venovenous (VV) ECMO returns oxygenated blood via a second venous cannula [4].
15.2 VV ECMO
The use of VV ECMO for acute respiratory distress syndrome (ARDS) has expanded dramatically following positive outcomes published in the CESAR trial [5] and the favorable experience during the H1N1 influenza pandemic of 2009–2010 [6,7,8]. More recent evidence clarifying its role in the management of adults with severe ARDS will likely contribute to increased use of VV ECMO in the future [9]. Despite the expanding role of VV ECMO for ARDS in the nontrauma patient population [5,6,7,8], VV ECMO use in the trauma patient population has been somewhat limited due to continued concerns over bleeding complications associated with systemic anticoagulation and the inflammatory response incited by the ECMO circuit [10,11,12], particularly in patients with traumatic brain injury (TBI) [4, 5, 13]. However, recent observational studies have demonstrated promising results for the use of VV ECMO in both the poly-trauma and TBI patient population with very few reported bleeding complications [18,19,20,21,22,23,24,25,26]. Table 15.1 summarizes the evidence for the use of VV ECMO for ARDS specific to the trauma patient population.
According to the 2017 Extracorporeal Life Support Organization (ELSO) guidelines [14], VV ECMO should be considered when risk of mortality exceeds 50% [14], and indicated when risk of mortality exceeds 80% [14]. 50% mortality in ARDS is associated with: (1) PaO2/FiO2 < 150 on FiO2 > 90% [15]; (2) Murray score of 2–3 (Table 15.2) [15]; (3) Age-adjusted oxygenation index (AOI) >60 [16]; and (4) ARDS prediction score (APPS) ≥5 [17]. 80% mortality in ARDS is associated with: (1) PaO2/FiO2 < 100 on FiO2 > 90% [15]; (2) Murray score of 3–4 (Table 15.2) [15]; (3) AOI >80 [16]; and (4) APSS ≥8 [17]. While ELSO states there are no absolute contraindications to VV ECMO [14], severely injured poly-trauma [10,11,12] or TBI [4, 13] are considered by many to have relative contraindication to the systemic anticoagulation used in VV ECMO. It is worth noting that the CESAR trial [5], the single randomized controlled trial demonstrating a survival benefit for VV ECMO referrals compared to no-ECMO (relative risk (RR) 0.69, [95% confidence interval (CI): 0.05–0.97]; p = 0.03), included trauma patients (6% of the ECMO cohort) but excluded patients with intracranial bleeding or any contraindication (relative or absolute) to systemic heparinization [5].
15.3 VV ECMO Circuit Management
15.3.1 VV ECMO Cannulation Strategies
The elements of a typical VV ECMO circuit and the three most common cannulation strategies employed in VV ECMO are shown in Fig. 15.1. The cannula orientation should maximize flow and minimize recirculation [14, 27,28,29] and should be placed under fluoroscopic and echocardiographic guidance if at all possible. In all cases, a bolus 5000 units of Heparin should be administered prior to cannulation to minimize the risk of clot formation and possible circuit thrombosis [1, 14].
Single site dual-lumen cannulation (AvalonElite Bi-caval Dual Lumen Catheter; Maquet, Gothenburg, Sweden) is performed with a 27 or 31 French cannula (depending on the patient’s size and cardiac output) typically using the right internal jugular (IJ) vein. The tip of the cannula is positioned in the mid-IVC a few centimeters below the hepatic veins with drainage occurring through side-ports in the SVC and IVC. The return lumen is approximately 10 cm above the distal tip and should be positioned such that the oxygenated return will flow through the tricuspid valve [29]. This cannulation strategy enables early ambulation but can be somewhat difficult to position.
The other cannulation strategies use single lumen catheters. In bilateral femoral cannulation, venous drainage occurs from a cannula introduced into the femoral vein with the tip placed 5–10 cm below the IVC-RA junction within the intra-hepatic vena cava (drainage side-holes positioned above the collapsible intra-abdominal vena cava). Oxygenated return occurs from a cannula introduced into the contralateral femoral vein with the tip in the RA at the level of the tricuspid valve [14, 27]. This strategy is commonly employed in urgent situations where access to the neck is limited and early ambulation is unlikely. This cannula orientation requires a large caliber vena cava to ensure adequate space for two cannulae. The other 2-site strategy is termed “bi-caval cannulation.” In this approach, venous drainage occurs from a cannula introduced into the femoral vein with the tip placed 5–10 cm below the IVC-RA junction, again within the intra-hepatic vena cava. Oxygenated return occurs through a small caliber, short cannula introduced into the right internal jugular (IJ) vein with the tip at the SVC-RA junction [14, 27, 28]. This approach is ideal for controlled cannulation in most trauma patients who will not be candidates for early ambulation.
15.3.2 Monitoring Targets
Following cannulation and heparinization, the VV ECMO circuit should be unclamped and flows gradually increased to the target flow range, typically ≥60% of the calculated cardiac output (CO) (approximately 50–80 mL/kg/min [3.5–5 L/min]) [14]. Inlet saturation (sampled from the drainage cannula immediately prior to the oxygenator) is a surrogate for SvO2 and should be maintained ≥70% [14, 36]. Outlet saturation (sampled from the return cannula immediately after the oxygenator) should be ≥95% with a PaO2 > 300 mmHg [14, 36]. If the outlet saturation is less than 95%, the oxygenator should be investigated for potential clot formation [14, 36]. FiO2 on the VV ECMO circuit should be titrated to achieve a patient-level arterial saturation of ≥88% [14, 36]. Sweep gas flow (oxygen flow through the gas exchange membrane) on the VV ECMO circuit should be titrated to achieve a patient-level PaCO2 between 30 mmHg and 40 mmHg [14, 36]. VV ECMO does not provide hemodynamic support and therefore will not mitigate the need for inotropic and/or vasopressor support. Inotropes are typically titrated to targets such as SvO2 ≥ 65% or cardiac index (CI) ≥2.0 L/min, and vasopressors titrated to a MAP ≥65 mmHg. In many cases, the patient’s hemodynamics will improve with decreased ventilator pressures and increased systemic oxygen levels.
15.4 VV ECMO Patient Management
15.4.1 Anticoagulation Range
In the absence of any contraindications to systemic anticoagulation, a heparin bolus of 5000 units should be administered prior to cannulation to minimize risk of clot formation while the circuit is clamped [1, 14]. A heparin infusion should then be initiated with a goal ACT of at least 160 s, [1, 14] ideally between 180 s and 220 s [14, 22]. Although aPTT may be used, ESLO guidelines do not recommend its use because it is susceptible to derangements in coagulation factor levels and platelet function which commonly occur in VV ECMO patients [14]. If aPTT is used to monitor ECMO anticoagulation, it should be maintained between 40 s and 50 s [14]. In the setting of TBI, heparin-boned circuitry [19, 30,31,32,33] and a period of heparin-free support have led to successful management of VV ECMO for ARDS in several case series [19, 33,34,35].
15.4.2 Ventilator Management
Ventilator FiO2 should be set on “lung rest” settings with an FiO2 ≤ 0.4 [14, 36], a plateau pressure of ≤25 cm H2O [5, 14, 36], and a PEEP between 5 and 10 cm H2O [5, 14, 36]. Although the ELSO guidelines [14, 36] and the CESAR Trial [5] promote pressure control ventilation (PCV) [5, 36], volume controlled ventilation (VCV) is acceptable, as long as tidal volumes are set at 4–6 mL/kg/ideal body weight and plateau pressures are maintained at ≤25 cm H2O [4]. Debate on the safety of allowing the lungs to “white out” by minimizing ventilator support continues. Regardless, PEEP levels should be decreased judiciously to avoid losing recruited alveolar units that may still be contributing to gas exchange.
15.4.3 Sedation Strategies
For the first 24–48 h after VV ECMO initiation, heavy sedation is recommended [14, 36]. After initial stabilization, a tapered sedation plan should be implemented to allow for early and frequent assessment of neurologic status [37]. Pharmacokinetic and pharmacodynamic changes in the critically ill result in significant variability between drug dosing and response [38]. These pharmacologic derangements are further exaggerated in ECMO patients [39]. The ECMO circuit increases the volume of distribution by either hemodilution and/or sequestration of drugs [39, 40], particularly highly lipophilic drugs [39,40,41,42]. Existing data for appropriate anesthesia and analgesia drug choices on ECMO remains somewhat sparse [43]. Initiating a continuous infusion of an opioid (e.g., fentanyl or hydromorphone) and a sedative (e.g., propofol) during VV ECMO is a reasonable first step [43]. Propofol buildup may start to appear as white streaks in the membrane lung after several days, but the impact of this on membrane efficiency is unknown. If hemodynamically stable, daily sedation interruptions are recommended, especially in anticipation of ECMO weaning and ultimately decannulation [44].
15.4.4 Peri-procedural Management
Surgical procedures can be done successfully while on VV ECMO. When possible, the heparin infusion should be discontinued 6 h prior. If urgent or emergent surgery is necessary, fresh frozen plasma (FFP) should be infused prior to and during surgery; however, pharmacologic reversal with protamine is never recommended because of risk of circuit thrombosis [14]. Electrocautery should be used liberally in surgical cases, and even in minor procedures such as chest tube insertion performed on VV ECMO, to minimize bleeding [14]. For patients who require open surgery while on ECMO, we recommended temporary cavitary closure with intermittent washouts until ECMO has been discontinued, as the patient is very likely to bleed significantly into the closed cavity during ECMO support.
15.4.5 Tracheostomy Timing and Technique
According to the 2017 ELSO guidelines [14], both “early” extubation and tracheostomy (i.e., at 3–5 days post-cannulation) are recommended for those on VV ECMO [14]. Candidates for endotracheal extubation (or no endotracheal intubation) [45] while on VV ECMO support are typically pre-operative lung transplantation cases [46,47,48,49,50]. Unlike pre-operative lung transplant patients, severely injured, polytrauma patients with ARDS are more likely to benefit from early tracheostomy airway management. Although early tracheostomy does not necessarily confer a mortality benefit or decreased duration of mechanical ventilation, it can permit decreased sedation and earlier mobilization [51,52,53]. Careful planning and meticulous hemostasis are essential to the success of a tracheostomy in a patient on VV ECMO and the advised technique differs from a standard tracheostomy [14]. A “hybrid” open/percutaneous technique minimizes the risk of bleeding: (1) hold heparin for 6 h, (2) set the ventilator to room air, (3) expose the anterior trachea through a small incision made with an electrocauter, (4) insert the tracheostomy using a percutaneous dilational technique with a Ciaglia Blue Rhino® (Cook Medical, Bloomington, IN) under bronchoscopic guidance, and (5) resume heparin at the previous infusion rate without a bolus once hemostasis is assured.
15.4.6 Early Mobilization and Physical Therapy
The literature for early physical therapy while on VV ECMO is accumulating [54]. Evidence for the efficacy and safety of early mobilization while on VV ECMO is in the pre-operative lung transplantation population [46,47,48,49,50] facilitated largely by using a dual-lumen cannula in the right IJ (AvalonElite Bi-caval Dual Lumen Catheter; Maquet, Gothenburg, Sweden). Recently, the scope of physical therapy during VV ECMO support has expanded and proven to be both efficacious [55] and safe [56].
15.5 VA ECMO
While the evidence for the use of VV ECMO for ARDS in the trauma patient population is accumulating with positive outcomes [18,19,20,21,22,23,24,25,26], the evidence for VA ECMO following cardiothoracic trauma or traumatic cardiac arrest from exsanguination is inadequate. Table 15.3 summarizes two retrospective, observational cohort studies investigating outcomes of a combined VV and VA ECMO cohort [30, 57]. VV ECMO cases in both studies had a survival benefit, but the VA patients in each study were very heterogeneous with respect to their underlying diagnoses [30, 57]. Future randomized controlled trials comparing VA ECMO to the current standard in a select trauma patient population are warranted.
To address this evidence gap for the utility of VA ECMO following traumatic arrest, Tisherman and colleagues are actively enrolling in a multicenter clinical trial [58]. This trial is an innovative, parallel assignment, interventional clinical trial comparing “usual care” to “emergency preservation and resuscitation (EPR)” in trauma patients who have exsanguinated to the point of cardiac arrest requiring resuscitative thoracotomy [58, 59]. The investigators define usual care as an emergency thoracotomy, open cardiac massage and fluid resuscitation, and EPR as going onto cardiopulmonary bypass (CPB) by central aortic cannulation in the ascending aorta and central venous cannulation in the right atrium for those patients who fail to achieve return of spontaneous circulation after aortic clamping [59]. These investigators plan to enroll 20 trauma patients (10 assigned to each arm) with a primary outcome of survival to hospital discharge without major disability, and secondary outcomes of (1) feasibility, (2) survival, (3) neurologic functional outcome, and (4) multiple organ dysfunction [58]. This trial represents an important first step in understanding how ECMO may be applied to the management of severely injured trauma patients outside of the typical indications of respiratory failure and the surgical management of tracheobronchial injuries.
15. Conclusions
VV ECMO for ARDS is feasible and safe in the trauma patient population and appears to confer a significant mortality benefit based on retrospective data. In the setting severe ARDS refractory to conventional mechanical ventilation, VV ECMO with delayed systemic anticoagulation is acceptable in those with TBI when combined with vigilant monitoring for circuit thrombosis. ECMO alters the pharmacokinetics and pharmacodynamics of lipophilic and protein-bound medications; so sedation strategies often need to be adjusted significantly during ECMO support. Surgical interventions can be performed, but the techniques used require modification to include liberal use of cautery and damage control techniques with open cavitary management. VA ECMO following traumatic arrest is being evaluated in a single pilot study. Taken together, use of both VV and potentially VA ECMO has the potential to substantially improve outcomes in the severely injured.
References
Kaplan JA, Augoustides JGT, Manecke GR, Maus T, Reich DL. Kaplan’s cardiac anesthesia: for cardiac and noncardiac surgery. 7th ed. Amsterdam: Elsevier; 2017.
Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, Paden ML, ELSO member centers. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(1):60–7.
Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry E. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59(3):202–10.
Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–14.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63.
Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I, Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–95.
Cianchi G, Bonizzoli M, Pasquini A, Bonacchi M, Zagli G, Ciapetti M, Sani G, Batacchi S, Biondi S, Bernardo P, et al. Ventilatory and ECMO treatment of H1N1-induced severe respiratory failure: results of an Italian referral ECMO center. BMC Pulm Med. 2011;11:2.
Beurtheret S, Mastroianni C, Pozzi M, D'Alessandro C, Luyt CE, Combes A, Pavie A, Leprince P. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome: single-centre experience with 1-year follow-up. Eur J Cardiothorac Surg. 2012;41(3):691–5.
Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–75.
Michaels AJ, Schriener RJ, Kolla S, Awad SS, Rich PB, Reickert C, Younger J, Hirschl RB, Bartlett RH. Extracorporeal life support in pulmonary failure after trauma. J Trauma. 1999;46(4):638–45.
Voelckel W, Wenzel V, Rieger M, Antretter H, Padosch S, Schobersberger W. Temporary extracorporeal membrane oxygenation in the treatment of acute traumatic lung injury. Can J Anaesth. 1998;45(11):1097–102.
Madershahian N, Wittwer T, Strauch J, Franke UF, Wippermann J, Kaluza M, Wahlers T. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. 2007;22(3):180–4.
Messing JA, Agnihothri RV, Van Dusen R, Najam F, Dunne JR, Honig JR, Sarani B. Prolonged use of extracorporeal membrane oxygenation as a rescue modality following traumatic brain injury. ASAIO J. 2014;60(5):597–9.
(ELSO) ELSO. ELSO Guidelines for cardiopulmonary extracorporeal life support. Ann Arbor, MI; 2017. Version 1.4. Available from: https://www.elso.org/resources/guidelines.aspx.
Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–3.
Dechert RE, Park PK, Bartlett RH. Evaluation of the oxygenation index in adult respiratory failure. J Trauma Acute Care Surg. 2014;76(2):469–73.
Villar J, Ambros A, Soler JA, Martinez D, Ferrando C, Solano R, Mosteiro F, Blanco J, Martin-Rodriguez C, Fernandez MM, et al. Age, PaO2/FIO2, and plateau pressure score: a proposal for a simple outcome score in patients with the acute respiratory distress syndrome. Crit Care Med. 2016;44(7):1361–9.
Cordell-Smith JA, Roberts N, Peek GJ, Firmin RK. Traumatic lung injury treated by extracorporeal membrane oxygenation (ECMO). Injury. 2006;37(1):29–32.
Muellenbach RM, Kredel M, Kunze E, Kranke P, Kuestermann J, Brack A, Gorski A, Wunder C, Roewer N, Wurmb T. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(5):1444–7.
Biderman P, Einav S, Fainblut M, Stein M, Singer P, Medalion B. Extracorporeal life support in patients with multiple injuries and severe respiratory failure: a single-center experience? J Trauma Acute Care Surg. 2013;75(5):907–12.
Ried M, Bein T, Philipp A, Muller T, Graf B, Schmid C, Zonies D, Diez C, Hofmann HS. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care. 2013;17(3):R110.
Guirand DM, Okoye OT, Schmidt BS, Mansfield NJ, Aden JK, Martin RS, Cestero RF, Hines MH, Pranikoff T, Inaba K, et al. Venovenous extracorporeal life support improves survival in adult trauma patients with acute hypoxemic respiratory failure: a multicenter retrospective cohort study. J Trauma Acute Care Surg. 2014;76(5):1275–81.
Bosarge PL, Raff LA, McGwin G Jr, Carroll SL, Bellot SC, Diaz-Guzman E, Kerby JD. Early initiation of extracorporeal membrane oxygenation improves survival in adult trauma patients with severe adult respiratory distress syndrome. J Trauma Acute Care Surg. 2016;81(2):236–43.
Munoz J, Santa-Teresa P, Tomey MJ, Visedo LC, Keough E, Barrios JC, Sabell S, Morales A. Extracorporeal membrane oxygenation (ECMO) in adults with acute respiratory distress syndrome (ARDS): a 6-year experience and case-control study. Heart Lung. 2017;46(2):100–5.
Ahmad SB, Menaker J, Kufera J, O'Connor J, Scalea TM, Stein DM. Extracorporeal membrane oxygenation after traumatic injury. J Trauma Acute Care Surg. 2017;82(3):587–91.
Menaker J, Tesoriero RB, Tabatabai A, Rabinowitz RP, Cornachione C, Lonergan T, Dolly K, Rector R, O’Connor JV, Stein DM, et al. Veno-venous extracorporeal membrane oxygenation (VV ECMO) for acute respiratory failure following injury: outcomes in a high-volume adult trauma center with a dedicated unit for VV ECMO. World J Surg. 2018;42(8):2398–403.
Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226(4):544–64. discussion 65-6
Javidfar J, Wang D, Zwischenberger JB, Costa J, Mongero L, Sonett J, Bacchetta M. Insertion of bicaval dual lumen extracorporeal membrane oxygenation catheter with image guidance. ASAIO J. 2011;57(3):203–5.
Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90(3):991–5.
Arlt M, Philipp A, Voelkel S, Rupprecht L, Mueller T, Hilker M, Graf BM, Schmid C. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81(7):804–9.
Perchinsky MJ, Long WB, Hill JG, Parsons JA, Bennett JB. Extracorporeal cardiopulmonary life support with heparin-bonded circuitry in the resuscitation of massively injured trauma patients. Am J Surg. 1995;169(5):488–91.
von Segesser LK, Turina M. Cardiopulmonary bypass without systemic heparinization. Performance of heparin-coated oxygenators in comparison with classic membrane and bubble oxygenators. J Thorac Cardiovasc Surg. 1989;98(3):386–96.
Reynolds HN, Cottingham C, McCunn M, Habashi NM, Scalea TM. Extracorporeal lung support in a patient with traumatic brain injury: the benefit of heparin-bonded circuitry. Perfusion. 1999;14(6):489–93.
Firstenberg MS, Nelson K, Abel E, McGregor J, Eiferman D. Extracorporeal membrane oxygenation for complex multiorgan system trauma. Case Rep Surg. 2012;2012:897184.
Yen TS, Liau CC, Chen YS, Chao A. Extracorporeal membrane oxygenation resuscitation for traumatic brain injury after decompressive craniotomy. Clin Neurol Neurosurg. 2008;110(3):295–7.
(ELSO) ELSO. ELSO guidelines for cardiopulmonary extracorporeal life support. Ann Arbor, MI; 2013. Version 1.3. Available from: https://www.elso.org/Portals/0/IGD/Archive/FileManager/929122ae88cusersshyerdocumentselsoguidelinesgeneralalleclsversion1.3.pdf.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–7.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–51. quiz 59
Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27(6):741.e9–18.
Shekar K, Roberts JA, McDonald CI, Fisquet S, Barnett AG, Mullany DV, Ghassabian S, Wallis SC, Fung YL, Smith MT, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16(5):R194.
Preston TJ, Hodge AB, Riley JB, Leib-Sargel C, Nicol KK. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol. 2007;39(4):234–7.
Preston TJ, Ratliff TM, Gomez D, Olshove VE Jr, Nicol KK, Sargel CL, Chicoine LG. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010;42(3):199–202.
Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit Care. 2017;21(1):66.
Dzierba AL, Brodie D, Bacchetta M, Muir J, Wasson L, Colabraro M, Gannon W, Connolly K, Biscotti M, Rietdijk W, et al. Ketamine use in sedation management in patients receiving extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(11):1822–3.
Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kuhn C, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant. 2010;10(9):2173–8.
Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–9.
Garcia JP, Kon ZN, Evans C, Wu Z, Iacono AT, McCormick B, Griffith BP. Ambulatory veno-venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142(4):755–61.
Hayes D Jr, Kukreja J, Tobias JD, Ballard HO, Hoopes CW. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros. 2012;11(1):40–5.
Rehder KJ, Turner DA, Hartwig MG, Williford WL, Bonadonna D, Walczak RJ Jr, Davis RD, Zaas D, Cheifetz IM. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58(8):1291–8.
Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145(3):862–7. discussion 7-8
Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, Miletto A, Mangione S, Sinardi AU, Pastorelli M, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303(15):1483–9.
Young D, Harrison DA, Cuthbertson BH, Rowan K, TracMan C. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–9.
Trouillet JL, Luyt CE, Guiguet M, Ouattara A, Vaissier E, Makri R, Nieszkowska A, Leprince P, Pavie A, Chastre J, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med. 2011;154(6):373–83.
Thiagarajan RR, Teele SA, Teele KP, Beke DM. Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med. 2012;5(1):47–52.
Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, Zemmel D, Galuskin K, Morrone TM, Boerem P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18(1):R38.
Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014;29(3):395–400.
Jacobs JV, Hooft NM, Robinson BR, Todd E, Bremner RM, Petersen SR, Smith MA. The use of extracorporeal membrane oxygenation in blunt thoracic trauma: a study of the Extracorporeal Life Support Organization database. J Trauma Acute Care Surg. 2015;79(6):1049–53. discussion 53-4
ClinicalTrials.gov, Medicine NUNLo. Emergency preservation and resuscitation (EPR) for Cardiac arrest from trauma (EPR-CAT) [updated: May 3, 2017; cited 2018]. ClinicalTrials.gov Identifier: NCT01042015; ClinicalTrials.gov Clinical Trial Registration. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01042015?term=Tisherman&rank=1.
Tisherman SA, Alam HB, Rhee PM, Scalea TM, Drabek T, Forsythe RM, Kochanek PM. Development of the emergency preservation and resuscitation for cardiac arrest from trauma clinical trial. J Trauma Acute Care Surg. 2017;83(5):803–9.
Bonacchi M, Harmelin G, Bugetti M, Sani G, Peris A. Indications of extracorporeal life support in poly-trauma. Br J Anaesth. 2014;113(6):1058–9.
Swol J, Brodie D, Napolitano L, Park PK, Thiagarajan R, Barbaro RP, Lorusso R, McMullan D, Cavarocchi N, Hssain AA, Rycus P, Zonies D, Extracorporeal Life Support Organization (ELSO). Indications and outcomes of extracorporeal life support in trauma patients. J Trauma Acute Care Surg. 2018;84(6):831–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Experts’ Comments by Emiliano Gamberini and Alessandro Circelli
Experts’ Comments by Emiliano Gamberini and Alessandro Circelli
There has been a significant increase in the use of extracorporeal life support (ECLS) in adult patients who are in a state of shock and pulmonary failure. It has been proven to be effective and safe in acute cardiopulmonary failure, even when conventional therapies fail. Advanced management of polytrauma patients should include extracorporeal membrane oxygenation (ECMO) in cases of persistent circulatory and/or respiratory failure despite adequate conventional treatments [30, 60, 61].
Technical advances and compact devices have led to the increased use of ECLS as an advanced option in severe trauma treatment. The improvements in devices allow safer and easier ECLS, for example, anticoagulation can be safely delayed for 48–72 h after trauma due to improved biocompatibility.
ECMO can be used in severe multiple trauma patients as a multi-approach management in respiratory failure (lung contusions, chest wall disruption, acute respiratory distress syndrome), traumatic brain injury (TBI) with associated respiratory failure and impossibility of maintaining normo-hypocapnia with lung protective strategies, post-traumatic cardiogenic shock (providing full hemodynamic support), and tracheobronchial injury.
In patients with severe TBI and hemodynamic instability, ECLS can be used with the purpose of saving time for brain death assessment, and should be continued in order to support an eventual organ donation program.
ECMO is also used to ensure adequate perfusion in cardiopulmonary failure in patients with severe trauma, even in the context of hemorrhagic shock. The surgeon can perform damage control surgery, and coagulation abnormalities can be treated according to the recommendations for blood component transfusion.
ECLS is also used in post-traumatic cardiac arrest requiring resuscitative thoracotomy, but the evidence for this is still inadequate.
The evidence for the benefits in terms of survival is still lacking, although we think that ECLS plays an important role in trauma patients, although the exact role is yet unknown. The use of ECMO in the treatment of trauma patients should be considered in patient populations where conventional treatments fail to result in more benefits than risks.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
MacKay, E.J., Cannon, J.W. (2020). Extracorporeal Membrane Oxygenation in the Unstable Trauma Patient. In: Hörer, T., DuBose, J., Rasmussen, T., White, J. (eds) Endovascular Resuscitation and Trauma Management . Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-25341-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-25341-7_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25340-0
Online ISBN: 978-3-030-25341-7
eBook Packages: MedicineMedicine (R0)