Abstract
Regenerative medicine, using stem cells with or without scaffolds, allows surgeons to treat congenital or acquired defects with normal structure and functions. Although stem cell and regenerative medicine are nowadays used quite often in some areas of medicine, it is obvious that they are in their crawling stage in reconstructive surgery and in rhinology. However, it is not difficult to predict that it will become popular in the next few decades and will be among the treatment regimens in the guidelines. The various developments in isolation, duplication, and differentiation of stem cells and three-dimensional scaffolds suggest that some of the more frequently applied therapies may be shelved in the close future. Stem cells are categorized as embryonic stem cells, induced pluripotent stem cells and adult stem cells. Adult stem cells are generally preferred in practice nowadays due to ease of isolation and differentiation stages. The purpose of this section is to provide information on stem cell applications and regenerative medicine in rhinology at present and in close future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The cells that have the potential to regenerate themselves and differentiation capacity are named as “stem cells.” Regenerative medicine allows engineering the damaged tissues and organs by stimulating human’s own repair mechanisms usually by using differentiated stem cells and scaffolds [1]. Regenerative medicine, using stem cells with or without scaffolds, allows surgeons to treat congenital or acquired defects with normal structure and functions. Although stem cell and regenerative medicine are nowadays used quite often in some areas of medicine, it is obvious that they are in their crawling stage in reconstructive surgery and in rhinology. However, it is not difficult to predict that it will become popular in the next few decades and will be among the treatment regimens in the guidelines. The various developments in isolation, duplication, and differentiation of stem cells and three-dimensional scaffolds suggest that some of the more frequently applied therapies may be shelved in the close future. The purpose of this section is to provide information on stem cell applications and regenerative medicine in rhinology at present and in close future.
Stem cells have been named accordingly to the origins they are obtained:
-
Embryonic stem cells (ESC).
-
Induced pluripotent stem cells (IPSC).
-
Adult stem cells (ASC) [2].
The regeneration potential of ESC is quite high. They have the ability to transform into three different germinal cells as ectodermal-, endodermal-, and mesodermal-originated cells [3]. The risk of malignancy development and low compliance with the immune system are the two disadvantages of the ESC [4]. Clinical use of ESC has severely been limited due to ethical problems [5]. IPSC can be generated from adult cells via reprogramming [6]. Their differentiation capacities are similar to ESC and can be differentiated into three different types of germinal cells [7]. Reprogramming of the cells can be done by viral vectors or nonviral factors [8, 9]. IPSC are used in the pharmaceutical industry, modeling of diseases, and regenerative medicine [10].
Adult stem cells are used for regenerative medicine frequently and derived from adult cells. The negative features of ASC are their limited differentiation capacities and traces from the origins of their textures. Isolation, differentiation, and autologous transplantation of ASC are easy, and the relatively small number of ethical considerations made it very popular in clinical practice [11, 12]. Mesenchymal stem cells (MSC) - a kind of ASC - are the most frequently used stem cell group in rhinology. MSC can be obtained from the tissues of adipose, bone marrow, muscle, or skin and generally referred to by their tissue origin as adipose-derived mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, etc. [13,14,15,16] Adipose-derived mesenchymal stem cells (ADMSC) are preferably used because of their ease of harvesting and limited morbidity in donor site [17]. ADMSC are not only the precursors of adipocytes but also multipotent progenitors of different type of cells. The presence of different progenitor cells allows differentiation ADMSC to osteoblast, chondrocyte, myocyte, epithelial cells, and neuronal cells [18, 19].
Studies about regenerative medicine in rhinology are still in their early stage, are growing at a rapid pace, and remain popular. Regenerative medicine consists of 3 parts: cells, scaffold, and adequate stimulus. There are 4 steps in development of tissue engineering: step 1, tissue growing in scaffold; step 2, tissue growing around the separation construction; step 3, lamination of 100 μ thick tissue layers grown in a special bioreactor; and step 4, tissue growing with blood vessels [20].
From a surgical point of view, the nose consists three different main layers: mucosa (epithelium), cartilage, and skin. Multiple- or single-layer tissues can be formed according to defects or surgeon’s desire. The next part of this chapter is to review the literature related of regenerative medicine in rhinology and present future perspectives.
2 Nasal Epithelium
Nasal epithelium is a complex structure that has many functions such as cleaning, unspecific defense (mucociliary clearance), humoral and cellular immune defense, cellular nasal cycle, microbiological colonization, and regeneration [21]. The defense mechanism of nasal mucosa includes ciliary epithelial cells, mucus glands, and production of various cytokines, chemokines and mediators. The mucosa needs to work in harmony with other nasal structures and components in order to obtain optimally effective nasal functions [19]. Any problem in these components or structures may result in mucosal inflammation leading to chronic nasal diseases. Nasal mucosa contains some differences compared to other respiratory tracts’ mucosa such as ciliary beat frequency and number of goblet cells. In vitro epithelial culture methods (air-liquid interface, ALI culture) have been developed to objectively evaluate the nasal mucosa functions or dysfunctions. ALI cultures mimic the nasal mucosa behaviors and allow the assessment of nasal mucosa functions such as nasociliary beat frequency, levels of cytokine, and mediators without any independent external factors. Nasal mucosa functions against possible pathogens and responses to pharmacological and/or toxic agents could be evaluated with these ALI cultures [22]. It is possible to show the effects of drugs on nasociliary beat frequency and pathophysiology of infection in the virus-infected ALI cultures [22, 23].
The treatment of septal perforation is a difficult and complex surgical procedure. Although the regenerative potential of nasal mucosa is high, local or free flaps are needed if the diameter of the perforation is greater than 2 cm. It is obvious that these flaps may also affect nasal functions in a negative manner. Therefore, regenerative medicine offering the same tissue properties may be an option while repairing septal perforation. There are studies in the literature with promising results showing that cultured nasal epithelial cells in tissue scaffolds may be used as nasal mucosal grafts in the future [24]. Nasal epithelial cells cultured on chitosan (a bioscaffold) may be used for treatment of septal perforation or CSF leaks [24]. In addition, acellular dermis (AlloDerm, LifeCell, NJ, USA), a bioscaffold, is shown to be an alternative when used as a interposition graft and induces the recovery of defect via secondary wound healing [25]. Scaffolds are the important components of the regenerative medicine and provide the opportunity for the cells to live and have desired mechanical properties. Therefore, bioscaffolds may be reinforced with other materials as collagen sponges with polypropylene. Fibroblasts and ADSC were seeded on these scaffold to build artificial tracheal tissue and transplanted into rats successfully [26]. Reinforced collagen sponge with crystalline polypropylene and high-density polyethylene was used to repair a tracheal defect, and after transplantation of 2 months, graft was surrounded with respiratory epithelium [27]. In 2008, Macchiarini et al. cultured respiratory mucosa and implanted it into cadaveric trachea. This artificial trachea was transplanted to a patient with advanced stenosis in the left main bronchus due to tuberculosis [28]. No major complications except for a necessity of dilatation were seen during a 5-year follow-up, and it was observed that it turned into a normally functioning trachea [29].
3 Cartilage
Cartilage is probably the most commonly used autograft in rhinology. Septal cartilage is usually preferred as a first-line graft because of its minimal donor site morbidity and mechanical properties. On the other hand, it may be difficult to obtain sufficient amount of septal cartilage in revision cases. Auricular and/or rib cartilage may be used, but the surgeons do not prefer them due to their mechanical properties or warping problems. Therefore, regenerative medicine is thought to be a common graft option for the treatment of nasal defects in the future. Three components are very important for the artificial cartilage: chondrocytes, scaffold, and growth factors.
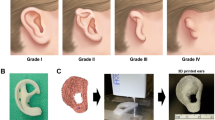
Chondrocytes can be multiplied or differentiated from mesenchymal adult stem cells. In a recent study, auricular chondrocytes were harvested and cultured and then implanted for augmentation into subcutaneous pocket of the nasal dorsum [30]. This procedure was successfully applied to 75 cases with a 6-year follow-up period. Two cases with septal perforation in which ADMSC seeded on chronOS Strip (Synthes Inc., USA) as a supportive material and bilateral mucosal flaps were used to close the defect [31]. In one patient, no perforation was seen in 28 months after surgery; on the other hand, the other patient continued the nasal picking and the scaffold was removed 12 months after surgery [31]. ADMSC are frequently used in stem cell-differentiated chondrogenesis due to low donor site morbidity and ease of isolation and differentiation stages. Isolation and differentiation stages could be made with readily avaliable culture media [49]. In a previous study, ADMSC was isolated and differentiated into chondrocytes with abovementioned culture media, then ADMSC derived chondrocytes were implanted on a bioactive glass scaffold and transplanted into the rat’s ear [32]. After a month, rats were sacrificed, and presence of chondrocytes was shown immunohistochemically in transplantation areas [32]. For the clinical application of chondrocytes derived from ADMSC, they were implanted into cadaveric trachea and transplanted into patients with advanced stage of left main bronchus malacia successfully, and long-term result was good [29].
For promoting mature cartilage, cells are induced with growth factors as transforming growth factors β1, β2, or β3 (β2 and β3 more potent than β1), bone morphogenic proteins, and fibroblast growth factors [33]. Growth factors are necessary to maintain cell phenotype and induce tissue growing.
In bioengineering of cartilage, the most important expectation in the future is to make cartilage with enough rigidity and easy shaping. Therefore, scaffold technology needs to be further developed and evaluated. Several scaffolds are used in regenerative medicine, but there is no consensus about the scaffold. In addition, the ideal scaffold should be biocompatible, biodegradable, non-immunogenic and non-toxigenic. Hydrogels have good chondrogenic features and, on the other hand, have not good mechanical properties. Also, cell viability problems may have been seen in some synthetic fiber scaffolds [34]. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) is a biodegradable and biocompatible scaffold and has good features for mechanical properties and chondrogenesis [35]. On the other hand, fragility and costing problems are the disadvantages of this scaffold. Cartilage growth was promoted by some scaffolds as collagen sponges and polyvinyl alcohol [36]. New skeleton designs are developing rapidly using the abovementioned materials by electrospinning and three-dimensional printing. Studies that evaluated the scaffold usually show short-term outcomes, but long-term outcomes are also very important and further investigations that show long-term outcomes of scaffold are needed.
4 Skin
Nose is in the most important location of the human face. Therefore, nasal defects should be repaired to achieve aesthetically acceptable results in accordance with the physiology and histology. Nasal skin defects usually occur as a result of tumor excision or trauma. Reconstruction can be difficult due to the size of defect area and aesthetic concerns. Paramedian forehead flaps are frequently used when repairing large defects, while most surgeons worry about the aesthetic outcomes (poor color matches, bulky, hair follicles) in these patients. Both dermis and epidermis must be reconstructed simultaneously.
Dermic templates that provide dermis regeneration are generally used in reconstructive surgery. Integra™ (Integra LifeSciences Corp., NJ, USA) was used for nasal dorsal augmentation and completely resorbed and displacement with autologous tissues [37]. This component is supposed to be superior to cadaveric acellular dermis.
It is thought that ADMSC will be very popular in skin repair in the future because it allows the differentiation of fibrovascular, endothelial, and epithelial tissues [38]. ADSMC have shown positive effects on the formation of new epidermis, prevention of skin aging, acceleration of angiogenesis, and proliferation of fibroblasts [39, 40]. In a rat model, mesenchymal stromal cell-seeded scaffolds significantly induced the epithelial thickness and hair follicle counts and reduced deposition of collagen compared to non-seeded cells on scaffolds in skin defects [41]. With today’s technology, it is not possible to create bioengineered skin that is fully functioning and color matched yet.
5 Olfaction
The olfactory epithelium is a specialized sensory area and responsible for olfaction in the nasal cavity. Olfactory epithelium consists of olfactory ensheathing cells (OEC), olfactory stem cells, olfactory sensory neurons, olfactory glands, supporting cells, and other cells. Olfactory system is quite different than the other sensory mechanism and could be regenerated its neurons by OEC. Releasing growth factor from OEC is very important for regeneration of olfactory epithelium [42]. Precursor cells were harvested from olfactory epithelium and could induce the regeneration of degenerated olfactory epithelium following implantation [43]. In vitro three-dimensional olfactory epithelial cultures can be created and then could be transplanted into degenerate epithelium [44]. In the future, olfaction disorders could be treated with development of the modification and cultivation of the olfactory epithelium cells.
Olfactory ensheathing cells have the potential to regenerate not only olfactory neurons but also other neurons in the other parts of body [45]. OEC are used for experimental treatment method in spinal cord lesions, peripheral nerve injuries, stroke, Parkinson’s disease, and amyotrophic lateral sclerosis due to its regenerative effects on neurons [42]. It is stated that the results of these studies are satisfactory and promising. Olfactory epithelial cells can be used not only for the treatment but also for diagnosis of schizophrenia, bipolar disorder, Alzheimer’s disease, and Parkinson’s disease [46,47,48].
6 Conclusion
Stem cells offer promising improvements in the treatment of diseases and malformations, especially in the reconstruction of tissue defects. Unfortunately, there is not enough scientific data available today to comment on clinical practice. For regenerative medicine, source of stem cells, efficacy of transplantation, integration with microenvironment, long-term results of transplanted cells, and effects of scaffolds on stem cells and target tissues will be one of the most emphasized topics in clinical practice in the near future.
References
Walia B, Satija N, Tripathi RP, Gangenahalli GU. Induced pluripotent stem cells: fundamentals and applications of the reprogramming process and its ramifications on regenerative medicine. Stem Cell Rev. 2012;8(1):100–15.
Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10.
Atala A. Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert Opin Biol Ther. 2005;5(7):879–92.
Hess PG. Risk of tumorigenesis in first-in-human trials of embryonic stem cell neural derivatives: Ethics in the face of long-term uncertainty. Account Res. 2009;16(4):175–98.
Camporesi S. The context of embryonic development and its ethical relevance. Biotechnol J. 2007;2(9):1147–53.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–53.
Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–5.
Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349(6):570–82.
McCormick JB, Huso HA. Stem cells and ethics: current issues. J Cardiovasc Transl Res. 2010;3(2):122–7.
Wu CH, Lee FK, Suresh Kumar S, Ling QD, Chang Y, Chang Y, et al. The isolation and differentiation of human adipose-derived stem cells using membrane filtration. Biomaterials. 2012;33(33):8228–39.
Mamidi MK, Nathan KG, Singh G, Thrichelvam ST, Mohd Yusof NA, Fakharuzi NA, et al. Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. J Cell Biochem. 2012;113(10):3153–64.
Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 2012;73(8):1305–17.
Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Skin-derived multipotent stromal cells--an archrival for mesenchymal stem cells. Cell Tissue Res. 2012;350(1):1–12.
Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11(57):160–70.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.
Uz U, Günhan K. Stem cell and regenerative medicine in rhinology: review. Turk J Rhinol. 2015;4(2):90–6.
Nose Y, Okubo H. Artificial organs versus regenerative medicine: is it true? Artif Organs. 2003;27(9):765–71.
Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2010;9. Doc07
Uz U, Chen B, Palmer JN, Cingi C, Unlu H, Cohen NA. Effects of thymoquinone and montelukast on sinonasal ciliary beat frequency. Am J Rhinol Allergy. 2014;28(2):122–5.
Wang DY, Li Y, Yan Y, Li C, Shi L. Upper airway stem cells: understanding the nose and role for future cell therapy. Curr Allergy Asthma Rep. 2015;15(1):490.
Huang TW, Young YH, Cheng PW, Chan YH, Young TH. Culture of nasal epithelial cells using chitosan-based membranes. Laryngoscope. 2009;119(10):2066–70.
Lee KC, Lee NH, Ban JH, Jin SM. Surgical treatment using an allograft dermal matrix for nasal septal perforation. Yonsei Med J. 2008;49(2):244–8.
Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, et al. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials. 2010;31(18):4855–63.
Omori K, Nakamura T, Kanemaru S, Asato R, Yamashita M, Tanaka S, et al. Regenerative medicine of the trachea: the first human case. Ann Otol Rhinol Laryngol. 2005;114(6):429–33.
Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–30.
Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet. 2014;383(9913):238–44.
Yanaga H, Imai K, Yanaga K. Generative surgery of cultured autologous auricular chondrocytes for nasal augmentation. Aesthet Plast Surg. 2009;33(6):795–802.
Sandor GK, Numminen J, Wolff J, Thesleff T, Miettinen A, Tuovinen VJ, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med. 2014;3(4):530–40.
Gunhan K, Bariskan S, Uz U, Vatansever S, Kivanc M. 13-93B3 bioactive glass: a new scaffold for transplantation of stem cell-derived chondrocytes. J Craniofac Surg. 2018;29(1):233–6.
Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15(7):1543–51.
Duda GN, Haisch A, Endres M, Gebert C, Schroeder D, Hoffmann JE, et al. Mechanical quality of tissue engineered cartilage: results after 6 and 12 weeks in vivo. J Biomed Mater Res. 2000;53(6):673–7.
Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, et al. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006;12(10):2729–38.
Gonzalez JS, Alvarez VA. Mechanical properties of polyvinyl alcohol/hydroxyapatite cryogel as potential artificial cartilage. J Mech Behav Biomed Mater. 2014;34:47–56.
Planas J. The use of Integra in rhinoplasty. Aesthet Plast Surg. 2011;35(1):5–12.
Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, et al. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27(1):250–8.
Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19(11–12):1327–35.
Zhao J, Hu L, Liu J, Gong N, Chen L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res Int. 2013;2013:578479.
Formigli L, Paternostro F, Tani A, Mirabella C, Quattrini A, Nosi D, et al. MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015.
Chou RH, Lu CY, Wei L, Fan JR, Yu YL, Shyu WC. The potential therapeutic applications of olfactory ensheathing cells in regenerative medicine. Cell Transplant. 2014;23(4–5):567–71.
Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9(7):1611–7.
Jang W, Lambropoulos J, Woo JK, Peluso CE, Schwob JE. Maintaining epitheliopoietic potency when culturing olfactory progenitors. Exp Neurol. 2008;214(1):25–36.
Mariano ED, Teixeira MJ, Marie SK, Lepski G. Adult stem cells in neural repair: Current options, limitations and perspectives. World J Stem Cells. 2015;7(2):477–82.
McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82(2–3):163–73.
Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67(4):462–9.
Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. 2010;3(11–12):785–98.
Uzdan Uz, Kivanc Gunhan, Seda Vatansever, Mujde Kivanc, Ali Vefa Yuceturk, Novel Simple Strategy for Cartilage Tissue Engineering Using Stem Cells and Synthetic Polymer Scaffold. Journal of Craniofacial Surgery 30 (3):940–943.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Günhan, K., Uz, U. (2020). Regenerative Medicine in Rhinology. In: Cingi, C., Bayar Muluk, N. (eds) All Around the Nose. Springer, Cham. https://doi.org/10.1007/978-3-030-21217-9_87
Download citation
DOI: https://doi.org/10.1007/978-3-030-21217-9_87
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21216-2
Online ISBN: 978-3-030-21217-9
eBook Packages: MedicineMedicine (R0)