Abstract
Renal insufficiency is a frequent comorbidity found in heart failure patients with reduced ejection fraction (HFREF). It is associated with hemodynamic alterations in HFREF, including reduced renal perfusion and venous congestion. When present, it poses significant challenges to clinicians treating these patients. Although evidence is lacking regarding their safety and efficacy when renal insufficiency is severe, it is plausible common evidence based therapies retain their beneficial effects, which may actually be larger considering absolute risk reductions. However, the downside is an increased risk of adverse events, counterbalancing possible beneficial effects. Care should be taken to evaluate each individual HFREF patients with renal dysfunction, including serial monitoring of renal function, carefully selecting, monitoring and uptitrating medical treatment and minimizing the risk for adverse events.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Case VignetteMr. Y is a 72 y/o man with an ischemic cardiomyopathy after suffering an anterior myocardial infarction at the age of 68 years. He is currently residing in New York Heart Association functional class II. His past medical record is also notable for poorly controlled diabetes with microvascular complications of retinopathy and nephropathy. Serum creatinine levels were normal at the time of his myocardial infarction, but have increased gradually up till 2.47 mg/dL now (estimated glomerular filtration rate 25 mL/min/1.73 m2). Mr. Y is taking aspirin, atorvastatin, metformin, insulin in a basal-bolus scheme, lisinopril 20 mg daily, carvedilol 25 mg twice daily, and eplerenone 50 mg daily. Serum potassium levels are slightly elevated at 5.2 mmol/L without other electrolyte disturbances. Blood pressure is well controlled at 132/58 mmHg. The electrocardiogram of Mr. Y shows a left bundle branch block with QRS width equal to 148 ms. On his latest echocardiography, left ventricular ejection fraction was 30%.
FormalPara Chapter Key Points-
Incidence and prognostic impact of chronic kidney disease (CKD) in heart failure with reduced ejection fraction (HFrEF)
-
Use of evidence-based medications for HFrEF in patients with CKD
-
Device therapy in HFrEF and CKD
-
Reno-protective strategies in HFrEF
Brief Discussion of the Case
The first thing that should come to mind in any clinician treating patients similar to the case presented here, is whether this patient is in stable condition. To do so, a detailed anamnesis, followed by physical examination and if necessary follow up diagnostic tests are warranted to do so. It is imperative to identify unstable patients before the clinical course is detrimental to such an extent only limited treatment options remain. If the patient is stable, this gives the clinician time to evaluate the patient closely, possibly seeing the patient in the outpatient clinic several times, and perhaps discuss this patients treatment with other physicians, consulting specialists and of course the patient and his caregivers. Fortunately, the gentleman in this case seems to be in reasonable shape, as he is in NYHA functional class II heart failure (HF). This means there is time to evaluate the current status, get a detailed picture of the medical situation, and decide on a treatment plan based on patients condition, laboratory and other diagnostic and functional test, as well as taking into consideration current HF guidelines [1,2,3].
This patient is suffering from HF with reduced ejection fraction (HFREF), probably caused by the (large) myocardial infarction 4 years ago. Immediately, a clinician familiar with the syndrome of HF will recognize that there is no available cure, which means all treatment options available are focused on improving quality of life, including extending length of life, as well as keeping the patient out of hospital [2]. When assessing such a patient with HFREF, it is important to evaluate whether any comorbidities exist that might further impair long term outcomes or increase the risk of decompensation, hospitalization or dying [4]. Furthermore, some comorbidities may interfere with treatment options. Certainly, the presence of comorbidities that by itself confer a substantial mortality risk (which could surpass the mortality risk of HF), could mean certain HF treatments should not be embarked on.
In general comorbidities that should interest a HF physician include among others: Diabetes Mellitus, Pulmonary Disease (including chronic obstructive pulmonary disease (COPD)), Coronary Artery Disease, Atrial Fibrillation, Cerebrovascular disease, Depression and perhaps most importantly for the current case: renal insufficiency [4,5,6,7].
Renal Function in HFREF
Why is renal function so important in HF? First, it is the organ that is in the end responsible for the maladaptive salt and water retention in response to neurohormonal activation when cardiac dysfunction (whatever is the cause) develops [8]. Second, because it is exactly there where evidence based treatments in HFREF exert their action (among other places). Thirdly, whatever the cause of renal dysfunction in HF, it is one of the strongest predictors of clinical outcome (and therefore risk marker) in HF [6, 9]. At the end of the twentieth century, this detrimental association between lower creatinine clearance and mortality was formally recognized in retrospective analyses from both SOLVD and PRIME II studies, sparking up more research in the field on why renal dysfunction was so important in HF [10, 11]. Ultimately, this culminated in a large study based meta-analysis, including over one million cases, where having chronic kidney disease (CKD) at baseline was associated with a more than two-fold mortality risk [6]. This risk was, surprisingly, similar in acute and chronic HF. These findings by itself should be sufficient reason to evaluate renal function closely in patients with HFREF.
Pathophysiology of Renal Insufficiency in Heart Failure
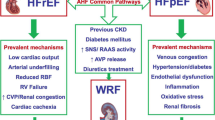
Even though we largely think we understand the importance of renal dysfunction in HFREF patients, the pathophysiology is still under debate (Fig. 6.1 shows most common concepts) [8, 12, 13]. However, we now know from small mechanistic studies that a reduction in cardiac output and increase in central venous pressure directly transmit to the kidney [14,15,16,17]. This means that in HFREF patients, with and without renin angiotensin aldosterone system (RAAS) blockade, there is a direct and strong relationship between renal blood flow and glomerular filtration rate (GFR) [14, 16]. When HF advances, and besides left sided filling pressures, also right sided pressures, especially central venous pressure start to rise and overt congestion develops, this also has it effects on renal function [18]. Importantly, high central venous pressure (directly transmits to renal venous pressure) contributes to a reduced GFR in two important ways. First it decreases the pressure gradient over de kidney (and glomerulus), thereby decreasing renal blood flow (this is an indirect way). Second, increased central and renal venous pressure leads to renal interstitial hypertension (high intracapsular pressure) [19,20,21]. On the long term this accelerates fibrosis and intrarenal damage, but on the short term it means pressure in the renal parenchymal tissues are high, resulting in collapsing of tubules, reducing the flow of ultrafiltrate from Bowman’s capsule to the collecting duct, which means lower filtration [22]. There are also signs that by itself, high renal venous pressure further promotes salt and water retention. It is therefore essential to get a feeling of congestive status of the HFREF patient with renal dysfunction to understand the cause of CKD in the individual patient (Tables 6.1, 6.2, and 6.3).
Overview of the pathophysiology of renal insufficiency in HFREF. (a) Organ-specific factors: Reduction in RBF and increased (renal) venous pressure, resulting in increased renal interstitial pressure (directly opposing filtration in Bowmans capsule (b)). Glomerular factors: Renal autoregulation preserves GFR, a process inhibited by RAAS inhibitors causing (pseudo) worsening renal function. Non-steroidal anti-inflammatory drugs inhibit prostaglandin synthesis, thereby impairing prostaglandin associated increase/dependent renal blood flow. Concomitant diseases have direct, but differential effect on glomerular filtration, glomerular integrity and podocyte function, as well as autoregulation. (c) Nephronic factors: the combination of increased interstitial pressure, reduced arterial perfusion, concomitant disease and therapies can cause tubular and glomerular injury. Increased renal interstitial pressure causes collapsing of renal tubules, thereby lowering GFR, and eventually leading to decreased urine output, sodium retention, and congestion. Abbreviations: ACEi, angiotensin-converting enzyme inhibitor: ARB, angiotensin II receptor blocker: FF, filtration fraction: GFR, glomerular filtration rate: MRA, mineralocorticoid receptor antagonist: NSAIDs, non-steroidal anti-inflammatory drugs: RAAS, renin–angiotensin–aldosterone system: RBF, renal blood flow. (From Damman et al. [21])
In the current case, this patient does have a strikingly reduced estimated GFR (25 mL/min/1.73 m2), more than might be expected from his age, creatinine and severity of HF. In such a situation, it is important to re-evaluate findings and medical history to understand the disproportional low eGFR. It could be that the hemodynamic status of the patient is more compromised than can be seen with minimal examination and anamnesis. If this is suspected, care should be taken to get objective evidence of to support this. More importantly, not only HF induces a decline in renal function, also many comorbidities exert detrimental effects, some of which contribute to the development of HF as well. Particularly, atherosclerosis, hypertension and diabetes mellitus are associated with worse renal function and more renal function decline in non HF populations, and all are associated with the development of HF by themselves [23, 24]. What this actually means is that in some patients, before the development of (overt) HF, renal function is often already compromised [25]. In the current case, this patient was already suffering from poorly controlled diabetes with end organ damage (retinopathy and nephropathy) probably long before HF occurred after the myocardial infarction. Although this did not translate in to an elevated serum creatinine level when the infarction occurred, diabetes can cause accelerated decline in renal function, cause glomerulosclerosis and tubular injury, as well as causing nephron loss [26]. Diabetic patients are also known to have renal hyperfiltration where filtration fraction (GFR divided by renal blood flow) actually increases; which is thought to be a sign of renal compensation, but also a sign of renal end organ damage [27]. This might have been the case with the current patient when serum creatinine was still normal at the time of the coronary event. Although hyperfiltration normally doesn’t occur in hypertension, this condition is also associated with accelerated decline in renal function and loss of nephrons [28]. It is also a major risk factor for HF, either directly or through promoting cardiovascular events [29]. Controlling blood pressure and optimizing diabetic regulation are therefore important treatment targets in patients at risk of HF, but also in HF patients themselves, since this might be associated with favourable renal outcomes. Although this has not been shown in an evidence based manner, it is unlikely that pathophysiological processes associated with early renal function decline in patients without (or before) HF are either halted, attenuated or even reversed when overt HF develops. Therefore, from a renal perspective, taking care of blood pressure and especially diabetic control, should be part of the treatment of HFREF patients with renal insufficiency.
Treatment of HFREF Patients with Renal Insufficiency
Besides diagnosing, controlling and treating comorbidities in HFREF, the primary focus of the treatment of HFREF patients – also in those with important renal insufficiency- should be initiation, uptitration and continuation of evidence based therapies as much as possible according to most recent HF guidelines [1,2,3]. As is the case for any HFREF patient, a patient with mild to moderate renal insufficiency (CKD stage 1–3, eGFR >30 mL/min/1.73 m2) should be treated with guideline recommended HF treatment (Fig. 6.2) [30]. The classes of drugs to consider in these patients include Angiotensin converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), Angiotensin receptor blocker neprilysin inhibitors (ARNI), beta-blockers and mineralocorticoid receptor antagonists (MRA), which all have a class I recommendation in clinical HF guidelines [2, 3]. ACEi and ARBs are often withheld in patients with modest to moderate (and severe) renal insufficiency in HF because of the perceived risk of worsening of renal function and hyperkaleamia [30]. In randomized clinical trials, where only patients were included with eGFR >30 ml/min/1.73m2 (so CKD stage 1 to 3b, but not stage 4 or 5 (dialysis)), there was no significant interaction between baseline CKD and the treatment effect of either ACEi or ARB. This means that the beneficial effects were maintained when baseline eGFR was lower. Since the absolute risk in these high risk patients was higher, this also meant that with similar relative risk reduction, the absolute risk reduction in these patients was actually greater [30]. However, this was offset by more frequent occurrence of hyperkaleamia and other adverse events, indicating that close monitoring of renal function and electrolytes is warranted, especially when renal function at baseline is already compromised. Similar results were found in post hoc analyses of both RALES and EMPHASIS-HF, showing that MRA therapy was beneficial also in patients with moderate renal insufficiency [31, 32]. The perceived risk of worsening renal function with RAAS inhibitors is actually true but should be seen in a different context [21]. As a response to a reduction in renal perfusion pressure, efferent vasoconstriction occurs in the kidney, which results in a stable GFR (at the cost of neurohormonal activation). With RAAS inhibition by ACEi, ARB and MRA’s, this efferent vasoconstriction is (partly) attenuated, which directly results in improvement of renal perfusion, but decline in GFR (and therefore lower FF) [14]. This decline in GFR called worsening renal function is seen in all studies with RAASi in HF [33]. However, when worsening renal function occurs in the setting of starting or uptitration of RAAS inhibitors, there is no associated detrimental effect on clinical outcome. Some increase in serum creatinine (or decrease in eGFR) should therefore be accepted, which could be up to 3 mg/dL or more than 50% increase in eGFR. Very large or steep increases in serum creatinine should always prompt more investigating and temporary halting the RAAS inhibitor, and in any circumstance, renal function and electrolytes should be checked regularly. The one exception within the group of RAAS inhibitors with regards to change in renal function is sacubitril/valsartan (ARNI). Compared with enalapril, sacubitril/valsartan resulted in a less pronounced decline in eGFR over time, while improving prognosis, even in patients with moderate CKD [34].
Evidence of guideline recommended treatments in HFREF according to CKD stages. Angiotensin blocker neprilysin inhibitor (ARNI) shows the same evidence as for ACEi, although only in one study. Abbreviations: ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, CKD: Chronic kidney disease, CRT: cardiac resynchronization therapy, ESRD: End stage renal disease, GFR: glomerular filtration rate, H-ISDN: hydralazine and isosorbide-dinitrate, ICD: implantable cardioverter-defibrillator, MRA: mineralocorticoid receptor antagonist, RAAS: renin angiotensin aldosterone system. (From Damman et al. [30])
In some situations, as is the case with the present patient, renal function could decline below the threshold of eGFR <30 ml/min/1.73 m2, either because of or despite starting treatment with RAAS inhibitors. It is imperative to try to keep patients on these life saving drugs, even though renal function is poor. Although we do not know whether discontinuation of these drug in these situation do any harm (or good), it is also very unlikely that the benefit of these therapies suddenly stops in patients with baseline eGFR <30 mL/min/1.73m2 [30]. However, what we do know is that more side effects such as hypotension occur, and the risk of hyperkaleamia rises [32]. With close monitoring and a case by case treatment plan, it is often possible to keep these patients on their evidence based therapies. Whether to pursue further uptitration (i.e. the present patient is treated with lisinopril 20 mg OD which could be uptitrated further) should also be decided on an individual basis. For instance, if a drop in eGFR was caused by the introduction of the ACEi, further uptitration might not be reasonable. On the other hand, if eGFR has remained stable over some period of time, and blood pressure permits, under close monitoring of vital signs and potassium, uptitration could be considered. In the circumstance this particular patient was RAAS inhibitor naïve and had the same laboratory results, a similar approach can be followed; use small dose steps, adjust according to changes in renal function and electrolytes and monitor vitals. For Beta-blocker therapy, although also in these trials patients with eGFR <30 ml/min/1.73 m2 were mostly excluded, there is even more consensus to treat HFREF patients with moderate/severe renal insufficiency according to general guidelines [30]. The reason is that there is no (detrimental) effect of beta-blocker therapy on renal function in HFREF patients, and the effect of the drugs were at least as strong (possibly stronger) in patients with more severe CKD stages. Whether or not other medical therapies such as digoxin, ivabradine, hydralazine or nitrates may be used in patients with moderate renal insufficiency has been extensively reviewed [30].
Device Therapy in HFREF Patients with Renal Insufficiency
After a HFREF patient with renal insufficiency has been treated with optimal medical therapy (highest tolerated dose), the question arises whether there is also an indication for device therapy [1, 3]. As is the case for medical treatment, large trials on implantable cardioverter defibrillator (ICD) treatment have excluded patients with severe renal dysfunction. But it is probable that the beneficial effect ICD therapy as observed in the entire HFREF population persists when renal dysfunction worsens. These patients might also be at increased risk of sudden death, especially given electrolyte abnormalities such as hyperkaleamia, and lower dosage of prescribed evidence based therapies [30]. Of course, whether or not an ICD should be implanted is not only dependent of cardiac status, but also of age, frailty, non cardiac life expectancy, comorbidities and patients preference.
Whereas all above mentioned treatment option should only be considered because of mortality or morbidity benefit (and not particularly for their benefit on renal function), this might not be the case for cardiac resynchronization therapy (CRT). The patient in the current case has a widened QRS complex (148 ms), with left bundle branch block morphology, which makes CRT a good option when on stable, high dose evidence based treatment (IIa B recommendation) [1]. This therapy is associated with improved long term outcomes in this patient category, including those with CKD stage 3 a/b, and it is plausible that it also improves outcome in patients with more severe stages of CKD (i.e. stage 4), although these were not included in the large trials. More importantly there is some evidence that CRT therapy may improve cardiac output and thereby improve renal perfusion, increasing GFR [35]. This might not be a direct reason to implant such a device, given also the risk of peri and post procedural complications, one of which could be contrast-induced nephropathy, but it at least suggests that renal impairment by itself should not be a reason not to implant a CRT in these patients.
Finally, HF patients who have severe renal dysfunction often have advanced HF. In selected patients, left ventricular assist devices (LVAD) implantation may be an option, either as bridge to transplant or as destination therapy. Conceptually, LVAD implantation will result in improvement of hemodynamics and most often result in improved renal function [36, 37]. However, there is increased risk of peri and direct posteroperative worsening of renal function on top of a compromised renal function already. The risk of dialysis is therefore real, but difficult to establish individually. Probably, renal impairment by itself (to some extent), should not be a reason not to implant a LVAD.
Renoprotective Strategies in Chronic HFREF
Certainly, no trial has been designed with the specific intent of improving renal function, although the Evaluation of Losartan in the Elderly Study (ELITE) specifically aimed to reduce the risk of worsening renal function [38]. However, losartan was similar to captopril with respect to renal function, but showed lower mortality risk, which was then not confirmed in ELITE II [39]. Renal dysfunction or worsening renal function has been part of most randomized clinical trials as adverse events. However the interpretation of these adverse events, especially in RAAS-inhibitor placebo controlled trials is difficult. In most if not all RAAS-inhibitor trials, the active compound was associated with more frequent renal adverse events [30]. However, we also know that despite this, mortality benefit was maintained suggesting that striving for improved or stable renal function when RAAS-inhibitor therapy is started or uptitrated really doesn’t necessarily translate into better outcomes [33]. Diuretics (mainly loop diuretics) have not been studied in a randomized, placebo controlled manner, but their use is advocated when congestion is present in any HF patient. From a renal perspective, diuretics probably have beneficial but also unwanted effects in HF. They improve and reduce (renal) venous congestion, thereby improving renal perfusion and reducing renal interstitial pressure. This may lead to improved renal function opening up the possibility for uptitration of evidence based treatments. On the other hand, reports suggest that long term use of (high dose loop) diuretics may be associated with worse (renal) outcomes, and even alterations on a nephron level [40,41,42]. However, it is extremely difficult to establish whether these associations are causative, considering confounding by indication where sicker patients are prescribed more (often) diuretics. The general consensus is however to prescribe a HFREF patients with as much diuretics as possible to achieve and maintain euvolemia, and as little diuretics as possible to preserve renal function and prevent common side effects such as gout like symptoms, cramps and intravascular depletion. By using a standardized approach, clinicians may be able to improve renal function (by altering dosing of ACEi, Diuretics, switching to clopidogrel), which might be especially useful in the frail, elderly population where also orthostatic hypotension and frequent multiple comorbidities are present [43]. Finally, it is important to prevent the use of certain (combination of) drugs to prevent (or treat) renal function decline. For instance, the use non-steroidal anti-inflammatory drugs (NSAIDs) should be minimized as their combination with RAAS-inhibitors can cause significant renal dysfunction. Combination therapy of ACEi and ARBs (or ARNI/Direct Renin inhibitors) is not advised, given the higher incidence of worsening renal function and hyperkaleamia, without robust evidence of improved outcomes. In any circumstance, frequent determination of renal function (serum creatinine, estimated GFR) and associated electrolytes (sodium, potassium, blood urea nitrogen) is indicated in any patients with HFREF, especially those who have changes in renal function, are unstable and/or are uptitrated with RAAS-inhibitors.
Conclusion
As the pivotal organ that induces the maladaptive salt and water retention in HF and is the target for therapy for most of our evidence based treatments, the kidney can never receive too much attention from HF clinicians. Although (severe) renal dysfunction in chronic HFREF should prompt concerns, it should not be a reason to withhold evidence based treatments. In any patient with chronic HFREF with renal dysfunction it is important to regularly monitor renal function and electrolytes, and to put effort into keeping or starting patients on these life saving therapies.
References
WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–51.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
van Deursen VM, Damman K, van der Meer P, Wijkstra PJ, Luijckx GJ, van Beek A, et al. Co-morbidities in heart failure. Heart Fail Rev. 2014;19(2):163–72.
Kajimoto K, Sato N, Takano T, investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Relationship of renal insufficiency and clinical features or comorbidities with clinical outcome in patients hospitalised for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2017;6(8):697–708.
Damman K, Valente MA, Voors AA, O’Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(1522-9645; 0195-668; 7):455–69.
Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281–93.
Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36(23):1437–44.
Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(1558-3597; 0735-1097; 10):1987–96.
Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35(0735-1097; 0735-1097; 3):681–9.
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, De ZD, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(1524-4539; 0009-7322; 2):203–10.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(1558-3597; 19):1527–39.
Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26(0195-668; 0195-668; 1):11–7.
Smilde TD, Damman K, van der Harst P, Navis G, Daan Westenbrink B, Voors AA, et al. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009;98(1861-0692; 2):121–9.
Damman K, van Deursen VM, Navis G, Voors AA, Van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(1558-3597; 0735-1097; 7):582–8.
Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39 Suppl 4(0012-6667; 0012-6667):10–21.
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(1558-3597; 7):589–96.
Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9(1388-9842; 9):872–8.
Burnett JC Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238(0002-9513; 0002-9513; 4):F279–82.
Fiksen-Olsen MJ, Strick DM, Hawley H, Romero JC. Renal effects of angiotensin II inhibition during increases in renal venous pressure. Hypertension. 1992;19(0194-911; 2):137–41.
Damman K, Tang WH, Testani JM, McMurray JJ. Terminology and definition of changes renal function in heart failure. Eur Heart J. 2014;35(48):3413–6.
Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16(2):133–42.
Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De ZD, et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int. 2008;74(1523-1755; 0085-2538; 4):505–12.
James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–12.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–77.
Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–39.
Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16(9):2557–64.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–62.
Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, et al. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014;63(1558-3597; 0735-1097; 9):853–71.
Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012;60(1558-3597; 0735-1097; 20):2082–9.
Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7(1):51–8.
Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16(1879-0844; 1388-9842; 1):41–8.
Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–98.
Boerrigter G, Costello-Boerrigter LC, Abraham WT, Sutton MG, Heublein DM, Kruger KM, et al. Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail. 2008;14(1532-8414; 7):539–46.
Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7(1):68–75.
Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59(1):26–36.
Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349(9054):747–52.
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial – the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355(9215):1582–7.
Damman K, Kjekshus J, Wikstrand J, Cleland JG, Komajda M, Wedel H, et al. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2016;18(3):328–36.
Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 2003;42(0735-1097; 0735-1097; 4):705–8.
Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017;377(20):1964–75.
de Silva R, Nikitin NP, Witte KK, Rigby AS, Loh H, Nicholson A, et al. Effects of applying a standardised management algorithm for moderate to severe renal dysfunction in patients with chronic stable heart failure. Eur J Heart Fail. 2007;9(4):415–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Damman, K. (2020). A Patient with Progressive Renal Insufficiency in Chronic Heart Failure with Reduced Ejection Fraction. In: Tang, W., Verbrugge, F., Mullens, W. (eds) Cardiorenal Syndrome in Heart Failure. Springer, Cham. https://doi.org/10.1007/978-3-030-21033-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-21033-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21032-8
Online ISBN: 978-3-030-21033-5
eBook Packages: MedicineMedicine (R0)