Abstract
Microalgae are important natural resources that can provide food, medicine, energy and various bioproducts for nutraceutical, cosmeceutical and aquaculture industries. Their production rates are superior compared to those of terrestrial crops. However, microalgae biomass production on a large scale is still a challenging problem in terms of economic and ecological viability. Microalgal cultivation system should be designed to maximize production with the least cost. Energy efficient approaches of using light, dynamic mixing to maximize use of carbon dioxide (CO2) and nutrients and selection of highly productive species are the main considerations in designing an efficient photobioreactor. In general, optimized culture conditions and biological responses are the two overarching attributes to be considered for photobioreactor design strategies. Thus, fundamental aspects of microalgae growth, such as availability of suitable light, CO2 and nutrients to each growing cell, suitable environmental parameters (including temperature and pH) and efficient removal of oxygen which otherwise would negatively impact the algal growth, should be integrated into the photobioreactor design and function. Innovations should be strategized to fully exploit the wastewaters, flue-gas, waves or solar energy to drive large outdoor microalgae cultivation systems. Cultured species should be carefully selected to match the most suitable growth parameters in different reactor systems. Factors that would decrease production such as photoinhibition, self-shading and phosphate flocculation should be nullified using appropriate technical approaches such as flashing light innovation, selective light spectrum, light-CO2 synergy and mixing dynamics. Use of predictive mathematical modelling and adoption of new technologies in novel photobioreactor design will not only increase the photosynthetic and growth rates but will also enhance the quality of microalgae composition. Optimizing the use of natural resources and industrial wastes that would otherwise harm the environment should be given emphasis in strategizing the photobioreactor mass production. To date, more research and innovation are needed since scalability and economics of microalgae cultivation using photobioreactors remain the challenges to be overcome for large-scale microalgae production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Algae are ubiquitous microscopic and macroscopic plants in both marine and freshwater ecosystems, and their biomass production is known to exceed those of terrestrial plants (Schenk et al. 2008; Kraan 2013; Guyon et al. 2018). Many microalgae species contain various high-value compounds with wide range of industrial applications. Thus, microalgae are important sources for various products including feedstocks of biofuels (Schenk et al. 2008; Pittman et al. 2011; Georgianna and Mayfield 2012; Medipally et al. 2015; Rastogi et al. 2018), biomass and pigments for aquaculture industry (Angeles et al. 2009; Alishahi et al. 2015; Liu et al. 2017), and commercially important compounds for food and health industries (Goh et al. 2014; Foo et al. 2015). Studies on biofuel production indicated that microalgae are more superior and sustainable source compared to terrestrial crops such as corns, coconut, jatropha and oil palm (Chisti 2007; Rastogi et al. 2018) due to their fast growth. In addition to biodiesel production, the use of wastewater and flue-gas for microalgae mass production helps to reduce water and air pollution, respectively (Cheah et al. 2015; Guldhe et al. 2017; Cao et al. 2017).
Microalgae are natural sources of valuable fatty acids and amino acids that can be utilized in food, nutraceutical, pharmaceutical and cosmeceutical industries (Pennington et al. 1988; Jin et al. 2003; Xia et al. 2013). Many species are capable of producing bioactives such as carotenoids, phenolic acids, flavonoids and highly unsaturated fatty acids (HUFAs) that can be used as additives and supplements for human health-promoting products and animal feeds (Natrah et al. 2007; Ebrahimi Nigjeh et al. 2013; Goh et al. 2014; Foo et al. 2017). These secondary metabolites produced in microalgae cells have been proven effective as antioxidant, antimicrobial, anti-inflammatory, anticancer and many other ailments (Ryckebosch et al. 2014; Foo et al. 2015; Guyon et al. 2018). In addition, they are useful as prebiotics and immunomodulatory agents. With valuable bioactive compounds in their cells, some microalgae commodities have been granted GRAS (generally regarded as safe) status as novel food products for health and medicines.
In aquaculture, microalgae have the potential to be used as colourants, prebiotics and enhancement of fish and invertebrate immunity (Peng et al. 2012; Liu et al. 2017). As a colourant source, carotenoids in microalgae such as canthaxanthin, astaxanthin and lutein have been regularly used as feed ingredients to enhance colour of the fish. In fact, β-carotene has been effectively used as pro-vitamin A (retinol) in multivitamin preparation and is usually included in the formulation of healthy feeds (Begum et al. 2016). Polyunsaturated fatty acids from microalgae, such as EPA (eicosapentaenoic acid, C20:5n-3) and DHA (docosahexaenoic acid, C22:6n-3), have been shown to positively affect immune responses in cultured fish and invertebrates by modulating fish immunity through enhancement of lymphocyte proliferation, cytokine production and natural killer (NK) cells activity (Vallejos-Vidal et al. 2016; Gbadamosi and Lupatsch 2018). Microalgae are also useful prebiotics that can act as stimulant for beneficial microbes (Panjiar et al. 2017) and inhibitor for pathogenic bacteria (Natrah et al. 2014). In addition, microalgae are an essential component of aquaculture system to ensure good water quality by efficient uptake of toxic compounds such as ammonia and nitrite (Mohamed Ramli et al. 2017). In general, the use of microalgae in aquaculture will improve water quality and provide protection of the cultured animals against various diseases through improvement of their diets and enhancement of their immune system. In addition, the current research effort to utilize microalgae as a vaccine carrier will further enhance not only the fish health but contribute to the sustainability of aquaculture industry.
At present, the production of microalgae biomass is still low, and adequate production to satisfy the increasing demand from various industries remained a challenging bottleneck. One of the main strategies of microalgae production is the use of appropriate microalgae cultivation system using natural or cheap resources such as wastewaters for nutrients, solar energy for light, flue-gas for CO2 and waves for mixing. There are many options for microalgal cultivation such as photobioreactors, raceways, tanks and ponds (Table 4.1). Among many types of microalgae production system, photobioreactors are key devices for pure single species culture where contaminants that occur in pond or raceway cultures can be controlled. However, like other photosynthetic systems, the success of photobioreactors will depend on all factors that affect energy consumption and maintenance of optimum culture condition. In mass microalgae cultivation, availability of water, light, nutrients and energy would be the main items to be factored into the production cost. The production can be further improved by species or strain selection and optimization of all related culture conditions. The use of wastes and natural resources for the culture would make the microalgae production more economical, and to some extent improves the pollution pressure on the environment.
4.2 Photobioreactor Development—Strategies
Conventional microalgae culture is mainly carried out in open space cultivation, especially in ponds, tanks or raceways. With comparatively lower construction and operating cost compared to closed system, open space cultivation is relatively easy to operate and relatively cheap as most utilize natural sunlight and aeration (Table 4.1). However, open system cultivation is prone to contamination which can affect the quality of the produced microalgae biomass and the extracted compounds such as astaxanthin and other carotenoids used in health and food industries. Thus, closed system cultivation is the better alternative for the production of high-value microalgae products (Table 4.1).
Photobioreactors have been developed since 1950s for biomass production of a specific microalgae species in order to overcome food supply crisis. Several configurations such as raceway system (Fig. 4.1), bubble column (Fig. 4.2), flat plate (Fig. 4.3) and tubular (Fig. 4.4) have been used (Olivieri et al. 2014). The early bioreactor design was very simple consisting of tubes and light sources. In the earlier years, bioreactors were relatively small, but the photobioreactor volume is getting bigger with more sophisticated design. Novoveská et al. (2016) designed a large microalgae photobioreactor in the offshore area to treat municipal wastewater, up to 50,000 gallons/day, whereby 75% of total nitrogen, 93% of total phosphorus and 92% of biological oxygen demand (BOD) of the influent wastewater was removed, and 3.5–22.7 g m−2 d−1 of microalgae biomass was produced.
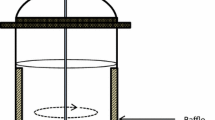
Photobioreactors are often categorized into (1) open and closed system, or (2) vertical and horizontal flow of culture media (Table 4.1). Most bioreactors have different specifications in terms of materials, light pass length, working volume and volume/surface ratio. Common features in bioreactors include (1) light receiver to capture light energy effectively, (2) loading ports for the culture media, carbon dioxide and harvesting and (3) mixing function to remove produced oxygen and to increase mass transfer efficiency in the culture media. Open raceway system is the most popular microalgae production system. The basic design was derived from oxidation pond in wastewater treatment. In general, the open raceway system has one or multiple paddles for circulating the media in the trough that has 20–30 cm water depth (Fig. 4.1a). The paddle mixing has higher energy efficiency compared to aeration mixing used in other closed photobioreactors due to low energy loss in the former. However, the lower cell density was often reported in raceway system due to the longer light path length (≈30 cm) compared to other closed photobioreactors. However, only the species that has low contamination risk can be cultured in this system.
To overcome the contamination issue, Dogaris et al. (2015) modified the raceway system to develop a new horizontal photobioreactor (HBR) that has thin light pass length of 5 cm with airlift pumps (Fig. 4.1a). The HBR system achieved a maximum biomass concentration of 4.3 g L−1 and average biomass productivity of 18.2 g m−2 d−1 over the course of 165 days without any contamination problem (Dogaris et al. 2015). Column and flat plate systems are categorized as vertical mixing photobioreactors, in which the agitation and mixing are accomplished by aeration. The main advantage of these bioreactors is the homogeneous and efficient mass transfer by entire mixing of the water column, while the raceway and tubular systems undergo partial mixing by paddle and airlift systems. To improve mixing efficiency, airlift column bioreactor was invented (Fig. 4.2). An airlift column bioreactor has a physical separation of the two interconnecting zones; the center column (dark zone) for upper flow and external side (light zone) for the downstream. The circulation of the dark and light cycles of overall media in the column provides constant light energy to all cells in the bioreactor.
To scale-up a column bioreactor, the reactor diameter increases and its surface/volume (S/V) ratio decreases, resulting in a decrease of cell density in the bioreactor. Lower biomass concentration in the harvested media requires higher cost and energy, when the harvested culture media is concentrated and dried. To avoid decreasing S/V ratio, the annular reactor was developed (Chini Zittelli et al. 2006; Posten 2009). The structure of the annular bioreactor is actually wrapped flat plate bioreactor with the appearance of a column bioreactor (Fig. 4.2c). The flat plate photobioreactor uses simple geometry and it can be designed to reduce light path length and keep high S/V ratio (Fig. 4.3a). The reactor is placed in a vertical or tilted inclination to receive sunlight energy effectively. The vertical mixing in column and flat plate bioreactors uses aeration which requires high energy consumption.
The performance of energy consumption in bioreactor is evaluated by net energy ratio (NER) that is the energy balance between total energy produced by the microalgae biomass (energy output) and energy requirement in the biomass production (energy input). Generally, the raceway system shows high NER ratio (>1.0) and high energy efficiency. On the other hand, vertical mixing reactor shows relatively low NER due to high energy consumption of aeration mixing (Burgess and Fernández-Velasco 2007; Huesemann and Benemann 2009; Jorquera et al. 2010). In order to improve the energy efficiency, the flat panel airlift (FPA) bioreactor with rectangular channel airlift which improves the efficiency of light utilization was designed (Degen et al. 2001) (Fig. 4.3b). Degen et al. (2001) reported that the FPA bioreactor showed 1.7 times higher productivity than the conventional flat plate reactor in Chlorella vulgaris cultivation.
Tubular reactor is one of the typical closed photobioreactors consisting of a tube and pump system (generally airlift pump system) to circulate culture media and works as degasser to remove oxygen produced by photosynthesis (Fig. 4.4). The advantage of the system is the high flexibility for the setting and it can be arranged horizontally, vertically and any other shape that is optimized to receive light source (Carlozzi 2003). However, the oxygen resulting from photosynthesis often increases up to an inhibitory level since it is only partially removed in the airlift system (Sánchez Mirón et al. 1999). In addition to the oxygen accumulation problem, the tubular system consumes high energy to circulate the culture media. Jorquera et al. (2010) reported that the tubular system requires >2500 W/m3 (NER = 0.2) to generate turbulent for suitable gas/liquid mixing and mass transfer in the systems while the raceway and flat plate systems consume 3.72 W/m3 (NER = 8.34) and 53 W/m3 (NER = 4.51) for the mixing and/or aeration, respectively. However, these energy consumption values greatly vary with the culturing conditions and assumptions made during the calculation of NER.
4.3 Strategies to Increase Efficiency of Photobioreactor Systems
Microalgae are flagged as the next generation biomass feedstock for bioenergy and biochemical for the growing world population. Since its production is associated with reducing the impacts of climate change and enhancing of food security, microalgae-based industries have high potential to assist the socio-economic development of the global community. Thus, upscaling of microalgae products should be pursued by improving its production systems.
There is a great need to develop efficient photobioreactors to satisfy the high demand for microalgae biomass. The strategy to design a highly efficient bioreactor system is to focus on all factors that affect the microalgae physiological responses and biomass quality. Microalgae require light, carbon dioxide and nutrients to produce biomass and biocompounds, the rates of which are governed by the metabolic properties of the cultured species itself and the culture conditions (Lucker et al. 2014). Optimizing the delivery of these factors to increase photosynthetic rates in photobioreactors would be the best strategy to obtain the maximum microalgae production. Thus, bioreactors have been designed to increase efficiencies in light, gas and nutrient utilization with increased outputs (Table 4.2).
4.3.1 Selection of Microalgae Species
Many microalgal species have variable contents of high-value compounds such as fatty acids, amino acids and carotenoids. Thus, for photobioreactor production, microalgae species with high yield biomass and rapid growth rate should be carefully selected to suit targeted products. For example, Haematococcus spp. have high carotenoids contents, especially astaxanthin (Guyon et al. 2018; Lim et al. 2018) and Chlamydomonas spp. are known sources for carbohydrates (Gifuni et al. 2017). In fact, some species have compounds that cannot be found in other species. For example, fucoxanthin is only found in brown seaweeds and diatoms (Foo et al. 2015). Molina-Miras et al. (2018) reported the production of amphidinols, a group of polyketides with high bioactivities from a marine dinoflagellate, Amphidinium carterae. Thus, concentration of a target compound can also be an important criterion for selecting an algal species for mass production in a photobioreactor.
Physiological parameters and biochemical composition of microalgae biomass also determine the productivity and quality. The culture environment has a high influence on the species physiological response. Zhang et al. (2017a, b) manipulated the glucose, nitrogen and light levels to enhance astaxanthin production in Chlorella zofingiensis. In a study of tropical microalgae, Rocha et al. (2017) reported that different chlorophyte strains of Scenedesmus, Chlamydomonas, Chlorella, Monoraphidium, Scenedesmus and Selenastrum have variable fatty acids, carbohydrate and protein contents and their metabolism and composition were closely related to the culture conditions. Guyon et al. (2018) also suggested that microalgae productivity and carotenoid contents are species-specific and influenced by a wide range of environmental parameters.
Different species require different light intensity and spectra to maximize their growth and productivity. Vadiveloo et al. (2015) showed that a green microalga, Nannochloropsis sp. produced the highest biomass when cultured under blue light (400–525 nm). Hidasi and Belay (2018) reported that biomass composition of Spirulina platensis showed diurnal changes with lower photosynthetic pigments during the light hours, but recovered during the night. In fact, optimal growth factors (light, CO2 and nutrients) are essential to achieve maximum production, but the exact requirements differ from one species to another. Mondal et al. (2017b) reported that light intensity of 80 µmol m−2 s−1 and photoperiod of 12L:12D were the optimal conditions for Chlorella sorokiniana culture, whereas other species require higher light intensity (Schultze et al. 2015). On the other hand, Holdmann et al. (2018) reported that Chlorella sorokiniana produced the highest biomass under strong light intensity and shorter photoperiod, probably due to different strains and culture conditions. Some species such as Chlorella sorokiniana and C. minutissima are capable of using pentoses which otherwise do not have any significant industrial application as a carbon source (Freitas et al. 2017). In fact, some species, such as Scenedesmus obliquus, was shown to sustain cell growth up to 2 h in the dark without affecting the photosynthetic rate (Maroneze et al. 2016).
Thus, one of the strategies for optimized photobioreactor production is to explore the vast sources of microalgae diversity and select those strains with high potential for different biotechnological applications. Gonçalves et al. (2016) showed that culture of mixed compatible species resulted not only in higher biomass production with higher nutrient removal, but also increased amount of lipids. Future research should focus on the selection and engineering of high-value species with robust characteristics and high growth rate. In addition, optimal culture conditions should be developed to enhance the microalgal biomass and high-value compounds production such as lipids, fatty acids, carotenoids and proteins (Rezvani et al. 2017; Zhuang et al. 2018). Manirafasha et al. (2018) demonstrated that supply of nitrogen source with metabolic stress resulted in high Arthrospira platensis growth with high accumulation of phycocyanin.
4.3.2 Aeration and Mixing
Aeration is important in providing adequate carbon dioxide and nutrients for microalgal cells to photosynthesize and synthesize organic compounds. In addition to delivering gas and nutrients, aeration also controls the mixing of the water column moving the algal cells to various parts of the reactors, from the light zone near the illumination surfaces to the darker-interior area. With mixing, algal cells are shuttled back and forth between the light and dark zone, enabling the microalgal cells to undergo short light–dark cycles that can promote faster growth and higher production of biomass compared to those bioreactors with limited optimized mixing. Ugwu et al. (2005, 2008) reported that short light–dark cycles could promote growth of microalgal cells. In addition, with regulated mixing and proper supply of carbon dioxide and removal of oxygen, microalgal cells are kept in suspension in suitable zones to efficiently harvest the light and nutrients for their growth. In general, mixing is one of the important aspects in photobioreactor development. Thawechai et al. (2016) optimized all interacting growth factors using Resonance Surface Methodology to enhance microalgae lipid and pigment production.
4.3.2.1 Carbon Dioxide
Carbon dioxide (CO2) is readily available in the atmosphere with concentrations ranging from 0.03–0.06% (v/v) depending on the area. There is a global trend of increasing CO2 from anthropogenic activities especially in congested urban and industrial areas where flue-gas can contribute significantly to the CO2 pool (Rahaman et al. 2011; Norhasyima and Mahlia 2018). Microalgae, on the other hand, can efficiently sequester CO2 at the rate of approximately 1.8 kg for every 1 kg of microalgae produced (Jiang et al. 2013). In addition, flue-gas which can be obtained from various industries can be utilized to enhance microalgae productivity to new production level and contribute to the reduction of greenhouse gases. Carbon dioxide uptake by microalgae can be enhanced in tandem with other growth factors, such as light (Mondal et al. 2017b) and nutrients (Yan et al. 2016) to promote high growth rates in microalgae. Schultze et al. (2015) reported that the increase of carbon dioxide together with light improved the production to 31–50 g m−2 d−1, using twin-layer biofilm photobioreactors (TL-PBRs), the highest microalgae dry biomass productivity reported to date (Table 4.2). Cheah et al. (2015) also reported the use of atmospheric CO2 and flue-gas for microalgae biomass production.
4.3.2.2 Nutrients
Carbon, nitrogen and phosphorus are the three major nutrients that are essential for microalgae growth. Carbon dioxide can be obtained from the atmosphere by aeration, but reactive nitrogen and phosphorus have to be supplied to the culture media. Microalgae are effective in consuming nutrients from wastewaters, such as domestic sewage, tannery wastewaters and aquaculture sludge which normally have organic contents (Table 4.3). da Fontoura et al. (2017) reported that Scenedesmus sp. showed a maximum biomass production of 210.5 mg L−1 d−1 when cultured in tannery wastewater with high uptake rate of ammoniacal nitrogen (85.6%) and phosphorus (96.9%). Other industries with discharges of nutrients can also use microalgae culture to reduce their nutrient loadings into the ecosystem. Yan et al. (2016) reported that removal efficiencies of total oxygen demand, total nitrogen and total phosphorus by Chlorella culture in a simultaneous biogas upgrading and nutrient reduction system were 93%, 81% and 80%, respectively, illustrating that microalgae can efficiently remove nutrients from wastewaters. Groundwater can also have high contents of nutrients. Rezvani et al. (2017) used groundwater to cultivate Ettlia sp. with biomass productivity of 0.2 g L−1 d−1.
Zhuang et al. (2018) reported that nitrogen and phosphorus were the two major determinants not only for microlagal biomass but also for improvement of protein synthesis. His idea was supported by many other studies that reported higher microalgae compounds are synthesized under adequate culture environment (Manirafasha et al. 2018). In fact, a culture consisting of a consortia of species showed higher nutrient removal compared to a single species culture (Gonçalves et al. 2016). Manipulations of major nutrients could enhance lipid production in marine microalgae (Adenan et al. 2016). In addition, light can also influence the production of lipids. Using a chemostat culture system at 1500 µmol m−2 s−1 light intensity, Seo et al. (2017) showed that high lipid productivity of 291.4 mg L−1 d−1 could be obtained. Some minerals also show effects on microalgae production. In a phototrophic culture, addition of calcium ions (Ca2+) would decrease the microalgae biomass production because the increase Ca2+ would increase the phosphate precipitation (Di Caprio et al. 2018).
4.3.3 Light and Temperature
In addition to carbon dioxide and nutrients, light is a critical factor in promoting microalgal growth and biomass/biocompound accumulation. Light does not only affect microalgae but also microbes. Nitrite oxidizers are light sensitive, and nitrite accumulation may occur if light intensity is increased (Vergara et al. 2016), and this might have some implication in photobioreactors using wastewater as the culture medium.
For photosynthetic-based industries, light is one of the main limiting factors for an efficient system. Thus, for the development of technological applications of producing energy from living biomass, the design of the culture vessels should ensure the availability of light to the producing cells both in terms of quantity and quality. Based on this premise, some models to predict the availability of light and its spectral distribution has been developed for microalgae bioreactors to increase biomass production and high-value compounds (Table 4.4). Fuente et al. (2017) developed a light field model to predict light attenuation in bioreactors which can be easily modified to accommodate different microalgae species in different photobioreactor types. The ability to predict the light intensity and spectral distribution are fundamental for productivity enhancement of these photobiological processes, the microalgal biomass production. In temperate countries when the growing season is short, photobioreactor engineering would focus on lengthening the photoperiod and maintaining a suitable temperature for the microalgae optimum growth and biomass production (Saeid and Chojnacka 2015).
Light distribution in a bioreactor depends on the incident light intensity, the configuration of the vessel and the algal biomass concentration (Zhang et al. 2017a, b). Naderi et al. (2017) developed a model of light distribution in a bioreactor based on the Beer–Lambert model which could provide useful information on light distribution and predict light reduction in the culture vessel. In bioreactors, light intensity attenuates sharply with the distance from the irradiated surface due to self-shading in the inner areas and light absorption by the dense microalgae cells. However, Hu and Sato (2017) proposed an internal light-limiting diode (LED) system that does not limit the volume of the reactor vessel, and light attenuation could be avoided by decreasing the light spacing (Table 4.4). In a bioreactor, not all zones are well lighted. Thus, strategies should be made such that the distance between the light source to the algal cells be optimized. Sun et al. (2016) illustrated the use of light guide to bring light close to the growing algal cells using hollow polymethyl methacrylate (PMMA) tubes embedded into a flat plate photobioreactor. In this way, the incident light can be transmitted and emitted to the interior of the PBR, providing a secondary light source for cells in light-deficient regions.
Different light spectrum has different effects on microalgae photosynthetic rates, which is further dependent on specific species (Vadiveloo et al. 2015). Schulze et al. (2016) suggested that LEDs emitting spectra between 390–450 (blue) and 630–690 nm (red) should be combined to increase high-quality microalgae biomass. Blue spectrum has been shown to be effective in increasing the microalgae productivity (Atta et al. 2013; Vadiveloo et al. 2015), in addition to the red spectrum (Detweiler et al. 2015; Schulze et al. 2014, 2016; Gao et al. 2017; Yan et al. 2016). Lima et al. (2018) showed that using LEDs with 70% red and 30% blue spectra with light intensity of 100 µmol m−2 s−1 provided relatively high biomass productivity of 0.145 g L−1 d−1 for Athrospira platensis cultured in modified Zarrouk’s medium. Thus, both red and blue spectrum are needed to boost the microalgae production. Interestingly, Leonardi et al. (2018) reported that it was not the blue or red spectrum individually that caused the increase in microalgal biomass (Scenedesmus quadricauda), but the interactions of all the photons in the absorption process. In addition to enhancing microalgae growth rates and biomass production, specific light spectrum can also influence the quantity and quality of biochemical compounds synthesized in microalgae cells. Vadiveloo et al. (2015) reported that the lipid content in Nannochloropsis sp. was highest under the blue spectrum.
However, increasing light intensity is not necessarily good for all microalgae. Naderi et al. (2017) demonstrated that increasing light intensity in dense cultures did not result in increased biomass due to light absorption and scattering. To accurately determine the light availability to microalgae cells, Kandilian et al. (2016) proposed a simple method to measure microalgal spectral absorption cross-section that can be used to predict and control light transfer and biomass production in a photobioreactor. Too strong light can cause photoinhibition. In their study of cyanobacteria culture in raceways, Hidasi and Belay (2018) reported that photosynthetic depression occurred at midday when the sunlight was highest. Aly et al. (2017) estimated that photoinhibition could cause 30–40% reduction in net microalgae biomass in an outdoor bioreactor. Yan et al. (2016), in their study of growing Chlorella sp. using biogas slurry nutrient, suggested that light intensity should be low (approximately 400 µmol m−2 s−1) during the early phase of the culture to avoid photoinhibition, and increase accordingly (to approximately 1000 µmol m−2 s−1) as the microalgae density increases. To prevent photoinhibition, Hidasi and Belay (2018) used flashing light in his raceway culture and showed that the microalgae growth rates were significantly higher compared to those that received continuous light. Application of flashing light approach by using different technological devices and/or by optimizing the mixing velocity of the culture at a suitable microalgae density, can also be integrated into the photobioreactor design to decrease the effect of photoinhibition and increase the microalgae biomass production (Abu-Ghosh et al. 2016).
4.3.3.1 Light Sources
Light can be obtained from the sun which is free but subjected to inconsistencies due to daily or seasonal, environmental and climate changes. In spite of the problems, solar energy should be fully utilized to decrease the cost of energy used. Zijffers et al. (2008) used Fresnel lenses to guide solar energy to focus on the microalgae cells in the photobioreactor. Vadiveloo et al. (2015) used blue photovoltaic filters to increase biomass production of Nannochloropsis sp. in large outdoor cultures as this species illustrated that blue light was the most efficient light to biomass conversion. In addition, trapped solar energy can be used as a source of electricity to run the microalgae cultivations system such as pumps and aerators (Parlevliet and Moheimani 2014). Thus, photobioreactor innovations should be strategized to fully exploit the natural, free and clean solar energy to drive large outdoor microalgae cultivation system, not only to increase the productivity of the cultivated microalgae, but also for electricity production to drive the cultivations system. On the other hand, the artificial light from lamps such as fluorescent tube, high intensity discharge lamp (HID) and light-limiting diode (LED), is costly, but consistent (Blanken et al. 2013). Thus, in designing an efficient microalgae production bioreactor, light factor, either from solar energy or artificial light, has to be optimized to ensure its availability to the photosynthesizing cells.
The effects of light of microalgae production also depend on other growth factors, such as the use of wastewater. Using a higher light intensity of 182.5 µmol m−2 s−1, da Fontoura et al. (2017) reported Scenedesmus biomass productivity of 0.211 g L−1 d−1 cultured in tannery wastewater. Thus, optimization of light, both in terms of intensity and spectral distribution with respect to other growth factors such as temperature, pH, aeration, nutrients and cultured species is the most important strategy to be considered in designing a photobioreactor (Mondal et al. 2017b; Seo et al. 2017; Lima et al. 2018). Willette et al. (2018) demonstrated that microalgae growth and photosynthetic rates declined at extreme temperatures (<15 °C), but the cold stress could boost the lipid and fatty acids production. In addition to temperature, photoperiods also play an important role in microalgae biomass production. Maroneze et al. (2016) showed that manipulations of photoperiod can reduce energy cost in Scenedesmus obliquus culture.
4.4 The Performances in Different Types of Photobioreactors
Upscaling of microalgae cultivation is crucial in the assessment of its economics and ecological viability. In assessing the performance of different types of photobioreactors, the cell density (g L−1) and biomass production rate (g m−2 d−1) are the most important parameters in terms of bioprocess engineering, although construction and running costs and energy expenditure are also crucial for the actual industrial process. High cell density culture has the merits of (1) efficient light utilization, (2) low energy consumption for pumping and circulating of culture media and (3) saving energy in dewatering and biomass concentration for downstream use of the biomass. Thus, high cell density culture is one of the keys for improvement of mass production of microalgae. Based on 48 previous works on outdoor microalgae culture in different countries, species and culture media (Table 4.5), there is a negative between the cell density (g-dw L−1) and light path length (m) in outdoor microalgae cultures (Fig. 4.5). The cell density increased with decreasing light pass length or volume/surface ratio (m) of the bioreactor. Doucha and Lívanský (2006) reported that high cell density of 43 g L−1 in the closed raceway system with 1 cm light path length. Ozkan et al. (2012) achieved extremely high cell density of 96.4 g L−1 in a biofilm reactor.

The data are collected from 48 previous studies on outdoor culture works listed in Table 4.1
The relationship between cell density (g-dw L−1) and light path length (m) of each reactor in outdoor culture.
For higher production rate, the bioreactor requires higher light intensity, since the production of microalgae are the conversion process of light energy to biomass energy. The areal production rate (g.m−2 day−1) seems to increase with daily solar radiation-PAR (MJ m−2 day−1) (Fig. 4.6). The areal production is not much different among bioreactor types and the rate tends to increase with higher daily solar radiation-PAR until around 13 MJ m−2 day−1 since the photosynthesis is the energy conversion process of light and biomass energy. However, lower production values were often reported even in the bioreactor that received higher solar radiations. These low values are causally related to (1) lack of nutrients and CO2, (2) insufficient mass transfer efficiency to distribute nutrients and CO2, (3) unsuitable environmental factor of pH and temperature, (4) non-optimal dilution rate and (5) variation of species-specific growth rate.

The data are collected from 48 previous studies on outdoor culture works listed in Table 4.1
The relationship between areal production rate (g-dw m−2 day−1) and daily solar radiation-PAR (MJ m−2 day−1) in outdoor culture.
To increase the light energy received by a photobioractor, the second generation of internally irradiated photobioreactors using optical fibers (Javanmardian and Palsson 1991; Ogbonna et al. 1999) and fresnel lenses (Ogbonna et al. 1999) as light-concentrating devices, were developed. Masojídek et al. (2003) used fresnel lenses to concentrate light energy on the surface of tubular reactor and achieved high light intensity of 7000 µE m−2 s−1 and 31.5 MJ m−2 day−1 (Masojídek et al. 2003), although the areal production was not the highest. The idea of the internal irradiation by light-concentrating device is not only to concentrate light energy but also to diffuse strong light in order to avoid photoinhibition. However, this bioreactor structure becomes complex and its cost of construction also increases. The strategy of using light concentration technology may not be suitable for mass production of microalgae that requires low cost and low energy consumption.
4.4.1 Technology Improvements
There are technologies to improve microalgae biomass production using photobioreactors by strategizing the use of growth factors especially increasing the efficiencies of light, carbon dioxide and nutrient utilization by different species (Table 4.6). Holdmann et al. (2018) illustrated an extremely effective technology using an airlift reactor showing 300% of production compared to the conventional method. To address the major problems in microalgae biomass and biomolecule production, Lee and Li (2017) proposed resonant ultrasound field incorporated dynamic photobioreactor (RUF-DPBS) that is labour-efficient, cost-effective and non-fouling. Huang et al. (2015) developed a novel internal mixers optimized with computational fluid dynamics to improve the performance of their flat plate photobioreactors to about 32.8% higher than the conventional mixer. In general, innovative and cost-effective technologies for microalgae biomass production are still urgently required to satisfy the market demand for microalgae biomass by microalgae-based industries. Conventional technologies cannot keep up with the increasing demand for microalgae.
4.4.2 Mathematical Modelling
Due to many interacting factors influencing microalgae biomass production, mathematical modelling becomes a useful tool in predicting the behaviour and impacts of different factors, which in turn affect the design of suitable culture vessels and microalgae production systems. Thus, integrated modelling of an efficient and strategic photobioreactor for optimum and sustainable production of microalgae should encompass light intensity and spectral distribution, carbon dioxide and nutrient supply and uptake, optimization of environmental factors in culture vessels, dissolved oxygen removal and growth biokinetics with reference to selected species (Al Ketife et al. 2016). Mondal et al. (2017a) used response surface methodology (RSM)-central composite design approach to model three interacting factors (light intensity, CO2 and temperature) to determine optimal culture conditions for Chlorellla sp. Gao et al. (2018) suggested a light distribution model to accurately predict the light intensity required for the fast growth of Haematococcus pluvialis culture under red LEDs. Aly et al. (2017) produced a mathematic model for the microalgae growth and CO2 sequestration in outdoor photobioractors, whereas Al Ketife et al. (2016) suggested a model that could permit optimization and scale-up of microalgae biomass production based on light, nutrients and carbon dioxide and their kinetics.
4.5 Conclusions and Future Perspectives
Microalgae are known to be sustainable feedstocks for biofuels and valuable compounds which are important in food, health and animal production industries. However, biomass production on a large scale is still an insurmountable challenge that need to be solved in terms of technological, economics and ecological viability. Photobioreactor is the best alternative to produce high-quality microalgae biomass but strategies are needed to build an economical, efficient and high-throughput microalgae production system. Efficient production of biomass through balancing the use of energy and reducing cost should be the focus in designing bioreactors. Microalgae growth factors including light, carbon dioxide and nutrients have to be technologically manipulated to develop a simple, efficient and cost-effective photobioreactor with high production rate but minimal construction and operation cost. Additional features to increase efficiency of the bioreactor such as efficient light harvesting with suitable light spectrum and adjustable photoperiod, suitable fluid dynamics to ensure optimised dispersion of microalgal cells, adjustable application of nutrient stress to trigger the production of high lipids contents in the algal cells, and automated oxygen discharge structure are necessary to overcome biomass production limitation. Natural light, gas and nutrient sources should be used to defray the operation cost. Strategic bioreactor should be flexible and adjustable to suit different species of microalgae and the target compounds and can be used in many areas with different climatic conditions. Large-scale photobioreactors should not be only technically improved but should be made economically feasible. Once technologically and economically improvised, photobioreactors could generate all the resources that are valuable and useful to global communities.
References
Abu-Ghosh S, Fixler D, Dubinsky Z, Iluz D (2016) Flashing light in microalgae biotechnology. Bioresour Tech 203:357–363
Acién Fernández FG, Fernández Sevilla JM, Sánchez Pérez JA, Molina Grima E, Chisti Y (2001) Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: assessment of design and performance. Chem Eng Sci 56(8):2721–2732
Adenan NS, Yusoff FM, Medipally SR, Shariff M (2016) Enhancement of lipid production in two marine microalgae under different levels of nitrogen and phosphorus deficiency. J Environ Biol 37:669–676
Al Ketife AMD, Judd S, Znad H (2016) A mathematical model for carbon fixation and nutrient removal by an algal photobioreactor. Chem Eng Sci 153:354–362
Alishahi M, Karamifar M, Mesbah M (2015) Effects of astaxanthin and Dunaliella salina on skin carotenoids, growth performance and immune response of Astronotus ocellatus. Aquac Int 23(5):1239–1248
Aly N, Tarai RK, Kale PG, Paramasivan B (2017) Modelling the effect of photoinhibition on microalgal production potential in fixed and trackable photobioreactors in Odisha. India Curr Sci 113(2):272–283
Angeles IP, Chien Y-H, Tayamen MM (2009) Effects of different dosages of astaxanthin on giant freshwater prawn Macrobrachium rosenbergii (De Man) challenged with Lactococcus garvieae. Aquac Res 41(1):70–77
Arashiro LT, Montero N, Ferrer I, Acien FG, Gomez C, Garfi M (2018) Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci Total Environ 622–623:1118–1130
Arora N, Patel A, Pruthi PA, Poluri KM, Pruthi V (2018) Utilization of stagnant non-potable pond water for cultivating oleaginous microalga Chlorella minutissima for biodiesel production. Renew Energ 126:30–37
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Tech 148:373–378
Becker EW (1984) Biotechnology and exploitation of the green alga Scenedesmus obliquus in India. Biomass 4(1):1–19
Becker EW, Venkataraman LV (1984) Production and utilization of the blue-green alga Spirulina in India. Biomass 4(2):105–125
Begum H, Yusoff FM, Banerjee S, Khatoon H, Shariff M (2016) Availability and utilization of pigments from microalgae. Crit Rev Food Sci 56(13):2209–2222
Blanken W, Cuaresma M, Wijffels RH, Janssen M (2013) Cultivation of microalgae on artificial light comes at a cost. Algal Res 2(4):333–340
Burgess G, Fernández-Velasco JG (2007) Materials, operational energy inputs, and net energy ratio for photobiological hydrogen production. Int J Hydrogen Energ 32(9):1225–1234
Cao W, Wang X, Sun S, Hu C, Zhao Y (2017) Simultaneously upgrading biogas and purifying biogas slurry using cocultivation of Chlorella vulgaris and three different fungi under various mixed light wavelength and photoperiods. Bioresour Tech 241:701–709
Carlozzi P (2000) Hydrodynamic aspects and Arthrospira growth in two outdoor tubular undulating row photobioreactors. Appl Microbiol Biot 54(1):14–22
Carlozzi P (2003) Dilution of solar radiation through “culture” lamination in photobioreactor rows facing south–north: a way to improve the efficiency of light utilization by cyanobacteria (Arthrospira platensis). Biotechnol Bioeng 81(3):305–315
Chang HX, Fu Q, Huang Y, Xia A, Liao Q, Zgu X, Zheng YP, Sun CH (2016) An annular photobioreactor with ion-exchange-membrane for non-touch microalgae cultivation with wastewater. Bioresour Tech 219:668–676
Cheah WY, Show PL, Chang J-S, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Tech 184:190–201
Chen BF, Yang HK, Wu CH, Lee TC, Chen B (2018) Numerical study of mixing in microalgae-farming tanks with baffles. Ocean Eng 161:168–186
Cheng-Wu Z, Zmora O, Kopel R, Richmond A (2001) An industrial-size flat plate glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture 195(1):35–49
Chini Zittelli G, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70(1):299–312
Chini Zittelli G, Rodolfi L, Biondi N, Tredici MR (2006) Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 261(3):932–943
Chinnasamy S, Bhatnagar A, Claxton R, Das KC (2010) Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour Tech 101(17):6751–6760
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
da Fontoura JT, Rolim GS, Farenzena M, Gutterres M (2017) Influence of light intensity and tannery wastewater concentration on biomass production and nutrient removal by microalgae Scenedesmus sp. Process Saf Environ 111:355–362
Degen J, Uebele A, Retze A, Schmid-Staiger U, Trösch W (2001) A novel airlift photobioreactor with baffles for improved light utilization through the flashing light effect. J Biotechnol 92(2):89–94
Del Campo JA, Rodriguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 85(3):289–295
Detweiler AM, Mioni CE, Hellier KL, Allen JJ, Carter SA, Bebout BM, Fleming EE, Corrado C, Prufert-Bebout LE (2015) Evaluation of wavelength selective photovoltaic panels on microalgae growth and photosynthetic efficiency. Algal Res 9:170–177
Di Caprio F, Altimari P, Pagnanelli F (2018) Effect of Ca2+ concentration on Scenedesmus sp. growth in heterotrophic and photoautotrophic cultivation. New Biotechnol 40:228–235
Dogaris I, Welch M, Meiser A, Walmsley L, Philippidis G (2015) A novel horizontal photobioreactor for high-density cultivation of microalgae. Bioresour Tech 198:316–324
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J Appl Phycol 18(6):811–826
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgal photobioreactor: potential productivity. J Appl Phycol 21(1):111–117
Doucha J, Straka F, Lívanský K (2005) Utilization of flue gas for cultivation of microalgae Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol 17(5):403–412
Ebrahimi Nigjeh S, Yusoff FM, Mohamed Alitheen NB, Rasoli M, Keong YS, Omar ARb (2013) Cytotoxic effect of ethanol extract of microalga, Chaetoceros calcitrans, and its mechanisms in inducing apoptosis in human breast cancer cell line. Biomed Res Int (Article ID 783690)
Feng P, Deng Z, Hu Z, Fan L (2011) Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour Tech 102(22):10577–10584
Foo SC, Yusoff FM, Ismail M, Basri M, Chan KW, Khong NMH, Yau SK (2015) Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res 12:26–32
Foo SC, Yusoff FM, Ismail M, Basri M, Yau SK, Khong NMH, Chan KW, Ebrahimi M (2017) Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J Biotechnol 241:175–183
Freitas BCB, Cassuriaga APA, Morais MG, Costa JAV (2017) Pentoses and light intensity increase the growth and carbohydrate production and alter the protein profile of Chlorella minutissima. Bioresour Tech 238:248–253
Fuente D, Keller J, Conejero JA, Rögner M, Rexroth S, Urchueguía JF (2017) Light distribution and spectral composition within cultures of micro-algae: quantitative modelling of the light field in photobioreactors. Algal Res 23:166–177
Gao X, Wang X, Li H, Roje S, Sablani SS, Chen S (2017) Parameterization of a light distribution model for green cell growth of microalgae: Haematococcus pluvialis cultured under red LED lights. Algal Res 23:20–27
García-González M, Moreno J, Cañavate JP (2003) Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. J Appl Phycol 15(2):177–184
García-González M, Moreno J, Manzano JC, Florencio FJ, Guerrero MG (2005) Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J Biotechnol 115(1):81–90
Gbadamosi OK, Lupatsch I (2018) Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia, Oreochromis niloticus. Algal Res 33:48–54
Georgianna DR, Mayfield SP (2012) Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 488(7411):329–335
Gifuni I, Olivieri G, Pollio A, Franco TT, Marzocchella A (2017) Autotrophic starch production by Chlamydomonas species. J Appl Phycol 29(1):105–114
Goh SH, Alitheen NB, Yusoff FM, Yap SK, Loh SP (2014) Crude ethyl acetate extract of marine microalga, Chaetoceros calcitrans, induces apoptosis in MDA-MB-231 breast cancer cells. Pharmacogn Mag 10(37):1–8
Goldman JC, Ryther JH, Williams LD (1975) Mass production of marine algae in outdoor cultures. Nature 254:594
Gonçalves AL, Pires JCM, Simões M (2016) Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour Tech 200:279–286
Guldhe A, Ansari FA, Singh P, Bux F (2017) Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol Eng 99:47–53
Gummert F, Meffert ME, Stratmann H (1953) Nonsterile large-scale culture of Chlorella in greenhouse and open air, In: Burlew JS (ed) Algae culture from laboratory to pilot plant. Carnegie Institution of Washington Publication 600, Washington D.C, pp 166–176
Guyon JB, Verge V, Schatt P, Lozano JC, Liennard M (2018) Bouget FY (2018) Comparative analysis of culture conditions for the optimization of carotenoid production in several strains of the picoeukaryote Ostreococcus. Mar Drugs 16:76. https://doi.org/10.3390/md16030076
Hall DO, Acién Fernández FG, Guerrero EC, Rao KK, Grima EM (2003) Outdoor helical tubular photobioreactors for microalgal production: Modeling of fluid-dynamics and mass transfer and assessment of biomass productivity. Biotechnol Bioeng 82(1):62–73
Hase R, Oikawa H, Sasao C, Morita M, Watanabe Y (2000) Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai city. J Biosci Bioeng 89(2):157–163
Hidasi N, Belay A (2018) Diurnal variation of various culture and biochemical parameters of Arthrospira platensis in large-scale outdoor raceway ponds. Algal Res 29:121–129
Hirata S, Hayashitani M, Taya M, Tone S (1996) Carbon dioxide fixation in batch culture of Chlorella sp. using a photobioreactor with a sunlight-collection device. J Ferment Bioeng 81(5):470–472
Holdmann C, Schmid-Staiger U, Hornstein H, Hirth T (2018) Keeping the light energy constant—Cultivation of Chlorella sorokiniana at different specific light availabilities and different photoperiods. Algal Res 29:61–70
Hu J-Y, Sato T (2017) A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energ Convers Manage 133:558–565
Hu Q, Guterman H, Richmond A (1996) A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnol Bioeng 51(1):51–60
Huang J, Feng F, Wan M (2015) Improving performance of flat-plate photobioreactors by installation of novel internal mixers optimized with computational fluid dynamics. Bioresour Tech 182:151–159
Huesemann M, Williams P, Edmundson S (2017) The laboratory environmental algae pond simulator (LEAPS) photobioreactor: validation using outdoor pond cultures of Chlorella sorokiniana and Nannochloropsis salina. Algal Res 26:39–46
Huesemann MH, Benemann JR (2009) Biofuels from microalgae: review of products, processes and potential, with special focus on Dunaliella sp. Science Publishers, New Hampshire
Javanmardian M, Palsson BO (1991) High-density photoautotrophic algal cultures: design, construction, and operation of a novel photobioreactor system. Biotechnol Bioeng 38(10):1182–1189
Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T (2013) Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Tech 128:359–364
Jiménez C, Cossı́o BR, Labella D, Xavier Niell F (2003) The feasibility of industrial production of Spirulina (Arthrospira) in Southern Spain. Aquaculture 217(1):179–190
Jin E-S, Polle JEW, Lee H-K, Hyun S-M, Chang M (2003) Xanthophylls in microalgae: from biosynthesis to biotechnological mass production and application. Korean Soc Appl Microbiol Biotech 13(2):165–174
Jorquera O, Kiperstok A, Sales EA, Embiruçu M, Ghirardi ML (2010) Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour Tech 101(4):1406–1413
Kandilian R, Jesus B, Legrand J, Pilon L, Pruvost J (2017) Light transfer in agar immobilized microalgae cell cultures. J Quant Spectrosc Ra 198:81–92
Kandilian R, Soulies A, Pruvost J, Rousseau B, Legrand J, Pilon L (2016) Simple method for measuring the spectral absorption cross-section of microalgae. Chem Eng Sci 146:357–368
Koller AP, Wolf L, Brück T, Weuster-Botz D (2018) Studies on the scale-up of biomass production with Scenedesmus spp. in flat-plate gas-lift photobioreactors. Bioproc Biosyst Eng 41(2):213–220
Kraan S (2013) Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strat Gl 18(1):27–46
Laws EA, Taguchi S, Hirata J, Pang L (1986a) Continued studies of high algal productivities in a shallow flume. Biomass 11(1):39–50
Laws EA, Taguchi S, Hirata J, Pang L (1986b) High algal production rates achieved in a shallow outdoor flume. Biotechnol Bioeng 28(2):191–197
Laws EA, Taguchi S, Hirata J, Pang L (1988a) Mass culture optimization studies with four marine microalgae. Biomass 16(1):19–32
Laws EA, Taguchi S, Hirata J, Pang L (1988b) Optimization of microalgal production in a shallow outdoor flume. Biotechnol Bioeng 32(2):140–147
Lee Y-H, Li P-H (2017) Using resonant ultrasound field-incorporated dynamic photobioreactor system to enhance medium replacement process for concentrated microalgae cultivation in continuous mode. Chem Eng Res Des 118:112–120
Leonardi RJ, Niizawa I, Irazoqui HA, Heinrich JM (2018) Modeling and simulation of the influence of fractions of blue and red light on the growth of the microalga Scenedesmus quadricauda. Biochem Eng J 129:16–25
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquacult 10(3):738–773
Lima GM, Teixeira PCN, Teixeira CMLL, Filócomo D, Lage CLS (2018) Influence of spectral light quality on the pigment concentrations and biomass productivity of Arthrospira platensis. Algal Res 31:157–166
Liu J, Wu Y, Wu C (2017) Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour Tech 241:1127–1137
López MCG-M, Sánchez EDR, López JLC (2006) Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J Biotechnol 123(3):329–342
López-Rosales L, García-Camacho F, Sánchez-Mirón A, Beato EM, Chisti Y, Grima EM (2016) Pilot-scale bubble column photobioreactor culture of a marine dinoflagellate microalga illuminated with light emission diodes. Bioresour Tech 216:845–855
Lucker BF, Hall CC, Zegarac R, Kramer DM (2014) The environmental photobioreactor (ePBR): an algal culturing platform for simulating dynamic natural environments. Algal Res 6:242–249
Manirafasha E, Murwanashyaka T, Ndikubwimana T (2018) Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresour Tech 255:293–301
Maroneze MM, Siqueira SF, Vendruscolo RG (2016) The role of photoperiods on photobioreactors—a potential strategy to reduce costs. Bioresour Technol 219:493–499
Marotta G, Scargiali F, Lima S, Caputo G, Grisafi F, Brucato A (2017) Vacuum air-lift bioreactor for microalgae production. Chem Eng Trans 57:925–930
Masojídek J, Papáček Š, Sergejevová M (2003) A closed solar photobioreactor for cultivation of microalgae under supra-high irradiance: basic design and performance. J Appl Phycol 15(2):239–248
Mayers JJ, Ekman Nilsson A, Albers E, Flynn KJ (2017) Nutrients from anaerobic digestion effluents for cultivation of the microalga Nannochloropsis sp.—impact on growth, biochemical composition and the potential for cost and environmental impact savings. Algal Res 26:275–286
Medipally SR, Yusoff FM, Banerjee S, Shariff M (2015) Microalgae as sustainable renewable energy feedstock for biofuel production. Biomed Res Int 2015:519513
Miranda JR, Passarinho PC, Gouveia L (2012) Bioethanol production from Scenedesmus obliquus sugars: the influence of photobioreactors and culture conditions on biomass production. Appl Microbiol Biot 96(2):555–564
Mirón AS, Garcı́a MCC, Gómez AC, Camacho FGa, Grima EM, Chisti Y (2003) Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Eng J 16(3):287–297
Mituya A, Nyunoya T, Tamiya H (1953) Re-pilot-plant experiments on algal mass culture. In: Burlew JS (ed) Algal culture: from laboratory to pilot plant. Carnegie Institution of Washington Publication 600, Washington D.C., pp 173–184
Mohamed Ramli N, Verdegem MCJ, Yusoff FM, Zulkifely MK, Verreth JAJ (2017) Removal of ammonium and nitrate in recirculating aquaculture systems by the epiphyte Stigeoclonium nanum immobilized in alginate beads. Aquacult Env Interac 9:213–222
Moheimani NR, Borowitzka MA (2006) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 18(6):703–712
Molina-Miras A, Morales-Amador A, de Vera CR (2018) A pilot-scale bioprocess to produce amphidinols from the marine microalga Amphidinium carterae: Isolation of a novel analogue. Algal Res 31:87–98
Mondal M, Ghosh A, Gayen K, Halder G, Tiwari ON (2017a) Carbon dioxide bio-fixation by Chlorella sp. BTA 9031 towards biomass and lipid production: optimization using central composite design approach. J CO2 Util 22:317–329
Mondal M, Ghosh A, Tiwari ON (2017b) Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energ Convers Manage 145:247–254
Morais MG, Radmann EM, Andrade MR, Teixeira GG, Brusch LRF, Costa JAV (2009) Pilot scale semi-continuous production of Spirulina biomass in southern Brazil. Aquaculture 294(1):60–64
Moreno J, Vargas MÁ, Rodrı (2003) Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomol Eng 20(4):191–197
Morita M, Watanabe Y, Saiki H (2002) Photosynthetic productivity of conical helical tubular photobioreactor incorporating Chlorella sorokiniana under field conditions. Biotechnol Bioeng 77(2):155–162
Murray AM, Fotidis IA, Isenschmid A, Haxthausen KRA, Angelidaki I (2017) Wirelessly powered submerged-light illuminated photobioreactors for efficient microalgae cultivation. Algal Res 25:244–251
Naderi G, Znad H, Tade MO (2017) Investigating and modelling of light intensity distribution inside algal photobioreactor. Chem Eng Process 122:530–537
Natrah FMI, Bossier P, Sorgeloos P, Yusoff FM, Defoirdt T (2014) Significance of microalgal–bacterial interactions for aquaculture. Rev Aquacult 6:48–61
Natrah FMI, Yusoff FM, Shariff M, Abas F, Mariana NS (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J Appl Phycol 19(6):711–718
Norhasyima R, Mahlia T (2018) Advances in CO2 utilization technology: a patent landscape review. J CO2 Util 26:323–335
Novoveská L, Zapata AKM, Zabolotney JB, Atwood MC, Sundstrom ER (2016) Optimizing microalgae cultivation and wastewater treatment in large-scale offshore photobioreactors. Algal Res 18:86–94
Ogbonna JC, Soejima T, Tanaka H (1999) An integrated solar and artificial light system for internal illumination of photobioreactors. In: Osinga R, Tramper J, Burgess JG, Wijffels RH (eds) Prog Ind M 35:289–297
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12(3):499–506
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15(2):249–257
Olivieri G, Salatino P, Marzocchella A (2014) Advances in photobioreactors for intensive microalgal production: configurations, operating strategies and applications. J Chem Technol Biot 89(2):178–195
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Tech 114:542–548
Panjiar N, Mishra S, Yadav AN, Verma P (2017) Functional foods from cyanobacteria: an emerging source for functional food products of pharmaceutical importance. In: Gupta VK, Treichel H, Shapaval VO, Oliveira LAd, Tuohy MG (eds) Microbial functional foods and nutraceuticals. Wiley, USA, pp 21–37. https://doi.org/10.1002/9781119048961.ch2
Parlevliet D, Moheimani NR (2014) Efficient conversion of solar energy to biomass and electricity. Aquat Biosyst 10:4
Peng J, Yuan JP, Wang JH (2012) Effect of diets supplemented with different sources of astaxanthin on the gonad of the sea urchin Anthocidaris crassispina. Nutrients 4(8):922–934
Pennington F, Guillard RRL, Liaaen-Jensen S (1988) Carotenoid distribution patterns in Bacillariophyceae (Diatoms). Biochem Syst Ecol 16(7):589–592
Pereira EG, Martins MA, Mendes MDSA, Mendes LBB, Nesi AN (2017) Outdoor cultivation of Scenedesmus obliquus BR003 in stirred tanks by airlift. J Braz Assoc Agric Eng. http://dx.doi.org/10.1590/1809–4430
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Tech 102(1):17–25
Posten C (2009) Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9(3):165–177 ENG LIFE SCI
Pushparaj B, Pelosi E, Tredici MR, Pinzani E, Materassi R (1997) As integrated culture system for outdoor production of microalgae and cyanobacteria. J Appl Phycol 9(2):113–119
Qin C, Lei Y, Wu J (2018) Light/dark cycle enhancement and energy consumption of tubular microalgal photobioreactors with discrete double inclined ribs. Bioresour. Bioprocess 5:28. https://doi.org/10.1186/s40643-018-0214-8
Rahaman MSA, Cheng L-H, Xu X-H, Zhang L, Chen H-L (2011) A review of carbon dioxide capture and utilization by membrane integrated microalgal cultivation processes. Renew Sust Energ Rev 15(8):4002–4012; Rastogi RP, Pandey A, Larroche C, Madamwar D (2018) Algal green energy—R&D and technological perspectives for biodiesel production. Renew Sust Energ Rev 82:2946–2969
Rezvani F, Sarrafzadeh MH, Seo SH, Oh HM (2017) Phosphorus optimization for simultaneous nitrate-contaminated groundwater treatment and algae biomass production using Ettlia sp. Bioresour Tech 244(Pt 1):785–792
Richmond A, Cheng-Wu Z (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85(3):259–269
Richmond A, Lichtenberg E, Stahl B, Vonshak A (1990) Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways. J Appl Phycol 2(3):195–206
Rocha RP, Machado M, Vaz MGMV (2017) Exploring the metabolic and physiological diversity of native microalgal strains (Chlorophyta) isolated from tropical freshwater reservoirs. Algal Res 28:139–150
Rodolfi L, Chini Zittelli G, Bassi N (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112
Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160:393–400
Saeid A, Chojnacka K (2015) Toward production of microalgae in photobioreactors under temperate climate. Chem Eng Res Des 93:377–391
Sánchez Mirón A, Cerón Garcı́a M-C, Garcı́a Camacho F, Molina Grima E, Chisti Y (2002) Growth and biochemical characterization of microalgal biomass produced in bubble column and airlift photobioreactors: studies in fed-batch culture. Enzyme Microb Tech 31(7):1015–1023
Sánchez Mirón A, Contreras Gómez A, Garcı́a Camacho F, Molina Grima E, Chisti Y (1999) Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. J Biotechnol 70(1):249–270
Schenk PM, Thomas-Hall SR, Stephens E (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1(1):20–43
Schultze LKP, Simon M-V, Li T, Langenbach D, Podola B, Melkonian M (2015) High light and carbon dioxide optimize surface productivity in a Twin-Layer biofilm photobioreactor. Algal Res 8:37–44
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32(8):422–430
Schulze PSC, Pereira HGC, Santos TFC (2016) Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res 16:387–398
Seo SH, Ha JS, Yoo C (2017) Light intensity as major factor to maximize biomass and lipid productivity of Ettlia sp. in CO2-controlled photoautotrophic chemostat. Bioresour Tech 244(Pt 1):621–628
Seshadri CV, Thomas S (1979) Mass culture of spirulina using low-cost nutrients. Biotechnol Lett 1(7):287–291
Sun Y, Huang Y, Liao Q, Fu Q, Zhu X (2016) Enhancement of microalgae production by embedding hollow light guides to a flat-plate photobioreactor. Bioresour Tech 207:31–38
Thawechai T, Cheirsilp B, Louhasakul Y, Boonsawang P, Prasertsan P (2016) Mitigation of carbon dioxide by oleaginous microalgae for lipids and pigments production: Effect of light illumination and carbon dioxide feeding strategies. Bioresour Tech 219:139–149
Torzillo G, Carlozzi P, Pushparaj B, Montaini E, Materassi R (1993) A two-plane tubular photobioreactor for outdoor culture of Spirulina. Biotechnol Bioeng 42(7):891–898
Torzillo G, Pushparaj B, Bocci F, Balloni W, Materassi R, Florenzano G (1986) Production of Spirulina biomass in closed photobioreactors. Biomass 11(1):61–74
Tredici MR, Carlozzi P, Chini Zittelli G, Materassi R (1991) A vertical alveolar panel (VAP) for outdoor mass cultivation of microalgae and cyanobacteria. Bioresour Tech 38(2):153–159
Ugwu C, Ogbonna J, Tanaka H (2002) Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Appl Microbiol Biot 58(5):600–607
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Tech 99(10):4021–4028
Ugwu CU, Ogbonna JC, Tanaka H (2005) Light/dark cyclic movement of algal culture (Synechocystis aquatilis) in outdoor inclined tubular photobioreactor equipped with static mixers for efficient production of biomass. Biotechnol Lett 27(2):75–78
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Vallejos-Vidal V, Reyes-Lopez F, Teles M, MacKenzie S (2016) The response of fish to immunostimulant diets. Fish Shellfish Immun 56:116–121
Vergara C, Muñoz R, Campos JL, Seeger M, Jeison D (2016) Influence of light intensity on bacterial nitrifying activity in algal-bacterial photobioreactors and its implications for microalgae-based wastewater treatment. Int Biodeter Biodegr 114:116–121
Watanabe A, Hattori A, Fujita Y, Kiyohara T (1959) Large scale culture of a blue-green alga, Tolypothrix tenuis, utilizing hot spring and natural gas as heat and carbon dioxide sources. J Gen Appl Microbiol 5(1–2):51–57
Willette S, Gill SS, Dungan B (2018) Alterations in lipidome and metabolome profiles of Nannochloropsis salina in response to reduced culture temperature during sinusoidal temperature and light. Algal Res 32:79–92
Xia S, Wang K, Wan L, Li A, Hu Q, Zhang C (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11(7):2667–2681
Yan C, Muñoz R, Zhu L, Wang Y (2016) The effects of various LED (light emitting diode) lighting strategies on simultaneous biogas upgrading and biogas slurry nutrient reduction by using of microalgae Chlorella sp. Energy 106:554–561
Ye Q, Cheng J, Guo W, Xu J, Li K, Zhou J (2018) Serial lantern-shaped draft tube enhanced flashing light effect for improving CO2 fixation with microalgae in a gas-lift circumflux column photobioreactor. Bioresour Tech 255:156–162
Zhang CW, Richmond A (2003) Sustainable, high-yielding outdoor mass cultures of Chaetoceros muelleri var. subsalsum and Isochrysis galbana in vertical plate reactors. Mar Biotechnol 5(3):302–310
Zhang J-Y, Qi H, He Z-Z, Yu X-Y, Ruan L-M (2017a) Investigation of light transfer procedure and photobiological hydrogen production of microalgae in photobioreactors at different locations of China. Int J Hydrogen Energ 42(31):19709–19722
Zhang T (2013) Dynamics of fluid and light intensity in mechanically stirred photobioreactor. J Biotechnol 168(1):107–116
Zhang Z, Huang JJ, Sun D, Lee Y, Chen F (2017b) Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour Tech 224:515–522
Zhu L, Wang Z, Takala J (2013) Scale-up potential of cultivating Chlorella zofingiensis in piggery wastewater for biodiesel production. Bioresour Tech 137:318–325
Zhuang L-L, Azimi Y, Yu D, Wu Y-H, Hu H-Y (2018) Effects of nitrogen and phosphorus concentrations on the growth of microalgae Scenedesmus. LX1 in suspended-solid phase photobioreactors (ssPBR). Biomass Bioenerg 109:47–53
Zijffers J-WF, Salim S, Janssen M, Tramper J, Wijffels RH (2008) Capturing sunlight into a photobioreactor: Ray tracing simulations of the propagation of light from capture to distribution into the reactor. Chem Eng J 145(2):316–327
Acknowledgements
This study is partially supported by the SATREPS-COSMOS Malaysia-Japan collaborative project under the auspices of the Department of Higher Education, Ministry of Education Malaysia.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yusoff, F.M., Nagao, N., Imaizumi, Y., Toda, T. (2019). Bioreactor for Microalgal Cultivation Systems: Strategy and Development. In: Rastegari, A., Yadav, A., Gupta, A. (eds) Prospects of Renewable Bioprocessing in Future Energy Systems. Biofuel and Biorefinery Technologies, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-030-14463-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-14463-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14462-3
Online ISBN: 978-3-030-14463-0
eBook Packages: EnergyEnergy (R0)