Abstract
Managed pollinators are often used in agriculture and horticulture to increase crop yield in greenhouses and open fields. Over the past 25 years, research has been conducted to investigate the potential of these pollinators to perform a second task, being the dispersal of biological control agents (BCO) alongside with their pollination service. Especially the suppression of grey mould, caused by Botrytis cinerea, has received considerable attention in multiple crops such as strawberries, raspberries and blueberries. Trials to suppress insect pest species, such as tarnished plant bug and western flower thrips, have also been conducted in crops such as tomato, sweet pepper and sunflowers. In this chapter, an overview is provided of the current literature with several case studies investigating the potential of entomovectoring to suppress plant pathogens and pest species under greenhouse and open field conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pollination plays a key role in the establishment of successful fruit setting in agriculture and horticulture, which is why managed pollinators are often relied upon to improve yield in greenhouses and open fields. Using the entomovectoring technology, pollinators can potentially provide a second service, being the dispersal of biological control agents (BCOs) to the crops to suppress pest species and plant pathogens. Starting with the first study by Peng et al. (1992) on the possibility to protect strawberries against grey mould using honey bees, multiple studies have investigated the potential of entomovectoring to protect crops. In this chapter, different case studies are presented both in open field and greenhouse conditions aiming to protect different target crops. These case studies give an overview on the knowledge that is available on using pollinators to vector BCOs to target crops and suppress diseases. It should be noted though that, because of the fact that the effectiveness of entomovectoring is determined by the interaction of many components, results cannot be extrapolated automatically to designs using different target crops or control agents (Fig. 1).
2 Entomovectoring for the Protection of Strawberries
Strawberry is a worldwide grown fruit crop in both open field and greenhouses. However, yields are often limited by diseases, the most destructive one being grey mould. Grey mould is caused by the airborne plant pathogen Botrytis cinerea and is most destructive on mature or senescent tissues of a variety of dicotyledonous hosts, including strawberry (Williamson et al. 2007). Symptoms become visible when fruits are ripening but infection with the pathogen occurs at flowering, as the stamens are considered to be the principal infection court. Therefore, treatment of the newly opened flowers seems to be the most effective strategy to prevent infection by B. cinerea (Mertely et al. 2002). As pollinators can potentially deliver control agents directly to the flower as soon as they are open and available for pollination, entomovectoring has been investigated as a way to protect strawberry plants against B. cinerea. The first study by Peng et al. (1992) investigated whether honey bees could disperse Gliocladium roseum, a fungus which suppresses spore production of Botrytis cinerea, to strawberry crops in open field and greenhouses by loading their Peng dispenser with a mix of talc-corn meal and spores. They found that honey bees emerged from the dispenser, carried the powder and transferred it successfully to the strawberry flowers. The amounts of transferred inoculum seemed sufficient to suppress B. cinerea, except when honey bee activity was reduced due to bad weather conditions.
The potential of another BCO, Trichoderma harzianum, was investigated in three different studies. The first one was conducted by Kovach et al. (2000) over a period of 4 years and used both honey bees and bumble bees as vectors. During the experiment, strawberry fields on several locations near New York (USA) were monitored and the effectiveness of Trichoderma harzianum 1295–22 spraying and vectoring was investigated. The authors reported that flowers in patches where the BCO was vectored by bees had lower concentrations of T. harzianum compared to flowers in patches that were treated with BCO’s through spraying application. However, it was apparent that the level of control achieved through entomovectoring with bees was higher than the spraying. It was also comparable or sometimes even higher to the control level as provided by commercial fungicides that were applied by spray at bloom. Moreover, it was remarked that the bee visits increased the seeds on collected strawberries with 22% and caused an increase in weight of up to 40% compared to strawberries in non-visited plots. Based on these findings, the authors concluded that the bee-vectored T. harzianum can be considered as a viable strategy for growers who wish to minimize the use of fungicides in the fight against B. cinerea. For more detailed information on the different experiments conducted during these 4 years, consult the original paper of Kovach et al. (2000).
A second study was conducted by Shafir et al. (2006) where a different strain, Trichoderma harzianum T39, was vectored by honey bees under open field conditions in Israel over two consecutive growth seasons. The authors compared the effect of the spraying of commercial fungicide with the vectoring of T. harzianum T39 (commercially developed as “Trichodex” for the control of grey mould). Honey bees were loaded with the powder formulation using the Triwaks dispenser as developed by Bilu et al. (2004).
Over the two seasons, the same protocol was used. It consisted of a randomized complete block design with four different treatments, being (1) fungicide only, (2) bee-vectored only, (3) both fungicide and bee-vectored, and (4) control. Sufficient levels of T. harzianum (104 CFU per flower) were found on flowers up to 200 meters from the hives in the bee-vectored treatments. It was concluded that the transmission of T. harzianum by honey bees is effective, but the ability to suppress grey mould was not constant throughout the season. The efficiency of both the fungicide and the vectored T. harzianum was best at the start of the season and started to fail towards the end, when the number of symptomatic fruits became too high. A third study was conducted by Albano et al. (2009) using honey bees (Apis mellifera) and bumble bees (Bombus impatiens) to vector the biofungicide “Rootshield” to strawberries in fields (honey bees) and greenhouses (bumble bees). They tested the ability of the vectors to get dusted with powder when walking through the Houle dispenser and deliver the powder to the strawberry crops. They found that both honey bees and bumble bees were capable of dispersing the powder efficiently. However, no data was reported on the level of disease suppression.
A third BCO that has been tested to protect strawberries against grey mould through entomovectoring is Gliocladium catenulatum, a fungus which is originally isolated from the soil. It is now produced by the Finnish company Verdera and commercially available as “Prestop”. The first study investigating the potential of “Prestop”, conducted by Hokkanen et al. (2012), started in 2005 and lasted over a period of 4 years. Research took place on different locations in Finland and used a newly developed dispenser, the BeeTreat dispenser, to load honey bees with “Prestop”. Experiments took place in open field conditions and compared the disease incidence and marketable yield between four different treatments, being (1) bee-vectored “Prestop”, (2) chemical fungicides, (3) chemical fungicides combined with bee-vectored “Prestop”, and (4) control group. Looking at disease control, the bee-vectored “Prestop” decreased the disease incidence on average by 50% compared to 65% for chemical fungicides and 80% for the combined treatment. Based on disease incidence, a combination of fungicides and bee-vectoring appeared to be the best option. However, when total marketable yield was investigated, the authors found that bee-vectored biocontrol was as effective, or in some years even more efficient, compared to the fungicides and the combined treatment. Comparing the marketable yields between the treatments collected in 2008 showed that it was only marginally larger in the fungicides treatment compared to the control. The bee-vectored biocontrol provided the highest yield with a 90% overall increase compared to the control group. Combining biocontrol with fungicides did not increase the yield any further despite the fact that disease suppression was better in this group (Fig. 2a). This suggests that sprays might have an impact on the yield potential of strawberry plants. The increased yield could also partially be attributed to the improved pollination of the flowers, as shown by the results of the trials on organic farms (Fig. 2b). Enhanced pollination by honey bees increased the yield by 58%, while combining pollination with bee-vectored biocontrol increased yield by 105% compared to the control group. All treatments were also reported to improve the shelf-life of the strawberries after harvesting, approximately doubling their durability, with the combination of fungicides and bee-vectored biocontrol increasing durability the most.
Overview of the marketable yield (red bars) and mouldy berries (grey bars) per 1 m of strawberry row. (a) compares marketable yield from the different treatment groups relative to the untreated control group (control yield = 100%) [Data from 2008 on 4 farms, each with 4 replicates]. (b) shows the yield on an organic strawberry farm in 2008. Compared treatments are untreated control (no disease control and only natural pollination), enhanced pollination (no disease control and increased pollination by honey bees) and enhanced pollination combined with bee-vectored biocontrol. Taken from Hokkanen et al. (2012)
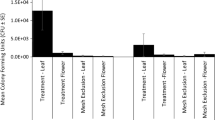
A second study was conducted under greenhouse conditions by Mommaerts et al. (2011) using the bumble bee Bombus terrestris. The authors used their own dispenser, the Mommaerts dispenser to load bumble bees with “Prestop-Mix” (Gliocladium catenulatum Strain J1446) and investigated the ability to suppress grey mould (B. cinerea) in manually infected strawberry plants. The experiments were conducted in a greenhouse with four fine-meshed tents, each subjected to a different treatment: (T1) Control (no pollination or biocontrol), (T2) “Maizena-Plus” (pollination and dissemination of Maizena-Plus), (T3) “Prestop-Mix” (pollination and dissemination of Prestop-Mix), and (T4) “Prestop-Mix” + “Maizena-Plus” (pollination and dissemination of a 1:1 “Prestop-Mix”:“Maizena-Plus” formulation). All plants were manually infected with 10 μl of a water solution with a concentration of 105 B. cinerea spores per ml (Fig. 3). A comparison with a control group that was inoculated with water only showed that manual inoculation can lead to the development of B. cinerea under the greenhouse conditions, which were considered optimal for the development of the fungus. The efficacy of the treatment was determined by comparing the numbers of flowers that were visited by the bumble bees during the first 4 weeks with the numbers of red fruits formed during the following 4 weeks (pre-harvest yield). Strawberries were also incubated for 2 days in the laboratory after picking and examined afterwards to determine post-harvest effects and yield of the treatments.
As essential results of this greenhouse test, the authors reported a preharvest yield which was higher for T3 and T4, with 72 ± 17% and 71 ± 9% of the visited flowers developing into strawberries, respectively. For T1 and T2, the yield was lower with 54 ± 21% and 51 ± 9%, respectively, indicating a positive effect of the vectored “Prestop-Mix” on preharvest yield. The post-harvest yield was also better for T3 and T4, as 67 ± 13% and 79 ± 17% of the harvested berries did not show any signs of rot after incubation, respectively, compared to 43 ± 13% for T1 and 50 ± 10% for T2. The total yields (calculated as % preharvest yield x % post-harvest yield) differed significantly between T3-T4 and T1-T2, being 47 ± 10% for T3 and 56 ± 10% for T4, compared to 24 ± 14% for T1 and 25 ± 8% for T2. The authors also found that the foraging activity of the bees was not affected by the powder formulation, which is an important condition for effective use of entomovectoring. A third three-year study using Prestop-Mix was conducted in Estonia by Karise et al. (2016), investigating the potential of bumble bees of B. terrestris to vector the powder under open field conditions and suppress B. cinerea infections in open field strawberries. The authors reported a significant reduction in grey mould infections during the first 2 years, but not in the third year, when the weather conditions were very favourable for Botrytis development, which resulted in a high disease level. Similar to the study of Shafir et al. (2006), the vectored BCO was not able to suppress the high levels of disease pressure under these very rainy conditions.
3 Entomovectoring Against Botrytis cinerea in Raspberry
Just like strawberries, raspberries can also suffer from yield loss caused by B. cinerea. Spraying applications are often not efficient due to the short lived flowers, as this makes it difficult or even impossible to time the applications so they can protect all flowers. Yu and Sutton (1997) investigated if Gliocladium roseum could be vectored by honey bees (A. mellifera) and bumble bees (B. impatiens) in open fields, to investigate if alternatives are available to replace spraying applications.
Field tests were conducted using two cultivars of raspberry, being the summer-bearing “Boyne” and fall-bearing “Redwing”, during the summer of 1993 and 1994. In both years, crops were divided into four treatments, being (T1) control, (T2) G. roseum spray application, (T3) G. roseum honey bee-vectored and (T4) G. roseum bumble bee-vectored. To assess treatment effects on the incidence of B. cinerea in the flowers, 16 flowers were taken from each plot and divided into 4 different groups which were sprayed with B. cinerea at concentrations of 0, 103, 104 or 105 conidia/ml.
The results of Yu and Sutton (1997) are shown in Fig. 4. Honey bee-vectored and bumble bee-vectored G. roseum seemed capable to suppress B. cinerea in both stamens and stigmas. Only on the first day after applying the pathogen (14th of June for “Boyne” and 10th of August for “Redwing”), spray applications resulted in a higher level of control. It should be noted that by looking at the results of each group separately, it was revealed that G. roseum vectored by honey bees and bumble bees was not able to control B. cinerea when a concentration of 105 conidia/ml was applied. To assess treatment effects on the incidence of grey mould on the fruits, 36 ripe berries were picked at random and incubated to check for the presence of the fungus (Yu and Sutton (1997). The application of G. roseum resulted in a significant decline of grey mould fruit rot in the cultivar “Boyne” in June 1994. There was a reduction from 90% in the control group to 41% for spray, 67% for bumble bees and 68% for honey bees. During the trails in June 1993 there was no significant reduction compared to the control group, which had an incidence of 65%. For the cultivar “Redwing”, neither of the trials in 1993 or 1994 found a significant reduction of the incidence compared to the control groups (50% incidence in 1993 and 60% in 1994). While stamens and stigmas were continuously protected by the bee-vectored G. roseum, control of fruit rot seemed to be inconsistent. Yu and Sutton (1997) attributed this to the fact that, despite the fact that B. cinerea often infects the fruits in an indirect way through the flowers, there is evidence that the conidia can also infect the ripe fruit surface, resulting in a grey mould infection on fruits that grew from protected flowers.
Sporulation incidence of Botrytis cinerea on stamens and stigmas in flowers of raspberry cv. Boyne and cv. Redwing in the different treatments. Data bars are pooled means of means for flowers that were challenge-inoculated with 0, 103, 104 and 105 conidia of B. cinerea per ml of water plus surfactant. Observations assigned with a different letter are significantly different. Taken from Yu and Sutton (1997)
4 Entomovectoring for Biological Control in Sweet Pepper
Tarnished plant bug (Lygus lineolaris, TPB) and western flower thrips (Frankliniella occidentalis, WFT) are two pest species found on greenhouse crops, including sweet peppers. While biological control measures can in some cases be efficient to fight pests as WFT, chemical insecticides are required for effective control on sweet peppers. TPB is also difficult to be kept under control with BCOs, making chemical pesticides the main, only control strategy (Al-mazra’awi et al. 2006). The first study on entomovectoring against these pest species in sweet pepper was conducted by Al-mazra’awi et al. (2006) and focused on the BCO Beauveria bassiana, a fungus that is active against both TPB and WFT. The bumble bee B. impatiens was selected as the vector to transfer the BCO to the sweet peppers and worker bumble bees were loaded using a slightly modified model of the Peng dispenser. The trials took place in a greenhouse using a randomized block design with each trial being replicated over time. The four treatments were (T1) bee-vectored B. bassiana + TPB, (T2) bee-vectored B. bassiana + WFT, (T3) bee-vectored heat-inactivated B. bassiana + TPB + WFT, and (T4) TPB+ WFT, without the presence of bumble bees or BCO.
The authors found that 90% of the flowers showed detectable amounts of B. bassiana, demonstrating a successful transfer from the dispenser to the target crops. The BCO was also recovered on the leaves of the crops. TPB and WFT were sampled on two different dates and mortality was assessed. During the first sampling, TPB individuals in treatment (T1) displayed a mortality of 33.6 ± 6.6% (with 90.0 ± 3.1% mycosed) compared to 9.2 ± 2.7% (6.0 ± 4.4%) mortality (mycosed) for treatment (T3) and 14.8 ± 4.1% (0%) for treatment (T4). For the second sampling, this was 45.0 ± 3.9% (91.0 ± 3.0%) for treatment (T1), 15.3 ± 3.2% (14.5 ± 7.5%) for treatment (T3) and 9.0 ± 1.9% (1.7 ± 1.7%) for treatment (T4). For both samplings, the mortality in treatment (T1) was significantly higher compared to the others, demonstrating a significant effect of the vectored B. bassiana on the mortality of the TPB. The same significant difference between the viable B. bassiana treatment and the controls was found for WFT. Treatment (T2) showed a mortality of 39.5 ± 11.8% (34.1 ± 6.9%) on the first sampling date and 34.1 ± 6.9% on the second sampling date. In comparison, treatment (T3) showed a mortality of 3.4 ± 2.6% and 3.1 ± 2.6%, respectively, whereas treatment (T4) had a mortality rate of 2.2 ± 2.2% and 0.5 ± 0.5%, respectively. The percentage of individuals showing mycosis was not reported for the WFT adults.
Kapongo et al. (2008a) investigated the optimal concentration for the vectored BCO powder containing B. bassiana (“BotaniGard 22WP”) to control TPB and green peach aphid (GPA) (Myzus persicae) on greenhouse sweet pepper, using bumble bees of B. impatiens as a vector. The experiment consisted of a randomized block design with 5 treatments, being (T1) low concentration of B. bassiana, (T2) middle concentration of B. bassiana, (T3) high concentration of B. bassiana, (T4) heat inactivated B. bassiana, and (T5) control treatment without bumble bees. For TPB, no mortality was found in treatment (T4) and (T5). Treatment (T1) resulted in the killing of 33.0 ± 5.0% of the adults, which was significantly lower compared to treatment (T2) and (T3), which had a mortality of 69.7 ± 3.6% and 67.1 ± 5.2%, respectively. Treatment (T2) and (T3) did not differ significantly from each other. For GPA, the same pattern was observed. Mortality in treatment (T1) (21.5 ± 3.5%) did differ significantly from the percentage found in treatment (T2) (33.5 ± 3.3%) and treatment (T3) (29.5 ± 5.3%), but no difference was found between the latter two. Treatment (T4) and (T5) both showed no mortality. Based on the data obtained, it looks that both the medium concentration (6.24×1010) and high concentration (2×1011) are able to affect the populations of both pest species.
A third experiment aimed to confirm the potential to co-vector B. bassiana and Clonostachys rosea using bumble bees of Bombus impatiens to control TPB and grey mould (B. cinerea) in sweet pepper simultaneously (Kapongo et al. 2008b). The experiment consisted of three treatments: (T1) mixed formulation of B. bassiana and C. rosea, (T2) heat-inactivated inoculum, and (T3) control treatment without inoculum or bumble bees. Plants were manually inoculated with B. cinerea. Treatment (T1) caused a mortality of 72.5 ± 1.4% of the adult TPB, which was significantly higher compared to the control treatments. The mortality in treatment (T2) was 10.8 ± 2.2%, and in treatment (T3) 10.8 ± 1.2%; the control groups showed no significant difference with (T2). In treatment (T1), grey mould on sweet pepper was suppressed by 58.9% in the flowers and by 46.8% on the leaves.
5 Entomovectoring for Biological Control in Tomato Plants
Another greenhouse crop which is grown around the world is tomatoes. Two pest species are frequently found on greenhouse tomatoes, being the greenhouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) (Lange and Bronson 1981). So far, no research has been done on the possibility to suppress two-spotted spider mite using entomovectoring, but two studies investigated the effect of bumble bee-vectored BCOs to control greenhouse whitefly (GWF) in tomato greenhouses. The first study by Kapongo et al. (2008a) vectored B. bassiana under different concentrations using bumble bees of B. impatiens, with the same design as described above for sweet pepper. The effects of different concentrations of B. bassiana were investigated by checking the mortality percentage of adult greenhouse whiteflies in each treatment. Both control treatments (no inoculum and heat inactivated inoculum) did not cause any mortality among the whiteflies. The low, middle and high concentration of B. bassiana resulted in 17.9 ± 2.1%, 53.9 ± 3.4% and 55.9 ± 4.2% mortality, respectively. The authors reported a significant difference between the low concentration and the middle or high concentration, but no significant difference between the middle and high concentration (Fig. 5).
A second study investigated the effect of the vectoring of a mix of B. bassiana and C. rosea to suppress both greenhouse whiteflies and grey mould at the same time (Kapongo et al. 2008b). The setup was identical as described above for the experiment with sweet pepper. Greenhouse whitefly adults in the B. bassianae + C. rosea treatment showed a significantly higher mortality percentage (59.1 ± 2.5%) compared to the ones in the heat-inactivated treatment and the control group (18.8 ± 6.6% and 20 ± 2.5%, respectively).
6 Entomovectoring Against Plant Pathogens in Blueberries
Among all diseases associated with blueberries, Monilinia vaccinii-corymbosi has de greatest economic impact on the industry (Scherm et al. 2001). M. vaccinii-corymbosi is a pathogenic fungus which infects open blueberry flowers and causes mummy berry disease, resulting in a yield decrease in blueberry fields. Since blueberries are dependent on sufficient pollination to ensure adequate fruit set, commercial blueberry producers often use supplemental bees to increase their yield (Dedej et al. 2004). However, pollinators are also the main vectors of the M. vaccinii-corymbosi conidia, leaving the growers with a dilemma as increasing pollination is also likely to increase the incidence of mummy berry disease. In search of a solution for this dilemma, Scherm et al. (2004) investigated if the biofungicide “Serenade”, a commercial formulation of Bacillus subtilis, was able to control flower infections when it was applied directly to the stigmas of open flowers. During tests in the lab, flowers were treated manually, but this would be unable to achieve in the field. In search of an alternative way to apply the “Serenade”, Dedej et al. (2004) used honey bees to deliver Serenade to the stigmas of rabbiteye blueberry bushes and suppress M. vaccinii-corymbosi. Honey bees were loaded with “Serenade” using the Gross dispenser and delivered the powder to plants under open field conditions. Treatments consisted of vectoring “Serenade” using different bee densities in the first year (0 bees, 1600 bees or 6400 bees) of the study. During the second and third year, additional treatments were added using the same bee densities, but no “Serenade” to vector. To assess the effect of the vectored “Serenade”, 30 fruit clusters were selected and bisected to assess the presence of mycelia or pseudosclerotia of M. vaccinii-corymbosi. It was found that disease levels increased with bee density and were lower when “Serenade” was vectored. Disease incidence in treatments with 6400 bees and no “Serenade” was highest among all treatments (21.1% in 2002 and 66.5% in 2003, compared to 14.2% and 30.5% for the control treatment in 2002 and 2003, respectively). Treatments with 6400 bees including “Serenade”, resulted in that the disease incidence dropped to 6.6% and 43.5% in 2002 and 2003, respectively. These results demonstrate that increasing the number of honey bees to improve pollination may increase the risk of spreading mummy berry disease in the field. Like strawberries and raspberries, blueberries can also suffer from grey mould caused by B. cinerea. Reeh et al. (2014) investigated the effect of bumble bee-vectored Clonostachys rosea (the commercial form “Origro’s Endophyte”) on the development of grey mould on lowbush blueberries under open field conditions. They found a significant reduction of the percentage of blossoms infected with B. cinerea, but total percentage of infected blossoms still remained high (up to 90% in some cases). The results demonstrated that entomovectoring alone might not be able to provide an economic advantage for blueberry growers suffering from grey mould, but it might be able to be effective when used as part of an integrated pest management plan.
7 Entomovectoring Against Pathogens and Pests in Sunflowers
Sunflower growers often suffer economic losses by pest species such as the banded sunflower moth (BSM) (Cochylis hospes). While chemical pesticides may control the damage afflicted by this species, it can also be detrimental for the honey bee populations visiting the sunflowers. As honey bees of A. mellifera are the main pollinator of sunflowers (Sosa 1988), alternatives were needed for an efficient control of the BSM that would not affect the honey bee populations that pollinated them. Jyoti and Brewer (1999) vectored Bacillus thuringiensis var. kurstaki, a BCO registered for use on sunflower to control BSM, with the use of honey bees under open field conditions. The potency of honey bee-vectored B. thuringiensis was compared with a spray application using a set-up with 3 treatments: (T1) sunflowers with bee-vectored BCA, (T2) sunflowers with spray application of the BCA, and (T3) control treatment. The experiment was performed twice, once in 1996 and once in 1997. Three sunflower heads were collected per sampling sites which radiated outward 7.6 m, 15.2 m and 22.8 m from the centre of each block and each assigned to a different treatment. The flower heads were infested with 50 BSM eggs and collected at physiological maturity. A first sample of 100 seeds was taken from each flower to determine the percentage of seeds damaged by BSM. A second sample of 100 seeds was taken to determine the weight of the seeds and seed oil concentration.
The authors found that bee-vectored B. thuringiensis resulted in a significantly lower amount of damaged seeds (1996: 12.1 ± 0.2%; 1997: 12.2 ± 0.4%) compared to the control group (1996: 21.1 ± 0.2%; 1997: 22.3 ± 0.4%). In 1997, there was also a significant difference between bee-vectoring and spray application, with bee-vectored B. thuringiensis resulting in a lower percentage of damaged seeds, but in 1996 no significant difference was found. The seed set (percentage of filled seeds) was also significantly higher in the bee-vectored treatment compared to the other two during both years. Seed oil content and seed yield always differed significantly between the bee-vectored treatment and the control group, indicating an overall positive effect of the presence of honey bees and the vectored B. thuringiensis. In most cases, bee-vectored control agent was also more effective compared to the spray application. These results demonstrate the positive influence of honey bees on sunflowers, both in the presence and absence of vectored B. thuringiensis. A second study conducted by Escande et al. (2002) in Argentina focused on entomovectoring to fight the plant pathogen Sclerotinia sclerotiorum, which causes sunflower head rot. The authors found that using honey bees to vector a mix containing various strains of Trichoderma sp. could significantly reduce head rot incidence in sunflowers. When combining the treatment with a resistant genotype of sunflowers, reductions of 90–23% were found. On top of that, the experiments were conducted under conditions where the incidence of Sclerotinia sclerotiorum could be as high as 86%, while under natural conditions the disease was found not to exceed 68%. Again however, no data was collected on the yield obtained in the presence or absence of honey bee-vectored S. sclerotiorum for determining the economic value of using entomovectoring to protect sunflowers against pathogens and pest species.
References
Al-mazra’awi MS, Shipp L, Broadbent B, Kevan P (2006) Biological control of Lygus lineolaris (Hemiptera: Miridae) and Frankliniella occidentalis (Thysanoptera: Thripidae) by Bombus impatiens (Hymenoptera: Apidae) vectored Beauveria bassiana in greenhouse sweet pepper. Biol Control 37:89–97
Albano S, Chagnon M, De Oliveira D, Houle E, Thibodeau P, Mexia A (2009) Effectiveness of Apis mellifera and Bombus impatiens as dispersers of the Rootshield® biofungicide (Trichoderma harzianum, strain T-22) in a strawberry crop. Hell Plant Prot J 2:57–66
Bilu A, Dag A, Elad Y, Shafir S (2004) Honey bee dispersal of biocontrol agents: an evaluation of dispensing devices. Biocontrol Sci Tech 14:607–617
Dedej S, Delaphane KS, Scherm H (2004) Effectiveness of honey bees in delivering the biocontrol agent Bacillus subtilis to blueberry flowers to suppress mummy berry disease. Biol Control 31:422–427
Escande AR, Laich FS, Pedraza MV (2002) Field testing of honeybee-dispersed Trichoderma spp. to manage sunflower head rot (Sclerotinia sclerotiorum). Plant Pathol 51:346–351
Hokkanen HMT, Menzler-Hokkanen I, Mustalahti AM (2012) Honey bees (Apis mellifera) for precision biocontrol of grey mould (Botrytis cinerea) with Gliocladium catenulatum on strawberries and raspberries in Finland. Arthropod-Plant Interactions (submitted)
Jyoti JL, Brewer GJ (1999) Honey bees (Hymenoptera: Apidae) as vectors of Bacillus thuringiensis for control of banded sunflower moth (Lepidoptera: Tortricidae). Environ Entomol 28:1172–1176
Kapongo JP, Shipp L, Kevan P, Broadbent B (2008a) Optimal concentration of Beauveria bassiana vectored by bumble bees in relation to pest and bee mortality in greenhouse tomato and sweet pepper. BioControl 53:797–812
Kapongo JP, Shipp L, Kevan P, Sutton JC (2008b) Co-vectoring of Beauveria bassiana and Clonostachys rosea by bumble bees (Bombus impatiens) for control of insect pests and suppression of grey mould in greenhouse tomato and sweet pepper. Biol Control 46:508–514
Karise R, Dreyersdorff G, Jahani M, Veromann E, Runno-Paurson E, Kaart T, Smagghe G, Mänd M (2016) Reliability of the entomovector technology using Prestop-Mix and Bombus terrestris L. as a fungal disease biocontrol method in open field. Sci Rep 6:31650
Kovach J, Petzoldt R, Harman GE (2000) Use of honey bees and bumble bees to disseminate Trichoderma harzianum 1295–22 to strawberries for Botrytis control. Biol Control 18:235–242
Lange WH, Bronson L (1981) Insect pests of tomatoes. Annu Rev Entomol 26:345–371
Mertely JC, MacKenzie SJ, Legard DE (2002) Timing of fungicide applications for Botrytis cinerea based on development stage of strawberry flowers and fruit. Plant Dis 86:1019–1024
Mommaerts V, Put K, Smagghe G (2011) Bombus terrestris as pollinator-and-vector to suppress Botrytis cinerea in greenhouse strawberry. Pest Manag Sci 67:1069–1075
Peng G, Sutton JC, Kevan PG (1992) Effectiveness of honey-bees for applying the biocontrol agent Gliocladium roseum to strawberry flowers to suppress Botrytis cinerea. Can J Plant Pathol Revue Can Phytopathol 14:117–129
Reeh KW, Hillier NK, Cutler GC (2014) Potential of bumble bees as bio-vectors of Clonostachys rosea for Botrytis blight management in lowbush blueberry. J Pest Sci 87:543–550
Scherm H, Nesmith DS, Horton DL, Krewer G (2001) A survey of horticultural and Pest management practices of the Georgia blueberry industry. Small Fruits Rev 1:17–28
Scherm H, Ngugi HK, Savelle AT, Edwards JR (2004) Biological control of infection of blueberry flowers caused by Monilinia vaccinii-corymbosi. Biol Control 29:199–206
Shafir S, Dag A, Bilu A, Abu-Toamy M, Elad Y (2006) Honey bee dispersal of the biocontrol agent Trichoderma harzianum T39: effectiveness in suppressing Botrytis cinerea on strawberry under field conditions. Eur J Plant Pathol 116:119–128
Smagghe G, Mommaerts V, Hokkanen H, Menzler-Hokkanen I (2012) Multitrophic interactions: the entomovector technology. In: Smagghe G, Diaz I (eds) Arthropod-plant interactions - novel insights and approaches for IPM. Springer, Dordrecht, pp 127–157
Sosa MA (1988) Hymenoptera pollinators on sunflower in North Dakota. North Dakota State University, Fargo
Williamson B, Tudzynsk B, Tudzynski P, van Kan JAL (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8:561–580
Yu H, Sutton JC (1997) Effectiveness of bumblebees and honeybees for delivering inoculum of Gliocladium roseum to raspberry flowers to control Botrytis cinerea. Biol Control 10:113–122
Acknowledgements
The author acknowledges support over the years by the EU-Core-Organic II (Bicopoll, Targeted precision biocontrol and pollination enhancement in organic cropping systems), the Special Research Fund of the Ghent University, the Flemish Agency for Innovation by Science and Technology (IWT-Flanders), and the Foundation Research-Flanders (FWO-Flanders) for his research on entomovectoring. Also Matti Pisman is gratefully thanked for his help with this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Smagghe, G. (2020). Case Studies on Entomovectoring in the Greenhouse and Open Field. In: Smagghe, G., Boecking, O., Maccagnani, B., Mänd, M., Kevan, P. (eds) Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops. Springer, Cham. https://doi.org/10.1007/978-3-030-18917-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-18917-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18916-7
Online ISBN: 978-3-030-18917-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)