Abstract
Pesticides are used to control any form of plant or organisms that can cause damage to human health or property. Agricultural products are attacked by a variety of pests during the production and storage. Despite usefulness and popularity of pesticides in controlling a variety of pests, they can cause many health risks arising from their exposure and residues in food and water. Also, the effective availabilities of traditional pesticide are usually less than 30% due to losses. Polymers, in the form of micro-nanocarriers, beads, granules and gels, are very important materials for the development of controlled release formulations (CRF) of pesticides which provide slow and controlled release of pesticides and also enhance the water-holding capacity of the soil. In this chapter, various types of natural and synthetic polymers used in the preparation of polymeric formulations and their release behavior are discussed. Various ways by which the diffusion of pesticides in polymer takes place and different formulation methods for controlled release pesticides were also discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Pesticides are the substances used to kill, suppress, or control any form of plant or organisms that can cause damage to human health or property. Agricultural products such as cereals, vegetables, and fruits are an essential part of the diet which provides a variety of nutrients that are required for metabolic reactions of the body. During the production and storage, these products are attacked by a variety of pests. Pests cause damaging of the agricultural products and reduce the quality and quantity as well. Various pesticides along with other pest management techniques are used to reduce the losses of these products and maintain their quality [1]. The uses of pesticides have attracted much attention as they have rapid action and require less labor than other pest control methods. Pesticides can also improve the nutritional value and quality of food [2]. Despite their usefulness and popularity, pesticides can cause many health risks arising from their exposure and from residues in food and drinking water [3]. Pesticide residues are the main source of soil and water pollution. The greater amount of pesticides is used for a longer period of time in conventional agrochemical application methods [4].
After the application of traditional pesticide formulations to the crops, the effective availabilities are usually less than 30% due to losses. More than 70% of pesticide does not reach to the target organisms and spreads in a wide area through water, soil, and air [5, 6]. These losses occur by wash off by rainwater, leaching, precipitation, and volatilization in the environment. Waxy cuticle and root surfaces of the plant also absorb pesticides, and they enter into the transport system of the plant. Degradation due to photolysis in the presence of sunlight, etc., is among the routes that reduce the amount of unused pesticide, and they also leave their residues in the environment and pose health hazards. The presence of pesticide residues in the environment is of great concern for researchers as pesticides have the potential to pose harmful effects and cause diseases to humans and other non-targeted organisms. Pesticides can also interfere with the reproductive systems thus initiating the fetal development. Pesticides also have the potential to cause cancer and asthma [7]. Some of the pesticides are sustained and remain in the body causing long-term exposure.
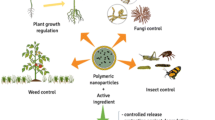
In the current scenario, the plant protection is based on the proper utilization of pesticide, and it is the most economical way of getting a high yield of good quality food but not compromising with the environment. The use of pesticides indiscriminately would result in harmful effects to the environment, people, and animals [8]. Researchers have been constantly trying to produce new formulations for the controlled release of pesticides for the protection of the environment and human health. Various mixtures of chemical agents to control pests are known as pesticide formulations. Special formulations of pesticides improve effectiveness, storage, safety, and handling. A pesticide formulation has an important role in its effectiveness and safety concerns. An appropriate formulation for a particular application can be obtained by considering type of pesticide, applicators safety, pest biology, available equipment for application, and final product cost. Various classes of polymers such as plastics, elastomers, and fibers are largely used in agriculture for increasing water-holding capacity and achieving controlled release of pesticides and nutrients [9, 10]. Polymers, in the form of micro-nanocarriers, beads, granules and gels, are very important materials for the development of controlled release formulations (CRF). CRF provide slow and controlled release of pesticides and also enhance the water-holding capacity of the soil [11]. After degradation, these formulations convert into compost and enhance soil nutrients [12]. CRF of pesticides are defined as depot systems which continuously release pesticide into the environment for a long time (months to years). According to this definition, such formulations can be successfully employed where a chronic exposure to biologically active compounds is required over a longer period. The use of polymeric formulations to solve the problem of pesticide loss and their accumulation in the environment is shown in Fig. 8.1.
A variety of polymers have been extensively used to control the release rates, molecular motilities, and the period of effectiveness depending on their end application. Polymeric dispersants offer superior stability by strong adsorption to the surface along with multiple anchoring points. The resultant effect is that the dispersant will not be disrupted by the incorporation of an adjuvant leading to a stable and highly efficacious formulation. Polymer-based controlled release systems offer various advantages over conventional formulations to avoid excessive use of active agrochemicals thus providing the most suitable technical solution of the pesticide residues [13]. The major benefit of the CRF is that if fewer amounts of agrochemicals are used for the protection of plants for a predetermined period, then it will reduce volatilization, leaching, and degradation of pesticide.
In 2016, the global market for controlled release pesticide size was estimated to be 1.7 billion USD, and up to 2025, it is expected to grow at a CAGR of 7.3%. The increasing demand of food for growing population is expected to drive more quanta of research in the field of CRF in the future [14].
8.2 Types of Polymers Used in Formulation
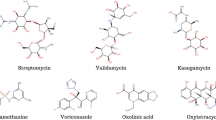
For the release of pesticides, many natural and synthetic polymers have been largely used in designing their formulations. Natural polymers are environment-friendly and easily degradable whereas synthetic polymers provide better stability to pesticide release carriers. Major classes of some natural and synthetic polymers used in making pesticide formulations are shown in Fig. 8.2.
List of some natural and synthetic polymers which are used in formulation for pesticides are given in Table 8.1.
8.2.1 Natural Polymers
Nowadays, natural polymers are gaining increasing attention over synthetic polymers in pesticide release formulations because of their easy availability, eco-friendly nature, cost-effectiveness, and biodegradability [35]. The following are major classes of natural polymers which are used in polymeric formulations of pesticides.
8.2.1.1 Polysaccharides
Polysaccharides are most widely used in controlled release pesticide formulations. Due to their ready availability, fast degradation, low cost and wide variety polysaccharides facilitate large-scale production of these formulations. Some of the known examples are amylose, cellulose, pectin, alginate, etc. On the basis of degradation behavior, polysaccharides are classified into (a) starch and systems based on amylase which are readily degradable, and (b) other polysaccharides such as cellulose and derivatives, dextran, chitin, chitosan, alginate, and guar gum which degrade slowly as compared to starches [36]. These macromolecules can be cyclic (cyclodextrin) or linear (chitosan) with positive or negative charges or even neutral [37]. Many polysaccharides can be used for the controlled release of agrochemicals by ionotropic gelation process by using multivalent metal ions [38,39,40]. Polysaccharides are suitable for all type of formulations including hydrogel, micro/nanoparticles, micro/nanocapsules, beads, and emulsions. Polysaccharides are hydrophilic in nature and are most suitable for hydrophilic pesticides as a matrix system. Hydrophobic pesticides can also be loaded into cross-linked micro/nanocapsules or used in the form of nano-emulsions.
8.2.1.2 Proteins
Proteins are another major class of natural polymers which are used in controlled release formulations of pesticides. Gelatin [29], casein, and albumin [31] are some of the common proteins which are largely used in these formulations. A herbicide [(4-chloro-2-methylphenoxy) acetic acid] formulation with gelatin clay composite was prepared and release of herbicide was investigated [41]. Proteins are also used in combination with polysaccharide to give structural stability.
8.2.1.3 Phospholipids
Many natural phospholipids from plants and animal origin such as lecithin are used in the preparation of liposomal delivery systems for pesticides. Natural phospholipids are preferred over synthetic phospholipids for the preparation of liposomes as they are cheap and largely available with reproducible results. Natural phospholipids obtained from vegetable sources such as soybeans, canola seed, wheat germ, sunflower, and flaxseed, and from animal sources, such as milk and egg yolk, can be used to produce low-cost liposomes for controlled release of pesticides. Natural phospholipids are also well accepted by regulatory authorities and are produced using less chemicals and solvents at higher yields [42].
8.2.1.4 Other Natural Polymers
Lignin which is a complex organic molecule found in support tissues of vesicular plants, and some algae are also widely used in agriculture for controlled release formulations [43]. Polylactic acid (PLA) which is a FDA-approved material is used as carriers for active agents [44, 45]. Liu et al. used polylactic acid for preparing carriers for controlled release of Lambda-Cyhalothrin via premix membrane emulsification. They prepared three types of carriers including microspheres, microcapsules, and porous microcapsules which are shown in Fig. 8.3.
SEM images of the a microspheres, b microcapsules, and c porous microspheres before and after cut by a super thin blade [21]
8.2.2 Synthetic Polymers
Many biodegradable synthetic polymers such as polyvinyl alcohol, polyacrylamide [46] are frequently used in the fabrication of slow-release formulations for pesticides. Although polyacrylamide gels are frequently used for encapsulation of pesticides, other polymers such as polyethylene, divynilbenzene, copolymers of acrylic acid and copolymers of cyclopentadiene with a glyceryl ester of an unsaturated fatty acid have also been used by researchers for designing pesticide release applications [47]. Some pesticides containing monomers are copolymerized with acrylamide and other hydrophilic co-monomers such as 4 vinyl pyridine [48]. Many pesticides such as pentachlorophenol (PCP), 2, 4-dichlorophenoxyacetic acid (2,4-D) and 4-chloro-2-methylphenoxyacetic acid are used as pendant groups in polymers [49]. Vinyl monomers containing pentachlorophenol pesticide via an ester linkage was prepared and homo- and copolymerized with 4-vinylpridine and styrene to induce hydrophilic and hydrophobic nature to the polymers [50]. Researchers combined chloropyriphos with polyethylene which can control mosquito larvae for about 18 months by one application itself [51]. Poly (vinyl alcohol) was widely used in agriculture for studying controlled release of agrochemicals [52]. Furthermore, the pesticides Azadirachtin A was physically bounded to both the PVA and poly (vinyl acetate) for its controlled release [53].
8.3 Diffusion of Pesticide into Polymer
Pesticides can diffuse into a polymer forming monolithic or reservoir-type formulations. In monolithic type, the pesticide is uniformly dispersed in a polymer matrix whereas in reservoir type the pesticide is covered by polymeric membrane as in case of capsules, laminates, liposome, coatings, etc.
8.3.1 Reservoir System
Reservoir-type formulations essentially consist of an enclosure system which exploits the diffusion of pesticides through a non-porous membrane as wall material for adjustment of the migration rates. Encapsulation ranges from macro to micro and nanoscales. In these systems, pesticides in the form of solid particles, liquids, or dispersions of solid particles in liquids, form a nucleus which is enclosed in a polymeric film. In reservoir-type formulation, the migration is controlled by membranes in which the transport of pesticides is controlled by Fick’s law
where J = Flux in g/cm
cm= Concentration of pesticide in g/cm2 of the polymeric membrane
dcm/dx = Concentration gradient
D = Diffusion coefficient of the pesticide in cm2/s in polymeric membrane
A = Surface area in cm2, through which diffusion takes place
M = Mass of agent releaseddM/
dt = steady-state release rate at time t.
8.3.1.1 Laminates
In laminate systems, a special type of membrane-controlled pesticide formulations can be produced. Laminates are obtained by the reciprocal bonding of different layers of sheet-like materials. For example in a three-layer formulation, the middle layer act as a reservoir containing pesticide in it and the other two outer layers act as barriers for controlling migration of pesticide. At the surfaces, the pesticides are released by leaching, evaporation, degradation, moisture wind dust, or mechanical contact by human or animals [54]. Laminates are used for the release of insect pheromones and insect attractants for insect control. A typical laminate system is shown in Fig. 8.4.
8.3.1.2 Capsules
Capsules are core–shell type structure where core is made up of pesticide which is surrounded by polymeric shell. They are usually in the range of nano to microsize. In the past decade, various pesticides were encapsulated into micro and nanocapsules [18, 33, 55]. Capsules can be mononuclear or polynuclear [56].
In the mononuclear capsules, only one depot of pesticide is present in core, whereas in multinuclear one, many small depots of pesticide are present. Chuxiang Sun et al. prepared nanocapsules of cross-linked carboxymethyl chitosan for the encapsulation of hydrophilic pesticide methomyl [18]. SEM and tem images of prepared nanocapsules are shown in Fig. 8.5 which clearly reveal core–shell nature of the capsules.
Latheef et al. prepared microcapsules of poly (methyl methacrylate) (PMMA), ethyl cellulose, poly (α-methylstyrene) and cellulose acetate with butyrate insecticide sulprofos contained in the core. The authors found the best results with ethyl cellulose formulations against eggs and larvae of the tobacco budworm Heliothis virescens in cotton plants [57].
Controlled release capsules of chitosan, alginate, and gelatin mixture were prepared by cross-linking biopolymers with glutaraldehyde. The capsules were loaded with the insecticides temephos [29], malathion and spinosad [54] and their pesticticidal activities were studied against Culex pipiens larvae.
8.3.1.3 Liposomes
Liposomes are lipid-based nonmaterial with hydrophic core encapsulated in a lipid bilayer [58]. Liposomes are also known as fatty acid vesicles [59], and it has been reported that the pesticides encapsulated in liposomes demonstrate superior action due to the prolonged persistence at reduced damage to other non-targeted organisms [60]. Liposomes can carry both hydrophilic and hydrophobic pesticide [61]. Hydrophobic pesticide can be encapsulated between lipid bilayer and hydrophilic in the core. Both types of liposomes can be made sticky so that they remain on leaves for longer times and do not wash off to the ground. Inexpensive liposomes can also be produced from synthetic lipids and natural lipids for the controlled release of pesticides. Hwang et al. prepared liposome of chitosan-coated lecithin for the controlled release of etofenprox [62].
8.3.1.4 Coatings
Mesoporous silica nanoparticles with polymer coatings have been developed to achieve controlled release of pesticides. The tunable pore size, low cost, large surface area, good-loading capacity, and low cost make them ideal carriers for controlled release of pesticides [63, 64]. Lidong et al. successfully coated a water-soluble chitosan (CS) derivative (N-(2-hydroxyl) propyl-3-trimethyl ammonium CS chloride on the surfaces of the mesoporous silica nanoparticle loaded with pyraclostrobin. The loading efficiency was greatly improved by the coating and the material showed excellent fungicidal activity against Phomopsis asparagi (Sacc.) in half dose [65]. Sato et al. developed a slow-release system with a certain lag time for Imidacloprid. They mixed Imidacloprid with bentonite and clay for the preparation of core granules. The core granules were coated with a mixture of high-density polythene and talc by using spouted bead coating system [66].
8.3.2 Matrix System
In matrix-type formulations, the pesticide is heterogeneously dispersed or dissolved in a solid polymeric matrix, and this can be either biodegradable or nonbiodegradable. The release of pesticide is generally controlled by diffusion through the matrix, chemical, or biological erosion, or through a combination of diffusion and erosion. The release by erosion is surface area-dependent, and the general expression that describes the rate of release Rr by an erosion mechanism is given as,
where KE= erosion rate constantA = exposed surface area
Co=concentration of loaded pesticide in the matrix.
Matrix systems are easy to produce than the reservoir systems, but the zero-order release cannot be obtained by these systems. These systems are easy to produce than the reservoir devices [67]. A major factor affecting the release process is the porosity or free volumes within the matrix of the plastic material the magnitude of which is controlled by the processing conditions and incorporated additives [68]. Volova and coworkers used poly(3-hydroxybutyrate) as a matrix for slow-release formulations of the herbicide metribuzin. Physical mixtures of polymer and pesticide in the form of solutions, powders, and emulsions were used to construct different metribuzin formulations in the form of granules, pellets, and microparticles [23].
8.3.2.1 Particles
Micro and nanoparticle prepared from a variety of natural and synthetic polymers have attracted attention of researchers in the area of controlled release of pesticide. The size of particles has great influence on the release behavior. Grillo et al. developed biodegradable polymeric microparticles of poly (hydroxybutyrate) (PHB) or poly(hydroxybutyrate-valerate) (PHBV), and the authors noticed that the herbicidal activity in microparticles formulation was found better as compared to the conventional formulations [69]. Faria et al. prepared calcium alginate microparticles for controlled release of tebuthiuron [70]. Liu et al. incorporated tebuconazole and chlorothalonil fungicides in polymeric nanoparticles prepared from polyvinyl pyridine and polyvinyl(pyridine-co-styrene) with 10 and 30% styrene, respectively. The mean diameter of particles was found in the range of 100–250 nm, and it increases with increase in the styrene content [71].
8.3.2.2 Pellets
PVA and 1,8-cineole mixture were prepared by dry mixing method. The aim of the present study was the evaluation of controlled release time and efficiency of 1 g insecticide pellets prepared using the eucalyptol, botanic constituent, and poly (vinyl alcohol), a biodegradable polymer, under laboratory condition [72].
8.3.2.3 Beads
Beads are the simplest formulations for the controlled release of pesticides. They are normally prepared by cross-linking of preformed polymers. The beads of polyacrylamide-g-guar gum and sodium alginate were prepared by cross-linking the IPN of grafted polymer and alginate with gluteraldehyde. The as-prepared beads were loaded with chlorpyrifos and liquid fenvelarate for their controlled release [16]. Hydrogel beads of calcium alginate and nickel alginate were prepared and assessed for the controlled release of carbaryl insecticide [15].
8.4 Formulation Methods
The release rate of pesticide from the polymer matrix is highly influenced by the following factors:
Chemical nature of the pesticide—polymer bond such as esters, amides, and acetals.
-
The distance of the pesticide molecule from the polymer backbone
-
Rate of breakdown of the bond between pesticide and the polymer by chemical, biological, or environmental agents.
-
Biodegradation of polymer.
-
Structure and dimension such as degree of polymerization, solubility, degree of cross-linking, co-monomers, and the stereochemistry of the polymer.
In order to control these parameters, the pesticides are either chemically bounded or physically incorporated into a polymer matrix by different techniques. The migration of the substances is, therefore, preceded by chemical reactions for bond cleavage or physical transport processes in and through polymers.
8.4.1 Physical Combination of Polymer and Pesticide
The active agent is dissolved, dispersed, or encapsulated within the polymeric matrix or coating. The release takes place through diffusion or biological/chemical degradation of the polymer. The polymer acts as a rate-controlling device in physical combination [73]. The physical combination broadly comprises laminated, reservoir, and monolithic systems [54].
8.4.1.1 Interfacial Polymerization
Morgan and co-researchers first described the principles of interfacial polymerization [74]. In interfacial polymerization, the polymerization reaction occurs at the interface of the two immiscible liquids [75]. For interfacial polymerization, two reactive monomers are dissolved in two immiscible solvents to facilitate their contact only at interface. In this way, the polymerization occurs only at the interface forming a polymeric film. Interfacial polymerization is largely employed for the formation of microcapsules consisting of liquid pesticide inside the polymeric membrane. In the formulation, generally one phase is an aqueous and the other phase is organic. If the aqueous phase is dispersed in organic phase, the core will be hydrophilic and can be used for encapsulation of hydrophilic pesticide whereas inverting the phases would result in a hydrophobic core which can be used for hydrophobic pesticides. Microcapsules of polyamide, polyurathanes, polyurease, and polyesters can be prepared by interfacial polymerization [76]. Moghbeli et al. prepared polyurea microcapsule containing ethion pesticide by interfacial polycondensation [77].
8.4.1.2 Coacervation
Encapsulation of pesticide by the coacervation phase-separation technique works in the liquid phase and generally consists of three steps.
-
Formation of three immiscible chemical phases
-
Deposition of coating
-
Hardening of the coating.
The pesticide and the polymer materials are initially present as different phases in the form of an emulsion or suspension. During mixing, the polymer encloses pesticide, through adsorption, around a nucleus of the pesticide. The polymer phase is subsequently precipitated (solidified) by means of thermal treatment, cross-linking reaction or desolvation [78]. The solvent used in this process must not dissolve the pesticide to obtain discrete capsules. Particle sizes of capsules are significantly affected by the mixing rate. The best example of simple coacervation is the encapsulation of core material in gelation [79].
The coacervation-phase separation can be divided into two categories, simple coacervation and complex coacervation. In simple coacervation, a strongly hydrophilic substance is added to a colloidal solution which results in the formation of two phases. The complex coacervation is a pH-dependent process where the microcapsules are produced by manipulating acidity or basicity of the system. The microcapsule formation takes place only above a critical pH. Complex coacervation deals with the system containing more than one colloid [80].
8.4.1.3 Ionic Gelation
Ionic gelation involves the interaction of an ionic polymer with oppositely charged ion to initiate cross-linking [81]. Pesticide-loaded matrix of various size ranges can be prepared by ionic gelation method in which the pesticide containing polymer solution is poured dropwise into the solution of metal ions. The best example of ionic gelation is the solution of sodium alginate and pesticide added drop-wise into calcium chloride solution. This method is very useful for the preparation of hydrogel-type release system where release of pesticide depends on swelling of polymer matrix. Isıklan encapsulated carbaryl in the alginate beads by the ionotropic gelation of sodium alginate (NaAlg) with calcium and nickel ions [15]. Kumbar prepared interpenetrating polymer network beads by ionic gelation technique. Solution of polyacrylamide-grafted-guar gum with sodium alginate containing chlorpyrophos/fenvelarate pesticides was dropped into the aqueous solution of gluteraldehyde and hydrochloric acid [82]. Micro and nanoparticles of polysaccharides were also prepared by ionic gelation process, but some defects such as improper surface morphology, high dispersibility index, and fragile particulate system can be encountered [83, 84].
8.4.1.4 Spray Drying and Spray Congealing
Spray drying is a single-step microencapsulation technique for different ingredients in various applications such as cosmetics, food materials, and agrochemicals [85,86,87]. In this technique, a liquid is rapidly transformed into a dried powder [88]. The four important steps of spray drying are atomization, contact of droplet with hot gas, evaporation, and powder separation. The main advantage of this technique is that one can use different forms such as emulsion, suspension, solution, and slurries via this technique [89, 90].
Spray drying has been extensively used for many years for microencapsulation of active agents. Both synthetic and natural polymers such as ethyl cellulose, gums, maltodextrin, polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA), chitosan, and poly(ɛ-caprolactone) (PCL), are widely used materials to encapsulate different pesticides [91, 92]. For microencapsulation of pesticide, the pesticide is dispersed in a liquid-coating material forming an emulsion. The emulsion is then sprayed into the heated chamber of a spray drier where rapid solidification of the coating takes place [93]. The size of the encapsulated particles depends upon the droplet size of emulsion. Spray drying process is beneficial for microencapsulation of biopesticides as spray-dried encapsulated biopesticides can be preserved for long time [94, 95].
Basic principal and equipment used for both spray drying and spray congealing are same and the only difference between the two methods is coating solidification. Coating solidification in the case of spray drying the polymeric material used for coating is dissolved in a suitable solvent whereas in spray congealing molten coating material is used. Waxes, fatty acids, and alcohols, polymers which are solids at room temperature but meltable at reasonable temperature are applicable to spray congealing [96].
8.4.2 Chemical Combination of Polymer and Pesticide
In chemical combination of polymer and pesticide, the pesticide is chemically attached to polymeric chain through covalent or ionic bonds. Here the pesticide either constitutes a part of the macromolecular backbone or is attached to it as a pendent group as shown in Fig. 8.6.
The polymer acts as a carrier for the pesticide in chemical combination [97] and the release of pesticide is dependent on the biological or chemical degradation by cleavage of the bonds between the polymer and the pesticide molecules [98]. The release of the pesticide is dependent on environmental conditions that break the linkages via chemical attack (hydrolytic by moisture; thermal/photo by sunlight) or biological degradation (enzymatic by microorganisms). pH of the medium, electrolyte concentration, temperature and ionic strength of the dissolution medium have great influence on degradation rate and hence on the release of pesticide also.
Only the pesticides containing a structural moiety with at least one reactive functional group can be used in this technique. For chemical combination, two approaches are utilized; in the first approach, a preformed polymer is used to form chemical linkage with pesticide; while in the second approach, a pesticide containing monomer unit or a pesticide and monomer mixture is polymerized. Amide, ester, and anhydride linkages are most common linkages formed between polymer and pesticide in their chemical combination [99].
8.4.2.1 Chemical Attachment of Pesticide with Preformed Polymer
It involves chemical modification of a preformed polymer with the pesticide via a chemical bond, leading to a polymer having the pesticide linked to the main chain as a pendant group. Pesticides containing carboxylic acid groups can be easily attached to a polymer containing labile hydrogen as found in polymers with alcohol or amine side chain. Here the pesticide is bonded to polymeric main chain as pendent group. This type of reaction is given in equation,
X can be sulfur, oxygen, or nitrogen.
8.4.2.2 Chemical Attachment of Pesticide with Polymerization of Monomer
This type of reaction leads to the formation of both type of polymers, either having pesticide group in main chain or pesticide group attached with main chain as pendent group. The main advantages of this method are the ability to control the molecular weight of the polymer, the weight ratio of polymer and pesticide, the hydrophobic-hydrophilic balance via appropriate co-monomers, and the distribution of groups along the backbone.
-
A.
Pesticide attached to polymer chain as pendent group
Copolymers containing pendant pentachlorophenol have been prepared by free radical copolymerization of the corresponding monomer. A series of vinyl monomers containing PCP via an ester linkage have been prepared which are shown in Scheme 8.1. These monomers have been homo-and copolymerized with styrene and 4-vinylpridine [100].
Examples of polymerization of biologically active monomers. [97]
-
B.
Polymers having the pesticide in the main polymeric chain
Heptachlor is a chlorinated dicyclopentadiene insecticide which is used to control soil insects and termites. Three copolymers of heptachlor with methacrylic acid (MAA), ethylene glycol dimethacrylate (EDMA), 4-vinyl pyridine (4-VP), divinyl-benzene (DVB) and styrene were prepared by Singh et al. The prepared copolymers are heptachlor-co-MAA-co-EDMA, heptachlor-co-4-VP-co-DVB, and heptachlor-co-Styrene-co-DVB [101].
References
Cooper J, Dobson H (2007) The benefits of pesticides to mankind and the environment. Crop Prot 26(9):1337–1348
Narayanasamy P (2006) Postharvest pathogens and disease management. John Wiley & Sons, New York
Maroni M, Fanetti AC, Metruccio F (2006) Risk assessment and management of occupational exposure to pesticides in agriculture. Med Lav 97:430–437
Bajpai AK, Giri A (2003) Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohyd Polym 53(3):271–279
Gamon M, Saez E, Gil J, Boluda R (2003) Direct and indirect exogenous contamination. Arch Environ Contam Toxicol 44:141–151
Shalaby SEM, Abdou GY (2010) The influence of soil microorganisms and bio-or-organic fertilizers on dissipation of some pesticides in soil and potato tube. J Plant Prot Res 50(1):86–92
Gilden RC, Huffling K, Sattler B (2010) Pesticides and health risks. J Obstet Gynecol Neonatal Nurs 39(1):103–110
CICOPLAFEST (2004). Catalogo Oficial de Plaguicidas. Comisión Intersecretarial para el Control de Proceso y Uso de plaguicidas, Fertilizantes y Sustancias Tóxicas
Maghchiche A, Haouam A, Immirzi B (2010) Use of polymers and biopolymers for water retaining and soil stabilization in arid and semiarid regions. J Taibah Univ Sci 4(1):9–16
Huang B, Chen F, Shen Y, Qian K, Wang Y, Sun C, Zhao X, Cui B, Gao F, Zeng Z, Cui H (2018) Advances in targeted pesticides with environmentally responsive controlled release. Nanomaterials 8(2):1–18
Roy A, Singh SK, Bajpai J, Bajpai AK (2014) Controlled pesticide release from biodegradable polymers. Cent Eur J Chem 12:453–469
Kumar S, Bhanjana G, Sharma A (2014) Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr Polym 101:1061–1067
Wang L, Li X, Zhang G, Dong J, Eastoe J (2007) Oil-in-water nanoemulsions for pesticide formulations. J Colloid Interface Sci 314:230–235
https://www.grandviewresearch.com/industry-analysis/slow-controlled-release-pesticides-market
Isıklan N (2007) Controlled release study of carbaryl insecticide from calcium alginate and nickel alginate hydrogel beads. J Appl Polym Sci 105:718–725
Kumbar SG, Ashok Dave AM, Aminabhavi TM (2003) Release kinetics and diffusion coefficients of solid and liquid pesticides through interpenetrating polymer network beads of polyacrylamide-g-guar gum with sodium alginate. J Appl Polymer Sci 90:451–457
Grillo R, Pereira ADES, Melo NFS, Porto RM, Feitosa LO, Tonello PS, Filho NLD, Rosa AH, Lima R, Fraceto LF (2011) Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J Hazard Mater 186(2–3):1645–1651
Sun C, Shu K, Wang W, Ye Z, Liu T, Gao Y, Zheng H, He G, Yin Y (2014) Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int J Pharm 463:108–114
Sun Y, Ma Y, Fang G, Fu Y (2016) Controlled pesticide release from porous composite hydrogels based on lignin and polyacrylic acid. Bio Res 11(1):2361–2371
Wang Y, Wang A, Wang C, Cui B, Sun C, Zhao X, Zeng Z, Shen Y, Gao F, Liu G, Cui H (2017) Synthesis and characterization of emamectin-benzoate slow-release microspheres with different surfactants. Sci Rep 7(1):1–9
Liu B, Wang Y, Yang F, Wang X, Shen H, Cui H, Wu D (2016) Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloid Surf B 144:38–45
Chen H, Huang G, Zhou H, Zhou X, Xu H (2018) Highly efficient triazolone/metal ion/polydopamine/MCM-41 sustained release system with pH sensitivity for pesticide delivery. R Soc Open Sci 5(7):1–10
Volova TG, Zhila NO, Vinogradova ON, Nikolaeva ED, Kiselev EG, Shumilova AA, Shershneva AM, Shishatskaya EI (2015) Constructing herbicide metribuzin sustained-release formulations based on the natural polymer poly-3-hydroxybutyrate as a degradable matrix. J Environ Sci Health B 51(2):113–115
Frandsen MV, Pedersen MS, Zellweger M, Gouin S, Roorda SD, Phan TQC (2010) Piperonyl butoxide and deltamethrin containing insecticidal polymer matrix comprising HDPE and LDPE. Patent Number WO 2010015256(A2):20100211
Cespedes FF, Flores CIF, Fernandez ID, Pena FV, Sanchez MV, Perez MF (2012) Preparation and characterization of imidacloprid lignin-polyethylene glycol matrices coated with ethylcellulose. J Agric Food Chem 60:1042–1051
Shakil NA, Singh MK, Pandey A, Kumar J, Parmar VS, Singh MK, Pandey RP, Watterson AC (2010) Development of poly(Ethylene Glycol) based amphiphilic copolymers for controlled release delivery of carbofuran. J Macromol Sci A Pure App Chem 47:241–247
Chin CP, Wu HS, Wang SS (2011) New approach to pesticide delivery using nanosuspensions: research and applications. Ind Eng Chem Res 50:7637–7643
Paula HCB, Sombra FM, Abreu FOMS, de Paula RCM (2010) Lippia sidoides essential oil encapsulation by angico gum/chitosan nanoparticles. J Brazilian Chem Soc 21:2359–2366
Badawy MEI, Taktak NEM, Awad OM, Elfiki SA, El-Ela NEA (2015) Larvicidal activity of temephos released from new chitosan/alginate/gelatin capsules against Culex pipiens. Int J Mosq Res 2(3):45–55
Celis R, Adelino MA, Hermosin MC, Cornejo J (2012) Montmorillonite—chitosan bionanocomposites as adsorbents of the herbicide clopyralid in aqueous solution and soil/water suspensions. J Hazard Mater 209–210:67–76
Sopena F, Villaverde J, Maqueda C, Morillo E (2011) Photostabilization of the herbicide norflurazon microencapsulated with ethylcellulose in the soil-water system. J Hazard Mater 195:298–305
Jerobin J, Sureshkumar RS, Anjali CH et al (2012) Biodegradable polymer based encapsulation of neem oil nanoemulsion for controlled release of Aza-A. Carbohydr Polym 90:1750–1756
Campos EVR, De Oliveira JL, Da Silva CMG, Pascoli M, Pasquoto T, Lima R, Abhilash PC, Fraceto LF (2015) Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications, Scientific Reports vol 5, Article number: 13809
Daems F, Béra F, Lorge S, Fischer C, Brostaux Y, Francis F, Lognay G, Heuskin S (2016) Impact of climatic factors on the release of E-β-caryophyllene from alginate beads. Biotechnol Agron Soc Environ 20(2):130–142
Azwa ZN, Yousif BF, Manalo AC, Karunasena W (2013) A review on the degradability of polymeric composites based on natural fibres. Mater Des 47:424–442
Wilkins RM (ed) (1990) Controlled delivery of crop-protection agents. Taylor and Francis Ltd, London
Hassani LN, Hendra F, Bouchemal K (2012) Auto-associative amphiphilic polysaccharides as drug delivery systems. DrugDiscov Today 7:608–614. https://doi.org/10.1016/j.drudis.2012.01.016
Fernandez-Perez M (2007) Controlled release systems to prevent the agroenvironmental pollution derived from pesticide use. J Environ Sci Health B 42(7):857–862
Singh B, Sharma DK, Dhiman A (2013) Environment friendly agar and alginate-based thiram delivery system. Toxicol Environ Chem 95(4):567–578
Wlodarczyk M, Siwek H (2013) Clomazone release kinetics from alginate matrix to the water environment. Przemysl Chem 92(8):1513–1516
Alromeed AA, Scrano L, Bufo S, Undabeytia T (2015) Slow-release formulations of the herbicide MCPA by using clay-protein composites. Pest Manage Sci 71(9):1303–1310
Hoogevest PV, Wendel A (2014) The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol 116(9):1088–1107
Chowdhury MA (2014) The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int J Biol Macromol 65:136–147
Huang D, Li W, Wang T, Shen H, Zhao P, Liu BX, You Y, Ma Y, Yang F, Wu D, Wang S (2015) Isoniazid conjugated poly (lactide-co-glycolide): long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials 52:417–425
Duan Y, Zhang B, Chu L, Tong HH, Liu W, Zhai G (2016) Colloids Surf B Biointerfaces 141:345–354
Rudzinski WE, Chipuk T, Dave AM, Kumbar SG, Aminabhavi TM (2003) pH-sensitive acrylic-based copolymeric hydrogels for the controlled release of a pesticide and a micronutrient. J Appl Polym Sci 87:394–403
Puoci F, Iemma F, Spizzirri UG, Cirillo G, Curcio M, Picci N (2008) Polymer in agriculture: a review. Am J Agric Biol Sci 3(1):299–314
Raheb A, Akelah A, Issa R, Solaro R, Chiellini E (1991) Herbicide containing methacrylate, synthesis, polymerization and release investigation. J Contr Release 17:113–122
Kashyap PL, Xiang X, Heiden P (2015) Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Bio Macromolecules 77:36–51
Sun G, Wheatley WB, Worley SD (1994) A new cyclic N-Halamine. Biocidal polymer. Indian Eng Chem Res 33:68–170
Miller T, Nelson LL, Young WW, Roberts LW, Roberts DR, Wilkinson RN (1973) Polymer formulations of mosquito larvicides. I. Effectiveness of polyethylene and polyvinyl chloride formulations of chlorpyrifos applied to artificial field pools. Mosq News 33(2):148–155
Chandra R, Rustgi R (1998) Biodegradable polymers. Prog Polym Sci 23(7):1273–1335
Riyajan SA, Sakdapipanich JT (2009) Encapsulated neem extract containing Azadirachtin-A within hydrolyzed Poly(vinyl acetate) for controlling its release and photodegradation stability. Chem Eng J 152(2–3):591–597
Scher HB (1999) Controlled-release delivery systems for pesticides. CRC Press, Boca Raton
Badawy ME, Taktak NE, Awad OM, Elfiki SA, Ela-Ela NEA (2016) Evaluation of released malathion and spinosad from chitosan/alginate/gelatin capsules against culex pipiens larvae. Res Rep Trop Med 7:23–38
Jyothi SS, Seethadevi A, Prabha KS, Muthuprasanna P, Pavitra P (2012) Microencapsulation: a review. Int J Pharma Bio Sci 3(1):509–531
Latheef MA, Dailey OD, Franz E (1993) Efficacy of polymeric controlled release formulations of sulprofos against tobacco budworm, heliothis virescens (Lepidoptera:Noctuidae) on cotton. In: Berger PD, Devisetty BN, Hall FR. (eds) Pesticide formulations and applications systems, vol 13, ASTM STP 1183. American Society for Testing and Materials, Philadelphia, pp 300–311
Gregoriadis G, Bacon A, Wanderley WC, Mccormack B (2002) A role for liposomes in genetic vaccination. Vaccine 20:B1–B9
WALDE 2006. Formation and properties of fatty acid vesicles (liposomes) In: Liposomes technology. Inform Healthcare, New-York
Tahibi A, Sakurai JD, Mathur R, Wallach DFH (1991) Novasome vesicles in extended pesticide formulation. Proc Symp Contr Rel Bioact Mat 18(1):231–232
Chrai SS, Murari R, Ahmed I (2002) Liposomes (a review). part two: drug delivery systems. Bipharm 15:40–43
Hwang C, Kim TH, Bang SH, Kim KS, Kwon HR, Seo MJ, Youn YN, Park HJ, Yasunaga-Aokt C, Yu YM (2011) Insecticidal effect of controlled release formulations of etofenprox based on nano-bio technique. J Fac Agric Kyushu Univ 56(1):33–40
Lehman SE, Larsen SC (2014) Zeolite and mesoporous silica nanomaterials: greener syntheses, environmental applications and biological toxicity. Environ Sci Nano 1(3):200–213
Sun R, Wang W, Wen Y, Zhang X (2015) Recent advance on mesoporous silica nanoparticles-based controlled release system: intelligent switches open up new horizon. Nanomaterials 5(4):2019–2053
Cao L, Zhang H, Cao C, Zhang J, Li F, Huang Q (2016) Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomaterials 6(7):1–13
Sato C, Watanabe M, Nakamura Y, Okuda S (1982) Jpn. Kokai Tokkyo Koho JP 57-126402 (in Japanese)
Anka Rao A, Narasimha Rao V, Seetha Devi A, Anil K, Vasu Naik V, Rajesh A (2015) Oral controlled release drug delivery system: an overview. Int J Pharma Chem Res 1(1):6–15
Sfirakis A, Rogers CE (1981) Sorption and diffusion of alcohols in amorphous polymers. Polym Eng Sci 21(9):542–547
Grillo R, Clemente Z, Oliveira JLD, Campos EVR, Chalupe VC, Jonsson CM, Lima RD, Sanches G, Nishisaka CS, Rosa AH, Oehlke K, Greiner R, Fraceto LF (2015) Chitosan nanoparticles loaded the herbicide paraquat: the influence of the aquatic humic substances on the colloidal stability and toxicity. J Hazard Mater 286:562–572
Faria DM, Junior SMD, Nascimento JPLD, Nunes EDS, Marques RP, Rossinoc LS, Moretoa JA (2016) Development and evaluation of a controlled release system of TBH herbicide using alginate microparticles. Mate Res 20(1):225–235
Liu Y, Yan L, Heiden P, Laks P (2001) Use of nanoparticles for controlled release of biocides in solid wood. J Appl Polym Sci 79(3):458–465
Sharifian I, Farahani S, Shafiei SE, Sanchooli M (2015) Evaluation of release time and efficiency of a botanical insecticide pellet under laboratory conditions. J Entomol Zool Stud 3(2):355–358
Kenawy ER (1998) Recent advances in controlled release of agrochemicals. J Macromol Sci C Polym Rev 38:365–390
Morgan PW, Kwolek SL (1959) Interfacial polycondensation. II. Fundamentals of polymer formation at liquid interfaces. J Polym Sci 11:299–327
Sinha VR, Goyal V, Bhinge JR, Mittal BR, Trehan A (2003) Diagnostic microspheres: an overview. Crit Rev Ther Drug Carrier Syst 20(6):431–460
Perignon C, Ongmayeb G, Neufeld R, Frere Y, Poncelet D (2014) Microencapsulation by interfacial polymerisation: membrane formation and structure. J Microencapsul 32(1):1–15
Moghbeli MR, Abedi V, Dekamin MG (2011) Microencapsulation of Ethion by interfacial polymerization utilizing potassium phthalimide-N-oxyl (PPINO) as a promoter. Iran J Chem Eng 8(4):34–42
Bakan JA (1975) Microencapsulation of pesticides and other agricultural materials. In: Harris
Mohanty B, Aswal VK, Kohlbrecher J, Bohidar HB(2005) Synthesis of Gelatin nanoparticles via simple coacervation. 21(3):149–160
Kiyoyama S, Shiomori K, Kawano Y, Hatate Y (2003) Preparation of microcapsules and control of their morphology. J Microencapsul 20(4):497–508
Ahirrao SP, Gide PS, Shrivastav B, Sharma P (2014) Ionotropic gelation: a promising cross linking technique for hydrogels. Res Rev J Pharma Nanotechnol 2(1):1–6
Kumbar SG, Dave AM, Aminabhavi TM (2003) Release kinetics and diffusion coefficients of solid and liquid pesticides through interpenetrating polymer network beads of polyacrylamide-g- Guar Gum with sodium alginate. J Appl Polymer Sci 90:451–457
Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El Dally M (2008) Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res 31(8):1040–1049
Wu P, He X, Wang K, Tan W, He C, Zheng M (2009) A novel methotrexate delivery system based on chitosan-methotrexate covalently conjugated nanoparticles. J Biomed Nanotechnol 5(5):557–564
Garza CB, Fernández JY, Huerta BEB (2013) Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res Int 54(1):641–649
Sen D, Khan A, Bahadur J, Mazumder S, Sapra BK (2010) Use of small-angle neutron scattering to investigate modifications of internal structure in self-assembled grains of nanoparticles synthesized by spray drying. J Colloid Interface Sci 347(1):25–30
Gong P, Zhang L, Han X, Shigwedha N, Song W, Yi H, Du Cao C M (2014) Injury mechanisms of lactic acid bacteria starter cultures during spray drying: a review. Drying Technol 32(7):93–800
Ribeiro RF, Motta MH, Flores FC, Beck RCR, Harter APG, Schaffazick SR, Silva CDBD (2016) Spray-dried powders improve the controlled release of antifungal tioconazole-loaded polymeric nanocapsules compared to with lyophilized products. Mater Sci Eng C 59:875–884
Abbaspourrad A, Datta SS, Weitz DA (2013) Controlling release from pH-responsive microcapsules. Langmuir 29(41):12697–12702
Li X, Anton N, Arpagaus C, Belleteix F, Vandamme T (2010) Nanoparticles by spray drying using innovative new technology: the Buchi Nano Spray Dryer B-90. J Control Release 147(2):304–310
Schafroth N, Arpagaus C, Jadhav UY, Makne S, Douroumis D (2012) Colloids Surf B Biointerfaces 90:8–15
Ungaro F, D’Angelo I, Coletta C, Di Villa Bianca RD, Sorrentino R, Perfetto B, Tufano MA, Miro A, Rotonda MIL, Quaglia F (2011) Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Controlled Release 157(1):149–159
Desai KGH, Park HJ (2005) Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agent on the properties of spray dried microspheres. J Microencapsul 22(4):377–395
Horaczek H, Viernstein H (2004) Beauveria brongniartii subjected to spray-drying in a composite carrier matrix system. J Microencapsul 21(3):317–330
Kurmen JEC, Alvarez MIG, Rivero LFV (2015) microencapsulation of a colombian spodoptera frugiperda nucleopolyhedrovirus with Eudragit® S100 by spray drying. Braz Arch Biol Technol 58(3):468–476
Albertini B, Passerini N, Sabatino MD, Vitali B, Brigidi P, Rodriguez L (2008) Polymerlipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci 36:591–601
Kenawy ER, Sherringtont DC (1992) Controlled release of agrochemicalmolecules chemically bound to polymers. Eur Polym J 28(8):841–862
Mitrus M, Wojtowicz A, Moscicki L (2009) Biodegradable polymers and their practical utility. In: Janssen LPBM, Leszek Moscicki L (eds) Thermoplastic starch: a green material for various industries, 1st edn. Wiley, Germany SAGARPA, SEDESOL, México, D. F, p 481
Allan GG, Chopra CS, Neogi AN, Wilkins R (1972) Design and synthesis of controlled release pesticide-polymer combinations. Nature 234(5328):349–351
Akelah A (1990) Applications of functionalized polymers in agriculture. J Islamic Acad Sci 3(1):49–61
Singh K, Pasha A, Rani BEA (2013) Preparation of molecularly imprinted polymers for heptachlor: an organochlorine pesticide. Chron Young sci 4(1):46–50
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mishra, A., Saini, R.K., Bajpai, A.K. (2020). Polymer Formulations for Pesticide Release. In: K. R., R., Thomas, S., Volova, T., K., J. (eds) Controlled Release of Pesticides for Sustainable Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-030-23396-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-23396-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23395-2
Online ISBN: 978-3-030-23396-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)