Abstract
Greenhouse cage trials were conducted to determine the optimal concentration of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) (BotaniGard 22WP® formulation) as vectored by the bumble bee, Bombus impatiens (Cresson) (Hymenoptera: Apidae) pollinator for control of greenhouse whitefly, Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) on greenhouse tomato, tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) and green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) on greenhouse sweet pepper. Three inoculum concentrations of B. bassiana: low, 9 × 109; middle, 6.24 × 1010; and high, 2 × 1011 conidia g−1 of inoculum and two controls (one with bees and heat-inactivated inoculum, and the other which contained only the host plants and pest species) were tested in a completely randomized block design. Beauveria bassiana killed 18, 54 and 56% of the adult T. vaporariorum and 33, 70 and 67% of the adult L. lineolaris, respectively, at the low, middle and high concentrations; but no infection from B. bassiana occurred in each of the control treatments. Internal infection rates after surface sterilization of the pest insects were 11, 34 and 35% for adult T. vaporariorum, 29, 54 and 58% for adult L. lineolaris, 22, 34 and 30% for nymphal M. persicae and 17, 29 and 32% for nymphal T. vaporariorum, respectively, at the low, middle and high concentrations. Significantly more bumble bees died at the high concentration of B. bassiana (42–45%) than at the other concentrations (9–15%) and the controls (5–7%). Spores of B. bassiana were collected throughout the plant canopy with the greatest numbers sampled from the top third of the canopy [ca. 1,200 colony forming units (CFU) per cm−2]. The middle concentration was selected as the optimal concentration because it provided the best pest control with the least impact on the bees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pests (arthropods and diseases), both native and exotic, are important problems for integrated pest management (IPM) of greenhouse crops. Over the last two decades in Canada, more than one new pest per year has invaded greenhouse vegetable crops (Ferguson and Shipp 2002; Gillespie 2002). The presence of these new pests necessitates implementation of control programs that are compatible with existing IPM strategies. Parasitoids and predatory arthropods are usually the main control measures in greenhouse vegetable crops (Albajes et al. 1999; Heinz et al. 2004). Among microbial control agents, Bacillus thuringiensis (Berliner) (Bacillales: Bacillaceae) has been the most popular control agent (Osborne et al. 2004).
Many entomopathogenic fungi also represent promising control measures for insect pests (Faria and Wraight 2001). The fungi invade their host insects through the external cuticle. Spores of the fungi attach to the cuticle, germinate and penetrate the host. They then proliferate in the host haemocoel as walled hyphal bodies or wall-less amoeboid protoplasts. The host insects die as a result of several modes of action, including depletion of nutrients, physical obstruction or invasion of organs, and toxinosis (Hajek 1997; Butt and Goettel 2000).
Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) is a potentially effective control agent for several greenhouse pests including whiteflies, Lygus, thrips and aphids (Gindin et al. 1996; Liu et al. 2003; Shipp et al. 2003; Al-mazra’awi et al. 2006a; Quesada-Moraga et al. 2006). Fortunately, B. bassiana can be used effectively in association with natural enemies, others pathogens, different types of pesticides and pollinators (Goettel et al. 1990; Steinkraus and Tugwell 1997; Jacobson et al. 2001; Shipp et al. 2003; Kouassi et al. 2003). Goettel and Jaronski (1997) found that <1% of the honey bee workers became infected and no infection was detected in the brood when treated with the equivalent of 5 × 1013 conidia of B. bassiana per hectare of canola three times at 5-day intervals. However, the bumble bees, Bombus pratorum (L.) and B. terrestris (L.) (Hymenoptera: Apidae) have been reported infected by B. bassiana (Goettel et al. 1990) and B. bassiana will infect B. impatiens without epizootics in the hive (Al-mazra’awi et al. 2006a). This latter relationship needs to be quantified.
Al-mazra’awi et al. (2006a) demonstrated for the first time that bumble bees can be used to vector B. bassiana for control of Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) and Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) in greenhouse sweet pepper. Mean infection mortalities for L. lineolaris ranged from 34–45% compared to 9–15% in the control. Mean infection rates for F. occidentalis ranged from 34–40% compared to 3% in the control. For outdoor crops, honey bees have been shown capable to deliver Metarhizium anisopliae (Metsch.) Sorokin for control of pollen beetle, Meligethes aeneus (F.) (Coleoptera: Nitidulidae) (>70% infection levels) (Butt et al. 1998; Carreck et al. 2007) and B. bassiana for L. lineolaris (22–56% infection levels) (Al-mazra’awi et al. 2006b) on canola. None of these studies investigated the optimal concentration of bee-vector entomopathogenic fungi for the pest control; distribution of the fungal control agent by the bee in the plant canopy; or quantified the effects of this fungus on bees.
Bumble bees are the main pollinator of greenhouse tomatoes and peppers worldwide (Kevan et al. 1991; Shipp et al. 1994; Velthuis and Van Doorn 2006). Combining the Bee Vector Technology and its use as a crop pollinator provides a new value added management strategy for pest management and crop production improvement in greenhouse tomato and sweet pepper. This technology has multiple potential benefits to growers such as: the delivery of the biological control agent (inoculum) to flowers, leaves and developing fruit; continuous dissemination of the inoculum to the crop 7 days a week; no involvement of manual application costs (labour) for the biological control agents; the combination of pollination and distribution of biological control agents; and the reduced negative impact on the environment as most of the inoculum lands on the plant and not in the soil or air (Kevan et al. 2007).
Thus, the objectives of the current studies were: (1) to quantify relationships among inoculum densities of B. bassiana as vectored by bumble bees on arthropod pest mortality [Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae), Myzus persicae (Sulzer) (Hemiptera: Aphididae) and L. lineolaris]; (2) to assess mortality of bumble bee vectors exposed to three inoculum densities of B. bassiana; and (3) to investigate the distribution of bee-vectored B. bassiana in the plant canopy.
Materials and methods
Plants
Tomato, Lycopersicon esculentum Mill. (Solanaceae) (cv Rapsodie), and sweet pepper, Capsicum annuum L. (Solanaceae) (cv Edison), plants were grown in a mixture of 1:1 sand and peat moss in 20 cm pots. The plants were maintained in a separate greenhouse compartment until they produced two sets of flowers. They were then transferred to fine-meshed experimental cages (520 × 240 × 220 cm). An Argus Computer Control System (Argus Control Systems Ltd., White Rock, BC, Canada) was used to maintain climatic conditions of 21–23°C and 80–85% RH for both crops. Plants were watered and fertilized with the Harrow Fertigation Manager (Labbate Climate Control Systems, Leamington, ON, Canada) following standard commercial practices (Ontario Ministry of Agriculture and Food 2005).
Insects
Adult T. vaporariorum and M. persicae used in the trials were collected from greenhouse colonies that were maintained on tomato and sweet pepper plants at the Agriculture and Agri-Food Canada (AAFC), Greenhouse and Processing Crops Research Centre (GPCRC), Harrow, ON, Canada. Second instar nymphs of T. vaporariorum for the cage trials were obtained by artificially infesting one top canopy leaf per plant on 12 randomly-selected tomato plants per treatment with 80 adult whiteflies inside an organdy sleeve cage (300 μm mesh) 2 weeks before each sampling date. The adults were left on the leaves for 3 days to allow for production of at least 50–100 eggs leaf−1. The late instar nymphs of M. persicae that were sampled in the trials were obtained from progeny of M. persicae that were released at the beginning of the trial.
Newly emerged adults of L. lineolaris (24–48-h old) were obtained from a laboratory culture maintained at AAFC, Southern Crop Protection and Food Research Centre (SCPFRC), London, ON, Canada. The colony of L. lineolaris was established from field-collected adult insects on alfalfa at the SCPFRC Research Farm. The laboratory colony of L. lineolaris was maintained on organic lettuce as a food source at 24°C, 60% RH and 16-h photoperiod.
Bumble bee colonies (B. impatiens), provided by Biobest Canada Ltd., Leamington, ON, Canada, were used in all trials. Each colony consisted of 1 queen and 50 workers. The colonies were anaesthetized by CO2 and transferred (bees + cells) from the commercial box to a wooden hive box (32 × 23 × 24 cm) fitted with an inoculum dispenser (Fig. 1). The bees were kept in the wooden boxes for 2 days and fed sugar solution (syrup) before using them in the cage trials to allow the bees to become familiar with the new hive box. The dispenser used was the same as Yu and Sutton (1997)’s model but as modified by Al-mazra’awi (2004).
Biological control agent
The commercial formulation of B. bassiana, GHA strain (BotaniGard 22WP®, Laverlam International Corporation, Butte, MT, USA) was used for all trials. The initial concentration of BotaniGard 22WP® was 2 × 1011 conidia of B. bassiana per g of product. Viability of the B. bassiana was determined before preparing the inoculum for each trial. This allowed for readjustment of the amount of product used to make the appropriate concentration for each treatment. To determine viability of the conidia, six 0.01 g samples of Botanigard 22WP® were suspended each in flask containing 100 ml distilled water and 0.1% Tween 80. Then 200 μl of the conidial suspension were added to 1 ml of Sabouraud’s dextrose broth with 1% yeast extract in a sterile test tube. The suspension was incubated at 24 ± 1°C for 24 h. After this, four groups each consisting of 200 conidia were examined for percentage germination using a hemacytometer and a compound microscope. Viability of the B. bassiana ranged from 95 to 99% for each of the trials. Three active concentrations of B. bassiana were prepared by mixing autoclaved corn flour, the diluent/carrier found to be most efficacious with the BotaniGard 22WP® (Al-mazra’awi et al. 2007). These concentrations were: 9 × 109 (low concentration), 6.24 × 1010 (middle concentration) and 2 × 1011 (high concentration) conidia of B. bassiana per g of the product.
Experimental design and procedures
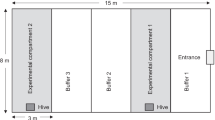
The cage trials were conducted in greenhouse compartments (13 × 8 m) containing two or three cages per greenhouse at the GPCRC. Fine mesh screening was used to contain the bees as well as the target pests. Each cage contained 64 potted sweet pepper (spring and summer 2004) or tomato plants (spring and summer 2005). The plants were arranged in two double rows of 16 plants per row in each cage. The environment within the cages was maintained at 21–23°C and 80–85% RH throughout the trials. A completely randomized block design was used for each of the five treatments with four replicates (sweet pepper) and five replicates (tomatoes) over time. The treatments included three inoculum densities: 9 × 109 conidia g−1, 6.24 × 1010 conidia g−1 and 2 × 1011 conidia of Beauveria g−1 (label rate for commercial formulation), heat inactivated B. bassiana treatment and a control treatment in which the inoculum was absent. One hive of 50 bumble bee workers (B. impatiens) was introduced into each treatment, except for the control treatment which had no bees.
Each colony of bumble bees was introduced 1 day after the plants were placed in the cages to allow acclimation to their new hive and the cage. On day 2, 256 L. lineolaris and 960 green peach aphids, M. persicae were released per treatment for the pepper trials and 1,000 T. vaporariorum in the tomato trials for each replicate. Wooden dispensers (21 × 12 × 8.5 cm) filled with 25 g of the appropriate inoculum concentration (BotaniGard 22WP® and corn flour mixture) were placed at the exit/entry opening of the hives on day 3, after which the bees were allowed to forage and disseminate the inoculum. The first samples (50 L. lineolaris and T. vaporariorum adults, and 48 nymphal T. vaporariorum or M. persicae) were collected on day 6 after which the dispensers were removed. The insect pests were collected to determine the percentage of mortality caused by B. bassiana, the number of B. bassiana propagules per Lygus or groups of five adult T. vaporariorum and groups of six immature T. vaporariorum and M. persicae, and the internal infection level of surface sterilized insect pests by B. bassiana. The hives were then closed and the bees (B. impatiens) fed pollen patties (pollen grains mixed with 50% wt./wt. sugar solution) until the dispensers were replaced. The dispensers were refilled and replaced on day 10 and a second set of samples of pest insects was collected 3 days later. After each sampling period, the remaining inoculum in the dispenser was dried and weighed using a Mettler AT250’s scale (Mettler-Toledo, Inc., USA) to determine the amount of inoculum that the bumble bees vectored during the sampling period. At each sampling date, five bees were collected per treatment as they exited from the dispenser to determine the number of B. bassiana propagules per bee.
Ten L. lineolaris, 10 T. vaporariorum adults and 24 nymphal M. persicae/T. vaporariorum per sample were surface sterilized to estimate internal infection levels (i.e. infection levels in the pests at the time that the samples were collected). The samples were surface-sterilized by immersing in 70% ethanol for 15–20 s, followed by 5% bleach containing 0.3% NaOCl amended with 0.05% Tween-20 for 3 min and two rinses of sterile distilled water amended with Tween-20 for 1–2 min (Shipp et al. 2003) The surface sterilized insects were then placed on water agar plates and incubated at 24 ± 1°C and 80% RH for 5 days to determine the incidence of mycosis.
Another 10 L. lineolaris, 10 T. vaporariorum adults 24 nymphal M. persicae/T. vaporariorum and 5 B. impatiens per treatment sample were washed to determine the amount of conidia [colony forming units (CFU)] that individual pests and bees carried. The samples were agitated individually in flasks containing 100 ml sterilized distilled water and 0.1% Tween 80 for the bees (in 50 ml of sterilized distilled water for groups of five individuals for adult T. vaporariorum and groups of six individuals for immature T. vaporariorum and M. persicae) on a rotary shaker at 125 rpm for 2 h. Three 0.1 ml aliquots of tenfold serial dilutions of each suspension were spread on oatmeal agar Petri plates amended with 550 μg ml−1 Dodine, 400 μg ml−1 penicillin G., 1,000 μg ml−1 streptomycin sulphate and 5 μg ml−1 crystal violet (Beilharz et al. 1982), incubated at 25 ± 1°C for 4–5 days, after which colonies of B. bassiana were counted and recorded.
The remaining 30 L. lineolaris and T. vaporariorum were used to determine the percentage mortality caused by B. bassiana over time. Each adult L. lineolaris was placed in an aerated Petri dish (9-cm ∅) and fed fresh organically grown lettuce leaves for 7 days. Individuals of T. vaporariorum were placed in an aerated sealed plastic vial on the growing point of a tomato plant for 7 days. The tomato growing points and organic lettuce were replaced every second day. All bioassay cages were inspected daily for dead insects. Dead insects were placed on moistened filter paper-lined Petri dishes and incubated at 25°C and 80% RH for 7 days. Presence of white mycelium on the dead insects was used to indicate the incidence of B. bassiana infection. Myzus persicae and T. vaporariorum nymphal mortalities could not be determined this way because of high handling mortality.
Mortality of the bumble bees (B. impatiens) exposed to the B. bassiana treatments was determined after the second sampling period. At this time, each bumble bee colony was transferred back into the commercial hive box and fed pollen patties for 3 weeks to simulate a typical time period that the hives would be kept in commercial greenhouses. The hives were maintained in controlled environment chambers at 23 ± 1°C. Incidence of bumble bee mortality was recorded weekly.
Twelve plants (three in each row) per treatment were selected randomly at each sampling period. One flower and three leaves were collected individually from the selected plant. The leaves were sampled at different positions of the plant canopy which was divided into three sections (top, middle and bottom). One leaf was collected from each of the three positions. The flower and leaf samples then were processed individually following the protocol described earlier for insect pests and bees to determine the number of CFU of B. bassiana. The leaves were washed in 100 ml sterilized distilled water and 0.1% Tween 80, and the flowers in 50 ml of sterilized distilled water.
Statistical analysis
Abbott’s formula (Abbott 1925) was used to determine percent mortalities of adult T. vaporariorum and L. lineolaris for the three Beauveria treatment concentrations. All mortality, mycosis and internal infection data for T. vaporariorum, L. lineolaris and M. persicae, and bee mortalities were subjected to arcsine square root transformation before statistical analysis. The transformed data for each insect species were first analysed using two-way analysis of variance (ANOVA). Mean percentages of killed insects and insects exhibiting mycosis were then compared using Tukey multiple comparison (SAS Institute 2001) to determine if significant differences occurred among them.
To compare the number of CFU on the insect and plant samples, and to determine the vertical distribution of B. bassiana in the crop canopy, the CFU counts of B. bassiana at each leaf position of the canopy and on the different insect and plant samples were arcsine transformed and subjected to PROC univariate, residual analysis and then analysed using a repeated measurement procedure (PROC MIXED covtest, SAS Institute 2001). The mean numbers of CFU of B. bassiana from each insect and plant sample and at each of the three leaf positions in the canopy were then compared using the F-test. Mortality and CFU data were back-transformed to their original scales for presentation in the tables.
Results
Mortality of pest insects by Beauveria bassiana
The highest percentages of dead insects were recorded at the concentrations of 6.24 × 1010 and 2 × 1011 conidia of B. bassiana per g of the inoculum (Table 1). A significant difference in percent mortality of insect pests was found among the three concentrations of B. bassiana (F 2, 21 = 39.41, P < 0.0001). Comparison between the middle and high concentrations indicated that there was no significant difference in mortality rates between these concentrations. The type of crop (pepper and tomato) did not have any impact on the performance of the different concentrations of B. bassiana (F 1, 21 = 0.16, P = 0.8524).
The rate of internal infection of B. bassiana increased with the concentration of B. bassiana (Table 1). On tomato, it varied from 11.0 to 35.0% in adult whiteflies and 17.0–32.0% in whitefly nymphs from low to high concentration. A similar trend was found on sweet pepper in which the infection rate varied between 29 and 58% in adult L. lineolaris and 22–30% in M. persicae. None of the insect pests in either the heat inactivated inoculum or the control treatments were infected (Table 1).
Effects of Beauveria bassiana on bumble bees (Bombus impatiens)
The highest percentage of bumble bee death occurred in the colonies in which the highest concentration of B. bassiana was used (Table 2). Comparison of treatments regarding the percentages of bee mortality showed a significant difference among treatments (F 4, 40 = 321.12, P < 0.0001) with the greatest percentage of dead bumble bees in the high concentration of B. bassiana compared to the other two concentrations. The percentages of mycosis among the dead bees were higher (73%) at the highest inoculum level than at the middle and lower levels (44–56%) (Table 2).
Vertical distribution of spores of Beauveria bassiana in the crop canopy
The numbers of spores of B. bassiana deposited by the bumble bees were not evenly distributed at each canopy height (Table 3) and each bee carried about 0.21 and 0.20 mg of inoculum per day in the tomato and pepper trials, respectively. The distribution of spores on the top, middle and bottom sections of tomato plants differed significantly (F 2, 81 = 11.13, P < 0.0001) with the highest number on the top leaves. Treatment effects on deposited spores also differed among canopy sections with the greatest number of CFU found on the top leaves at the highest concentration of Beauveria (F 2, 81 = 57.86, P < 0.0001). No interaction was found between inoculum density and density of spores of B. bassiana on the leaves (F 4, 81 = 1.25, P = 0.2959). Similar results were obtained in sweet pepper. As in tomato, the distribution of spores of B. bassiana differed significantly among the three levels in the leaf canopy in peppers (F 2, 63 = 28.42, P < 0.0001) with the highest number of spores deposited on the top leaves. There was a significant difference among the three concentrations of spores of B. bassiana applied (F 2, 63 = 29.24, P < 0.0001). The results also indicated that there was no interaction between treatments and distribution of spores in the canopy (F 4, 63 = 0.12, P = 0.9761).
Number of CFU of Beauveria bassiana deposited by bumble bees on leaves and flowers
Colony forming units of B. bassiana were detected on a majority of the tomato leaf samples from the treatments in which the bees vectored high, middle and low concentrations of the pathogen. On pepper, essentially all leaves had CFU of B. bassiana from the high, middle and low concentrations. Counted CFU/cm−2 ranged from 714 to 76 on tomato leaf samples and from 596 to 172 on sweet pepper, for the high, middle and low concentrations of B. bassiana (Tables 4, 5). No CFU of B. bassiana was detected on the leaves in the heat inactivated inoculum and control treatments.
Colony forming unit counts of B. bassiana on the tomato flower samples varied from 30.7 × 103 to 2.8 × 103 for the high, middle and low concentration treatments. The percentages of flowers having CFU of B. bassiana were again high (>75%) for the high, middle and low concentration treatments. In the pepper trials, 22.0 × 103 to 2.1 × 103 CFU of B. bassiana were found on flowers from the high, middle and low concentration treatments. Those CFU of B. bassiana were detected on 85–58% of the flower samples corresponding to high, middle and low concentration treatments (Tables 4, 5).
Number of CFU of Beauveria bassiana on insects (pests and pollinator)
The numbers of CFU of B. bassiana found on the different insects and the percentage of those insects that had detectable CFU are presented in Tables 4 and 5, respectively, for the tomato and sweet pepper trials. Approximately 96 to 76% of the bumble bees from the high, middle and low concentration treatments from both crops carried spores of B. bassiana after leaving the dispenser. On tomato, bumble bees had 1.6 × 106 to 1.0 × 104 CFU of B. bassiana for the high, middle and low concentration treatments. On pepper, they carried 1.6 × 106 to 3.3 × 105 CFU of B. bassiana from the high, middle and low concentration treatments. On the pest insects, the numbers of CFU ranged from 544 to 69 for groups of five adult T. vaporariorum and from 253 to 77 per adult L. lineolaris, for the high, middle and low concentration treatments. Colony forming units of B. bassiana varied from 267 to 30 for groups of six T. vaporariorum nymphs, and from 230 to 39 for groups of six M. persicae, for the high, middle and low concentration treatments. No B. bassiana was found on any of the insects from the heat inactivated inoculum and control treatments.
Discussion
In the current study, individual bumble bees carried ca. 0.20 mg of inoculum per day when exiting the dispenser which was approximately half of what Al-mazra’awi et al. (2006a) and Yu and Sutton (1997) recorded. Al-mazra’awi et al. (2007) reported that the quantity of conidia of B. bassiana that was picked up by honey bees was less when the moisture content of the inoculum was lower. In the trials by Al-mazra’awi et al. (2006a), the humidity was 70% compared to 80–85% RH in our trials.
Distribution of spores in the plant canopy in our study showed a difference among the three plant heights (top, middle and bottom). Foraging behaviour of the bees and the growth phenology of the plant were probably the main determinants for the vertical distribution pattern of spore deposition. Bumble bees are attracted to the flowers for pollen and nectar (Kevan 2005), and flowers in general occur in the top third of the plant canopy. Thus, the leaves in the top section of the canopy would receive also larger amounts of inoculum from the bees. In addition, inoculum powder probably was shed also from the bees by buzz pollination of the flowers whereby high frequency muscle vibrations of the bees are used to shake the pollen off the flowers and grooming by the bees on the leaves. This inoculum ended up being deposited throughout the plant canopy. The horizontal distribution of inoculum among plants could not be assessed because of the small size of the cage (5.20 × 2.40 m) and bumble bees are able to fly more than 200 m (Heinrich 1979; Loose et al. 2005).
The spores of B. bassiana vectored by the bumble bees and deposited on the flowers and leaves infected the T. vaporariorum and L. lineolaris present on the plants. Adult L. lineolaris mortalities were greater than for adult T. vaporariorum (68% vs. 55%) for the pooled middle and high concentration treatments, and 33% vs. 18% for the low concentration treatment. There are several possible reasons for these differences. First, L. lineolaris are more mobile insects than whiteflies (Howard et al. 1994). The latter only move a short distance before settling down on another leaf (Arnó et al. 2006). By frequently moving, L. lineolaris is more likely to contact spores of B. bassiana either in the air or on the plant surface. Second, L. lineolaris feed on the upper parts of the plants such as leaves, flowers and growing points where most of the infective spores of B. bassiana were deposited. In contrast, whiteflies feed on the underside of the plant leaves (Arnó et al. 2006) which are less exposed to deposition of B. bassiana’s spores carried by the bumble bees.
Individual aphid and whitefly nymphal mortalities were not determined because mortality from handling these insects was too great. However, the internal infection rates recorded for the aphids and whitefly nymphs were high enough to show that the B. bassiana induced mortality among them. The internal infection level for adults was always lower than the bioassay mortalities (Table 1). This can be explained by the fact that B. bassiana spores must first attach, then penetrate the insect cuticle and multiply in the haemocoel. This infection process may take several hours to 2 days (Jaronski 1997). In the present study, insect pests were immediately processed after their collection to determine their internal infection levels. Thus, the spores attached to insects used in the bioassay trials had a longer time to germinate and penetrate the host compared to those samples that were processed for surface sterilization shortly. Similar internal infection levels for immature T. vaporariorum in complete greenhouse cucumber spray trials at GPCRC resulted in >60% reduction in adult T. vaporariorum population levels after four weekly spray applications of B. bassiana (Shipp et al. 2003). In addition, Wraight et al. (2000) reported >90% control of immature Bemisia argentifolii Bellow and Perring in field trials on cucumber, melon and cantaloupe where B. bassiana was applied using a portable air-assisted sprayer.
Findings from our study indicate that B. bassiana can have an impact on bumble bee pollinators. Vandenberg (1990) reported that B. bassiana reduced honey bee longevity at concentrations of 9 × 107 and 9 × 108 spores per bee when sprayed on the bees or mixed in 50% sucrose syrup and fed to the bees. However, when whole-hives of honey bees were exposed to the same concentration of B. bassiana (strain GHA), as used in the middle concentration of our study, for three times at 5-day intervals, infection level was <1% for workers and no infection occurred in the brood (Goettel and Jaronski 1997). Brood temperature for honey bees is ca. 32–36°C which is too high for germination and development of B. bassiana (Ekesi et al. 1999). The hive temperatures for B. impatiens were not measured in the current study, but may also impact the susceptibility of B. impatiens to infection by B. bassiana, especially when colony size exceeds the initial stocking rate of 50 bees per hive. In commercial greenhouses, hives populations will grow to >100 bees per hive before the hive is replaced.
In our study, exposure of B. impatiens to the high concentration of B. bassiana for 3 days and then repeated again for another 3-day exposure period after 4 days resulted in 42–45% mortality compared to the same exposure periods for the other treatments after a 5 weeks period. However, the percentage mortality of bumble bees exposed to the middle and low concentrations were not statistically different (9–15%) and, under practical commercial production conditions, not much higher than the control mortalities (5–7%). Evaluation of these bee-vectored B. bassiana concentrations under commercial production conditions for greenhouse tomato over a 7 weeks period in 2006 found that pollination level and fruit yield and quality were all within acceptable commercial standards (J.P.K., unpublished data). The high and middle concentrations of B. bassiana also killed the highest percentages of T. vaporariorum and L. lineolaris (ca. 62%). Therefore, the middle concentration of B. bassiana as vectored by bumble bee pollinators was considered the optimal concentration level.
Our study has shown that bumble bees can be used to vector effectively B. bassiana to greenhouse tomato and sweet pepper to infect greenhouse pests such as T. vaporariorum, M. persicae and L. lineolaris. This is a new technique for the application of microbial control agents which combines pest control with flower pollination (Kevan et al. 2005). Future research will test if other control agents compatible with B. bassiana could replace some or all of the corn flour that was added in the preparation of the inoculum, so that bumble bees could vector multiple agents for potential control of arthropod pests and suppression of plant diseases.
References
Abbott WS (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Albajes R, Gullino ML, van Lenteren JC, Elad Y (eds) (1999) Integrated pest and disease management in greenhouse crops. Kluwer, Dordrecht, The Netherlands
Al-mazra’awi MS (2004) Biological control of tarnished plant bug and western flower thrips by Beauveria bassiana vectored by bee pollinators. Ph.D. thesis, University of Guelph, Guelph, ON, Canada
Al-mazra’awi MS, Shipp L, Broadbent B, Kevan P (2006a) Biological control of Lygus lineolaris (Hemiptera: Miridae) and Frankliniella occidentalis (Thysanoptera: Thripidae) by Bombus impatiens (Hymenoptera: Apidae) vectored Beauveria bassiana in greenhouse sweet pepper. Biol Control 37:89–97
Al-mazra’awi MS, Shipp L, Broadbent B, Kevan P (2006b) Dissemination of Beauveria bassiana by honey bees (Hymenoptera: Apidae) for control of tarnished plant bug (Hemiptera: Miridae) on canola. Environ Entomol 35:1569–1577
Al-mazra’awi MS, Kevan PG, Shipp L (2007) Development of Beauveria bassiana dry formulation for vectoring by honey bees Apis mellifera (Hymenoptera: Apidae) to flowers of crops for pest control. Biocontrol Sci Technol 17:733–741
Arnó J, Albajes R, Gabana R (2006) Within-plant distribution and sampling and mixed infestations of Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) in winter tomato crops. J Econ Entomol 99:331–340
Beilharz VC, Parbery DG, Swart HJ (1982) Dodine: a selective agent for certain soil fungi. T Brit Mycol Soc 79:507–511
Butt TM, Goettel MS (2000) Biossays of Entomogenous fungi. In: Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. CABI Publishing, New York, pp 141–191
Butt TM, Carreck NL, Ibrahim L, Williams IH (1998) Honey bee mediated infection of pollen beetle (Meligethes aeneus Fab.) by the insect-pathogenic fungus, Metarhizium anisopliae. Biocontrol Sci Technol 8:533–538
Carreck NL, Butt TM, Clark SJ, Ibrahim L, Isger EA, Pell JK Williams IH (2007) Honey bees can disseminate a microbial control agent to more than one inflorescence pest of oilseed rape. Biocontrol Sci Technol 17:179–191
Ekesi S, Maniania NK, Ampong-nyarko K (1999) Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci Technol 9:177–185
Faria M, Wraight SP (2001) Biological control of Bemisia tabaci with fungi. Crop Prot 20:767–778
Ferguson G, Shipp L (2002) New pests in Ontario greenhouse vegetables. IOBC/WPRS Bull 25(1):69–72
Gillespie DR (2002) Biological and integrated control in vegetables in British Columbia: the challenge of success. IOBC/WPRS Bull 25(1):73–76
Gindin G, Barash I, Raccah B, Singer S, Ben-Zeev IS, Klein M (1996) The potential of some entomopathogenic fungi as biocontrol agents against the onion thrips, Thrips tabaci and the western flower thrips, Frankliniella occidentalis. Folia Ent Hung 57(Suppl):37–42
Goettel MS, Jaronski ST (1997) Safety and registration of microbial agents for control of grasshoppers and locusts. Mem Entomol Soc Can 171:83–99
Goettel MS, Poprawski TJ, Vandenberg JD, Li Z, Roberts DW (1990) Safety to non target invertebrates of fungal biocontrol agents. In: Laird M, Lacey LA, Davidson EW (eds) Safety of microbial insecticides. CRC, Boca Raton, FL, pp 209–232
Hajek AE (1997) Ecology of terrestrial fungal entomopathogens. Adv Microbiol Ecol 15:193–249
Heinz KM, van Driesche RG, Parrellla MP (eds) (2004) Biocontrol in protected culture. Ball Publishing, Batavia, IL, pp 552
Heinrich B (1979) Bumblebee economics. Harvard University Press, Cambridge, MA
Howard RJ, Garland JA Seaman WL (eds) (1994) Diseases and pests of vegetable crops in Canada. The Canadian Phytopathological Society and the Entomological Society of Canada, Ottawa, pp 554
Jacobson RJ, Chandler D, Fenlon J Russell KM (2001) Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci Technol 11:391–400
Jaronski ST (1997) New paradigms in formulating myco-insecticides. Am Soc Test Mater 1328:99–112
Kevan PG (2005) Advertisement in flowers. In: Dafni A, Kevan PG, Husband BC (eds) Practical pollination biology. Enviroquest, Cambridge, ON, Canada, pp 147–229
Kevan PG, Straver WA, Offer M, Laverty TM (1991) Pollination of greenhouse tomatoes by bumble bees in Ontario. Proc Entomol Soc Ontario 122:15–19
Kevan PG, Shipp L, Kapongo JP, Al-mazra’awi MS (2005) Bee pollinators vector biological control agents against insect pests of horticultural plants. In: Guerra Sanz JM, Roldán Serrano A, Mena Granero A (eds) First short course on pollination of horticulture plants. IFAPA, Consejería de Inovación, Ciencia y Impresa, La Mojonera, Almería, Spain, pp 77–95
Kevan P, Sutton J, Shipp L (2007) Pollinators as vectors of Biocontrol agents—the B52 story. In: Vincent C, Goettel M, Lazarovits G (eds) Biological control: a global perspective—case studies from around the world. CABI Publishing, UK, pp 319–327
Kouassi M, Coderre D, Todorova SI (2003) Compatibility of zineb, dimethoate and Beauveria bassiana (Balsamo) Vuillemin against tarnished plant bug (Hemiptera: Miridae). J Entomol Sci 38:359–367
Liu H, Skinner M, Brownbridge M, Parker BL (2003) Characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae). J Invert Pathol 82:139–147
Loose JL, Drummond FA, Stubbs C, Woods S, Hoffmann S (eds) (2005) Conservation and management of native bees in cranberry. Agricultural and forest experimental station. The University of Maine. Bull 191:27
Ontario Ministry of Agriculture and Food (2005) Growing greenhouse vegetables. Publication 371. Queen’s Printer, Toronto, Canada, p 159
Osborne LS, Bolckmans K, Landa Z, Peńa J (2004) Kinds of natural enemies. In: Heinz KM, van Driesche RG, Parrellla MP (eds) Biocontrol in protected culture. Ball Publishing, Batavia, IL, pp 95–127
Quesada-Moraga E, Maranhao EAA, Valverde-Garcia P, Santiago-Alvarez C (2006) Selection of Beauveria bassiana isolates for the control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biol Control 36:274–287
SAS Institute (2001) PROC user’s manual, version (6th edn). SAS Institute, Cary, NC
Shipp JL, Whitfield GH, Papadopoulos AP (1994) Effectiveness of the bumble bee, Bombus impatiens Cresson (Hymenoptera: Apidae), as a pollinator of greenhouse sweet pepper. Sci Hortic 57:29–39
Shipp JL, Zhang Y, Hunt DWA, Ferguson G (2003) Influence of humidity and greenhouse microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pests. Environ Entomol 32:1154–1163
Steinkraus DC, Tugwell NP (1997) Beauveria bassiana (Deuteromycotina: Moniliales). J Entomol Sci 32:79–90
Vandenberg JD (1990) Safety of four entomopathogens for caged adult honey bees (Hymenoptera: Apidae). J Econ Entomol 83:755–759
Velthuis HHW, Van Doorn A (2006) A century of advances in bumble bee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37:421–451
Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentofolii. Biol Control 17:203–217
Yu H, Sutton JC (1997) Effectiveness of bumblebees and honeybees for delivering inoculum of Gliocladium roseum to raspberry flowers to control Botrytis cinerea. Biol Control 10:113–122
Acknowledgements
We thank the Improved Farming Systems and Practices Initiative, Pest Management Centre, Agriculture and Agri-Food Canada for financial support. We also thank Laverlam International Corporation, Butte, MT, USA and Biobest Canada Limited, Leamington, ON, Canada for providing us with Beauveria bassiana and colonies of bumble bees, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapongo, J.P., Shipp, L., Kevan, P. et al. Optimal concentration of Beauveria bassiana vectored by bumble bees in relation to pest and bee mortality in greenhouse tomato and sweet pepper. BioControl 53, 797–812 (2008). https://doi.org/10.1007/s10526-007-9142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9142-9