Abstract
Vitamin D deficiency is common in multiple sclerosis (MS) patients. This review explores the potential benefits and limitations of high-dose vitamin D supplementation in MS management. We reviewed relevant literature on the effects of high-dose vitamin D supplementation on relapse rates, disability progression, quality of life, and MRI markers of disease activity in MS patients. Additionally, we discussed the mechanisms by which vitamin D might influence MS, potential adverse effects, and future research directions. Studies suggest that high-dose vitamin D supplementation may reduce relapse rates and improve MRI markers of disease activity in MS. However, the evidence for its impact on disability progression and quality of life remains inconclusive. Vitamin D’s immunomodulatory properties are well-documented, and its potential for neuroprotection and neurogenesis warrants further investigation. High-dose vitamin D supplementation holds promise as a complementary or disease-modifying therapy for MS. However, further robust research is required to solidify its role in clinical practice. Exploring vitamin D’s multifaceted effects on the immune system, neuroprotection, and neurogenesis paves the way for novel therapeutic strategies to improve the lives of individuals with MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiple sclerosis (MS) is a chronic, inflammatory demyelinating disease of the central nervous system (CNS) characterized by autoimmune attacks on the myelin sheath [1]. This demyelination disrupts nerve impulses, leading to a wide range of symptoms that can vary depending on the affected areas of the brain and spinal cord [2]. The exact cause of MS remains unknown, but it is believed to be a complex interplay between genetic predisposition and environmental factors. Affecting over 2.8 million individuals worldwide, MS is the leading cause of non-traumatic neurological disability in young adults [2]. The disease course is highly variable, with symptoms ranging from mild fatigue and muscle weakness to severe paralysis, vision problems, and cognitive impairment [3]. The significant burden of MS on individuals and healthcare systems shows the urgent need for effective therapeutic strategies to manage disease progression and improve quality of life.
Vitamin D, a fat-soluble vitamin essential for bone health and calcium homeostasis, has emerged as a potential player in the immunomodulatory landscape [4]. Vitamin D exerts its effects through interaction with the vitamin D receptor (VDR), a ligand-activated transcription factor expressed in various immune cells [5]. Studies suggest that vitamin D can modulate immune function by suppressing T cell proliferation, promoting regulatory T cell activity, and influencing the production of inflammatory cytokines [6, 7]. This immunomodulatory potential has sparked interest in exploring the role of vitamin D supplementation in MS, a disease with a well-established autoimmune component. This narrative review aims to examine the current evidence on the impact of high-dose vitamin D supplementation on MS.
2 Methodology
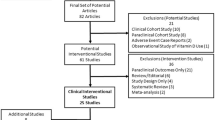
We searched PubMed, Google Scholar, Cochrane Library, and ScienceDirect, wielding a combination of keywords like “vitamin D,” “high-dose supplementation,” “multiple sclerosis,” and crucial outcomes like “relapses” and “disability progression.” (Fig. 1). Additionally, we employed the precision of Boolean operators (AND, OR, NOT). Our search focused on articles from 2000 to March 2024. Only studies investigating the effects of high-dose vitamin D supplementation (exceeding recommended daily intake) on people with MS, and employing human study designs (observational studies or randomized controlled trials), qualified for inclusion. Studies solely focused on vitamin D deficiency or insufficiency, or those utilizing animal models or in vitro experiments, were excluded.
Two independent reviewers (N.A and A.E.B) searched through titles and abstracts. Studies deemed potentially relevant were then retrieved for a deeper examination. Once the chosen studies were identified, we embarked on the critical task of data extraction. Due to the anticipated diversity in study designs, outcomes measured, and participant demographics, we opted for a narrative synthesis approach. This approach allows us to weave together the findings from both observational and RCT studies, critically evaluating their strengths and weaknesses.
3 Mechanisms of action of vitamin D in multiple sclerosis
Several potential mechanisms by which vitamin D might impact MS are under investigation. Table 1. Vitamin D’s influence on MS pathogenesis likely hinges on its interaction with the vitamin D receptor (VDR), a protein expressed in various immune cells [8,9,10]. VDR acts as a molecular switch, and when vitamin D binds to it, a cascade of cellular responses is triggered [11, 12]. These responses can modulate the immune system in several ways, potentially impacting MS development and progression [13]. One key area of influence is the regulation of T cells, the foot soldiers of the adaptive immune system [14]. Studies suggest that vitamin D-VDR interaction can suppress the proliferation and activity of Th1 and Th17 cells [15, 16]. These T cell subsets are known to play a central role in driving autoimmune responses [16]. Vitamin D might offer a potential shield against the immune system’s attack on the myelin sheath in MS [17]. It achieves this by dampening the activity of specific T cells involved in the autoimmune response [18].
In contrast, vitamin D appears to support regulatory T cells (Tregs) [19]. These specialized T cells act as immune system moderators, actively suppressing the activity of other T cells and promoting immune tolerance [20]. Vitamin D supplementation holds promise for reducing the inflammatory response in MS by enhancing Treg function [21]. Tregs are regulatory T cells that act like immune system moderators, and boosting their activity could create a more balanced immune environment, potentially lessening the inflammation that characterizes MS [22]. Cytokines are signalling molecules that orchestrate communication between immune cells [23]. In MS, the production of pro-inflammatory cytokines, such as interferon-gamma and interleukin-17, is elevated [24]. These cytokines further activate immune cells and exacerbate inflammation. Evidence suggests that vitamin D might influence the production of these pro-inflammatory cytokines, potentially leading to a less inflammatory cytokine profile [25,26,27]. This shift in the cytokine landscape could contribute to a more subdued immune response in MS.
Epidemiological studies have observed a fascinating trend: individuals residing in regions with less sunlight exposure, and consequently lower vitamin D synthesis, tend to exhibit a higher prevalence of MS [28]. This geographic correlation, while not establishing a direct cause-and-effect relationship, certainly warrants further investigation. As discussed earlier, vitamin D possesses immunomodulatory properties [29, 30]. Low vitamin D levels might lead to a state of immune dysregulation, characterized by an imbalance between pro-inflammatory and anti-inflammatory responses. This dysregulation could create a more susceptible environment for the development of autoimmune diseases like MS.
Sunlight exposure is the primary source of vitamin D synthesis in humans. Geographic regions with less sunshine may have a higher population burden of vitamin D deficiency [31]. However, these regions might also share other environmental factors, such as dietary habits or exposure to specific infectious agents, that could potentially contribute to MS risk [32]. Therefore, disentangling the independent effect of vitamin D deficiency from other environmental factors remains a challenge. Vitamin D’s immunomodulatory effects might also influence Epstein–Barr virus (EBV), a virus linked to MS development [33]. Vitamin D suppresses EBV replication and reactivation, potentially reducing its contribution to the autoimmune response in MS. However, further research is needed to understand how VDR polymorphisms might influence this specific mechanism [34].
The ability of vitamin D to modulate various aspects of the immune system, particularly those relevant to autoimmune responses, provides a compelling rationale for exploring its potential therapeutic role in MS [33]. While the exact mechanisms by which vitamin D might impact MS remain under investigation, these initial findings offer a promising avenue for future research [34].
4 Clinical evidence: high-dose vitamin D supplementation and MS outcomes
Mounting evidence explores the potential of high-dose vitamin D supplementation as a therapeutic strategy in MS management (Table 2).
4.1 Relapse rates
Several RCTs have yielded promising results regarding the impact of high-dose vitamin D supplementation on relapse rates in MS patients. Sotirchos et al. conducted a trial with 40 relapsing–remitting MS patients [1]. Their findings demonstrated that high-dose vitamin D3 supplementation (10,400 IU/day) significantly reduced annualized relapse rates compared to placebo (0.19 vs 0.41, p = 0.04) over an 18-month treatment period [1]. Similarly, Golan et al. reported a statistically significant association between high-dose vitamin D3 supplementation (20,000 IU/week) and a lower proportion of patients experiencing relapses compared to placebo (24% vs 37%, p = 0.03) in a study involving 229 patients over 96 weeks [19]. Supporting these observations, Essa et al. observed a significant decrease in annualized relapse rates with high-dose vitamin D3 (10,000 IU/day) compared to placebo (0.17 vs 0.41, p < 0.001) over 24 months [18].
However, some studies have yielded contradictory results. Hupperts et al. and Kampman et al. did not detect any statistically significant differences in relapse rates between high-dose vitamin D3 supplementation (14,000 IU/day and 20,000 IU/week, respectively) and placebo groups [20, 22]. These inconsistencies highlight the need for further investigation to reconcile these findings and determine optimal dosages and treatment durations for high-dose vitamin D supplementation in reducing relapse rates for MS patients.
4.2 Disability progression
The evidence regarding vitamin D’s impact on disability progression in MS remains inconclusive. Essa et al. did not observe any significant differences in Expanded Disability Status Scale (EDSS) scores between the high-dose vitamin D3 and placebo groups [18]. Similarly, studies by Hupperts et al., Camu et al., and Kampman et al. reported no significant effect of high-dose vitamin D supplementation on disability progression as measured by EDSS scores [4, 20, 23]. Additionally, Dörr et al. did not detect a significant impact of high-dose vitamin D3 (20,000 IU/day) on EDSS scores over 96 weeks in their study with 229 patients [21]. These findings suggest that high-dose vitamin D supplementation might not significantly alter the course of disability progression in MS.
4.3 MRI outcomes
Several studies have assessed the effects of vitamin D on MRI markers of disease activity. Camu et al. found that high-dose vitamin D3 (100,000 IU/month) was associated with a lower number of new gadolinium-enhancing lesions compared to placebo (0.1 vs 0.6, p = 0.03) [4]. Similarly, Grimaldi et al. reported a significant reduction in new T2 lesions with high-dose vitamin D3 (20,000 IU/day) versus placebo (1.5 vs 4.1, p < 0.001) [24]. These findings suggest the potential benefits of high-dose vitamin D supplementation in reducing MRI activity, which might correlate with reduced inflammatory processes in the central nervous system. However, Hupperts et al. did not detect any significant differences in MRI outcomes between the high-dose vitamin D3 and placebo groups [20]. Furthermore, a trial by Stein et al. found that high-dose vitamin D3 (10,400 IU/day) significantly reduced new gadolinium-enhancing lesions compared to placebo (0.3 vs 1.5, p < 0.001) in 40 patients over 18 months [12]. These inconsistencies warrant further investigation to clarify the role of high-dose vitamin D on MRI outcomes in MS.
4.4 Quality of life
The impact of high-dose vitamin D supplementation on quality of life in MS patients remains a relatively unexplored area. While some RCTs have investigated this aspect, the evidence must be more conclusive. For instance, a trial by Rolf et al. involving 229 patients with MS found no significant difference in Multiple Sclerosis Impact Scale (MSIS-29) scores between the high-dose vitamin D3 (20,000 IU/week) and placebo groups over 96 weeks [25]. Similarly, Kampman et al. conducted a trial that evaluated the impact of vitamin D supplementation on quality-of-life measures but did not observe any significant differences between the high-dose vitamin D3 and placebo groups [23].
4.5 Factors influencing vitamin D status and response
Several factors influence a person’s vitamin D status and response to supplementation. Sunlight exposure remains the primary source of vitamin D for most individuals [26]. During sun exposure, ultraviolet B (UVB) radiation triggers the conversion of 7-dehydrocholesterol in the skin to previtamin D3, which isomerizes into vitamin D3 [26]. The amount of previtamin D synthesized depends on the duration and surface area of exposed skin. However, recommendations for sun exposure caution against overexposure and its associated risks. Optimal sun exposure involves short periods (5–30 min) most days of the week, without sunscreen, as sunscreens with SPF 8 or higher can significantly reduce UVB penetration and vitamin D synthesis [26].
Dietary sources contribute to vitamin D status, although to a lesser extent than sunlight exposure. Fatty fish, fish liver oil, and egg yolks are naturally rich in vitamin D [27]. Additionally, some food products are fortified with vitamin D, such as milk in the United States [27]. The recommended dietary allowance (RDA) for vitamin D in adults (19 years and older) is 600 IU daily for both men and women. This recommendation increases to 800 IU daily for adults over 70 years old [28]. In addition, immunomodulatory drugs, commonly used to manage autoimmune diseases like MS, can influence vitamin D status. These medications, such as azathioprine, methotrexate, and cyclophosphamide, modulate the immune system by altering antibody production [29]. While no interactions have been identified between azathioprine and vitamin D3 [30], other immunomodulatory drugs can affect vitamin D levels. For instance, cyclophosphamide, a chemotherapy drug sometimes used for MS, can increase the risk of severe vitamin D deficiency by accelerating vitamin D catabolism (breakdown) [31].
Variations in an individual’s genetic makeup, known as polymorphisms, can influence vitamin D status. Polymorphisms within specific genes can affect vitamin D metabolism. CYP24A1 is a gene that encodes an enzyme responsible for the hydroxylation (modification) of the active form of vitamin D (1,25-dihydroxyvitamin D3) [33]. While variations in CYP24A1 are believed to influence vitamin D levels, the exact relationship requires further investigation [34]. Also, certain medical conditions can predispose individuals to vitamin D deficiency or worsen existing deficiencies. Obesity is associated with lower vitamin D levels because the fat tissue in obese individuals acts as a reservoir for vitamin D, leading to lower serum (blood) concentrations [35]. Additionally, an inverse relationship exists between vitamin D levels and both systolic blood pressure and blood glucose levels. This means that higher blood pressure and blood sugar are associated with lower serum vitamin D concentrations. Notably, pre-diabetes may also be linked to vitamin D deficiency [35].
4.6 Optimal dosing strategies for achieving therapeutic vitamin D levels in MS
While vitamin D supplementation can be a valuable tool for managing MS, it is not a one-size-fits-all approach. Generally, older adults and those with underlying health conditions like obesity, high blood pressure, or pre-diabetes are more likely to benefit from supplementation due to their increased risk of vitamin D deficiency. Additionally, people on long-term steroids or with a history of fractures may also require supplementation to support bone health. However, there are some situations where vitamin D supplementation can be harmful. Individuals with hypercalcemia (excessively high blood calcium levels), hypervitaminosis D (vitamin D toxicity), impaired kidney function, or malabsorption syndrome (difficulty absorbing nutrients) should not take vitamin D supplements without close medical supervision.
The goal of supplementation is to achieve a therapeutic level of vitamin D in the bloodstream, typically considered above 75 nmol/L for MS patients. Doctors may initially recommend a high loading dose, such as 50,000 IU per week for up to 12 weeks, to rapidly reach this target level. Once achieved, a lower maintenance dose (usually between 2000 and 5000 IU daily) is typically prescribed for long-term management [36]. The Multiple Sclerosis Clinic of Canada suggests a more cost-effective approach: starting with a daily dose of 2000 IU for 4 months and then increasing to 3000 IU daily upon reaching the desired vitamin D level [37, 38].
4.7 Adverse effects and safety considerations
Certain patient populations with underlying diseases such as lymphoma and granulomatous disorders may be more susceptible to vitamin D toxicity [39]. Toxicity from excessive cholecalciferol (vitamin D3) supplementation is primarily characterized by hypercalcemia (elevated blood calcium), hypercalciuria (excessive calcium in the urine), elevated serum 25(OH)D levels (> 150 ng/mL or > 375 nmol/L), and normal or slightly increased 1,25(OH)2D concentrations [40]. The vast array of clinical manifestations associated with vitamin D overdose is largely attributable to hypercalcemia and can affect various organ systems [39, 40]. Known cardiovascular adverse effects include hypertension, shortened QT interval (ECG abnormality), ST-segment elevation (ECG abnormality), and bradyarrhythmia with first-degree heart block. Hypercalciuria is the earliest detectable renal manifestation, but polyuria (excessive urination), nephrocalcinosis (calcium deposits in the kidneys), and renal failure can also occur. Other reported symptoms of vitamin D toxicity caused by hypercalcemia include hearing loss, band keratopathy (corneal deposits), and painful periarticular calcinosis (calcium deposits around joints) [40]. A recent concern suggests that uncontrolled intake of ultra-high doses of vitamin D may mimic the progression of primary progressive MS, potentially causing a delay in diagnosis until the side effects become irreversible or even fatal [41]. This highlights the need for further research on the safety of high-dose vitamin D supplementation (exceeding 1000 IU/kg/day) in the context of MS management [42].
5 Clinical implications and future directions
Vitamin D plays a critical role in regulating the immune system, particularly by influencing T cell and dendritic cell function [43]. In MS, the disease pathology is driven by dysregulated immune responses targeting myelin antigens, the protective sheath around nerve fibres [44]. Several studies suggest a link between vitamin D deficiency and an increased risk of developing MS, as well as a more aggressive disease course [44]. Supplementation with vitamin D holds promise for modulating this immune dysregulation in MS patients.
Vitamin D’s potential extends beyond immunomodulation. Experimental evidence suggests its involvement in neuroprotection and neurogenesis, the generation of new neurons [45]. Studies have shown that vitamin D can promote remyelination (repair of damaged myelin), safeguard neurons from oxidative stress, and influence neurotrophic factors that support nerve cell growth and survival [45]. Given the progressive neurodegeneration characteristic of MS, these neuroprotective properties of vitamin D are particularly intriguing for long-term disease management.
Furthermore, vitamin D is a fat-soluble vitamin with well-established immunomodulatory properties [3]. It exerts these effects through binding to the Vitamin D Receptor (VDR) expressed on immune cells [4]. Polymorphisms potentially affect its function and how it interacts with vitamin D. These VDR polymorphisms can influence how individuals with MS utilize vitamin D. Some polymorphisms decrease the VDR’s ability to bind vitamin D, reducing its effectiveness in modulating the immune system in MS. For instance, the Taq I (T/C) polymorphism has been linked to a weaker response to vitamin D [46]. Studies suggest a potential link between VDR polymorphisms and MS risk and progression [47, 48]. Certain polymorphisms, like FokI (C/T) variations, are associated with increased MS susceptibility [49]. Additionally, some research suggests that individuals with specific VDR genotypes respond better to vitamin D supplementation in terms of reducing relapse rates [50, 51].
MS patients grapple with a variety of symptoms such as fatigue, spasticity, and cognitive decline, significantly impacting their quality of life [46]. While disease-modifying therapies (DMTs) target the underlying disease process, managing symptoms remains a crucial aspect of MS care [46]. The potential of vitamin D supplementation to alleviate some of these symptoms has been investigated, although current evidence is limited and inconclusive.
The current standard of care for MS includes DMTs like interferon-beta, glatiramer acetate, and newer monoclonal antibodies [47]. While DMTs are crucial for managing MS, some have unintended consequences on vitamin D levels or its metabolism in the body. For example, medications like glatiramer acetate (Copaxone) decrease intestinal absorption of vitamin D, while medications like interferon beta-1a (Avonex) affect enzymes involved in vitamin D metabolism [52]. Existing research suggests a potential interaction between DMTs and vitamin D levels in MS patients. Studies have shown that some DMTs, like fingolimod (Gilenya), may be associated with a decrease in serum vitamin D levels [33]. Due to this potential impact, it is crucial for MS patients receiving DMTs to have regular monitoring of their vitamin D levels. Early detection and treatment of vitamin D deficiency can help ensure optimal health outcomes.
Moreover, these medications have limitations, including variable effectiveness, side effects, and high costs [47]. Vitamin D supplementation has been proposed as an adjunctive therapy to complement existing treatments and potentially enhance their efficacy [43]. Preclinical and clinical data suggest that vitamin D might work synergistically with DMTs to modulate immune responses and improve treatment outcomes [43]. Beyond its adjunctive role, vitamin D supplementation is being explored as a potential disease-modifying intervention in MS [43]. While current DMTs primarily target the immune system, vitamin D’s neuroprotective effects offer a unique opportunity to address the neurodegenerative aspect of the disease [45]. Additionally, the favourable safety profile and affordability of vitamin D make it an attractive candidate for long-term use as a disease-modifying agent [45].
Despite promising findings from observational studies and smaller clinical trials, there is a lack of strong evidence from well-designed randomized controlled trials (RCTs) [46]. Many existing studies have limitations such as small sample sizes, short follow-up periods, and heterogeneity in patient populations and vitamin D dosing regimens [46]. Large-scale RCTs with long-term follow-up are necessary to definitively assess the efficacy and safety of vitamin D supplementation in MS management.
The optimal dosing regimen for vitamin D supplementation in MS remains unclear [46]. While higher doses might be necessary to achieve therapeutic effects, concerns regarding potential toxicity and hypercalcemia exist [45]. Additionally, the optimal treatment duration to observe significant clinical benefits is not well-established [45]. Future research should focus on establishing individualized dosing strategies based on patient characteristics and disease stages.
MS is a heterogeneous disease with patients exhibiting variability in clinical presentation, disease course, and response to treatment [43]. Personalized approaches to therapy, including vitamin D supplementation, hold promise for optimizing treatment outcomes [43]. Genetic, environmental, and clinical factors may influence individual responses to vitamin D, necessitating personalized dosing strategies [45]. Identifying biomarkers that predict treatment response could help determine which patients are most likely to benefit from vitamin D supplementation.
The review explores various aspects of vitamin D supplementation in MS, including its impact on disease activity, quality of life, and potential as an adjunctive or disease-modifying therapy. While the review mentions existing clinical trials, it highlights the lack of strong evidence from well-designed RCTs.
6 Conclusion
Mounting evidence suggests a potential role for high-dose vitamin D supplementation in MS management. Studies have shown promising results regarding its impact on relapse rates and MRI markers of disease activity. However, the evidence for its effect on disability progression and quality of life remains inconclusive. While vitamin D’s immunomodulatory properties are well-established, its potential for neuroprotection and neurogenesis opens exciting avenues for further exploration. Despite the encouraging preliminary findings, limitations exist. The current evidence is largely based on observational studies and smaller clinical trials. Well-designed, large-scale RCTs with long-term follow-up are necessary to definitively assess the efficacy and safety of high-dose vitamin D supplementation in MS patients. Furthermore, establishing optimal dosing regimens tailored to individual characteristics and disease stages is crucial. Personalized medicine approaches that consider genetic, environmental, and clinical factors influencing vitamin D response hold promise for optimizing treatment outcomes.
High-dose vitamin D supplementation shows promise as a complementary or disease-modifying therapy for MS. However, further robust research is needed to solidify its role in clinical practice. Continued exploration of vitamin D’s multifaceted effects on the immune system, neuroprotection, and neurogenesis paves the way for the development of novel therapeutic strategies for improving the lives of individuals with MS.
Data availablity
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Abbreviations
- MS:
-
Multiple sclerosis
- EBV:
-
Epstein–Barr virus
- IU:
-
International Unit (of vitamin D)
- UVB:
-
Ultraviolet B (radiation)
- RDA:
-
Recommended dietary allowance
- CYP24A1:
-
Cytochrome P450 family 24 subfamily A member 1 (a gene)
- RCTs:
-
Randomized Controlled Trials
- DMTs:
-
Disease-modifying therapies
References
Shirvani-Farsani Z, Behmanesh M, Mohammadi SM, Naser MA. Vitamin D levels in multiple sclerosis patients: association with TGF-β2, TGF-βRI, and TGF-βRII expression. Life Sci. 2015;134:63–7. https://doi.org/10.1016/j.lfs.2015.05.017. (Epub 2015 May 31).
Seyedebrahimi R, Yang P, Azimzadeh M, Farsani ME, Ababzadeh S, Kalhor N, Sheykhhasan M. Deep learning approaches for early diagnosis of neurodegenerative diseases. Hershey: IGI Global; 2024.
Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, Gocke A, Steinman L, Mowry EM, Calabresi PA. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86(4):382–90. https://doi.org/10.1212/WNL.0000000000002316. (Epub 2015 Dec 30).
Camu W, Lehert P, Pierrot-Deseilligny C, Hautecoeur P, Besserve A, Jean Deleglise AS, Payet M, Thouvenot E, Souberbielle JC. Cholecalciferol in relapsing-remitting MS: a randomized clinical trial (CHOLINE). Neurol Neuroimmunol Neuroinflamm. 2019;6(5): e597. https://doi.org/10.1212/NXI.0000000000000597. (Erratum in: Neurol Neuroimmunol Neuroinflamm. 2019;7(1)).
Åivo J, Hänninen A, Ilonen J, Soilu-Hänninen M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol. 2015;280:12–5. https://doi.org/10.1016/j.jneuroim.2015.01.005. (Epub 2015 Jan 21).
Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–6.
Røsjø E, Steffensen LH, Jørgensen L, Lindstrøm JC, ŠaltytėBenth J, Michelsen AE, Aukrust P, Ueland T, Kampman MT, Torkildsen Ø, Holmøy T. Vitamin D supplementation and systemic inflammation in relapsing-remitting multiple sclerosis. J Neurol. 2015;262(12):2713–21. https://doi.org/10.1007/s00415-015-7902-5. (Epub 2015 Oct 1).
Duan S, Lv Z, Fan X. Vitamin D status and the risk ofmultiple sclerosis: a systematic review and meta-analysis. Neurosci Lett. 2014;570:108–13.
Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72:234–40.
Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-Dihydroxyvitamin D3 selectively and reversibly im-pairs T helper-cell CNS localization. Proc Natl Acad Sci USA. 2013;110:21101–6.
Bhargava P, Gocke A, Calabresi PA. 1,25-Dihydroxyvitamin D3 impairs the differentiation of effector memory T cells in vitro in multiple sclerosis patients and healthy controls. J Neuroimmunol. 2015;279:20–4.
Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, Egan GF, Mitchell PJ, Harrison LC, Butzkueven H, Kilpatrick TJ. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77(17):1611–8. https://doi.org/10.1212/WNL.0b013e3182343274.
Muris AH, Smolders J, Rolf L, Thewissen M, Hupperts R, Damoiseaux J, SOLARIUM study group. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J Neuroimmunol. 2016;300:47–56. https://doi.org/10.1016/j.jneuroim.2016.09.018. (Epub 2016 Oct 3).
Burton JM, Kimball S, Vieth RA. Phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74(23):1852–9.
Thouvenot E, et al. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur J Neurol. 2015;22:564–9.
Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. NeuroImmunoModulation. 2015;22(6):400–4. https://doi.org/10.1159/000439278. (Epub 2015 Sep 25).
Pakpoor J, Ramagopalan S. Evidence for an association between vitamin D and multiple sclerosis. Curr Top Behav Neurosci. 2015;26:105–15. https://doi.org/10.1007/7854_2014_358.
Holmøy T, Røsjø E, Zetterberg H, Blennow K, Lindstrøm JC, Steffensen LH, et al. Vitamin D supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol Scand. 2018;139(2):172–6.
Golan D, Halhal B, Glass-Marmor L, Staun-Ram E, Rozenberg O, Lavi I, et al. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-β: a randomized controlled trial assessing the effect on flu-like symptoms and immunoregulatory responses. BMC Neurol. 2013;13:60.
Essa S, Falah M, Alkharashi A, Alrasheed H, Curtius D, Bakhiet M. Effect of high-dose vitamin D supplementation on relapse rate and disability progression in patients with relapsing-remitting multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord. 2023;71: 103627.
Dörr J, Bäcker-Koduah P, Wernecke KD, Becker E, Hoffmann F, Faiss J, et al. High-dose vitamin D supplementation in multiple sclerosis—results from the randomized EVIDIMS trial. Mult Scler. 2020;26(2):179–90.
Hupperts R, Smolders J, Vieth R, Holmøy T, Marhardt K, Schluep M, et al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology. 2019;93(20):e1906–16.
Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomized controlled trial. Mult Scler. 2012;18(8):1144–51.
Grimaldi LM, Palmeri B, Salemi G, Sangiorgi S, Parrinello G, Laudanna C, et al. High-dose vitamin D3 supplementation in relapsing-remitting multiple sclerosis. A randomized, placebo-controlled trial. J Neurol Sci. 2015;348(1–2):80–4.
Rolf L, Muris AH, Hupperts R, Damoiseaux J. Vitamin D effects on B cell function in autoimmunity. Ann N Y Acad Sci. 2014;1317:84–91.
Srivastava SB. Vitamin D: do we need more than sunshine? Am J Lifestyle Med. 2021;15(4):397–401.
Ross AC, Taylor CL, Yaktine AL. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
Boston 677 HA, Ma 02115 +1495‑1000. Vitamin D. The nutrition source. 2012. https://www.hsph.harvard.edu/nutritionsource/vitamin-d/#:~:text=RDA%3A%20The%20Recommended%20Dietary%20Allowance.
Avorn J. Learning about the safety of drugs—a half-century of evolution. N Engl J Med. 2011;365(23):2151–3.
Schäffler H, Schmidt M, Huth A, Reiner J, Glass Ä, Lamprecht G. Clinical factors are associated with vitamin D levels in IBD patients: a retrospective analysis. J Dig Dis. 2018;19(1):24–32.
Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2008;24(2):219–24.
Gunter C. Polymorphism. Genome.gov. https://www.genome.gov/genetics-glossary/Polymorphism#:~:text=Polymorphism%2C%20as%20related%20to%20genomics.
Munger K, Ascherio A. Understanding the joint effects of EBV and vitamin D in MS. Mult Scler. 2013;19(12):1554–5. https://doi.org/10.1177/1352458513503723.
Disanto G, Handel AE, Damoiseaux J, Hupperts R, Giovannoni G, Smolders J, Ramagopalan SV. Vitamin D supplementation and antibodies against the Epstein–Barr virus in multiple sclerosis patients. Mult Scler. 2013;19(12):1679–80. https://doi.org/10.1177/1352458513494494. (Epub 2013 Jul 4).
Al Zarooni AAR, Al Marzouqi FI, Al Darmaki SH, Prinsloo EAM, Nagelkerke N. Prevalence of vitamin D deficiency and associated comorbidities among Abu Dhabi Emirates population. BMC Res Notes. 2019;12(1):1–6.
Pludowski P, Grant WB, Karras SN, Zittermann A, Pilz S. Vitamin D supplementation: a review of the evidence arguing for a daily dose of 2000 international units (50 µg) of vitamin D for adults in the general population. Nutrients. 2024;16(3):391.
Vitamin D Choices booklet. MS-UK. https://ms-uk.org/vitamin-d-and-multiple-sclerosis-choices-booklet/. Accessed 9 Apr 2024.
Vitamin D might improve MS symptoms. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/expert-answers/vitamin-d-and-ms/faq-20058258#:~:text=Research%20studies%20have%20shown%20that.
Janoušek J, Pilařová V, Macáková K, Nomura A, Veiga-Matos J, Silva DDD, Remião F, Saso L, Malá-Ládová K, Malý J, Nováková L, Mladěnka P. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit Rev Clin Lab Sci. 2022;59(8):517–54. https://doi.org/10.1080/10408363.2022.2070595.
Feige J, Moser T, Bieler L, Schwenker K, Hauer L, Sellner J. Vitamin D supplementation in multiple sclerosis: a critical analysis of potentials and threats. Nutrients. 2020;12(3):783. https://doi.org/10.3390/nu12030783.
Feige J, Salmhofer H, Hecker C, Kunz AB, Franzen M, More E, Sellner J. Life-threatening vitamin D intoxication due to intake of ultra-high doses in multiple sclerosis: a note of caution. Mult Scler. 2019;25:1326–8.
Gandhi F, Jhaveri S, Avanthika C, Singh A, Jain N, Gulraiz A, Shah P, Nasir F. Impact of vitamin D supplementation on multiple sclerosis. Cureus. 2021;13(10): e18487. https://doi.org/10.7759/cureus.18487.
Smolders J, Torkildsen Ø, Camu W, Holmøy T, Demir A. Vitamin D in multiple sclerosis: trends, updates, and challenges. Mult Scler J. 2020;26(2):181–92.
Ascherio A, Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2021;41(03):259–67.
Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. J Intern Med. 2020;287(6):680–95.
Imani D, Razi B, Motallebnezhad M, Rezaei R. Association between vitamin D receptor (VDR) polymorphisms and the risk of multiple sclerosis (MS): an updated meta-analysis. BMC Neurol. 2019;19(1):339. https://doi.org/10.1186/s12883-019-1577-y.
Zhang YJ, Zhang L, Chen SY, Yang GJ, Huang XL, Duan Y, Yang LJ, Ye DQ, Wang J. Association between VDR polymorphisms and multiple sclerosis: systematic review and updated meta-analysis of case–control studies. Neurol Sci. 2018;39(2):225–34. https://doi.org/10.1007/s10072-017-3175-3. (Epub 2017 Nov 6).
Guerini FR, Agliardi C, Oreni L, Groppo E, Bolognesi E, Zanzottera M, Caputo D, Rovaris M, Clerici M. Vitamin D receptor gene polymorphism predicts the outcome of multidisciplinary rehabilitation in multiple sclerosis patients. Int J Mol Sci. 2023;24(17):13379. https://doi.org/10.3390/ijms241713379.
CancelaDíez B, Pérez-Ramírez C, Maldonado-Montoro MDM, Carrasco-Campos MI, Sánchez Martín A, Pineda Lancheros LE, Martínez-Martínez F, Calleja-Hernández MÁ, Ramírez-Tortosa MC, Jiménez-Morales A. Association between polymorphisms in the vitamin D receptor and susceptibility to multiple sclerosis. Pharmacogenet Genom. 2021;31(2):40–7. https://doi.org/10.1097/FPC.0000000000000420.
Hosni HA, Fouad AM, Ibrahim NW, et al. Investigating the role of VDR gene variants in multiple sclerosis susceptibility: a case–control study in Egypt. Egypt J Neurol Psychiatry Neurosurg. 2024;60:51. https://doi.org/10.1186/s41983-024-00794-z.
Tizaoui K, Kaabachi W, Hamzaoui A, et al. Association between vitamin D receptor polymorphisms and multiple sclerosis: systematic review and meta-analysis of case–control studies. Cell Mol Immunol. 2015;12:243–52. https://doi.org/10.1038/cmi.2014.47.
Salari M, Farajzadegan Z, Sadeghi K. Vitamin D therapy in patients with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci. 2019;400:228–35.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
NA conceptualised the study; all authors were involved in the literature review; NA and A.E.B extracted the data from the reviewed studies; all authors wrote the final and first drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aderinto, N., Olatunji, G., Kokori, E. et al. High-dose vitamin D supplementation in multiple sclerosis: a systematic review of clinical effects and future directions. Discov Med 1, 12 (2024). https://doi.org/10.1007/s44337-024-00023-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44337-024-00023-9