Abstract
Synthetic seeds are artificially encapsulated propagules that mimic true seeds in agriculture. Although a variety of plant materials, such as shoot tips, axillary buds, callus, micro cuttings, and protocorm-like bodies, are used in the production of synthetic seeds, somatic embryos are the most widely used explants in the production of these seeds. Synthetic seeds compete with traditional approaches to preserve the germplasm of threatened plant species. The resulting progenies are the true clones of the main plant, thus preserving the intactness of the genetic background. Due to poor germination and low seed amount, wild Beta species are exposed to the risk of extinction. Wild relatives of Beta have agronomically important properties such as resistance to diseases and abiotic stresses. Numerous attempts have been made to give these traits to sugar beet crop through conventional breeding methods. Despite the importance of synthetic seed for wild beets, it has not yet been investigated. The production of synthetic seeds ensures the conservation and availability of wild germplasm of the genus Beta for cytogenetic and breeding studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Seeds are zygotic embryonic plants produced after fertilization in flowering plants. Seeds connect different generations of plants and ensure the maintenance and transfer of plant genetic material in nature. These structures are enclosed with protective layers to keep the embryo safe during storage and dispersal (Bewley and Black 1985).
Seeds are essentially composed of an embryo and protective layers. True seeds contain endosperm tissues that provide the nutrients necessary for germination. Endosperm stores various substances mainly including starch, proteins, and oils. However, in some species, cotyledonary leaves are the source of these components. Seeds are the basis of agriculture and, when the necessary factors for germination are provided, they produce a plant similar to the main plant.
Depending on plant species and conditions, the production of true seeds can be time-consuming, arduous, impossible, or costly. Synthetic seeds have great potential as an alternative to true seeds, especially due to low production costs and long-term storage (Roy and Mandal 2008). These seeds can be handled, stored, transported, and planted like true seeds (Sharma et al. 2013).
The term “synthetic seed” was first defined as an encapsulated single somatic embryo by Murashige (1977). In the early studies, the concept was limited to plants where somatic embryogenesis could be demonstrated; however, it was later on extended to any in vitro-derived propagule due to the recalcitrance of some species to somatic embryogenesis (Bapat et al. 1987). Synthetic seeds are also known as artificial seeds, synseeds, and manufactured seeds. Somatic embryos are often used as propagule in the production of synthetic seeds; however, cell aggregates, shoot buds, auxiliary buds, or any other structure capable of giving rise to a plant can also be used. Furthermore, these seeds can hold this ability for a long time and can be stored (Ara et al. 2000; Saiprasad 2001; Daud et al. 2008).
Synthetic seed technology is a suitable alternative method for proliferation of commercially important plants. These tissue culture-originated artificial seeds are economically preferred for the propagation of hybrids generated through breeding approaches (Rihan et al. 2017). Synthetic seeds, however, can be used to proliferate the species that hardly reproduce through generative propagation and are at risk of extinction.
The wild relatives of sugar beet (Beta vulgaris spp. vulgaris) are invaluable germplasm reservoirs that are in danger of extinction. Although synthetic seeds have been produced for more than 30 years, no attempt has been made to utilize synthetic seed technology for wild beet species. However, in situ and ex situ efforts have been made to protect these invaluable genotypes (Frese et al. 2001).
In this chapter, we emphasize the importance of wild beet relatives in the genus Beta and review the synthetic seed technology to evaluate the use of this approach for wild beet accessions.
2 Origin and Development of Synthetic Seeds
When Steward et al. (1958) and Reinert (1958), at the same time, reported the first somatic embryogenesis in the carrot plant (Daucus carota), most probably the idea of synthetic seed production created. However, the first report of plant propagation by somatic embryos was presented by Murashige (1977). He succeeded to obtain the surviving plants originating from somatic embryos in vitro conditions. Studies continued to accelerate the process of developing plants from somatic embryos for commercial applications. Drew (1979) delivered the carrot somatic embryos in a liquid drilling system; however, the results were disappointing as he developed only three carrot plants in carbohydrate-free media. The first synthetic seed production goes back to a report by Kitto and Janick (1982). They encapsulated multiple somatic embryos of carrot in a polyoxyethylene glycol and desiccated the embryos. Polyoxyethylene is a readily soluble chemical in water and after drying forms a thin layer. In addition, it is not toxic to the embryo and does not allow growth of the microorganism and, therefore, later was also used to encapsulate the celery (Apium graveolens) embryos (Kitto and Janick, 1985). A polyethylene glycol (PEG)-based mixture was also applied to coat the carrot somatic embryos and embryonic calli. Following the dehydration procedure, the survival rate was scored by placing the coated embryos on culture medium allowing them to rehydrate (Janick et al. 1993). Since the first studies, many efforts have been made to produce synthetic seed in various plant species (Ravi and Anand 2012). Survival percentage of encapsulated embryos was enhanced by modifications applied in the encapsulation matrix and speed of dehydration. Various propagules have been utilized to establish the system for the species recalcitrant to somatic embryogenesis.
3 Types of Synthetic Seeds
The somatic embryos used for the production of synthetic seeds may or may not be encapsulated. The state of quiescence in uncoated somatic embryos defines the usage of these seeds. If the uncoated embryo is non-quiescent, it is used for the in vitro micropropagation of plant, and if quiescent, it is used for the germplasm storage. The encapsulated somatic embryos are divided into two types of hydrated and dried seeds. The encapsulated somatic embryos may be non-quiescent or quiescent if the seed coat is hydrated or desiccated, respectively. The quiescent somatic embryos coated with dehydrated artificial coatings are the most resembling synthetic seeds to the conventional ones in terms of handling and storage.
The vigor of the seedlings obtained from the desiccated embryos is higher than that obtained from the hydrated embryos. It is ideal if the synthetic seed is produced through encapsulated desiccated embryos (Pond and Cameron 2003). Prior to encapsulation process, somatic embryos are hardened to tolerate the desiccation which induces the quiescence. However, desiccated seeds are only produced if the somatic embryos are tolerant to dehydration. In some species, the generated somatic embryos are sensitive to water deficiency; thus, the embryo ought to be coated with hydrogels (Magray et al. 2017). Desiccation degree is determinative and depends on the developmental stage of the embryo. If the embryo is mature, the drying process should be carried out rapidly, and if it is immature, it is opted to implement the process slowly (Senaratna et al. 1990).
4 Wild Beets: Synthetic Seed and Applications
4.1 Species of the Genus Beta
Based on the crossing ability between cultivated beet and other genotypes, the genus Beta divides into three gene pools: the primary gene pool contains the cultivated beet and other species which can easily cross; the secondary gene pool includes the species that despite the crossability with cultivated beet generate sterile progenies; and the tertiary gene pool members generate hybrids only by human intervention and artificial crossing (Jassem 1992; Kadereit et al. 2006; Frese 2010) (Table 1). However, an earlier classification had placed B. nana in a separate section, thus dividing the genus Beta into four sections as Beta, Corollinae, Nanae, and Procumbentes (Ford-Lloyd 2005). The Eastern Anatolia-Western Caucasus crossing region is accepted as the origin and resource of genetic diversity for Beta and Corollinae (Boughey 1981). Section Nanae is distributed in the mountainous regions of Greece, whereas the members of the section Procumbentes spread far away in the Canary Islands, coastal areas of North-West Africa, and Southeastern Spain (Frese 2010).
4.2 Employment of the Wild Beet Genotypes
The interest in wild beet genotypes as the genetic resources for improving the sugar beet germplasm has been increasing since the late 1800s, and the values of these genotypes have been well-demonstrated (De Bock 1986; Doney and Whitney 1990; Van Geyt et al. 1990; Lewellen and Skoyen 1991; Doney 1993). Since that time, breeders have been employing these germplasms to improve the agronomic traits of the sugar beet crop. There are committees established to study and protect the germplasm of the genus Beta. Very comprehensive information on the genotypes of Beta is available at the Genetic Resources Information Network (GRIN) Database of National Plant Germplasm System (NPGS). More than 22% of the available Beta accessions are the B. maritima accessions which are well-characterized and most useful accessions in the breeding of sugar beet (Biancardi et al. 2010).
4.3 Wild Beets Are the Reservoir for Resistance Genes
Sugar beet yield is affected by different biotic and abiotic factors. The cyst nematode (Heterodera schachtii Schm.), leaf spot disease (Cercospora beticola Sacc.), and Beet necrotic yellow vein virus (BNYVV) are among the most destructive pests and disease agents of sugar beet crop that reduce root yield and sugar content (Weiland and Koch 2004; McGrann et al. 2009; Biancardi et al. 2010). Sugar beet cyst nematode is spread over more than 40 countries and causes major product losses (McCarter 2008). However, some Beta species carry cyst nematode resistance genes that can be employed in breeding programs. C. beticola is a fungal pathogen known as the most damaging sugar beet disease worldwide, corresponding to leaf spot disease in sugar beet (Weiland and Koch 2004). Depending on the severity of the disease, C. beticola reduces the sugar yield by 1–55% (Rossi et al. 1995; Altınok 2012). The soil-borne disease of rhizomania is caused by BNYVV, which is transmitted by Polymyxa betae Keskin (Keskin 1964). Rhizomania causes yield losses of up to 80% in the sugar beet crop and the only way to control the disease is to cultivate resistant varieties (Tamada and Baba 1973).
Wild relatives of genus Beta contain agronomically important characteristics and can be exploited in introgression of desirable traits into cultivated beet crop (Table 2). Being a member of the section Beta, B. v. ssp. maritima is a very close and cross-compatible relative of B. v. ssp. vulgaris (Biancardi et al. 2012). It owns valuable traits such as resistance to H. schachtii and is the widely deployed wild-type genotype in sugar beet breeding studies (Panella and Lewellen 2007). In addition, B. maritima is a source of resistance to Cercospora leaf spot disease, so it has been used to develop resistant varieties (Munerati et al. 1913). Although resistance to Cercospora has been reported in all of the Corollinae, Procumbentes, and Beta sections, the resistance degree is highly different between them. Among species, B. maritima has the most pronounced degree of resistance, so it is widely used in sugar beet breeding approaches (Munerati 1932; Panella and Frese 2000; Skaracis and Biancardi 2000; Luterbacher et al. 2004). In contrast to B. maritima, B. nanae and B. macrorhiza accessions are susceptible to Cercospora (Coons 1954). B. maritima also could be a reliable resource for resistance genes of rhizomania, and among these genes, Rz1 and Rz2 are generally used in the commercial development of resistant varieties (Scholten et al. 1999). Wild beet genotypes are also invaluable resources of tolerance to abiotic stresses such as drought, salt, and frost which could be exploited to improve the quality of growth in sugar beet under adverse climatic conditions (Frese et al. 2001). The Corollinae section, in particular B. corolliflora, is thought to be an important germplasm against abiotic stresses. However, the low homology percentage of chromosomes between different gene pools may be an obstacle in the transmission of the desired genes (Jung and Wricke 1987; Van Geyt et al. 1990).
4.4 The Contribution of Synthetic Seeds to Breeding Programs
The first production of the sugar beet crop was restricted to Northern Europe, a temperate climate and a relatively disease-free zone; therefore, small selection pressure was applied against the pathogens. Following the cultivation in other regions with different climatic conditions, various diseases emerged and affected crop yield (Lewellen 1992). Although numerous genetic improvements have been made to date, susceptibility to disease still threatens crop yields and production worldwide (Table 2). Wild beet accessions have great potential to expand the germplasm pool of sugar beet. Wild Beta species are evaluated in terms of important agronomic characteristics to be used in sugar beet breeding. These accessions indicate different degrees of resistance to biotic and abiotic factors. Initial efforts to screen wild beet genotypes for pathogen resistance were made in the early 1900s. The first documented report of using the B. vulgaris ssp. maritima in breeding studies of cultivated beet belongs to Munerati et al. (1913) who found B. maritima as a source of resistance to Cercospora leaf spot disease (Biancardi et al. 2010). However, various resistance genes were later on transferred to B. vulgaris through interspecific hybridization. The obtained interspecific hybrids were resistant to different biotic factors (Table 2). The synthetic seed technology contributes to the breeding of cultivated accessions. The preparation of synthetic seeds for Beta wild relatives ensures the presence of these accessions for breeding programs.
Sugar beet is a biennial crop and has to be exposed to the prolonged cold of winter for flowering and seed production (Letschert 1993). However, wild-type relatives are naturally annual, and production of the flowering stem is triggered in the first year (Biancardi et al. 2010). Depending on the geographical origin and altitude, a perennial growth pattern is also possible in wild beet genotypes (Marlander et al. 2011). In vitro production of synthetic seeds allows researchers to shorten the breeding process by applying equivalent but controlled conditions to stimulate vernalization.
Simultaneous flowering time is very important for successful plant-pollinator interactions (Elliott and Weston 1993; Alcaraz et al. 1998). The lack of synchronicity in flowering, especially in different genotypes, makes the hybridization of wild and cultivated species difficult (Alibert et al. 2003; Cuguen et al. 2005). Application of synthetic seeds and human intervention to adjust the cultivation time enhance the chance of fertilization and seed production. The mass production of clonal plants with homogeneity in the genetic background causes simultaneous flowering in the population. In addition to homogeneity in flowering, the production of large amounts of pollen is another feature that breeders will appreciate when they intend to convey the desired characteristics between species. Compared to the monogerm seeds, the multigerm Beta accessions produce more pollen (Alibert et al. 2003; Biancardi et al. 2010). Therefore, the selection of superior genotypes that match the breeding objectives is an important step before hybridization.
The production of inbred lines is necessary for the development of hybrid seeds. However, inbreeding is sometimes not possible due to genetic barriers and the presence of allogamy in many species. Sugar beet is primarily self-incompatible, and the self-pollination is rare among wild beets. This feature was used by breeders to increase and maintain heterosis in multigerm varieties prior to the discovery of cytoplasmic male sterility (Owen 1942). Large-scale conservation and micropropagation of selective rare hybrid genotypes have attracted interest in the production of synthetic seeds. The production of synthetic seeds can eliminate the genetic segregation because of the participation of only one parent, as well as the use of somatic cells in seed production. These seeds are the actual clones of the sampled plant, so it can also be considered in the maintenance of the unstable sterile genotypes, genotypes that have difficulty in germination and transgenic plants (Gantait et al. 2015).
Hybridization of species distributed in different gene pools is limited due to existing genetic barriers (Abe and Tsuda 1987; Jung and Wricke 1987; Van Geyt et al. 1990). However, there are successful hybridization reports between the species of different sections (Table 2). The obtained interspecific hybrids might lack one or more chromosomes leading to aneuploidy. These individuals will be used in cytogenetic and biotechnological studies of Beta species (Savitsky 1960, 1975; Yu 1983, 2005; Heijbroek et al. 1988; Sandal et al. 1997). Thus, the protection of the desired genotypes between such hybrids requires an asexual multiplication technique. Artificial seed technology can protect and reproduce the desired progeny for future uses in breeding programs.
5 Germplasm Conservation of Wild Beet Species
True seeds are consisting of an embryo, nutritive tissues, and protective layers. The embryo and nutritive tissues of true seeds are covered with a seed coat which keeps the embryo quiescent and tolerant to adverse climatic conditions that have been the source of inspiration for the preservation of the germplasm in gene banks. Zygotic seeds are reliable sources to preserve germplasms in repositories. Furthermore, the incidence of pathogenic infestations and high metabolic activities affect the period of seed storage. Unlike the true seeds, synthetic seeds do not necessarily contain nutritive tissues or seed protective layers and the state of quiescence might be different in these seeds. Therefore, depending on the purpose of use, the structural complexity of synthetic seeds is defined. Seed coats of synthetic seeds not only help to the keep the propagules from pathogenic diseases but also establish a safe seedbed in the soil. It protects the propagules from drought and other unfavorable conditions and also keeps the seed safe during the transportation and storage (Ara et al. 2000).
True seeds of the wild beet species are coated with a thick and excessively indented testa which causes poor germination (Coons 1975). The perennial pattern of growth and the low germination rate affect the multiplication of wild beet relatives in natural habitats (McGrath et al. 2007; Marlander et al. 2011). The difficulties in germination and proliferation, especially among the species of Corollinae, challenge the survival of wild beet species (Frese et al. 2001). To date, in situ and ex situ efforts have been made to save these genotypes in their natural distributed areas or botanical gardens (Ren et al. 2012). However, the need to accelerate and improve the propagation process and efficiency is still felt.
Unlike the true seeds which produce following the sexual reproduction and undergo the genetic recombination process, the intactness of the parental genetic background is protected in synthetic seeds. Propagules used in the production of synthetic seeds are generated in aseptic conditions; therefore, the resulting seeds are pathogen-free and thus are superior to the traditional methods in which diseases encounter severe threats to the stoked genotype. Moreover, the international exchange of plant material would be easier and faster across the country borders and successfully contribute to the control of plant diseases (Daud et al. 2008; Nyende et al. 2005). Recently the in vitro multiplication of wild beet genotypes was investigated to assess the in vitro regeneration potential of different species of the genus Beta (Ergül et al. 2018). Results indicated the multiplication capability of the selected genotypes when were subjected to cytokinin and gibberellin growth regulators. In an early study, the callus induction potential of the wild genotypes of Beta sections was evaluated (Yu 1989). Despite the conducted studies, the assessment of somatic embryogenesis in these species yet remains. Somatic embryos are frequently used as proper explants in the production of synthetic seeds; therefore, the importance of studies over the somatic embryogenesis in wild beet species is emphasized (Skaracis 2005).
Production of synthetic seeds will provide a sufficient quantity of wild beet accessions to ensure the germplasm conservation. This technique will ease the renewal of germplasm in gene banks, and the germplasm continuity will be ensured; moreover, the costs of germplasm maintenance in the gene banks will be reduced efficiently.
6 Synthetic Seed Production and Storage Methods
6.1 Explant Materials
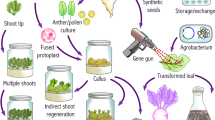
A variety of propagules have been utilized in the production of synthetic seeds (Fig. 1). Propagules are divided into two categories of unipolar and bipolar. Both types are used in the production of synthetic seeds and have pros and cons. Bipolar propagules such as somatic embryos contain shoot and root apical meristems and generate the plant in a single stage (Ara et al. 2000). Such seeds can be used as hydrated or dried. Seeds carrying the somatic embryos have a strong capacity for reproduction and can maintain their regenerative potential for a long time leading to a uniform plant production (Leroy et al. 2000). The germination rate of the somatic embryos is different from that of zygotic embryos so that the zygotic embryos are superior to synthetic seeds in germination rate (Lulsdorf et al. 1993; Attree et al. 1994). Nevertheless, the encapsulation method and the plant genotype are determining factors in germination rate of the seeds (Cartes et al. 2009). The resulting plants originating from bipolar propagules may cause somaclonal variations. Moreover, asynchronous and late maturation features of embryonic poles are among the factors restricting the usage of somatic embryos in the production of synthetic seeds (Castellanos et al. 2004). On the other hand, unipolar propagules contain only a shoot or a root pole and are superior to the bipolar ones in terms of genetic fidelity and a vast range of vegetative explants. Unipolar explants can be any of the apical tips, axillary buds, micro shoots, micro bulbs, microtubers, corms, rhizomes, meristemoids, cell aggregates, and primordia biological materials with different levels of complexity and conversion ratios (Sharma et al. 2013).
The pie chart depicts the propagules used in synthetic seed production and their relative contribution to synthetic seed production studies (Reddy et al. 2012)
Although propagules such as shoot apical meristems, axillary buds, and micro shoots do not contain root apical meristem, these are encapsulated to generate seeds. However, prior to the encapsulation process, explants are subjected to the root inducing chemicals. Some studies reported the possibility of root induction and conversion of the buds to plantlets when cultivated on white’s rooting medium without chemical pretreatments (Bapat and Rao 1990; Ganapathi et al. 1992). Based on a study, the conversion of the encapsulated apical tips was more than axillary buds; however, a later study indicated the conversion of 100% for axillary buds when encapsulated in a suitable matrix (Capuano et al. 1998; Lata et al. 2009).
Plant species respond differently to synthetic seed production that emphasizes the effect of genotype and plant species. The literature review shows that somatic embryos as the most corresponding propagule have been employed in the majority of the studies including vegetable crops, spices, and plantation crops, ornamental plants and orchids, medicinal plants, forage legumes, fruit crops, and forest trees (Reddy et al. 2012). Following somatic embryos, shoot tip explants are the second most widely used propagule; however, other explant sources have been less frequently used (Fig. 1). Both somatic embryos and shoot tip explants were used as plant material in the production of synthetic seeds for B. vulgaris, which can also be recommended for wild relatives of Beta (Saunders and Tsai 1999; Rizkalla et al. 2012).
6.2 Encapsulation Chemicals and Processes
Agents such as agar, agarose, alginate, polyox, polyco 2133, guar gum, tragacanth gum, gelrite, carrageenan, carboxymethyl cellulose, polyacrylamide, nitrocellulose, and sodium pectate ethyl cellulose have been tested for the encapsulation of synthetic seeds till date (Ara et al. 2000; Saiprasad 2001; Lambardi et al. 2006). Among these chemicals, sodium alginate has been found as the most suitable agent for encapsulation of somatic embryos due to its low cost, low toxicity, and quick gelling properties (Saiprasad 2001). This chemical was used to encapsulate the B. vulgaris somatic embryo and shoot tip propagules at concentrations of 2% and 4%, respectively (Saunders and Tsai 1999; Rizkalla et al. 2012). Sodium alginate can protect the biologic material for a longer time when compared to other agents such as agar. The firmness of the seed coat is determined by the ratio of sodium ions exchanged with calcium in CaCl2·H2O solution (Daud et al. 2008). The literature review indicates that the most satisfying results are obtained once explants are encapsulated with 3% sodium alginate and 100 mM CaCl2·H2O. In the majority of these studies, the regeneration frequency was noted more than 90% (Singh and Chand 2010; Ahmad et al. 2012; Sakhanokho et al. 2013; Varshney and Anis 2014).
Structure of the synthetic seeds imitates that of true seeds. The biologic material in synthetic seeds represents the zygotic embryo in true seeds (Cartes et al. 2009). Synthetic endosperms are comprised of MS culture medium supplemented with growth regulators such as cytokinin and auxins, minerals and vitamins, gelling chemical, and anti-pathogenic components (Ravi and Anand 2012).
Synthetic seeds are single-layered, double-layered, or hollow bead structures. To produce single-layered seeds, the in vitro originated plant materials are mixed with a proper hydrogel. Alginate is the most frequently used coating agent employed in the concentration of 0.5–5%. After alginate dissolves in double distilled water or liquid nitrogen, the solution is utilized in the production of the beads containing propagules. The beads are then treated with a complexion agent such as calcium chloride (CaCl2·2H2O). It is important to obtain round and firm calcium alginate beads.
The concentration of sodium and calcium together with the complexion time affect the permeability and rigidity of the beads and may be different between plant species. Generally, treatment of beads with 3% (w/v) sodium alginate and 100 mM calcium chloride for 20–30 min is reported as the most suitable combination for seed production (Sarkar and Naik 1998; Tabassum et al. 2010; Ahmad and Anis 2010; Ozudogru et al. 2011; Alatar and Faisal 2012; Hung and Trueman 2012a, b). Low alginate concentrations (<3%) interfere with solidification and high concentrations (5–6%) result in a very hard coating that delays germination (Larkin et al. 1998; Ahmad and Anis 2010; Sharma et al. 2009a, b).
The content of the matrix, such as nutrients and growth regulators, influences the success of germination and conversion of the encapsulated explant (Chand and Singh 2004; Sundararaj et al. 2010). Synthetic seeds are highly susceptible to microbial infections, so various antimicrobial agents are added to the gel matrix to reduce infection (Saiprasad 2001; Wang et al. 2007).
The addition of activated carbon to the matrix gel enhances explant conversion and vigor. Activated carbon adsorbs toxic products such as phenolic compounds that can damage encapsulated propagule; moreover, it helps the diffusion of nutrients and gases. It contributes to decomposition of alginate and enhanced respiration of the biological material, thereby prolongs the storage time. Additionally, the activated carbon retains the nutrients and releases them gradually which provides a long-term supply of essential nutrients for the propagule. Pretreatment of synthetic seeds with potassium nitrate makes an impressive contribution to the production of shoots and roots from coated propagules (Sharma et al. 2013).
Double layering the plant material is proposed to increase the protection of encapsulated propagules. Once the single layer seeds are produced, they can be coated with the same concentration of sodium alginate and then coated with the treatment of CaCl2·2H2O. Double-layered synthetic seeds have the same properties of the single-layered ones; however, double encapsulation provides better protection (Micheli et al. 2002; Pinker and Abdel-Rahman 2005).
When the position of the propagule in the bead is compared between conventional synthetic seeds and true seeds, the adjacent position of the propagule to bead surface makes the protection fragile in synthetic seeds. Hollow beads are promising tools to assure the protection of encapsulated propagules; however, the process is laborious and costly, and the success of this method is still controversial (Winkelmann et al. 2004; Pourjavadi et al. 2006).
6.3 Storage of Synthetic Seeds
Storage of synthetic seeds is carried out for a variety of purposes, such as the germplasm transport between countries, proliferation, and protection of invaluable germplasms. Researchers investigate the ideal conditions for the storage of synthetic seeds. Storage temperature and matrix components are the most important factors affecting the conversion rate; however, the effect of species on the storage is also decisive. Generally, 4 °C is the most suitable temperature for short-term storage of synthetic seeds including B. vulgaris (Saiprasad and Polisetty 2003; Kavyashree et al. 2006; Singh et al. 2007; Faisal and Anis 2007; Pintos et al. 2008; Sharma et al. 2009a, b; Ikhlaq et al. 2010; Tabassum et al. 2010). However, several studies on some tropical and subtropical crops have reported that higher temperature (25 °C) is required for bead storage (Srivastava et al. 2009; Sundararaj et al. 2010; Mishra et al. 2011). Although wild Beta species have not been investigated for synthetic seeds, there are reported studies over B. vulgaris spp. vulgaris. In vitro storage of sugar beet synthetic seeds was evaluated after addition of osmotic agents to the MS medium (Rizkalla et al. 2012). According to the results, the addition of 0.05 M mannitol or sorbitol to the medium increased seed survival during in vitro storage but disrupted growth quality. This result is consistent with Westcott (1981), which previously reported the toxic effects of mannitol. In an early study, the effect of cold storage of the beads was investigated, and the results showed that the cold treatment did not improve the conversion rate and also slowed down the rate of development (Saunders and Tsai 1999).
For long-term storage of synthetic seeds, dehydration and cryopreservation storage techniques are used. Cryopreservation of the propagules can only be useful if the formation of intracellular ice crystals is avoided; otherwise, harmful effects prevent cell survival. Various techniques of cryopreservation inclusion of simple desiccation, encapsulation-dehydration, the two-stage freezing, vitrification, and encapsulation-vitrification have been employed to date. Pretreatment of encapsulated biological tissues in a medium supplemented with high concentrations of sucrose results in progressive water withdrawal of the coated propagule. Additional dehydration of the encapsulated explants increases the concentration of sucrose results in a glass transition during cooling to ultralow temperatures (–196 °C) and subsequently fatal damages to the cells (Engelmann and Takagi 2000). Figure 2 summarizes the approaches used to store synthetic seeds.
7 Difficulties in the Production of Synthetic Seeds
Large-scale and cost-effective synthetic seed production requires investigations of high-quality propagules and encapsulation methods. Since the first synthetic seed production report, many improvements have been made. Despite the impressive advantages, this technique is currently facing limitations that challenge commercial production. Lack of dormancy in synthetic seeds causes limitations during the storage period. Especially because these seeds are stored at lower temperatures, the vitality and conversion rate reduces over time. Among the biological materials studied, somatic embryos are superior to others because of their potential in the production of the shoot and root system in one step. However, in addition to improper maturation of somatic embryos, synchronic deficits in the development of somatic embryos make the application of somatic embryos difficult (Reddy et al. 2012; Hung and Trueman 2012a, b). Somatic embryogenesis may be difficult due to the existing recalcitrance of some plant species, thus encouraging the use of other explant sources in the production of synthetic seeds. Although such explants are promising tools, the creation of the root system is a complex challenge in this approach. In most woody plant species, single-step rooting is the major obstacle to non-embryogenic coated propagules (Chand and Singh 2004; Naik and Chand 2006; Hung and Trueman 2012a).
One of the main problems of synthetic seeds is the practicality of these seeds during sowing under ex vitro conditions. In addition to the oxygen supply and nutrient deficiency, the presence of various pathogens in commercial substrates such as soil or vermiculite causes infection risks and other limitations (Jung et al. 2004; Rihan et al. 2012). However, the successful conversion of encapsulated propagules into vigorous seedlings remains one of the important factors that hamper commercial production (Sharma et al. 2013). Adjusting the matrix composition of the beads will overcome the barriers that exist in storage and direct sowing. The hydrated calcium alginate-based or dried polyethylene glycol-based encapsulation will also provide the benefit of synthetic seed technology for long-term preservation of germplasms by cryopreservation (Ara et al. 2000).
The in vitro responses of wild beet species are not well studied yet. For instance, the somatic embryogenesis capability of these species is still unknown, whereas somatic embryo is the most widely used explant in synthetic seed production. Lack of in vitro studies along with frequently encountered difficulties may cause similar risks in the artificial seed production of wild Beta species.
8 Conclusion
Synthetic seeds have the potential to take part in the protection of endangered germplasms or to produce economic plants on commercial scales. Despite the importance of wild beet genotypes, synthetic seed technology for these species has not yet been investigated. The development of in vitro protocols for somatic embryogenesis of these species is particularly important in the first step. The encapsulation procedure, as well as storage, should be examined for wild beet accessions.
References
Abe J, Tsuda C (1987) Genetic analysis for isozyme variation in the section Vulgares, genus Beta. Jpn J Breed 37(3):253–261
Ahmad N, Anis M (2010) Direct plant regeneration from encapsulated nodal segments of Vitex negundo. Biol Plant 54:748–752
Ahmad N, Faisal M, Fatima N et al (2012) Encapsulation of microcuttings for propagation and short-term preservation in Ruta graveolens L.: a plant with high medicinal value. Acta Physiol Plant 34:2303–2310
Alatar A, Faisal M (2012) Encapsulation of Rauvolfia tetraphylla microshoots as artificial seeds and evaluation of genetic fidelity using RAPD and ISSR markers. J Med Plants Res 6:1367–1374
Alcaraz G, Geuter T, Laillet G et al (1998) Sugar beet pollen biology. In: Proceedings of 61th IIRB congress, Brussels, pp 393–399
Alibert B, Sellier H, Souvré A (2003) Flux de gènes entre la betteravesucrière GM et lesbetteravesrudéralesdansles conditions de la production de semencesau champ. In: Proceedings of 66th IIRB congress, Brussels
Altınok HH (2012) The disease prevalence and severity of Cercospora leaf spot in sugar beet cultivations in Kayseri. Derim 29(2):33–45
Ara H, Jaiswal U, Jaiswal VS (2000) Synthetic seed: prospects and limitations. Curr Sci 78:1438–1444
Attree SM, Pomeroy MK, Fowke LC (1994) Production of vigorous, desiccation tolerant white spruce (Picea-glauca (moench) voss) synthetic seeds in a bioreactor. Plant Cell Rep 13:601–606
Bapat VA, Rao PS (1990) In vivo growth of encapsulated axillary buds of mulberry (Morus-indica L.). Plant Cell Tissue Organ Cult 20:69–70
Bapat VA, Mhatre M, Rao PS (1987) Propagation of Morus indica L. (Mulberry) by encapsulated shoot buds. Plant Cell Rep 1987(6):393–395
Bewley JD, Black M (1985) Seeds: physiology of development and germination. Plenum, New York, p 367
Biancardi E, De Biaggi M (1979) Beta maritima L. in the Po Delta. In: Atti Convegno Tecnico Internazionale sulla Bieticoltura in Commemorazione di Ottavio Munerati. ISCI, Rovigo, pp 183–185
Biancardi E, McGrath JM, Panella LW et al (2010) Sugar beet. In: Bradshaw JE (ed) Root and tuber crop. Springer, New York, pp 173–219
Biancardi E, Panella LW et al (2012) Beta maritima: the origin of beets. Springer, New York
Boughey CL (1981) Evolutionary and taxonomic studies on wild and cultivated beets. PhD Thesis, University of Birmingham
Capuano G, Piccioni E, Standardi A (1998) Effect of different treatments on the conversion of M.26 apple rootstock synthetic seeds obtained from encapsulated apical and axillary micropropagated buds. J Hortic Sci Biotechnol 73:299–305
Cartes P, Castellanos H, Ríos D et al (2009) Encapsulated somatic embryos and zygotic embryos for obtaining artificial seeds of rauli-beech (Nothofagus alpina (Poepp. and Endl.) oerst.). Chil J Agric Res 69:112–118
Castellanos H, Sánchez-Olate M, Ríos YD (2004) Embriogénesis somática recurrente en raulí (Nothofagus alpine (Poepp. et Endl.) Oerst). Segundo Congreso Chileno de Ciencias Forestales, Valdivia, Chile. 10–12 de noviembre. Universidad Austral de Chile, Valdivia, p 36
Chand S, Singh AK (2004) Plant regeneration from encapsulated nodal segments of Dalbergia sissoo Roxb, a timber-yielding leguminous tree species. J Plant Physiol 161:237–243
Coons GH (1954) The wild species of Beta. Proc Am Soc Sugar Beet Technol 8:142–147
Coons GH (1975) Interspecific hybrids between Beta vulgaris L. and the wild species of Beta. J Am Soc Sugar Beet Technol 18:281–306
Cuguen J, Arnaud JF, Delescluse M et al (2005) Gene flow within the Beta species complex: genetic diversity of weed and wild sea beet populations within the French sugar beet production area. Adv Sugar Beet Res 6:103–115
Daud M, Taha MZ, Hasbullah AZ (2008) Artificial seed production from encapsulated micro shoots of Saintpaulia ionantha Wendl. (African Violet). J Appl Sci 8:4662–4667
De Bock TSM (1986) The genus Beta: domestication, taxonomy and interspecific hybridization for plant breeding. Acta Hortic 182:335–343
Doney DL (1993) Broadening the genetic base of sugarbeet. J Sugar Beet Res 30:209–220
Doney DL, Whitney ED (1990) Genetic enhancement in Beta for disease resistance using wild relatives: a strong case for the value of genetic conservation. Econ Bot 44:445–451
Drew RLK (1979) A cheap, simple apparatus for growing large batches of plant tissue in submerged liquid culture plant. Sci Lett 17:227–236
Elliott MC, Weston GD (1993) Biology and physiology of the sugar beet plant. In: Cooke DA, Scott RK (eds) The sugar beet crop: science into practice. Chapman and Hall, London, pp 37–66
Engelmann F, Takagi H (2000) Cryopreservation of tropical plant germplasm: current research progress and application. In: Proceedings of an international workshop. International Plant Genetic Resources Institute (IPGRI), Tsukuba, Japan
Ergül A, Khabbazi SD, Oğuz MÇ et al (2018) In vitro multiplication of wild relatives in genus Beta conserves the invaluable threatened germplasms. Plant Cell Tissue Organ Cult:1–7
Faisal M, Anis M (2007) Regeneration of plants from alginate-encapsulated shoots of Tylophora indica (Burm. F.) Merrill., an endangered medicinal plant. J Hortic Sci Biotechnol 82:351–354
Ford-Lloyd BV (2005) Sources of genetic variation, genus Beta. In: Biancardi E, Campbell LG, Skaracis GN, De Biaggi M (eds) Genetics and breeding of sugar beet. Science, Enfield, pp 25–33
Frese L (2010) Conservation and access to sugarbeet germplasm. Sugar Technol 12(3–4):207–219
Frese L, Desprez B, Ziegler D (2001) Potential of genetic resources and breeding strategies for base-broadening in Beta. Broadening the genetic base of crop production. IPGRI/FAO, Rome, pp 295–309
Ganapathi TR, Suprasanna P, Bapat VA et al (1992) Propagation of banana through encapsulated shoot tips. Plant Cell Rep 11:571–575
Gantait S, Kundu S, Ali N et al (2015) Synthetic seed production of medicinal plants: a review on influence of explants, encapsulation agent and matrix. Acta Physiol Plant 37(5):98
Gidner S, Lennefors BL, Nilsson NO et al (2005) QTL mapping of BNYVV resistance from the WB41 source in sugar beet. Genome 48:279–285
Grimmer MK, Kraft T, Francis SA et al (2008a) QTL mapping of BNYVV resistance from the WB258 source in sugar beet. Plant Breed 127(6):650–652
Grimmer MK, Bean KMR, Qi A et al (2008b) The action of three Beet yellows virus resistance QTLs depends on alleles at a novel genetic locus that controls symptom development. Plant Breed 127(4):391–397
Heijbroek W, McFarlane JS, Doney DL (1977) Breeding for tolerance to beet-cyst eelworm Heterodera schachtii Schm. in sugarbeet. Euphytica 26(3):557–564
Heijbroek W, Roelands AJ, De Jong JH et al (1988) Sugar beets homozygous for resistance to beet cyst nematode (Heterodera schachtii Schm.), developed from monosomic additions of Beta procumbens to B. vulgaris. Euphytica 38:121–131
Hung CD, Trueman SJ (2012a) Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana x C. citriodora. Acta Physiol Plant 34:117–128
Hung CD, Trueman SJ (2012b) Preservation of encapsulated shoot tips and nodes of the tropical hardwoods Corymbia torelliana × C. citriodora and Khaya senegalensis. Plant Cell Tissue Organ Cult 109:341–352
Ikhlaq M, Hafiz IA, Micheli M et al (2010) In vitro storage of synthetic seeds: effect of different storage conditions and intervals on their conversion ability. Afr J Biotechnol 9:5712–5721
Janick J, Kim YH, Kitto S et al (1993) Desiccated synthetic seed. In: Redenbaugh K (ed) Synseeds. CRC, Boca Raton, FL, pp 11–33
Jassem B (1992) Species relationship in the genus Beta as revealed by crossing experiments. In: International beta genetic resources network. In: Report on the 2nd international beta genetic resources workshop held at the Institute for Crop Science and Plant Breeding, Braunschweig, Germany, 24–28 June 1991. International Crop Network Series No, vol 7, pp 55–61
Jung C, Wricke G (1987) Selection of diploid nematode-resistant sugar beet from monosomic addition lines. Plant Breed 98(3):205–214
Jung SJ, Yoon ES, Jeong JH et al (2004) Enhanced post-germinative growth of encapsulated somatic embryos of Siberian ginseng by carbohydrate addition to the encapsulation matrix. Plant Cell Rep 23:365–370
Kadereit G, Hohmann S, Kadereit JW (2006) A synopsis of Chenopodiaceae subfam. Betoideae and notes on the taxonomy of Beta. Willdenowia 36:9–19
Kavyashree R, Gayatri MC, Revanasiddaiah HM (2006) Propagation of mulberry variety-S54 by synseeds of axillary bud. Plant Cell Tissue Organ Cult 84:245–249
Keskin B (1964) Polymyxa betae n. sp., einParasit in den Wurzeln von Beta vulgaris Tournefort, besonders während der Jugendentwicklung der Zuckerrübe. Arch Microbiol 49(4):348–374
Kitto SL, Janick J (1982) Polyox as an artificial seed coat for a sexual embryo. HortScience 17:448
Kitto SL, Janick J (1985) Hardening increases survival of synthetically-coated asexual embryos of carrot. J Am Soc Hortic Sci 110:283–286
Lambardi M, Benelli C, Ozudogru EA (2006) Synthetic seed technology in ornamental plants. In: Teixeira da Silva JA (ed) Floriculture, ornamental and plant biotechnology, vol 2. Global Science Books, London, pp 347–354
Lange W, De Bock TS, Van Geyt JPC et al (1988) Monosomic additions in beet (Beta vulgaris) carrying extra chromosomes of B. procumbens. Theor Appl Genet 76(5):656–664
Lange W, Muller J, De Bock TS (1993) Virulence in the beet cyst nematode (Heterodera schachtii) versus some alien genes for resistance in beet. Fundam Appl Nematol 16:447–454
Larkin PJ, Davies PA, Tanner GJ (1998) Nurse culture of low number of Medicago and Nicotiana protoplasts using calcium alginate beads. Plant Sci 58:203–210
Lata H, Chandra S, Khan IA et al (2009) Propagation through alginate encapsulation of axillary buds of Cannabis sativa L. – an important medicinal plant. Physiol Mol Biol Plants 15:79–86
Leroy XJ, Leon K, Charles G et al (2000) Cauliflower somatic embryogenesis and analysis of regenerant stability by ISSRs. Plant Cell Rep 19:1102–1107
Letschert JPW (1993) Beta section Beta: biogeographical patterns of variation and taxonomy. Thesis. Wageningen Agricultural University Papers 93–1, p 155
Lewellen RT (1992) Use of plant introductions to improve populations and hybrids of sugarbeet. In: Use of plant introductions in Cultivar Development, Part 2. Crop Science Society of America, Madison, WI, pp 117–135
Lewellen RT (2000) Registration of powdery mildew resistant sugarbeet germplasms CP01 and CP02. Crop Sci 40:1515
Lewellen RT (2004) Registration of CP07 and CP08 sugarbeet germplasms with resistance to powdery mildew, rhizomania, and other diseases. Crop Sci 44(6):2276
Lewellen RT (2006) Registration of CN12 and CN72 sugarbeet germplasm populations with resistance to cyst nematode. Crop Sci 46(3):1414
Lewellen RT, Skoyen IO (1991) Improvement and performance of populations of sugarbeet × Beta maritima. J Sugar Beet Res 28:79
Lewellen RT, Whitney ED (1993) Registration of germplasm lines developed from composite crosses of sugarbeet× Beta maritima. Crop Sci 33(4):882–883
Lulsdorf MM, Tautorus TE, Kikcio SI et al (1993) Germination of encapsulated embryos of interior spruce (Picea-glauca-engelmannii complex) and black spruce (Picea-mariana Mill). Plant Cell Rep 12:385–389
Luterbacher M, Asher M, Deambrogio E et al (2004) Sources of resistance to diseases of sugar beet in related Beta germplasm: I. Foliar diseases. Euphytica 139:105–121
Magray MM, Wani KP, Chatto MA et al (2017) Synthetic seed technology. Int J Cur Microbiol Appl Sci 6(11):662–674
Marlander B, Lange T, Wulkow A (2011) Dispersal principles of sugar beet from seed to sugar with particular relation to genetically modified varieties. J Kult (J Cul Plants) 63:349–373
McCarter JP (2008) Molecular approaches toward resistance to plant-parasitic nematodes. In: Plant cell monographs. Springer, Berlin
McFarlane JS (1984) Final report: breeding for resistance to sugarbeet yellow wilt. Cooperative Agreement No. 58-9AHZ-0-520 between USDA-ARS, Washington, DC and Beet Sugar Development Foundation, Denver, CO
McGrann GR, Grimmer MK, Mutasa-Göttgens ES et al (2009) Progress towards the understanding and control of sugar beet rhizomania disease. Mol Plant Pathol 10(1):129–141
McGrath JM, Saccomani M, Stevanato P et al (2007) Beet. In: Kole C (ed) Genome mapping and molecular breeding in plants, vol 5. Springer, Berlin, pp 191–207
Mesbah M, Scholten OE, De Bock TS et al (1997) Chromosome localisation of genes for resistance to Heterodera schachtii, Cercospora beticola and Polymyxa betae using sets of Beta procumbens and B. patellaris derived monosomic additions in B. vulgaris. Euphytica 97(1):117–127
Micheli M, Pellegrino S, Piccioni E et al (2002) Effects of double encapsulation and coating on synthetic seed conversion in M.26 apple rootstock. J Microencapsul 19:347–356
Mishra J, Singh M, Palni LMS et al (2011) Assessment of genetic fidelity of encapsulated microshoots of Picrorhiza kurroa. Plant Cell Tissue Organ Cult 104:181–186
Munerati O (1932) Sull’incrocio della barbabietola coltivata con la beta selvaggia della costa adriatica. L’Industria Saccarifera Italiana 25:303–304
Munerati O, Mezzadroli G, Zapparoli TV (1913) Osservazioni sulla Beta maritima L. nel triennio 1910–1912. Staz Sper Agric Ital 46:415–445
Murashige T (1977) Plant cell and organ culture as horticultural practice. Acta Hortic 78:17–30
Naik SK, Chand PK (2006) Nutrient-alginate encapsulation of in vitro nodal segments of pomegranate (Punica granatum L.) for germplasm distribution and exchange. Sci Hortic 108:247–252
Nyende AB, Schittenhelm S, Mix-Wagner G et al (2005) Yield and canopy development of field grown potato plants derived from synthetic seeds. Eur J Agron 22:175–184
Owen FV (1942) Inheritance of cross- and self-sterility and self-fertility in Beta vulgaris. J Agric Res 64:679–698
Ozudogru EA, Kaya E, Kirdok E et al (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell Dev Biol Plant 47(2):309–320
Panella L, Frese L (2000) Cercospora resistance in Beta species and the development of resistant sugarbeet lines. In: Asher MJC, Holtschulte B, Molard MR, Rosso F, Steinrücken G, Beckers R (eds) Cercospora beticola Sacc. biology, agronomic influence and control measures in sugar beet, Belgium, pp 163–176
Panella L, Lewellen RT (2007) Broadening the genetic base of sugarbeet: introgression from wild relatives. Euphytica 154:383–400
Pelsy F, Merdinoglu D (1996) Identification and mapping of random amplified polymorphic DNA markers linked to a rhizomania resistance gene in sugar beet (Beta vulgaris L.) by bulked segregant analysis. Plant Breed 115(5):371–377
Pinker I, Abdel-Rahman SSA (2005) Artificial seed for propagation of Dendranthema × grandiflora (Ramat.). Prop Orn Plants 5:186–191
Pintos B, Bueno MA, Cuenca B et al (2008) Synthetic seed production from encapsulated somatic embryos of cork oak (Quercus suber L.) and automated growth monitoring. Plant Cell Tissue Organ Cult 95:217–225
Pond S, Cameron S (2003) Tissue culture artificial seed. In: Thomas B (ed) Encyclopedia of applied plant sciences. Elsevier, Oxford, pp 1379–1388
Pourjavadi A, Barzegar SH, Mahdavinia GR (2006) MBA-cross linked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr Polym 66:386–395
Ravi D, Anand P (2012) Production and applications of artificial seeds: a review. Int Res J Biol Sci 1(5):74–78
Reddy MC, Murthy KSR, Pullaiah T (2012) Synthetic seeds: a review in agriculture and forestry. Afr J Biotechnol 11:14254–14275
Reinert J (1958) Morphogenese und ihre Kontrolle an Gewebekulturenaus Carotten. Naturwissenschaften 45(14):344–345
Ren H, Zhang Q, Lu H et al (2012) Wild plant species with extremely small populations require conservation and reintroduction in China. Ambio 41(8):913–917
Richardson KL (2018) Registration of sugar beet germplasm lines CN921–515 and CN921–516 with sugar beet cyst nematode resistance from Beta vulgaris subsp. maritima. J Plant Regist 12(2):264–269
Rihan HZ, Al-Issawi M, Al-Swedi F et al (2012) The effect of using PPM (plant preservative mixture) on the development of cauliflower microshoots and the quality of artificial seed produced. Sci Hortic 141:47–52
Rihan H, Kareem F, El-Mahrouk M et al (2017) Artificial seeds (principle, aspects and applications). Agronomy 7(4):71
Rizkalla AA, Badr-Elden AM, Ottai MES et al (2012) Development of artificial seed technology and preservation in sugar beet. Sugar Tech 14(3):312–320
Rossi V, Racca P, Giosue S (1995) Geophytopathological analysis of Cercospora leaf spot on sugarbeet in the Mediterranean area. Phytopathol Med:69–82
Roy B, Mandal AB (2008) Development of synthesis seed involving androgenic and pro-embryos in elite indica rice. Indian J Biotechnol 7:515–519
Saiprasad G (2001) Artificial seeds and their applications. Resonance 6:39–47
Saiprasad GVS, Polisetty R (2003) Propagation of three orchid genera using encapsulated protocorm-like bodies. In Vitro Cell Dev Biol Plant 39:42–48
Sakhanokho HF, Pounders CT, Blythe EK (2013) Alginate encapsulation of Begonia microshoots for short-term storage and distribution. Sci World J 13:1–7
Salentijn EMJ, Sandal NN, Lange W et al (1992) Isolation of DNA markers linked to a beet cyst nematode resistance locus in Beta patellaris and Beta procumbens. Mol Gen Genet MGG 235(2–3):432–440
Sandal NN, Salentijn EMJ, Kleine M et al (1997) Backcrossing of nematode-resistant sugar beet: a second nematode resistance gene at the locus containing Hs1pro–i. Mol Breed 3:471–480
Sarkar D, Naik PS (1998) Synseeds in potato: an investigation using nutrient-encapsulated in vitro nodal cutting segments. Sci Hortic 73:179–184
Saunders JW, Tsai CJ (1999) Production of somatic embryos and shoots from sugarbeet callus: Effects of abscisic acid, other growth regulators, nitrogen source, sucrose concentration and genotype. In Vitro Cell Dev Biol Plant 35(1):18–24
Savitsky H (1960) Viable diploid, triploid, and tetraploid hybrids between Beta vulgaris and species of the section Patellares. J Am Soc Sugar Beet Technol 11:215–235
Savitsky H (1975) Hybridization between Beta vulgaris and B. procumbens and transmission of nematode (Heterodera schachtii) resistance to sugar beet. Can J Genet Cytol 17:197–209
Scholten OE, De Bock TS, Klein-Lankhorst RM et al (1999) Inheritance of resistance to beet necrotic yellow vein virus in Beta vulgaris conferred by a second gene for resistance. Theor Appl Genet 99(3–4):740–746
Senaratna T, McKersie BD, Bowley SR (1990) Artificial seeds of alfalfa (Medicago sativa L.). Induction of desiccation tolerance in somatic embryos. In Vitro Cell Dev Biol Plant 16:85–90
Sharma S, Shahzad A, Jan N et al (2009a) In vitro studies on shoot regeneration through various explants and alginate-encapsulated nodal segments of Spilanthes mauritiana DC., an endangered medicinal herb. Int J Plant Dev Biol 3:56–61
Sharma S, Shahzad A, Sahai A (2009b) Artificial seeds for propagation and preservation of Spilanthes acmella (L.) Murr., a threatened pesticidal plant species. Int J Plant Dev Biol 3:62–64
Sharma S, Shahzad A, da Silva JAT (2013) Synseed technology—a complete synthesis. Biotechnol Adv 31(2):186–207
Singh AK, Chand S (2010) Plant regeneration from alginate encapsulated somatic embryos of Dalbergia sissoo Roxb. Indian J Biotechnol 9:319–324
Singh B, Sharma S, Rani G et al (2007) In vitro response of encapsulated and non-encapsulated somatic embryos of Kinnow mandarin (Citrus nobilis Lour × C. deliciosa Tenora). Plant Biotechnol Rep 1:101–107
Skaracis GN (2005) Molecular biology and biotechnology, genetic engineering. In: Biancardi E, Campbell LG, Skaracis GN, De Biaggi M (eds) Genetics and breeding of sugar beet. Science, Enfield, NH, pp 255–268
Skaracis GN, Biancardi E (2000) Breeding for Cercospora resistance in sugar beet. In: Asher MJC, Holtschulte B, Molard MR, Rosso F, Steinrücken G, Beckers R. (eds) Cercospora beticola Sacc. biology, agronomic influence and control measures in sugar beet. International Institute for Beet Research (IIRB), Brussels. Adv Sugar Beet Res Ser 2:177–195
Srivastava V, Khan SA, Banerjee S (2009) An evaluation of genetic fidelity of encapsulated microshoots of the medicinal plant: Cineraria maritima following six months of storage. Plant Cell Tissue Organ Cult 99:193–198
Steward FC, Mapes MO, Smith J (1958) Growth and organized development of cultured cells I. Growth and division of freely suspended cells. Am J Bot 45(9):693–703
Sundararaj SG, Agrawal A, Tyagi RK (2010) Encapsulation for in vitro short-term storage and exchange of ginger (Zingiber officinale Rosc.) germplasm. Sci Hortic 125:761–766
Tabassum B, Nasil IA, Farooq AM et al (2010) Viability assessment of in vitro produced synthetic seeds of cucumber. Afr J Biotechnol 9:7026–7032
Tamada T, Baba T (1973) Beet necrotic yellow vein virus from rhizomania affected sugar beet in Japan. Ann Phytopathol Soc Jpn 39:325–332
Van Geyt JPC, Oleo M, Lange W et al (1988) Monosomic additions in beet (Beta vulgaris) carrying extra chromosomes of Beta procumbens. Theor Appl Genet 76(4):577–586
Van Geyt JPC, Lange W, Oleo M et al (1990) Natural variation within the genus Beta and its possible use for breeding sugar beet: a review. Euphytica 49(1):57–76
Varshney A, Anis M (2014) Synseed conception for short-term storage, germplasm exchange and potentialities of regeneration genetically stable plantlets of desert date tree (Balanites aegyptiaca Del.). Agrofor Syst 88:321–329
Wang WG, Wang SH, Wu XA et al (2007) High frequency plantlet regeneration from callus and artificial seed production of rock plant Pogonatherum paniceum (Lam.) Hack. (Poaceae). Sci Hortic 113:196–201
Weiland J, Koch G (2004) Sugarbeet leaf spot disease (Cercospora beticola Sacc.). Mol Plant Pathol 5(3):157–166
Westcott RJ (1981) Tissue culture storage of potato germplasm. Use of growth retardants 2. Potato Res 24:343–352
Winkelmann T, Meyer L, Serek M (2004) Germination of encapsulated somatic embryos of Cyclamen persicum. Hortic Sci 39:1093–1097
Yu MH (1983) Sugar beet germplasm resistant to sugar beet nematode. Crop Sci 23:1021–1022
Yu MH (1989) Callus induction and differentiation from leaf explants of different species of the genus Beta. Crop Sci 29:205–209
Yu MH (1997) Registration of Mi-1 root-knot nematode resistant beet germplasm line. Crop Sci 37(1):295–295
Yu MH (2002) Registration of sugarbeet germplasm M1-3 resistant to root-knot nematode. Crop Sci 42(5):1756
Yu Y (2004) Genetics of Aphanomyces disease resistance in sugarbeet (Beta vulgaris), AFLP mapping and QTL analyses. PhD Dissertation. Michigan State University
Yu MH (2005) Cyst nematode. In: Biancardi E, Campbell LG, Skaracis GN, De Biaggi M (eds) Genetics and breeding of sugar beet. Science, Enfield, NH, pp 103–109
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khabbazi, S.D., Yüksel Özmen, C., Ergül, A. (2019). Synthetic Seeds of Wild Beet: Basic Concepts and Related Methodologies. In: Faisal, M., Alatar, A. (eds) Synthetic Seeds . Springer, Cham. https://doi.org/10.1007/978-3-030-24631-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-24631-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24630-3
Online ISBN: 978-3-030-24631-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)