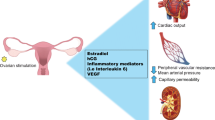
Abstract
A decrease in the birth rate and an increase in maternal age have been showed in the last decades. Alternatively, advances in assisted reproductive technology (ART) allows pregnancy at any age, and we face new ethical and health challenges regarding the question of what is the limit for a woman to become pregnant. Now that ARTs are common throughout the world, the medical, legal, moral, and ethical debates unleashed since their inception has been globalized. On the other hand, genetic counseling is a communication process that deals with the human problems associated with the occurrence, or the risk of occurrence, of a genetic disorder in a family. It is based on medical, reproductive, and family history and must always be carried out. In assisted reproduction, risk assessment includes not only the embryo and the fetus but also the parent itself.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Fertility and Aging
1.1.1 Age as a Social Factor of Infertility
If we analyze the demographic progression that took place in Europe in the last century, we observe a clear secular trend toward a decrease in the birth rate and an increase in maternal age. As an explanation of this, it seems that a purely cultural reason stands out: the postponement in the desire for genesis, which, in many cases, occurs for a variety of employment, social, and economic reasons. On the other hand, advances in assisted reproduction allow pregnancy at any age, and we face new ethical and health challenges regarding the question of what is the limit for a woman to become pregnant. It is shown that age decreases fertility due to factors such as [1, 2]:
-
A decrease in the number of oocytes
-
A decrease in the frequency of intercourse
-
A decrease in oocyte quality
-
A decreases in sperm quality
1.1.2 Problems in Fertility and Pregnancy Derived from Age
Fertility declines with the passage of time, particularly after 35 years. A decline is seen in both the number and quality of the reserve of ovules, which increases the difficulty for pregnancy and the risk of spontaneous abortions (more than 50%) and of fetuses with chromosomal abnormalities (e.g., Down’s syndrome) [3].
As age itself is the factor that most influences the rate of spontaneous pregnancy and the outcomes of fertility treatments, in people over 35 years of age, it is not recommended to wait a year to see a specialist; instead it is advisable to take matters into their hands after 6 months of trying to conceive. In people aged over 40 years, it is recommended to seek help immediately [4].
The application of assisted reproductive techniques (ARTs) is very common in this age group, with a strong tendency to resort to egg donation. This also prevents the increased risk of fetuses with chromosomal abnormalities (the age of the oocyte is that of the donor), but not that of other pregnancy complications such as gestational diabetes, hypertension, intrauterine growth restriction, placental pathology, and prematurity, which continue to depend on the mother’s age. Multiple pregnancies are also more frequent, because of the greater number of embryos transferred. As a consequence, the numbers of operative or instrumental deliveries and perinatal and maternal morbidity/mortality are all increased during perimenopause [5].
1.1.3 Up to What Age Is Pregnancy Allowed with Fertility Treatments?
Progress in reproductive medicine and in obstetrics itself has raised another important debate regarding age: what is the age limit to conceive or to apply an ART? Certain healthy habits and the feeling of staying young and being prepared for all eventualities have triggered the demand for fertility treatments for women over 40. Oocyte donation makes this a possibility even for those who have crossed the border into menopause. Since uterine age does not correspond to ovarian age, the maternity departments are now full of “older” women, an example of how advances in medicine appear to have developed ahead of the necessary and slow process of legislation and ethics. An older woman, even being postmenopausal, may not feel too old to have a child and may offer the infant a better education now that she no longer has the financial or emotional needs of younger women [6].
However, one of the immediate consequences of an increase in pregnancies in older women is the greater demand for medical and psychological assistance, since there are fewer requests for prenatal diagnostic methods, practices to reduce stress, and voluntary interruptions of pregnancy, along with an increase in perinatal and maternal morbidity and mortality, not to mention the concern for raising grandchildren instead of children.
There is an open debate on whether or not a child’s upbringing is optimal at these ages. In particular, some people are shocked to see older women breastfeeding their babies in the nonscientific press, while others warn of the high risk of leaving them as orphans at a young age. In the future vision of our own wellbeing, there are even those who argue that it is preferable to have children—regardless of the age at which they are conceived—to increase the birth rate. In their arguments in favor it is pointed out that they will take care of our care when we are elderly. And even when the argument seems to have tilted on the side of those who accept as logical, and even normal, a pregnancy over 40 years, common sense should still prevail when it comes to putting a cap on this demand. Although there is no clear limit, it seems to be ethical and medically advisable to place this limit before the age of 55, but it is more reasonable to place it at 50 years, given the high risk of cardiovascular morbidity from that age [7].
1.1.4 Social Controversies of Infertility Treatments
Of all the areas of medicine and all the attributes that have conditioned the evolution of our species, it is without doubt that sexuality and reproduction are those that are most loaded with social singularities. In addition, the rapid advance of reproductive technology has introduced significant changes in the conception of families that is sometimes difficult to assimilate within less advanced societies and those most impregnated with extreme conservatism. Some common practices in reproductive medicine have also generated controversies, such as the donation or freezing of gametes and embryos, embryonic reduction, or the costs of these treatments for the health system.
1.1.5 The Sociocultural Acceptance of the New Models of Families that Emerged with ARTs
From the first birth achieved with ART, what appears to have been most scandalous certain groups is the change in the classic conception of family, that is, two-parent, heterosexual, within a religious or civil commitment, and with the transmission of one’s genes to the offspring.
We can see then how far the concept of family to which we have become accustomed has shifted in the new millennium. It is now seen as nothing more than cohabitation with single-parent families, those of homosexual couples with no legal ties or where genes other than those of legal parents are transmitted. In this regard, in countries where surrogacy is allowed, it can be possible for a baby to theoretically have five parents: the donor of the ovule, the sperm donor, the woman who has gestated the child in surrogacy, and the legal parents who have requested the treatment. Improvements in laboratory techniques for the cryopreservation of gametes or embryos have even made it possible for one parent to be deceased. There is no doubt that popular fantasies have been triggered and that continued discussion of these issues will cause perplexity.
Now that ARTs are common throughout the world, the medical, legal, moral, and ethical debates unleashed since their inception have been globalized. Some countries, like Spain, have regulated these by taking into account the clinical recommendations, but in other latitudes and within the groups of immigrants with whom we live, there are opinions of a religious nature that prevail in the deemed suitability and use of these methods [8].
1.2 Infertility Generalities
Infertility is a generic term that refers to the problems that reduce human fertility and that, in the strictest sense, is considered a disease. From an epidemiological perspective, infertility is considered a frequent phenomenon. It is estimated that (i) infertility affects some 70–80 million couples around the world, that 15% of those living in Western countries will consult for it, and (ii) that in these more advanced societies, there is a growing group of men and women who already have at least one child but wish to have another. There is a direct relationship between certain social and lifestyle factors and fertility and infertility: age; use of tobacco, alcohol, caffeine, marijuana, cocaine, and other drugs; use of anabolics; obesity; and psychological stress.
1.2.1 Diagnosis of Infertility
1.2.1.1 When to Diagnose Infertility?
In the absence of previous indications, couples who have been trying to become pregnant for more than 1 year should begin testing and therapeutic measures. An exam should be conducted when the woman is over 35 years old or if there is a history of menstrual rhythm disturbances or suspicion of uterine, tubal, or endometriosis pathology, as well as when the male has a history of cryptorchidism or other testicular pathology.
1.2.1.2 What Is the Basic Infertility Test?
This test consists of establishing a complete clinical history, a menstrual history, a general exam, preconceptional advice, and coital counseling. The exam must be given to both members of the couple. During the infertility test, cost-effective measures should be considered, being as minimally invasive as possible and conducted in accordance with the wishes of the two partners. The causes of infertility are varied and, in many cases, are multiple. This almost always involves both members of a couple. The tests included for infertility are gynecological exam and cytology, ultrasound of the uterus and ovaries, basic seminogram, tubal X-ray (hysterosalpingography), and hormone and ovarian reserve study. In cases where another alteration is suspected and other fertility treatments have failed, other tests may be performed, such as advanced hormonal study, cervical or vaginal cultures, hysteroscopy, advanced seminogram and sperm DNA fragmentation test, and study of coagulation disorders.
1.2.1.3 Monitored Anamnesis in Reproductive Medicine
For female patients, evaluation of anamnesis in reproductive medicine considers parity, obstetrical outcomes, age at first menstruation, menstrual formula, dysmenorrhea, contraceptive methods used, number of sexual relationships, length of infertility and previous treatments, previous surgeries and illnesses, gynecological history, allergies to medication, lifestyle habits (such as use of tobacco, alcohol, drugs, diet, and frequency of exercise), occupation (and any associated stress), and family history. For male patients, anamnesis includes information about children from a previous partner (having other children does not exclude the potential for infertility), length of infertility and results from previous studies and treatments, previous surgeries and illnesses, medication allergies, lifestyle habits (such as use of tobacco, alcohol, drugs), occupation (and any associated stress), and family history.
1.2.1.4 Diagnosis of Ovarian Function and the Hypothalamic-Gonadal Axis
Evaluate ovarian function, menstrual history, basal temperature, cervical mucus, serum progesterone, urinary luteinizing hormone (LH), follicular development, endometrial thickness and appearance, and analysis of androgens, thyroid hormones, prolactin, and gonadotropins. Conduct a transvaginal ultrasound.
1.2.1.5 Study of Ovarian Function
The evaluation of ovulatory function is an important part of the basic infertility test. However, we do not have any evidence that accurately assures us that ovulation has occurred except, obviously, pregnancy. This says a lot about the variability and false-positives of many of the tests that are routinely used in the clinic. A history of regular cycles corresponds to correct ovulation in 97% of cases.
1.2.1.6 The Test of the Ovarian Reserve
Although age is the main prognostic factor of female fertility, in recent decades, motherhood has been possible for older women, which has changed our usual practice in the fertility clinic such that the analysis of the ovarian reserve has become one of the basic pillars upon which an adequate diagnosis and reproductive prognosis can be reached. Parameters for evaluating the ovarian reserve include biochemical markers such as follicle-stimulating hormone (FSH), estradiol (E2), inhibins A and B, and, more recently, the anti-Müllerian hormone (AMH). Ultrasound markers include the ovarian volume, the number of antral follicles, and the flow of the uterine artery. In addition, some dynamic tests have been designed to improve the prognosis of those using drugs commonly used in ovarian stimulation (clomiphene, exogenous FSH, or gonadotropin-releasing hormone (GnRH) analogues). These tests measure the variation of endogenous FSH, estradiol, and inhibin. Although they have been able to improve the sensitivity of the test, the increase is not sufficient to justify the expense and exposure to the established drug. AMH derives its name from its capacity to cause the regression of the conduits of Müller during masculine differentiation. In women, the AMH has a great paracrine power. The function of AMH is to inhibit the growth of nondominant follicles, with its local concentration increasing until reaching maximum levels in the antral follicles. Consequently, the measurement of AMH is a reflection of follicular activity, and as its peripheral blood values do not fluctuate as much as other hormones, it is used as an excellent ovarian reserve marker.
1.2.1.7 Indicators of Ovarian Reserve
Biochemical indicators of ovarian reserve include FSH, estradiol, inhibins A and B, testosterone, and AMH. Indicators observable by ultrasound include the number of antral follicles and the volume of the ovary. Dynamic tests may include clomiphene testing, exogenous FSH ovarian reserve testing, response of inhibin and E2 to exogen FSH, and testing the response of inhibin and E2 GnRH analogues. These various tests comprise the different ovarian reserve indicators. In summary, the most accurate are the ultrasound counts of antral follicles and the measurement of AMH; the least expensive are the ultrasound counts of antral follicles and the basal value of FSH and E2 (between the first and third day of the cycle); dynamic tests do not provide benefits compared with biochemical indicators or ultrasound tests and are expensive while remaining unable to predict the age of menopause presentation. Dynamic tests are only useful for offering a reproductive prognosis in women who plan to undergo fertility treatment.
1.2.2 Causes of Infertility
1.2.2.1 Ovarian Factors
The evaluation of ovulatory function is important as a first measure in basic infertility testing because it corresponds to 15–25% of the causes of infertility. A history of regular cycles corresponds to ovulation in 97% of the cases. A confirmed pregnancy is the only way to establish that ovulation actually occurred due to the great variability and false-positives involved with other tests. When an ovulatory dysfunction is diagnosed and a pharmacological treatment is indicated after 3–6 months without getting pregnant, another possible cause of infertility must be investigated. As a general rule, when the woman has regular cycles, ovulation is likely to be correct. When irregular cycles are presented, we can measure progesterone in the second phase or request a graph of basal temperature.
A prolactin measurement routine is not necessary unless there are menstrual abnormalities, galactorrhea, or suspected pituitary tumor. Similarly, patients with anovulation have a higher proportion of presenting with thyroid disease, but thyroid-stimulating hormone will only be measured when this disease is suspected. The assessment of the ovarian reserve is made in certain cases of patients over 35 years of age or with the intention of providing them with a prognosis or additional information to decide on possible treatments. An FSH lower than 10 mIU/mL with E2 less than 30 pg/mL reflects a normal follicular reserve status.
1.2.2.2 Uterine Factors
The cervical factor is a rare cause of infertility. The postcoital test is the classic test that determines it, but there is great interobserver variability, and it is not necessary to routinely carry this out because it has no prognostic value and is not indicative of any type of therapy. Although uterine malformations are not usually a cause of infertility, examining the uterine cavity should be part of a basic infertility test. This should be done in an individualized manner, according to other previous findings, and should be based on a transvaginal ultrasound. In case of suspicion of organic pathology (polyps, submucosal fibroids, hyperplasia), a hysterosonography or a hysteroscopy will be requested.
1.2.2.3 Tubal and Peritoneal Factor
Tubal obstruction is responsible for infertility, either as a single cause or accompanied by other causes in 30% of cases. The tubal factor should be investigated when other infertility factors have been ruled out because the test that determines it, called the hysterosalpingography (HSG), is an invasive and often painful technique. Of course, if in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is planned, HSG is not needed. For the study of tubal factor, the HSG is the least invasive and most cost-effective form, allowing diagnosis of tubal obstructions (proximal or distal) and evaluation of the uterine cavity. It is done in the first phase of the cycle before ovulation. If screening for chlamydial infection has not been done, antibiotic prophylaxis must be carried out. However, it is not precise in detecting peritubal adhesions and for diagnosing a peritoneal endometriosis, in which case it is indicated to perform a laparoscopy if there are strong suspicions of endometriosis, tuboperitoneal adhesions, or important tubal pathology.
1.2.2.4 Male Factor
The minimum evaluation of the male should include a complete medical history, a physical exam, and at least two seminograms separated 3 months from each other that should be initiated before subjecting the woman to any type of invasive exam, such as HSG. The seminogram is the main test in the study of the male factor, and abstinence is recommended for 2–3 days. The seminogram offers basic information on seminal volume, concentration, mobility, and sperm morphology. Unless the laboratory has its own criteria, it is recommended to follow the 2010 WHO guidelines.
1.2.3 Techniques for Assisting Reproduction
1.2.3.1 What Is Artificial Insemination (AI)?
Broadly speaking, AI involves the introduction of semen into the uterus, which is why it is also called intrauterine insemination. We distinguish the conjugal AI (CAI), meaning it is from the male partner, from donor AI (DAI). We have used the word “capacitated,” which is an important part of these ART. Indeed, for a sperm to acquire the ability to cross the membrane that surrounds the ovum and fertilize it, it must first undergo biochemical modifications in the most distal part of its head in a region called the acrosome. This phenomenon occurs naturally when the acrosome passes through the cervix and is imitated in the laboratory before being deposited inside the uterine cavity. Sometimes we use it as a diagnostic method known as a training test, and it helps us assess whether a patient’s semen is valid for proposing a conjugal AI.
1.2.3.2 What Are the Indications of AI?
It can be supposed that all semen qualifies for CAI. In each clinic or unit of human reproduction, there are criteria to decide if the seminogram, as the analysis is called, is normal or has some alteration and also if it is valid or not for a CAI or even IVF or ICSI. Some centers have their own criteria for defining seminograms, and although few do, they must be centers where there is a researcher who has previously published such criteria in specialized journals. For this reason, the majority of reproduction laboratories use the WHO’s criteria, which are periodically renewed. The latest revision is available online.
1.2.3.3 What Is IVF and ICSI?
When IVF or an ICSI is proposed, the two gametes (oocytes and sperm) are needed in the reproduction laboratory to perform the fertilization, which is why it is called in vitro. According to the latest data collected by the Spanish Society of Fertility, the pregnancy rate per transfer is close to 40%. These techniques require the training and accreditation of the personnel in charge (gynecologists and embryologists) and are planned in a series of steps:
-
1.
Follicular development: recruitment (rescue) and growth of one or more follicles, the structures of the ovary where the oocytes mature. Development is usually stimulated by a medication that contains gonadotropins, the natural female hormones responsible for follicular development. The process may be controlled with ultrasound and hormonal analysis.
-
2.
Obtaining the oocytes. The vagina is punctured, and the process of obtaining the oocytes is guided by ultrasound. The follicles are punctured, and their liquid content (follicular fluid) is suctioned where the oocytes supernate. Although it can be performed under local anesthesia, sedation of the patient is preferred in many centers so that the patient does not suffer pain from the puncture.
-
3.
In vitro fertilization (IVF). Fertilization itself occurs when the microinjection (ICSI) or its modern variant is performed with the extension of the microscopic vision and the selection of the sperm with better morphology (intracytoplasmic morphologically selected sperm injection).
-
4.
Embryo transfer. Once the oocytes are fertilized, the resulting embryo or embryos are transferred into the uterus in a maneuver similar to that of AI. They are usually scheduled 2–6 days after the follicular puncture, and those that are not chosen for the transfer are cryopreserved for another attempt. The criteria to decide which are transferred and which are frozen are morphological, and each reproduction center usually has its own scale to catalog its quality. The number of embryos to be transferred is controversial and generates uncertainty in patients. The transfer of more than three embryos is not allowed and for ethical reasons it is often recommended to limit this to only one, although this restricts the percentage of pregnancies.
1.2.3.4 What Is Preimplantation Genetic Diagnosis?
Preimplantation genetic diagnosis (PGD) was developed as a technique to find out the sex of embryos with a test that detects the Y chromosome in the selected embryonic cells. Evidently, the determination of sex is not the purpose of this technique, but rather its purpose is the location of genetic defects transmitted by the X chromosome. Since the 1990s then, its use has expanded to other genetic diseases. Undoubtedly, the PGD has proved to be an extraordinary step, both for the knowledge of embryonic development and for the infertility and infertility clinic.
1.3 Genetics in Assisted Reproduction
1.3.1 Genetic Counseling and Consultation of Clinical Genetics
Genetic counseling “is a communication process that deals with the human problems associated with the occurrence, or the risk of occurrence, of a genetic disorder in a family” [9]. It is based on medical, reproductive, and family history and must always be carried out. In assisted reproduction, risk assessment includes not only the embryo and the fetus but also the parent itself. In this way, it is necessary to assess the possibilities of giving birth to a child with congenital malformations or genetic diseases and even their long-term appearance (e.g., genomic imprinting diseases), but for this it is necessary to study thoroughly the causes of infertility of the couple and their genetic, personal, and family clinical history. The information must be obtained in a systematic way, and, although the autonomy of the couple prevails, in cases of doubt, the interests of the future children must be put before them. Genetic counseling is always nondirective and is an essentially medical act, although other health professionals may be involved. Today it is not possible to carry out any genetic study detached from genetic counseling, and, in turn, genetic counseling should not be carried out outside the context of medical consultation. This consultation must be conducted by a clinical geneticist and has a multiple purpose—diagnostic, prognostic, preventive, and therapeutic—since it helps to choose the ideal reproduction technique in each case. The consultation also serves the purpose of performing comprehensive genetic counseling and obtaining informed consent for any genetic measure that is to be adopted. In a structured way, the pretest objectives are as follows:
-
Obtaining the genetic medical history
-
Report and evaluation of the genetic-reproductive risks of the couple
-
Forecast report of future genetic tests
-
Evaluation of the results of genetic and complementary tests
-
Report of the reproductive genetic counsel
-
Genetic eligibility report
-
Obtaining the informed consent of genetic tests
Similarly, the posttest objectives are:
-
Interpret the results obtained with the genetic analysis carried out pre- and postimplantation (prenatal)
-
Establish embryo-fetal viability and the absence of genetic disease in the newborn
-
Validate the genetic diagnostic strategy used
-
Establish genetic counseling for future pregnancies
In assisted reproduction, pretest genetic counseling falls primarily on the couple and consists of studying the possible genetic etiology of the causes of infertility that have led to it, along with the genetic makeup of the parents. Posttest genetic counseling, in contrast, focuses on the embryo (preimplantation study techniques), fetus (prenatal study techniques), and newborn (postnatal study techniques).
As observed, the work that is carried out in the clinical genetic consultation is extensive and very specialized and is currently considered essential. The various types of genetic reports each fulfill a different but complementary function: genetic risk assessment report, pretest genetic counseling report, posttest genetic counseling report, genetic-reproductive counseling report, estimation report of future genetic tests, and suitability genetic report (in the case in which the carrying out of a preimplantation genetic study is recommended). We must not lose sight of the fact that all these reports are made at the preconception stage. If these genetic reports are not taken into account, this will lead to a situation of poor counseling of couples, a higher failure rate of reproductive techniques, an increase in the number of abortions, and possible clinical and legal repercussions due to the transmission of hereditary diseases, along with an increase in economic spending. It should also be noted that assisted reproduction units are, on many occasions, the first point of contact for a couple with an infertility consultation so, as a first step, the possible genetic etiology of infertility should be studied.
1.3.2 Genetic Diagnosis vs. Preimplantation Genetic Screening
Among the most genuinely “reproductive” genetic diagnostic techniques, there are two available procedures; these are very similar to each other but have very different indications.
The so-called preimplantation genetic test (PGT) is a procedure that consists of obtaining one or two blastomeres from an embryo in the stage of 6–8 cells (usually on the 3rd day postfertilization). The cells obtained can be studied genetically by means of different types of tests, and, depending on the outcome of the study, the decision can me made regarding whether or not to transfer the embryo of origin to the patient. The objective of the study is, of course, to avoid the transmission of a genetic disease to offspring and can be carried out from two different and complementary perspectives. If the aim is to avoid transmitting a known genetic disease, we would speak of a clearly diagnostic procedure (PGD). In contrast, the preimplantation genetic screening procedure (PGS) is a technique similar to the previous one, whereby the embryo is selected in certain population groups initially “not affected” by a known genetic disease, but with a high risk of having offspring with some type of inherited disease, usually chromosomal abnormalities. The exposed groups are usually women of advanced age, males with different degrees of oligospermia, couples with repeated failures using reproductive techniques, and couples with recurrent miscarriages and normal karyotypes.
This distinction between PGD and PGS has been losing relevance with changes in molecular diagnostic methods. In a recent review, Harper et al. (2018) have shown that the increasingly frequent use of next-generation sequencing (NGS) technology makes it unnecessary to propose a differentiated study between the various chromosomes among themselves or between the different indications of study. Using current terminology we would replace the use of the term PGT-A techniques (preimplantation genetic test of aneuploidies) with PGS, and PGT-M techniques would be replaced with the PGD (preimplantation genetic test on Mendelian diseases). The PGT-M techniques are no longer reduced to the analysis of a single locus, one or two genes, or a genomic region, but with the use of NGS techniques, it is possible to implement the study of an increasing genomic variability. Even haplotyping techniques, through analysis of CNVs (copy number variations) and SNVs (single-nucleotide variants), can be applied to almost any genetic disease [10].
The changes in the embryonic material used for the analysis favor the evolution of the preimplantation diagnosis, since frozen embryos are frequently used with various vitrification techniques, which make more time available for the study. The blastocoel fluid that can genetically represent the internal cell mass is also under investigation, while the study of the trophectoderm cells only represents the extraembryonic tissue. The possibility of studying the DNA present in the embryonic culture media (as a noninvasive source) has even been pointed out, but doubts persist as to whether these culture media are really free of any trace of human DNA or if the presence of cells of the maternal oophore cluster persists in them [11].
One question that has not yet been clarified is whether these embryo selection techniques based on chromosomal screening (PGT-A) are really useful, that is, if they have clinical utility. In this context this is measured by their ability to improve clinical outcomes in terms of embryo survival and the birth of children without chromosomal abnormalities in different types of populations (advanced maternal age, infertile, with severe male factors) and with different stages of development (cleavage, blastocyst). This will not be known until enough controlled and randomized clinical trials are published. Therefore, it is preferable to consider these PGT-A techniques as a quantitative selection (ranking tool) rather than a qualitative selection (screening tool) [12].
We have previously warned that no genetic test can be considered outside the context of genetic counseling, particularly these complex preimplantation techniques that require a thorough knowledge of the materials studied, the times in which they are made, the possible techniques to be applied, and the possible results to be obtained, along with the need to explain all of this to the couple in the form of pretest and posttest genetic counseling.
1.3.3 Epigenetic Effects of Assisted Reproduction Techniques
A question of great interest is how can assisted reproduction techniques affect in vitro the epigenome of the developing embryo. The epigenome is the set of chemical processes that affect the genetic material of a cell or organism without altering its sequence, but modifying its expression. The reprogramming moments of the epigenome represent stages that are potentially sensitive to certain effects from outside the embryo. Two stages of reprogramming are clearly distinguished: the period of formation of the reproductive cells (gametogenesis) and postfertilization embryonic development (preimplantation) [13].
Regarding gametogenesis, the mechanisms of epigenetic regulation such as methylation—which can help to stabilize the germline DNA or to silence certain genes—are poorly understood. The degree of methylation in oocytes appears to be half that observed in sperm. Another element of regulation at this stage is the control of the expression of transposons (short and mobile sequences of DNA), which could be involved in the development of some genetic diseases. During initial embryonic development, there are many changes produced by DNA methylation in the form of silencing (or not) of certain genes involved in it. The environmental agents suspected to interfere with this reprogramming are, among others, multiple ovulation [14], the composition of the culture media [15], the vibration to which the embryonic cultures are subjected, light, temperature, and the same genetic manipulation [16]. One of the possible consequences under study is the appearance of genetic diseases in relation to genomic imprinting phenomena, such as the Beckwith-Wiedemann, Prader-Willi, Angelman, and Silver-Russell syndromes. In a recent study on the REMERA (Registre des Malformations en Rhône-Alpes) registry, Uk et al. found up to three times more risk of these diseases among children born by assisted reproduction techniques than those born of natural gestations [17]. Currently, it is too early to establish an unequivocal relationship with these diseases, and the studies carried out are insufficient. The disturbing transgenerational effects that may exist cannot yet be determined until a good number of generations have elapsed. Thus, although in general it could be estimated that the risk is low, no firm conclusions can be drawn until the outcomes of future research are known [18].
1.3.4 Congenital Defects, Population Genetics, and Assisted Reproduction
Currently, it has been accepted that the use of assisted reproduction techniques involves a slight increase in the risk of developing congenital defects, as well as an increase in the genetic causes of infertility in future pregnancies. Although there is evidence for these suggestions, studies that include larger populations and specific designs are necessary to further validate them.
With regard to congenital defects, Chen et al. [19] in a recent review and meta-analysis concluded that there is a significant association between a high prevalence of congenital defects and single pregnancies achieved after the use of assisted reproduction techniques such as IVF/ICSI (both considered in absolute numbers, as for all categories of congenital defects including musculoskeletal, urogenital, circulatory, digestive anomalies, chromosomal defects, face, neck, eyes, auricular pavilions, cleft lip, cleft palate, respiratory system) except for malformations of the nervous system. These authors concluded that the risk of congenital defects is greater after the ICSI technique than after IVF. With regard to the specific case of cardiac defects, Giorgione et al. [20] also concluded after an extensive review and meta-analysis that cardiac defects are more prevalent (up to 50% more) after obtaining pregnancies with ART techniques. This leads to the recommendation of a systematic echocardiographic study of all pregnancies obtained after the use of these techniques. These results, although very indicative of the high risk of congenital defects following assisted reproduction techniques, should be considered with caution [20]. Further cohort studies and a more uniform methodology are needed in order to confirm this correlation.
Finally, the use of assisted reproduction techniques allows couples with abnormal karyotypes, mutations for cystic fibrosis, and microdeletions of the Y chromosome to achieve pregnancy and potentially transmit these genetic defects to their offspring, which represents an increased risk for the fertility of future generations. Harper et al. [10] ask “Is this a true risk?” Will it matter if, simultaneously, it improves our ability to treat infertility? Again we have to conclude that there is not yet a sufficient time perspective to evaluate these possible transgenerational modifications, but we must be vigilant to assess their possible consequences [10].
1.3.5 Regenerative Medicine
Among the potential genetic uses of assisted reproduction techniques there is regenerative medicine, which consists of curing damaged organs by introducing healthy cells. Current techniques allow human embryonic stem cells to be obtained at the blastocyst stage (5–6 days) or even earlier (3rd day). Surprisingly, we now know that completely differentiated somatic cells (such as fibroblasts) can be reprogrammed into pluripotent cells by means of the induced expression of only four key genes [21], OCT4, KLF4, SOX2, and C-MYC, which opens the door to its use as an alternative to embryonic cells, as it is devoid of other ethical and legal considerations. The utility of these non-embryonic cells is confirmed as a possible source of highly differentiated tissues or for research, generating disease models from people affected by unique mutations.
Finally, it should be noted that cultures of pluripotent cells have shown to be at risk of generating genomic instability [22] by developing alterations (trisomies of chromosomes 12 and 17 and small recurrent amplifications of chromosome 20 [23], mitochondrial mutations [24], and epigenomic changes [25]) that could be considered precursors of certain types of cancer, particularly those of germ cells, which requires a thorough study to improve the conditions of the crops.
1.3.6 Genetic Therapy and Assisted Reproduction
We will cite two examples of the possible therapeutic uses of the genetic techniques of assisted reproduction: the prevention of the transmission of mitochondrial diseases and the genomic edition.
It is well known that the mitochondria that accompany the pronuclei are exclusively maternal, which gives rise to a type of inheritance with very specific characteristics, the so-called mitochondrial inheritance. Given that this type of disease is currently incurable, solutions are sought through assisted reproduction. To avoid the transmission of known mitochondrial mutations, three types of techniques have been developed, known as mitochondrial replacement techniques (TRM): transmission of the achromatic spindle, transmission of the pronuclei, and transmission of the polar corpuscles. All of these techniques are designed to transmit the genome of an egg/zygote containing the abnormal mitochondria to an egg/zygote with normal (donated) mitochondria. An interesting review of this topic has recently been published [26].
Genomic editing is a procedure that involves the use of molecular scissors, capable of identifying a specific sequence of the genome, cutting it and inserting another DNA sequence into it to repair a damaged DNA or modify its expression. These scissors or tools are diverse, the most outstanding of which include zinc finger nucleases (ZFN), transcription activating nucleases (TALEN), and the revolutionary nucleases of reverse repeated palindromic sequences (CRISPR-Cas) [27]. There are still no safe routes of clinical application of this technique directly on the embryo [28], but nevertheless its application to germ cells—both spermatogonia and mature oocytes—is being investigated. However, the actual indications for its possible application are currently limited. Scientific societies such as the ASHG, the ESHG, and the ESHRE2 are making recommendations on this matter [29].
1.3.7 Ethical and Legal Issues
There are many ethical issues that arise when we address the genetic aspects within the field of assisted reproduction, and in this chapter, we do not intend to analyze them exhaustively although it seems convenient, at least, to list them to have a perspective of the problems that arise and the need to generate adequate responses to them. We are going to stay with genetic issues without entering into other specific ethical debates (even though these are equally necessary) linked to the use of reproductive techniques.
One of the aspects most questioned from the genetic point of view is the preimplantation diagnosis: Who can access it? There is no closed list of possible genetic conditions, although it is considered reasonable that they are diseases that significantly reduce quality of life. In Spain, the consideration of whether or not to study a disease depends on the respective Autonomic Commission of Assisted Reproduction, and each country deals with its own legislation. It is important to harmonize legislation within the European environment. A second question would be whether these types of genetic studies should be carried out for predisposing mutations to the development of common long-term diseases, including the genetic predisposition to cancer. Regarding the predisposition to cancer, there are numerous records in many European countries, although the absoluteness of this indication decreases when we refer to other common diseases such as diabetes and hypertension. Digging deeper into this idea, we could arrive at the concept of the designer baby, in which we would have excluded all possible mutations (deleterious or predisposing) in order to seek the best possible development, hand in hand with the new massive sequencing technologies (WES/WGS). This also occurs with the most commonly accepted baby medication (savior sibling) and the corresponding HLA determinations. Derived from this general approach of preimplantation study, new doubts arise as to whether it is ethical to transfer an affected embryo (in the case of not having healthy embryos) in very specific cases or about the use of preimplantation genetic screening.
There is also debate about the necessary genetic studies in gamete donors and their implications, not only referring to the future embryos thus conceived but also to the individuals who donate them.
Genetic cascade studies—offered in a general way to certain families that transmit a genetic trait—may clash with the “right to not know” principle and create a new ethical conflict.
On the other hand, there are ethical questions concerning the possible prevention of mitochondrial diseases that, due to their specific type of transmission, represent an unknown on their expression—yes or no—in offspring and the degree of intensity of mitochondrial diseases.
All these and many others are issues of relevance to which we must respond in a consensual and unitary manner. We must ensure that there are no differences in attention and interpretation in reproductive genetic medicine while also preventing the promotion of inequality, or, where appropriate, the increasing disembarkation of private companies, with their offer of the direct tests offered to the consumer (TDC) and the end of anonymity for gamete donors [30] or the even more recent cases of cross-border reproductive care (transboundary reproductive care) [31].
References
Liu XJ. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res. 2016;363(1):57–68.
Wilding M. Potential long-term risks associated with maternal aging (the role of the mitochondria). Fertil Steril. 2015;103(6):1397–401.
Yoldemir T. Fertility in midlife women. Climacteric. 2016;19(3):240–6.
Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103(5):1136–43.
Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–7.
Braverman AM. Old, older and too old: age limits for medically assisted fatherhood? Fertil Steril. 2017;107(2):329–33.
MacArthur T, Bachmann G, Ayers C. Menopausal women requesting egg/embryo donation: examining health screening guidelines for assisted reproductive technology. Menopause. 2016;23(7):799–802.
van den Akker O. A review of family donor constructs: current research and future directions. Hum Reprod Update. 2006;12(2):91–101.
American Society of Human Genetics. Genetic counseling. Am J Hum Genet. 1975;27:240–2.
Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S, Macek M Jr. Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur J Hum Genet. 2018;26:12–33. https://doi.org/10.1038/s41431-017-0016-z.
Kuznyetsov V, Madjunkova S, Antes R, Abramov R, Motamedi G, Ibarrientos Z, et al. Evaluation of a novel non-invasive preimplantation genetic screening approach. PLoS One. 2018;13(5):e0197262.
Maxwell SM, Colls P, Hodes-Wertz B, et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil Steril. 2016;106:1414–19.e5.
Hoeijmakers L, Kempe H, Verschure PJ. Epigenetic imprinting during assisted reproductive technologies: the effect of temporal and cumulative fluctuations in methionine cycling on the DNA methylation state: susceptibility to epigenetic defects in art. Mol Reprod Dev. 2016;83:94–107.
Marshall KL, Rivera RM. The effects of superovulation and reproductive aging on the epigenome of the oocyte and embryo. Mol Reprod Dev. 2018;85:90–105. https://doi.org/10.1002/mrd.22951
Koscinski I, Merten M, Kazdar N, Guéant J-L. Culture conditions for gametes and embryos: which culture medium? Which impact on newborn? Gynécol Obstét Fertil Sénol. 2018;46:474–80.
Ventura-Juncá P, Irarrázaval I, Rolle AJ, Gutiérrez JI, Moreno RD, Santos MJ. In vitro fertilization (IVF) in mammals: epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol Res. 2015;48:68. https://doi.org/10.1186/s40659-015-0059-y.
Uk A, Collardeau-Frachon S, Quentin Scanvion LM, Amarf E. Assisted reproductive technologies and imprinting disorders: results of a study from a French congenital malformations registry. Eur J Med Genet. 2018;61(9):518–23. https://doi.org/10.1016/j.ejmg.2018.05.017.
Miranda RC, Salem NA, Fincher AS, Mahnke AH, Burrowes SG. Epigenetic mechanisms and inheritance of acquired susceptibility to disease. In: Medical epigenetics; 2016. p. 531–52. https://doi.org/10.1016/B978-0-12-803239-8.00030-2.
Letao Chen B, Yang T, Zheng Z, Hong Y, Wang H, Qin J. Birth prevalence of congenital malformations in singleton pregnancies resulting from in vitro fertilization/intracytoplasmic sperm injection worldwide: a systematic review and meta-analysis. Arch Gynecol Obstet. 2018;297:1115–30. https://doi.org/10.1007/s00404-018-4712-x.
Giorgione V, Parazzini F, Fesslova V, Cipriani S, Candiani M, Inversetti A, Sigismondi C, Tiberio F, Cavoretto P. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:33–42. https://doi.org/10.1002/uog.18932.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Pasi CE, Dereli-O A, Negrini S, Friedli M, Fragola G, Lombardo A, Van Houwe G, Naldini L, Casola S, Testa G, Trono D, Pelicci PG, Halazonetis TD. Genomic instability in induced stem cells. Cell Death Differ. 2011;18:745–53.
Spits C, Mateizel I, Geens M, et al. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol. 2008;26:1361–3.
Van Haute L, Spits C, Geens M, Seneca S, Sermon K. Human embryonic stem cells commonly display large mitochondrial DNA deletions. Nat Biotechnol. 2013;31:20–3.
Amps K, Andrews PW, Anyfantis G, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Greenfield A, Braude P, Flinter F, Lovell-Badge R, Ogilvie C, Perry ACF. Assisted reproductive technologies to prevent human mitochondrial disease transmission. Nat Biotechnol. 2017;35:1059–68. https://doi.org/10.1038/nbt.3997.
Mojica FJ, Montoliu L. On the origin of CRISPR-Cas technology: from prokaryotes to mammals. Trends Microbiol. 2016;24(10):811–20. https://doi.org/10.1016/j.tim.2016.06.005.
Ma H, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–9. https://doi.org/10.1038/nature23305.
De Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG FF, Goddijn M, Howard HC, Radojkovic D, Rial-Sebbag E, Dondorp W, Tarlatzis BC, Cornel MC. Responsible innovation in human germline gene editing. Background document to the recommendations of ESHG and ESHRE. Eur J Hum Genet. 2018;26(4):450–70. https://doi.org/10.1038/s41431-017-0077-z.
Harper JC, Kennett D, Reisel D. The end of donor anonymity: how genetic testing is likely to drive anonymous gamete donation out of business. Hum Reprod. 2016;31:1135–40.
Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P, Westphal LM, Inhorn MC, Patrizio P. Cross border reproductive care (CBRC): a growing global phenomenon with multidimensional implications (a systematic and critical review). J Assist Reprod Genet. 2018;35(7):1277–88. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mendoza Ladrón de Guevara, N., Motos Guirao, M.A. (2019). Assisted Reproductive Technology in Perimenopausal Women. In: Pérez-López, F. (eds) Postmenopausal Diseases and Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-13936-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-13936-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13935-3
Online ISBN: 978-3-030-13936-0
eBook Packages: MedicineMedicine (R0)