Abstract
Progenitor stem cells have been identified, isolated and characterized in numerous tissues and organs. However, their therapeutic potential and the use of these stem cells remain elusive except for a few progenitor cells from bone marrow, umbilical cord blood, eyes and dental pulp. The use of bone marrow-derived hematopoietic stem cells (HSC) or mesenchymal stem cells (MSCs) is restricted due to their extreme invasive procedures, low differentiation potential with age and rejection. Thus, we need a clinical grade alternative to progenitor stem cells with a high potential to differentiate, naïve and is relatively easy in in vitro propagation. In this review, we summarize cell populations of adherent and floating spheres derived from different origins of skin, or correctly foreskin, by enzymatic digestion compared with established MSCs. The morphology, phenotype, differentiation capability and immunosuppressive property of the adherent cell populations are comparable with MSCs. Serum-free cultured floating spheres have limited mesodermal but higher neurogenic differentation potential, analogous to neural crest stem cells. Both the populations confirmed their plethora potential in in vitro. Together, it may be noted that the skin-derived adherent cell populations and floating cells can be good alternative sources of progenitor cells especially in cosmetic, plastic and sports regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells or progenitor cells have remained a promising source of regenerative cells for more than four decades, with prominent innovations to suit life science, medicine and biomedical engineering (Bianco and Robey 2001; Campisi and d'Adda di Fagagna 2007; Caplan 2007; Ikada 2006). Progenitor cells have revolutionized medicine and improved the quality of human life worldwide by repairing, preserving, or enhancing tissue and organ function. This has been classified under cell-based therapy with a great deal of debate on the use of scaffold or modes of tissue engineering that may or may not be detrimental in human applications (Bianco and Robey 2001; Ikada 2006). However, the avenues of tissue engineering are not closed and there is now a large literature source that mediates better understanding on the mechanistic functions of the stem cells (Bianco and Robey 2001; Ikada 2006). Eventually, what makes the difference in the obvious use of adult stem cells over embryonic stem cells is the ethical concerns (Robertson 2010) and fear of teratoma (Blum and Benvenisty 2008) observed predominantly in a later source. Currently, there is much need of progenitor cells, mostly in organ repair, either in regenerating a portion or to condition the graft (Rafii and Lyden 2003).
A major setback is the lack of organ donors for regenerative medicine (Stock and Vacanti 2001). This has motivated the researchers to hunt for a non-invasive source of stem cells or to reprogram fibroblasts to produce diverse and mature progenies with the potential to self-sustain and -maintain (Yamanaka 2008; Ramesh et al. 2009). Several experimental studies have addressed the potential of different types of stem cells and their ability to repair or regenerate damaged tissues or organs (Bianco and Robey 2001; Campisi and d'Adda di Fagagna 2007; Caplan 2007; Hipp and Atala 2008; Ikada 2006; Rafii and Lyden 2003).
Skin has long been researched for its use as a potential source of regenerative cells (Tables 1, 2). For the past decade, several works have elaborated the differentiation potential of skin progenitor cells or reprogramming of the skin fibroblast to progenitor-like cells (Yamanaka 2008; Ramesh et al. 2009).
The presence of regenerative cells in tissues and organs can be attributed to sustain homeostasis and respond to damage. The presence of stem cells regulates the matured cell population constant pool of progenitors, which in turn provides the microenvironment for regulatory signal communications and regeneration. (Walker et al. 2009). For instance, an established study shows bone marrow as a provider of fibroblast-like cells in the dermis and keratinocytes in the epithelia (Aoki et al. 2004). Recently, the need for stem cells in various therapies has led to revisiting less invasive ways to obtain an abundance of progenitor cells. The applications today vary from systemic to plastic or sports surgery; the plasticity of the cells allowing easy grafting at the site of application remains the target for whatever type or source from which the progenitors are isolated (Clover et al. 2012; Moseley et al. 2006). Skin has been long debated to be a potential progenitor cell source. However, in the past decade, ample evidence has successfully characterized skin harboring progenitor cells (Tables 1, 2). A few recent reports have shown that foreskin, a designated biological waste material removed from infants, has been an excellent source of progenitor-like cells (Al-Nbaheen et al. 2012; Vishnubalaji et al. 2012b).
Although adult stem cells have been isolated from different sources, recent studies have demonstrated that skin stromal cells have similar properties as mesenchymal stem cells (Al-Nbaheen et al. 2012; Vishnubalaji et al. 2012b). According to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), these skin-derived stromal cells are plastic adherent and positive for mesenchymal stem cell markers CD29, CD44, CD71, CD73, CD105, CD90 and CD166 and negative for CD45, CD14, CD34, CD133 and HLA-DR. The skin-derived mesenchymal cells or stromal cells exhibit these stem cell markers confirming their mesenchymal lineage. In addition, they can differentiate into adipogenic and osteogenic lineages (Al-Nbaheen et al. 2012; Dominici et al. 2006; Haniffa et al. 2007; Lorenz et al. 2008; Vishnubalaji et al. 2012b).
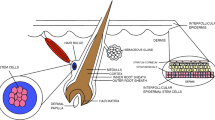
Skin is an abundant human tissue in regenerative science. It consists of epidermis and dermis layers, which are under a constant renewal process and contain a number of cell populations that originate from both mesoderm and ectoderm (Tsatmali et al. 2002). The epidermis is constantly regenerated throughout life by epidermal stem cells with their rapid self-regenerating ability. The cyclic development of hair follicles is reliant on precursor cells that exist in the dermal papillae and the bulge. Following an injury, the skin cells in the dermis contribute to the route of healing (Blanpain and Fuchs 2006). However, there may be other sites within the skin that harbor multipotent progenitors. Dermal dissociation followed by a mixture of specialized cultivation systems have supported the culturing of multipotential cells (Tables 1, 2).
The presence of precursor or mesenchymal stem cells in the skin was perhaps one of the most captivating and therapeutically significant findings of the last few years in the field of adult stem cell research. Presently, investigators assume that there may be several types of adult skin stem cells or progenitors found in both layers of the skin such as dermal stem cells (Al-Nbaheen et al. 2012; Vishnubalaji et al. 2012b), epidermal stem cells (Blanpain and Fuchs 2006) and melanocyte stem cells (Steingrimsson et al. 2005). Although epidermal skin stem cells were discovered first and remain better characterized, non-epidermal stem cells may also have enormous potential and are yet to be explored.
In this review, we provide an insight into the morphological, physiological and biochemical characteristics of skin-derived stromal cell population. Furthermore, this review examines the available literature for its localization, derivation and multipotency, which better correlates with conventional MSCs.
Uses in cell therapy
Cell-based therapy is still a promising choice of treatment for several diseases and superficial injuries (Aldahmash et al. 2012). The most important applications are seen with rheumatoid arthritis, psoriasis, multiple sclerosis and insulin-dependent diabetes mellitus, which are autoimmune disorders. In addition, the progenitors cells are used in spinal cord injury and meniscal tears or in bathing the menisci in transplantations, aiding better menisci homing and also promoting tissue regeneration.
Though various types of stem cells have been utilized in cell-based therapy, there has been a wide gap in the use of in vitro scale-up cells in therapeutic applications. We have previously shown that progenitor cells derived from specific tissues differentiated in vitro such as adipose tissue, with bone marrow stromal cells differentiating into melanocytes and chondrocytes (Fernandes et al. 2004; Kern et al. 2006; Vishnubalaji et al. 2012a b). Evidently, a ranking of cells applied for cell-based therapy will not only be based on the assessment of the functional competences of the cells but also on the safety and achievability of the treatment in the clinical setting. Significantly, the study of skin progenitors or stem cells has evolved as a base for the treatment of skin loss, wound healing, skin disease and hair loss (Lorenz et al. 2008; Shih et al. 2005). Human skin-derived stromal cells (hSDSCs) can be identified in vivo by specific label retention or in vitro by clonogenicity (Chen et al. 2007).
Mesenchymal stem cells
Regarding generic characters and potency of MSCs, their merits and demerits have been illustrated sufficiently in abundant research articles with clinical trial support (Kebriaei and Robinson 2011; Trounson et al. 2011). In this section, we will cover these points. Mesenchymal stem cells or marrow stromal cells are primitive, unique, multipotent stem cells residing mainly in the stromal compartment of the bone marrow but they have also been isolated from niches in other tissues, like adipose tissue, umbilical cord blood, placenta, umbilical cord, amniotic fluid and membrane, dental pulp and from second trimester fetal tissues (liver, lung, and spleen) (Alviano et al. 2007; Biernaskie et al. 2006; Gronthos et al. 2000; in 't Anker et al. 2003; Kebriaei and Robinson 2011; Kern et al. 2006; Li et al. 2005; Vishnubalaji et al. 2012a b; Wang et al. 2004; You et al. 2008). MSCs are positive for stromal-associated markers and negative for endothelial and hematopoietic markers (Kern et al. 2006; Pawitan 2009; Pittenger et al. 1999; Vishnubalaji et al. 2012a b). Stromal cells are a group of clonogenic cells and can proliferate without differentiation, or differentiate into any of the mesenchymal tissues (i.e. the tissues of the body that support the elements, comprising adipose, osseous, cartilaginous, elastic and fibrous connective tissues) depending on various influences from cytokines or growth factors including epidermal growth factor (EGF), platelet-derived growth factors (PDGF) and basic fibroblast growth factor (FGF-2) (Martin et al. 1997). Bone marrow, a complex tissue composed of a highly organized network of hematopoietic, endothelial and mesenchymal cells, has also been used to treat bone or cartilage defects and degeneration (Caplan et al. 1997; Vacanti et al. 1993). Apart from their multilineage differentiation ability, MSCs have the added advantage of having inherent host compatibility, immunosuppressive ability, susceptibility to gene modification and extensive capacity for in vitro expansion. MSCs are known to migrate to some tissues, particularly when injured or under pathological conditions and are capable of secreting a broad spectrum of bioactive macromolecules by themselves that are both immunoregulatory and help to structure the regenerative microenvironments. All this has led to important approaches of utilizing MSCs for tissue engineering as well as clinical trials for gene therapy for a variety of congenital and acquired diseases (Kebriaei and Robinson 2011; Sotiropoulou et al. 2006; Trounson et al. 2011).
The differentiation capability of MSCs in combination with their immunosuppressive effect makes them an ideal challenger for assorted cellular transplantation therapies. Plenty of animal studies have investigated MSCs regeneration ability in various tissues with low or transient levels of engraftment. The bioactive factors secreted by MSCs have both paracrine and autocrine activities. These secreted bioactive factors can alter the local immune system and thereby rejuvenate or repair the diseased or injured tissues with their trophic effects (Caplan and Dennis 2006).
However, current findings suggest that stromal cells from other sources may not be able to substitute bone marrow stromal cells. Stromal cell populations obtained from different tissues showed significant differences in their proliferation, differentiation and molecular phenotype, which should be taken into consideration when planning their use in clinical protocols (Al-Nbaheen et al. 2012; Sakaguchi et al. 2005).
Skin-derived stromal cells
There are several published papers describing the isolation of skin-derived precursors or multipotent stem cells from the skin and foreskin of human, pig, rodents, embryo, or neonates. Particularly, dermal skin is obtained from the face, dorsal back, scalp and thigh (Tables 1, 2). Investigators observed significant alterations in cell morphology depending on culture conditions after isolation of cells with enzymatic digestion such as trypsin, collagenase, dispase and liberase blendzymes (Tables 1, 2). These potential stromal cells were named as SKP (skin derived precursor) cells (Fernandes et al. 2004; Toma et al. 2005), multipotent adult stem cells (Toma et al. 2001) and skin-derived mesenchymal stem cells (Riekstina et al. 2008).
Stromal cells are generated from primitive mesenchyme, like all connective tissue cells. Vimentin expression indicates their mesodermal origination (Huang et al. 2010). The core function of fibroblasts is to sustain the structural integrity of connective tissue by constantly introducing precursors from the extracellular matrix. The dermis of the connective tissue is constructed by collagen and elastin fibers immersed in glycosaminoglycans. The fibroblast progenitors or multipotent stem cells are developed from studies on connective tissues that had revealed evidence of pluripotent or multipotent MSC-like cells. These tissues include cord stroma, adipose tissue, skeletal muscle and placenta, among others (Kern et al. 2006; Pawitan 2009; Peng and Huard 2004; Vishnubalaji et al. 2012b). In addition, investigations on hair development had suggested that there were specialized fibroblast populations in both the dermal papilla and the hair shaft (Biernaskie et al. 2009; Fernandes et al. 2004; Hunt et al. 2008; Jahoda et al. 2003; Ohyama et al. 2006).
In our experience, the foreskin-derived stromal cells (FDSCs) expressed CD90, CD73, CD105, CD166, SH3 and SH4, which is similar to the phenotypes of human bone marrow MSCs. However, in comparison with commercial foreskin fibroblast line (Hs68), FDSCs were positive for CD105 and negative for CD54 antigen. The differentiation of FDSCs into adipocytes, osteocytes, neural cells, Schwann-like cells, smooth muscle cells and hepatocyte- and endothelial- like cells were all uniform, consistent with published mesenchymal lineages (Table 1) (Huang et al. 2010; Manini et al. 2011; Vishnubalaji et al. 2012b).
Stromal cells have immunosuppressive properties that are functionally comparable to mesenchymal stem cells. They share mesenchymal stem cell phenotype, transdifferentiation and are thus difficult to discriminate from MSCs. Dermal fibroblasts inhibit allogeneic T cell activation by autologously derived cutaneous antigen-presenting cells (APCs) and other stimulators under in vitro conditions (Haniffa et al. 2007). Although these progenitor or multipotent stem cell population imitate mesenchymal stem cells, still none of the studies have identified and shown specific markers for histological localization in the adjacent area. Furthermore, in the latest disclosure, even regularly grown fibroblasts can be reprogrammed into pluripotential (iPS) cells by retrovirus-mediated transfection with four transcription factors (Oct-3/4, Sox2, KLF4 and c-Myc) that are highly expressed in embryonic stem cells (ESCs) (Yamanaka 2008; Ramesh et al. 2009). Stromal cells may be a remarkable element for tissue regeneration which further mimic the mesenchymal phenotype.
Skin-derived precursor cells
Several structures are present in the skin apart from the three layers, such as hair follicles, blood vessels, sensory receptors and nerve endings. The precursors maintain the skin integrity by regenerating the different structures. The properties of SKP are analogous to the embryonic neural crest stem cells (NCSCs); they have been isolated from both rodent and human skin (Toma et al. 2001, 2005).
SKPs, if cultured in the presence of EGF and FGF-2 under non-adherent condition, will generate floating spheres (like embryoid bodies) that can be consecutively passaged. They have the ability to generate adipocyte, osteoblast, chondrocyte, neuron, Schwann cells, melanocyte, glial cells, smooth muscle cells and pericyte under appropriate conditions (Fig. 1; Table 1). By contrast, under adherence culture condition without growth factors they will form adherent spindle-shaped cells that have the capability to generate hepatocyte and insulin-producing cells in suitable condition, besides the ectodermal and mesodermal lineages (Fig. 1; Table 1). These cells express vimentin, not nestin (Chen et al. 2007; Shi and Cheng 2004). Immunogenic characterization of floating SKP spheres by flow cytometric analysis proposes that SKPs are bright positive for CD29 (β1-integrin), dim positive for CD49f (transferrin) and CD71, and negative for E-Cadherin, CD34 (hematopoietic marker) and Thy-1.2 (Kawase et al. 2004).
In addition, SKPs cultured as individual single cells (Toma et al. 2001, 2005) or separated skin cells plated at low density such as 25,000 skin cells/ml (Fernandes et al. 2004) produce clonal spheres that can be enlarged, thus causing clonal SKP cultures. The clonality was shown by co-culturing skin cells derived from a mouse with those isolated from an YFP-expressing transgenic mouse. The resulting spheres had cells that were either all fluorescent or all non-fluorescent, thus representing that, beneath these circumstances, SKP spheres are generated by the division of the cells not from the clustering of cells (Fernandes et al. 2004).
Upholding of the stem cell self-renewal capacity is a major dispute in regenerative medicine. SKPs can self-renew for a long period of time. Rodent SKPs can be expanded for at least 5 months and then differentiate into both mesodermal and neural origin (Toma et al. 2001) and human SKPs can be expanded for more than 1 year and retain a normal karyotype for 15 months, as assessed by G-banding (Toma et al. 2005). A similar outcome was also observed in porcine SKPs (Dyce et al. 2004).
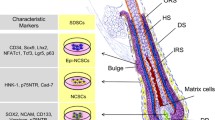
Numerous studies have established that there are at least three different sorts of stem cells in the skin: hair follicle, bulge, and dermal precursor cells. These cells have the ability to proliferate for many passages with unspecialized phenotype and they can differentiate into specialized cell types under specific conditions (Blanpain and Fuchs 2006; Fernandes et al. 2004; Ohyama et al. 2006). The mammalian skin contains a multipotent adult precursor cell that shares characteristics with embryonic NCSCs, including a neural crest-like differentiation capability in vitro and in vivo. It can be isolated and expanded as SKPs, when they maintain their multipotency. These endogenous precursors first arise during embryogenesis and they persist into adulthood, with a niche in the papillae of hair and whisker follicles (Fernandes et al. 2004). SKPs have also been derived from human dental pulp (Fernandes et al. 2004; Miura et al. 2003). These precursors are multipotent, nestin-positive, fibronectin-positive stem cells and can be produced from both juvenile and adult skin and are distinct from mesenchymal stem cells. Individual clones of SKPs and hair follicle precursor cells can differentiate into both neuroectodermal and mesodermal lineage, including neurons, glia, smooth muscle cells and adipocytes (Toma et al. 2001, 2005).
Concerts of skin-derived precursor cells
Dermal fibroblasts are the conventional negative control for the assessment of cell multipotency in many experiments, as they are considered as terminally differentiated cells. In 1993, studies showed that the pluripotent stem cell can be derived from connective tissues associated with heart, skeletal muscle and dermis and had the capacity of mesodermal lineage differentiation. With a different concentration of dexamethasone, cells from different clones were differentiated into muscle, fat, cartilage and bone. In this study, approximately 70% of the population had a pure stellate phenotype, while the balance was lineage committed (Young et al. 1995, 2001).
In 2001, another group supported the existence of resident self-renewing, multipotency cells in the dermal skin of rodents and humans (Toma et al. 2001). They were described as progenitor SKP by their differentiation potential into mesenchymal, neural and neural crest origins (Toma et al. 2001). Further studies from the same group confirmed that SKPs express properties like neural crest cells (Fernandes et al. 2004).
The developmental capability of SKPs was tested in chick embryos (Fernandes et al. 2004). Some of the injected SKPs migrated to the dorsal root ganglion (DRG), peripheral nerves and particularly to the dermal layer of the skin from the neural crest stream, as has been experimentally proven by an EYFP-labeled system (Fernandes et al. 2004). Primary and passaged SKP populations express nestin and extracellular matrix proteins fibronectin, vimentin, versican, precursor cell marker Sca-1, NGFRp75 and OCT4 (Biernaskie et al. 2006; Fernandes et al. 2004; Li et al. 2010). Early stage SKPs are negative for Schwann cell markers p75NTR, myelin-associated protein MBP, Sox10 and P0 and the melanocyte markers c-kit, trp1 and dct and the neuronal DβH and peripherin (Fernandes et al. 2004). Embryonic NCSCs express the transcription factors slug, snail, twist, Pax3 and Sox9, which are also expressed in SKPs. In addition, Dermo-1 and SHOX2 are expressed in SKPs that are associated with mesenchymal competence of cranial neural crest cells (Fernandes et al. 2004; Toma et al. 2005).
The adherent dermal mesenchymal stem cells, which are positive for CD90, CD105 and vimentin and negative for CD34, c-kit, CD133 and nestin have been isolated from human foreskin. This population could be expanded for over 100 doublings while holding their chromosomal balance and multipotency. Later, it was found that all single-cell-derived clones were multipotent and could differentiate into adipogenic, osteogenic and myogenic lineages (Bartsch et al. 2005). In a follow-up publication, 6.4% clones were found to be tripotent, 19.1% clones were bipotent, while 10.6% were unipotent (Chen et al. 2007). Restoration of multipotent rather than pluripotent fibroblast-derived cells gave rise to granulocytic, monocytic, megakaryocytic and erythroid lineages by OCT4 transduction supplemented with hematopoietic cytokines (Szabo et al. 2010). Therefore, skin contains heterogeneous populations with various levels of differentiation potential progenitors. The correlation between multipotent dermal stromal cells and SKPs remains unclear and deserves further investigation.
CD45+FibsOCT4 cells from any source can give rise to efficient hematopoietic progenitor-like cells that have the ability to mature into myeloid lineages under in vitro and in vivo conditions. This finding displays regeneration of multipotency from human stromal cells and suggests an alternative for human pluripotent cells in autologous cell-replacement therapies (Szabo et al. 2010).
In vivo studies
Adult stem cells represent an inevitable tool for clinical trials in supporting cellular therapy (Trounson et al. 2011). Bone marrow was the first source reported to be rich in MSCs; however, bone marrow requires invasive procedures and hospitalization with a great deal of donor/recipient rejection and low differentiation potential with increasing age (Vishnubalaji et al. 2012a). Recently, adipose tissue, achievable by a less invasive method, was launched as an alternative source for adult stem cells (Kern et al. 2006; Vishnubalaji et al. 2012a).
Today, stem cell therapy has already reached the bedside from the bench, although there is an incomplete knowledge of the control programs motivating their fate and plasticity (Rafii and Lyden 2003). While adult stem cells have been used in therapy for three decades, embryonic stem cell research has yet to yield any successful clinical trial results. Recently, the FDA endorsed the Geron Biopharmaceuticals hESC-derived oligodendrocyte progenitor cells for human embryonic stem cell clinical trials for acute spinal cord injury (neurosciencenews.com, 2011, February 24).
hSDSCs constitute a leading candidate for autologous transplantation in patients with glioblastoma. This has been proved by their anti-tumor activity in vivo, localized around the tumor and migratopn into the tumor site when injected either into the brain controlateral hemisphere or into the tail vein of mice (Pisati et al. 2007). hSDSCs migrated efficiently and condensed the tumor vessel solidity suppressing angiogenesis when implanted into chick embryo experimental glioma and transgenic Tyrp1-Tag mice models (Pisati et al. 2007).
Are skin-derived progenitor cells comparable with or different from MSCs?
A good number of individual studies propose self-renewal and differentiation capabilities of skin-derived precursors and MSCs (Tables 1, 2). However, there has been no comparison available. Since both are mesenchymal in origin, we give a comparative note on various properties including differentiation and self-renewal. Dissociation of skin cells are frequently done with enzymatic digestion and the population is distinct. MSCs are grown in the presence of serum, while SKPs are cultured without serum (Riekstina et al. 2008). The discrepancy in cultural environments may contribute to the variation in marker expression and morphology. Adherent MSCs are negative for nestin and positive for fibronectin, vimentin and collagen type I and rarely express cytokeratin, though another study showed them less positive for alpha smooth muscle actin and nestin and rarely expressed cytokeratins (Chen et al. 2007; Lorenz et al. 2008; Shi and Cheng 2004). In contrast, floating spheres express nestin, vimentin, versican and fibronectin and not cytokeratin (Fernandes et al. 2004, 2008; Toma et al. 2001, 2005).
Adherent cells (MSCs), when cultured in serum-free conditions with epidermal growth factor (EGF) and fibroblast growth factor (FGF2), typically form floating spheres. This study revealed that adherent cells that are incapable of forming any floating sphere-like cells survived with a low proliferation rate (Hoogduijn et al. 2006; Shih et al. 2005). Morphology of skin-derived cells was quite similar when cultured in the presence of serum with or without growth factors (Shih et al. 2005). Both the populations differentiated into several types of cell lineages like conventional MSCs with appropriate culture conditions and induction (Fig. 1).
In our experimental study, we focused on human adult dermal skin and neonatal foreskin regardless of age limits and location. We cultured them in DMEM-HG containing penicillin and streptomycin, 100μg/ml each with 10% fetal bovine serum (FBS) (Riekstina et al. 2008) and we derived the cells by explant culture without enzymatic digestion. We characterized migrated adherent fibroblast-like cells that derived from explant culture. Morphologic analysis and surface markers expression suggested the mesenchymal origin. Adherent cell populations were spindle-shaped and positive for vimentin (Fig. 2). Skin-derived stromal cells showed a higher CFU-F frequency and higher cell proliferation rate in long-term cultures compared with adipose-derived MSCs (Al-Nbaheen et al. 2012). Phenotype analysis showed positive for stromal cell-associated markers CD13 (>98%), CD29 (>93%), CD44 (>99%), CD73 (>98%), CD90 (>99%) and CD105 (>98%) and negative for hematopoietic endothelial markers CD34, CD45, CD14, CD31 and HLA-DR (Al-Nbaheen et al. 2012; Vishnubalaji et al. 2012b). Microarray-based gene expression revealed that transcriptome of human foreskin stromal cell was much closer to that of bone marrow MSCs, followed by adult skin and then adipose-derived MSCs (Al-Nbaheen et al. 2012). After in vitro expansion, the cells were successfully induced into adipocyte (Fig. 3), osteoblast (Fig. 3) and endothelial lineages (Fig. 4), which demonstrated their multipotency (Al-Nbaheen et al. 2012; Aldahmash et al. 2012; Huang et al. 2010; Vishnubalaji et al. 2012b). Overall, these results prove that skin-derived floating spheres (SKPs) and adherent cells (MSCs) are two distinct populations from the same origin with overlapping properties and differentiation.
Morphology of stromal cells derived from human adult dermal skin (hADSSCs) and neonatal foreskin (hNFSSCs). Cells were plastic-adherent with a fibroblast-like spindle-shaped morphology (a, b). Immunofluorescent staining primary cultures (c, d) and cells for vimentin; nuclei were visualized with DAPI (4'-6-diamidino-2-phenylindole). Bar 100 μm. Reproduced from Vishnubalaji et al. (2012b)
Adipocyte and osteoblast differentiation potential of stromal cells derived from human adult dermal skin (hADSSCs) and neonatal foreskin (hNFSSCs). Cytochemical analyses of non-induced and induced cells, Oil red O stain for Adipocyte (a, b, e, f) and alkaline phosphatase stain for osteoblast (c, d, g, h). Analysis by quantitative reverse transcription with the polymerase chain reaction (qRT-PCR) of adipocyte- (i) and osteoblast- (j) associated gene expression in induced and non-induced cells. Bar 100 μm. Reproduced from Vishnubalaji et al. (2012b)
Immunofluorescence staining for endothelial associated markers 72 h post-induction on matrigel. Stromal cells derived from human adult dermal skin (hADSSCs) and neonatal foreskin (hNFSSCs) were induced for 7 days and then plated on matrigel-coated wells. Expression of endothelial associated markers (CD31 (a, f), VE-cadherin (b, g), eNOS (c, h), VEGF165 (d, i) and vWF (e, j) was assessed using immunofluorescence microscopy. 4,6 Diamidino-2 phenylindole (DAPI) was used to counter stain for cell nuclei. Notably, hNFSSCs exhibited tremendous tightly packed capillary tube-like structures (g, n). In vitro angiogenic potential of stromal cells. Phase contrast image analysis of undifferentiated and differentiated hADSSCs (k, l) and hNFSSCs (m, n). Bar 100 μm. Analysis by quantitative reverse transcription with the polymerase chain reaction (qRT-PCR) of endothelial-associated gene expression in induced (WO MG without Matrigel, MG with Matrigel) and non-induced (control) cells (o–v). Reproduced from Vishnubalaji et al. (2012b)
Future directions
For the past 10 years, scientific research reports on stem cells have cast a new light on the capability of skin-derived cells to replenish tissues. SKPs’ well-identified mesodermal differentiation is limited to their in vitro differentiation into smooth muscle cells, adipocytes and chondrocytes (Tables 1, 2). SKPs express neural crest cell markers and differentiate into cell types that are derived from NCSCs and also migrate like neural crest cells when introduced into chick embryos. Thus, SKPs will have a role to play in spinal cord injury and nervous system and dysmyelinating disorders.
Skin-derived precursors and stromal cells will either serve as an alternative or will add up to the cell source currently listed for regenerative therapies. In the near future, skin can serve as a precious source of multipotent cells with the capabilities for ectodermal, endodermal and mesodermal cells.
Finally, we see considerable activity in networking on new stem cell products for potential therapies in a scalable expansion and cost-effective xeno-free condition. Hopefully, serum-free cultured human SKPs will be available in future stem cell transplantation.
Conclusion
MSCs have emerged as an indispensable tool in regenerative medicine. In cell-based therapies, numerous sources have been identified and cultured by various methods. However, much work is still needed for an optimal clinical grade product, where regulatory and higher safety standards are to be fulfilled. Previous reports discussed above show that floating spheres and adherent cells are multipotent precursors/stem cells from skin, while adherent populations share MSC properties and prove their lineage in differentiation and immunosuppressive capabilities. Skin-derived floating spheres (SKPs) are easily accessible and can be the potential autologous source for stem cell transplantation in regenerative medicine. They have been derived by non-enzymatic methods (cost effective and time saving) and cultured with or without animal serum and both populations proved their potency under in vitro conditions. We believe skin-derived progenitors/multipotent stem cells are one of the promising protagonists that might act as a key step in the successful development of future stem cell therapies in the upcoming century.
References
Aldahmash A, Zaher W, Al-Nbaheen M, Kassem M (2012) Human stromal (mesenchymal) stem cells: basic biology and current clinical use for tissue regeneration. Ann Saudi Med 32:68–77
Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M, Aldahmash A (2012) Human Stromal (Mesenchymal) Stem Cells from Bone Marrow, Adipose Tissue and Skin Exhibit Differences in Molecular Phenotype and Differentiation Potential. Stem Cell Rev Rep. doi:10.1007/s12015-012-9365-8
Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP (2007) Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7:11
Aoki S, Toda S, Ando T, Sugihara H (2004) Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell 15:4647–4657
Bartsch G, Yoo JJ, De Coppi P, Siddiqui MM, Schuch G, Pohl HG, Fuhr J, Perin L, Soker S, Atala A (2005) Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev 14:337–348
Bi D, Chen FG, Zhang WJ, Zhou GD, Cui L, Liu W, Cao Y (2010) Differentiation of human multipotent dermal fibroblasts into islet-like cell clusters. BMC Cell Biol 11:46
Bianco P, Robey PG (2001) Stem cells in tissue engineering. Nature 414:118–121
Biernaskie JA, McKenzie IA, Toma JG, Miller FD (2006) Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc 1:2803–2812
Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W (2007) Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 27:9545–9559
Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD (2009) SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5:610–623
Blanpain C, Fuchs E (2006) Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22:339–373
Blum B, Benvenisty N (2008) The tumorigenicity of human embryonic stem cells. Adv Cancer Res 100:133–158
Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM (1997) Principles of cartilage repair and regeneration. Clin Orthop Relat Res 342:254–269
Chen FG, Zhang WJ, Bi D, Liu W, Wei X, Chen FF, Zhu L, Cui L, Cao Y (2007) Clonal analysis of nestin(-) vimentin(+) multipotent fibroblasts isolated from human dermis. J Cell Sci 120:2875–2883
Clover AJ, Lane O'Neill B, Kumar AH (2012) Analysis of attitudes toward the source of progenitor cells in tissue-engineered products for use in burns compared with other disease states. Wound Repair Regen 20:311–316
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Dyce PW, Zhu H, Craig J, Li J (2004) Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun 316:651–658
Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD (2004) A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6:1082–1093
Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD (2006) Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol 201:32–48
Fernandes KJ, Toma JG, Miller FD (2008) Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B 363:185–198
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625–13630
Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP (2007) Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 179:1595–1604
Hipp J, Atala A (2008) Sources of stem cells for regenerative medicine. Stem Cell Rev 4:3–11
Hoogduijn MJ, Gorjup E, Genever PG (2006) Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev 15:49–60
Huang HI, Chen SK, Ling QD, Chien CC, Liu HT, Chan SH (2010) Multilineage differentiation potential of fibroblast-like stromal cells derived from human skin. Tissue Eng Part A 16:1491–1501
Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, Compston A, Chandran S (2008) A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells 26:163–172
Ikada Y (2006) Challenges in tissue engineering. J R Soc Interface 3:589–601
in 't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE (2003) Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88:845-852
Jahoda CA, Whitehouse J, Reynolds AJ, Hole N (2003) Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol 12:849–859
Kawase Y, Yanagi Y, Takato T, Fujimoto M, Okochi H (2004) Characterization of multipotent adult stem cells from the skin: transforming growth factor-beta (TGF-beta) facilitates cell growth. Exp Cell Res 295:194–203
Kebriaei P, Robinson S (2011) Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy 13:262–268
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, Mao N (2005) Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res 15:539–547
Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, Herlyn M (2010) Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci 123:853–860
Lorenz K, Sicker M, Schmelzer E, Rupf T, Salvetter J, Schulz-Siegmund M, Bader A (2008) Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp Dermatol 17:925–932
Manini I, Gulino L, Gava B, Pierantozzi E, Curina C, Rossi D, Brafa A, D'Aniello C, Sorrentino V (2011) Multi-potent progenitors in freshly isolated and cultured human mesenchymal stem cells: a comparison between adipose and dermal tissue. Cell Tissue Res 344:85–95
Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R (1997) Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138:4456–4462
McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD (2006) Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci 26:6651–6660
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807–5812
Moseley TA, Zhu M, Hedrick MH (2006) Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 118:121S–128S
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC (2006) Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 116:249–260
Pawitan JA (2009) Prospect of Adipose Tissue Derived Mesenchymal Stem Cells in Regenerative Medicine. Cell Tissue Transplant Therapy 2:7–9
Peng H, Huard J (2004) Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol 12:311–319
Pisati F, Belicchi M, Acerbi F, Marchesi C, Giussani C, Gavina M, Javerzat S, Hagedorn M, Carrabba G, Lucini V, Gaini SM, Bresolin N, Bello L, Bikfalvi A, Torrente Y (2007) Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res 67:3054–3063
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Rafii S, Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9:702–712
Ramesh T, Lee SH, Lee CS, Kwon YW, Hyun-Jai Cho HJ (2009) Somatic cell dedifferentiation/reprogramming for regenerative medicine. Int J Stem Cells 2:18–27
Riekstina U, Muceniece R, Cakstina I, Muiznieks I, Ancans J (2008) Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 58:153–162
Robertson JA (2010) Embryo stem cell research: ten years of controversy. J Law Med Ethics 38:191–203
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52:2521–2529
Shi CM, Cheng TM (2004) Differentiation of dermis-derived multipotent cells into insulin-producing pancreatic cells in vitro. World J Gastroenterol 10:2550–2552
Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, Shen EY, Chiu WT (2005) Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells 23:1012–1020
Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M (2006) Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24:462–471
Steingrimsson E, Copeland NG, Jenkins NA (2005) Melanocyte stem cell maintenance and hair graying. Cell 121:9–12
Stock UA, Vacanti JP (2001) Tissue engineering: current state and prospects. Annu Rev Med 52:443–451
Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M (2010) Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468:521–526
Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784
Toma JG, McKenzie IA, Bagli D, Miller FD (2005) Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 23:727–737
Trounson A, Thakar RG, Lomax G, Gibbons D (2011) Clinical trials for stem cell therapies. BMC Med 9:52
Tsatmali M, Ancans J, Thody AJ (2002) Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem 50:125–133
Vacanti CA, Kim W, Upton J, Vacanti MP, Mooney D, Schloo B, Vacanti JP (1993) Tissue-engineered growth of bone and cartilage. Transplant Proc 25:1019–1021
Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T (2012a) Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res 341:419–427
Vishnubalaji R, Manikandan M, Al-Nbaheen M, Kadalmani B, Aldahmash A, Alajez NM (2012b) In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev Biol 12:7
Walker MR, Patel KK, Stappenbeck TS (2009) The stem cell niche. J Pathol 217:169–180
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 22:1330–1337
Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, Beermann F, Barrandon Y, Sommer L (2006) Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol 175:1005–1015
Yamanaka S (2008) Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif 41(Suppl 1):51–56
You Q, Cai L, Zheng J, Tong X, Zhang D, Zhang Y (2008) Isolation of human mesenchymal stem cells from third-trimester amniotic fluid. Int J Gynaecol Obstet 103:149–152
Young HE, Ceballos EM, Smith JC, Mancini ML, Wright RP, Ragan BL, Bushell I, Lucas PA (1993) Pluripotent mesenchymal stem cells reside within avian connective tissue matrices. In Vitro Cell Dev Biol Anim 29A:723–736
Young HE, Mancini ML, Wright RP, Smith JC, Black AC Jr, Reagan CR, Lucas PA (1995) Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn 202:137–144
Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC Jr (2001) Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec 264:51–62
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vishnubalaji, R., Al-Nbaheen, M., Kadalmani, B. et al. Skin-derived multipotent stromal cells – an archrival for mesenchymal stem cells. Cell Tissue Res 350, 1–12 (2012). https://doi.org/10.1007/s00441-012-1471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1471-z