Abstract
The study of combined biomechanical and neurological approaches to anterior cruciate ligament (ACL) injury rehabilitation is a relevantly new area of research and clinical implementation. The classical view of orthopedic-focused ACL rehabilitation has been to treat the local knee joint and proximal hip and core structures to create improved stability and mobility for enhanced joint control. This approach has led to a predominately biomechanical centric focus on ACL rehabilitation that has yielded immense strides in understanding the mechanics associated with injury, yet ACL reinjury rates still remain high. The inability of current biomechanically focused interventions to fully optimize movement is readily apparent when athletes engage in cognitively challenging and sensory rich sport environments, because the rate of motor coordination errors increases under these complex field-based conditions. In this chapter, an overview is provided for the foundation of neuroscience integration into musculoskeletal rehabilitation. The initial overview begins with the association of neuromuscular control/injury and musculoskeletal knee injury. Next, we review the current status of neurocognitive and neurophysiological influences on ACL injury mechanism and its implication for movement control. Novel neurocognitive training approaches utilizing visual-motor function, multi-task paradigms, and sport specific neuroloading will be explored to provide sports medicine clinicians with advanced integrative practical knowledge to formulate new techniques for ACL rehabilitation and injury prevention plans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Anterior Cruciate Ligament Injury
An anterior cruciate ligament (ACL) rupture is a debilitating activity-related knee injury that usually requires surgical reconstruction and extensive rehabilitation to restore knee stability and function [1,2,3]. The current best evidence suggests that targeting the neuromuscular control system is the key to effective rehabilitation to restore patient function and reduce reinjury risk [4, 5]. The current standard of care for ACL post-surgical rehabilitation is to engage in neuromuscular training, yet a failure rate up to 25% remains following return to activity in young active individuals [6,7,8]. This high failure rate is further compounded by the majority of individuals not even returning to preinjury levels of activity [9]. This leaves an opportunity to improve current neuromuscular training interventions to ensure return to physical activity levels with optimal outcomes and reduced second injury risk [10,11,12,13,14].
Although evidence supports neuromuscular training for effective injury prevention and rehabilitation, many of these approaches primarily target biomechanical factors such as muscle strength, balance, and plyometric function with less consideration for cognitive or neurological components [4, 5, 15, 16]. While rectifying the biomechanical profile and restoring muscle strength are vital components of the rehabilitation process, there may be potential to further improve function and decrease reinjury risk [17, 18]. Recent reports demonstrate unresolved neuroplastic alterations after injury, reconstruction, and rehabilitation that may be limiting function and the return to sport (RTS) participation [19,20,21]. These data stem from the foundational concept that the ACL is not only an intra-articular ligament providing mechanical stability to the knee joint, but is also highly innervated with mechanoreceptors that provide afferent signals to the central nervous system (CNS) and injury/reconstruction causes the loss of these mechanoreceptors [22,23,24]. A simple analogy for ACL reconstruction (ACLR) is that a torn electrical cord can be appropriately put back together, but the cord does not properly conduct electricity in its previous fashion. By targeting cognitive-associated neurological factors during neuromuscular rehabilitation progressions, it may be possible to improve the transfer of sensorimotor adaptations from the clinic to activity, and ultimately improve patient outcomes [25, 26].
2 Limitations of the Classic Structural-Mechanical Model
The very nature of the noncontact ACL injury mechanism illustrates the vital role of the CNS to restore function and prevent second ACL injury [27, 28]. The noncontact ACL mechanism is due to a loss of neuromuscular control during activities that can range from simple running to jump-landing and rapid direction changes [29,30,31]. This noncontact injury scenario demonstrates the need to challenge a broad spectrum of sensorimotor control contributions. The noncontact mechanism has repeatedly been associated with a failure to maintain knee neuromuscular control, while attending to an external focus of attention, involving highly complex dynamic visual stimuli, variable surfaces, movement planning, rapid decision-making, variable player positions and environment interactions, and unanticipated perturbations [32,33,34,35].
While many factors, including hormonal [36, 37], gender [38, 39], anatomical [40,41,42,43], and even genetic [44,45,46] influences, have been implicated in injury risk, the primary focus of physical rehabilitation has been dynamic neuromuscular control, since it is modifiable [5, 15, 16, 47,48,49,50,51] and a prospective predictor of primary [52,53,54,55] and secondary [7] injury. A great deal of evidence suggests targeting the neuromuscular control system is the key to intervention effectiveness, and the ability to mitigate injury risk may be to optimize the biomechanical-neurological integrated system [4, 5, 48, 56]. However, despite a great deal of biomechanical data to support altered movement strategies that continue to exist despite intervention, orthopedic medicine has only just begun to examine how joint injury influences the nervous system.
Recent research has demonstrated that CNS changes may be more important to sustained optimization of movement strategies than reliance on biomechanical post-test measures alone [21, 57,58,59,60,61,62,63]. This suggests that the CNS underlies any modification of injury risk, and to decrease risk, a motor control adaptation is required to adjust the requisite neuromuscular and biomechanically measured change [47, 61, 64,65,66]. The sustainment of movement strategies to reduce injury risk is highly associated with a neuroplastic motor learning adaptation [67,68,69,70,71]. However, due to limitations of the biomechanical model of musculoskeletal injury assessment, current interventions focus on adaptations made in primarily biomechanical terms that have been shown to revert to pre-intervention levels or not induce improvement at all [10, 12, 66, 72,73,74].
Current standard of care interventions that target the neuromuscular control system may be missing vital aspects of sensorimotor function because significant deficits in neuromuscular function remain during RTS [4, 5, 75,76,77]. The best practice neuromuscular control focused programs may be insufficient to fully address reinjury risk or restore patient function [7, 12, 64, 78,79,80]. It is likely that aspects of sensorimotor function that are affected by the injury are not adequately addressed in therapy, allowing suboptimal neuroplastic compensations to occur [81,82,83]. Consideration of neurological post-injury adaptations, in addition to restoring mechanical stability, is needed to formulate adjunct therapeutic strategies to improve neuromuscular control.
3 Neuromuscular Control
The term neuromuscular control is meant to encompass a spectrum of human function, ranging from the afferent input, the processing of that input, generation of the efferent output, and the overall coordination of the system [84]. Neuromuscular control also has a temporal component in the continuous feedback loops between sensory and motor processing that contribute to the final measurable output [85]. As the muscles contract and bodily segments move, the afferent system is constantly sending new signals to the motor system to update the position, force generation, environmental representation, and other factors relative to the output. This constantly updating system represents the neuromuscular control profile so important to movement control and performing motoric tasks.
To experimentally capture the neuromuscular control system, a largely behaviorist and functionalist methodology has dominated the field with reliance on a postural-structural-biomechanical approach [86]. This prevailing method is concerned primarily with measuring the final output of the system in the form of joint biomechanics without any quantification of the underlying mechanisms that generate those mechanics [9, 87]. This behaviorist or outcome-oriented approach does not account for the extensive neural computations associated with sensory processing along vestibular, visual, and somatosensory pathways which in turn allow for stability and control in the presence of a changing environment [87, 88]. The proprioception, force control, and kinesthetic contributions of the sensory system are vital to the organization of motor output and maintaining neuromuscular control integrity [88]. The ACL is unique compared to most ligamentous structures in that it has robust afferent connections with the spinal cord [89, 90] and cerebrum [24, 91]. This is due to the high volume of mechanoreceptors such as free nerve endings, Ruffini end organs, Pacinian corpuscles, and Golgi receptors in the synovial lining of the ACL that contribute a great deal to afferent function [92,93,94,95,96]. Restoration of these important neurological features has not been well established in the clinical setting, yet may prove to be vitally important to the future function post ACL injury.
The interaction between proprioceptive inputs, such as that from the ACL, and visual input plays a crucial role in providing overall afferent input to the CNS to regulate movement control feedback loops [88, 97,98,99,100]. The brain receives somatosensory information in the thalamus and primary somatosensory cortex (via Brodmann’s areas 3-1-2), and then integrates that afferent information caudally in the posterior parietal cortex, areas 5 and 7. This is also where the temporal lobe processed vestibular and visual information integrates with somatosensation before transmitting to the premotor cortex (area 8) and finally to the motor cortex (area 6) to achieve motor drive [85].
Musculoskeletal injuries may alter this flow of somatosensory [19, 81, 101,102,103], vestibular [104,105,106], and visual [82, 107, 108] processing in the CNS to sustain motor control. To maintain neuromuscular integrity in the presence of joint injury, the CNS may compensate with altered motor planning [105], regulation of integrated sensory information reaching the motor areas [19, 101], increased reliance on visual feedback or memory [82, 109], and/or alter the cortical-spinal drive [110, 111]. This CNS functional reorganization is most likely due to the mechanoreceptors lost in the damaged tissue contributing to decreased afferent input [92, 93]. This diminished sensory function is present despite years after the injury and normalized strength of the surrounding musculature [81, 83]. This is a likely source of neuroplasticity post musculoskeletal injury; thus, examining methods to address the sensory-visual-motor system along with the neuromuscular system in rehabilitation may improve patient function and decrease recurrent injury risk.
4 Neuromechanical Principles of Performance and Injury Risk
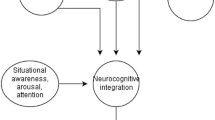
Action is the expression of cognitive processes [112], which integrate expectations derived from previous experiences with perceptions of changing conditions in both internal to the body and with respect to the external environment [113]. The term perception-action coupling refers to the interdependent nature of neural processes that link sensory inputs to motor outputs [113,114,115,116]. The efficiency of perception-action coupling may also be referred to as neuromechanical coupling , which has specifically been related to supraspinal modulation in muscle tone to create an optimal state of readiness to respond [117]. Thus, the term neuromechanical responsiveness is a designation for the combination of neurocognitive and biomechanical factors that influence the effectiveness of neuromuscular responses to rapidly changing environmental circumstances [118].
A list of common neurocognitive dimensions important to neuromechanical responsiveness can be found in Table 16.1. Vision is the source of sensory input that is primarily relied upon to make decisions about alternative responses to a given external environmental scenario [119], but cognitive processes that interpret visual inputs do not necessarily produce an internal representation that perfectly reproduces every element of the actual scene [120]. Visual-spatial working memory is required to synthesize discrete “snapshot” visual inputs for brain perception of a continuous stream of visual information, with processing informed by memories of past experiences in similar scenarios [121]. Simultaneously, an athlete will process a continuous stream of internal sensory information regarding motor performance, such as balance, proprioception, and force output. The athlete is not only required to attend to these different streams of information simultaneous (i.e., dual-tasking), but typically must be able to process and react as fast as possible in order to maximize their task performance. Because the brain of a given individual provides finite neural resources, rapidly changing circumstances in a highly demanding situation can require selective attention to a limited number of key information processing requirements [119,120,121,122]. If cognitive load exceeds neural processing capacity, an athlete may be required to narrow the range of sensory information to which they attend. This may result in “blindness” to unattended visual stimuli or inattention to errors in motor control output [123]. Conversely, when a primary focus of attention does not exhaust processing resources, a larger scope of sensory stimuli may be used to maximize performance [124]. The relative levels of cognitive demand during an athletic task and the cognitive capacity of the athlete may contribute to the overall injury risk of the athlete.
A practical example of this dynamic may be seen in the case of a running back in American football who is attempting to advance the ball downfield. The running back must use visual and spatial cognitive resources to attend to an evolving field of play, such as the location of his blocking linemen, the angles of pursuit of opposing linebackers, and his own position relative to the boundaries of play or first down marker. This information is continually compared to prior memory formed by practice of the play or prior game experience. At the same time, he is processing this external information, the running back is processing internal feedback of his own motor performance and interaction with the environment, such as a wet playing field, and then responding as needed to make alterations to his motor control. The player also needs to be able to process and react to these streams of information as fast as possible in order to maximize the yardage gained on the play. If there is a significant mismatch between his capacity for cognitive processing and the cognitive load imposed during task performance, the running back may be at increased risk for injury. This may manifest by a contact injury mechanism, whereby the running back is unable to adequately prepare to receive a hit in a safe manner from a player in his peripheral vision to whom he was unable to devote attentional resources. Similarly, this increased risk of injury could result from a noncontact mechanism, possibly due to a lack of attention to or errors in processing of internal feedback of motor control while prioritizing the processing of external sensory information.
This concept of neuromechanical responsiveness has been demonstrated in prior studies. Normal individuals ranging from military recruits to high-level collegiate football players who perform in the lower range of neurocognitive reaction time have been demonstrated to be at increased risk for musculoskeletal injuries [125, 126]. This effect has also been demonstrated with respect to ACL injury risk specifically, with one study finding that ACL-injured collegiate athletes demonstrated lower levels of preinjury performance across a range of neurocognitive domains, including visual and verbal memory, visual motor speed, and reaction time [127]. Similarly, biomechanical performance of athletic tasks associated with ACL injury risk has been shown to degrade with cognitive loading [128, 129], while athletes with poor memory, reaction time, and visual processing scores demonstrate worse biomechanical performance on biomechanical measures associated with an increased risk of ACL injury compared to athletes with good neurocognitive scores [130]. This relationship can be complicated by fatigue, which is a well-known risk factor for injury and has been shown to interact with cognitive demands to adversely affect the lower extremity biomechanics of female athletes during single-leg jump landing [114, 131]. Fatigue may exacerbate the adverse effects of other conditions or injuries on neural processing capacity, thereby increasing susceptibility to lapses in attention, distractibility, and inattentional blindness to environmental stimuli in the peripheral visual fields.
The dynamics of the relationship between cognition and biomechanics are further strained after ACL injury. Rupture of the ACL eliminates an important source of mechanoreceptor input to the CNS [24, 132, 133], and may have profound implications for maintenance of dynamic knee stability [117]. First, increased activation of brain areas that focus attention and process sensory information suggests that a greater volume of neural resources are required to control knee displacements [101]. Second, brain reweighting of sensory inputs increases reliance on vision for motor programming [107, 134], which has also been demonstrated in other ligamentous injuries such as chronic ankle instability [135]. These increased demands on neural resources may impose critical limitations on the ability to perform simultaneous visual, cognitive, and motor processes, thereby compromising neuromechanical responsiveness. Susceptibility to a poor functional outcome from an ACL injury or to a second ACL injury may be increased by low preinjury neurocognitive performance, the subsequent neural maladaptation from the injury, or a combination of the two [136, 137].
While brain activation patterns can exhibit dramatic changes following injury, activation patterns can similarly respond to training and open a new pathway for rehabilitation subsequent to ACL injury [138,139,140,141,142]. Training approaches may be used to enhance cognitive processing and diminish neural maladaptation from the injury. Due to the value of visual information during athletic tasks for performance purposes and the increased reliance on visual information in the absence of proprioceptive information from the ACL, visual-cognitive training during rehabilitation may be important for the attainment of desirable neuroplastic adaptions. Choice responses to visual stimuli that involve whole-body movements may be advantageous for strengthening of functional connectivity that integrates neural networks for visual-cognitive and motor tasks [138,139,140,141,142,143,144,145,146,147,148]. Such functional network integration may explain enhanced automaticity of multi-task responses that coincide with reduced neural activation of circuits linking the primary visual cortex, primary motor cortex, and cerebellum [139, 149, 150]. Thus, assessment and training activities should combine focused attention, visual stimulus discrimination for rapid decision making, and execution of compound motor skills [116, 150,151,152]. A number of computerized systems are now available to clinically assess and train the multiple interrelated aspects of neuromechanical responsiveness, including the capability for motion tracking of whole-body reactive responses to visual targets appearing within a virtual reality environment [58, 116, 152].
5 ACL Specific Neurological Adaptations
The overarching theory underpinning neuroplasticity from ACL injury is that the CNS afferent input is disrupted due to the lost somatosensory signals from the ruptured ligament and increased nociceptor activity associated with pain, swelling, and inflammation. The disrupted sensory input and injury-associated joint instability, muscle atrophy, and movement compensations combine to induce motor control adaptations. The reconstruction process leads to further deafferentation of the joint, causing continued neuroplastic modifications that result in maladapted efferent neuromuscular output [136].
In animal models, the ACL mechanoreceptor and afferent connections can be traced within the nervous system to the spinal cord, brain stem, and cerebral regions that contribute to proprioceptive, nociceptive, and reflex function [89, 91]. The initial sensorimotor neuroplasticity after ACL injury is likely caused by the abrupt loss of this connection that once provided the nervous system with continuous feedback [92,93,94,95,96, 153]. In human studies, the afferent loss is demonstrated by altered or absent somatosensory-evoked potentials with stimulation of the common peroneal nerve [24, 102, 103, 133] or, in surgery, of the ACL directly [154]. The loss of primary afferent information, combined with the pain and inflammatory responses, contributes to fundamentally alter the somatosensory feedback [107, 155,156,157]. The disrupted input, combined with mechanical changes and compensations [158, 159] (contralateral loading [80, 160], hip or ankle strategies [17, 161]), facilitates the adaptations for motor control [134, 162, 163]. On a foundational level, the altered motor output is displayed by disrupted gamma motor neuron function [163,164,165] and perturbation reflexes [162, 166] that play a key role in the ability to maintain neuromuscular integrity in a changing environment, requiring rapid and precise muscle stiffness or activation strategies [167,168,169]. The lost ability to rely on reflex and gamma motor neuron drive to prepare alpha motor neuron function requires the CNS to engage in supplementary mechanisms such as increased utilization of visual feedback to maintain the required sensory input for motor control [136, 170, 171]. As such, neuromuscular control after ACL injury may require enhanced visual feedback or memory reliance, depriving the CNS of resources once used for managing environmental interaction to maintain knee joint stability.
These deficits in neural function are not rectified with ACLR, as they may in fact become even more pronounced and/or present bilaterally [24, 110, 111, 165, 172,173,174]. The bilateral motor control, reflex, and proprioceptive changes are theorized to be due to both spinal [89, 91] and supraspinal [103, 175] mechanisms [176]. This ongoing neuroplasticity and altered mechanical and biological function of the joint combines to reduce proprioception acuity as measured by joint position sense [177, 178], movement detection [179, 180], and force sense [181]. To investigate the neurological adaptions of functional sensory loss, Baumeister et al. used electroencephalography (EEG), during force and joint sense tasks, and found that those with ACLR had greater brain activation in attentional and sensory areas [19, 101]. The increased activation may be attributed to less neural efficiency, or increased neural load to complete the same task; interestingly, despite increased cortical activation, proprioceptive performance was still worse in those with ACLR as compared to controls [19, 101]. These results indicate the loss of the native ACL not only constitutes a mechanical instability but a degree of nervous system deafferentation that is not rectified with reconstructive surgery and rehabilitation [153]. This partial deafferentation is further illustrated by investigations utilizing transcranial magnetic stimulation (TMS) to assess the CNS efferent pathway between the quadriceps and the brain [175, 177, 182, 183]. Heroux and Tremblay reported enhanced resting corticomotor excitability in those with ACL injury [182]. A potential mechanism for increased resting motor cortex excitability may be the altered sensory feedback, as the brain attempts to maintain motor output with attenuated sensory input. This increase in excitability may increase potential feed-forward mechanisms by decreasing the threshold for connections with motor planning areas, or allowing for increased input from other sensory sources (vision, vestibular) [184,185,186,187].
A neuroimaging investigation by Kapreli et al. [107] provided initial evidence of the neuroplastic effects of ACL injury. They performed functional magnetic resonance imaging (fMRI) of the brain during knee extension-flexion and found those with an ACL injury had increased activation of the pre-supplementary motor area, posterior secondary somatosensory area, and the posterior inferior temporal gyrus (pITG), compared to matched controls [107]. The pre-supplementary motor area is highly involved in complex motor planning [188, 189], and despite the relative simplicity of the movement task (single joint movement of 40° of knee extension-flexion while laying supine), those with an ACL injury needed to engage higher level motor control areas to a greater degree to execute the movement. This increased activation possibly indicates that on a neural-control level, simple movements are more taxing to those with a previous ACL injury [190]. The increase in posterior secondary somatosensory area provides further evidence of sensory-based neuroplasticity after injury, as this area is involved in regulating painful stimuli, but highly interconnected with the anterior secondary somatosensory area that integrates somatosensory inputs [169, 191, 192]. Interestingly, the participants in the study did not report pain during the movement, conceivably indicating a sensory processing adaptation from the initial increase in nociceptive input from the traumatic nature of the injury and not an acute effect. Alternatively, the prolonged nature of the rehabilitation, chronic pain, or joint instability may continue to disrupt typical somatosensory system afferent integration. The pITG plays a role in many cerebral functions [193, 194] but may primarily be involved with visual processing of movement [169]. As such, an increase in pITG activation during movement may indicate that in response to ACL injury there is an increased utilization of visual processing and motor-planning resources for movement concurrent with depression of somatosensory function [24, 82, 102, 103, 107, 133]. The findings of Kaperli et al. were also confirmed in ACLR patients with similar altered visual-motor and sensory-motor brain activation, potentially indicating shifts in cortical-subcortical processing and sensory reweighting [137, 171].
6 Neuroplasticity in Sport Rehabilitation
The transition from rehabilitation to sport activity is challenged by complex environmental interactions that place high demand on cognitive and sensorimotor processes and, in turn, increase ACL reinjury risk [32,33,34,35]. In a constantly changing environment, the primary afferent pathways (vestibular, visual, and somatosensory) interact to integrate and contextualize the feedback necessary for the efferent neuromuscular control system to maintain adequate stability and control [87, 88]. One area of sensorimotor function that may uniquely be affected by ACL injury is motor control requiring visual feedback [87]. The visual system provides a fundamental mechanism for coordination, regulation, and control of movement while managing environmental interactions (external focus) [109, 195, 196]. The need for visual feedback is especially true in executing movement sequences [189, 197] and with increases in task complexity and variability [196, 198,199,200]. The interplay between vision and somatosensation is particularly vital to provide sufficient afferent input to the CNS to regulate motor control and maintain neuromuscular integrity during action and environmental interaction [88, 97,98,99,100]. In this sensory-to-motor feedback loop, changes to visual or sensory feedback lead to subsequent alterations in neuromuscular control during movement (closed-loop processing) [23, 87, 88, 97, 99, 196].
Rehabilitative exercises are typically completed with an internal focus of control, meaning full attention is being directed to the internal aspects of the movement only (e.g., avoidance of excessive knee valgus or increasing knee flexion) [5, 22, 201]. Such an internal focus can offer positive benefits early in rehabilitation, when the need to develop or restore a motor pattern or muscle contraction ability is vital. However, function in the athletic environment, or even activities of daily living, requires constant interactions with the dynamic and constantly changing visual environment. Sport and activities of daily living, therefore, require an external focus of control, where attention is directed to the environment and the body relies on automatic motor control to maintain joint-to-joint integrity [200, 202, 203].
The need to challenge a broad spectrum of sensorimotor control is demonstrated by the noncontact ACL injury scenario itself: a failure to maintain knee neuromuscular control, while attending to an external focus of attention, involves highly complex dynamic visual stimuli, variable surfaces, movement planning, rapid decision-making, variable player positions and environment interactions, and unanticipated perturbations [32,33,34,35]. The need to bridge the intense neurocognitive and motor control demands of sport during rehabilitation may, therefore, benefit from specific interventions that target these neurological factors in addition to the biomechanical techniques that are already widely addressed.
Trauma to the ACL has been shown to modify how the nervous system processes the integration between vision and somatosensation [81, 82, 107, 108, 204]. By targeting injury-induced sensory-motor plasticity, a unique opportunity exists to improve the translation of neuromuscular system enhancements from the rehabilitation environment to the return to sport environment [58, 114, 131, 205]. The combined afferent neuroplasticity due to the lost mechanoreceptors of the ACL [94,95,96] and efferent neuroplasticity due to arthrogenic muscle inhibition [206] and disrupted gamma-motor neuron feedback loops [173] may induce specific central nervous system compensations. We have found that the CNS will increase reliance on visual feedback to program motion [136, 137, 171, 207,208,209]. Despite the injury, the nervous system continues to sustain motor output in the presence of depressed proprioceptive input [81, 82, 210] which may force increased use of visual-related feedback (memory or directly) by the motor cortex. This may also be partially induced, during rehabilitation, as therapy is strongly targeted at increased quadriceps activation immediately after surgery with a constant focus of attention on the knee joint; thus, the nervous system may create this visual-motor link during recovery.
Courtney et al. [102, 103, 162] in a series of works demonstrated that ACL-deficient individuals that went on to become copers (positive outcome without surgery) and adapted their movement strategy with increased hamstring activation to compensate for the instability had absent somatosensory-evoked potentials in the brain from the ACL. This was in opposition to non-copers or those that needed surgery or had a poor outcome having intact somatosensory-evoked potentials and no adaptation in motor control strategy. This work indicates that, if the brain does not receive the disrupted or absent afferent signal from a damaged ACL, no motor adaptation will occur. Any peripheral or spinal adaptations that mitigate the loss of the somatosensory-evoked potential at the brain actually resulted in a poorer outcome [211, 212]. This is further supported by recent work of Pietrosimone and colleagues who demonstrated that, after ACLR, those that have the lowest quadriceps activation failure, highest strength, and best reported outcomes have the greatest increase in cortical excitability [175, 178, 211, 212]. This may indicate that unique cortical mechanisms underpin recovery from injury and increased top-down and feed-forward mechanisms can compensate to a degree the resulting instability and depressed afferent feedback form the injury.
7 ACL Injury Induced Sensory-Visual-Motor Processing Compensations
Neuroplastic observations following ACL injury are supported by biomechanical evidence, suggesting that with increased task complexity, neuromuscular control is deteriorated in individuals with an ACL injury or reconstruction to a greater extent than controls, possibly due to overload of motor planning resources [213, 214]. The specific neuroplastic visual-motor control adaptation is observed during static balance as those with ACL injury have significantly diminished postural control when vision is obstructed (blindfold or eyes closed) [108, 215], but limited to no degradation in postural control with eyes open, as they are able to use vision to compensate and maintain balance [216, 217]. A more pronounced effect on neuromuscular control is observed when disrupting visual-motor processing during complex landing and cutting maneuvers that play an even greater role in injury risk [218,219,220]. The simple addition of a target, during a jump-landing task, increased injury risk mechanics [221]and altered muscle activation, decreasing postural stability [222]. The effects of forcing visual focus on the environment during more complex cutting or direction change tasks further degrades neuromuscular control capability in healthy athletes with the addition of a defender [219], a virtual soccer interface [223], or a level of unanticipated decision making during the task (selecting direction) [224, 225]. The effect of occupying the visual system with environmental cues during landing or change of direction has an even greater effect on those with ACL injury history [28, 213]. Furthermore, adding an anticipatory component that integrates visual processing and reaction time further demonstrates a reduction in knee neuromuscular control [226]. The inclusion of short-term memory and online decision-making also demonstrates specific adaptations in the maintenance of joint-to-joint neuromuscular integrity during complex athletic maneuvers such as cutting or sidestepping [114, 225, 227,228,229,230]. Recently, examination of injury risk, comparing ball-handling or offensive action (considered anticipatory and feedforward in nature) vs. defending (considered unanticipatory and responsive in nature), demonstrated a higher risk with defensive action [231]. This large-scale epidemiological data further support the possible increased injury risk movement strategies when unanticipated, rapid decision-making and/or visual-motor feedback is altered during the laboratory biomechanical studies.
These findings, taken together, suggest that ACL injury may lead to a cascade of neuroplastic and neuromuscular alterations that increase reliance on visual feedback and cortical motor planning for the control of knee movement. The post-injury disrupted sensory feedback, combined with the observed motor compensations, contributes to fundamentally alter the CNS mechanisms for motor control [19, 24, 92, 94, 96, 101, 111, 133]. In attempting to regulate neuromuscular control in the presence of decreased somatosensory input, the nervous system supplements with increased motor planning, conscious cortical involvement, and greater reliance on visual feedback. This ACL injury induced neuroplasticity can have consequences for function and further injury risk as the visual feedback and motor planning neural mechanisms become overloaded in the athletic environment. Specific additions to current neuromuscular interventions, targeting these neuroplastic imbalances, may play a significant role to induce sensory-motor adaptations to decrease dependence on visual feedback when transitioning to more demanding activities [232, 233].
The application of neuroplastic constructs during neuromuscular rehabilitation to optimize musculoskeletal therapy interventions is a new frontier for orthopedic care. The opportunity to supplement traditional interventions by further targeting neuroplastic, cognitive, and visual-motor capabilities is an exciting time for research and clinical practice. These new approaches allow clinicians to approximate the neurocognitive demands of higher intensity athletic activity in a safe, controlled, and most importantly feedback rich environment before reintegration into sport. Recognition of the visual-motor implications in neuromuscular control, injury recovery, and prevention, combined with new technologies, may help to mitigate post-injury movement dysfunction and decrease injury risk when returning to activity.
The training, and even restoration, of primarily biomechanical factors relative to ACL injury risk [52, 234] may not be addressing all the physiologic consequences of the injury, as even years post injury, patient-reported dysfunction and poor movement control persist [79, 80, 83, 159, 235, 236]. The impaired physical performance and patient-reported dysfunction might in part have a neurological origin [107, 173, 175]. The capacity for neuroplasticity, after injury and during therapy, presents an avenue to close a gap between rehabilitation and activity by targeting a broader spectrum of sensorimotor function during neuromuscular training [12, 16, 64, 79]. Alternative approaches and adjunct therapies may help to address the neurological system functions associated with the faulty movement patterns underlying ACL reinjury risk [7, 101, 111, 155].
A possibly overlooked factor in ACL injury prevention and rehabilitation design is visual-motor control associated with maintaining neuromuscular joint-to-joint integrity while engaging in the complex athletic environment [35, 237]. As physical activity and athletic participation require high demand on the visual-motor system to maintain environmental interaction as well as neuromuscular integrity, visual disruption in rehabilitation may be a promising tool to more closely mimic sport demands. The ability to sustain motor control in the variable sport environment demands a complex CNS integration of a constantly changing profile of sensory inputs including visual feedback, proprioception, and vestibular equilibrium to maintain neuromuscular control [87, 88].
The increased visual-motor activation in those with ACL injury suggests an adapted motor control strategy that may not be rectified with current rehabilitation methods. Advancing the neuromuscular control challenge during rehabilitation and prevention strategies can facilitate neuroplasticity not only for the motor regions, but also improve sensory integration and, thereby, address the visual processing bias. The key to this training is to consider the focus of attention, task complexity, visual input, and cognitive load during rehabilitation [114, 225]. Many mechanisms are available, including incorporating reaction time components [225], ball tracking, engaging other players [217], adding decision making [114] or anticipatory aspects [225] and having the patient dual task [214] by engaging the upper extremity while doing lower extremity exercises, or simply occupying the mind with memory or related tasks, can all increase the neural demand of our neuromuscular training strategies. Additionally, as eyes closed or blindfolded conditions have a greater effect on balance and movement performance in those with ACL injury, incorporating them during rehabilitation may address the visual-motor neuroplasticity [82, 108]. New technologies such as stroboscopic glasses provide a means to directly perturbate the visual-motor system under a variety of novel conditions that may help the transition back to the athletic environment, where visual attention is constantly distracted [238, 239]. Previous research using vision obstruction (blindfold) demonstrates alterations in landing neuromuscular control that may increase injury risk [240, 241]. Due to the method of limiting vision, these investigations lacked generalizability and sport specificity as the tasks were simple single movements without environmental interaction. The development of stroboscopic glasses that disrupt vision, without completely removing it, now allows visual-motor assessment during dynamic movements and target acquisition tasks. Stroboscopic glasses technology allows the patient to engage in neuromuscular training under depressed visual feedback and increased cognitive load in a safe clinical environment. This ability to train under a visually disrupted or knockdown stress may provide a means to target unique neuroplastic factors in rehabilitation [242, 243]. The consideration of visual-motor approaches during injury prevention and rehabilitation programs may provide a means to further improve intervention effectiveness. These approaches can be paired with foundational neuromuscular techniques for optimizing strength, multiplanar knee and trunk control, and movement asymmetries [244]. The use of a direct visual disruption technology such as stroboscopic glasses provides an opportunity to supplement traditional interventions [214, 242]. The clinician can add another training area that may decrease injury risk by targeting visual-motor processing along with the traditional neuromuscular, strength, and movement dysfunctions [170, 245]. The cognitive approximation of the demands involved in higher intensity athletic activity under the supervision of a well-trained clinician may further decrease musculoskeletal injury risk. Recognition of the visual-motor implications for maintaining neuromuscular control and injury avoidance may help to mitigate injury risk.
While the suggestions above provide a direct method to challenge the visual-motor system during high level dynamic movements, training the visual processing system in isolation may also have a beneficial effect on neuromuscular control. Swanik et al. provided prospective evidence for decreased visual processing speed as a risk factor for primary ACL injury [127]. Swanik et al. [127] prospectively reported that decreased aspects of neurocognitive function increased the risk of experiencing a noncontact ACL injury. Specifically, reaction time, visual processing, and memory, measured via a computerized concussion baseline assessment (IMPACT), were significantly lower than matched controls [127]. The role of visual-motor function and reaction time to facilitate preparation of the neuromuscular system in anticipation of high-risk situations, maneuvers, or incoming players, provides the theorized mechanism for neurocognition to influence musculoskeletal injury risk [246, 247]. Faster reaction time or processing speed may increase the potential to prepare for incoming perturbations or cognitively manage the complex athletic environment, while maintaining neuromuscular control. Visual training has been shown to improve reaction time and visual processing ability related to sport performance and may be worth considering as an aspect of neuromuscular reeducation [238].
If visual-motor processing ability is suboptimal, this may decrease the ability to compensate for external stimuli and/or attenuate the rapid and sometimes unanticipated maneuvers that depend on quick visual-motor interaction [222, 226, 248]. Visual-motor processing is imperative to successful sport function, whereby complex sensory and visual feedback must be handled with minimal preparation time [35, 246]. Visual memory ability may also assist in motor planning during activity as the constantly changing environment (player or ball positions) must be kept in short-term visual memory when planning movement sequences [243]. While limited connections exist relating biomechanical, visual-motor function, and changes induced by ACL injury, previous reports indicate altered neuromuscular control during visual-motor environmental interaction that may influence injury risk mechanics in healthy active participants [114, 219, 221, 226, 227].
8 Use of Neuromechanical Principles in Clinical Settings
Traditionally, ACL rehabilitation has focused on remediation of peripheral biomechanical impairments such as ligament laxity, restricted joint motion, and muscle weakness through techniques involving strength, flexibility, balance, and plyometric training in order to return athletes to competition after injury and reduce risk of ACL reinjury [249]. Utilization of neurocognitive training techniques is less common and presents unique challenges to the clinician and/or coach. Not only do athletes present with high variability in physical ability, especially in youth sports, but neurocognitive ability may vary even among athletes with similar physical attributes. In addition, many of the published studies to date using the computerized systems noted previously are potentially cost prohibitive and may be unavailable to most athletes with ACL injuries. Even if such resources are available, the volume of practice likely required to develop neurocognitive skills may compete with already busy training schedules. Finally, tailoring training programs to the unique abilities of the individual athlete as opposed to mass application of neuromuscular programs may hinder large-scale implementation of such strategies. Nonetheless, the massing body of evidence linking neurocognitive function to injury rink cannot be ignored and clinicians must consider all variables when developing programs intended to reduce risk of ACL injury or reinjury.
When implementing neurocognitive training alongside traditional training techniques, care must be taken to monitor task complexity as to not compromise performance. It is well documented that as cognitive demands increase physical performance will decrease [250, 251]. Prior to placing challenging neurocognitive demands on an athlete, a baseline musculoskeletal profile must be established, taking into consideration the athlete’s age, skill level, sport, and position. A youth athlete without a basic understanding of body mechanics and movement strategies cannot be expected to maintain the desired knee position while undergoing a high degree of cognitive load. It is therefore advisable that adequate neuromuscular control be achieved prior to progressing cognitive demands. In addition, inexperienced athletes may also perform more poorly in neurocognitive tasks [252] and may have varying ability to process neurocognitive demands, especially if out of context with their sport.
As athletes develop motor skill and move from the cognitive to associative and autonomous stages of motor learning, the training environment should transition from achieving desired performance to facilitating long-term motor learning. As such, the amount and type of feedback should be systematically reduced while simultaneously increasing the complexity of the task environment. One such strategy involves adding cognitive challenges to be performed in conjunction with the physical task (Table 16.2). Often used cognitive tasks include serial sevens, serial threes, spelling words backwards, controlled word association (COWA), and the Stroop task. While these tasks are not specific to sports, they are commonly used to assess an individual’s concentration and memory and serve to simulate the volume of information that must be processed during athletic competition.
In addition to cognitive load, an athlete’s ability to respond to stimuli may be influenced by their ability to visualize their environment and detect moving targets. In the context of ACL injury, the athlete’s ability to react to varying visual or auditory stimuli and then execute the desired motor pattern at high speeds is vitally important. In a training or rehabilitation setting, simple oculomotor exercises may be implemented to ensure precise visual skills. The most common trained movements are pursuits, saccades, and convergence. In a subset of individuals, oculomotor impairments have been linked to deficits in neurocognitive scores [253]. Oculomotor training progressions may include self-paced saccades, Hart Charts, pencil push-ups, and Brock strings (Table 16.3). In addition to oculomotor tasks, one cannot neglect the degree of head movement that occurs in sport, as such vestibular training may be of added benefit. Head velocities of up to 6000 deg/s have been detected in running, with an error as small as one degree resulting in visual distortion thus impairing an athlete’s ability to detect stimuli [254]. Vestibular training techniques include balance training and adaption exercises, termed vestibular ocular reflex (VOR) exercises. The effectiveness of such exercises has been well documented in individuals with inner ear pathology [255]; however, the effect on athletic performance has not yet been established. Both oculomotor and vestibular exercises may be progressed by manipulating the environment from simple to busy and transitioning targets from predictable/stationary to unpredictable/moving. Coaches and clinicians may use simple hand gestures, cue cards, computer programs, or actual sports equipment/balls as visual targets. As athletes master each task, additional physical demands should be placed on the athlete to simulate the demands of their sport.
One criticism of sports vision training is a potential lack of transfer to performance on the field. It is therefore essential, in current context, that visual and cognitive training be made to replicate the unique demands of the sport and position. For example, a soccer goalie will require a high degree of hand-eye coordination, but may not need the ball-handling skills of a mid-fielder. In contrast, all positions in basketball require some degree of hand-eye coordination in addition to lower body quickness and agility. It would not be expected for the soccer midfielder to perform at the same level as the goalie in an object detection and interception task, but the midfielder may exhibit a higher degree of lower extremity control in the presence of cognitive loads.
Table 16.4 represents a sample progression of basic movements often used in athletic training routines. The base movement is first made more difficult by the addition of a single visual or cognitive task, and then by the combination of visual and cognitive tasks (Fig. 16.1). If the athlete can accomplish the movement within the desired parameters, the movement may be progressed; in our example, a squat is progressed to depth jump and the sequence is repeated. The exercise continues progressing towards more sports-specific movements to include directional jumping, responding to a variety of cues. Ideally, these movements are progressed to on-field practice with actual opponents as visual cues.
9 Case Examples
9.1 Case 1
A 22-year-old female presented status-post ACL repair from an injury occurring during intramural soccer, and with a history of contralateral ACL repair 4 years previously. The patient reported that her rehabilitation and RTS progression after her first surgery only included predictable motor demands and that she never fully regained confidence in her ability to protect herself from future injury, even during simple running tasks. Despite this, she returned to soccer, only to tear her contralateral ACL. For her current injury, the patient progressed as expected through initial phases of the rehabilitation protocol. The addition of cognitive tasks was used to further challenge automaticity of the skills being developed. After exhibiting sufficient strength and balance, agility drills were implemented with progression to unanticipated direction changes utilizing a flanker and Stroop task to challenge concentration. After completing rehabilitation for her second ACL reconstruction, the patient stated her confidence was significantly higher and she planned to continue to include the cognitive demands in her training routine.
9.2 Case 2
A 12-year-old female was referred to physical therapy for patellofemoral pain syndrome, with worsening of her injury that occurred during a vault performed during gymnastics practice. Upon initial evaluation, the patient was determined to have inadequate neuromuscular control to maintain a neutral patellofemoral during simple squatting tasks. After 4 weeks of physical therapy, the patient demonstrated the ability to perform depth jumps intended to simulate landing from various heights while maintaining patellofemoral neutral and without pain. However, when a cognitive task (word association) was added to the landing task, the patient immediately reverted to her pre-training movement pattern. The patient was seen for three more weeks with a focus placed on dual task training, specifically during landing tasks. Upon discharge, the patient demonstrated the ability to land with the desired patellofemoral position while attending to various cognitive tasks.
References
Gottlob CA, Baker CL Jr. Anterior cruciate ligament reconstruction: socioeconomic issues and cost effectiveness. Am J Orthop. 2000;29(6):472–6.
Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;367:272-282.
Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135–42.
Grindstaff TL, Hammill RR, Tuzson AE, Hertel J. Neuromuscular control training programs and noncontact anterior cruciate ligament injury rates in female athletes: a numbers-needed-to-treat analysis. J Athl Train. 2006;41(4):450–6.
Yoo JH, Lim BO, Ha M, et al. A meta-analysis of the effect of neuromuscular training on the prevention of the anterior cruciate ligament injury in female athletes. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):824–30.
Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–21.
Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–78.
Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–57.
Reinecke K, Cordes M, Lerch C, et al. From lab to field conditions: a pilot study on EEG methodology in applied sports sciences. Appl Psychophysiol Biofeedback. 2011;36(4):265–71.
DiStefano LJ, Blackburn JT, Marshall SW, Guskiewicz KM, Garrett WE, Padua DA. Effects of an age-specific anterior cruciate ligament injury prevention program on lower extremity biomechanics in children. Am J Sports Med. 2011;39(5):949–57.
Heidt RS Jr, Sweeterman LM, Carlonas RL, Traub JA, Tekulve FX. Avoidance of soccer injuries with preseason conditioning. Am J Sports Med. 2000;28(5):659–62.
Herman DC, Weinhold PS, Guskiewicz KM, Garrett WE, Yu B, Padua DA. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop-jump task. Am J Sports Med. 2008;36(4):733–40.
Hewett TE, Ford KR, Hoogenboom BJ, Myer GD. Understanding and preventing ACL injuries: current biomechanical and epidemiologic considerations - update 2010. NAJSPT. 2010;5(4):234–51.
Steffen K, Myklebust G, Olsen OE, Holme I, Bahr R. Preventing injuries in female youth football--a cluster-randomized controlled trial. Scand J Med Sci Sports. 2008;18(5):605–14.
Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60.
Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;22(3):987–1014.
Goerger BM, Marshall SW, Beutler AI, Blackburn JT, Wilckens JH, Padua DA. ACL injury alters pre-injury coordination of the hip and knee: the JUMP ACL study. Med Sci Sports Exerc. 2013;45(5):221.
McLean SG. The ACL injury enigma: we can’t prevent what we don’t understand. J Athl Train. 2008;43(5):538–40.
Baumeister J, Reinecke K, Schubert M, Weiss M. Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Res. 2011;29(9):1383–9.
Baumeister J. What the brain can tell us in musculoskeletal rehabilitation. J Sports Med Doping Stud. 2012;2(3):1–2.
Beck S, Taube W, Gruber M, Amtage F, Gollhofer A, Schubert M. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007;1179:51–60.
Benjaminse A, Otten E. ACL injury prevention, more effective with a different way of motor learning? Knee Surg Sports Traumatol Arthrosc. 2011;19(4):622–7.
Berthoz A, Lacour M, Soechting J, Vidal P. The role of vision in the control of posture during linear motion. Prog Brain Res. 1979;50:197–209.
Valeriani M, Restuccia D, Di Lazzaro V, Franceschi F, Fabbriciani C, Tonali P. Clinical and neurophysiological abnormalities before and after reconstruction of the anterior cruciate ligament of the knee. Acta Neurol Scand. 1999;99(5):303–7.
Baumeister J, Reinecke K, Liesen H, Weiss M. Cortical activity of skilled performance in a complex sports related motor task. Eur J Appl Physiol. 2008;104(4):625–31.
Hall KG, Magill RA. Variability of practice and contextual interference in motor skill learning. J Mot Behav. 1995;27(4):299–309.
Kirkendall DT, Garrett WE Jr. The anterior cruciate ligament enigma. Injury mechanisms and prevention. Clin Orthop Relat Res. 2000;372:64–8.
Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–12.
Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–50.
McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103(901):537–9.
Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. J Athl Train. 2008;43(4):396–408.
Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252–9.
Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–22.
Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–25.
Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball - video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–67.
Hewett TE, Zazulak BT, Myer GD. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35(4):659–68.
Ruedl G, Ploner P, Linortner I, et al. Are oral contraceptive use and menstrual cycle phase related to anterior cruciate ligament injury risk in female recreational skiers? Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1065–9.
Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–9.
Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13(3):184–8.
Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior cruciate ligament-injured subjects have smaller anterior cruciate ligaments than matched controls: a magnetic resonance imaging study. Am J Sports Med. 2009;37(7):1282–7.
Everhart JS, Flanigan DC, Simon RA, Chaudhari AM. Association of noncontact anterior cruciate ligament injury with presence and thickness of a bony ridge on the anteromedial aspect of the femoral intercondylar notch. Am J Sports Med. 2010;38(8):1667–73.
Jamison ST, Flanigan DC, Nagaraja HN, Chaudhari AM. Side-to-side differences in anterior cruciate ligament volume in healthy control subjects. J Biomech. 2010;43(3):576–8.
Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43(9):1702–7.
Hewett TE, Lynch TR, Myer GD, Ford KR, Gwin RC, Heidt RS Jr. Multiple risk factors related to familial predisposition to anterior cruciate ligament injury: fraternal twin sisters with anterior cruciate ligament ruptures. Br J Sports Med. 2010;44(12):848–55.
Posthumus M, Collins M, van der Merwe L, et al. Matrix metalloproteinase genes on chromosome 11q22 and the risk of anterior cruciate ligament (ACL) rupture. Scand J Med Sci Sports. 2012;22(4):523–33.
Raleigh SM, Posthumus M, O'Cuinneagain D, van der Merwe W, Collins M. The GDF5 gene and anterior cruciate ligament rupture. Int J Sports Med. 2013;34(4):364–7.
Greska EK, Cortes N, Van Lunen BL, Onate JA. A feedback inclusive neuromuscular training program alters frontal plane kinematics. J Strength Cond Res. 2011;26(6):1609–19.
McCann R, Nelson C, Van Lunen B, Greska E, Ringleb S, Onate J. Neuromuscular changes following an injury prevention program for ACL injuries. Int J Athl Ther Train. 2011;14(4):16–20.
Noyes FR, Barber-Westin SD, Smith ST, Campbell T. A training program to improve neuromuscular indices in female high school volleyball players. J Strength Cond Res. 2011;25(8):2151–60.
Pollard CD, Sigward SM, Powers CM. Injury prevention training results in biomechanical changes consistent with decreased knee loading in female athletes during landing. Knee. 2009;4:5.
Zemkova E, Hamar D. The effect of 6-week combined agility-balance training on neuromuscular performance in basketball players. J Sports Med Phys Fitness. 2010;50(3):262–7.
Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501.
Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3–8.
Padua DAMS, Beutler AI, Garrett WE. Prospective cohort study of biomechanical risk factors of ACL injury: The JUMP-ACL Study. American Orthopaedic Society of Sports Medicine Annual Meeting; 2009; Keystone.
Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35(7):1123–30.
Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J Knee Surg. 2005;18(1):82–8.
Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15(2):161–7.
Grooms DR, Kiefer AW, Riley MA, et al. Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: initial findings from the train the brain project. J Sport Rehabil. 2018;27(5):1–5. https://doi.org/10.1123/jsr.2017-0241.
Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99(4):1558–68.
Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136(4):431–8.
Powers CM, Fisher B. Mechanisms underlying ACL injury-prevention training: the brain-behavior relationship. J Athl Train. 2010;45(5):513–5.
Schubert M, Beck S, Taube W, Amtage F, Faist M, Gruber M. Balance training and ballistic strength training are associated with task-specific corticospinal adaptations. Eur J Neurosci. 2008;27(8):2007–18.
Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78(3):553–64.
Gokeler A, Benjaminse A, Hewett TE, et al. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Med. 2013;43(11):1065–74.
Onate JA, Guskiewicz KM, Marshall SW, Giuliani C, Yu B, Garrett WE. Instruction of jump-landing technique using videotape feedback: altering lower extremity motion patterns. Am J Sports Med. 2005;33(6):831–42.
Padua DA, DiStefano LJ, Marshall SW, Beutler AI, de la Motte SJ, DiStefano MJ. Retention of movement pattern changes after a lower extremity injury prevention program is affected by program duration. Am J Sports Med. 2012;40(2):300–6.
Emanuel M, Jarus T, Bart O. Effect of focus of attention and age on motor acquisition, retention, and transfer: a randomized trial. Phys Ther. 2008;88(2):251–60.
Herman DC, Onate JA, Weinhold PS, et al. The effects of feedback with and without strength training on lower extremity biomechanics. Am J Sports Med. 2009;37(7):1301–8.
Onate JA, Guskiewicz KM, Sullivan RJ. Augmented feedback reduces jump landing forces. J Orthop Sports Phys Ther. 2001;31(9):511–7.
Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol. 2008;99(4):1836–45.
Wood CA, Ging CA. The role of interference and task similarity on the acquisition, retention, and transfer of simple motor skills. Res Q Exerc Sport. 1991;62(1):18–26.
Grandstrand SL, Pfeiffer RP, Sabick MB, DeBeliso M, Shea KG. The effects of a commercially available warm-up program on landing mechanics in female youth soccer players. J Strength Cond Res. 2006;20(2):331–5.
Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg. 2006;88(8):1769–74.
Soderman K, Werner S, Pietila T, Engstrom B, Alfredson H. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):356–63.
Gardinier ES, Di Stasi S, Manal K, Buchanan TS, Snyder-Mackler L. Knee contact force asymmetries in patients who failed return-to-sport readiness criteria 6 months after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(12):2917–25.
Larsen JB, Farup J, Lind M, Dalgas U. Muscle strength and functional performance is markedly impaired at the recommended time point for sport return after anterior cruciate ligament reconstruction in recreational athletes. Hum Mov Sci. 2015;39:73–87.
Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a delaware-oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(12):914–23.
Knoll Z, Kiss RM, Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol. 2004;14(3):287–94.
Myer GD, Martin L Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40(10):2256–63.
Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–62.
Ageberg E, Friden T. Normalized motor function but impaired sensory function after unilateral non-reconstructed ACL injury: patients compared with uninjured controls. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):449–56.
Friden T, Roberts D, Movin T, Wredmark T. Function after anterior cruciate ligament injuries - influence of visual control and proprioception. Acta Orthop Scand. 1998;69(6):590–4.
Roberts D, Ageberg E, Andersson G, Friden T. Clinical measurements of proprioception, muscle strength and laxity in relation to function in the ACL-injured knee. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):9–16.
Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;27(3):353–70.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, vol. 4. New York: McGraw-Hill; 2000.
Lederman E. The fall of the postural-structural-biomechanical model in manual and physical therapies: exemplified by lower back pain. J Bodyw Mov Ther. 2011;15(2):131–8.
Magill RA. Motor learning and control: concepts and applications, vol. 11. New York: McGraw-Hill; 2007.
Winter DA. Biomechanics and motor control of human movement. Waterloo: Wiley; 2009.
Gomez-Barrena E, Martinez-Moreno E, Munuera L. Segmental sensory innervation of the anterior cruciate ligament and the patellar tendon of the cat’s knee. Acta Orthop Scand. 1996;67(6):545–52.
Gomez-Barrena E, Nunez A, Martinez-Moreno E, Valls J, Munuera L. Neural and muscular electric activity in the cat’s knee. Changes when the anterior cruciate ligament is transected. Acta Orthop Scand. 1997;68(2):149–55.
Park HB, Koh M, Cho SH, Hutchinson B, Lee B. Mapping the rat somatosensory pathway from the anterior cruciate ligament nerve endings to the cerebrum. J Orthop Res. 2005;23(6):1419–24.
Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop. 2002;73(3):330–4.
Fremerey RW, Lobenhoffer P, Zeichen J, Skutek M, Bosch U, Tscherne H. Proprioception after rehabilitation and reconstruction in knees with deficiency of the anterior cruciate ligament: a prospective, longitudinal study. J Bone Joint Surg. 2000;82(6):801–6.
Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10(6):329–35.
Schutte MJ, Dabezies EJ, Zimny ML, Happel LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg. 1987;69(2):243–7.
Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204–9.
Santello M, McDonagh MJN, Challis JH. Visual and non-visual control of landing movements in humans. J Physiol. 2001;537(1):313–27.
Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol. 2005;93(6):3200–13.
Thompson HW, McKinley PA. Landing from a jump: the role of vision when landing from known and unknown heights. Neuroreport. 1995;6(3):581–4.
Wade MG, Jones G. The role of vision and spatial orientation in the maintenance of posture. Phys Ther. 1997;77(6):619–28.
Baumeister J, Reinecke K, Weiss M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: an EEG study. Scand J Med Sci Sports. 2008;18(4):473–84.
Courtney C, Rine RM, Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69–74.
Courtney CA, Rine RM. Central somatosensory changes associated with improved dynamic balance in subjects with anterior cruciate ligament deficiency. Gait Posture. 2006;24(2):190–5.
Ergen E, Ulkar B. Proprioception and ankle injuries in soccer. Clin Sports Med. 2008;27(1):195–217.
Hopper DM, Creagh MJ, Formby PA, Goh SC, Boyle JJ, Strauss GR. Functional measurement of knee joint position sense after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84(6):868–72.
Hopper DM, Strauss GR, Boyle JJ, Bell J. Functional recovery after anterior cruciate ligament reconstruction: a longitudinal perspective. Arch Phys Med Rehabil. 2008;89(8):1535–41.
Kapreli E, Athanasopoulos S, Gliatis J, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37(12):2419–26.
Okuda K, Abe N, Katayama Y, Senda M, Kuroda T, Inoue H. Effect of vision on postural sway in anterior cruciate ligament injured knees. J Orthop Sci. 2005;10(3):277–83.
Peuskens H, Vanrie J, Verfaillie K, Orban G. Specificity of regions processing biological motion. Eur J Neurosci. 2005;21(10):2864–75.
Konishi YU. ACL repair might induce further abnormality of gamma loop in the intact side of the quadriceps femoris. Int J Sports Med. 2011;32(4):292–6.
Madhavan S, Shields RK. Neuromuscular responses in individuals with anterior cruciate ligament repair. Clin Neurophysiol. 2011;122(5):997–1004.
Araújo D, Hristovski R, Seifert L, et al. Ecological cognition: expert decision-making behaviour in sport. Int Rev Sport Exerc Psychol. 2017;15:1–25.
Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–29.
Borotikar BS, Newcomer R, Koppes R, McLean SG. Combined effects of fatigue and decision making on female lower limb landing postures: central and peripheral contributions to ACL injury risk. Clin Biomech. 2008;23(1):81–92.
Farrow D, Abernethy B. Do expertise and the degree of perception—action coupling affect natural anticipatory performance? Perception. 2003;32(9):1127–39.
Hadlow SMPD, Mann DL, Portus MR, Abernethy B. Modified perceptual training in sport: a new classification framework. J Sci Med Sport. 2018;21(9):950–8.
Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–88.
Wilkerson GB, Grooms DR, Acocello SN. Neuromechanical considerations for postconcussion musculoskeletal injury risk management. Curr Sports Med Rep. 2017;16(6):419–27.
Wahn B, König P. Is attentional resource allocation across sensory modalities task-dependent? Adv Cogn Psychol. 2017;13(1):83–96.
Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23(1):315–41.
Pisella L. Visual perception is dependent on visuospatial working memory and thus on the posterior parietal cortex. Ann Phys Rehabil Med. 2017;60(3):141–7.
Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12(12):739–51.
Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–8.
Puglisi G, Leonetti A, Landau A, et al. The role of attention in human motor resonance. PLoS ONE. 2017;12(5):e0177457.
Taimela S, Kujala UM, Osterman K. The relation of low grade mental ability to fractures in young men. Int Orthop. 1991;15:75–7.
Taimela S, Osterman L, Kujala U, et al. Motor ability and personality with reference to soccer injuries. J Sports Med Phys Fitness. 1990;30:194–201.
Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35(6):943–8.
Dai B, Cook RF, Meyer EA, Sciascia Y, Hinshaw TJ, Wang C, Zhu Q. The effect of a secondary cognitive task on landing mechanics and jump performance. Sports Biomech. 2018 Jun;17(2):192–205.
Kim AS, Needle AR, Thomas SJ, Higginson CI, Kaminski TW, Swanik CB. A sex comparison of reactive knee stiffness regulation strategies under cognitive loads. Clin Biomech. 2016;35:86–92.
Herman DC, Barth JT. Drop-jump landing varies with baseline neurocognition: implications for anterior cruciate ligament injury risk and prevention. Am J Sports Med. 2016;44(9):2347–53.
McLean SG, Borotikar B, Lucey SM. Lower limb muscle pre-motor time measures during a choice reaction task associate with knee abduction loads during dynamic single leg landings. Clin Biomech. 2010;25(6):563–9.
An YW, DiTrani Lobacz A, Lehmann T, et al. Neuroplastic changes in ACLR patients from neuromechanical decoupling. Scand J Med Sci Sports. 2018;29:251–8. https://doi.org/10.1111/sms.13322.
Valeriani M, Restuccia D, Di Lazzaro V, Franceschi F, Fabbriciani C, Tonali P. Central nervous system modifications in patients with lesion of the anterior cruciate ligament of the knee. Brain. 1996;119(Pt 5):1751–62.
Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sport Phys. 2017;47(3):180–9.
Song K, Burcal CJ, Hertel J, Wikstrom EA. Increased visual use in chronic ankle instability: a meta-analysis. Med Sci Sports Exerc. 2016;48(10):2046–56.
Grooms D, Appelbaum G, Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015;45(5):381–93.
Grooms DR, Page SJ, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: neuroimaging in musculoskeletal medicine. J Athl Train. 2015;50(10):1005–10.
Churchill N, Hutchison MG, Leung G, et al. Changes in functional connectivity of the brain associated with a history of sport concussion: a preliminary investigation. Brain Inj. 2017;31(1):39–48.
Parasuraman R, Jiang Y. Individual differences in cognition, affect, and performance: behavioral, neuroimaging, and molecular genetic approaches. NeuroImage. 2012;59(1):70–82.
Serrien DJ, Ivry RB, Swinnen SP. The missing link between action and cognition. Prog Neurobiol. 2007;82(2):95–107.
Taubert M, Draganski B, Anwander A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30(35):11670–7.
Wu T, Liu J, Hallett M, et al. Cerebellum and integration of neural networks in dual-task processing. NeuroImage. 2013;65:466–75.
Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–90.
Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2012;12(9):509–23.
Heinen K, Feredoes E, Ruff CC, et al. Functional connectivity between prefrontal and parietal cortex drives visuo-spatial attention shifts. Neuropsychologia. 2017;99:81–91.
Kahn AE, Mattar MG, Vettel JM, et al. Structural pathways supporting swift acquisition of new visuomotor skills. Cereb Cortex. 2017;27(1):173–84.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Nat Acad Sci. 2008;105(34):12569–74.
Weissman DH, Roberts K, Visscher K, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971.
Rémy F, Wenderoth N, Lipkens K, et al. Dual-task interference during initial learning of a new motor task results from competition for the same brain areas. Neuropsychologia. 2010;48(9):2517–27.
Shuggi IM, Oh H, Shewokis PA, et al. Mental workload and motor performance dynamics during practice of reaching movements under various levels of task difficulty. Neuroscience. 2017;360:166–79.
Nakata H, Yoshie M, Miura A, et al. Characteristics of the athletes’ brain: evidence from neurophysiology and neuroimaging. Brain Res Rev. 2010;62(2):197–211.
Wilkerson GB, Nabhan DC, Prusmack CJ, et al. Detection of persisting concussion effects on neuromechanical responsiveness. Med Sci Sports Exerc. 2018;50(9):1750–6.
Kapreli E, Athanasopoulos S. The anterior cruciate ligament deficiency as a model of brain plasticity. Med Hypotheses. 2006;67(3):645–50.
Pitman MI, Nainzadeh N, Menche D, Gasalberti R, Song EK. The intraoperative evaluation of the neurosensory function of the anterior cruciate ligament in humans using somatosensory evoked potentials. Arthroscopy. 1992;8(4):442–7.
Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett. 2004;366(1):63–6.
Im HJ, Kim JS, Li X, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62(10):2995–3005.
Levine JD, Dardick SJ, Basbaum AI, Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985;5(5):1380–6.
Myer GD, Schmitt LC, Brent JL, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377–87.
Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44(10):1948–53.
Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–41.
Ernst GP, Saliba E, Diduch DR, Hurwitz SR, Ball DW. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000;80(3):251–60.
Courtney CA, Durr RK, Emerson-Kavchak AJ, Witte EO, Santos MJ. Heightened flexor withdrawal responses following ACL rupture are enhanced by passive tibial translation. Clin Neurophysiol. 2011;122(5):1005–10.
Konishi Y, Fukubayashi T, Takeshita D. Possible mechanism of quadriceps femoris weakness in patients with ruptured anterior cruciate ligament. Med Sci Sports Exerc. 2002;34(9):1414–8.
Konishi Y, Konishi H, Fukubayashi T. Gamma loop dysfunction in quadriceps on the contralateral side in patients with ruptured ACL. Med Sci Sports Exerc. 2003;35(6):897–900.
Konishi Y, Aihara Y, Sakai M, Ogawa G, Fukubayashi T. Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Scand J Med Sci Sports. 2007;17(4):393–9.
Di Fabio RP, Graf B, Badke MB, Breunig A, Jensen K. Effect of knee joint laxity on long-loop postural reflexes: evidence for a human capsular-hamstring reflex. Exp Brain Res. 1992;90(1):189–200.
Boudreau SA, Farina D, Falla D. The role of motor learning and neuroplasticity in designing rehabilitation approaches for musculoskeletal pain disorders. Man Ther. 2010;15(5):410–4.
Knecht S, Henningsen H, Elbert T, et al. Cortical reorganization in human amputees and mislocalization of painful stimuli to the phantom limb. Neurosci Lett. 1995;201(3):262–4.
Torquati K, Pizzella V, Babiloni C, et al. Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: an MEG study using fMRI constraints. NeuroImage. 2005;26(1):48–56.
Grooms DR, Chaudhari A, Page SJ, Nichols-Larsen DS, Onate JA. Visual-motor control of drop landing after anterior cruciate ligament reconstruction. J Athl Train. 2018;53(5):486–96.
Grooms DR, Page SJ, Nichols-Larsen DS, et al. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017;47(3):180–9.
Biedert R, Zwick E. Ligament-muscle reflex arc after anterior cruciate ligament reconstruction: electromyographic evaluation. Arch Orthop Trauma Surg. 1998;118(1-2):81–4.
Konishi Y, Fukubayashi T, Takeshita D. Mechanism of quadriceps femoris muscle weakness in patients with anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2002;12(6):371–5.
Roberts D, Friden T, Stomberg A, Lindstrand A, Moritz U. Bilateral proprioceptive defects in patients with a unilateral anterior cruciate ligament reconstruction: a comparison between patients and healthy individuals. J Orthop Res. 2000;18(4):565–71.
Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013;22(1):1–6.
Pietrosimone BG, McLeod MM, Lepley AS. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4(1):31–5.
Lepley AS, Bahhur NO, Murray AM, Pietrosimone BG. Quadriceps corticomotor excitability following an experimental knee joint effusion. Knee Surg Sports Traumatol Arthrosc. 2013;23(4):1010–7.
Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21(3):736–42.
Bonfim TR, Jansen Paccola CA, Barela JA. Proprioceptive and behavior impairments in individuals with anterior cruciate ligament reconstructed knees. Arch Phys Med Rehabil. 2003;84(8):1217–23.
Friden T, Roberts D, Ageberg E, Walden M, Zatterstrom R. Review of knee proprioception and the relation to extremity function after an anterior cruciate ligament rupture. J Orthop Sports Phys Ther. 2001;31(10):567–76.
Heroux ME, Tremblay F. Weight discrimination after anterior cruciate ligament injury: a pilot study. Arch Phys Med Rehabil. 2005;86(7):1362–8.
Heroux ME, Tremblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):823–33.
Norte GE, Pietrosimone BG, Hart JM, Hertel J, Ingersoll CD. Relationship between transcranial magnetic stimulation and percutaneous electrical stimulation in determining the quadriceps central activation ratio. Am J Phys Med Rehabil. 2010;89(12):986–96.
Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. NeuroImage. 2006;31(2):710–20.
On AY, Uludag B, Taskiran E, Ertekin C. Differential corticomotor control of a muscle adjacent to a painful joint. Neurorehabil Neural Repair. 2004;18(3):127–33.
Tinazzi M, Rosso T, Zanette G, Fiaschi A, Aglioti SM. Rapid modulation of cortical proprioceptive activity induced by transient cutaneous deafferentation: neurophysiological evidence of short-term plasticity across different somatosensory modalities in humans. Eur J Neurosci. 2003;18(11):3053–60.
Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115(6):1264–75.
Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. NeuroImage. 1999;10(6):682–94.
Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36(Suppl 2):T155–63.
Meister I, Krings T, Foltys H, et al. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: implications for cortical motor organization. Hum Brain Mapp. 2005;25(3):345–52.
Chen TL, Babiloni C, Ferretti A, et al. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: an fMRI study. NeuroImage. 2008;40(4):1765–71.
Ferretti A, Babiloni C, Gratta CD, et al. Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: an fMRI study. NeuroImage. 2003;20(3):1625–38.
Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–36.
Bonner MF, Price AR. Where is the anterior temporal lobe and what does it do? J Neurosci. 2013;33(10):4213–5.
Smith WM, Bowen KF. The effects of delayed and displaced visual feedback on motor control. J Mot Behav. 1980;12(2):91–101.
Warren WH Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4(2):213–6.
Amador N, Fried I. Single-neuron activity in the human supplementary motor area underlying preparation for action. J Neurosurg. 2004;100(2):250–9.
Dault MC, Frank JS, Allard F. Influence of a visuo-spatial, verbal and central executive working memory task on postural control. Gait Posture. 2001;14(2):110–6.
Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res. 1993;97(1):139–44.
Remaud A, Boyas S, Lajoie Y, Bilodeau M. Attentional focus influences postural control and reaction time performances only during challenging dual-task conditions in healthy young adults. Exp Brain Res. 2013;231(2):219–29.
Wulf G. Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exer Psychol. 2013;6(1):77–104.
Babiloni C, Marzano N, Infarinato F, et al. “Neural efficiency” of experts’ brain during judgment of actions: a high-resolution EEG study in elite and amateur karate athletes. Behav Brain Res. 2010;207(2):466–75.
Del Percio C, Babiloni C, Marzano N, et al. “Neural efficiency” of athletes’ brain for upright standing: a high-resolution EEG study. Brain Res Bull. 2009;79(3-4):193–200.
Ageberg E, Roberts D, Holmstrom E, Friden T. Balance in single-limb stance in patients with anterior cruciate ligament injury: relation to knee laxity, proprioception, muscle strength, and subjective function. Am J Sports Med. 2005;33(10):1527–35.
Jarus T, Gutman T. Effects of cognitive processes and task complexity on acquisition, retention, and transfer of motor skills. Can J Occup Ther. 2001;68(5):280–9.
Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97.
Grooms D, Simon JE, Onate JA; Athletic Trainers Osteoarthritis Consortium. Neuroplasticity and patient-reported outcomes after anterior cruciate ligament reconstruction. Ohio University Athletic Training. 2016.
Grooms DR, Myer GD. Upgraded hardware horizontal line What about the software? Brain updates for return to play following ACL reconstruction. Br J Sports Med. 2017;51(5):418–9.
Grooms DR, Onate JA. Neuroscience application to noncontact anterior cruciate ligament injury prevention. Sports Health. 2015;8(2):149–52.
Roberts D, Friden T, Zatterstrom R, Lindstrand A, Moritz U. Proprioception in people with anterior cruciate ligament-deficient knees: comparison of symptomatic and asymptomatic patients. J Orthop Sports Phys Ther. 1999;29(10):587–94.
Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–39.
Pietrosimone BG, Lepley AS, Ericksen HM, Clements A, Sohn DH, Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(6):665–74.
Houck JR, Wilding GE, Gupta R, De Haven KE, Maloney M. Analysis of EMG patterns of control subjects and subjects with ACL deficiency during an unanticipated walking cut task. Gait Posture. 2007;25(4):628–38.
Negahban H, Ahmadi P, Salehi R, Mehravar M, Goharpey S. Attentional demands of postural control during single leg stance in patients with anterior cruciate ligament reconstruction. Neurosci Lett. 2013;556:118–23.
O’Connell M, George K, Stock D. Postural sway and balance testing: a comparison of normal and anterior cruciate ligament deficient knees. Gait Posture. 1998;8(2):136–42.
Hoffman M, Schrader J, Koceja D. An investigation of postural control in postoperative anterior cruciate ligament reconstruction patients. J Athl Train. 1999;34(2):130–6.
Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC. Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–8.
McLean SG, Neal RJ, Myers PT, Walters MR. Knee joint kinematics during the sidestep cutting maneuver: potential for injury in women. Med Sci Sports Exerc. 1999;31(7):959–68.
McLean SG, Lipfert SW, van den Bogert AJ. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004;36(6):1008–16.
Swanik CB, Lephart SM, Giraldo JL, DeMont RG, Fu FH. Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. J Athl Train. 1999;34(2):121–9.
Ford KR, Myer GD, Smith RL, Byrnes RN, Dopirak SE, Hewett TE. Use of an overhead goal alters vertical jump performance and biomechanics. J Strength Cond Res. 2005;19(2):394–9.
Wikstrom E, Tillman M, Schenker S, Borsa P. Failed jump landing trials: deficits in neuromuscular control. Scand J Med Sci Sports. 2008;18(1):55–61.
Cortes N, Blount E, Ringleb S, Onate JA. Soccer-specific video simulation for improving movement assessment. Sports Biomech. 2011;10(1):22–34.
McLean SG, Huang X, van den Bogert AJ. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clin Biomech. 2005;20(8):863–70.
Pollard CD, Heiderscheit BC, van Emmerik RE, Hamill J. Gender differences in lower extremity coupling variability during an unanticipated cutting maneuver. J Appl Biomech. 2005;21(2):143–52.
Brown TN, Palmieri-Smith RM, McLean SG. Sex and limb differences in hip and knee kinematics and kinetics during anticipated and unanticipated jump landings: implications for anterior cruciate ligament injury. Br J Sports Med. 2009;43(13):1049–56.
Pollard CD, Davis IM, Hamill J. Influence of gender on hip and knee mechanics during a randomly cued cutting maneuver. Clin Biomech. 2004;19(10):1022–31.
Almonroeder TG, Kernozek T, Cobb S, et al. Cognitive demands influence lower extremity mechanics during a drop vertical jump task in female athletes. J Orthop Sports Phys Ther. 2018;48(5):381–7.
Besier TF, Lloyd DG, Ackland TR, et al. Anticipatory effects on knee joint loading during running and cutting maneuvers. Med Sci Sports Exerc. 2001;33(7):1176–81.
Meinerz CM, Malloy P, Geiser CF, et al. Anticipatory effects on lower extremity neuromechanics during a cutting task. J Athl Train. 2015;50(9):905–13.
Monfort SM, Comstock RD, Collins CL, Onate JA, Best TM, Chaudhari AM. Association between ball-handling versus defending actions and acute noncontact lower extremity injuries in high school basketball and soccer. Am J Sports Med. 2015;43(4):802–7.
Dingenen B, Janssens L, Luyckx T, Claes S, Bellemans J, Staes FF. Lower extremity muscle activation onset times during the transition from double-leg stance to single-leg stance in anterior cruciate ligament injured subjects. Hum Mov Sci. 2015;44:234–45.
Dingenen B, Janssens L, Luyckx T, Claes S, Bellemans J, Staes FF. Postural stability during the transition from double-leg stance to single-leg stance in anterior cruciate ligament injured subjects. Clin Biomech. 2015;30(3):283–9.
Sadoghi P, von Keudell A, Vavken P. Effectiveness of anterior cruciate ligament injury prevention training programs. J Bone Joint Surg. 2012;94(9):769–76.
Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40(1):41–8.
Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013;46(3):515–20.
Wilkerson GB. Neurocognitive reaction time predicts lower extremity sprains and strains. Int J Athl Ther Train. 2012;17(6):4–9.
Appelbaum LG, Cain MS, Schroeder JE, Darling EF, Mitroff SR. Stroboscopic visual training improves information encoding in short-term memory. Atten Percept Psychophys. 2012;74:1–11.
Appelbaum LG, Schroeder JE, Cain MS, Mitroff SR. Improved visual cognition through stroboscopic training. Front Psychol. 2011;2:276.
Swanik CBSK, Huxel KC, Tiemey RT, Hamstra KL, Hillstrom HJ. EMG and kinematic analysis of drop jumps from an unknown height: a mechanism for non-contact injuries. J Athl Train. 2003;38(2):S18.
Swanik K, Swanik C, Huxel K, Huston K, Fierney R, Hillerstrom H. Gender difference in drop jumps from unknown heights: an EMG and kinematic analysis. J Athl Train. 2004;39:S14–5.
Mitroff SFP, Bennett D, Yoo H, Reichow A. Enhancing ice hockey skills through stroboscopic visual training: a pilot study. Athl Train Sports Health Care. 2013;5(6):261–4.
Smith TQ, Mitroff SR. Stroboscopic training enhances anticipatory timing. Int J Exerc Sci. 2012;5(4):4.
Di Stasi S, Myer GD, Hewett TE. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2013;43(11):777–92.
Kim KM, Kim JS, Grooms DR. Stroboscopic vision to induce sensory reweighting during postural control. J Sport Rehabil. 2017;26:1–11.
Harpham JA, Mihalik JP, Littleton AC, Frank BS, Guskiewicz KM. The effect of visual and sensory performance on head impact biomechanics in college football players. Ann Biomed Eng. 2013;42(1):1–10.
Mihalik JP, Blackburn JT, Greenwald RM, Cantu RC, Marshall SW, Guskiewicz KM. Collision type and player anticipation affect head impact severity among youth ice hockey players. Pediatrics. 2010;125(6):E1394–401.
Houck JR, De Haven KE, Maloney M. Influence of anticipation on movement patterns in subjects with ACL deficiency classified as noncopers. J Orthop Sports Phys Ther. 2007;37(2):56–64.
Pelletier R, Higgins J, Bourbonnais D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet Disord. 2015;16(1):25.
Ross LM, Register-Mihalik JK, Mihalik JP, et al. Effects of a single-task versus a dual-task paradigm on cognition and balance in healthy subjects. J Sport Rehabil. 2011;20:296–310.
Teel EF, Register-Mihalik JK, Troy Blackburn J, Guskiewicz KM. Balance and cognitive performance during a dual-task: preliminary implications for use in concussion assessment. J Sci Med Sport. 2013;16:190–4.
Verburgh L, Scherder EJ, Van Lange PA, Oosterlaan J. Do elite and amateur soccer players outperform non-athletes on neurocognitive functioning? A study among 8-12 year old children. PLoS One. 2016;11:e0165741.
Alhilali LM, Yaeger K, Collins M, Fakhran S. Detection of central white matter injury underlying vestibulopathy after mild traumatic brain injury. Radiology. 2014;272:224–32.
Schubert MC, Minor LB. Vestibulo-ocular physiology underlying vestibular hypofunction. Phys Ther. 2004;84:373–85.
Hillier S, McDonnell M. Is vestibular rehabilitation effective in improving dizziness and function after unilateral peripheral vestibular hypofunction? An abridged version of a Cochrane Review. Eur J Phys Rehabil Med. 2016;52:541–56.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Onate, J., Herman, D., Grooms, D., Sutton, Z., Wilkerson, G. (2019). Neuroscience Principles for ACL Rehabilitation and Reinjury Risk Reduction. In: Noyes, F., Barber-Westin, S. (eds) Return to Sport after ACL Reconstruction and Other Knee Operations. Springer, Cham. https://doi.org/10.1007/978-3-030-22361-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-22361-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22360-1
Online ISBN: 978-3-030-22361-8
eBook Packages: MedicineMedicine (R0)