Abstract
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system. Much has been learned about the role of vitamin D in MS, although our understanding remains incomplete. While the precise etiology of MS remains incompletely understood, low vitamin D status is one factor that appears to predispose to the development of MS, and patients with low 25(OH)D levels may be at greater risk of disease activity. Clinical trials are currently underway to more directly address the role of vitamin D supplementation in MS, yet further investigations are needed. This chapter reviews the role of vitamin D in the pathophysiology of MS and the evidence related to clinical outcomes in patients with MS who have vitamin D deficiency.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system and the most common cause of nontraumatic disability among young adults [1, 2]. The incidence and prevalence of MS are increasing, and the medical, social, and economic burden of the disease is significant [3]. While the precise etiology of MS remains to be completely elucidated, it appears to arise from a combination of genetic and environmental factors. Vitamin D, “the sunshine vitamin,” and sunlight have been implicated among several other environmental factors thought to contribute to an individual’s risk of developing MS, others including smoking, obesity, and Epstein-Barr virus infection. The interaction between vitamin D and MS has been the subject of significant investigative efforts, and much has been learned. This chapter will discuss the role of vitamin D in the pathophysiology of MS and review the evidence related to clinical outcomes in patients with MS who have vitamin D deficiency.
Background
Source and Metabolism of Vitamin D
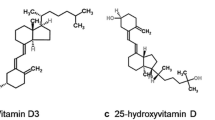
Vitamin D is technically a prohormone that is synthesized in the skin from 7-dehydrocholesterol as a result of exposure to solar ultraviolet-B radiation (UVB) or obtained through ingestion. UVB radiation photolysis of 7-dehydrocholesterol to pre-vitamin D3 which is subsequently isomerized by a nonenzymatic membrane enhanced catalysis to vitamin D3 [4]. Although sun exposure is capable of yielding substantial amounts of vitamin D, a number of individual factors (e.g., age, increased skin pigmentation, use of sunscreen, time spent indoors) and environmental factors (e.g., time of day, latitude, climate) limit sunlight as a source of vitamin D [5]. Dietary sources of vitamin D such as salmon, tuna, egg yolk, shiitake mushrooms, and other mushrooms exposed to sunlight for UVB radiation as well as fortified milk, orange juice, and some cereals can provide modest amounts (between 100 and 200 international units (IU) per day) of vitamin D in the form of vitamin D3 (cholecalciferol, animal sources) or vitamin D2 (ergocalciferol, yeast, mushrooms, and plant sources) [6,7,8] (Fig. 10.1).
Although vitamin D2 and vitamin D3 have no known intrinsic biological activity on calcium metabolism or on non-calcimimetic genomic activities, there is some evidence that vitamin D3 itself may play a fundamental role in stabilizing endothelial membranes reducing inflammatory activity. Vitamin D (D represents D2 or D3) is weakly bound to the vitamin D-binding protein and transported via the bloodstream to the liver for enzymatic conversion to 25-hydroxyvitamin D [25(OH)D]. This is subsequently hydroxylated to 1,25-dihydroxyvitamin D [1,25(OH)2D] in the kidneys for regulating calcium, phosphate, and bone metabolism [7, 8]. 1,25(OH)2D interacts with its nuclear vitamin D receptor (VDR) in the small intestine resulting in the enhancement of dietary calcium and phosphate absorption. In the bone, this hormone interacts with its receptor in osteoblasts resulting in the increased expression of receptor activator of NFkB (RANKL), which in turn interacts with monocytes to become mature osteoclasts. These cells are responsible for removing calcium from the skeleton to help maintain calcium homeostasis.
There are a variety of tissues and cells that also have the capacity to convert 25(OH)D to 1,25(OH)2D including macrophages, monocytes, breast, colon, brain, and prostate among other tissues. It is believed that the local production of 1,25(OH)2D acts in an autocrine or paracrine fashion to regulate a wide variety of genes controlling DNA synthesis, apoptosis, and cellular maturation among many other activities. In addition to these genomic activities, locally produced 1,25(OH)2D initiates its own destruction by markedly enhancing the expression of the 25-hydroxyvitamin D-24-hydroxylase. This enzyme causes oxidation of the side chain producing a water-soluble inactive vitamin D metabolite calcitroic acid. When healthy adults ingested 2000 IUs/day of vitamin D for 12 weeks, 291 genes responsible for regulating more than 100 different metabolic processes were altered in their peripheral white blood cells [8]. These non-calcemic genomic activities may be responsible for the importance of vitamin D in such diverse roles as cancer prevention [9], as well as immune [10], and cardiovascular disease [11].
Immunomodulatory Effects of Vitamin D
The role of vitamin D in immune function has been the subject of extensive investigation since the discovery of VDRs in activated human T and B lymphocytes [12, 13]. VDRs have now been identified on virtually all immune cells, many of which are also capable of converting 25(OH)D into 1,25(OH)2D [12,13,14,15,16,17], allowing 1,25(OH)2D to modulate immune function at sites of inflammation [18]. Vitamin D modulates the response of both innate and adaptive immune cells [18]. Vitamin D does not appear to be immunosuppressive, but rather immunomodulatory, with pleotropic effects on immune function. The immunomodulatory mechanisms of vitamin D have been recently reviewed extensively [18,19,20], so we will only discuss a few key findings pertinent to MS and experimental autoimmune encephalomyelitis (EAE).
Treatment with the active form of vitamin D, 1,25(OH)2D, promotes a tolerogenic state among dendritic cells, characterized by decreased production of inflammatory cytokines (e.g., interleukin-12 [IL-12]) and increased production of anti-inflammatory cytokines (e.g., IL-10, transforming growth factor-β) [21]. These tolerogenic dendritic cells are less capable of activating alloreactive T-cells and promote the differentiation of regulatory T-cells [21, 22]. The effects of 1,25(OH)2D on macrophages are more complex. Early in the course of an inflammatory stimulus, 1,25(OH)2D produced by macrophages promotes inflammatory and antimicrobial mechanisms essential for pathogen clearance [23, 24]. However, 1,25(OH)2D also attenuates toll-like receptor-mediated inflammation through enhancing negative feedback [25] which promotes decreased production of inflammatory cytokines such as IL-6 and TNF, increases production of IL-10, and impairs macrophage activation of T-cells [18]. Thus while 1,25(OH)2D promotes the inflammatory M1 macrophage phenotype, it also abrogates inflammation through favoring the anti-inflammatory M2 macrophage phenotype.
Historically, vitamin D was thought to modulate adaptive immunity through its effects on innate immune function as outlined above. More recently, however, the role of vitamin D in directly modulating adaptive immunity has attracted interest and is beginning to be elucidated [20]. The effects of vitamin D on B-cells remain incompletely understood. 1,25(OH)2D reduces the proliferation of B-cells, inhibits immunoglobulin class switching, induces B-cell apoptosis, and decreases antibody production [20]. However, 1,25(OH)2D also stimulates terminally differentiating B-cell to become plasma cells and promotes B-cell migration to sites of inflammation [20].
T-cells are directly and indirectly affected by vitamin D, although the effects of 1,25(OH)2D differ between different T-cell subsets [26]. For example, 1,25(OH)2D inhibits the differentiation and activity of Th17 cells, and impairs the development of experimental autoimmune encephalomyelitis (EAE) by MOG-specific Th17 cells in a VDR-dependent fashion [27, 28]. 1,25(OH)2D reduces T-cell production of IL-2, IL-17, and IFNγ and abrogates the cytotoxic activities of CD4+ and CD8+ T-cells [29]. In vivo 1,25(OH)2D has been found to enhance the development of IL-10-producing T-cells, reduce the number of IL-6- and IL-17-secreting cells, and increase the number of CD4+CD25+ T-regulatory cells [30].
While 1,25(OH)2D is capable of preventing EAE in either male or female mice, even at high doses, vitamin D3 supplementation was only protective in female mice (in a 17-β-estradiol-dependent manner) [31, 32]. A sex-specific effect of vitamin D has not been observed in key epidemiological studies, however, as discussed below. In North America it is well documented that living below an approximate latitude equivalent to Atlanta, Georgia, for the first 10 years of life reduces risk of developing MS substantially, regardless of where the person locates after this time, suggesting that sun exposure possibly through the action of vitamin D has some benefit [5]. UVB exposure also appears to inhibit the development of EAE [33], although it remains unclear whether vitamin D or other effects of UVB on immune function mediate this observation [34, 35]. In addition, neither UVB nor 25(OH)D appear capable of altering the course of EAE after the development of initial symptoms, while 1,25(OH)2D is capable of exerting immunomodulatory effects even after EAE onset [36].
One pilot study including 40 patients with MS randomized to 800 or 10,400 IU daily vitamin D3 noted pleotropic immunomodulatory effects of high-dose supplementation [37]. High-dose vitamin D3 reduced the proportion of IL-17-producing CD4+ T-cells, and reductions in IL-17 production correlated with increases in serum 25(OH)D levels. CD4+IL-17+ T-cells (Th17) have been implicated in the immune pathogenesis of EAE and MS and IL-17 gene expression is increased in MS lesions [38]. The study also noted a reduction in the proportion of effector memory CD4+ T-cells and an increase in central memory CD4+ cells and naïve CD4+ T-cells in the high-dose group. No differences were noted in the serum levels of 51 other cytokines evaluated in the study.
Measuring Vitamin D Status
The renal production of 1,25(OH)2D is tightly regulated and has a relatively short half-life (4 h), while 25(OH)D has a longer half-life (20–60 days) [39, 40]. As an integrated measure of vitamin D produced by solar UVB exposure, dietary intake, and release from adipose tissues, serum levels of 25(OH)D are the best indicator of an individual’s overall vitamin D status [41]. The 1,25(OH)2D levels in circulation are 1000 times lower than 25(OH)D levels, and they are often normal or elevated in patients with vitamin D deficiency because of the renal production of this hormone in response to increasing blood levels of parathyroid hormone. Thus the measurement of 1,25(OH)2D is of no value in determining a person’s vitamin D status but is helpful in the differential diagnosis of acquired and inherited disorders in calcium, phosphate, and bone metabolism [7]. It should also be noted that extrarenal production is not tightly regulated. This is the reason why patients with granulomatous disorders developed hypercalciuria and hypercalcemia due to the unregulated production of 1,25(OH)D by activated macrophages and its release into the circulation [7]. The 25(OH)D serum levels are reported in ng/mL or nmol/L with 1 ng/mL equal to 0.4006 nmol/L.
Vitamin D Deficiency and Insufficiency
Nutritional rickets associated with vitamin D deficiency was widespread from the industrial age until the mid-twentieth century. Age of onset determines clinical manifestations but generally includes short stature, bone pain, bowing deformities of the legs and widening of the joints (epiphyseal plates), and severe proximal muscle weakness. In the 1920s, the value of cod liver oil, which contains adequate levels of vitamin D3, was recognized. At the same time, it was demonstrated that sun exposure could cure rickets, and initially the precursor of vitamin D2 was added to milk and then exposed to ultraviolet radiation, which imparted antirachitic activity. In the 1930s when vitamin D was commercially produced, this process was eliminated and vitamin D2 was added directly to the milk [5]. As a consequence, nutritional rickets was virtually eliminated in the United States by the 1940s. The importance of vitamin D in bone health and calcium homeostasis is now well-recognized and has been recently reviewed [42].
In 2011, the Institute of Medicine released guidelines for the general population which recommended a dietary vitamin D intake of 600 IU/day for those aged 1–70, and 800 IU/day for those >70 years old, corresponding to serum levels of 25(OH)D of 16 ng/mL [43]. The report did not recommend that all individuals attempt to achieve levels of 20 ng/mL or higher; rather it emphasized that most (97.5%) individuals’ nutritional needs would be met at serum levels of 25(OH)D <20 ng/mL. However, the Endocrine Society whose guidelines were for the treatment and prevention of vitamin D deficiency in children and adults defined deficiency as <20 ng/mL, insufficiency as 21–29 ng/mL, and sufficiency as ≥30 ng/mL for maximum musculoskeletal health [44]. The definitions proposed by these two groups have been debated in the literature and will not be explored in detail herein [45,46,47].
Vitamin D Status and Risk of Developing MS
Several key observations form the foundation for the hypothesis that hypovitaminosis D is an MS risk factor [48]. First, the prevalence of MS has been observed to increase with greater distance from the equator, which is strongly inversely correlated with duration and intensity of UVB exposure and 25(OH)D levels [5, 49,50,51,52,53]. Second, populations at high latitudes but with higher consumption of vitamin D-rich fatty fish have a lower than expected prevalence of MS [49, 52]. And third, the risk of MS appears to decrease with early migration from higher to lower latitudes [54, 55]. The final observation appears to have decreased in recent decades, possibly related to increasing tendency to avoid sun exposure and stay indoors for greater portions of the day even in warmer climates [56]. MS risk varies by latitude. Vitamin D status is inversely related to latitude [5], but other potentially involved factors also have a latitudinal gradient. For example, Epstein-Barr virus prevalence shows a direct latitude gradient, whereas parasite infections show an inverse relationship. How each of these factors contribute to overall risk of MS requires further characterization.
Serum Levels of 25-Hydroxyvitamin D
If hypovitaminosis D has an effect on MS risk, we should observe MS incidence to increase with lower serum levels of 25(OH)D. Longitudinal studies examining 25OH(D) levels before the onset of MS are crucial because it is well established that serum 25(OH)D levels decrease after the onset of MS [57, 58]. Studies that only look at vitamin D status after the development of MS leave open the potential for reverse causation as a confounder.
Although controversial [59], the risk of MS appears to be higher for individuals born in the spring (serum vitamin D status are lowest over the winter and early spring) than autumn, an observation that is most prominent in high-risk areas (higher latitude/less sunlight) and does not hold true in areas with higher sunlight exposure [60, 61]. Higher milk intake, dietary vitamin D consumption, and maternal predicted 25(OH)D were all associated with a decreased risk of MS in children of mothers from the Nurses Mother’s Study, a prospective, longitudinal cohort study [62]. This suggests that maternal hypovitaminosis D during pregnancy may contribute to risk in the offspring. Further corroborating these results, a prospective, nested case-control study evaluated whether maternal serum 25(OH)D levels in early pregnancy are associated with MS risk in offspring [63]. The authors found that offspring of mothers with serum 25(OH)D <12 ng/mL during early pregnancy had a nearly twofold increased risk of MS compared to offspring from mothers with normal 25(OH)D levels. Another study found that low concentrations of neonatal 25(OH)D were associated with an increased risk of MS, with the greatest risk in the lowest quintile (<8.3 ng/mL) and lowest in the highest quintile (≥19.6 ng/mL) suggesting a dose-response effect [64]. Taken together these studies suggest that low levels of vitamin D in utero and in neonates may increase the risk of MS.
Hypovitaminosis D also appears to increase risk of MS in adulthood. A prospective, nested case-control study of US military personnel found that high levels of serum vitamin D were associated with a decreased risk of MS [57]. A nested case-control study from another group including individuals in northern Sweden noted similar decreased risk of MS with higher vitamin D levels [65]. Munger and colleagues recently reported the results of a nested case-control study of 1092 women diagnosed with MS in the Finnish maternity cohort [66]. 25(OH)D was quantified in serum obtained prior to MS diagnosis, and subjects were matched with up to three controls on date of birth and area of residence. Conditional logistic regression adjusted for year of sample collection, gravidity, and parity were used to estimate relative risks and 95% confidence intervals. They found that women with 25(OH)D levels <12 ng/mL had a 43% higher risk of MS compared to those with levels ≥20 ng/mL.
Genetic Studies
Several recent studies have utilized Mendelian randomization to estimate the effect of vitamin D on the risk of MS. This is a method that uses measured variation in genes with known function to estimate the association of modifiable exposures in the risk of disease. Studies using this approach reduce the chance of reverse causation because inherited alleles are not affected by most confounding variables or disease status. In one study, genome-wide data of genetic variants shown to predict levels of serum 25(OH)D were applied to the International MS Genetics Consortium [67]. The authors found that alleles known to decrease levels of serum 25(OH)D predicted an increased susceptibility to MS. Another study found similar results in two separate populations, including white, non-Hispanic Americans and members of a Swedish population study [68]. A study of patients with pediatric-onset MS found independent effects of low vitamin D and high BMI [69]. These data further support the hypothesis that low levels of vitamin D exert independent causal effects on MS.
There are, however, a few studies with discrepant results worth considering. A population-based, multicenter, case-control study in Sweden investigated the link between vitamin D status at birth and the risk of MS using stored neonatal dried blood [70]. This study included 459 persons with MS and 663 controls and found no association between neonatal serum 25(OH)D quintiles and the risk of MS. Results were not appreciably different when adjusted for confounding factors such as month of birth, latitude of birth, breastfeeding or adult sun exposure, vitamin D intake, smoking, etc. The results of this study were viewed critically on several grounds, including that blood samples were not well preserved with degradation of 25(OH)D noted in the study. Additionally, the range of 25(OH)D levels was narrow and mostly low (mean 11.9 ng/mL, median 10.3, interquartile range 6.8–15.4 ng/mL) [71].
Vitamin D Status MS Disease Activity
Several studies have shown a correlation between relapse rates and vitamin D status. Although these are confounded by the possibility of reverse causation, they lend support to the possible role of vitamin D supplementation in MS. A retrospective study of pediatric patients with MS, after adjusting for several factors including age, race, ethnicity, disease duration, and treatment, found that every 10 ng/mL increase in 25(OH)D levels was associated with a 34% decrease in relapse rate [72]. Similar results were seen in adult-onset MS, where one study observed relapse rate to decrease by 27% for every doubling of 25(OH)D levels [73], and another noted that every increase in 25(OH)D by 4 ng/mL was associated with up to 12% reduction in relapse rate [74].
A 5-year longitudinal cohort study did not find a statistically significant correlation between vitamin D status and relapse rate in patients with relapsing-remitting multiple sclerosis (RRMS) or clinically isolated syndrome (CIS) , but did observe a 15% lower risk of new T2 lesions and a 32% lower risk of new enhancing lesions on MRI for every 10 ng/mL increase in 25(OH)D [75]. A follow-up study found a tendency for an inverse relationship between average 25(OH)D levels and the composite endpoint of ≥3 new brain T2 lesions or ≥1 relapse within a year in patients with CIS [76]. A retrospective study of 100 patients with CIS from another group found that lower levels of serum 25(OH)D were associated with an increased risk of conversion to clinically definite MS, an association that was even stronger when controlling for additional risk factors for conversion [77]. Multiple studies have also correlated lower vitamin D status with greater disability and disease severity, although reverse causation remains a concern in such studies.
Two MS treatment trials examined 25(OH)D levels and risk of MS progression. A possible advantage to these studies is that clinical outcomes such as relapses and MRI activity were more systematically ascertained than in observational cohorts. The BENEFIT (Betaferon/Betaseron in Newly Emerging Multiple Sclerosis For Initial Treatment) study was a randomized trial designed to evaluate the effect of early vs. delayed treatment with interferon beta-1b (IFNB-1b) in patients with CIS. As part of the study, serum 25(OH)D levels were tested at 0, 6, 12, and 24 months from randomization. Ascherio and colleagues analyzed the relationship between serum 25(OH)D levels and MS activity/progression using clinical and radiological data [78]. Low 25(OH)D levels were found to be a strong risk factor for long-term MS activity and progression with increased hazard of conversion to clinically definite MS (by radiologic or clinical criteria), higher rate of new lesion formation on MRI, higher rate of clinical relapses, and higher rate of brain atrophy on MRI with lower levels of 25(OH)D.
The BEYOND (Betaferon/Betaseron Efficacy Yielding Outcomes of a New Dose) study included measurements of 25(OH)D every 6 months, and a post hoc analysis of the data demonstrated that higher levels of 25(OH)D were associated with lower rates of MS activity on MRI; however, there was no significant association between serum 25(OH)D levels and rate of brain atrophy or clinical outcomes [79]. The association between 25(OH)D and MS activity was stronger in the early treatment (with IFNB-1b) group than in the delayed treatment group, suggesting that there may be an additive effect of IFNB-1b with vitamin D.
The aforementioned studies demonstrating that individuals with higher vitamin D levels experienced more MS disease control with IFNB-1b led to further exploration of possible immunomodulatory mechanisms [80]. Enhanced regulation of genes involved in immunomodulation was advanced as a possible explanation [81]. Lending support to this finding, an independent team of investigators observed a greater production of vitamin D from sunlight in patients treated with IFNB [81]. In that study, every 10 ng/mL increase in 25(OH)D was associated with a 10% decrease in relapse rates, and interestingly IFNB was only protective against relapse in patients with higher levels of 25(OH)D. Patients with inadequate levels were at increased risk of relapse, despite IFNB treatment. However, another prospective cohort study of 88 patients with RRMS in Norway found that pre-IFN treatment, higher levels of 25(OH)D were associated with less radiologic disease activity, but this effect was no longer detected after IFNB treatment [82]. No associations were noted for relapses or EDSS progression.
The relationship between vitamin D status and other disease-modifying therapies was explored in the CLIMB (Comprehensive Longitudinal Investigation of Multiple Sclerosis at Brigham and Women’s Hospital) study, a prospective cohort study that began enrolling patients in 2000 [83]. Rotstein and colleagues investigated the effect of vitamin D status on clinical and MRI outcomes in patients treated with IFN (n = 96), glatiramer acetate (GA) (n = 151), or fingolimod (n = 77) [84]. Serum 25(OH)D levels were adjusted for season and patients were divided into subgroups based on 25(OH)D tertile. The primary endpoint was time to first inflammatory event, defined as either clinical relapse or gadolinium-enhancing lesion on MRI. The authors found that higher 25(OH)D levels were associated with longer time to the combined endpoint for patients on IFN or fingolimod, but not glatiramer. There was a significant association with gadolinium-enhancing lesions in both the IFN and GA groups, although the effect was greater for the IFN group, but no significant association with relapses was seen in either group. In the fingolimod group, there was a significant association for the combined endpoint and relapses, but not for gadolinium-enhancing lesions. These results suggest that patients with robust vitamin D status might experience a greater benefit from this with some disease-modifying therapies than others. It makes sense in theory that some medications may duplicate many of vitamin D’s effects and show less benefit when combined, while others might work primarily on separate pathways with an additive effect. Further research is necessary to better understand the interactions between MS drugs and vitamin D.
Vitamin D Supplementation
Vitamin D Supplementation as a Means to Preventing MS Development
Prospective non-randomized studies investigating whether vitamin D supplementation may lower the risk of developing MS are limited and have been conflicting. One study that included two large cohorts of women (the Nurses’ Health Study with 92,253 women followed from 1980 to 2000 and Nurses’ Health Study II with 95,310 women followed from 1991 to 2001) found a decreased pooled, age-adjusted relative risk of MS for subjects in the highest quintile of total vitamin D intake compared to the lowest quintile (RR = 0.67) [85]. The same study noted a reduced relative risk with vitamin D intake through supplements. Women who took ≥400 IU/day had a relative risk of 0.59 compared to those who did not take supplementation; however, no association was observed between MS incidence and vitamin D intake from food sources. Another study using the data from both the Nurses’ Health Study and Nurses’ Health Study II study examined the association of vitamin D intake specifically during adolescence with risk of MS and found no significant effect associated with vitamin D intake, including through supplementation [85, 86]. However, there was a non-statistically significant trend toward decreased risk of MS with supplementation ≥400 IU/day. Some have advocated for a more proactive approach consisting of vitamin D supplementation in hopes of preventing MS, at least for individuals at high risk including smokers, the obese, and those with a family history of MS [87].
Vitamin D Supplementation as a Means to Decreasing MS Disease Severity
Studies investigating the role of vitamin D supplementation in MS are conflicting and no consensus has been reached regarding the use of vitamin D. A systematic review of randomized, controlled trials published in 2013 noted problems with small sample sizes (23–68 patients), heterogeneity in dosing, form of vitamin D (cholecalciferol vs. ergocalciferol), and outcome clinical measures [88]. Four of the five trials demonstrated no effect of vitamin D in MS, while one showed a reduction of the number of enhancing lesions only. The authors concluded that the evidence for vitamin D supplementation in MS is inconclusive and that larger studies are warranted [88]. A meta-analysis also from 2013 was only able to include 129 high-dose vitamin D-treated patients and 125 controls and found no correlation between high-dose vitamin D treatment and clinical relapses and similarly concluded that the existing studies were methodologically limited and further investigation was warranted in the form of larger, more prolonged studies [89]. Below we comment on some particular studies, attempting to offer some perspective about trials that have been conducted to date.
Two studies explored the effect of a 20,000 IU/week (equivalent = approximately 3000 IU daily) of vitamin D3 on clinical and MRI outcomes. A 1-year randomized controlled study including 66 patients with MS randomized patients to a weekly dose of 20,000 IU vitamin D3 or placebo. Eighty-four percent of patients in the treatment arm achieved levels of 25(OH)D >34 ng/mL and had fewer enhancing lesions on MRI. However the study was not powered to assess clinical outcomes. A 96-week trial originally designed to assess the effects of high-dose vitamin D3 supplementation on bone density in patients with MS found that a weekly dose of 20,000 IU of vitamin D3 was also not powered to assess clinical outcomes, but did not appear to affect the course of the disease [90].
One study randomized patients with clinically active RRMS to either a dose of 6000 or 1000 IU ergocalciferol daily in patients with clinically active RRMS [91]. The authors found no difference between groups in MRI-based outcome measures. A higher exit EDSS and a higher proportion of relapse were noted in the high-dose arm. A few methodologic limitations are worth mentioning, however. Only 23 patients were enrolled initially and 3 patients withdrew from the study. A patient in the high-dose group included in the analysis had 38 enhancing lesions at baseline, while all others had 2–5, and the low-dose group was on average 5–10 years younger than the high-dose group. Another study including 50 patients randomized to 8000 IU vitamin D3 or placebo daily found no effect on clinical or MRI metrics [92]. Mosayebi and colleagues randomized 62 patients to 300,000 IU (equivalent = 10,000 IU daily) once monthly to either vitamin D3 injection or placebo and no difference in clinical or radiological measures of disease activity, although lymphocyte proliferation rates were lower in the treatment arm [93].
The SOLAR study, another randomized, double-blind, placebo controlled phase 2 study including patients who were on subcutaneous IFNB-1a with 25(OH)D levels <60 ng/mL, did not find significant differences in clinical outcomes between groups, but was technically limited by poor recruitment [94, 95]. The study did note differences in MRI findings. The study included 229 patients who were randomized to treatment with 14,000 IU/day cholecalciferol or placebo. The primary endpoint was freedom from disease activity as measured by relapses, progression on EDSS, or new unique enhancing or T2 lesions. Only available in abstract format, the primary endpoint was changed due to delayed recruitment, allowing for reductions in study size and duration. Compared with placebo, vitamin D supplementation did not affect freedom from disease activity, but did reduce the number of new active lesions overall and new T1 hypointense lesions in patients aged 18–30 years. Another randomized, placebo-controlled phase 2 study included 129 patients with MS on IFNB-1a who were randomized to either 100,000 IU vitamin D3 twice monthly (daily equivalent of 7143 IU) or placebo noted no effect on clinical parameters, but did observe a protective effect of vitamin D on MRI parameters. The authors found no statistically significant effect on clinical parameters in the intention-to-treat analysis, but the study may have been underpowered as a consequence of an unexpectedly low relapse rate among the control patients [96].
Ongoing Studies
Three ongoing studies are registered with www.clinicaltrials.gov. The Efficacy of Vitamin D Supplementation in Multiple Sclerosis (EVIDIMS) study is a randomized, controlled, double-blind stratified phase 2 clinical trial of patients with CIS or MS on IFNB-1b in Germany [97]. Patients are randomized to high-dose (average 10,200 IU daily) or low-dose (average 200 IU daily) cholecalciferol for 18 months. The primary outcome measure is the number of new T2 lesions on brain MRI. Secondary endpoints include other MRI and OCT parameters, clinical metrics, and patient-reported outcomes such as quality of life and fatigue. Results are anticipated in 2018 or 2019.
Another ongoing study, the Vitamin D to Ameliorate Multiple Sclerosis (VIDAMS) study, is a randomized, controlled phase 3 study with a target recruitment of 172 patients with MS in the United States [98]. Patients will be randomized to high-dose (5000 IU daily) or low-dose (600 IU daily) vitamin D3 as add-on therapy to glatiramer acetate. The primary outcome is proportion of patients experiencing a relapse, and secondary outcomes include additional clinical and radiological metrics. The study will terminate in 2018. Finally, an actively recruiting, double-blind, randomized, controlled trial including 100 patients with MS will randomize patients to 1000 or 4000 IU vitamin D3 daily for 4 months. The primary outcome is the change in 25(OH)D levels, with goals including improving the understanding of immunomodulatory effects of vitamin D in vivo.
Translating Data into Clinical Practice
Offering Vitamin D Supplementation to Patients
Although substantial evidence has demonstrated the safety and tolerability of even relatively high doses of vitamin D, the absence of definitive data from large randomized controlled trials has limited the application of vitamin D supplementation for patients with MS. Similarly, the role of vitamin D supplementation for the prevention of MS in the general public, as well as for higher-risk individuals, such as family members of persons with MS, remains incompletely defined.
It is unclear whether D2 or D3 might perform better as a supplement or if they are equivalent. In a meta-analysis of seven randomized trials evaluating serum 25(OH)D concentrations in patients requiring supplementation with D2 vs. D3, D3 increased serum 25(OH)D more efficiently than D2 [99]. This result should be interpreted cautiously given different dosing frequencies, doses, and time periods used in this study. It is also unclear how this might apply to patients considered to be at a normal level who are supplemented to further increase their serum vitamin D 25OH [99].
Despite the limitations in our current understanding of whether vitamin D supplementation might alter MS disease course, we routinely evaluate the vitamin D status of patients with radiologically isolated syndrome (RIS), CIS, and MS and provide supplementation to target a level of 40–70 ng/mL with oral cholecalciferol, if necessary (Fig. 10.2). We choose 40 ng/mL as the lower bound based on an increased risk of disease development observed in the military nested case-control cohort [57] and worsening of MS observed in the post hoc analysis of the BENEFIT trial [78] in individuals with levels <40 ng/mL. We recheck serum levels 3–6 months after making any adjustments to supplementation, and once serum levels have been relatively stable on a consistent regimen, we check levels every 6–12 months for monitoring. Patients with a BMI >30 require 2–3 times more vitamin D to satisfy their requirements [100]. Similarly, those who are treated chronically with steroids or who have gut absorption problems or are treated with antiepileptic medications such as phenytoin or phenobarbital may require higher supplemental doses [101].
Offering Vitamin D to Those at Risk for MS
The evidence supporting a preventative effect of vitamin D is more compelling than the evidence suggesting a therapeutic effect. In addition to correcting low levels of 25(OH)D for patients with RIS, CIS, or MS, we generally recommend that first-degree relatives of persons with MS have their vitamin D status evaluated and corrected with oral supplementation if needed [87].
Safety and Toxicity
When considering supplementation, an understanding of the safety profile of vitamin D and signs of intoxication are important. A double-blind, randomized pilot study demonstrated the safety and tolerability of high-dose vitamin D in MS patients over a 6-month study period [37]. The authors randomized 40 study participants to receive supplemental cholecalciferol at doses of 10,400 IU daily and 800 IU daily for 6 months. Adverse events were minor and did not differ between treatment groups. Three patients (one in the low-dose and two in the high-dose group) developed nausea that resolved after discontinuing supplementation. Baseline serum 25(OH)D levels did not differ between treatment groups, but increased to a greater extent in the high-dose than the low-dose group: the mean change from baseline was 34.9 ng/mL in the high-dose and 6.9 ng/mL in the low-dose group. One patient in the high-dose group was found to have a serum calcium level of 10.6 mg/dL (reference range 8.4–10.5 mg/dL; the participant’s baseline level was 10.0 mg/dL) with a normal urine calcium. That participant completed the study, and at the 6-month follow-up, after stopping supplementation, the serum calcium level had normalized. Dosing frequency was reduced to every other day in one patient from each treatment group for elevated urine calcium/creatinine ratio.
An open-label, phase I/II dose-escalation trial of vitamin D found that high-dose vitamin D did not significantly increase serum calcium levels compared to patients not on high doses. Patients with MS were matched for demographics and disease characteristics and randomized to control or treatment with vitamin D [102]. Treatment arm patients were given escalating doses up to 40,000 IU daily. The study was not statistically precise enough nor designed to assess clinical outcomes, but did provide class II evidence that high-dose vitamin D given to patients with MS for 52 weeks does not significantly increase serum calcium levels compared to patients not on high-dose supplementation.
Toxicity from vitamin D is most often related to hypercalcemia, and associated serum 25(OH)D levels are typically well above 150 ng/mL in these patients [44]. Doses above 10,000 IU daily are generally required to achieve these levels [103]. A study in Canada reported that healthy adults taking as much as 20,000 IUs of vitamin D per day maintain blood levels of 25(OH)D in the range of 60–80 ng/mL without any evidence of toxicity [104]. The effects of long-term relatively high-dose vitamin D have not been well studied, however. A few reports suggest a correlation between serum levels of 25(OH)D >60 ng/mL and increased risks of any-cause mortality, pancreatic cancer, and vascular calcification, but one cannot assess cause and effect given the observational nature of these studies [105,106,107].
Vitamin D intoxication clinically manifests with confusion, polyuria, polydipsia, anorexia, emesis, and muscle weakness related to hypercalcemia. Hyperphosphatemia also occurs due to the suppression of PTH, and increased intestinal absorption of dietary phosphate and chronic vitamin D intoxication can cause nephrocalcinosis, vascular calcification, and bone demineralization. A recent systematic review and meta-analysis did not find an increased risk of cardiovascular events with vitamin D supplementation, and a Cochrane database review found a decreased risk of death in the elderly with vitamin D3 (but not vitamin D2 supplementation) [108, 109]. The level at which hypercalcemia occurs is undefined, but many experts define intoxication as serum levels of 25(OH)D3 ≥150 ng/mL [7, 44]. Our practice is to monitor serum 25(OH)D levels every 6 months while adjusting vitamin D3 supplementation and annually for patients with stable levels. Adjustments can be made based on the patient’s serum 25(OH)D levels according to Fig. 10.2.
Conclusion
Vitamin D has pleotropic effects, some of which are beneficial to the immune system. It is our view that there is good evidence that in utero and beyond low vitamin D status predispose to the development of multiple sclerosis. It is also likely that MS patients with low 25(OH)D levels are at greater risk of MS disease activity. The data supporting the role vitamin D in the treatment of MS are not as compelling as those in the prevention of MS, but better prospective studies are needed. The trials to date are limited by methodological issues such as their small size and short duration, but the association between 25(OH)D levels and MS activity is stronger for MRI than for clinical outcomes. This may be related to higher sensitivity of MRI to disease activity than clinical metrics. In practice we recommend vitamin D supplementation to our MS patients and first-degree relatives employing the rationale that in our geographic area, oftentimes 25(OH)D levels are low and that it is unlikely to cause harm if moderate doses are used. Clinical trials are currently underway to more directly address the role of vitamin D supplementation in MS, yet further investigations are needed. As most studies have included primarily older Caucasians, it is important to evaluate the role of vitamin D in younger persons of non-Caucasian ethnicity.
References
Anderson DW, Ellenberg JH, Leventhal CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31(3):333–6.
Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, Vecsei L, et al. The epidemiology of multiple sclerosis in Europe. Eur J Neurol. 2006;13(7):700–22.
Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–32.
Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88(2):296–307.
Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51–108.
Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–64S.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–55.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57.
Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: a systematic review. Semin Arthritis Rheum. 2011;40(6):512–31.e8.
Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–17.
Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–3.
Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–10.
Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61(4):457–61.
Morgan JW, Kouttab N, Ford D, Maizel AL. Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endocrinology. 2000;141(9):3225–34.
Chen L, Cencioni MT, Angelini DF, Borsellino G, Battistini L, Brosnan CF. Transcriptional profiling of gamma delta T cells identifies a role for vitamin D in the immunoregulation of the V gamma 9V delta 2 response to phosphate-containing ligands. J Immunol. 2005;174(10):6144–52.
Jeffery LE, Qureshi OS, Gardner D, Hou TZ, Briggs Z, Soskic B, et al. Vitamin D antagonises the suppressive effect of inflammatory cytokines on CTLA-4 expression and regulatory function. PLoS One. 2015;10(7):e0131539.
Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2016;7:697.
Rolf L, Muris AH, Hupperts R, Damoiseaux J. Illuminating vitamin D effects on B cells—the multiple sclerosis perspective. Immunology. 2016;147(3):275–84.
Peelen E, Knippenberg S, Muris AH, Thewissen M, Smolders J, Tervaert JW, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10(12):733–43.
Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–11.
Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39(11):3147–59.
Xu H, Soruri A, Gieseler RK, Peters JH. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38(6):535–40.
Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–3.
Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, et al. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013;190(7):3687–95.
Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286(2):997–1004.
Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–69.
Meehan TF, DeLuca HF. The vitamin D receptor is necessary for 1alpha,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys. 2002;408(2):200–4.
Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther. 2018;7(1):59–85.
Correale J, Ysrraelit MC, Gaitan MI. Vitamin D-mediated immune regulation in multiple sclerosis. J Neurol Sci. 2011;311(1–2):23–31.
Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175(6):4119–26.
Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009;183(6):3672–81.
Hauser SL, Weiner HL, Che M, Shapiro ME, Gilles F, Letvin NL. Prevention of experimental allergic encephalomyelitis (EAE) in the SJL/J mouse by whole body ultraviolet irradiation. J Immunol. 1984;132(3):1276–81.
Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006;92(1):140–9.
Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Nataf S, Garcion E, Darcy F, Chabannes D, Muller JY, Brachet P. 1,25 Dihydroxyvitamin D3 exerts regional effects in the central nervous system during experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1996;55(8):904–14.
Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86(4):382–90.
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172(1):146–55.
Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387–91.
Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–8.
Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97(1–2):13–9.
Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nat Rev Dis Primers. 2017;3:17101.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency – is there really a pandemic? N Engl J Med. 2016;375(19):1817–20.
Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–65.
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–52.
Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain. 2010;133(Pt 7):1869–88.
Swank RL, Lerstad O, Strom A, Backer J. Multiple sclerosis in rural Norway its geographic and occupational incidence in relation to nutrition. N Engl J Med. 1952;246(19):722–8.
van der Mei IA, Ponsonby AL, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology. 2001;20(3):168–74.
Vukusic S, Van Bockstael V, Gosselin S, Confavreux C. Regional variations in the prevalence of multiple sclerosis in French farmers. J Neurol Neurosurg Psychiatry. 2007;78(7):707–9.
Westlund K. Distribution and mortality time trend of multiple sclerosis and some other diseases in Norway. Acta Neurol Scand. 1970;46(4):455–83.
Orton SM, Wald L, Confavreux C, Vukusic S, Krohn JP, Ramagopalan SV, et al. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology. 2011;76(5):425–31.
Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47(4–5):425–48.
Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology. 1985;35(5):672–8.
Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61(6):504–13.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–8.
van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254(5):581–90.
Fiddes B, Wason J, Kemppinen A, Ban M, Compston A, Sawcer S. Confounding underlies the apparent month of birth effect in multiple sclerosis. Ann Neurol. 2013;73(6):714–20.
Grytten N, Torkildsen O, Aarseth JH, Benjaminsen E, Celius EG, Dahl OP, et al. Month of birth as a latitude-dependent risk factor for multiple sclerosis in Norway. Mult Scler. 2013;19(8):1028–34.
Torkildsen O, Grytten N, Aarseth J, Myhr KM, Kampman MT. Month of birth as a risk factor for multiple sclerosis: an update. Acta Neurol Scand Suppl. 2012;(195):58–62.
Mirzaei F, Michels KB, Munger K, O’Reilly E, Chitnis T, Forman MR, et al. Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol. 2011;70(1):30–40.
Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel HM, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol. 2016;73(5):515–9.
Nielsen NM, Munger KL, Koch-Henriksen N, Hougaard DM, Magyari M, Jorgensen KT, et al. Neonatal vitamin D status and risk of multiple sclerosis: a population-based case-control study. Neurology. 2017;88(1):44–51.
Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79(21):2140–5.
Munger KL, Hongell K, Aivo J, Soilu-Hanninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology. 2017;89(15):1578–83.
Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, et al. Vitamin D and risk of multiple sclerosis: a Mendelian Randomization Study. PLoS Med. 2015;12(8):e1001866.
Rhead B, Baarnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2(5):e97.
Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88(17):1623–9.
Ueda P, Rafatnia F, Baarnhielm M, Frobom R, Korzunowicz G, Lonnerbro R, et al. Neonatal vitamin D status and risk of multiple sclerosis. Ann Neurol. 2014;76(3):338–46.
Ascherio A, Munger KL. Not too late to take vitamin D supplements. Ann Neurol. 2014;76(3):321–2.
Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67(5):618–24.
Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79(3):261–6.
Simpson S Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68(2):193–203.
Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–40.
Mowry EM, Pelletier D, Gao Z, Howell MD, Zamvil SS, Waubant E. Vitamin D in clinically isolated syndrome: evidence for possible neuroprotection. Eur J Neurol. 2016;23(2):327–32.
Martinelli V, Dalla Costa G, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, et al. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult Scler. 2014;20(2):147–55.
Ascherio A, Munger KL, White R, Kochert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71(3):306–14.
Fitzgerald KC, Munger KL, Kochert K, Arnason BG, Comi G, Cook S, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72(12):1458–65.
Munger KL, Kochert K, Simon KC, Kappos L, Polman CH, Freedman MS, et al. Molecular mechanism underlying the impact of vitamin D on disease activity of MS. Ann Clin Transl Neurol. 2014;1(8):605–17.
Stewart N, Simpson S Jr, van der Mei I, Ponsonby AL, Blizzard L, Dwyer T, et al. Interferon-beta and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology. 2012;79(3):254–60.
Loken-Amsrud KI, Holmoy T, Bakke SJ, Beiske AG, Bjerve KS, Bjornara BT, et al. Vitamin D and disease activity in multiple sclerosis before and during interferon-beta treatment. Neurology. 2012;79(3):267–73.
Gauthier SA, Glanz BI, Mandel M, Weiner HL. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev. 2006;5(8):532–6.
Rotstein DL, Healy BC, Malik MT, Carruthers RL, Musallam AJ, Kivisakk P, et al. Effect of vitamin D on MS activity by disease-modifying therapy class. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e167.
Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5.
Munger KL, Chitnis T, Frazier AL, Giovannucci E, Spiegelman D, Ascherio A. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol. 2011;258(3):479–85.
Marrie RA, Beck CA. Preventing multiple sclerosis: to (take) vitamin D or not to (take) vitamin D? Neurology. 2017;89(15):1538–9.
Pozuelo-Moyano B, Benito-Leon J, Mitchell AJ, Hernandez-Gallego J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis. Neuroepidemiology. 2013;40(3):147–53.
James E, Dobson R, Kuhle J, Baker D, Giovannoni G, Ramagopalan SV. The effect of vitamin D-related interventions on multiple sclerosis relapses: a meta-analysis. Mult Scler. 2013;19(12):1571–9.
Kampman MT, Steffensen LH, Mellgren SI, Jorgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012;18(8):1144–51.
Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77(17):1611–8.
Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin D on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial. Mult Scler Int. 2012;2012:452541.
Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Investig. 2011;40(6):627–39.
Smolders J, Hupperts R, Barkhof F, Grimaldi LM, Holmoy T, Killestein J, et al. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011;311(1–2):44–9.
Hupperts R, Smolders J, Vieth R, Holmøy T, Marhardt K, Schluep M, Killestein J, Barkhof F, Grimaldi LM, Beelke M. High dose cholecalciferol (vitamin D3) oil as add-on therapy in subjects with relapsing-remitting multiple sclerosis (RRMS) receiving subcutaneous interferon β-1a (scIFNβ-1a). Neurology. 2017;88(16 Suppl):S44.005.
Camu W, Pierrot-Deseilligny C, Hautecoeur P, Besserve A, Deleglise A-SJ, Lehert P, Souberbielle J-C. Cholecalciferol supplementation in relapsing multiple sclerosis patients treated with subcutaneous interferon beta-1a: a randomized controlled trial. Neurology. 2017;88(16 Suppl):S44.004.
Dorr J, Ohlraun S, Skarabis H, Paul F. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized controlled trial. Trials. 2012;13:15.
Bhargava P, Cassard S, Steele SU, Azevedo C, Pelletier D, Sugar EA, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials. 2014;39(2):288–93.
Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–64.
Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9(11):e111265.
Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54.
Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74(23):1852–9.
Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S–6S.
Pham TM, Ekwaru JP, Loehr SA, Veugelers PJ. The relationship of serum 25-hydroxyvitamin D and insulin resistance among nondiabetic Canadians: a longitudinal analysis of participants of a preventive health program. PLoS One. 2015;10(10):e0141081.
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11.
Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, Qi D, Patel AV, Helzlsouer KJ, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81–93.
Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37.
Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–55.
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bradshaw, M.J., Holick, M.F., Stankiewicz, J.M. (2020). Vitamin D and Multiple Sclerosis. In: Rizvi, S., Cahill, J., Coyle, P. (eds) Clinical Neuroimmunology. Current Clinical Neurology. Humana, Cham. https://doi.org/10.1007/978-3-030-24436-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-24436-1_10
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-24435-4
Online ISBN: 978-3-030-24436-1
eBook Packages: MedicineMedicine (R0)