Abstract
Bradykinesia is the cardinal symptom of Parkinson’s disease (PD) related to slowness of movement. The causes of PD bradykinesia are not known largely, because there are multiple brain areas and pathways involved from the neuronal degeneration site (dopamine (DA) neurons in substantia nigra pars compacta (SNc) and ventral tegmental area (VTA)) to the muscles. A neurocomputational model of basal ganglio-thalamo-cortico-spino-muscular dynamics with dopamine of PD bradykinesia is presented as a unified theoretical framework capable of producing a wealth of neuronal, electromyographic, and behavioral movement empirical findings reported in parkinsonian human and animal brain studies. The model attempts to uncover how information is processed in the affected brain areas, what role does DA play, and what are the biophysical mechanisms giving rise to the observed slowness of movement in PD bradykinesia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Parkinson’s disease
- Slowness of movement
- Bradykinesia
- Akinesia
- Computer model
- Dopamine
- Basal ganglia
- Motor cortex
- Spinal cord
- Triphasic pattern of muscle activation
1 Introduction
Bradykinesia is the hallmark and most disabling symptom of PD. Early in the disease, the most notable manifestation of bradykinesia is difficulty with walking, speaking, or getting into and out of chairs [24]. Individuals fail to swing an arm during walking or lack facial expression [1, 24, 33]. Later in life, bradykinesia affects all movements and, at its worst, results in a complete inability to move. Patients require intense concentration to overcome the apparent inertia of the limbs that exists for the simplest motor tasks. Movement initiation is particularly impaired when unnatural or novel movements are attempted [9] or when combining several movements concurrently [4, 29].
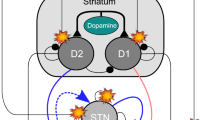
The causes of bradykinesia are not known, in part because there are multiple pathways from the sites of neuronal degeneration to the muscles. Figure 4.1 shows three of the most important pathways: (1) the pathway from SNc and VTA to the striatum and from the striatum to the substantia nigra pars reticulata (SNr) and the globus pallidus internal segment (GPi) and from there to the thalamus and the frontal cortex, (2) the pathway from SNc and VTA to the striatum and from the striatum to the SNr and the GPi and from there to the brainstem, and (3) the pathway from the SNc/VTA to cortical areas such as the supplementary motor area (SMA), the parietal cortex, and the primary motor cortex (M1), and from there to the spinal cord.
One of the popular views is that cortical motor centers are inadequately activated by excitatory circuits passing through the basal ganglia (BG) [2]. As a result, inadequate facilitation is provided to motor neuron pools, and hence movements are small and weak [2]. The implication of this view is that cells in the cortex and spinal cord are functionally normally. This paper will show otherwise.
The paper’s view is that disruptions of the BG output and of the SNc’s DA input to frontal and parietal cortices and spinal cord are responsible for delayed movement initiation. Elimination of DA modulation from the SNc disrupts, via several pathways, the buildup of the pattern of movement-related responses in the primary motor and parietal cortex and results in a loss of directional specificity of reciprocal and bidirectional cells in the motor cortex as well as in a reduction in their activities and their rates of change. These changes result in delays in recruiting the appropriate level of muscle force sufficiently fast and in an inappropriate scaling of the dynamic muscle force to the movement parameters. A repetitive triphasic pattern of muscle activation is sometimes needed to complete the movement. All of these result in an increase of mean reaction time and a slowness of movement (i.e., bradykinesia).
2 Empirical Signatures of PD Bradykinesia
PD bradykinesia has been linked with the degeneration of DA neurons in SNc and VTA. Bradykinesia manifests only when 80–90% of DA neurons die. All motor cortical and subcortical areas are innervated by SNc and VTA DA neurons [6, 23, 35]. The degeneration of DA neurons leads to a number of changes relevant to bradykinesia in the neuronal, electromyographic (EMG), and movement parameters reported in parkinsonian human and animal brains:
-
Reduction of peak neuronal activity and rate of development of neuronal discharge in the primary motor cortex and premotor area [26, 32].
-
Abnormal oscillatory GP (external and internal) neuronal responses [31].
-
Disinhibition of reciprocally tuned cells [22]. Reciprocally tuned cells are cells that discharge maximally in one movement direction but pause their activities in the opposite direction.
-
Significant increase in mean duration of neuronal discharge in motor cortex preceding and following onset of movement [3, 22, 26].
-
Multiple triphasic patterns of muscle activation [22, 27]. Triphasic pattern of muscle activation is a characteristic electromyographic (EMG) pattern characterized by alternating bursts of agonist and antagonist muscles. The first agonist burst provides the impulsive force for the movement, whereas the antagonist activity provides the braking force to halt the limb. Sometimes a second agonist burst is needed to bring the limb to the final position. In PD patients, multiple such patterns are observed in order for the subjects to complete the movement.
-
Reduction in the rate of development and peak amplitude of the first agonist burst of EMG activity [5, 10, 22, 25, 27, 32].
-
Co-contraction of muscle activation [3]. In PD patients, the alternating agonist-antagonist-agonist muscle activation is disrupted resulting in the coactivation of opponent muscle groups.
-
Increases in electromechanical delay time (time between the onset of modification of agonist EMG activity and the onset of movement) [3, 21, 22].
-
Asymmetric increase in acceleration (time from movement onset to peak velocity) and deceleration (time from peak velocity till end of movement) times of a movement.
-
Decrease in the peak value of the velocity trace [3, 8, 21, 22, 25, 30, 34].
-
Significant increases in movement time [3, 21, 22, 30, 32, 34].
Neural architecture of the dopamine modulated basal ganglio-thalamo-cortico-spino-muscular model. Top: basal ganglia module (GO signal representing the opening of the thalamo-cortical gate via inhibition of GPi response). Middle: cortical module for trajectory formation. Bottom: opponent-processing spino-muscular module for agonist-antagonist-agonist muscle activation. Arrow black lines, excitatory projections; solid dot black lines, inhibitory projections; diamond-dotted green lines, dopamine modulation; solid arrow gray lines, excitatory feedback pathways from muscle spindles. Solid dot gray lines: inhibitory feedback pathways from muscle spindles. GO globus pallidus internal segment (GPi) output signal, P bidirectional co-contractive signal, T target position command, V difference vector (DV) activity, u desired velocity vector (DVV) activity, A current perceived position vector (PPV) activity, M alpha motoneuronal activity, R Renshaw cell activity, X spinal type-b inhibitory interneuronal activity, I spinal type-a inhibitory interneuronal activity, S static gMN activity, D dynamic gMN activity, i and j antagonist cell pair
3 Basal Ganglio-thalamo-cortico-spino-muscular Model of PD Bradykinesia
Figure 4.2 depicts the basal ganglio-thalamo-cortico-spino-muscular model with dopamine of PD bradykinesia dynamics. The mathematical formalism of the model has been detailed in [11, 16, 20]. The model is composed of three modules coupled together: (1) the basal ganglio-thalamic module, (2) the cortical module, and (3) the spino-muscular module. All modules and their components are modulated by DA. The basal ganglio-thalamic module generates a scalable voluntary GO signal that gates volitional-sensitive velocity motor commands in the cortical module, which activate the lower spinal centers in the spino-muscular module. In the cortical module, an arm movement difference vector (DV) is computed in cortical parietal area 5 from a comparison of a target position vector (TPV) with a representation of the current position called perceived position vector (PPV). The DV signal then projects to area 4 (primary motor cortex), where a desired velocity vector (DVV) and a non-specific co-contractive signal (P) [28] are formed. The DVV and P signals correspond to two partly independent neuronal systems within the motor cortex. DVV represents the activity of reciprocal neurons [22] and is organized for the reciprocal activation of antagonist muscles. P represents the activity of bidirectional neurons (i.e., neurons whose activity decreases or increases for both directions of movement [22]) and is organized for the co-contraction of antagonist muscles. Whereas the reciprocal pattern of muscle activation serves to move the joint from an initial to a final position, the antagonist co-contraction serves to increase the apparent mechanical stiffness of the joint, thus fixing its posture or stabilizing its course of movement in the presence of external force perturbations [7, 28]. The spino-muscular module is an opponent-processing muscle control model of how spinal circuits afford independent voluntary control of joint stiffness and joint position. It incorporates second-order dynamics, which play a large role in realistic limb movements.
4 Results
The model can account for all empirical signatures of PD bradykinesia as they have been described in the previous section and reported in previous publications of the author [11,12,13,14,15,16,17,18,19,20]. To assist the readers of this paper, a subset of these simulation results are reported here. Reduction of DA in cortical and subcortical motor areas disrupts, via several pathways, the rate of development and peak neuronal activity of primary motor cortical cells (reciprocal and bidirection neurons) (see Figs. 4.3 and 4.4 for comparison). A clear triphasic AG1-ANT1-AG2 pattern marked with arrows which is evident in control case PSTH of reciprocally and bidirectionally organized neurons (Fig. 4.3) disappears in the dopamine-depleted case (Fig. 4.4). The same triphasic pattern is evident in simulated control DVV cell activities (Fig. 4.3) that disappears in the DA-depleted case (Fig. 4.4).
Comparison of peristimulus time histograms (PSTH) of reciprocally organized neurons (column 1; reproduced with permission from Doudet et al. [22, Fig. 4.4a, p. 182], Copyright Springer-Verlag) in area 4, simulated area’s 4 reciprocally organized phasic (DVV) cell activities (column 2), PSTH of area’s 4 bidirectional neurons (column 3; reproduced with permission from [22, Fig. 4.4a, p. 182], Copyright Springer-Verlag), and simulated area’s 4 co-contractive (P) cells activities (column 4) for a flexion (row 1) and extension (row 2) movements in normal monkey. The vertical bars indicate the onset of movement. Note a clear triphasic AG1-ANT1-AG2 pattern marked with arrows is evident in PSTH of reciprocally and bidirectionally organized neurons. The same triphasic pattern is evident in simulated DVV cell activities. The second peak in simulated activities marked with an arrow arises from the spindle feedback input to area’s 5 DV activity
Comparison of PSTH of reciprocally organized neurons (column 1; reproduced with permission from [22, Fig. 4.4a, p. 182], Copyright Springer-Verlag) in area 4, simulated area’s 4 reciprocally organized phasic (DVV) cell activities (column 2), PSTH of area’s 4 bidirectional neurons (column 3; reproduced with permission from [22], Fig. 4.4a, p. 182, Copyright Springer-Verlag), and simulated area’s 4 co-contractive (P) cells activities (column 4) for a flexion (a and c) and extension (b and d) movements in MPTP-treated monkey. The vertical bars indicate the onset of movement. Note that the triphasic pattern is disrupted: Peak AG1 and AG2 bursts have decreased, and ANT pause is shortened
These changes lead in delays in recruiting the appropriate level of muscle force sufficiently fast and in a reduction of the peak muscle force required to complete the movement (see Fig. 4.5).
Comparison of simulated GO signals (row 1) and α-MN activities (row 2) in normal (column 1) and dopamine-depleted (column 2) conditions. (Row 2) Blue solid curve, agonist α-MN activity; Red-dashed curve, antagonist α-MN activity. Note in PD (DA-depleted) case, the triphasic pattern is disrupted, and it is replaced by a biphasic pattern of muscle activation. Also, the peaks of agonist and antagonist bursts are decreased
Repetitive and sometimes co-contractive patterns of muscle activation are needed to complete the movement (see Fig. 4.6).
Comparison of the experimental GPi PSTH (column 1), GO signals (column 2), and α-MN activities (column 3) in normal (row 1) and dopamine-depleted (row 2) conditions. (Column 3, rows 1 and 2) Blue-colored solid curve, agonist α-MN unit; Red-colored dashed curve, antagonist α-MN unit. Note in dopamine-depleted case, the α-MN activity is disrupted and replaced by repetitive and co-contractive agonist-antagonist bursts (row 2, column 3). (Column 1, row 1) GPi PSTH in intact monkey reproduced with permission from Tremblay et al. [49, Fig. 4.4, p. 6], Copyright Elsevier. (Column1, row 2) GPi PSTH in MPTP monkey reproduced with permission from [49, Fig. 4.2, p. 23], Copyright Elsevier
These disruptions result in an abnormal slowness of movement (see Fig. 4.7).
Comparison of experimentally obtained (column 1; adapted from [26], Fig. 4.5, p. 189) and simulated (column 2) forearm displacement (position) in normal (a) and Parkinson’s disease (b) conditions. Shaded area: representation of neuronal change related to movement. In a and b, (column 1) the vertical bar indicates the onset of forearm displacement; S auditory cue, CRT mean value of cellular reaction time, T A changes of neuronal activity preceding onset of movement (OM), T B changes of neuronal activity following OM, T C changes of neuronal activity from T B till movement end (ME). (column 2) The time interval between vertical-dashed lines starting from left to right indicates CRT, TA, TB, and TC, respectively
References
Abbs JH, Hartman DE, Vishwanat B (1987) Orofacial motor control impairment in Parkinson’s disease. Neurology 37:394–398
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. TINS 12:366–375
Benazzouz A, Gross C, Dupont J, Bioulac B (1992) MPTP induced hemiparkinsonism in monkeys: behavioral, mechanographic, electromyographic and immunohistochemical studies. Exp Brain Res 90:116–120
Benecke R, Rothwell JC, Dick JPR (1986) Performance of simultaneous movements in patients with Parkinson’s disease. Brain 109:739–757
Berardelli A, Dick JPR, Rothwell JC, Day BL, Marsden CD (1986) Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 49:1273–1279
Bjorklund A, Lindvall O (1984) Dopamine containing systems in the CNS. In: Bjorklund A, Hokfelt T (eds) Handbook of chemical neuroanatomy. Classical transmitters in the CNS, Part 1, vol 2. Elsevier, Amsterdam, pp 55–121
Bullock D, Contreras-Vidal JL (1993) How spinal neural networks reduce discrepancies between motor intention and motor realization. In: Newel K, Corcos D (eds) Variability and motor control. Human Kinetics Press, Champaign., 1993, pp 183–221
Camarata PJ, Parker RG, Park SK, Haines SJ, Turner DA, Chae H et al (1992) Effects of MPTP induced hemiparkinsonism on the kinematics of a two-dimensional, multi-joint arm movement in the rhesus monkey. Neuroscience 48(3):607–619
Connor NP, Abbs JH (1991) Task-dependent variations in parkinsonian motor impairments. Brain 114:321–332
Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC (1996) Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol 39(1):79–88
Cutsuridis V (2006a) Biologically inspired neural architectures of voluntary movement in normal and disordered states of the brain. Unpublished PhD dissertation
Cutsuridis V (2006b) Neural model of dopaminergic control of arm movements in Parkinson’s disease bradykinesia. Kolias S, Stafilopatis A, Duch W ICANN 2006: artificial neural networks. LNCS, 4131. Springer, Berlin, 583–591
Cutsuridis V (2007) Does reduced spinal reciprocal inhibition lead to cocontraction of antagonist motor units? A modeling study. Int J Neural Syst 17(4):319–327
Cutsuridis V (2010a) Neural network modeling of voluntary single joint movement organization. I. Normal conditions. In: Chaovalitwongse WA et al (eds) Computational neuroscience. Springer, New York, pp 181–192
Cutsuridis V (2010b) Neural network modeling of voluntary single joint movement organization. II. Parkinson’s disease. In: Chaovalitwongse WA, Pardalos P, Xanthopoulos P (eds) Computational neuroscience. Springer, New York, pp 193–212
Cutsuridis V (2011) Origins of a repetitive and co-contractive pattern of muscle activation in Parkinson’s disease. Neural Netw 24:592–601
Cutsuridis V (2013a) Bradykinesia models of Parkinson’s disease. Scholarpedia 8(9):30937
Cutsuridis V (2013b) Bradykinesia models. In: Jaeger D, Jung R (eds) Encyclopedia of computational neuroscience. Springer, New York
Cutsuridis V (2018) Bradykinesia models. In: Jaeger D, Jung R (eds) Encyclopedia of computational neuroscience, 2nd edn. Springer, New York
Cutsuridis V, Perantonis S (2006) A neural model of Parkinson’s disease bradykinesia. Neural Netw 19(4):354–374
Doudet DJ, Gross C, Lebrun-Grandie P, Bioulac B (1985) MPTP primate model of Parkinson’s disease: a mechanographic and electromyographic study. Brain Res 335:194–199
Doudet DJ, Gross C, Arluison M, Bioulac B (1990) Modifications of precentral cortex discharge and EMG activity in monkeys with MPTP induced lesions of DA nigral lesions. Exp Brain Res 80:177–188
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Gibberd FB (1986) The management of Parkinson’s disease. Practitioner 230:139–146
Godaux E, Koulischer D, Jacquy J (1992) Parkinsonian bradykinesia is due to depression in the rate of rise of muscle activity. Ann Neurol 31(1):93–100
Gross C, Feger J, Seal J, Haramburu P, Bioulac B (1983) Neuronal activity of area 4 and movement parameters recorded in trained monkeys after unilateral lesion of the substantia nigra. Exp Brain Res 7:181–193
Hallett M, Khoshbin S (1980) A physiological mechanism of bradykinesia. Brain 103:301–314
Humphrey DR, Reed DJ (1983) Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. In: Desmedt JE (ed) Motor control mechanisms in health and disease. Raven Press, New York. 1983
Lazarus JC, Stelmach GE (1992) Inter-limb coordination in Parkinson’s disease. Mov Disord 7:159–170
Rand MK, Stelmach GE, Bloedel JR (2000) Movement accuracy constraints in Parkinson’s disease patients. Neuropsychologia 38:203–212
Tremblay L, Filion M, Bedard PJ (1989) Responses of pallidal neurons to striatal stimulation in monkeys with MPTP-induced parkinsonism. Brain Res 498(1):17–33
Watts RL, Mandir AS (1992) The role of motor cortex in the pathophysiology of voluntary movement deficits associated with parkinsonism. Neurol Clin 10(2):451–469
Weiner WJ, Singer C (1989) Parkinson’s disease and non-pharmacologic treatment programs. J Am Geriatr Soc 37:359–363
Weiss P, Stelmach GE, Adler CH, Waterman C (1996) Parkinsonian arm movements as altered by task difficulty. Parkinsonism Relat Disord 2(4):215–223
Williams SM, Goldman-Rakic PS (1998) Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex 8:321–345
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cutsuridis, V. (2019). Basal Ganglio-thalamo-cortico-spino-muscular Model of Parkinson’s Disease Bradykinesia. In: Cutsuridis, V. (eds) Multiscale Models of Brain Disorders. Springer Series in Cognitive and Neural Systems, vol 13. Springer, Cham. https://doi.org/10.1007/978-3-030-18830-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-18830-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18829-0
Online ISBN: 978-3-030-18830-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)