Abstract
Extracorporeal life support encompasses various techniques that support patients with severe cardiac and/or pulmonary failure. Extracorporeal membrane oxygenation (ECMO) is provided for stabilization of salvageable patients when death is imminent and other treatment options are not available. Advancements in ECMO technology have improved its safety and portability, which has led to a dramatic increase in its use. ECMO is an extension of cardiopulmonary bypass technique that has been routinely used in cardiac surgery. Today, ECMO is applied in multiple different scenarios, but more clinical research is needed to establish practice guidelines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
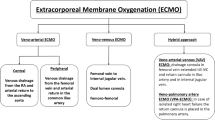
Extracorporeal life support (ECLS) encompasses various techniques that support patients with severe cardiac and/or pulmonary failure. In recent years, there has been a substantial increase in the use of ECLS in a broad population of patients with cardiopulmonary failure [1]. Extracorporeal membrane oxygenation (ECMO) may be configured as venoarterial ECMO (VA-ECMO) for cardiac support, veno-venous ECMO (VV-ECMO) for respiratory support, veno-arterial-veno ECMO (VAV-ECMO) for cardiac and respiratory failure, or extracorporeal carbon dioxide removal (ECCO2R) for lung protection [2]. ECMO to restore circulation during cardiac arrest is referred to as extracorporeal cardiopulmonary resuscitation (ECPR). In recent years, there has been a dramatic increase in the use of VA-ECMO for cardiogenic shock. This variation of ECMO is the primary focus of discussion in this chapter.
ECMO support is provided for stabilization of salvageable patients when death is imminent and other treatment options are not available. ECMO techniques are a modification of the standard cardiopulmonary bypass technique and allow for support durations (typically days to weeks) longer than usual cardiac surgery. ECMO support allows time for recovery of cardiac or pulmonary function or bridges patients to other definitive therapy, such as transplantation or durable left ventricular assist device (LVAD) support.
ECMO is a high-risk and complex therapy that requires substantial resources for initiation and continuing support. Effective ECMO requires an infrastructure or system of care with medical and technical expertise in all phases of advanced cardiopulmonary care. Ideally, a network consisting of a tertiary care center that provides all facets of advanced care is partnered with community healthcare providers to allow treatment of a broad range of patients in various communities [3,4,5]. Patient access to advanced cardiopulmonary care (including ECMO, transplant, durable LVAD, and other devices) with optimal medical therapy is an important challenge today.
Since the introduction of ECMO in the mid-1970s, its use has varied widely, with application predominantly in pediatrics until the 2009 H1N1 influenza epidemic, which affected a large number of adults [6,7,8]. Advancements in ECMO technology has improved its safety and portability, which has led to a dramatic increase in its use [9]. The ECMO circuit is smaller than the usual cardiopulmonary bypass, cannulae designed for ECMO are readily available, and the entire system is portable. These advances have allowed expansion into multiple hospital environments, such as the intensive care unit, cardiac catheterization laboratory, emergency department, and operating room, but also have allowed ECMO therapy to be instituted in community hospitals, permitting stabilization and safe transport to a tertiary care center. The application of VA-ECMO for cardiogenic shock has increased considerably in recent years. The ability to implement support in a variety of settings is important for the rapid restoration of circulation with adequate oxygenation. Prompt access to ECMO support avoids multiple organ failure, which is key to successful outcomes of the therapy [10,11,12]. ECMO support stabilizes the patient’s condition, allowing time for necessary transport, accurate diagnosis of underlying pathology, and definitive therapy in a controlled environment.
The current growth rate of ECMO is outpacing the evidence supporting the current clinical applications and management practices [13, 14]. Standards of care for ECMO have not been established, and most practices are not supported by randomized, controlled clinical trials [15]. The goal of this chapter is to provide a contemporary review of the use of ECMO for support during profound cardiogenic shock and pulmonary failure.
ECMO Indications and Contraindications
ECMO may be indicated for support of patients with severe pulmonary or cardiac failure, or a combination of both. Because ECMO is not intended to resolve the primary etiology of cardiac or pulmonary failure, it is a temporary bridge to recovery or other definitive therapy [16]. Indications for VA-ECMO in cardiac failure include cardiogenic shock, refractory ventricular arrhythmia, ongoing cardiopulmonary resuscitation for cardiac arrest, and acute decompensated heart failure (Table 15.1) [15]. The leading causes of cardiogenic shock are acute myocardial infarction (70% to 80%) and acute decompensated chronic heart failure (up to 30%), and these conditions comprise the majority of patients needing ECMO support. It is about 50% in our multi-institutional high-volume shock network [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Patients with witnessed cardiac arrest with cardiopulmonary resuscitation (CPR) in progress may be candidates for VA-ECMO if there is a reasonable possibility that neurologic function is intact. In many patients, ECMO support as a bridge to decision is necessary to determine viability for other treatments. Patients with irreversible end-organ function may not be suitable candidates for transplantation or durable mechanical circulatory support. Patients may need a period of stabilization and assessment of neurologic function. Bridging to recovery for cardiac failure may result in successful weaning without further need for mechanical assistance, and patients are treated medically. A bridge-to-bridge approach may be appropriate for patients who cannot be weaned from support and are candidates for a durable LVAD or artificial heart. Bridge to transplant is an option for a few patients, but donor availability limits this treatment to a small number of patients.
Use of VV-ECMO for primary pulmonary failure with adequate cardiac function is almost always considered a bridge to recovery while the lungs recover from infection or other cause of severe adult respiratory distress syndrome (ARDS) (Table 15.1). Etiologies for severe respiratory failure that may require VV-ECMO include viral, bacterial, or fungal lung infections, primary lung disease (e.g., cystic fibrosis, primary pulmonary hypertension, idiopathic fibrosis), chest trauma, and bronchiolitis obliterans. Patients with severe air leak from any cause, when adequate oxygenation and CO2 removal cannot be maintained, should be promptly supported with VV-ECMO. Acute severe respiratory failure in asthma or chronic obstructive pulmonary disease (COPD) may also benefit from this therapy.
ECMO Contraindications
Most contraindications to ECMO support are relative, while the risks are considered together with the potential benefits for individual patients. Relative contraindications include irreversible organ function, particularly the brain; conditions decreasing quality of life (neurologic damage, malignancy, severe risk of bleeding with anticoagulation); older age; patients who have been on conventional therapy for long duration; and aortic dissection. Although older age is associated with higher mortality, it should not be considered an absolute contraindication [20]. Lack of exit strategies should be considered as a contraindication.
The ECMO System
The basic ECMO system has a centrifugal blood pump, a membrane oxygenator, heat exchanger, inflow and outflow cannula, and tubing to establish a circuit between these components and the patient’s circulatory system (Fig. 15.1). Pumps most commonly used include the CentriMag (Abbott, Chicago, Illinois, USA), Rotaflow (Maquet, Rastatt, Germany), or TandemHeart (TandemLife, Pittsburgh, Pennsylvania, USA). Blood is drained from the inflow cannula and is pumped through the oxygenator and back to the patient through the outflow cannula. The gas flow rate and the fraction of inspired oxygen (FiO2) through the oxygenator control the exchange of oxygen and carbon dioxide with the blood. VA-ECMO draws blood from the venous circulation and returns oxygenated blood to the arterial circulation; it is used primarily for cardiac support while providing pulmonary assist. VV-ECMO differs by returning oxygenated blood to the pulmonary circulation and is used for severe pulmonary failure in the presence of adequate native cardiac function (Fig. 15.2).
Management of ECMO
Cannulation
Initiation of VA-ECMO support with open chest cannulation may take place in the operating room for cases of postcardiotomy shock; however, the majority of cases are initiated outside of the operating room [21]. VA-ECMO support can be initiated quickly using percutaneous cannulation in multiple hospital environments, such as the cardiac catheterization laboratory, operating room, emergency department, and intensive care unit. Appropriate cannulation for ECMO is critical for successful outcomes. Central cannulation usually is performed for postcardiotomy support when the chest is open; peripheral cannulation is most often employed in nonsurgical cases. Cannulation is initiated only after all team members and system components are at the bedside. A bolus of heparin, 50–100 units per kilogram, is given just before cannula placement. Cannulation for VA-ECMO is most commonly via a femoral vein (inflow) and a femoral artery (outflow). Venous cannulas are 19–25 Fr in diameter with a length up to 60 cm and may be of single- or multistage configuration. The outflow cannula placed in the arterial system is 15–19 Fr in diameter and 20–25 cm long. Cannula sizes vary with the size of the patient, and the proper cannula diameter can be determined by assessment of the vessel diameter using vascular ultrasound. The Fr gauge size used should be 3 times the size of the target vessel in mm [22]. Vascular ultrasound is useful to locate the target vessel and to assess for anatomic problems. The femoral arterial cannula ideally is at least more than 2 mm smaller than the vessel diameter to allow flow to the lower extremity. A properly selected cannula size allows enough blood flow to the leg and avoids ischemia. Near-infrared spectroscopy provides an assessment of tissue oxygenation and should be used to assess for leg ischemia [23].
Except when ECPR has been initiated, cannulation to provide blood flow distal to the cannulation site should be performed. Distal cannulation is completed by insertion of a 5–7 Fr sheath connected to the arterial outflow. Retrograde and antegrade cannulation can be accomplished by placement of a T-shaped Dacron graft on the femoral artery. This should be used when large cannula sizes are inserted or when femoral unilateral arterial and venous cannulation is performed. When distal arterial blood flow to the leg is inadequate, a separate perfusion cannula should be placed in the distal superficial femoral artery by cutdown or to the posterior tibial artery for retrograde perfusion.
When venous drainage is inadequate, an additional venous drainage cannula through a different vein may be necessary. It may be possible to change the cannula to a larger size, although removal and replacement of the cannula is sometimes very challenging. A damaged, kinked, or clotted cannula must be exchanged with a new cannula.
Initiating ECMO Support
The implementation of VA-ECMO for cardiac failure is usually more urgent than implementing VV-ECMO for respiratory failure [24, 25]. After a thorough assessment of the patient’s condition is completed by the ECMO team, the risk and benefits are carefully considered to avoid fruitless use of resources. Once the decision to proceed with ECMO support has been made, a lead physician coordinates cannulation and initiation of support. The initiation of ECMO therapy is extremely time sensitive, and numerous activities take place concurrently or in rapid succession until the patient’s condition is stabilized [26]. Perfusionists prepare the ECMO circuit, and nurses provide medical therapy as directed. The implanting physician determines the cannulation locations and cannula sizes to be inserted. Appropriate selection of cannula size should allow for full-support flow rate of 50–70 ml/kg/min in adults. After initiating support, VA-ECMO flow rate should be maintained in appropriate range to allow for adequate tissue perfusion or oxygenation, which is usually 4–6 l/min.
Monitoring
Patients should be monitored with an arterial pressure line and a pulmonary artery catheter. Continuous monitoring of the mixed venous oxygen saturation provides a real-time assessment of system perfusion. Pulmonary artery pressures are useful in assessing for left ventricular distension and the need for venting of the ventricle. Liberal use of echocardiography is invaluable in assessing for ventricular distension and the need for left ventricular venting [27]. It is also important to assess valvular function and thrombus formation within the heart.
Blood Gases
Oxygen delivery from ECMO should provide an arterial saturation greater than 95% (VA-ECMO) or at least 80% (VV-ECMO) with nominal ventilatory support. The difference between arterial and venous oxygen saturation is normally in the range of 20% to 30% when there is adequate oxygen delivery and consumption. A hematocrit of at least 40% assures adequate oxygen delivery. Removal of CO2 is controlled by the sweep gas flow rate through the ECMO membrane. The ratio of gas flow rate to blood flow rate is initially set at 1:1 and then titrated to maintain the partial pressure of CO2 (PaCO2) within the desired range. Carbogen (5% CO2/95% O2) may be used as the sweep gas to help maintain the outlet PaCO2 at about 40 mmHg. If the PaCO2 is greater than 70 mmHg, increasing the sweep gas gradually over several hours will help prevent rapid change in the arterial pH.
Anticoagulation
Most of the modern ECMO circuits require low level of systemic anticoagulation to prevent thrombosis within the circuit. Heparin is the most common anticoagulant and is monitored by activated thromboplastin time, (40–60 seconds), activated clotting time (1.5–2.5 times baseline), or indirect heparin concentration with anti-factor Xa (0.3–0.5 IU/ml) [28]. Thromboelastography (TEG) may also be useful to assess the time and density of clot formation in response to kaolin. Clot density is affected by clotting factors, platelets, and fibrinolysis, so TEG provides more information than activated clotting time. TEG can be performed with or without an agent that inactivates heparin to separate the anticoagulant effect of heparin from other factors. In patients with heparin-induced thrombocytopenia (HIT), direct thrombin inhibitors, such as parenteral bivalirudin and argatroban, may be used [29]. When alternative anticoagulants are used, the activated partial thromboplastin time should be maintained between 50 and 60 seconds.
Ventilator Management
Since ECMO provides full gas exchange, mechanical ventilation is less critical during full support. However, adequate respiratory function is necessary before weaning is begun. Endotracheal intubation is often necessary to maintain the airway. Mechanical ventilation with low tidal volume (3–5 ml/kg) with low airway pressure is desired to prevent lung injury. Positive end-expiratory pressure (PEEP) of 10–15 mmHg helps to maintain alveolar expansion. The FiO2 should be kept below 0.40.
Complications of ECMO
Bleeding
Bleeding is a common complication of ECMO support due to the necessary anticoagulation therapy and hematologic abnormalities. Blood transfusion should be used to maintain a hemoglobin concentration of at least 10 mg/dl. Bleeding can be minimized by careful attention to hemostasis at the cannula insertion sites and careful monitoring of anticoagulation therapy. In some cases, anticoagulation therapy may be decreased or stopped to help control excessive bleeding [30, 31].
Stroke
The rate of ischemic and hemorrhagic stroke during ECMO is approximately 4% [32]. The causes of stroke are due to anticoagulation therapy, the artificial surfaces of the ECMO circuit, and hemodynamic instability. Maintaining adequate flow rates and careful monitoring of anticoagulation status with adjustments in therapy are vital. Anticoagulation should be increased in low-flow states and during weaning, and excessive dosing of anticoagulants must be avoided. Cannulation of the femoral artery is associated with a much lower stroke rate than cannulation of the carotid artery.
Infection
Infection is common in patients being supported by ECMO, with over 50% of patients acquiring bacteremia, which has a mortality rate greater than 60% [16]. As much as possible, sterile techniques should be used during emergent cannulation, and antibiotic therapy should be provided throughout support. Usual surgical infection-control measures must be employed.
Limb Ischemia
A potential complication of femoral arterial cannulation occurs when the outer cannula diameter is near equal to the inner diameter of the blood vessel, resulting in very low or absent distal blood flow [31]. Although this complication occurs in less than 5% of cases, it can be effectively treated with proper recognition and treatment [23]. When time permits, an ultrasound procedure is performed on the cannulation target vessel to measure its diameter, permitting optimal cannula size selection. A proper cannula size will allow enough blood flow to the leg. Leg ischemia can be monitored using near-infrared spectroscopy on the leg, which provides an assessment of tissue oxygenation. Retrograde and antegrade cannulation is performed by placement of a T-shaped Dacron graft on the femoral artery, with cannula directed in both directions.
Left Ventricular Overload
In certain conditions, aortic retrograde blood flow during VA-ECMO may increase left ventricular afterload, which increases left ventricular end-diastolic pressure, left atrial pressure, and pulmonary wedge pressure [23, 33]. The elevated left-sided pressure can lead to pulmonary edema, hemoptysis, and poor gas exchange [34]. Hypoxemia may become severe, and poorly oxygenated blood from the left ventricle enters the cerebral and coronary circulation, causing neurologic dysfunction and worsening myocardial function. Decreased output from the left ventricle due to high afterload may inhibit aortic valve opening, increasing the potential for clot formation within the left ventricle or aortic root [35].
Venting of the left ventricle is used in 15%–20% of patients supported with VA-ECMO [36]. Patients should be closely monitored with a pulmonary artery catheter and a right radial arterial pressure line to assess for excessive afterload and prevent left ventricle overload. The pulmonary artery diastolic pressure should be maintained less than 22 mmHg. Left ventricular contractility, pulse pressure, and aortic valve opening are assessed with the right radial arterial pressure waveform. A low or absent pulse pressure and no dicrotic notch indicates that VA-ECMO flow and left ventricular afterload exceed the ability of the left ventricle to eject blood, and the aortic valve remains closed. Echocardiography should be used to assess left ventricle and left atrial size and to aid in intravascular volume management.
Minimizing VA-ECMO flow may help to avert left ventricular distension, but a flow rate should always be maintained at a level that achieves adequate systemic perfusion assessed by lactate level, arterial pH, and central venous oxygen saturation. Left ventricular afterload may also be controlled by adjusting VA-ECMO flow with cautious dosing of vasodilators and inotropes and appropriate balance of intravascular volume.
Various methods have been used for left heart decompression during peripheral VA-ECMO. Intra-aortic balloon pump (IABP) support is easy to implant and is effective in certain cases. Transseptal placement of an 8- to 15-Fr cannula into the left atrium with blood drained into the venous inflow has been effective [34]. Another percutaneous method to decompress the left ventricle uses a blade-balloon septostomy to create a left-to-right shunt [37]. Atrial stenting decreases left atrial volume but requires surgical closure following termination of VA-ECMO. Draining the pulmonary artery into the ECMO inflow through a percutaneously placed 15-Fr cannula has been effective in two reported cases [38]. Direct cannulation at the apex through a mini-thoracotomy or a subcostal approach with placement of a 21- to 23-Fr cannula in the left ventricle is another effective means [35, 39, 40]. The Impella 2.5 and 5.0 ventricular assist devices (Abiomed Inc., Danvers, MA, USA) may be used for either primary mechanical support in cardiogenic shock or for left ventricular unloading during VA-ECMO support. Single-center studies have shown improved hemodynamics; however, controlled multicenter trials evaluating this technique have not been conducted [41,42,43,44]. It is important to note that there are no studies comparing the various techniques for left ventricle decompression. The technique used should be based on the level of expertise and training at the individual center.
Harlequin Syndrome
Harlequin syndrome is a complication that may occur during VA-ECMO with peripheral cannulation when respiratory function is poor and deoxygenated blood from the left ventricle is pumped into the arterial circulation and is the primary blood flow source to the coronary and carotid arteries [23, 45]. In severe cases, myocardial recovery is deterred, and cerebral ischemia may cause neurologic deficit. The presence of Harlequin syndrome can be detected by monitoring the arterial oxygen saturation at the right radial artery, as this is the most distal point from the VA-ECMO blood flow. A finger pulse oximeter on the right hand may give an early indication of oxygen desaturation. In cases of Harlequin syndrome, mechanical ventilation with appropriate FiO2 and PEEP may help to maintain an arterial oxygen saturation of at least 90%. When the right radial oxygen saturation is less than 88%, the VA-ECMO flow may be too low and can be increased. Decreasing inotropic medications may also be useful. Use of beta-blockers to decrease heart rate may help decrease left heart output. When these measures fail to resolve the low radial artery saturation, cannulation of the ascending aorta for the VA-ECMO outflow should be employed. Also, the VA-ECMO outflow can be split between the femoral artery and a new cannula in the superior vena cava (SVC). When splitting the outflow, the cannula size in the femoral artery should be 17–19 Fr and should be 15–17 Fr in the SVC location [(31].)
Recirculation
Recirculation may occur with VV-ECMO—oxygenated blood from the ECMO outflow enters the drainage cannula without passing through the systemic circulation [46]. Recirculated blood does not contribute to oxygen delivery and decreases the overall efficiency of ECMO support. The pump speed and location of the inflow and outflow cannulas need to be assessed when recirculation occurs. Increasing the distance between the two cannulas and lowering flow rate may minimize the recirculation effects. Also, larger cannulas may allow high flow rates with minimal recirculation. Adding a second drainage cannula in a different location may also resolve the problem.
Weaning ECMO
Weaning from VA-ECMO versus VV-ECMO is very different, and techniques for both vary widely among institutions [47]. The optimal method for ECMO weaning is yet to be determined and is usually based on experience. Assessment for weaning begins soon after the initial stabilization, and daily trials of weaning should be performed. When considering weaning in patients, the primary cause of cardiac or pulmonary failure must be reversible, and other organ failure must be resolved or resolving [48]. Patients who do not have recoverable etiologies must be considered for transplantation, durable mechanical circulatory support, or hospice. Weaning from VV-ECMO requires return of adequate pulmonary function, whereas removal of VA-ECMO requires adequate cardiac function. Generally, weaning from VV-ECMO is more gradual, and careful attention to the adequacy of mechanical ventilation is given [47]. VA-ECMO weaning requires that patients maintain adequate cardiac output and blood pressure throughout the weaning process.
Weaning from VA-ECMO requires patients to maintain satisfactory hemodynamics before and throughout the weaning process. Inotropic support should be minimal, and mechanical support with an IABP or pVAD should be weaned. A weaning trial most often is performed in the operating room, and decannulation with proper hemostasis is performed for successful weaning trials. Echocardiography is used to assess for any ventricular distension, and blood pressure is continuously assessed. VA-ECMO flow is decreased in increments of 25% to 33% of the baseline flow rate, while the mean arterial blood pressure is maintained at greater than 65 mmHg. The patient should maintain a mixed venous saturation of at least 65% and an arterial saturation greater than 90% when ECMO flow is less than 1.5 l/min. Some centers advocate a slow weaning period of a few hours or even days, whereas others will perform the weaning trial in just an hour or two [16, 48].
Weaning from VV-ECMO begins once the patient has been stabilized, and minimal blood flow and sweep gas are set according to the patient’s condition. The level of VV-ECMO support is decreased as the patient’s condition improves and respiratory function returns. When VV-ECMO support is less than 30% of the maximum or initial amount of support, cardiac and respiratory function may allow removal of support. With the patient spontaneously breathing at an FiO2 of less than 50%, the ECMO flow is decreased in increments of 1 l/min to a minimum of 1 l/min. When the patient maintains a PaCO2 less than 50 mmHg and an arterial oxygen saturation greater than 95% for at least 1 hour, support can be removed [49].
ECMO Program Organization
ECMO is a complex therapy that should be managed at centers with appropriate experience and resources to ensure it is used effectively [24, 50]. Patients requiring ECMO support need the highest level of intensive care from a multidisciplinary team. New developing programs need to partner with experienced programs to provide a state-of-the-art system of care. Comprehensive advanced heart failure care programs at tertiary care centers serve as hub ECMO centers that are associated with regional centers. ECMO programs organized regionally can provide the high quality of care to as many patients as possible. Centers participating in a hub-and-spoke system of care should adhere to standardized protocols that detail criteria for the initiation of ECMO support, contraindications, follow-up care, and exit strategies.
ECMO programs should have a board-certified director with expertise in critical care, advanced heart failure, thoracic, cardiac, vascular, or trauma surgery or other board-certified specialist with specific training and experience in ECMO [24, 51, 52]. An ECMO coordinator, perfusionists, critical care nurses, and respiratory therapists provide technical and medical care. Ideally, institutional protocols guide team organization and individual team member responsibilities. Team members should complete ECMO training and periodically demonstrate competency [53]. An ECMO team coordinator is an essential part of the organization and communications. ECMO programs may use physician specialists, nurse practitioners, or RN to coordinate team activities and to provide triage between hub-and-spoke centers. Specialties needed to support the ECMO program include interventional cardiology, cardiothoracic surgery, pulmonology, neurology, nephrology, radiology, infectious disease, social workers, chaplains, palliative care, financial counselor, and hospital administrators. The responsibilities of each team member must be defined and agreed upon [15].
Quality assurance of the ECMO program must be a priority for the institution. Before starting the ECMO program, essential training and proficiency testing for all team members must be completed. Routine meetings with team members for review of training and equipment needs, staffing levels, patient volume, and case reviews are important. Referring spoke centers should participate in these review meetings. Morbidity and mortality meetings should be held to review any serious complication or death during ECMO support. Participation in the Extracorporeal Life Support Organization (ELSO) registry is useful for data comparison between a center and other institutions. New ECMO programs should complete a thorough analysis of the potential patient volume to assure that an appropriate amount of support exists. Hospital administration should be committed to financial support of the program costs and be prepared to adjust resources as the volume of cases varies.
ECMO Transport
There is an increasing number of patients that are being treated with ECMO in community hospitals. Ideally, patients with severe refractory cardiogenic shock or pulmonary failure will have ECMO support initiated at a major center that offers comprehensive advanced heart failure care [24]. However, many patients in need of emergent cardiopulmonary support who present at community hospitals may be initiated on-site by the local specialist or the ECMO team from a hub center. Regardless of the location at which ECMO support is initiated, the patient’s follow-up care should take place at a center with complete advanced heart failure care and/or pulmonary care.
The ELSO has published ECMO transport guidelines, which should be considered by centers participating in transport programs [54]. Efficient advanced cardiac care systems, including high-volume hub hospitals, emergency medical services, and community-based spoke centers, may impact the outcomes of patients with profound cardiac and/or pulmonary failure [50]. A hub-and-spoke regional network entails three levels of care (Fig. 15.3) [55]. Level 1 centers provide comprehensive care, including heart transplant, long-term LVAD, total artificial heart, and short-term circulatory support. Level 2 centers offer cardiac catheterization and surgery and percutaneous short-term mechanical support. Level 3 centers provide resuscitation and medical therapy to stabilize patients. Effective participation of this type of network requires commitment and communication from a variety of healthcare professionals. Protocols that outline communications, triage and patient selection, patient management, and the transport process must be established and strictly followed by all participating centers. Established patient selection criteria will help to minimize debate during the triage process. The Level 1 hospital is responsible for the overall coordination of the ECMO program.
A mobile ECMO team from the Level 1 center, with personnel trained in cannulation, transport of patients, initiation of support, and patient management, must be available 24 hours a day. Transport of critically ill patients with ECMO support, or those in need of support, must be prompt and efficient, as delays increase mortality [56]. The mobile ECMO team should consist of a combination of an ECMO specialist, ECMO coordinator, perfusionists, paramedics, and respiratory therapists. The team must be available for immediate transport to the patient’s location. The ECMO coordinator at the hub center must have the ability and authority to choose the most appropriate means of transportation. Depending on distance and size of the metropolitan area, ground ambulance, helicopter, or fixed-wing aircraft are used as appropriate. Patients with severe cardiogenic shock or pulmonary failure at Level 3 centers should be promptly transported to the hub center if their condition is stable. For unstable cases, an ECMO transport team is dispatched to initiate therapy on-site. After the mobile ECMO team stabilizes the patient, safe transport to the hub hospital can take place.
Future Directions
ECMO support is unique in comparison to other healthcare technologies, as it was not developed specifically for current clinical applications. ECMO is an extension of cardiopulmonary bypass that has been routinely used in cardiac surgery. Today, ECMO is being applied in multiple different scenarios without randomized controlled clinical trials supporting the indications for use. Consequently, indications and contraindications need to be defined, and standards of care need to be established. Because patients with refractory cardiac or pulmonary failure present in extreme conditions, it is often difficult or impossible to discuss care with the patients or their next of kin. Ethical dilemmas related to this therapy need more thorough consideration in future research.
Extracorporeal cardiopulmonary resuscitation (ECPR) with ECMO may offer survival benefit for the large number of patients experiencing in-hospital and pre-hospital cardiac arrest [57, 58]. ECMO offers a means to rapidly restore circulation, which is the key component to achieving survival in patients with profound cardiogenic shock or cardiac arrest. Preliminary reports present the possible benefit of pre-hospital ECMO support initiated by emergency medical services [59]. Pre-hospital ECMO support is particularly challenging due to the absence of the multidisciplinary team present in the hospital environment.
As more patients with acute cardiopulmonary failure are being treated in community hospitals, more advanced care networks need to be established [4, 5, 60]. This includes the use of ECMO technology in use at more hospitals by more physician specialties. Presently, there are a large number of patients without access to this life-saving therapy; this situation will improve as technology advances and more medical professionals are trained to provide the therapy. Outreach programs at tertiary care centers can initiate the development of networks; societies need to promote these activities as well.
Clinical research is necessary to better define populations of patients that will benefit from this therapy. Because patients are at high risk of death, randomized controlled trials are nearly impossible to conduct; however, studies that focus on assessing risks and benefits are needed to better define patient selection. Protocols for management and weaning from support need to be better defined to establish more uniform practices. The Extracorporeal Life Support Organization (ELSO) registry provides some useful information on practices and outcomes but needs to be expanded to provide more precise guidelines.
References
Stentz MJ, Kelley ME, Jabaley CS, et al. Trends in extracorporeal membrane oxygenation growth in the United States, 2011-2014. ASAIO J. 2018;65:712.
Conrad SA, Broman LM, Taccone FS, et al. The extracorporeal life support organization Maastricht treaty for nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med. 2018;198:447–51.
El-Banayosy A, Cobaugh D, Zittermann A, et al. A multidisciplinary network to save the lives of severe, persistent cardiogenic shock patients. Ann Thorac Surg. 2005;80:543–7.
Dini CS, Lazzeri C, Chiostri M, Gensini GF, Valente S. A local network for extracorporeal membrane oxygenation in refractory cardiogenic shock. Acute Card Care. 2015;17:49–54.
Aubin H, Petrov G, Dalyanoglu H, et al. Four-year experience of providing mobile extracorporeal life support to out-of-center patients within a suprainstitutional network-outcome of 160 consecutively treated patients. Resuscitation. 2017;121:151.
Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57.
Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30.
Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15.
Steimer DA, Hernandez O, Mason DP, Schwartz GS. Timing of ECMO initiation impacts survival in influenza-associated ARDS. Thorac Cardiovasc Surg. 2018;67:212.
Chillcott S, Stahovich M, Earnhardt C, Dembitsky W. Portable rapid response extracorporeal life support: a center's 20-year experience with a registered nurse-run program. Crit Care Nurs Q. 2008;31:211–5.
Durinka JB, Bogar LJ, Hirose H, et al. End-organ recovery is key to success for extracorporeal membrane oxygenation as a bridge to implantable left ventricular assist device. ASAIO J. 2014;60:189–92.
Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63:2769–78.
Morris AH. Exciting new ECMO technology awaits compelling scientific evidence for widespread use in adults with respiratory failure. Intensive Care Med. 2012;38:186–8.
Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717.
Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:698–716.
Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–30.
Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–9.
Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57:688–96.
Lorusso R, Gelsomino S, Parise O, et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: trends in application and outcome from the Extracorporeal Life Support Organization (ELSO) registry. Ann Thorac Surg. 2017;104:62–9.
McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg. 2015;27:81–8.
Nanjayya VB, Murphy D. Ultrasound guidance for extra-corporeal membrane oxygenation: general guidelines. extracorporeal life support Organization; https://www.elso.org/Portals/0/Files/elso_Ultrasoundguideance_ecmogeneral_guidelines_May2015.pdf.
Meuwese CL, Ramjankhan FZ, Braithwaite SA, et al. Extracorporeal life support in cardiogenic shock: indications and management in current practice. Neth Heart J. 2018;26:58–66.
Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–96.
Kon ZN, Bittle GJ, Pasrija C, et al. Venovenous versus venoarterial extracorporeal membrane oxygenation for adult patients with acute respiratory distress syndrome requiring precannulation hemodynamic support: a review of the ELSO registry. Ann Thorac Surg. 2017;104:645–9.
Kilic A, Shukrallah BN, Kilic A, Whitson BA. Initiation and management of adult veno-arterial extracorporeal life support. Ann Transl Med. 2017;5:67.
Donker DW, Meuwese CL, Braithwaite SA, et al. Echocardiography in extracorporeal life support: a key player in procedural guidance, tailoring and monitoring. Perfusion. 2018;33:31–41.
Delmas C, Jacquemin A, Vardon-Bounes F et al. Anticoagulation monitoring under ECMO support: a comparative study between the activated coagulation time and the anti-Xa activity assay. J Intensive Care Med. 2018:885066618776937.
Sanfilippo F, Asmussen S, Maybauer DM, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2016;32:312.
Chung YS, Cho DY, Sohn DS, et al. Is stopping heparin safe in patients on extracorporeal membrane oxygenation treatment? ASAIO J. 2017;63:32–6.
Koerner MM, Harper MD, Gordon CK, et al. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): prevention and management of acute complications. Ann Cardiothorac Surg. 2019;8:66–75.
Nasr DM, Rabinstein AA. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. 2015;11:383–9.
Prasad A, Ghodsizad A, Brehm C, et al. Refractory pulmonary edema and upper body hypoxemia during veno-arterial extracorporeal membrane oxygenation-a case for atrial Septostomy. Artif Organs. 2018;42:664.
Aiyagari RM, Rocchini AP, Remenapp RT, Graziano JN. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med. 2006;34:2603–6.
Centofanti P, Attisani M, La Torre M, et al. Left ventricular unloading during peripheral extracorporeal membrane oxygenator support: a bridge to life in profound cardiogenic shock. J Extra Corpor Technol. 2017;49:201–5.
Meani P, Gelsomino S, Natour E, et al. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail. 2017;19(Suppl 2):84–91.
Johnston TA, Jaggers J, McGovern JJ, O'Laughlin MP. Bedside transseptal balloon dilation atrial septostomy for decompression of the left heart during extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 1999;46:197–9.
Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J. 2011;57:38–40.
Guirgis M, Kumar K, Menkis AH, Freed DH. Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg. 2010;10:672–4.
Eudailey KW, Yi SY, Mongero LB, Wagener G, Guarrera JV, George I. Trans-diaphragmatic left ventricular venting during peripheral venous-arterial extracorporeal membrane oxygenation. Perfusion. 2015;30:701–3.
Moazzami K, Dolmatova EV, Cocke TP, et al. Left ventricular mechanical support with the Impella during extracorporeal membrane oxygenation. J Tehran Heart Cent. 2017;12:11–4.
Cheng A, Swartz MF, Massey HT. Impella to unload the left ventricle during peripheral extracorporeal membrane oxygenation. ASAIO J. 2013;59:533–6.
Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella(R) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–12.
Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J. 2011;57:169–76.
Tak VM, Holecek WF 3rd. Harlequin effect in angiography on extracorporeal membrane oxygenation mimicking aortic dissection. Ann Thorac Surg. 2018;106:e97.
Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–21.
Grant AA, Hart VJ, Lineen EB, et al. A weaning protocol for venovenous extracorporeal membrane oxygenation with a review of the literature. Artif Organs. 2018;42:605.
Aissaoui N, El-Banayosy A, Combes A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015;41:902–5.
(ELSO) ELSO. Guidelines for adult respiratory failure. www.elso.org. 2017.
van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–68.
Gutsche JT, Vernick WJ. Cardiac and critical care anesthesiologists may be ideal members of the Mobile ECMO team. J Cardiothorac Vasc Anesth. 2016;30:1439–40.
Lawson DS, Lawson AF, Walczak R, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of extracorporeal life support organization (ELSO) centers. J Extra Corpor Technol. 2008;40:166–74.
Salna M, Chicotka S, Biscotti M 3rd, et al. Management of Surge in extracorporeal membrane oxygenation transport. Ann Thorac Surg. 2018;105:528–34.
Dirnberger DR, Fiser R, Harvey C et al. Extracorporeal Life Support Organization (ELSO): Guidelines for ECMO transport. https://www.elso.org/2015.
Tchantchaleishvili V, Hallinan W, Massey HT. Call for organized statewide networks for management of acute myocardial infarction-related cardiogenic shock. JAMA Surg. 2015;150:1025–6.
Jaroszewski DE, Kleisli T, Staley L, et al. A traveling team concept to expedite the transfer and management of unstable patients in cardiopulmonary shock. J Heart Lung Transplant. 2011;30:618–23.
Tonna JE, Johnson NJ, Greenwood J, et al. Practice characteristics of emergency department extracorporeal cardiopulmonary resuscitation (eCPR) programs in the United States: the current state of the art of emergency department extracorporeal membrane oxygenation (ED ECMO). Resuscitation. 2016;107:38–46.
ELSO. Guidelines for ECPR cases. https://www.elso.org/2013..
Lamhaut L, Jouffroy R, Soldan M, et al. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84:1525–9.
Morshuis M, Bruenger F, Becker T, et al. Inter-hospital transfer of extracorporeal membrane oxygenation-assisted patients: the hub and spoke network. Ann Cardiothorac Surg. 2019;8:62–5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
El Banayosy, A. (2020). Extracorporeal Membrane Oxygenation Techniques and Modern Considerations. In: Karimov, J., Fukamachi, K., Starling, R. (eds) Mechanical Support for Heart Failure . Springer, Cham. https://doi.org/10.1007/978-3-030-47809-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-47809-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47808-7
Online ISBN: 978-3-030-47809-4
eBook Packages: MedicineMedicine (R0)