Abstract
Purpose of Review
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome of exertional intolerance, cardiac dysfunction, and fluid overload and is associated with significant morbidity and mortality.
Recent Findings
As our understanding of this syndrome has evolved, we are beginning to recognize the similarities and associations with chronic kidney disease (CKD). Salt and fluid retention are common in CKD and may be the sentinel event leading ultimately to the syndrome of HFpEF. Mechanisms linking both disease states include hypervolemia, inflammation, and endothelial dysfunction, which are also common to comorbidities that drive both HFpEF and CKD.
Summary
In this review, we will discuss recent clinical research focusing on HFpEF, CKD, and comorbidities including hypertension and diabetes mellitus. We will review strategies for volume management and novel therapeutic approaches with new classes of drugs, including sodium-glucose cotransporters and angiotensin receptor/neprilysin inhibitors, which may work through targeting of both the heart and the kidney. Lastly, we emphasize why focusing on the alleviation of factors provoking renal injury and slowing the progression of renal dysfunction may provide the most therapeutic benefit in patients who have been diagnosed with HFpEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an aging population, the prevalence of chronic diseases is rising [1]. This includes both heart failure with preserved ejection fraction (or HFpEF) and chronic kidney disease (CKD) [2, 3••]. The presence of either HFpEF or CKD may accelerate progression of the other condition [4]. In this review, we will discuss recent literature on the epidemiology, pathophysiology, diagnosis, and treatment strategies for both HFpEF and CKD. Inappropriate salt and fluid retention is central to the clinical consequences of HFpEF. As we discuss, HFpEF in fact may be a “vicious cycle” disorder of the kidneys and cardiovascular system.
Heart Failure with Preserved Ejection Fraction
HFpEF is subtype of heart failure which represents approximately half of the cases of HF and is associated with comorbidities including older age, obesity, CKD, atrial fibrillation, and hypertension [5, 6]. These conditions, especially CKD, can exacerbate sodium and fluid retention leading to congestion. The clinical diagnosis of HFpEF generally requires signs and symptoms of congestion in the setting of preserved left ventricular ejection fraction (LVEF) [7•]. Despite this relatively straightforward concept, the heterogeneity of HFpEF is readily apparent in the multiple definitions that have been proposed. In a 2019 analysis of 461 patients presenting with exertional dyspnea, the application of different HFpEF professional societal definitions to these patients demonstrated significant heterogeneity in clinical profiles and outcomes [5]. This variability was also apparent from an analysis of three large randomized clinical HFpEF trials: (TOPCAT (Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function), I-PRESERVE (Irbesartan in Heart Failure With Preserved Systolic Function), and CHARM Preserved (Candesartan Cilexetil in Heart Failure Assessment of Reduction in Mortality and Morbidity)). Two phenotypes of HFpEF emerged: (1) obese young non-Caucasian patients and (2) older patients with hypertension, CKD, and atrial fibrillation [8]. To provide a more practical definition that integrates this clinical challenge, scoring systems have been derived, which rely upon clinical and echocardiographic findings for the diagnosis (e.g., H2PEF score) [9]. Interestingly, CKD is highly specific (90%) for the diagnosis of HFpEF using the H2PEF score.
Chronic Kidney Disease
CKD and end-stage renal disease (ESRD) have a growing prevalence among the elderly with approximately 38% of US adults 65 years or older with a diagnosis of CKD [2]. Risk factors include hypertension, diabetes mellitus, and obesity [2]. CKD is defined on the basis of persistently reduced estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 or at least 1 marker of kidney damage for >3 months [10]. As stated, the definition can be either based on renal injury markers or traditional eGFR equations, which rely on serum creatinine. The use of novel biomarkers is relevant; in one cohort of patients with HFpEF, serum cystatin-C and urinary albumin were better at predicting future HF as compared with creatinine-based eGFR [11, 12]. Moreover, CKD may be more common in HFpEF, in contrast to other forms of HF. In the Swedish Heart Registry, when HF was separated into HFpEF, mid-range (HFmrEF), and reduced ejection fraction (HFrEF), the prevalence of CKD was greatest among those with HFpEF [13]. Moreover, occult renal dysfunction may be underappreciated by measures of eGFR. In a study of 90 patients with HF, biomarkers of renal injury (neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-beta-d-glucosaminidase (NAG) and kidney injury molecule 1 (KIM-1), and urinary albumin) were measured [14]. In patients with “normal’ renal function (as determined by eGFR), increased urinary concentrations of these biomarkers were associated with mortality and hospitalization for decompensated HF. Some patients with a diagnosis of HFpEF have concomitant obesity and diabetes mellitus which can predispose to glomerular hyperfiltration, causing renal dysfunction with a “normal” eGFR [15, 16]. However, in these cases, abnormal urine findings such as albuminuria are associated with biventricular dysfunctional remodeling. Findings such as these suggest renal injury is common in patients with known HF despite normal estimated GFR by commonly invoked equations.
Pathophysiology and Mechanisms
The pathophysiology of HFpEF and CKD have overlapping characteristics [7•]. Both conditions are commonly associated with hypertension, diabetes, and obesity, which lead to systemic inflammation, oxidative stress, arterial stiffening, and endothelial dysfunction [7•, 15, 16]. In the heart, these pathways lead to diastolic dysfunction, microvascular disease, and subtle contractile abnormalities. In the case of kidney, these same processes lead to nephron loss, with compensatory hypertrophy and hyperfiltration of the remaining nephrons in an attempt to maintain GFR. These processes then drive further renal dysfunction.
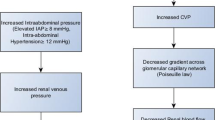
The interplay between cardiac and renal dysfunction is often described as the cardiorenal syndrome (CRS), where there is concomitant presence of both renal dysfunction and heart failure [17]. Some have proposed subtypes categorized by acuity and whether cardiac or renal dysfunction predisposes to the other organ’s injury [17]. For example, in a primary cardiovascular insult, such as acute myocardial infarction and cardiogenic shock, the acute decreased blood flow and pressure to the kidney produces acute renal injury, with resultant sodium and fluid avidity. In the case of primary renal dysfunction, concomitant volume retention leads to a volume-overloaded state, independent of ejection fraction, and the myocardial contractile state. Furthermore, an increased CVP, often characteristic of HFpEF, leads to a cascade of elevated intra-abdominal pressure, pulmonary hypertension, and endothelial dysfunction. In addition, the gradient across the glomerular capillary network decreases, leading to decreased renal perfusion and lower glomerular filtration. In a recent cohort study, similar degrees of venous congestion by echocardiography and renal dysfunction were seen in both HFpEF and HFrEF [18].
Endothelial dysfunction as a result of renal impairment is likely one mechanism linking HFpEF and CKD [19]. Circulating factors related to the activated inflammatory cascade in renal injury likely leads to downstream myocardial dysfunction, specifically myocyte stiffening and subsequent fibrosis. Mediators including fibroblast growth factor 23 (FGF23) have been implicated as triggers for endothelial dysfunction [20]. Furthermore, renal dysfunction is also associated with low levels of erythropoietin, which can have subsequent impact on endothelial function and inflammation [21]. As renal function worsens to ESRD, rising levels of uremic toxins are associated with inflammation which likely have a direct cardiotoxic effect [22]. Overall, the presence and progression of CKD provokes the inflammatory cascade and endothelial dysfunction, predisposing to myocardial dysfunction seen in HFpEF.
Hypertension is a critical risk factor for both CKD and HFpEF. The absence of hypertension (as well as obesity and diabetes mellitus) is associated with an 86% lower risk of HF [23]. Traditionally, the cardiac remodeling with concentric hypertrophy secondary to pressure overload has been thought to be responsible for the diastolic abnormalities in classic HFpEF. Other primary causes of hypertension can also serve as a mediator of the CRS and volume overload. In the Pickering syndrome, renal artery stenosis leads to malignant hypertension and subsequent flash pulmonary edema [24]. This is an example of cardiorenal syndrome type 3, in which a renal injury predisposes to cardiac dysfunction [25•]. With respect to the renal system, prolonged hypertension leads to disruptive injury despite autoregulatory mechanisms. The mechanisms of injury are primarily related to pressure differentials across the glomerular bed as well as oxidative stress leading to fibrogenic mediators.
Diabetes mellitus (DM) is another major contributor to both HFpEF and CKD. DM leads to maladaptive cardiac remodeling and predisposes to all subtypes of HF [16]. The prevalence of DM in HF is high (up to 50%) and is more often seen in HFpEF than HFrEF [26]. In DM, hyperinsulinemia, circulating adipokines and cytokines, and oxidative stress contribute to the microvascular disease, low-grade vascular inflammation, and endothelial dysfunction that are characteristic of HFpEF and CKD [27, 28]. For example, in endomyocardial biopsy samples from patients with HFpEF, increased levels of inflammatory cells are seen [29]. DM is a major cause of CKD, also known as diabetic nephropathy (DN). DN is often associated with glomerular changes including glomerulosclerosis and thickening of the basement membrane with eventual fibrosis and tubular atrophy. The mechanisms at play include oxidative stress, renin-angiotensin system (RAS) activation, and TGF-β activation. Glycemic control to prevent development and progression of CKD has been a major endpoint for many therapeutics for DM.
Therapies
In terms of therapies for HFpEF and CKD, prevention remains the cornerstone of management. Specifically, control of comorbidities which are common to both disease states is imperative. Recent data from the EPIC-NL (European Prospective Investigation Into Cancer and Nutrition–Netherlands) cohort demonstrated that individuals with ideal scores of smoking cessation, physical activity, body mass index, diet, blood pressure control, total cholesterol, and blood glucose (the American Heart Association’s Life’s Simple 7) had a 55% reduced long-term risk of HF [30]. Blood pressure can be controlled in many cases of HFpEF with agents including diuretics, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and vasodilating β-blockers such as carvedilol. In the SPRINT trial, treatment to a systolic blood pressure of 120 mmHg was associated with a 38% reduction in the risk of HF [31]. The use of sacubitril/valsartan as a therapeutic agent has been a significant addition to the HF armamentarium in patients with HFrEF as demonstrated by the PARADIGM-HF trial [32]. In more recent studies, the UK-HARP-III trial demonstrated sacubitril/valsartan preserved renal function in those with CKD and had improved blood pressure control as compared with irbesartan [33]. The ongoing PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction) trial will answer whether sacubitril/valsartan is superior to angiotensin receptor blockade alone in patients with chronic symptomatic HFpEF [34]. Trial results and subgroup analyses will provide critical information to further understand which phenotypes of HFpEF may benefit from ARNI therapy.
The prevention of hypervolemia in HF is another important target in the concept of HFpEF as a kidney disorder. Salt and water retention are a part of the clinical syndrome of decompensated HFpEF and the use of diuretic therapy is a mainstay of volume removal. In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, spironolactone did not reduce the incidence of CV death or HF hospitalization in patients with HFpEF [35]. However, subsequent analyses demonstrated marked heterogeneity within enrolled sites and a signal for possible benefit for patients in the USA [36]. Across eGFR ranges, spironolactone demonstrated benefit when close laboratory surveillance is possible [37]. New data from TOPCAT has reinforced the role of spironolactone as a diuretic or diuretic sparing agent in HFpEF [38, 39]. As a result of the inconclusive results from TOPCAT, the Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction (SPIRRIT) will evaluate if spironolactone can provide mortality and hospitalization benefit in patients with HFpEF, focusing on patients in the Swedish Heart Registry and the USA [40]. Finally, mineral corticoid receptor antagonists may slow the progression of renal insufficiency: two large prospective trials are testing this hypothesis.
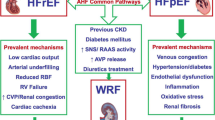
Beyond the management of hypertension and hypervolemia, novel therapies targeting hyperglycemia have shown promise in patients with HFpEF and CKD. The sodium-glucose cotransporter-2 inhibitors (SGLT2i) demonstrate favorable effects on diabetes-related hyperglycemia, obesity, and hypertension. These drugs block sodium-glucose cotransport in the proximal tubule and increase delivery of sodium chloride to the macula densa. This leads to downstream vasoconstriction of afferent arterioles and reduces hyperfiltration seen in patients with diabetes mellitus and obesity. Importantly, these drugs impact cotransporters fundamentally limited to the kidney, e.g., a renal-specific therapy. Findings from the EMPA-REG (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial demonstrated a 35% risk reduction for HF hospitalization in patients with DM2 and cardiovascular disease. When examining renal outcomes, patients receiving empagliflozin have a lower incidence of acute renal injury and worsening nephropathy. In patients with CKD, empagliflozin reduced the risk of CV death by 29% compared with placebo [41]. Additionally, empagliflozin attenuated cardiac hypertrophy and improved diastolic function in mouse models, suggesting a possible role for SGLT2i to alleviate the predisposing mechanisms in HFpEF [42]. Moreover, empagliflozin appears to block the adenosine-mediated tubuloglomerular feedback that leads to glomerular hyperfiltration [43]. Canagliflozin, another SGLT2i, demonstrated a decreased incidence of albuminuria and worsening renal function in the CANVAS trial [44]. Clearly, this novel class of therapies, originally designed to treat patients with DM has shown benefit in both HF and CKD. Future incorporation of trial endpoints, including MARCE (major adverse renocardiac events), in future trials studying SGLT2i and other DM therapies will likely demonstrate benefit in both CKD and HFpEF. In fact, these hypotheses are so compelling that randomized trials are underway that are testing this class of drugs in patients with HFpEF in the absence of diabetes. Figure 1 summarizes the comorbidities common to both HFpEF and CKD and novel therapeutic targets being actively studied.
A framework for the comorbidities leading to HFpEF and CKD and novel therapeutic targets being studied. ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid antagonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor; HFpEF, heart failure with preserved ejection fraction; CKD, chronic kidney disease
Conclusions
HFpEF has become one of the most challenging modern cardiovascular diseases to establish effective therapies. We are beginning to understand the multiple pathophysiologic processes at play, resulting in a heterogeneous disease state for which refined phenotyping will likely help identify effective therapies. In doing so, it will be helpful to take “a step back” and scrutinize the conditions which accompany and likely contribute to HFpEF. This broad perspective includes CKD and accompanying fluid retention which may ultimately provoke a renocardiac syndrome, e.g., HFpEF. If we step back from our cardiocentric approach to disease understanding, we can examine how mechanisms including endothelial dysfunction, inflammation, and volume retention provoke renal injury that leads to subclinical cardiac dysfunction over time. Novel therapies including SGLT2is, ARNIs, and new strategies to improve hypertension and hypervolemia will likely improve renal function and subsequent cardiac function.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
An empirical study of chronic diseases in the United States: a visual analytics approach to public health. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5876976/. Accessed 26 Jul 2019.
Age-adjusted prevalence of CKD stages 1–4. [Online]. Available: https://nccd.cdc.gov/CKD/detail.aspx?Qnum=Q10. Accessed 20 Jul 2019.
•• WRITING COMMITTEE MEMBERS, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. This comprehensive guideline statement covers the management of heart failure.
Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574–85.
Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions | Circulation. [Online]. Available: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.039136. Accessed 29 Jul 2019.
Shah KS, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86.
• Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507–15. This review article discusses the pathophysiology and hemodynamics seen in HFpEF.
Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction | JACC: J Am Coll Cardiol [Online]. Available: http://www.onlinejacc.org/content/74/5/601. Accessed 29 Jul 2019.
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–70.
Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference - Kidney International. [Online]. Available: https://www.kidney-international.org/article/S0085-2538(19)30276-5/fulltext. Accessed 22 Jul 2019.
Risk factors for heart failure in patients with chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) Study | J Am Heart Assoc. [Online]. Available: https://www.ahajournals.org/doi/10.1161/JAHA.116.005336. Accessed 23 Jul 2019.
Shchekochikhin D, Nikiforova T, Shilova A, Nesterov A, Baturina O, Gognieva D, et al. Evaluation of discriminative capacity of two formulas of CKD-EPI to predict complications after the first episode of heart failure with preserved ejection fraction. Int J Nephrol Renov Dis. 2019;12:113–8.
Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1606–14.
Damman K, van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TDJ, Westenbrink BD, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96(16):1297–302.
Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2014;2(6):586–96.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71.
Agrawal A, Naranjo M, Kanjanahattakij N, Rangaswami J, Gupta S. Cardiorenal syndrome in heart failure with preserved ejection fraction—an under-recognized clinical entity. Heart Fail Rev. 2019;24(4):421–37.
Van Aelst LNL, et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20(4):738–47.
Ter Maaten JM, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18(6):588–98.
Fibroblast growth factor-23: what we know, what we don’t know, and what we need to know. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/23625971?dopt=Abstract. Accessed 28 Jul 2019.
Progenitor cells and vascular function are impaired in patients with chronic kidney disease. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/20083473?dopt=Abstract. Accessed 28 Jul 2019.
Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/23941724?dopt=Abstract. Accessed 28 Jul 2019.
Hypertension, obesity, diabetes, and heart failure–free survival | JACC: heart failure. [Online]. Available: http://heartfailure.onlinejacc.org/content/4/12/911.abstract. Accessed 28 Jul 2019.
Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/2900930?dopt=Abstract. Accessed 29 Jul 2019.
• Cardiorenal syndrome. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/24656104. Accessed 29 Jul 2019. This review discusses the subtypes of cardiorenal syndrome and current management strategies.
Boonman-de Winter LJM, Rutten FH, Cramer MJM, Landman MJ, Liem AH, Rutten GEHM, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55(8):2154–62.
Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/25116954. Accessed 29 Jul 2019.
Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/24548662. Accessed 29 Jul 2019.
Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. - PubMed - NCBI. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/21075869. Accessed 29 Jul 2019.
Uijl A, Koudstaal S, Vaartjes I, Boer JMA, Verschuren WMM, van der Schouw YT, et al. Risk for heart failure: the opportunity for prevention with the American Heart Association’s Life’s simple 7. JACC Heart Fail. 2019;7(8):637–47.
A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
McMurray JJV, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Richard H, et al. Effects of sacubitril/valsartan versus Irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–14.
Solomon SD, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. 2018;11(7):e004962.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42.
Beldhuis IE, et al. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Fail. 2018;7(1):25–32.
Kalogeropoulos Andreas P, Jincy T, Javed B, Fang James C. Abstract 11286: spironolactone lowers loop diuretic requirements without adversely affecting renal function and electrolyte balance in patients with heart failure and preserved ejection fraction: a TOPCAT trial analysis. Circulation. 2018;138(Suppl_1):A11286.
Selvaraj S, et al. Prognostic value of albuminuria and influence of spironolactone in heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11(11):e005288.
Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction - Full Text View - ClinicalTrials.gov. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT02901184. Accessed 29 Jul 2019.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Empagliflozin Improves Diastolic Function in a Nondiabetic Rodent Model of Heart Failure With Preserved Ejection Fraction | JACC: Basic to Translational Science. [Online]. Available: http://basictranslational.onlinejacc.org/content/4/1/27.abstract. Accessed 30 Jul 2019.
Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140(4):303–15.
Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr James Fang served on the Steering Committee for the EVALUATE-HF Trial and as a co-lead investigator for the DELIVER trial.
Dr Shah has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mechanisms of Hypertension and Target-Organ Damage
Rights and permissions
About this article
Cite this article
Shah, K.S., Fang, J.C. Is Heart Failure with Preserved Ejection Fraction a Kidney Disorder?. Curr Hypertens Rep 21, 86 (2019). https://doi.org/10.1007/s11906-019-0993-0
Published:
DOI: https://doi.org/10.1007/s11906-019-0993-0