Abstract
Electrophysiology and catheter ablation in the pediatric population is currently undergoing a rapid evolution. The availability of three-dimensional mapping systems has lead to a significant reduction in radiation exposure for patients and staff. As the technology continues to improve, there is opportunity for radiation to be eliminated from routine procedures. This chapter will review the history of the mapping systems that have been at the forefront thus far, as well as outline approaches to ablating specific arrhythmias without fluoroscopy, from the simplest to the most difficult. Finally, important reasons to pursue a zero-fluoroscopy approach in this population will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Given the known risks of ionizing radiation, particularly relevant in the pediatric population, there has been an increasing effort to limit its use. Indeed, the era of low and even fluoroscopy-free electrophysiology study and ablation procedures has arrived [1]—D. Bradley
The above statement highlights the change currently in progress in the profession of pediatric electrophysiology. In little more than one decade, there has been a revolution in the treatment of arrhythmias in children. This revolution has been facilitated entirely by the development and refinement of three-dimensional mapping systems , which allow for the nonfluoroscopic visualization of catheter electrodes. The current millennium began with catheter ablation being the treatment of choice for arrhythmia management in children. Catheter guidance at that time was entirely dependent upon fluoroscopy. Today, three -dimensional mapping tools have allowed dramatic decreases in radiation exposure in nearly all-pediatric electrophysiology (EP) labs. This “fluoroless” movement, away from radiologic guidance, has been spurred by reports revealing the significant increase in medical radiation exposure, as well as the potential associated health risks [2,3,4]. Between 1987 and 2009 there was a 730% increase in medical radiation exposure in the United States. As a result, patient tracking of lifetime radiation dosing is becoming prevalent.
Ten years ago, fluoroscopy was the sole means of catheter guidance in the vast majority of pediatric electrophysiology labs. Today, virtually all EP labs use some form of three-dimensional guidance. Ten years from now, fluoroscopic guidance for routine pediatric ablation procedures will be rare.
Pediatric electrophysiologists have been the leaders in this revolution. As the fluoroless procedure becomes more common in the pediatric world, the techniques will spill over into the adult arena. The purpose of this chapter is to discuss the role of no-fluoro ablation techniques in the treatment of pediatric arrhythmias. Data relating to the performance and outcomes of this new approach are presented to help the practitioner incorporate the techniques into his or her daily practice. For many of the studies cited, we will also quote the rationale given for the importance of the particular study.
There has been a steady improvement in fluoroscopy times since the start of catheter ablation. Data from the pediatric registry (1998) showed the mean fluoroscopy time to be 47.6 min. Data from the earliest years of that registry (1991–1992) showed a mean of 60 min [5]. The next large study was the PAPCA registry . It was published in 2004, and showed a mean fluoro time of 38.3 min for supraventricular tachycardia (SVT) ablation in children [6].
The interest in minimizing radiation dose is due to the associated risks. Clay et al. using radiation dosimeters during EP procedures estimated that a single 15 min biplane fluoroscopy exposure during pediatric ablation conferred a lifetime risk to the patient of about 1:5000 for development of a fatal malignancy [7]. Extrapolating that to the early registry data would suggest that those patients could carry a risk as high as 1:1000 per procedure, for lifetime cancer. For a single procedure, the risk is greater for the patient than for the physician. However, over an entire career, the risk of radiation exposure for the staff can also be significant. The Expert Consensus Document on Radiation Safety in the Practice of Cardiology estimates that a busy interventional cardiologist, working an entire career, has about a 4% risk of fatal malignancy due to occupational radiation exposure [3]. That document recommends radiation doses, “As Low As Reasonably Achievable” (ALARA) .
For these reasons, broad interest remains in lowering radiation dose. Some of these efforts have gone toward optimizing fluoroscopy use. Gellis et al. developed an “ALARA ” protocol to help minimize radiation dose when fluoro was being used [8]. But the greatest benefit would come from nonfluoroscopic guidance systems.
Three-Dimensional Mapping: What Does It Involve?
Broadly, nonfluoroscopic mapping , or electroanatomic mapping systems (EAM) , use computers to localize electrodes in three-dimensional space. The location is then displayed on a screen. Relevant blood vessels and cardiac chambers can be drawn, creating a three-dimensional shell within which the catheter can be manipulated.
There have been two mapping systems which have impacted the direction of patient care: the CARTO® system (Biosense-Webster) and the EnSite system (Abbott, Abbott Park, Ill, USA). The CARTO system was the first system clinically available. The first report of its use was by Worley in 1998 [8]. That system works on magnetic detection. The patient is positioned on the cath table, lying on an array of magnets. A proprietary catheter contains a magnetic detector in its tip. By detection of the magnetic field strength at the tip, a computer can triangulate the position of the tip in three-dimensional space. The EnSite system functions by electrical impedance measurement. The patient has three sets of patches positioned on the torso to create three orthogonal electrical fields, anterior-posterior, superior-inferior, and right-left. Any electrode from any catheter can detect the three fields, and the computer can then triangulate the location of that electrode relative to a reference electrode, usually on the patient’s abdomen.
Each system has its own strengths. The CARTO system does not need a reference electrode, and shows good geometry stability provided the patient remains stationary on the magnet pad. However, geometry creation in the early versions was a slow, tedious process, with little resemblance to anatomic structures (Fig. 14.1). It requires a proprietary ablation catheter with a magnetic detector, which makes it poorly functional with cryoablation catheters. The EnSite system can create anatomically detailed geometry in a very short period of time (Fig. 14.2). However, being impedance based, anything that changes electrical impedance can cause the geometry to shift. These include air volume within the lung, and fluid volume and distribution within the body. It can function with any brand or type of catheter, and therefore makes cryoablation a readily available option.
In 2002, using the CARTO system , Drago et al. were the first to report catheter ablation without fluoroscopy [9]. In their report, they performed ablations on 21 children with right-sided manifest accessory pathways. In nine of the patients no fluoroscopy was used. While this marked a milestone in catheter ablation technique, it was never duplicated by another laboratory. The reason why is that the original CARTO system was only compatible with one catheter, a radiofrequency ablation catheter. To perform an ablation without fluoroscopy meant completing the procedure using just one catheter. The only patients who were potentially eligible would be patients with right-sided manifest accessory pathways. All other arrhythmias would require more than one catheter, and therefore were not candidates for a fluoroless procedure. It would be five more years before any other groups would report fluoroless procedures, and those would all utilize EnSite.
The CARTO system , in its early versions, had many weaknesses which prohibited it from functioning as a viable tool for fluoroless procedures. With the release of CARTO 3 , those handicaps had mostly been resolved. As the second decade of the new millennium unfolded, some labs were beginning to assess CARTO 3’s utility for decreasing fluoroscopy.
As noted by Pass, “Efforts to reduce fluoroscopic dose are of great potential benefit to both patients and staff to reduce both deterministic and stochastic risk” [10].
As previously stated, Gellis reported the experience in Dr. Pass’ lab of an “ALARA ” protocol, which attempted to decrease radiation exposure using only fluoroscopic guidance of catheters. They reported less total radiation dose, with fluoroscopy alone, than another published study, which utilized CARTO 3 in addition to fluoroscopy [11]. It is important to note that, while the study clearly demonstrates radiation reduction through manipulation of fluoro equipment, it also demonstrates the importance of experience with EAM systems. Just 1 year after publishing his data demonstrating lower radiation exposure than a current EAM system, Pass reported his labs experience with that same EAM system (CARTO 3), while still following their own ALARA protocol. In his own words: “When CARTO 3 was added… we observed a nearly sevenfold drop in average dose from 93 to 13 mGy/case” [10]. Others reporting a benefit from the newer CARTO system included Dr. Berul, who noted “The use of high levels of radiation is potentially dangerous, especially in a pediatric population undergoing rapid somatic growth and cellular maturation.” They report a 60–70% decrease in all measures of radiation exposure after introduction of CARTO 3 [12].
The competitor of CARTO was EnSite. EnSite was the mapping system that triggered the revolution. Being a nonproprietary, “open” system, it allowed the use of all available mapping and ablation catheters. It also localized transseptal needle tips, and certain guidewires. All of those characteristics made it the leading system for broad application of fluoroless interventions. Tuzcu was the first to begin that process. He reported his early experience in 2007, taking note of the fact that, “Patients and staff can be exposed to a significant amount of fluoroscopy during these (fluoroscopic) procedures.” His study involved 28 children with right-sided SVT mechanisms undergoing ablation. Of the 28 procedures, 24 were completed without the use of fluoroscopy. He had acute success in 22 of the 24. There were no complications [13].
That same year, our group reported our experience using EnSite in a pediatric lab. Of 30 patients undergoing SVT ablation, 80% required no fluoroscopy. Five of the six patients requiring fluoro needed it only for transseptal puncture. There was 100% acute success and no complications [14]. For the next 5 years, all publications describing fluoroless procedures utilized the Ensite system. With the release of CARTO 3, reports began to be published around 2013 of its utility to eliminate fluoro. Today, both systems are viable for fluoroless procedures in the majority of instances. One of the benefits of both systems is the continuous localization of electrodes throughout the entire procedure. One does not need to activate fluoroscopy to determine if the catheter has moved. However, both systems can still be improved.
Arrhythmias Amenable to Fluoroless Ablation in Pediatric Labs
Right-Sided Accessory Pathways
While all arrhythmias are approachable without fluoroscopy, there are differing levels of difficulty. Right-sided accessory pathways are the obvious first choice for a fluoroless intervention in children. As a catheter leaves the femoral sheath and enters the femoral vein, it is visible on the EAM. Its movement and direction can be followed as it ascends the IVC. If the catheter redirects into a renal or hepatic vein, that is easily seen. When the catheter enters the liver, it takes a slight leftward direction. With a couple more inches of travel, the catheter enters the right atrium and atrial electrograms will be visible from the electrodes. After optimizing the system, and collecting respiratory compensation, the relevant geometry can then be drawn, using a steerable catheter. The usual procedure for SVT ablation is to draw Superior Vena Cava (SVC), Inferior Vena Cava (IVC), right atrium, coronary sinus (cs), and tricuspid valve. The location of the His electrogram is identified and marked. In our early experience, we drew the tricuspid valve by taking points with good atrial and ventricular electrograms. However, with time, one usually finds that, by letting the catheter advance across the tricuspid valve into the ventricle and moving it within the ventricle, the tricuspid valve draws itself. Into this geometry, one can place as many catheters as is deemed appropriate for the procedure. Pacing, EP study, mapping, and ablation can all proceed normally. Since all structures can be directly accessed from the femoral vein, the only reason for the use of fluoroscopy with right-sided accessory pathways is inexperience with the equipment. Fluoroscopy does not localize the His bundle better than EAM . Ergul published Tuzcu’s results using EnSite for fluoroless ablation of anteroseptal accessory pathways. In 24 patients, they reported a 96% acute success, an 8% recurrence, and no complications [15].
Gaita noted, in explaining their use of CARTO 3 for right-sided accessory pathways, that, “The use of fluoroscopy exposes children to the potential harmful effects of radiation due to the longer life expectancy of this subset of patients.” His work, reported by Scaglione, describes 47 accessory pathways in 44 children. They had 100% acute success, 15% recurrence, and no complications [16].
Atrioventricular Nodal Reentrant Tachycardia
AV nodal reentrant tachycardia (AVNRT) is the next simplest arrhythmia to approach without fluoroscopy. Catheter introduction and geometry creation are the same as described, but more attention is paid to Koch’s triangle. The His bundle is identified and either marked, or a catheter is left in place for continuous recording of the His electrogram. The coronary sinus is drawn, and usually a catheter is left in place. This, then, gives an excellent real-time orientation of Koch’s triangle. If the EnSite system is used, then either radiofrequency (RF) or cryoenergy can be employed, at the physician’s discretion. If CARTO 3 is used, the system will make RF the preferred energy, due to relative incompatibility of cryo with CARTO, as Dr. Ceresnak notes in 2016: “The CARTO 3 system requires the use of specific, proprietary Biosense-Webster ablation catheters . This can be particularly problematic when using the CARTO 3 system if cryoenergy is needed for ablation.” Fortunately, his group has been able to outline a workaround for the problem. By e mploying a “jumper cable,” they were able to “trick” CARTO into visualizing the cryocath during ablation [17]. As an alternative to jumper cables, Scaglione used CARTO 3 to position the cryocatheter in the slow pathway location, and then disconnected it from CARTO and plugged into the cryo console for immediate lesion delivery. This approach results in loss of visualization of the catheter during the lesion. Given the high safety factor of cryo, the authors felt this was an acceptable solution. They report 21 ablation procedures of AVNRT. Nineteen were completed without fluoro. Two patients required fluoro to navigate the catheters from the femoral vein to the heart, which is a potential remaining handicap of the CARTO system . They had 100% acute success, with 5 recurrences at 2 years, and no complications [18]. Alvarez reported their experience comparing a traditional fluoro approach to EnSite for ablation of AVNRT. With 25 patients in each group, they achieved zero fluoroscopy in 24/25 with EnSite. All other outcomes, including success, complications, procedure time, and recurrence, were the same between the two groups [19]. Gist reported our group’s learning curve for fluoroless ablation of AVNRT using EnSite (Table 14.1). Comparing our first 2 years experience to the most recent 2 years, we noted a 20% improvement in procedure time. No patient required fluoro in either the early or late era. The success, complication, and recurrence rates were equal [20].
Other Right-Sided Mechanisms of Tachycardia
Less common mechanisms of tachycardia can also be approached without fluoroscopy. These include atrial flutter, ectopic tachycardia, and intra-atrial reentry. Typical atrial flutter is uncommon in children, but easily managed. The tricuspid annulus and isthmus are readily defined. Creating a line of block is documented by standard EP protocols.
Ectopic atrial tachycardia (EAT) may be better treated with EAM than with standard fluoroscopy. This is because EAM systems have the added benefit of being able to create an activation map , which can graphically show the wavefront of depolarization within the atrium (Figs. 14.3 and 14.4) demonstrates this in a patient with an ectopic tachycardia from the left atrial appendage. Dieks reported her group’s experience of the Ensite system for guiding ablation of focal atrial tachycardia . Over a 10-year period, they attempted 16 ablations, with acute success in 14/16. Compared to other published series’, they found higher success rate and lower fluoroscopy times [21]. Intra-atrial reentrant tachycardia (IART) is a troublesome rhythm to deal with regardless of approach. It is most often seen in complex congenital heart disease. Because of this, it is more likely to require fluoroscopy. However, because these patients are exposed to multiple procedures over their lifetime, it is also the reason to attempt to minimize radiation exposure.
Same patient as Fig. 14.3, after ablation. Notice earliest activation now maps to area near sinus node
Three-dimensional mapping tools can increase the success of the procedure due to their ability to provide both activation maps and voltage maps. Voltage maps can help in defining areas of scar that might be serving to support the reentrant circuit. There is not a set approach to ablation in this population, and each patient needs to be assessed and treated individually. However, there are reports of fluoroless ablations being performed for this arrhythmia in complex congenital heart disease (CHD) [22].
Left-Sided Mechanisms
Left-sided arrhythmias have all the same treatment issues that right-sided mechanisms do, plus the added factor of access. To access left-sided tachycardias, there are three options available in the usual EP lab: patent foramen ovale, transseptal puncture, or retrograde arterial. These will be discussed individually.
The patent foramen ovale (PFO) is somewhat of a buried treasure to the electrophysiologist. Few things bring the sense of good personal fortune like discovering ready-made access to a left-sided accessory pathway. When present, a PFO is easily identified on EAM . When drawing geometry, the catheter will take an unmistakable course. With the catheter facing the atrial septum, as it is advanced superiorly, it will take a course aiming leftward and posteriorly. There will generally still be excellent atrial electrograms noted, unless the catheter is advanced into a pulmonary vein. When a PFO is discovered during geometry creation, the operating physician should pause and say a short prayer that the patient’s tachycardia mechanism is a left-sided concealed pathway. The remainder of the procedure can then continue according to standard protocol.
In the absence of a PFO, the options become transseptal or retrograde. If patient size allows a retrograde approach, then the ablation can proceed without fluoro. Advancing the ablation catheter into the left ventricle (LV) is not technically different with EAM guidance than with fluoroscopy. Positioning of the catheter tip on the mitral annulus is aided by electrograms, and the presence of a coronary sinus catheter. It will not take long to develop a comfort level with the 3D images, which replace the fluoro images to which we are accustomed. Gaita has embraced this approach, noting that, “the use of fluoroscopy exposes children to the potential harmful effects of radiation due to the longer life expectancy of this subset of patients.” His lab has reported fluoroless ablation in 21 children with left-sided accessory pathways. Two patients had PFO, and the remainder a retrograde approach. None of the cases required fluoroscopy. They had 100% acute success and no complications [16].
If a transseptal approach is needed, then the current options are transesophageal (TEE) or intracardiac echo (ICE) . We prefer TEE because it is not restricted by venous access issues, and can be performed on any size patient. However, the downsides of TEE are that it is more time consuming, and requires the assistance of a second physician. For those individuals wishing to learn the TEE approach, it is relatively simple. A guidewire is advanced from the femoral vein to the SVC. A bicaval TEE view confirms the position of the guidewire. The transseptal sheath and dilator are advanced to the SVC, with visualization by TEE. The guidewire is then removed and replaced with the transseptal needle. Continuing with a bicaval view, the sheath and dilator are pulled down to engage the atrial septum. The view is then changed to an aortic valve en-face view. From this angle, the thin membranous septum is well visualized. The dilator is manipulated to demonstrate tenting of the membranous atrial septum, with care being taken to visualize the tip of the dilator (Fig. 14.5). The needle is then advanced into the left atrium. Saline is injected to confirm left atrial position of the needle, and then the sheath is advanced over the needle. Ablation then continues per standard protocols. We began this approach in 2006, and published our data in 2008 [23]. Since that time, it has remained our standard approach. Others have reported use of ICE to accomplish the same goal [24].
Many labs are now approaching all arrhythmias with a zero-fluoroscopy intention. In Gersak’s lab, they performed 43 consecutive ablations for SVT in children, with an intention of zero fluoroscopy. Utilizing EAM and ICE, they achieved 100% success with 0 fluoro and no complications. They concluded that stochastic and deterministic effects potentially express a greater risk in this population. Therefore, there seems to be some rationale to go even beyond the utilization of the ALARA principle in pediatric catheter ablation procedures [25].
In Aziz’s lab, they report an 88% decrease in fluoro time, and 54% of cases achieving zero fluoroscopy for SVT ablation in children. These numbers are what they achieved when first attempting fluoroless procedures, having not yet gone through a learning curve [26]. Wan reported very similar results as they went through their learning curve [27].
Ventricular Arrhythmias
Ventricular arrhythmia ablation presents additional challenges if the intention is to reach zero fluoroscopy . These procedures often require special catheters and sheaths to successfully ablate the substrate. It takes a little more practice to reach zero fluoroscopy in this setting. A common situation for ventricular arrhythmia ablation is to place a spiral catheter in the right ventricular outflow tract (RVOT) . This requires a long sheath. But, sheaths are not visible on EAM systems. To deal with this issue, one must take note of the fact that the sheath insulates the electrodes from the electrical fields generated by the surface patches. Translated, that means that whenever an electrode is pulled back into a sheath, the catheter is distorted on the EAM . It is, therefore, possible to identify when the tip of the sheath is right at the proximal electrode of a catheter. With that fact understood, it becomes possible to place a spiral catheter into the RVOT. Start by inserting the particular sheath needed. Through this sheath, advance a steerable catheter into the main pulmonary artery (MPA) . Next, advance the sheath over the catheter until you see the distortion of the proximal electrode. This will inform you that the tip of the sheath is now positioned in the MPA. Remove the steerable catheter and advance the spiral catheter through the sheath. As the distal electrode emerges from the sheath, hold the catheter steady and pull the sheath back. This will allow the catheter to coil in the MPA . It can then be pulled back and positioned in the RVOT. This technique can allow a zero fluoroscopy procedure for those wishing to pursue it. Utilizing this technique, Von Bergen et al. were able to achieve zero fluoroscopy in three out of five ventricular arrhythmia ablations [28]. In Tuzcu’s lab, they achieved 6/17 fluoroless ablations of ventricular tachycardia in their early results, and 19/35 in their later report [29, 30]. Figure 14.6 demonstrates the three-dimensional anatomy of a patient with left ventricular outflow tract (LVOT) tachycardia, mapped from both the RV and the LV.
Special Considerations
Other issues of particular import to the pediatric electrophysiologist are congenital heart disease and small patient size. As previously noted, patients with significant CHD require more radiologic procedures over their lifetime, and require more fluoroscopy per procedure. They may be the group of patients who will benefit the most from EAM systems. Most reports of fluoroless ablation in patients with CHD are isolated case reports [22, 31]. We have reported on two neonates with WPW and CHD undergoing fluoroless ablation [32]. One patient had Ebstein’s anomaly , and the other had pulmonary atresia. Both procedures were accomplished without fluoroscopy, and neither patient has had recurrence at 3 years follow-up.
Small size is a known risk for complication in catheter ablation, with patients <15 kg at greater risk [5]. Mapping and ablation catheters are developed for adult-sized patients. At the lower limits of our patient size, the curves and stiffness of the available catheters become a detriment to patient care. This is true regardless of the method of catheter guidance. The EnSite system suffers from similar technical difficulties. The total surface area of all patches and electrodes for an EnSite procedure is 1380 cm2, while the typical torso surface area of a newborn is 900 cm2. However, since most of the navigation patches are gel, with only a small portion of it being the wire mesh, it is possible to remove up to 90% of the gel pad without interfering with the wiring of the patch. Viewing the patches under fluoroscopy can identify the extent of the wire mesh antenna. This has allowed us to create a template for removing excess gel pad from the patch as a less than ideal, but systematic work-around.
Benefits
The benefits of a no-fluoro approach in pediatric electrophysiology include avoidance of lead protective equipment, elimination of stochastic complication risk, allowing safer performance of procedures during pregnancy, improved outcomes, and potential portability of the procedure.
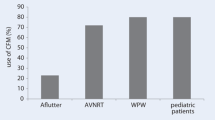
Wearing lead aprons, thyroid shields, and goggles is held to be an absolute requirement within the profession of electrophysiology. But the utility of EAM systems raises the possibility of removing those cumbersome defenses. Stec et al. report their experience from 188 consecutive SVT ablations performed with an intention of zero fluoroscopy [33]. Of the 188, 179 were completed without fluoro. Based on this data, and noting that the “need for lead aprons and eye protection to protect medical staff increases workplace discomfort and the risk of neck, back and joint complications,” they have recommended a “no lead” approach. We have followed this approach for over 10 years, and the increased comfort associated with it will very likely be the impetus that ultimately drives most labs to adopt a no-fluoro technique.
Long-term stochastic complication risks are low enough that electrophysiologists are habituated to ignore them. However, a recent publication makes a sobering point. Roguin et al. reported on 31 physicians with head and neck tumors [34]. The physicians were interventional cardiologists, radiologists, and electrophysiologists. Of the 31 tumors, the sidedness of the tumor could be defined in 26. Of the 26 in which the sidedness of the tumor was identified in the chart, 22 of them were left sided, one was midline, and only three were right sided. Given that the X-ray source is always to the physician’s left, it is unsettling to find that 85% of head and neck tumors in this population were on the left side.
Arrhythmias during pregnancy are another uncommon but troublesome scenario. There are a number of case reports now demonstrating the utility of EAM to perform fluoroless procedures during pregnancy [35,36,37,38,39,40,41,42,43,44]. Given the risks of fluoroscopy, and the risks of antiarrhythmic medications during pregnancy, fluoroless ablation may eventually become the treatment of choice for arrhythmia management in pregnancy. In addition to benefits to the pregnant patient, there are also potential benefits to staff. In the last 12 years in our lab, we have had multiple staff members who were able to maintain their jobs in the EP lab throughout pregnancy because we were not using fluoroscopy.
Portability of electrophysiology procedures remains just over the horizon. Routine fluoroless procedures will first need to become commonplace before the profession will be ready to accept routine portability. However, the reason to consider it is due to the rare but difficult scenario of tachycardia-induced cardiomyopathy in the patient who requires ECMO support. In that setting, ablation is the treatment of choice, and can be life saving. But, transporting an ECMO patient to the EP lab is fraught with risk. Eliminating the need for fluoroscopy has the potential to eliminate the need for the stationary EP lab. It is technically feasible to perform an ablation in the intensive care unit (ICU). While we have not yet performed an ablation on an ECMO patient in an ICU, we have developed our service in a way that will easily allow it when needed. Our system is maintained on a portable cart, which can be transported to any room where a procedure is necessary. Thus far, we have performed ablations in various operating rooms within the hospital. We have performed more than 100 ablations outside of the cath lab. Our early data was recently published [45]. While this is unique at the moment, it will become more common as greater worldwide experience is obtained.
In addition to minimizing radiation exposure, there may also be other benefits to 3D mapping. Ceresnak et al. reported improved acute success when EAM was used for catheter guidance in ablation of WPW, as opposed to fluoroscopy alone [46]. This was a multicenter, retrospective review. Tuzcu reported similar findings from a single institution [47].
The Future
The future of pediatric electrophysiology is fascinating to consider from this moment in time. Unquestionably, fluoroscopy will be eliminated from all but the most difficult or complex cases. As Giuseppe Stabile notes, “Once the decision has been made to use a navigation system to reduce fluoroscopy time, the next step is just a question of learning and confidence” [48]. What is not clear about the future is, what will be the changes in the tools and technology that will expedite the transformation, and who will lead the revolution. Immediate needs include sheaths, dilators, guidewires, and needles that are visible on EAM systems. Those simple tools would markedly increase the acceptance of fluoroless procedures. Once the elimination of fluoroscopy has become commonplace, it is only a little further down the road before we reach the point of elimination of the traditional EP lab. As L Szydlowski notes, “Growing experience with this radiation-free approach should lead to the complete elimination of fluoroscopy during SVT ablations, and in the training of a new generation of invasive electrophysiologists” [33].
Indeed, the era of fluoroscopy-free ablation procedures has arrived.
References
Kean AC, LaPage MJ, Yu S, Dick M 2nd, Bradley DJ. Patient and procedural correlates of fluoroscopy use during catheter ablation in the pediatric and congenital electrophysiology lab. Congenit Heart Dis. 2015;10(3):281–7.
Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. Gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120(19):1903–9.
Limacher MC, Douglas PS, Germano G, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. American college of cardiology. J Am Coll Cardiol. 1998;31(4):892–913.
Broga D. Ionizing radiation exposure of the population of the united states. National Council on Radiation. 1st edn. Wiley: Hoboken, NJ: 2009. https://doi.org/10.1118/1.3245881.
Kugler JD, Danford DA, Houston K, Felix G. Radiofrequency catheter ablation for paroxysmal supraventricular tachycardia in children and adolescents without structural heart disease. Pediatric EP society, radiofrequency catheter ablation registry. Am J Cardiol. 1997;80(11):1438–43.
Van Hare GF, Javitz H, Carmelli D, et al. Prospective assessment after pediatric cardiac ablation: demographics, medical profiles, and initial outcomes. J Cardiovasc Electrophysiol. 2004;15(7):759–70.
Clay MA, Campbell RM, Strieper M, Frias PA, Stevens M, Mahle WT. Long-term risk of fatal malignancy following pediatric radiofrequency ablation. Am J Cardiol. 2008;102(7):913–5.
Gellis LA, Ceresnak SR, Gates GJ, Nappo L, Pass RH. Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era. Pacing Clin Electrophysiol. 2013;36(6):688–94.
Drago F, Silvetti MS, Di Pino A, Grutter G, Bevilacqua M, Leibovich S. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. 2002;13(8):778–82.
Pass RH, Gates GG, Gellis LA, Nappo L, Ceresnak SR. Reducing patient radiation exposure during paediatric SVT ablations: use of CARTO(R) 3 in concert with “ALARA” principles profoundly lowers total dose. Cardiol Young. 2015;25(5):963–8.
Miyake CY, Mah DY, Atallah J, et al. Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm. 2011;8(4):519–25.
Clark BC, Sumihara K, McCarter R, Berul CI, Moak JP. Getting to zero: impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J Interv Card Electrophysiol. 2016;46(2):183–9.
Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol. 2007;30(4):519–25.
Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. 2007;30(4):510–8.
Ergul Y, Tola HT, Kiplapinar N, Akdeniz C, Saygi M, Tuzcu V. Cryoablation of anteroseptal accessory pathways in children with limited fluoroscopy exposure. Pediatr Cardiol. 2013;34(4):802–8.
Scaglione M, Ebrille E, Caponi D, et al. Zero-fluoroscopy ablation of accessory pathways in children and adolescents: CARTO3 electroanatomic mapping combined with RF and cryoenergy. Pacing Clin Electrophysiol. 2015;38(6):675–81.
Ceresnak SR, Nappo L, Janson CM, Pass RH. Tricking CARTO: cryoablation of supraventricular tachycardia in children with minimal radiation exposure using the CARTO3 system. Pacing Clin Electrophysiol. 2016;39(1):36–41.
Scaglione M, Ebrille E, Caponi D, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol. 2013;36(12):1460–7.
Alvarez M, Tercedor L, Almansa I, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009;6(12):1714–20.
Gist K, Tigges C, Smith G, Clark J. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: early versus late experience. Pacing Clin Electrophysiol. 2011;34(3):264–8.
Dieks JK, Muller MJ, Schneider HE, et al. Catheter ablation of pediatric focal atrial tachycardia: ten-year experience using modern mapping systems. Pediatr Cardiol. 2016;37(3):459–64.
Zambito MP, Samuel BP, Vettukattil JJ, Ratnasamy C. Fluoroless catheter ablation of intraatrial reentrant tachycardia status post fontan procedure: fluoroless catheter ablation in fontan patient. Int J Cardiol. 2015;201:126–8.
Clark J, Bockoven JR, Lane J, Patel CR, Smith G. Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin Electrophysiol. 2008;31(3):283–9.
Mah DY, Miyake CY, Sherwin ED, et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace. 2014;16(2):277–83.
Jan M, Zizek D, Rupar K, et al. Fluoroless catheter ablation of various right and left sided supra-ventricular tachycardias in children and adolescents. Int J Cardiovasc Imaging. 2016;32(11):1609–16.
Nagaraju L, Menon D, Aziz PF. Use of 3D electroanatomical navigation (CARTO-3) to minimize or eliminate fluoroscopy use in the ablation of pediatric supraventricular tachyarrhythmias. Pacing Clin Electrophysiol. 2016;39(6):574–80.
Wan G, Shannon KM, Moore JP. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol. 2012;35(2):235–42.
Von Bergen NH, Bansal S, Gingerich J, Law IH. Nonfluoroscopic and radiation-limited ablation of ventricular arrhythmias in children and young adults: a case series. Pediatr Cardiol. 2011;32(6):743–7.
Ozyilmaz I, Ergul Y, Akdeniz C, Ozturk E, Tanidir IC, Tuzcu V. Catheter ablation of idiopathic ventricular tachycardia in children using the EnSite NavX system with/without fluoroscopy. Cardiol Young. 2014;24(5):886–92.
Akdeniz C, Gul EE, Celik N, Karacan M, Tuzcu V. Catheter ablation of idiopathic right ventricular arrhythmias in children with limited fluoroscopy. J Interv Card Electrophysiol. 2016;46(3):355–60.
Giaccardi M, Chiodi L, Del Rosso A, Colella A. ‘Zero’ fluoroscopic exposure for ventricular tachycardia ablation in a patient with situs viscerum inversus totalis. Europace. 2012;14(3):449–50.
Bigelow AM, Arnold BS, Padrutt GC, Clark JM. Non-fluoroscopic cardiac ablation of neonates with CHD. Cardiol Young. 2017;27(3):592–6.
Stec S, Sledz J, Mazij M, et al. Feasibility of implementation of a “simplified, no-X-ray, no-lead apron, two-catheter approach” for ablation of supraventricular arrhythmias in children and adults. J Cardiovasc Electrophysiol. 2014;25(8):866–74.
Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111(9):1368–72.
Szumowski L, Szufladowicz E, Orczykowski M, et al. Ablation of severe drug-resistant tachyarrhythmia during pregnancy. J Cardiovasc Electrophysiol. 2010;21(8):877–82.
Wu H, Ling LH, Lee G, Kistler PM. Successful catheter ablation of incessant atrial tachycardia in pregnancy using three-dimensional electroanatomical mapping with minimal radiation. Intern Med J. 2012;42(6):709–12.
Hogarth AJ, Graham LN. Normal heart ventricular tachycardia associated with pregnancy: successful treatment with catheter ablation. Indian Pacing Electrophysiol J. 2014;14(2):79–82.
Raman AS, Sharma S, Hariharan R. Minimal use of fluoroscopy to reduce fetal radiation exposure during radiofrequency catheter ablation of maternal supraventricular tachycardia. Tex Heart Inst J. 2015;42(2):152–4.
Bigelow AM, Crane SS, Khoury FR, Clark JM. Catheter ablation of supraventricular tachycardia without fluoroscopy during pregnancy. Obstet Gynecol. 2015;125(6):1338–41.
Chen G, Sun G, Xu R, et al. Zero-fluoroscopy catheter ablation of severe drug-resistant arrhythmia guided by ensite NavX system during pregnancy: two case reports and literature review. Medicine (Baltimore). 2016;95(32):e4487.
Prolic Kalinsek T, Jan M, Rupar K, Razen L, Antolic B, Zizek D. Zero-fluoroscopy catheter ablation of concealed left accessory pathway in a pregnant woman. Europace. 2017;19(8):1384.
Kozluk E, Piatkowska A, Kiliszek M, et al. Catheter ablation of cardiac arrhythmias in pregnancy without fluoroscopy: a case control retrospective study. Adv Clin Exp Med. 2017;26(1):129–34.
Rossi L, Penela D, Villani GQ. Intracardiac echocardiography catheter-guided zero fluoroscopy transeptal puncture technique for ablation of left-sided accessory pathway in a pregnant woman. Europace. 2017;19(11):1825.
Lahiri A, Srinath SC, Chase D, Roshan J. Zero fluoroscopy radiofrequency ablation for typical atrioventricular nodal reentrant tachycardia (AVNRT). Indian Pacing Electrophysiol J. 2017;17(6):180–2.
Bigelow AM, Smith PC, Timberlake DT, et al. Procedural outcomes of fluoroless catheter ablation outside the traditional catheterization lab. Europace. 2017;19(8):1378–84.
Ceresnak SR, Dubin AM, Kim JJ, et al. Success rates in pediatric WPW ablation are improved with 3-dimensional mapping systems compared with fluoroscopy alone: a multicenter study. J Cardiovasc Electrophysiol. 2015;26(4):412–6.
Tuzcu V. Significant reduction of fluoroscopy in pediatric catheter ablation procedures: long-term experience from a single center. Pacing Clin Electrophysiol. 2012;35(9):1067–73.
Solimene F, Donnici G, Shopova G, et al. Trends in fluoroscopy time during radiofrequency catheter ablation of supraventricular tachycardias. Int J Cardiol. 2016;202:124–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bigelow, A.M., Clark, J.M. (2019). The Demise of Fluoroscopy in Pediatric Electrophysiology. In: Proietti, R., Wang, Y., Yao, Y., Zhong, G., Lin Wu, S., Ayala-Paredes, F. (eds) Cardiac Electrophysiology Without Fluoroscopy. Springer, Cham. https://doi.org/10.1007/978-3-030-16992-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-16992-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16991-6
Online ISBN: 978-3-030-16992-3
eBook Packages: MedicineMedicine (R0)