Abstract
The family history is the cornerstone of the genetic risk assessment. Taking a detailed family history helps ensure that important genetic information is not overlooked and that any appropriate testing and/or information is provided to the patient prior to pregnancy. Guidelines from the American Society for Reproductive Medicine (ASRM) suggest a review of personal and family history of genetic disease and prior genetic test results that may affect the course of treatment, with patients being counseled about additional genetic testing that may be indicated before starting treatment relating to their personal or family history. When issues arise as a result of this evaluation, referral to a genetics specialist is recommended. As the following cases demonstrate, implementation of a routine genetic counseling screening program for all patients using assisted reproductive technology (ART) provides immense benefits so that important indications for referral to a genetic counselor are not missed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The family history is the cornerstone of the genetic risk assessment. Taking a detailed family history helps ensure that important genetic information is not overlooked and that any appropriate testing and/or information is provided to the patient prior to pregnancy. Genetic counselors are professionals trained to collect and analyze detailed family history information for genetic disorders.
Guidelines from the Practice Committee of American Society for Reproductive Medicine (ASRM) [1, 2] suggest a thorough evaluation including a physical exam, laboratory testing, review of personal and family history of genetic disease, as well as prior genetic test results (e.g., chromosome analysis, carrier screening) that could affect the course of treatment. The guidelines further state that patients should be counseled about additional genetic testing that may be indicated before starting treatment relating to their personal or family history. When issues arise as a result of this evaluation, only then is referral to a genetics specialist recommended. Despite the growing use of genetic counseling related to genetic testing in the IVF setting, it is still not routine for clinics to utilize the genetic counselor’s skills for family history assessment for couples pre-IVF.
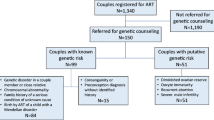
The value of routine family history risk assessment by a genetic counselor has been demonstrated in a study of almost 700 couples undergoing IVF at one fertility center, comparing questionnaire results with family history obtained by a genetic counselor [3]. Similar findings occurred when evaluating family histories of over 700 ovum donors from a single agency [4]. A study utilizing genetic counselors to perform chart review for the purpose of identifying oncology patients who would benefit from a genetics evaluation showed an increase of 129 patients over 10 months which was significant [5].
As the following cases demonstrate, implementation of a routine genetic counseling screening program for patients using assisted reproductive technology (ART) provides immense benefits, rather than relying on less rigorous methods of family history collection, so that important indications for referral to a genetic counselor are not missed.
Case 1
Couple referred for routine genetic counseling before in vitro fertilization (IVF). Upon family history review, the male partner revealed that his brother has polycystic kidney disease (PKD). His mother has renal failure due to PKD, diagnosed in her 50s. His maternal uncle died of PKD. The male partner had not had a renal ultrasound. There has been no genetic testing done in the family.
ADPKD is a relatively common autosomal dominant disease, affecting about 1/500–1/1000 people. In rare cases, renal cysts may be apparent in fetal life but more typically first develop in adolescence or early adulthood.
Genetic testing is best performed on an affected individual; there is more than one causative gene and not all affected individuals will have an identifiable mutation. The recommended strategy was to either offer genetic testing to the patient’s affected brother or for the patient to have a renal ultrasound which if positive would be diagnostic. He chose to have a renal ultrasound, which revealed numerous cysts in both kidneys. Genetic testing can now be offered to the male partner since he is affected. PGT-M is available if a mutation is found. Risk to his offspring is 50%—the couple is interested in selecting out affected embryos. This information was not in the patient family history questionnaire and would have been missed. It is not uncommon for patients with genetic diseases in their family to be unaware of the availability of genetic testing either for themselves or for their embryos.
Case 2
Couple referred for genetic counseling before in vitro fertilization (IVF). Female partner reports during routine family history evaluation that her mother had died of a cerebrovascular accident at age 50. Upon further inquiry, she mentioned that her mother had hypertension. This history prompted a direct question about whether her mother had autosomal dominant polycystic kidney disease (since her mother died young and hypertension is associated with PKD), which she confirmed. This information would have been missed unless a specific question was asked because the patient did not mention the specific underlying diagnosis. Genetic counselors evaluate family history for unusual occurrences and have genetic diseases on their radar as a potential cause. The patient has not had a renal ultrasound, which was offered. If she were identified as affected, this information would inform her personal healthcare, possibly her IVF treatment course, and the couple’s options for screening their embryos.
Case 3
Couple referred for preconception counseling to explore options for genetic testing since male partner’s father was diagnosed with retinitis pigmentosa (RP) in the 1970s at age 42. No medical records were available to confirm the diagnosis. Family history assessment revealed that the male partner’s father also has congenital hearing loss; he has not had genetic testing. The male partner had an ophthalmology evaluation for RP at age 42 that was negative. He has not had an audiology exam.
RP is a group of inherited disorders in which abnormalities of the photoreceptors or the retinal pigment epithelium of the retina lead to progressive visual loss. Patients first experience defective dark adaptation or “night blindness,” followed by constriction of the peripheral visual field, and, eventually, loss of central vision late in the course of the disease.
At least 35 different genes or loci are known to cause non-syndromic RP.
Since the affected father has both RP and congenital hearing loss, it was suspected that he had Usher syndrome, characterized by congenital sensorineural hearing loss and RP.
Genetic testing was arranged for the affected father and he tested positive for a mutation for Usher syndrome type 2. This is an autosomal recessive condition. The male partner is thus an obligate carrier, and testing was done to confirm carrier status. The female partner is currently being tested. If she is a carrier, the risk to their offspring for Usher would be 25%. PGT-M could be offered, which the couple was interested in pursuing to avoid an affected offspring.
If the family history had not been evaluated, the congenital hearing loss would have been missed which was the tipoff to ordering testing for Usher instead of RP. An additional important point is that testing the affected individual for the relevant gene/mutation is imperative as illustrated in this case. If testing for RP was ordered for the male partner (and was negative), he would have been tested for the incorrect gene.
Case 4
Couple referred for genetic counseling to discuss results of genetic carrier screening. Both partners were identified as carriers for a mild variant for alpha-1 antitrypsin deficiency, an autosomal recessive condition associated with lung and liver disease. Routine assessment of the family history revealed that the male partner’s mother has neurofibromatosis, type 1 (NF1). NF1 is characterized by multiple café au lait spots, axillary and inguinal freckling, dermal neurofibromas, and Lisch nodules. Learning disabilities are frequent. Less common but potentially more serious manifestations include plexiform neurofibromas, optic and other central nervous system gliomas, malignant peripheral nerve sheath tumors, osseous lesions, and vasculopathy. NF1 is an autosomal dominant condition with 50% risk to offspring of affected persons. The gene is nearly 100% penetrant; however, the phenotype is variable. NF1 is one of the more common genetic diseases, with a prevalence of approximately 1 in 3000.
Further discussion of the family history reveled that the male partner has “bumps on his skin” and has had an MRI revealing plexiform neurofibromas. The physician who ordered the MRI was aware of the family history of NF1 but did not refer him to a geneticist or ophthalmologist nor did he diagnose him with NF1 (which he likely has). The patient is interested in confirming whether he has NF1. Part of the genetic counseling process is to help patients make informed decisions about whether they are interested in genetic testing and how resulting information may be useful in the context of their lives or reproductive decision-making. It is important to note that not all affected patients are interested in genetic testing.
Patient was referred for a clinical genetics evaluation (which includes physical exam with ancillary referrals to specialists such as ophthalmology, further CT or MRI scans) to obtain a diagnosis. If a diagnosis is made, genetic testing can be offered. If a mutation is identified, PGT-M can be offered. Risk is 50% to his offspring if he is affected. Although the referral of this couple was to discuss carrier screening, had the family history not been taken, this important information would have been missed.
Conclusion
Genetic conditions are relatively rare individually, but it is not uncommon to identify genetic conditions during routine family history evaluation. It is estimated that over 10,000 human diseases are monogenic, or caused by a single gene. The global prevalence of monogenic diseases is estimated as 10/1000 [6]. Genetic counselors are well versed with features of genetic conditions and patterns in families, allowing targeted questions to be asked of the patient during family history discussion. The family history is an important basic component of a pre-IVF screening protocol, and genetic counselors are poised to collect the most comprehensive history from patients and guide the clinician on appropriate work-up when genetic conditions are identified in a family. As with any screening program, it is best applied to all patients in the clinic in order to capture all relevant cases.
Given the number of genetic issues that may arise in a family history alone, as well as the broader fertility setting (e.g., genetic carrier screening, PGT and mosaic embryo discussion, male and female fertility issues, chromosome translocations and other abnormalities, gamete donor family history evaluation), the expertise of a genetic counselor is particularly beneficial for ART clinics. Incorporating genetic counselors into the ART clinic to routinely assess patients’ family history further ensures that genetic conditions that could affect the health of the patient or the treatment plan are not missed and that indicated genetic testing based on the family history is offered to patients.
References
Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44–50. https://doi.org/10.1016/j.fertnstert.2015.03.019 Epub 2015 Apr 30.
Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18–25. https://doi.org/10.1016/j.fertnstert.2014.12.103 Epub 2015 Jan 15.
Vance A, Zouves C. The importance of family history risk assessment in the infertility setting. Fertil Steril. 2005;84(Supplement 1):S125.
Vance A. Family history risk assessment: data for 723 consecutive ovum donors from a single agency demonstrates the value of the genetics consult. Fertil Steril. 2011;96(3):S218.
J Genet Couns. 2014;23(3):323–9. https://doi.org/10.1007/s10897-013-9664-5 Epub 2013 Oct 25.PMID: 24155015.
Genes and human diseases. eWHO.int/genomics/public/geneticdiseases.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vance, A. Family history risk assessment by a genetic counselor is a critical step in screening all patients in the ART clinic. J Assist Reprod Genet 37, 2279–2281 (2020). https://doi.org/10.1007/s10815-020-01870-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01870-y