Abstract
Reproductive aging is an increasingly pressing problem facing women in modern society, due to delay in child bearing. According to Statistics Canada, 52% of all Canadian births in 2011 were by women aged 30 years and older, up from 24% in 1981 (http://www.statcan.gc.ca/pub/91-209-x/2013001/article/11784-eng.htm). Women older than 35 years of age experience significantly increased risks of infertility, miscarriage and congenital birth defects, mostly due to poor quality of the eggs. Increasingly sophisticated, and often invasive, assisted reproductive technologies (ARTs) have helped millions of women to achieve reproductive success. However, by and large, ARTs do not address the fundamental issue of reproductive aging in women: age-related decline in egg quality. More importantly, ARTs are not, and will never be, the main solution for the general population. Here, I attempt to review the scientific literature on age-related egg quality decline, based mostly on studies in mice and in humans. Emphasis is given to the brief period of time called oocyte maturation, which occurs just prior to ovulation. The rationale for this emphasis is that oocyte maturation represents a critical window where unfavorable ovarian conditions in older females contribute significantly to the decline of egg quality, and that science-based intervention during oocyte maturation represents the best chance of improving egg quality in older women. Finally, I summarize our own work in recent years on peri-ovulatory putrescine supplementation as a possible remedy for reproductive aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women experience increasing reproductive difficulties starting in their late 30s, including infertility, increased risks of spontaneous abortion and congenital birth defects. Decreasing quantity and, especially, poor quality of eggs are the leading cause of these premenopausal reproductive problems. These woman-specific reproductive aging problems stem from the protracted oogenesis in mammalian females: primordial oocytes are generated at the fetal stage but mature to fertilizable eggs at ovulation. The time lapse of 10–50 years in the making of a mature human egg appears to be an insurmountable challenge to the age-related decline of egg quality. Apart from long-term egg freezing and egg donation, there are no scientifically proven remedies for these problems. Furthermore, both egg freezing and egg donation have their own technical and ethical difficulties, in addition to their limited applicability (i.e., only in expensive IVF procedures). In this review, I focus on the process of oocyte maturation, a brief time during which the oocyte undergoes dramatic changes, expelling exactly half of the chromosomes and reorganizing other cellular components, to get ready to be united with a haploid sperm to start fetal development. I hypothesize that a significant proportion, if not the majority, of the aneuploidy and poor egg quality is due to unfavorable oocyte maturation in aged oocytes. Furthermore, it is possible, through dietary or medical means, to restore a robust oocyte maturation process in older women, to reduce aneuploidy and improve the quality of the mature eggs leading to better reproductive outcome.
Oogenesis in mammals including humans
In mammals, oogenesis begins during the embryonic stage when primordial germ cells initiate meiosis and develop into primary oocytes (Baltus et al. 2006). By birth, females have developed a finite number of primary oocytes arrested in meiotic prophase that comprise their lifetime egg supply (Lei and Spradling 2013; Zhang et al. 2014). The prophase oocytes contain replicated sister chromatids bound together lengthwise by a ring-shaped protein complex called cohesin, similar to prophase in somatic cells. However, in primary oocytes, all four sister chromatids, two maternal and two paternal, are linked together. This happens because, in germ cells, the duplicated maternal and paternal chromosomes synapse and undergo one or more homologous recombination events (aka crossover) between non-sister chromatids, resulting in homolog interlocking (Fig. 1, top). The four sisters remain linked through postnatal development while supporting transcription activity required for oocyte growth and the accompanying follicle development. In sexually mature females, cohorts of their follicles, each containing a prophase oocyte, are recruited to enter the ovulation cycle throughout the reproductive life. Prior to ovulation, the fully grown oocyte, still in prophase with an intact nucleus and with its four sisters of each chromosome linked, undergoes oocyte maturation (meiosis I): chromosome condensation into bivalents (Fig. 1, top), nuclear envelop breakdown (aka germinal vesicle breakdown or GVBD), spindle assembly and expelling of one homolog (two sisters) of each chromosome to the first polar body. Subsequently, in fertilization (meiosis II), the sisters are segregated into the 2nd polar body and the fertilized egg (Fig. 1).
Two unique and critical features of meiosis ensure the differential segregation of homologous chromosomes in meiosis I and sister chromatids in meiosis II. The first is sister kinetochore co-orientation during assembly of the metaphase I spindle. In yeast, this is achieved by a protein complex dubbed monopolin (Corbett et al. 2010), which promotes fusion of the two sister kinetochores (Sarangapani et al. 2014), although the equivalent protein complex in animal cells has not yet been identified (McCollum 2012). The second is the stepwise removal of chromosome cohesin. In meiosis I, cohesin on chromosome arms is cleaved by a protease, separase but cohesin near the centromeres is protected, thus “unlocking” chromosome bivalents allowing chromosome homologs to separate while keeping sisters together (Fig. 1). Protection of centromeric cohesin in meiosis I requires a kinetochore protein called Shugoshin (Kitajima et al. 2004; Rabitsch et al. 2004), which appears to recruit protein phosphatase 2A to protect centromeric cohesin (specifically its Rec8 subunit) against the action of separase (Riedel et al. 2006). Cohesin near the centromeres is cleaved by separase in meiosis II to allow sister chromatid separation (Kudo et al. 2006; Lee et al. 2006).
Egg aneuploidy rates of humans and of mice
Aneuploidy is defined as a cellular condition where its chromosome number is not the exact multiple of the monoploid (haploid) of that organism. Specifically, it refers to the gain or loss of the entire chromosome(s) due to chromosome segregation errors during anaphase of the cell division cycle. Other common chromosome errors such as triploidy (3 sets of monoploids) or chromosome translocation are not aneuploidy. This distinction is important because chromosome aneuploidy has a unique etiology (anaphase errors) and is associated with maternal aging. On the other hand, the majority of triploidies appear to be due to paternal factors, especially dispermy (fertilized by two sperms), in humans (Kajii and Niikawa 1977; Zaragoza et al. 2000) and in mice (Maudlin and Fraser 1977). Chromosome translocation errors are caused by chromosome breakage and rejoining and such errors, unlike aneuploidies and triploidies, are heritable.
Chromosome segregation errors are typically caused by non-disjunction, failure of sister chromatids to disjoin in (mitotic) anaphase. When applied to segregation errors in meiosis, meiosis I non-disjunction results in gain or loss of a whole chromosome (both sister chromatids) and meiosis II non-disjunction results in gain or loss of a sister chromatid. Decades of genetic studies have indicated that the vast majority of human aneuploidy has a maternal origin (egg aneuploidy) (Hassold and Hunt 2001; Nagaoka et al. 2012). A trisomic (3 copies of a single chromosome) offspring with two maternal chromosomes that retain (grand)parental heterozygosity is considered a meiosis I error (homologous chromosome non-disjunction). A trisomic offspring with two maternal chromosomes of identical sequence is considered a meiosis II error (sister chromatids non-disjunction). However, it is now clear that the majority of meiotic chromosome segregation errors are premature separation of sister chromatids (PSSC) in meiosis I (Fig. 1) (Angell 1991; Tao and Liu 2013; Kuliev et al. 2011; Gabriel et al. 2011; Yun et al. 2014; Christopikou et al. 2013; Fragouli et al. 2013; Handyside et al. 2012; Liu and Tao 2012), making this distinction problematic. The aforementioned offspring with two maternal chromosomes of identical sequence (i.e., “meiosis II error”) could be a meiosis I error in which the sister chromatids prematurely separated (due to the loss of centromeric cohesin) but are both retained in the mature egg, likely because the two sister kinetochores are attached to the same pole (Fig. 1; PSSC 1). The lack of sister cohesin renders the random segregation of the two sisters in meiosis II (in this case, being retained in the fertilized egg).
Accurate assessment of aneuploidy rates in the general population is not possible due to ethical conditions. Earlier studies involved metaphase chromosome spread (Evans 1987) of surplus human eggs and invariably had small sample sizes. This technique suffers from many deficiencies including procedural loss of chromosomes and inadequate chromosome spreading. As summarized by Pellestor et al. (2005), the reported aneuploidy rates for human eggs have a mean of 35.9 %, with significant variations among the individual studies. With the availability of biopsied polar bodies, some investigators have employed fluorescence in situ hybridization (FISH) using chromosome-specific probes to assess chromosome copy number. These studies generally reported very high aneuploidy rates in human eggs (32.1–52.1 %), despite the small number of chromosomes (3–5) that were examined (Pellestor et al. 2006). Most notable is a study of more than 20,000 human eggs (via FISH analyses of five chromosomes, 13, 16, 18, 21 and 22, most commonly featured in human trisomies at birth or spontaneously aborted fetuses) reporting an overall 48.6 % aneuploidy rates (average donor age of 38.8 years) and clear maternal age association with higher aneuploidy rates in older donors (Kuliev et al. 2011). An extrapolation of this number to all 23 chromosomes would suggest that egg aneuploidy rates in these IVF patients must be extraordinarily high.
Complete and reliable chromosome copy number can now be readily analyzed by an increasing array of single cell (polar bodies, eggs and blastomeres) genomic methods, microarray-based comparative genomic hybridization (aCGH) (Fishel et al. 2010), quantitative SNP (single nucleotide polymorphism) arrays (Northrop et al. 2010) and next generation sequencing (Baslan et al. 2012; Hou et al. 2013). Several studies using aCGH have been published (Gabriel et al. 2011; Fragouli et al. 2011, 2013; Geraedts et al. 2011; Handyside et al. 2012) and provide the most accurate view of human egg aneuploidy, at least for IVF patients. The results of these recent studies are remarkably consistent among themselves. In three relatively large studies with IVF patients (average age between 40.0 and 40.8 years), the overall aneuploidy rates of combined meiosis I and meiosis II (both 1st and 2nd polar bodies examined) were 70 % (Fragouli et al. 2011), 72 % (Geraedts et al. 2011) and 74 % (Fragouli et al. 2013), respectively. If only the first polar bodies are examined, 40 % (Fragouli et al. 2011), 58 % (Geraedts et al. 2011), 57 % (Fragouli et al. 2013) and 52.4 % (Gabriel et al. 2011) were found to be aneuploid. Many of these samples contain errors in both the 1st and 2nd polar bodies and many contain complex aneuploidies (with more than one chromosome error).The accuracy of this approach is further validated by the excellent concordance when the corresponding embryos are karyotyped (Geraedts et al. 2011; Christopikou et al. 2013). It is also clear from these latest studies that older donor ages are associated with higher egg aneuploidy rates and higher proportions that are complex aneuploidies.
Efforts in karyotyping mouse eggs appear to lag behind those in humans and are restricted to cytological analyses. It is generally agreed that egg aneuploidy rates in young mice are very low (~1 %), meaning at least an order of magnitude lower than that in young women. However, the reported egg aneuploidy rates for older mice are quite variable. Golbus (1981) reported 2–3 % hyperploid eggs (hypoploidy is excluded because of technical loss of chromosomes) in 12- to 15-month-old Swiss-Wester random bred or CBA inbred mice. Koehler et al. (2006) reported a similarly low (2.6 %) aneuploidy rate in 8- to 11-month-old C57BL/6 mice. On the other hand, others have reported much higher egg aneuploidy rates in old mice, although generally with a relatively small number of eggs analyzed (less than 100 per group). For example, Pan et al. (2008) reported a 25 % egg hyperploidy rate in 17-month-old D6D2F1 mice. Selesniemi et al. (2011) reported a 30 % aneuploidy rate in 12-month-old C57BL/6 mice, with the majority being hyperploids. One unique technical challenge in counting the telocentric mouse chromosomes in the eggs is the difficulty in discerning a small chromosome dyad from a single sister chromatid. To overcome this, we included additional staining of the centromeres (Hodges and Hunt 2002) in our study and reported an egg aneuploidy rate of 12.7 % in 8-month-old C57BL/6 mice, with the vast majority being PSSC and very few being whole chromosome nondisjunction (1.4 %) (Tao and Liu 2013). Merriman et al. (2012), using a novel karyotyping technique via in situ centromere counting of fixed intact eggs (Chiang et al. 2010), reported aneuploidy rates of 37.5 % (n = 40) and 60 % (n = 30) for 12- and 15-month-old CD1 females, respectively.
Egg aneuploidy, causes and maternal age association
Decades of genetic studies of human trisomic offspring (or aborted fetuses) and their parents have yielded a great deal of information on the origin and maternal age association of human aneuploidies (Hassold and Hunt 2001; Nagaoka et al. 2012). A two-hit hypothesis (Lamb et al. 1996; Orr-Weaver 1996; Koehler et al. 1996) seems to best summarize these findings. It is hypothesized that a prenatal crossover anomaly represents the first hit, predisposing the affected chromosomes to a greater risk of mis-segregation in meiosis I and/or meiosis II. The eventual manifestation of aneuploidy, however, requires a second hit, under conditions that render the vulnerable chromosomes to actually mis-segregate during meiosis. Three types of vulnerable chromosomes have been recognized: achiasmate chromosomes, chromosomes with a single crossover near the telomere and chromosomes with a crossover near the centromere. While all chromosomes in human spermatocytes have at least one crossover (Lynn et al. 2002), both genetic (MacDonald et al. 1994; Fisher et al. 1995; Bugge et al. 1998; Oliver et al. 2008) and cytological (Cheng et al. 2009) studies have indicated that significant numbers of chromosomes in human oocytes are achiasmate. As much as 25 % of human fetal oocytes contain at least one achiasmate chromosome, as determined by staining pachytene oocytes with antibodies against the DNA mismatch repair protein MLH1 (Cheng et al. 2009). Achiasmate chromosomes in mammals are expected to randomly segregate in meiosis I and this has been implicated as an important mechanism for human trisomy 18, trisomy 21 and aneuploidy involving the X chromosome (Nagaoka et al. 2012). In addition to achiasmate chromosomes, a single crossover near the telomere and a crossover near the centromere also predispose the affected chromosomes to mis-segregation. In general, achiasmate or a single crossover near the telomere predisposes the affected chromosomes to meiosis I errors. A crossover near the centromere predisposes the chromosome to meiosis II errors. Most importantly, all three types of abnormality exhibit a greater risk of mis-segregation with increasing maternal age (Nagaoka et al. 2012).
Maternal aging is indisputably linked to increased aneuploidies in humans and in mice. The reason behind the maternal aging effect (or the mechanisms of the second hit), however, is far from clear.
It is thought that the amount of cohesin, which is established during chromosome replication and which appears to be non-replenishable in the prolonged prophase arrest (Revenkova et al. 2010; Tachibana-Konwalski et al. 2010), progressively decreases through aging, therefore rendering oocytes more susceptible to nondisjunction in older females. This is a particularly attractive notion for chromosomes with a single crossover near the telomere, since the loss of cohesin distal to the crossover would render the chromosome practically “achiasmate”. Indeed, it has been demonstrated in both mice (Lister et al. 2010; Chiang et al. 2010; Liu and Keefe 2008) and in humans (Tsutsumi et al. 2014; Duncan et al. 2012) that maternal aging is associated with reduced levels of chromosome cohesin but curiously not overall cohesin in the oocyte cytoplasm (it is not clear why oocytes retain cytoplasmic cohesin if not for replenishing chromosome cohesin). However, despite an almost complete loss of biochemically detectable chromosome cohesin (Rec8) in aged mouse oocytes (Chiang et al. 2010; Liu and Keefe 2008), chromosomes in old metaphase I oocytes remain invariably in bivalent configuration (Lister et al. 2010; Chiang et al. 2010). Therefore, age-related loss of chromosome cohesin alone is not sufficient to disrupt the chromosome linkage established in fetal stages. It is intriguing that as many as 25 % of oocytes in human fetuses have at least one achiasmate chromosome (Cheng et al. 2009). If, as expected, these achiasmate chromosomes randomly segregate in meiosis I, more than 10 % of all human eggs (regardless of age) should gain or lose at least one whole chromosome. This is clearly inconsistent with clinical observations, which suggest that only ~5 % of human aneuploidy (not 5 % of eggs!) is whole chromosome non-disjunction in meiosis I (Angell 1991; Kuliev et al. 2011; Gabriel et al. 2011; Handyside et al. 2012). Therefore, it is possible that MLH1 foci underestimate human oocyte recombination rates (Cheng et al. 2009), or that nature is more complex than our current simplistic interpretation.
As for the majority of age-related aneuploidy, PSSC in meiosis I, the best explanation might be the combined (partial) loss of chromosome cohesin and Shugoshins (Lister et al. 2010; Dupont et al. 2012; Yun et al. 2014). Therefore, instead of selective removal of cohesin on chromosome arms allowing separation of chromosome homologs, cohesin in vulnerable chromosomes is completely removed in anaphase I (Fig. 1) (Liu and Tao 2012). Without centromeric cohesin, the two sisters can co-segregate if they are attached to the same pole (PSSC 1, aka “balanced predivision”; Angell 1997; Dailey et al. 1996), or prematurely segregated into the first polar body and the mature egg (PSSC 2). These prematurely separated sisters in the mature eggs, either present singly (PSSC 2) or in pairs (PSSC 1), will randomly segregate in meiosis II.
Aside from the loss of cohesin and Shugoshins, the most obvious factor that might influence mis-segregation errors is the fitness of the meiotic spindle. There are numerous studies that indicate age-associated abnormality in spindle morphology in mice (Eichenlaub-Ritter et al. 2004). The scarcity of human eggs does not permit large-scale analyses of spindle morphology by invasive immunofluorescence microscopy. Nonetheless, an early study of human oocytes indicated that the majority of eggs (10/12) from young women (20–25 years) had normal MII spindle with all chromosomes aligned at the metaphase plate. In contrast, the majority of eggs (11/14) from older women (40–45 years) had abnormal spindle and misaligned chromosomes (Battaglia et al. 1996). More recently, many investigators have taken advantage of non-invasive polarization microscopy (PolScope) to visualize spindles in human eggs. These studies generally concluded that eggs from older patients have spindles with lower light retardance (indicative of lower density and fewer ordered microtubule fibers and hence less normal spindles) (Shen et al. 2006; Rama Raju et al. 2007; De et al. 2005). Others, however, have questioned the reliability of these PolScope measurements, because many abnormal spindles have high microtubule density and exhibit high light retardance (Coticchio et al. 2010). Clearly, defects in spindle function will affect chromosome segregation. This is particularly true in animal oocytes where the quality control mechanism, spindle assembly checkpoint, is either entirely absent (Shao et al. 2013) or does not provide strict surveillance on chromosome misalignment (Nagaoka et al. 2011; Duncan et al. 2009). The question, though, is why do aged oocytes make inferior spindles?
Egg quality is not just chromosomal
Analyses of spontaneous abortions in humans have indicated an overall 35 % (30–40 %) being chromosomal abnormal. The majority (~2/3) of chromosome anomaly are trisomies (Hassold and Hunt 2001; Carp et al. 2001; Kroon et al. 2010). Autosomal monosomies are rarely found among spontaneously aborted human fetuses, suggesting that they likely die before or shortly after implantation. Indeed, earlier experiments in mice have indicated that autosomal monosomies die much earlier (E3–E4) than trisomies of the corresponding chromosomes (≥E10) (Epstein and Travis 1979). Nonetheless, the majority (~65 %) of human spontaneous abortuses are euploid, which die of causes other than these gross chromosomal abnormalities. Although these studies do not identify single gene mutations or small chromosomal deletions, it is safe to conclude that a significant proportion of these deaths are due to poor egg quality not related to egg aneuploidy. In addition, a large proportion of human conceptions, particularly of older women, are lost before clinical recognition of pregnancy due to aneuploidy (particularly monosomies and multiple chromosomal errors) and poor egg quality.
It appears that this is also true, if not more so, in mice. For example, we reported a 12.7 % of egg aneuploidy rate in 8-month-old C57BL/6 mice (Tao and Liu 2013) and yet the fetal death rate of similar aged C57BL/6 mice is as high as 60 % (Tao et al. 2015). We karyotyped these dead fetuses and found that the vast majority are euploid (Tao and Liu, unpublished). Clearly, aging-related decline of egg quality and developmental potential is more than just egg aneuploidy. What are these other factors that impact the developmental potential of the eggs?
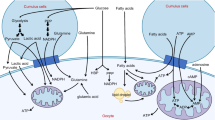
A fully grown mouse oocyte contains about 100,000 mitochondria (Piko and Taylor 1987). Although the number does not increase during oocyte maturation, mitochondria translocate to the peri-nuclear area and form aggregates (Dumollard et al. 2006). This translocation/remodeling is correlated with distinct bursts of ATP production (Yu et al. 2010). It has been hypothesized by many that compartmentalized ATP production may serve a specific energetic need (e.g., spindle formation) while minimizing the production of reactive oxygen species (ROS) associated with mitochondrial respiration (Eichenlaub-Ritter et al. 2011; Dumollard et al. 2004; Van Blerkom 2011). Although mitochondria produce most of the cellular ROS, they also play a vital role in regulating cellular redox balance (Dumollard et al. 2009). Mammalian oocytes synthesize millimolar quantities of the antioxidant glutathione, an ATP-dependent process involving substrates provided by the surrounding cumulus cells (Luberda 2005). In addition, mitochondria also produce the TCA cycle intermediate, NADPH, another major cellular-reducing equivalent (Dumollard et al. 2007). Oocyte maturation is accompanied by an increase in glutathione and eggs matured in vivo contain much higher glutathione levels than those matured in vitro, likely contributing to the superior developmental potential of in vivo eggs. A high concentration of glutathione in the mature egg is thought to be critical for pre-implantation development when the embryo is relying on itself after losing cumulus cell support. Finally, in addition to endoplasmic reticulum, oocyte mitochondria are also important intracellular calcium stores and participate in calcium signaling after fertilization (Dumollard et al. 2003). Therefore, mitochondrial dysfunction has been postulated as a major mechanism in reproductive aging (Eichenlaub-Ritter et al. 2011; Van Blerkom 2011). Examining human oocytes from IVF patients have indicated a significant inverse correlation between maternal age and mtDNA copy number (as a surrogate of the mitochondrial number) (May-Panloup et al. 2005; Santos et al. 2006). However, there are no compelling studies to indicate that simply boosting mitochondrial numbers in the oocytes can reduce egg aneuploidy or improve its developmental potential, particularly given the possible importance of maturation-specific mitochondrial translocation (Yu et al. 2010; Dumollard et al. 2006). Genetic (Johnson et al. 2007) and pharmacological (Zeng et al. 2007; Zhang et al. 2006) depletion of ATP in the oocytes disrupts spindle formation but this total disruption of ATP synthesis does not equate the possible energy deficiency in aged oocytes. On the contrary, measuring ATP contents of individual oocytes from IVF patients has not revealed an age-dependent decline (Van Blerkom et al. 1995; Van Blerkom 2011). Furthermore, genetic studies in mice have indicated that as much as a 60 % reduction in the mitochondrial number has no noticeable effect in embryonic development (Wai et al. 2010). Nonetheless, it remains probable that mitochondrial health, in energy supply and particularly with regards to cellular redox regulation, may be a key factor in the developmental potential of the eggs (Eichenlaub-Ritter et al. 2011).
In addition to mitochondrial dysfunction, age-related deficiency of histone deacetylation (and other histone modifications; Gu et al. 2010) may also contribute to both chromosome segregation errors and poor developmental potential. Immature mouse oocytes are highly acetylated on multiple lysine residues of histone H3 and H4 and undergo global histone deacetylation during oocyte maturation (Kim et al. 2003). Inhibition of histone deacetylation using trichostatin A (TSA), an inhibitor of class I and class II histone deacetylases (HDACs), during oocyte maturation results in lagging chromosomes in anaphase (Wang et al. 2006), increased aneuploidy and poor embryonic development after the treated eggs are fertilized in vitro (Akiyama et al. 2006). Interestingly, incomplete histone deacetylation has been reported in eggs of older mice (Akiyama et al. 2006; Suo et al. 2010) and older women (van den Berg et al. 2011), as well as in eggs following IVM (Huang et al. 2012). It is not clear why insufficient histone deacetylation increases egg aneuploidy. A possible reason might be that insufficient histone deacetylation interferes with chromatin remodeling and chromosome condensation, which is required for proper kinetochore function and spindle attachment (Yang et al. 2012). This is likely an oversimplification, since mitosis (which has a similar requirement for chromosome condensation) in somatic cells and early embryos does not involve global histone deacetylation (Kim et al. 2003). More importantly, global histone deacetylation during oocyte maturation is likely essential for re-establishing embryonic histone acetylation patterns for normal embryonic development, since histone acetylation is an important epigenetic mechanism that generally activates transcription of the targeted gene (Verdin and Ott 2015).
It seems that global histone deacetylation during oocyte maturation is accomplished by activation of class 1 HDACs in mice (Ma and Schultz 2013; Kim et al. 2003), although one study has suggested that other classes of HDACs are responsible in porcine oocytes (Endo et al. 2008). Importantly, why is histone deacetylation deficient during maturation of aged oocytes? The obvious answer might be a diminished expression of HDACs in aged oocytes, although no evidence has yet been published. On the other hand, using prolonged in vitro incubation after maturation (so-called postovulatory aging) as a maternal aging model, Cui et al. (2011) suggested an increased expression of histone acetyltransferase 1 (HAT1) and increased ROS as the main reasons for histone hyperacetylation in porcine oocytes. Similarly, Huang et al. (2007) demonstrated a gradual re-acetylation of multiple lysin residues of H3 and H4 during postovulatory aging in mice, suggesting the presence of HAT activity in mature eggs. Therefore, histone hyperacetylation in aged oocytes could be due to increased HAT activity, reduced HDAC activity, or both.
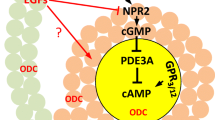
Like insufficient histone deacetylation in aged oocytes that likely contributes to egg aneuploidy (therefore, a component of “second hit”) and poor developmental potential, we have recently discovered another biochemical deficiency, peri-ovulatory deficiency of putrescine, in aged oocytes that might be an equally important contributor to egg aneuploidy and poor developmental potential. Luteinizing hormone (LH) surge plays a central role in triggering oocyte maturation and the subsequent rupture of ovarian follicles to release the mature eggs. It has been known for more than four decades that mammalian ovaries produced a LH-mediated transient rise of ornithine decarboxylase (ODC) and its product putrescine, concurrent with in vivo oocyte maturation (Fig. 2). This metabolic change has received relatively little attention, likely because its inhibition in adult rats did not impair ovulation or normal pregnancy (Fozard et al. 1980b). We have now demonstrated that deficiency in this metabolic pathway in older mice has significant reproductive consequences, pointing to a promising remedy for reproductive aging (Tao and Liu 2013; Tao et al. 2015; Liu and Tao 2012).
Peri-ovulatory ovarian ODC deficiency as a key factor in reproductive aging
ODC is the first and rate-limiting enzyme in cellular synthesis of biogenic polyamines, putrescine (a diamine), spermidine (a triamine) and spermine (a tetraamine). In eukaryotic cells, putrescine is produced via decarboxylation of ornithine, catalyzed by ODC (Fig. 2). Putrescine serves as a substrate for spermidine synthesis in which spermidine synthase transfers a propyl amine moiety from decarboxylated S-adenosylmethionine (dcSAM) to putrescine. Spermine is synthesized in a similar fashion by transferring a propyl amine moiety from dc-SAM to spermidine, catalyzed by spermine synthase (Gerner and Meyskens 2004).
Biochemically, spermine can be converted back to spermidine and spermidine to putrescine, catalyzed by a polyamine oxidase and involving prior acetylation of the substrates (spermine and spermidine). However, the physiological significance of this “reverse” pathway to generate putrescine is not clear, since in most animal tissues/cells the level of putrescine is low or undetectable while spermidine and spermine are at high steady state levels (>1 mM) (Nishimura et al. 2006). In addition, animals readily take up polyamines from food sources, as well as from the symbiotic bacteria primarily residing in the gut (Gerner and Meyskens 2004).
ODC is an essential gene, from yeast to mammals. Yeast deficient in ODC growth-arrests unless exogenous polyamines are added (Schwartz et al. 1995). Pregnant mice exhibit high ODC activity in the uterus/fetuses during early gestation (E5–E8); ingestion of the highly selective ODC inhibitor α-difluoromethylornithine (DFMO) during this period causes immediate growth arrest and death of the embryos (Fozard et al. 1980a). Mice homozygous for the ODC gene die before implantation (Pendeville et al. 2001). Given the essential role of ODC and polyamines in cell proliferation, many aspects of male and female reproduction, as well as embryogenesis, require this metabolic pathway (Lefevre et al. 2011).
The essential role of polyamines in cell proliferation is indisputable, although the precise mechanisms by which polyamines participate in cell proliferation remain unknown. This review, however, focuses on the peri-ovulatory rise of ODC (Fig. 2) and putrescine (not polyamines in general) in the ovaries and their function in healthy oocyte maturation. Transient rise of ovarian ODC activity has been demonstrated in many mammalian species (Channing and Tsafriri 1977), including rats (Kobayashi et al. 1971; Fozard et al. 1980b), mice (Bastida et al. 2005), hamsters (Persson et al. 1986), rabbits (Bieniarz et al. 1983) and pigs (Veldhuis and Hammond 1979). Inhibition of the transient ODC rise in mice by DFMO diminishes ovarian ODC activity, resulting in reduction of progesterone production and reduction of vascularization in the corpus luteum, suggesting a role in luteinization of granulosa cells (Bastida et al. 2005). However, similar DFMO treatment in rats does not reduce the number of implantation sites or litter size (Fozard et al. 1980b). Furthermore, a similar transient rise of ovarian ODC activity and putrescine in non-mammalian species such as Xenopus (Younglai et al. 1980; Sunkara et al. 1981) and hens (Armstrong 1994) clearly indicates additional function(s) unrelated to luteinization. In Xenopus oocytes, this transient rise of ODC activity is eliminated by antisense morpholino oligos targeting Xenopus ODC mRNA. Inhibition of the transient ODC rise does not interfere with first polar body emission but the mature eggs exhibit elevated levels of ROS, Cytochrome c leakage from mitochondria and caspase 3 activation (Zhou et al. 2009).
Given these results in Xenopus oocytes, we investigated a potential role of ODC in mouse oocyte maturation. Injecting young mice with hCG stimulates a transient rise of ODC (Tao and Liu 2013) and putrescine (Tao et al. 2015) in the ovaries, peaking 5 h post-injection, similar to the transient rise of ovarian ODC and putrescine in rats (Fozard et al. 1980b). Adding 1 % DFMO in mouse drinking water for 48 h including the period of hCG stimulation eliminates ODC activity (Tao and Liu 2013) and putrescine increase (Tao et al. 2015). DFMO treatment does not affect the number of ovulated eggs but increases egg aneuploidy. Similarly, adding DFMO in IVM medium does not affect oocyte maturation rates but increases egg aneuploidy. The effect of DFMO on egg aneuploidy is not restricted to CF1 mice. The F1 hybrid mice from crossing C57BL/6 and SPRET exhibit a “basal” egg aneuploidy rate much greater than those found in the parental mice, likely due to suboptimal homologous recombination in germ cells of the hybrid (Koehler et al. 2006). DFMO treatment of oocytes from these F1 mice further increases aneuploidy rates (Tao and Liu 2013).
Older mice exhibit reduced levels of ovarian ODC (Fig. 2) (Tao and Liu 2013) and putrescine (Tao et al. 2015). A combination of putrescine supplementation of mouse drinking water up to the time of oocyte retrieval and putrescine supplementation in IVM medium reduces egg aneuploidy in older mice (Tao and Liu 2013). Remarkably, peri-ovulatory putrescine supplementation of mouse drinking water significantly improves egg quality, as indicated by the greater cell number of the resulting blastocysts, reduces early embryo death and increases the number of live birth (Tao et al. 2015).
The mechanism by which age-related ODC/putrescine deficiency increases egg aneuploidy and decreases egg developmental potential remains unknown. In Xenopus oocytes, inhibition of ODC during oocyte maturation significantly increases the level of oxidative stress, which is rescued by the addition of excess putrescine in the medium (Zhou et al. 2009).
Remedies to reduce aneuploid conceptions and to improve egg quality in older women
With the apparent reliability of genomic approaches to karyotype polar bodies and single cells biopsied from fertilized eggs and early embryos, respectively, many IVF clinics have started to apply them in selecting euploid embryos for transfer (Gardner et al. 2015). Some groups have used polar body screening to select euploid zygotes for transfer (Fishel et al. 2010; Geraedts et al. 2011). Most others have used blastomere biopsy of cleavage embryos (day 3 biopsy for day 5 transfer) or trophectoderm cell biopsy of blastocysts (day 5 biopsy for day 6 transfer, or for next cycle transfer after freezing) to select euploid embryos. Most of these studies, including several randomized control trials, have reported better reproductive outcome for the interventions (Gardner et al. 2015). However, others have warned against wide-spread and indiscriminate application of these invasive preimplantation genetic diagnosis (PGD) approaches in the general IVF population (Gleicher et al. 2014; Mastenbroek and Repping 2014). Regardless, PGD is limited to women undergoing IVF procedures. For women undergoing natural conception, there are no proven remedies to reduce age-related aneuploid conceptions.
It is clear that oocytes and oocyte chromosomes are not born equal. A proportion of the endowed primary oocytes of each female contain “vulnerable” chromosomes susceptible for mis-segregation (achiasmate chromosomes, chromosomes with a single crossover near the telomere and those with a crossover near the centromere). Recent egg karyotyping by aCGH have indicated that some, particularly smaller, chromosomes are more prone to mis-segregation (Gabriel et al. 2011; Fragouli et al. 2011, 2013; Handyside et al. 2012), confirming the conclusion of decades of genetic and cytogenetic studies (Nagaoka et al. 2012). However, the chance of all vulnerable chromosomes, including achiasmate chromosomes, to mis-segregate increases with maternal age, suggesting that all vulnerable chromosomes need a second hit to mis-segregate and therefore have a “second chance” to properly segregate. I hypothesize that oocyte maturation represents the best window of opportunity to intervene to improve fertility of older women. Specifically, an unfavorable oocyte maturation condition represents the most important “second hit” in age-related aneuploidy. What about meiosis II errors, as some have suggested that in older women the majority of chromosome segregation errors occur in meiosis II (Fragouli et al. 2013)? In my opinion, a significant proportion, if not the majority, of these “meiosis II errors” are likely meiosis I errors in which the prematurely separated sister chromatids co-segregate (Fig. 1; PSSC 1). If so, reducing meiosis I errors will likely significantly reduce these “meiosis II errors”.
Perhaps just as important, if not more so, an unfavorable oocyte maturation condition contributes greatly to the poor developmental potential of aged eggs, irrespective of karyotype. This is perhaps most evident by the fact that despite decades of research on the mechanisms of mammalian oocyte maturation and refining of IVM conditions, human IVM remains the most challenging assisted reproductive technologies (ART) procedure due to the poor developmental potential of IVM eggs, even for young women (Smitz et al. 2011). As proposed long ago by Eppig et al. (1994), oocyte “cytoplasmic maturation” is critical for ensure optimal post-fertilization development. The biochemical nature of oocyte cytoplasmic maturation remains poorly defined but should include the biochemical changes discussed here. Strategic mitochondrial translocation and mitochondrial health in energetic, redox and post-fertilization calcium signaling and global histone deacetylation are likely important components of oocyte cytoplasmic maturation. Peri-ovulatory rise of ovarian ODC and putrescine may be an evolutionally conserved mechanism to safeguard this critical process in vertebrates.
Peri-ovulatory putrescine supplementation represents an ideal intervention in reproductive aging. First, putrescine is naturally produced during ovulation. Second, exogenous putrescine is readily absorbed, rapidly cleared after cessation of supplementation and safe for both the mother and fetuses (Tao et al. 2015). Third, it entails a very short period of intervention. Finally, it is applicable both in IVF and in natural conception.
Concluding remark
Reproductive aging in women is a serious societal problem in industrialized countries, with women increasingly struggling with fertility due to delayed child bearing. Increasingly sophisticated and invasive ART have helped millions of older women to conceive but these do little to tackle the fundamental problem of female reproductive aging, increased aneuploidy and the poor developmental potential of aged eggs. Studies in model organisms, mainly mice, have identified several likely culprits underlining the age-related decline in egg quality. These include loss of chromosome cohesin and Shugoshins, mitochondrial dysfunction, defective spindles and insufficient histone deacetylation. However, most are not likely “druggable”, especially for reproductive aging. Unlike most human diseases in which a potential drug needs to be nontoxic to the affected person only, a potential drug for reproductive aging needs to be safe for the woman and her conceptus and a developing fetus is extremely sensitive to many otherwise benign chemicals. Peri-ovulatory putrescine supplementation represents a unique and compelling candidate. Further work, however, will be required to validate its efficacy and safety in humans.
References
Akiyama T, Nagata M, Aoki F (2006) Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci U S A 103:7339–7344
Angell RR (1991) Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet 86:383–387
Angell R (1997) First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet 61:23–32
Armstrong DG (1994) The effect of LH, FSH and pregnant mares’ serum gonadotrophin on ornithine decarboxylase activity in thecal and granulosa tissue during follicular growth and atresia in laying hens (Gallus domesticus). J Reprod Fertil 100:273–278
Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC (2006) In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38:1430–1434
Baslan T, Kendall J, Rodgers L, Cox H, Riggs M, Stepansky A, Troge J, Ravi K, Esposito D, Lakshmi B, Wigler M, Navin N, Hicks J (2012) Genome-wide copy number analysis of single cells. Nat Protoc 7:1024–1041
Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Tejada F, Penafiel R (2005) Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization. Endocrinology 146:666–674
Battaglia DE, Goodwin P, Klein NA, Soules MR (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 11:2217–2222
Bieniarz A, Berger T, Nishimura K, diZerega GS (1983) Ibuprofen modulation of human chorionic gonadotropin-induced ornithine decarboxylase activity and ovulation in the rabbit ovary. Am J Obstet Gynecol 147:327–332
Bugge M, Collins A, Petersen MB, Fisher J, Brandt C, Hertz JM, Tranebjaerg L, de Lozier-Blanchet C, Nicolaides P, Brondum-Nielsen K, Morton N, Mikkelsen M (1998) Non-disjunction of chromosome 18. Hum Mol Genet 7:661–669
Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G (2001) Karyotype of the abortus in recurrent miscarriage. Fertil Steril 75:678–682
Channing CP, Tsafriri A (1977) Mechanism of action of luteinizing hormone and follicle-stimulating hormone on the ovary in vitro. Metabolism 26:413–468
Cheng EY, Hunt PA, Naluai-Cecchini TA, Fligner CL, Fujimoto VY, Pasternack TL, Schwartz JM, Steinauer JE, Woodruff TJ, Cherry SM, Hansen TA, Vallente RU, Broman KW, Hassold TJ (2009) Meiotic recombination in human oocytes. PLoS Genet 5, e1000661
Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA (2010) Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 20:1522–1528
Christopikou D, Tsorva E, Economou K, Shelley P, Davies S, Mastrominas M, Handyside AH (2013) Polar body analysis by array comparative genomic hybridization accurately predicts aneuploidies of maternal meiotic origin in cleavage stage embryos of women of advanced maternal age. Hum Reprod 28:1426–1434
Corbett KD, Yip CK, Ee LS, Walz T, Amon A, Harrison SC (2010) The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell 142:556–567
Coticchio G, Sciajno R, Hutt K, Bromfield J, Borini A, Albertini DF (2010) Comparative analysis of the metaphase II spindle of human oocytes through polarized light and high-performance confocal microscopy. Fertil Steril 93:2056–2064
Cui MS, Wang XL, Tang DW, Zhang J, Liu Y, Zeng SM (2011) Acetylation of H4K12 in porcine oocytes during in vitro aging: potential role of ooplasmic reactive oxygen species. Theriogenology 75:638–646
Dailey T, Dale B, Cohen J, Munne S (1996) Association between nondisjunction and maternal age in meiosis-II human oocytes. Am J Hum Genet 59:176–184
De SL, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, Coticchio G (2005) Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online 11:36–42
Dumollard R, Hammar K, Porterfield M, Smith PJ, Cibert C, Rouviere C, Sardet C (2003) Mitochondrial respiration and Ca2+ waves are linked during fertilization and meiosis completion. Development 130:683–692
Dumollard R, Marangos P, FitzHarris G, Swann K, Duchen M, Carroll J (2004) Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131:3057–3067
Dumollard R, Duchen M, Sardet C (2006) Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol 17:314–323
Dumollard R, Ward Z, Carroll J, Duchen MR (2007) Regulation of redox metabolism in the mouse oocyte and embryo. Development 134:455–465
Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K (2009) Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol 20:346–353
Duncan FE, Chiang T, Schultz RM, Lampson MA (2009) Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 81:768–776
Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK (2012) Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell 11:1121–1124
Dupont C, Harvey AJ, Armant DR, Zelinski MB, Brenner CA (2012) Expression profiles of cohesins, shugoshins and spindle assembly checkpoint genes in rhesus macaque oocytes predict their susceptibility for aneuploidy during embryonic development. Cell Cycle 11:740–748
Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R (2004) Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online 8:45–58
Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T (2011) Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 11:783–796
Endo T, Kano K, Naito K (2008) Nuclear histone deacetylases are not required for global histone deacetylation during meiotic maturation in porcine oocytes. Biol Reprod 78:1073–1080
Eppig JJ, Schultz RM, O'Brien M, Chesnel F (1994) Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol 164:1–9
Epstein CJ, Travis B (1979) Preimplantation lethality of monosomy for mouse chromosome 19. Nature 280:144–145
Evans EP (1987) Karyotying and sexing of gametes, embryos and fetuses and in situ hybridization to chromosomes. In: Monk M (ed) Mammalian Development: a practical approach. IRL, Washington
Fishel S, Gordon A, Lynch C, Dowell K, Ndukwe G, Kelada E, Thornton S, Jenner L, Cater E, Brown A, Garcia-Bernardo J (2010) Live birth after polar body array comparative genomic hybridization prediction of embryo ploidy-the future of IVF? Fertil Steril 93:1006
Fisher JM, Harvey JF, Morton NE, Jacobs PA (1995) Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am J Hum Genet 56:669–675
Fozard JR, Part ML, Prakash NJ, Grove J, Schechter PJ, Sjoerdsma A, Koch-Weser J (1980a) L-Ornithine decarboxylase:an essential role in early mammalian embryogenesis. Science 208:505–508
Fozard JR, Prakash NJ, Grove J (1980b) Ovarian function in the rat following irreversible inhibition of L-ornithine decarboxylase. Life Sci 27:2277–2283
Fragouli E, Alfarawati S, Goodall NN, Sanchez-Garcia JF, Colls P, Wells D (2011) The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod 17:286–295
Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, Enciso M, Wells D (2013) The origin and impact of embryonic aneuploidy. Hum Genet 132:1001–1013
Gabriel AS, Thornhill AR, Ottolini CS, Gordon A, Brown AP, Taylor J, Bennett K, Handyside A, Griffin DK (2011) Array comparative genomic hybridisation on first polar bodies suggests that non-disjunction is not the predominant mechanism leading to aneuploidy in humans. J Med Genet 48:433–437
Gardner DK, Meseguer M, Rubio C, Treff NR (2015) Diagnosis of human preimplantation embryo viability. Hum Reprod. doi:10.1093/humupd/dmu064
Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, Harper J, Schmutzler A, Collins J, Goossens V, van der Ven H, Vesela K, Gianaroli L (2011) Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod 26:3173–3180
Gerner EW, Meyskens FL Jr (2004) Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4:781–792
Gleicher N, Kushnir VA, Barad DH (2014) Preimplantation genetic screening (PGS) still in search of a clinical application: a systematic review. Reprod Biol Endocrinol 12:22
Golbus MS (1981) The influence of strain, maternal age, and method of maturation on mouse oocyte aneuploidy. Cytogenet Cell Genet 31:84–90
Gu L, Wang Q, Sun QY (2010) Histone modifications during mammalian oocyte maturation: dynamics, regulation and functions. Cell Cycle 9:1942–1950
Handyside AH, Montag M, Magli MC, Repping S, Harper J, Schmutzler A, Vesela K, Gianaroli L, Geraedts J (2012) Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet 20:742–747
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291
Hodges CA, Hunt PA (2002) Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma 111:165–169
Hou Y, Fan W, Yan L, Li R, Lian Y, Huang J, Li J, Xu L, Tang F, Xie XS, Qiao J (2013) Genome analyses of single human oocytes. Cell 155:1492–1506
Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY (2007) Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod 77:666–670
Huang J, Li T, Ding CH, Brosens J, Zhou CQ, Wang HH, Xu YW (2012) Insufficient histone-3 lysine-9 deacetylation in human oocytes matured in vitro is associated with aberrant meiosis. Fertil Steril 97:178–184
Johnson MT, Freeman EA, Gardner DK, Hunt PA (2007) Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod 77:2–8
Kajii T, Niikawa N (1977) Origin of triploidy and tetraploidy in man: 11 cases with chromosomes markers. Cytogenet Cell Genet 18:109–125
Kim JM, Liu H, Tazaki M, Nagata M, Aoki F (2003) Changes in histone acetylation during mouse oocyte meiosis. J Cell Biol 162:37–46
Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427:510–517
Kobayashi Y, Kupelian J, Maudsley DV (1971) Ornithine decarboxylase stimulation in rat ovary by luteinizing hormone. Science 172:379–380
Koehler KE, Boulton CL, Collins HE, French RL, Herman KC, Lacefield SM, Madden LD, Schuetz CD, Hawley RS (1996) Spontaneous X chromosome MI and MII nondisjunction events in Drosophila melanogaster oocytes have different recombinational histories. Nat Genet 14:406–414
Koehler KE, Schrump SE, Cherry JP, Hassold TJ, Hunt PA (2006) Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr Biol 16:R579–R580
Kroon B, Harrison K, Martin N, Wong B, Yazdani A (2010) Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertil Steril 95:1827–1829
Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu H, Helmhart W, Kudo H, McKay M, Maro B, Ellenberg J, de Boer P, Nasmyth K (2006) Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126:135–146
Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak JJ (2011) Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod Biomed Online 22:2–8
Lamb NE, Freeman SB, Savage-Austin A, Pettay D, Taft L, Hersey J, Gu Y, Shen J, Saker D, May KM, Avramopoulos D, Petersen MB, Hallberg A, Mikkelsen M, Hassold TJ, Sherman SL (1996) Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet 14:400–405
Lee J, Okada K, Ogushi S, Miyano T, Miyake M, Yamashita M (2006) Loss of Rec8 from chromosome arm and centromere region is required for homologous chromosome separation and sister chromatid separation, respectively, in mammalian meiosis. Cell Cycle 5:1448–1455
Lefevre PL, Palin MF, Murphy BD (2011) Polyamines on the reproductive landscape. Endocr Rev 32:694–712
Lei L, Spradling AC (2013) Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A 110:8585–8590
Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Hoog C, Herbert M (2010) Age-related meiotic segregation errors in Mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20:1511–1521
Liu L, Keefe DL (2008) Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online 16:103–112
Liu XJ, Tao Y (2012) Peri-ovulatory putrescine to reduce aneuploid conceptions. Aging (Albany NY) 4:723–725
Luberda Z (2005) The role of glutathione in mammalian gametes. Reprod Biol 5:5–17
Lynn A, Koehler KE, Judis L, Chan ER, Cherry JP, Schwartz S, Seftel A, Hunt PA, Hassold TJ (2002) Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296:2222–2225
Ma P, Schultz RM (2013) Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet 9, e1003377
MacDonald M, Hassold T, Harvey J, Wang LH, Morton NE, Jacobs P (1994) The origin of 47, XXY and 47, XXX aneuploidy: heterogeneous mechanisms and role of aberrant recombination. Hum Mol Genet 3:1365–1371
Mastenbroek S, Repping S (2014) Preimplantation genetic screening: back to the future. Hum Reprod 29:1846–1850
Maudlin I, Fraser LR (1977) The effect of PMSG dose on the incidence of chromosomal anomalies in mouse embryos fertilized in vitro. J Reprod Fertil 50:275–280
May-Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P (2005) Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod 20:593–597
McCollum D (2012) Monopolin. Curr Biol 22:R937–R938
Merriman JA, Jennings PC, McLaughlin EA, Jones KT (2012) Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol Reprod 86:49
Nagaoka SI, Hodges CA, Albertini DF, Hunt PA (2011) Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol 21:651–657
Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13:493–504
Nishimura K, Shiina R, Kashiwagi K, Igarashi K (2006) Decrease in polyamines with aging and their ingestion from food and drink. J Biochem (Tokyo) 139:81–90
Northrop LE, Treff NR, Levy B, Scott RT Jr (2010) SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod 16:590–600
Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL (2008) New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet 4, e1000033
Orr-Weaver T (1996) Meiotic nondisjunction does the two-step. Nat Genet 14:374–376
Pan H, Ma P, Zhu W, Schultz RM (2008) Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 316:397–407
Pellestor F, Anahory T, Hamamah S (2005) The chromosomal analysis of human oocytes. An overview of established procedures. Hum Reprod Update 11:15–32
Pellestor F, Andreo B, Anahory T, Hamamah S (2006) The occurrence of aneuploidy in human: lessons from the cytogenetic studies of human oocytes. Eur J Med Genet 49:103–116
Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, Cleveland JL (2001) The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol 21:6549–6558
Persson L, Isaksson K, Rosengren E, Sundler F (1986) Distribution of ornithine decarboxylase in ovaries of rat and hamster during pro-oestrus. Acta Endocrinol (Copenh) 113:403–409
Piko L, Taylor KD (1987) Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol 123:364–374
Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K (2004) Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr Biol 14:287–301
Rama Raju GA, Prakash GJ, Krishna KM, Madan K (2007) Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online 14:166–174
Revenkova E, Herrmann K, Adelfalk C, Jessberger R (2010) Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol 20:1529–1533
Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Galova M, Petronczki M, Gregan J, Cetin B, Mudrak I, Ogris E, Mechtler K, Pelletier L, Buchholz F, Shirahige K, Nasmyth K (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441:53–61
Santos TA, El SS, St John JC (2006) Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril 85:584–591
Sarangapani KK, Duro E, Deng Y, Alves FL, Ye Q, Opoku KN, Ceto S, Rappsilber J, Corbett KD, Biggins S, Marston AL, Asbury CL (2014) Sister kinetochores are mechanically fused during meiosis I in yeast. Science 346:248–251
Schwartz B, Hittelman A, Daneshvar L, Basu HS, Marton LJ, Feuerstein BG (1995) A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem J 312(Pt 1):83–90
Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL (2011) From the cover: prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A 108:12319–12324
Shao H, Li R, Ma C, Chen E, Liu XJ (2013) Xenopus oocyte meiosis lacks spindle assembly checkpoint control. J Cell Biol 201:191–200
Shen Y, Stalf T, Mehnert C, De SL, Cino I, Tinneberg HR, Eichenlaub-Ritter U (2006) Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod Biomed Online 12:737–751
Smitz JE, Thompson JG, Gilchrist RB (2011) The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med 29:24–37
Sunkara PS, Wright DA, Nishioka K (1981) An essential role for putrescine biosynthesis during meiotic maturation of amphibian oocytes. Dev Biol 87:351–355
Suo L, Meng QG, Pei Y, Yan CL, Fu XW, Bunch TD, Zhu SE (2010) Changes in acetylation on lysine 12 of histone H4 (acH4K12) of murine oocytes during maternal aging may affect fertilization and subsequent embryo development. Fertil Steril 93:945–951
Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K (2010) Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 24:2505–2516
Tao Y, Liu XJ (2013) Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell 12:42–49
Tao Y, Liu D, Mo G, Wang H, Liu XJ (2015) Peri-ovulatory putrescine supplementation reduces embryo resorption in older mice. Hum Reprod 30:1867–1875
Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, Ohye T, Kato T, Fujii T, Kurahashi H (2014) Age-related decrease of meiotic cohesins in human oocytes. PLoS ONE 9, e96710
Van Blerkom J (2011) Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 11:797–813
Van Blerkom J, Davis PW, Lee J (1995) ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod 10:415–424
van den Berg I, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, Laven JS, van Doorninck JH (2011) Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod 26:1181–1190
Veldhuis JD, Hammond JM (1979) Role of ornithine decarboxylase in granulosa-cell replication and steroidogenesis in vitro. Biochem Biophys Res Commun 91:770–777
Verdin E, Ott M (2015) 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol 16:258–264
Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA (2010) The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 83:52–62
Wang Q, Yin S, Ai JS, Liang CG, Hou Y, Chen DY, Schatten H, Sun QY (2006) Histone deacetylation is required for orderly meiosis. Cell Cycle 5:766–774
Yang F, Baumann C, Viveiros MM, De La FR (2012) Histone hyperacetylation during meiosis interferes with large-scale chromatin remodeling, axial chromatid condensation and sister chromatid separation in the mammalian oocyte. Int J Dev Biol 56:889–899
Younglai EV, Godeau F, Mester J, Baulieu EE (1980) Increased ornithine decarboxylase activity during meiotic maturation in Xenopus laevis oocytes. Biochem Biophys Res Commun 96:1274–1281
Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K (2010) Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol 224:672–680
Yun Y, Lane SI, Jones KT (2014) Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development 141:199–208
Zaragoza MV, Surti U, Redline RW, Millie E, Chakravarti A, Hassold TJ (2000) Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet 66:1807–1820
Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL, Liang XY (2007) Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod 22:1681–1686
Zhang X, Wu XQ, Lu S, Guo YL, Ma X (2006) Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res 16:841–850
Zhang H, Liu L, Li X, Busayavalasa K, Shen Y, Hovatta O, Gustafsson JA, Liu K (2014) Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci U S A 111:17983–17988
Zhou Y, Ma C, Karmouch J, Katbi HA, Liu XJ (2009) Antiapoptotic role for ornithine decarboxylase during oocyte maturation. Mol Cell Biol 29:1786–1795
Acknowledgments
Research in the author’s laboratory is supported by grants from March of Dimes (6-FY13-126) and the Canadian Institute of Health Research (MOP-89973). I would like to thank Mr. Guolong Mo for providing Figure 2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X.J. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res 363, 57–68 (2016). https://doi.org/10.1007/s00441-015-2264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2264-y