Abstract
The diagnosis of leptomeningeal metastasis (LM) is based on a clinical assessment, cerebrospinal magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) cytological analysis. The diagnosis can be challenging since LM can cause a variety of neurological symptoms and signs and since there are multiple differential diagnoses to consider as a cause of neurological problems in patients that are often in advanced stages of the disease. The EANO ESMO guideline defines the diagnosis of LM as confirmed if malignant cells are found in the CSF or by biopsy. The diagnosis is probable in the case of typical clinical symptoms and signs and typical neuroimaging findings in patients with metastatic cancer. Repeated assessments may be necessary if the diagnosis of LM remains doubtful. The diagnostic role of novel molecular liquid biopsy markers is under evaluation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Leptomeningeal metastasis (LM) is defined as the spread of malignant cells in the subarachnoid space and in the leptomeninges. It is sometimes denoted as carcinomatous meningitis, in case of carcinoma, or neoplastic meningitis, but this term is misleading since it suggests a disorder that is primarily of inflammatory origin. LM may be observed in approximately 10% of patients with metastatic cancer [1].
The risk of experiencing LM in the course of systemic cancer today is probably higher than that figure, given that patients survive much longer, that diagnostic approaches have changed dramatically with the introduction of magnetic resonance imaging (MRI) and advanced cytology and even liquid biopsy techniques to detect cancer cells in the cerebrospinal fluid (CSF), and that the cerebrospinal compartment may be more difficult to control using systemic therapies than other body compartments. In up to 70%, the diagnosis of LM is made in the context of systemic disease progression. Breast cancer, lung cancer and melanoma are the three main causes of LM.
The median survival is limited to a few months and once neurological signs are present, they are fixed and rarely improved by therapeutic interventions. Thus, the diagnosis should be made as soon as possible in case of suspicion of LM in order to prevent neurological deterioration. The diagnosis is based on clinical evaluation, cerebrospinal MRI and CSF analysis [2].
2 Risk Factors
Risk factors for LM include an opening of the ventricular system during brain metastasis surgery, resection of cerebellar metastases especially when using a piece-meal resection [3,4,5,6,7,8] and primary tumor-related factors. In breast cancer patients, lobular subtype and triple negative status (absence of estrogen receptors, absence of progesterone receptors, absence of HER2 expression) have been reported as risk factors of LM [9]. HER2 overexpression alone has been shown to be a risk factor of brain metastases; however, its role as a risk factor of LM is less clear. In lung cancer patients, EGFR mutation has been reported as being a risk factor of LM in a large retrospective cohort of 5387 non-small-cell lung (NSCLC) patients, where 184 cases of LM were identified [10]. The role of other driver mutations for LM risk has not been defined.
Only limited data are available on melanoma LM patients, and no risk factor has been identified.
3 Clinical Presentation
Symptoms and signs depend on the neuroanatomical regions involved by LM and are often multifocal. Headache, nausea and vomiting, mental changes, gait difficulties, cranial nerve palsies, sensori-motor deficits, cauda equina syndrome, and radicular and back pain, depending on the distribution of tumor cells in the CNS, are considered typical signs of LM [2]. The clinical presentation can be subtle with discrete and isolated symptoms and signs. Thus, a detailed clinical evaluation is required at diagnosis and during follow-up. Symptoms and signs of LM should be differentiated from those related to concomitant brain metastases and neurological complications of the cancer and its treatment. A standardized scorecard has been proposed by the RANO group [11], but it has not been validated yet.
4 Radiological Presentation
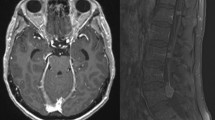
LM may be a diffuse disease of the entire central nervous system and cerebrospinal imaging is thus required for the staging of LM [2, 12]. Cranial computed tomography (CT) should be performed only in patients with contra-indications to MRI and has its limitations in particular for the assessment of the spinal cord. The radiologic assessment of LM can be challenging. Some technical aspects should be considered when evaluating LM patients, such as slice positioning and slice thickness, and time interval between injection of contrast agent and acquisition of images. Contrast agent should be injected 10 min before image acquisition and the slice thickness should be 1 mm or less in the brain and 3 mm or less for the spinal cord [2]. Lumbar punctures should be performed after MRI since they may induce a meningeal enhancement. The most sensitive sequence for the detection of LM is the contrast-enhanced T1-weighted sequence [13, 14]. The follow-up should be performed on the same device or on an MRI scanner with identical field strength.
Typical MRI findings include linear or nodular leptomeningeal enhancement on the leptomeninges. These findings can be observed at sulcal, ependymal, cranial nerve or cauda equina levels. Communicating hydrocephalus can also be observed in LM because of poor CSF resorption. Differential diagnosis includes focal dural enhancement after surgery, pachymeningitis, meningioma en plaque, brain metastases, CNS vasculitis, Moyamoya disease, neuro-sarcoidosis, and various inflammatory and infectious diseases.
A scorecard to rate neuroimaging findings in LM has been proposed by the RANO group, but this has not been validated and is therefore currently under revision.
The radiological presentation of LM help to guide clinical decision making. Four subtypes have been delineated in the EANO ESMO guidelines [2]: A, diffuse linear leptomeningeal disease, B nodular leptomeningeal disease, C a combination of A and B, and D no focal lesions, but potentially hydrocephalus (see Table 14.1).
Parenchymal brain metastases are associated with LM in 31–66% of patients with breast cancer [15,16,17,18,19,20,21,22,23], 56–82% of patients with lung cancer [24,25,26,27,28,29,30] and 57–87% of patients with melanoma [31,32,33].
18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET-CT) is not helpful for the diagnosis or follow-up of LM. CSF flow studies using 111indium-DTPA or 99technetium macro-aggregated albumin have been recommended in candidates for intra-CSF pharmacotherapy if CSF flow blocks are suspected.
5 CSF Cytology
Indirect, but non-diagnostic pathological findings are frequently observed in the CSF of LM patients. An increased opening pressure (>200 mm H2O) is noted in 21–42% [28, 34], high protein levels (>50 mg/dL) in 56–91% [16, 21, 28, 34, 35], decreased glucose levels (<60 mg/dL) in 22–63% [21, 28, 34, 35] and increased leukocyte counts (>4/mm3) in 48–77.5% of the patients [21, 28, 34, 35].
CSF standard cytology is the gold standard to confirm the diagnosis of LM. The identification of malignant cells in the CSF during standard CSF cytology confirms the diagnosis of LM. The CSF should be considered as negative only in the unequivocal absence of tumor cells. In the presence of suspicious or atypical cells, the CSF should be reported as equivocal.
The sensitivity of standard cytology is moderate to low. Simple measures should be taken to facilitate the detection of malignant cells in the CSF, such as obtaining at least 5 mL of CSF, ideally more than 10 mL, processing the CSF within 30 minutes after sampling and avoiding blood contamination of the CSF [2, 11, 36,37,38]. If the first CSF cytology is negative or equivocal, a second sample should be obtained which reportedly increases the sensitivity to 80%. The usefulness of further CSF samples remains unclear. CSF fixation in dedicated tubes has been shown to increase the diagnostic yield in hematological diseases, but the usefulness of this approach remains to be established for solid tumors [39].
Novel technologies using epithelial cell adhesion molecule (Ep-CAM) antibodies or other tumor-specific antibody-covered magnetic nanoparticles such as high-molecular weight-melanoma-associated antigen/melanoma-associated chondroitin sulfate proteoglycan (HMW-MAA/MCSP) can identify circulating tumor cells and should contribute in the future to a higher sensitivity of detecting malignant cells in the CSF.
The Veridex Cellsearch® assay has been approved by FDA for the detection of tumor cells in peripheral blood [40]. Different adaptations of the technique have been developed for the detection and quantification of tumor cells in the CSF [41,42,43,44,45,46], but no standard has been established until now. Tumor cells can be identified using flow cytometry with fluorescently labelled antibodies against membrane-bound proteins of tumor cells coupled with fluorescence-activated cell sorting (FACS) for the quantification of tumor cells [47, 48].
Cell-free circulating tumor DNA (ctDNA) represents a fraction of total cell-free DNA originating from necrotic and apoptotic cells. Genomic alterations can be detected by micro-arrays [49], digital/real-time polymerase chain reaction (RT-PCR), targeted amplicon sequencing and whole exome sequencing [50,51,52,53]. Analysis of ctDNA in the CSF may help the diagnosis when the standard CSF cytology is negative, detect actionable genomic targets and monitor the response to treatment [54]. CSF ctDNA is probably more sensitive than CSF standard cytology for the detection of LM [55]. However, the detection of ctDNA in the CSF may be caused by concomitant brain parenchymal metastases or by blood contamination during CSF sampling and should be interpreted cautiously for the diagnosis and follow-up of LM [2]. In NSCLC, the determination of EGFR and T790M status at LM diagnosis and during the follow-up can help to guide the therapeutic strategy. Promising results were observed after treatment with osimertinib, an oral third-generation EGFR tyrosine kinase inhibitor that is active in tumors expressing the EGFR T790M resistance mutation [56]. DNA methylation profiling in the CSF represents another promising tool for the diagnosis and the management of LM [57] .
6 Diagnosis of LM
According to EANO ESMO guidelines, the diagnosis of LM can be either confirmed, in the presence of tumor cells in the CSF, or probable, or possible, or there may be lack of evidence [2] (see Table 14.1). Two major criteria define the LM classification: (1) the confirmation of the diagnosis by CSF cytology (confirmed LM, type I) versus not confirmed (type II), and (2) the MRI presentation: linear disease for type A, nodular disease for type B, a combination of both linear and nodular disease for type C and no neuroimaging evidence of LM except hydrocephalus for type D. This classification aims at guiding the therapeutic strategy and requires confirmation in prospective studies.
7 Conclusion
The diagnosis of LM is based on clinical manifestation, cerebrospinal MRI findings and standard CSF cytology and is often challenging. Standardized scorecards should be used for the clinical and imaging follow-up of patients; however, no such scorecard has been validated yet. Characterization of genomic alterations and methylation profiles may improve the sensitivity of CSF analysis in the future.
References
Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–92.
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(suppl_4):iv84–99.
Roelz R, Reinacher P, Jabbarli R, Kraeutle R, Hippchen B, Egger K, et al. Surgical ventricular entry is a key risk factor for leptomeningeal metastasis of high grade gliomas. Sci Rep. 2015;5:17758.
Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg. 2012;116(5):984–93.
Elliott JP, Keles GE, Waite M, Temkin N, Berger MS. Ventricular entry during resection of malignant gliomas: effect on intracranial cerebrospinal fluid tumor dissemination. J Neurosurg. 1994;80(5):834–9.
van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66(2):225–7.
Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: a potentially preventable complication. J Neurooncol. 1997;32(3):215–23.
Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64(4):664–74.. discussion 674–676
Abouharb S, Ensor J, Loghin ME, Katz R, Moulder SL, Esteva FJ, et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014;146(3):477–86.
Li Y-S, Jiang B-Y, Yang J-J, Tu H-Y, Zhou Q, Guo W-B, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2016;11(11):1962–9.
Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro-Oncol. 2017;19:484–92.
Chamberlain M, Soffietti R, Raizer J, Rudà R, Brandsma D, Boogerd W, et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro-Oncol. 2014;16(9):1176–85.
Singh SK, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol. 2002;23(5):817–21.
Mahendru G, Chong V. Meninges in cancer imaging. Cancer Imaging Off Publ Int Cancer Imaging Soc. 2009;9:S14–21.
de Azevedo CRAS, Cruz MRS, Chinen LTD, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–72.
Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol Off J Eur Soc Med Oncol. 2010;21(11):2183–7.
Meattini I, Livi L, Saieva C, Franceschini D, Marrazzo L, Greto D, et al. Prognostic factors and clinical features in patients with leptominengeal metastases from breast cancer: a single center experience. J Chemother Florence Italy. 2012;24(5):279–84.
Lara-Medina F, Crismatt A, Villarreal-Garza C, Alvarado-Miranda A, Flores-Hernández L, González-Pinedo M, et al. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012;18(3):233–41.
Le Rhun E, Taillibert S, Zairi F, Kotecki N, Devos P, Mailliez A, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92.
Torrejón D, Oliveira M, Cortes J, Sanchez-Olle G, Gómez P, Bellet M, et al. Implication of breast cancer phenotype for patients with leptomeningeal carcinomatosis. Breast Edinb Scotl. 2013;22(1):19–23.
Yust-Katz S, Garciarena P, Liu D, Yuan Y, Ibrahim N, Yerushalmi R, et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol. 2013;114(2):229–35.
Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–8.
Niwińska A, Pogoda K, Michalski W, Kunkiel M, Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. 2018;138(1):191–8.
Umemura S, Tsubouchi K, Yoshioka H, Hotta K, Takigawa N, Fujiwara K, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer Amst Neth. 2012;77(1):134–9.
Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2012;7(2):382–5.
Park JH, Kim YJ, Lee J-O, Lee K-W, Kim JH, Bang S-M, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer Amst Neth. 2012;76(3):387–92.
Gwak H-S, Joo J, Kim S, Yoo H, Shin SH, Han J-Y, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2013;8(5):599–605.
Lee SJ, Lee J-I, Nam D-H, Ahn YC, Han JH, Sun J-M, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2013;8(2):185–91.
Riess JW, Nagpal S, Iv M, Zeineh M, Gubens MA, Ramchandran K, et al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer. 2014;15(3):202–6.
Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, Thunnissen E, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer Amst Neth. 2015;89(3):255–61.
Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro-Oncol. 2008;10(6):1010–8.
Geukes Foppen MH, Brandsma D, Blank CU, van Thienen JV, Haanen JB, Boogerd W. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(6):1138–42.
Glitza IC, Rohlfs M, Guha-Thakurta N, Bassett RL, Bernatchez C, Diab A, et al. Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open. 2018;3(1):e000283.. [Internet] [cited 2018 Dec 30]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5786950/
Kwon J, Chie EK, Kim K, Kim HJ, Wu H-G, Kim IH, et al. Impact of multimodality approach for patients with leptomeningeal metastases from solid tumors. J Korean Med Sci. 2014;29(8):1094–101.
Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis—the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62.
Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–9.
Rogers LR, Duchesneau PM, Nunez C, Fishleder AJ, Weick JK, Bauer LJ, et al. Comparison of cisternal and lumbar CSF examination in leptomeningeal metastasis. Neurology. 1992;42(6):1239–41.
Dux R, Kindler-Röhrborn A, Annas M, Faustmann P, Lennartz K, Zimmermann CW. A standardized protocol for flow cytometric analysis of cells isolated from cerebrospinal fluid. J Neurol Sci. 1994;121(1):74–8.
Quijano S, López A, Manuel Sancho J, Panizo C, Debén G, Castilla C, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(9):1462–9.
CellSearch™ circulating tumor cell kit premarket notification—expanded indications for use—colorectal. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf7/k071729.pdf
Nayak L, Fleisher M, Gonzalez-Espinoza R, Lin O, Panageas K, Reiner A, et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology. 2013;80(17):1598–605.. discussion 1603
Lee JS, Melisko ME, Magbanua MJM, Kablanian AT, Scott JH, Rugo HS, et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat. 2015;154(2):339–49.
Tu Q, Wu X, Le Rhun E, Blonski M, Wittwer B, Taillandier L, et al. CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer Amst Neth. 2015;90(2):352–7.
Patel AS, Allen JE, Dicker DT, Peters KL, Sheehan JM, Glantz MJ, et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget. 2011;2(10):752–60.
Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt MDC, Faure GC. Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol. 2012;12:21.
Le Rhun E, Tu Q, De Carvalho Bittencourt M, Farre I, Mortier L, Cai H, et al. Detection and quantification of CSF malignant cells by the CellSearch technology in patients with melanoma leptomeningeal metastasis. Med Oncol Northwood Lond Engl. 2013;30(2):538.
Campoli MR, Chang C-C, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24(4):267–96.
van Bussel MTJ, Pluim D, Bol M, Beijnen JH, Schellens JHM, Brandsma D. EpCAM-based assays for epithelial tumor cell detection in cerebrospinal fluid. J Neurooncol. 2018;137(1):1–10.
Magbanua MJM, Roy R, Sosa EV, Hauranieh L, Kablanian A, Eisenbud LE, et al. Genome-wide copy number analysis of cerebrospinal fluid tumor cells and their corresponding archival primary tumors. Genomics Data. 2014;2:60–2.
Sasaki S, Yoshioka Y, Ko R, Katsura Y, Namba Y, Shukuya T, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig. 2016;54(1):14–9.
Li Y, Pan W, Connolly ID, Reddy S, Nagpal S, Quake S, et al. Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J Neurooncol. 2016;128(1):93–100.
Shingyoji M, Kageyama H, Sakaida T, Nakajima T, Matsui Y, Itakura M, et al. Detection of epithelial growth factor receptor mutations in cerebrospinal fluid from patients with lung adenocarcinoma suspected of neoplastic meningitis. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2011;6(7):1215–20.
Yang H, Cai L, Zhang Y, Tan H, Deng Q, Zhao M, et al. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn JMD. 2014;16(5):558–63.
Seoane J, De Mattos-Arruda L, Le Rhun E, Bardelli A, Weller M. Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann Oncol Off J Eur Soc Med Oncol. 2018;30:211–8.
De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martínez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839.
Yang JC-H, Kim D-W, Kim S-W, Cho BC, Lee J-S, Ye X, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol. 2016;34(15_suppl):9002.
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Le Rhun, E., Weller, M. (2020). Clinical, Imaging, and CSF Cytological Presentation of Leptomeningeal Metastases from Solid Non-CNS Primary Tumors. In: Ahluwalia, M., Metellus, P., Soffietti, R. (eds) Central Nervous System Metastases. Springer, Cham. https://doi.org/10.1007/978-3-030-23417-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-23417-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23416-4
Online ISBN: 978-3-030-23417-1
eBook Packages: MedicineMedicine (R0)