Abstract
Carboxylic acid production from food waste by mixed culture fermentation is an important future waste management option. Obstacles for its implementation are the need of pH control and a broad fermentation product spectrum leading to increased product separation costs. To overcome these obstacles, the selective production of lactic acid (LA) from model food waste by uncontrolled pH fermentation was tested using different reactor configurations. Batch experiments, semi-continuously fed reactors and a percolation system reached LA concentrations of 32, 16, and 15 gCODLA/L, respectively, with selectivities of 93%, 84%, and 75% on COD base, respectively. The semi-continuous reactor was dominated by Lactobacillales. Our techno-economic analysis suggests that LA production from food waste can be economically feasible, with LA recovery and low yields remaining as major obstacles. To solve both problems, we successfully applied in situ product extraction using activated carbon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The economic treatment of food waste is a major challenge for the development of sustainable waste management systems . Traditional waste management treatment options include landfilling, composting, or anaerobic digestion for biogas production. In recent years, alternative technologies have been developed to produce value-added products from waste, for example, via the carboxylate platform (Bastidas-Oyanedel et al. 2015). In the carboxylate platform, carboxylic acids are produced via an undefined mixed culture of bacteria. To produce volatile fatty acids (VFAs), e.g., acetic, propionic, or butyric acid (all pKa ~ 4.8), a pH of around 5 is considered the lower boundary because below that pH, solvent production overtakes acid production due to inhibition (Bastidas-Oyanedel et al. 2015; Aceves-Lara et al. 2008). A possible explanation is that the protonated VFA form is the more inhibitory form for microorganisms (Penumathsa et al., 2008) and the lower the pH, the more VFAs are present in their protonated form.
Controlling pH can be achieved either by chemical means or co-fermentation with other wastes (Wu et al. 2016). The need to control pH in these systems imposes further economic and logistic constraints and is therefore a drawback for the production of platform chemicals from food waste. Ideally, carboxylic acids are produced using the intrinsic buffer capacity of food waste without pH control or co-fermentation, while retaining a high final product concentration. Another obstacle for the production of carboxylic acids from food waste is that mixtures of acids are often produced, e.g., acetic and butyric acid that have to be separated before utilization (Zhang et al. 2014). Separation and recovery of carboxylic acids from fermentation broths can be achieved by adsorption, either as direct addition of adsorbent in the fermenter or in a separated step (Yousuf et al. 2016). There are other techniques, different to adsorption, that include solvent extraction, membrane-based solvent extraction, electro dialysis, and membrane separation (Bastidas-Oyanedel et al. 2016, Lopez-Garzon and Straathof 2014).
The production of a single acid at a high concentration is highly desirable considering downstream processing (Zhang et al. 2014). Lactic acid (LA) is a promising carboxylic acid that can potentially fulfill all the requirements mentioned above. Its pKa of 3.9 is lower than the pKa of VFAs, and therefore, fewer acids are in their protonated form at a low pH. This reduces product inhibition by protonated acids at low pH and allows for higher product concentrations at a low pH compared to VFAs. In addition, previous studies suggest that a high selectivity can be achieved for LA over other products. Kim et al. (2012), for example, achieved a selectivity of LA over total fermentation products from the fermentation of food waste of over 90% using food waste pretreatment at 60 °C. LA can serve as precursor for the production of many useful chemicals and products, such as biodegradable polymers, pyruvic acid, acrylic acid, 1,2-propanediol, and lactate ester (Bastidas-Oyanedel et al. 2015; Gao et al. 2011). Today, it is already produced using bacterial fermentation (López-Garzón and Straathof 2014).
As mentioned before, microbial communities of mixed culture fermentation can be affected/modified by process parameters, e.g., pH, product inhibition. Recent advancements and increased access to novel sequencing technologies have resulted in the generation of a large amount of data regarding microbial populations and their dynamics in environmental and industrial settings (Ju and Zhang 2015). These data could help applied microbiologists and engineers in optimizing bioreactor technologies with an emphasis on process parameters and/or inoculum selection and maintenance. In order to do this, it is necessary to first establish correlations between efficient well-operated reactors and the microbial communities driving them.
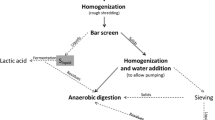
The aim of this study was to explore different reactor configurations for LA production by pH-uncontrolled fermentation of food waste. In addition, we were interested in determining the microbial communities that develop under different reactor configurations. Model food waste was fermented mesophilically in three reactor configurations: (a) semi-continuous feeding, (b) batch, and (c) percolation systems. Percolation systems are attractive in the context of waste management because they are easy to handle and allow for subsequent composting (Bonk et al. 2015). In these systems, the hydraulic retention time (HRT) can be separated from food waste retention time and possibly also the retention time of microorganisms if they are able to attach to the food waste. Similar systems already exist on industrial scale for biomethane production from waste, such as the Aikan® system (Bastidas-Oyanedel et al. 2015). Since the percolation system is a practical setting for industrial waste treatment, the economic feasibility of its application to LA production from food waste was also estimated in this study. A major obstacle of all the tested reactor configurations is the recovery of the product. Therefore, in situ product extraction was tested using activated carbon.
2 Materials and Methods
2.1 Substrate and Inoculum
In order to have reproducible conditions, a model food waste was used as substrate, consisting of 50% cooked rice (Thai Pathumthani Fragrant Rice) and 50% dry dog food (Purina® Dog Chow Complete and Balanced) based on dry mass (Yousuf et al. 2016). It was prepared fresh for each experiment and feeding. Based on manufacturer’s information on the product composition, the model food waste contained 12% protein, 69% carbohydrates, and 5% fat based on dry mass (Yousuf et al. 2016). The theoretical maximum LA yield was estimated to 0.74–1 gCODLA/gTSfood waste fed, based on Castillo Martinez et al. (2013) and Grootscholten et al. (2013). Generally, no pretreatment was applied to the model food waste if not indicated.
The semi-continuous fed reactors were originally inoculated with anaerobic sludge from the Mafraq wastewater treatment plant in Abu Dhabi, and fed for 5 months with food waste from the Masdar Institute campus canteen, Abu Dhabi, under varying feeding and pH control strategies before the conditions described below were established. After those 5 months, inoculum for the batch experiments was taken from the semi-continuously fed reactors and kept at 37 °C for 4 days before starting the batch experiments.
2.2 Reactors Configurations
2.2.1 Semi-Continuously Fed Reactors
Three fermenters were run in parallel with a working volume of 13 L, 100 rpm stirring, an organic loading rate (OLR) of 5 gTS/L/day, a HRT of 15 days, and a semi-continuous feeding interval of model food waste of 10.5 days. The temperature was controlled with a heating bath at 35 °C. To prevent a possible organic overload, half of the corresponding dog food amount was added with 3–4 days delay. Under these conditions, the reactors were run for 8 months.
2.2.2 Batch Experiments
Batch experiments were conducted in duplicates in glass bottles with a total volume of 327 mL and a working volume of 115 mL. Table 1 shows the experimental conditions of most important batch experiments. As standard conditions, substrate concentrations of 52 gTS/L and 35 mL inoculum from the semi-continuously fed reactors were chosen. Tap water was used to bring the volume up to 115 mL. At the start of the experiment, the headspace was flushed with nitrogen, and the bottles were incubated without any shaking at 37 °C for 11 days.
In addition to these standard conditions, the influence of shaking of the batch bottles at 100 rpm was tested. Furthermore, the model food waste was grinded using pestle and mortar to test the influence of mechanical pretreatment. To test the possibility of nutrient limitation, a mineral solution was added (Angelidaki et al. 2009; Bastidas-Oyanedel et al. 2010). To test the difference between the uncontrolled pH fermentation and controlled pH fermentation, the pH of the inoculum was raised to 7, and sodium bicarbonate was added with final concentrations of 15 and 30 g/L. Substrate concentrations were varied between 10 and 156 gTS/L, and inoculum amounts between 0 and 35 mL. To investigate the impact of an increased retention time of the fermenting bacteria, a sequential batch experiment and a fixed bed batch experiment were run.
2.2.3 Percolation System
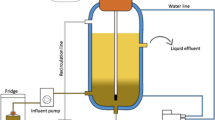
The percolation system consisted of a plastic tube with a total volume of 2.7 L and a height of 0.72 m. The percolation tube was completely filled with model food waste mixed with wood chips (size ~5*2*2 cm) as structural material. The fermentation liquid entered at the top of the column, percolated through the mixture of food waste and wood chips and exited at an outlet at the bottom of the tube. To prevent a clogging of tubes by particular matter, this outlet was protected by a sieve with <1 mm diameter and a kitchen sponge. The percolate flowed by gravity into a bioreactor with a working volume of 2 L, a temperature of 35 °C, and stirring of 50 rpm. A peristaltic pump recirculated the fermentation liquid with a dilution rate of 2.76 day−1 to the top of the percolation tube. Each experiment was run for 1 week and used 218 gTS of model food waste. After starting the experiment, tap water was added as needed to the reactor to keep a working volume of 2 L. The first experiment was started without the addition of inoculum.
2.2.4 In Situ and Online Extraction with Activated Carbon
Batch experiments with activated carbon were conducted to measure if an increase in lactic acid yield is possible by in situ product removal. Forty-six grams of activated carbon pellets (DoPhin® FM902) were washed before use with warm tap water as recommended by the manufacturer. The activated carbon was put in a fine textile net (~30 mesh) to keep the activate carbon granules in contact with the fermentation broth while preventing granules from mixing with the food waste for simpler separation after the fermentation. The activated carbon was submerged into the fermentation broth at the start of the experiment. After the experiment, the activated carbon was placed without any pretreatment into 115 mL acetone to desorb the fermentation products. Acetone desorption allows recovering the carboxylic acids in an organic solvent, rather than in aqueous solution, offering multiple application in thermochemical/catalytic down streaming of the carboxylic acids (Zanella et al. 2014).
2.3 Analytical Methods
Chemical oxygen demand (COD) was measured using spectrophotometric tests (LCK 014, range 1–10 g/L, Hach Lange GmbH) and a Hach DR 2800 spectrophotometer. Reactor samples were prepared by centrifugation for 5 min at 14,000 rpm, filtration (0.45 μm), and dilution with DI water to fit in the cuvette test linear range.
The concentrations of organic acids and alcohols were measured by high-performance liquid chromatography (HPLC) . Samples were prepared by centrifugation for 5 min at 14,000 rpm and filtration (0.45 μm). In addition, samples were diluted five times with 0.01 M H2SO4. In the case of the activated carbon experiments, the fermentation products containing acetone samples were diluted 20 times. Samples were run in an Agilent 1260 at 65 °C, using an Agilent Hi-Plex H column, 5 mM H2SO4 as mobile phase at 0.6 mL/min, a UV detector at 210 mm for organic acids, and a RID detector at 35 °C for glucose and ethanol. Ethanol could not be quantified for the activated carbon extraction because it eluted at a retention time close to the one of acetone. Error bars in all figures represent the standard error of mean (SEM).
2.4 Microbial Community Analysis
2.4.1 DNA Isolation
To extract DNA from the fermentation broth samples, a method for DNA extraction from high-strength wastewater sludge was adopted (Tabatabaei et al. 2010).
2.4.2 16S rRNA Gene Amplicon Sequencing
The extracted DNA samples were sent to Macrogen Inc. (Seoul, South Korea) where 16S rRNA gene libraries were prepared using the universal primers 337F (GAC TCC TAC GGG AGG CWG CAG) and 805R (GAC TAC CAG GGT ATC TAA TC) which amplify the third and fourth variable regions. The barcoded libraries were combined together and sequenced using Illumina MiSeq (Control Software v2.2). The average throughput per sample was approximately 52.5 million bases and 245,000 reads in total (forward and reverse reads combined). The raw sequences were uploaded to the NCBI sequence read archive (SRA), and the accession numbers for the percolation reactor samples are SRX2672533, SRX2672534. The accession numbers for the semi-continuous fed reactors are SRX2672535, SRX2672536, and SRX2672537.
2.4.3 Bioinformatics Pipeline and Analysis
FastQ files were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME V1.9.1) software tool (Kuczynski et al. 2012). The sequences were aligned with the August 2013 version of the Greengenes Database (The Greengenes Database Consortium). Note that the relative abundancies shown were not corrected for differences in 16S rRNA gene copy numbers.
2.5 Techno-Economic Analysis
The techno-economic analysis was based on Bonk et al. (2015), a percolation system followed by a composting stage. The data used for the techno-economic analysis consisted on the product concentrations of the first percolation experiment in this study. All other process parameters and economic assumptions were left identical to the non-pretreatment scenario used by Bonk et al. (2015). No gate fee was applied, i.e., food waste does neither bring cost nor income to the LA producing waste treatment plant. To estimate the separation cost of LA from water, an energy cost of 0.0068 USD/kgwater (López-Garzón and Straathof 2014) was used. The income from the by-products (ethanol and carboxylic acid) was neglected.
3 Results and Discussion
3.1 First Process Insights Based on Semi-Continuously Fed Reactor
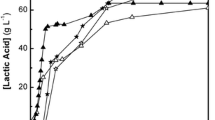
Figure 1 shows several sampling points of the uncontrolled pH fermentation study, with a semi-continuous fed reactor. LA concentrations reached a maximum of 20.1 gCODLA/L (18.6 gLA/L) after 10.5 days (before the next feeding). This concentration is much lower than for pH-controlled fermentation of food waste that lead to concentrations of 38 gCODLA/L (Zhang et al. 2008) or pure culture pH-controlled fermentations leading to 227 gCODLA/L (Castillo Martinez et al. 2013). A high product concentration is important to decrease the cost of removing water by, for example, evaporation during the product during the product recovery process (López-Garzón and Straathof 2014). The reason for the lower concentration does not lie in a substrate limitation (see Sect. 3.2). Rather, it is probably caused by product inhibition. In comparison to these pH-controlled experiments (minimum pH 5), the pH in our reactors was 2.96. At this low pH, more molecules of LA are protonated and therefore lead to more product inhibition, assuming that protonated carboxylic acids are the more damaging form (Rodríguez et al. 2006). On the other hand, the low pH might have the advantage that a highly stereoisomer-selective L-LA production could occur as previously discovered by Zhang et al. (2008). However, we could not discriminate l and d-lactic isomers with our analytical method. Therefore, further investigations are required in this regard. In conclusion, lactic acid concentration in our pH-uncontrolled fermentation were lower than in pH-controlled experiments and might never reach such high concentrations due to a higher product inhibition at a low pH.
Another important aspect is the selectivity of lactic acid production, i.e., the amount of side products in the fermentation that increase the cost of product separation. In relation to all measured compounds, LA made up 49–67% (gCODLA/gCODtotal products). Ethanol was found as the major by-product (<4.9 gCOD/L). The contamination by ethanol might impose only minor practical challenges on the recovery of pure LA because its boiling point (78 °C) is quite different to the LA boiling point (122 °C). In addition, ethanol could be used for esterification of LA to yield valuable lactate esters (Castillo Martinez et al. 2013). On the other hand, contamination by other carboxylic acids can result in more problems for the recovery of pure LA, because some compounds have a very close boiling point to LA, for example, acetic acid (118 °C). LA made up 63–88% (gCODLA/gCODtotal acids) of the total acids, and acetic acid was the major acid by-product. Therefore the contamination of LA by carboxylic acids, and in particular by acetic acid, needs to be reduced.
Concerning the data quality, difference in the sum of all compounds measured by HPLC and the measured SCOD was up to 30%. This could be due to undetected compounds and/or measurement errors. Here, it is unclear if this is due to a measurement error or real differences in the biological replicates. The latter was not expected because no changes in the operation of the reactors were done nor was there any problem with the reactors detected.
To explore the composition of the microbial community, genomic DNA of all three reactors was extracted from samples from March19, 2015. Figure 2 shows the microbial community composition on order level. The semi-continuously fed reactors were dominated by Lactobacilalles. Note that no correction was done for the different 16S rRNA gene copy numbers in the genomes of the involved microorganisms. That being said, Lactobacilalles represented approximately 53% of aligned sequences in the semi-continuously fed reactors. In this order, species of the genus Lactobacillus of the family Lactobacillaceae were the most abundant species. The dominance of Lactobacilalles in the present study is in accordance with the recent discovery of the dominance of Lactobacillus in pH-uncontrolled mixed culture fermentation of agricultural peel wastes (Liang et al. 2016). Fermentation of fruit and vegetable waste was also recently found to result in dominance of Lactobacillus when controlled at pH = 4 (Wu et al. 2015). The raw FastQ sequencing files were also uploaded to Genbank.
In conclusion, selective lactic acid production was achieved at pH-uncontrolled conditions under semi-continuous feeding. The dominant order was Lactobacillus. Yet, higher concentrations, yields, and selectivities for lactic acid would be preferable. Therefore, batch experiments were conducted to evaluate how to improve this performance.
3.2 Process Understanding and Optimization Based on Batch Experiments
Batch experiments were conducted to understand better the factors influencing lactic acid production under pH-uncontrolled conditions and finally find conditions to improve concentrations, yields, and selectivities for lactic acid compared to the semi-continuously fed reactor. Only the most important batch results are presented below.
Figure 3 compares the product concentration of the batch experiment No. 3 (no shaking) after 11 days with the concentration of the inoculum (taken from the semi-continuously fed reactor 10.5 days after feeding plus 4 days in incubator). The relative amount of inoculum and food waste to working volume per feeding is the same for this batch and the semi-continuously fed reactor. The batch experiment resulted with 13.95 gCODLA/L in lower LA concentrations than the inoculum from the semi-continuous reactor (16.53 gCODLA/L). On the other hand, selectivity was higher for the batch experiment with 0.93 gCODLA/gCODtotal products compared to 0.8 gCODLA/gCODtotal products for the inoculum. A main difference lies in the higher ethanol and acetic acid concentration measured in the inoculum from the semi-continuously fed reactor. Acetic acid in the batch experiment was below detection limit, leading to a 96% (gCODLA/gCODtotal acids) selectivity of LA over all other acids. The LA yield was 0.28 gCODLA/gTS food waste fed. The reason for the higher ethanol concentration in the semi-continuously fed reactors is not clear. One possible explanation is that after 7 months of operation, there might have been a biofilm on the walls of the semi-continuously fed reactors, and this biofilm might be enriched for ethanol producing microbes. But we did not directly check for the existence of a biofilm to confirm this hypothesis. In support of this hypothesis, both the sequential batch and the fixed bed batch experiments show higher ethanol concentrations after 2 months (>4.4 gCOD/L) than at the start of the experiment (~0.3 gCOD/L). A potential biofilm formation leading to unwanted side products is an important issue and therefore should be followed up in future research. In conclusion, using the same food waste concentration, batch experiment No. 3 resulted in general in similar fermentation products as the semi-continuously fed reactor, and in detail in higher LA selectivity and lower LA concentrations.
Comparison of semi-continuously fed reactors with batch No. 3. Semi-continuous reactor sampled 10.5 days after feeding plus 4 days in incubator. Batch sampled at day 11. Error bars: 1 SEM (technical duplicate semi-continuously fed reactor, biological duplicate batch; error bar of first bar for each date is the SEM of the sum of products). Succinate, Formate, Valerate, Hexanoate below detection limit
The batch experiment at 50 °C (batch No. 4) resulted in a lower LA concentration than the mesophilic control (batch No. 1). Shaking (batch No. 2) and shaking plus grinding (batch No. 5) increased the LA concentration by 27% and 20%, respectively. The batch experiment with additional mineral medium (batch No. 15) did not show any improvements ruling out nutrient limitation. This is further supported by the pH-controlled batch experiment (batch No. 7) that resulted in much higher product yields (up to 0.79 gCODtotal products/gTS food waste fed) than the pH-uncontrolled experiments. Therefore, product inhibitions by protonated acids and ethanol as well as the low pH seem to be the limiting factors to higher product concentrations, rather than substrate or nutrient limitations. Increasing the initial food waste concentration to 158 gTS/L (batch No. 17) leads to LA concentration of 31.8 gCOD/L but lowered the yield (to 0.2 gCODLA/gTSfood waste fed). The selectivity over the total fermentation products remained high (0.93 gCODLA/gCODtotal products, 0.95 gCODLA/gCODtotal acids). The higher LA concentration might be the result of the higher buffer capacity resulting from the high initial food waste concentration. The final pH of 3.3 was higher the pH in most other batch experiments and the semi-continuously fed reactors. The higher pH lowers product inhibition by protonated acids. Fixed bed and sequential batch experiments (batch No. 11 and No. 18) lead to increased ethanol concentrations but also to higher selectivities of LA over other organic acids. Evaluating the impact of inoculum is challenging because the inoculum itself contains LA and other products and has an impact on the initial pH. Therefore a batch with inoculum (batch No. 10, initial LA concentration 6.1 gCODLA/L, initial pH 3.25) was compared with a batch without inoculum but with LA addition (batch No. 6, initial LA concentration 5.4 gCODLA/L, initial pH 3.23). Both resulted in the same selectivity for LA (93% gCODLA/gCODtotal products) and a similar LA concentration (15.1 gCODLA/L for batch 10 and 16.2 gCODLA/L for batch 6). Therefore, it seems like the microorganisms present in the inoculum did not play a crucial role. The microorganisms present in the model food waste seemed to be sufficient for the fermentation. Nevertheless, the use of inoculum lowers the initial pH resulting in higher LA selectivities.
From a techno-economic point of view, the most interesting result is that a simple batch fermentation system with a high food waste load without shaking resulted in high LA concentrations and selectivities. The high LA concentration combined with a high selectivity over acetic acid is important to reduce product separation costs. The low yield is disadvantageous, especially if food waste becomes a resource in the future that needs to be paid for. In that case, a higher yield strategy will become economically critical.
3.3 Percolation System as Practicable Large Scale Reactor Configuration
3.3.1 Experimental Results
Figure 4 shows the fermentation products of the percolation system after 3 and 7 days. After 7 days of percolation, the final LA concentration reached 16.4 gCOD/L with a yield of 0.15 gCODLA/gTSfed. The calculated product COD concentration differed less than 1% from the measured SCOD at day 7. The LA selectivity of 78% (gCODLA/gCODtotal acids) was much lower compared to the batch experiments. We hypothesize that this is due to the higher buffer capacity and the higher final pH of 3.74, both resulting from the higher food waste amount per fermentation broth. A higher buffer capacity leads to a slower pH drop and therefore results in a longer time period favorable for VFA production. Possible improvements to increase the selectivity are to change the food waste—fermentation liquid ratio, leave some of the percolation liquid for the next batch, or to have a continuous liquid exchange disconnected to the percolation tube. Nevertheless, even with the simple percolation set-up used in this study, lactic acid can be produced as major fermentation product in concentrations similar to batch and semi-continuous experiments.
3.3.2 Techno-Economic Assessment
Section 3.3.1 has shown that LA can be produced in a pH-uncontrolled percolation system but that further process optimization is still required. Nevertheless, this is a practicable system for industrial scale implementation, and therefore a techno-economic evaluation is already interesting at this stage. Excluding the cost of lactic acid separation and purification, the minimum selling price of LA was estimated to 362 USD/tLA. This is low compared to the market price of 1000–1800 USD/tLA (Bastidas-Oyanedel et al. 2015), but the separation and purification of LA from the fermentation broth remains a challenge and uncertainty in the techno-economic assessment. For a first estimate, purification of LA can be neglected assuming that LA selectivities in the fermentation can be further optimized in the future. In that case, the separation cost of LA from the fermentation broth can be roughly estimated by the cost of removing water. Evaporation is a standard process to achieve this separation, but the costs are high for dilute aqueous solution (López-Garzón and Straathof 2014) such as the fermentation broth from the percolation reactors. Therefore, assuming evaporation as separation technology will result in a conservative separation cost. For the LA concentration of percolation experiment (16.4 gCODLA/L, 15.2 gLA/L), the energy costs to evaporate the necessary 65 twater/tLA were estimated to 442 USD/tLA. This results in a LA production cost of 804 USD/tLA, excluding equipment cost for evaporation. This is still 200–1000 USD/tLA below today’s market price. Assuming that the performance of the batch experiments (batch No. 17) can also be achieved in the percolation system, the total LA production cost including separation from water would be 514 USD/tLA.
Evaporation is rather an unpracticable separation technology in our case because of the high energy requirement of evaporation. Therefore, the techno-economic feasibility of other separation processes should be evaluated in the future, for example, chromatography (Thang and Novalin 2008), reverse osmosis (Diltz et al. 2007), electrodialysis (Wang et al. 2013), or adsorption as presented in the experimental results. In particular, technologies are preferable that allow for small-scale decentralized waste management plants. In this context, the idea of a fermentation process that requires no pH control is particularly attractive.
LA can be produced via two different metabolic routes, leading to the isomers L and/or D (Hoelzle et al. 2014). Using pure culture LA fermentation, a higher selectivity for L-LA was found for pH-uncontrolled fermentation (Abdel-Rahman et al. 2013). Unfortunately, the isomeric purity of the LA produced in this study could not be discriminated. But if this is the case, the economic feasibility would again increase for producing a more valuable pure isomer with a price of about 2400–3400 USD/t (Taian Health Chemical Co. Ltd. 2016).
This estimate on the techno-economic feasibility showed that the production of LA from food waste has a realistic potential to become a waste treatment option, but before it can be realized, further research and development in the field of separating LA from the fermentation broth in an energy efficient and sustainable way is required.
3.4 In Situ for Product Removal and Yield Increase
The techno-economic analysis showed that the recovery of LA from the fermentation broth remains a major challenge. In addition, the experiments resulted in LA yields as low as 0.15 gCODLA/gTSfood waste fed for the percolation system and 0.2 gCODLA/gTSfood waste fed for the batch experiments with the highest LA concentration (batch No. 17). A low yield also has a negative impact on the process economics. In situ extraction using activated carbon could be a solution for both problems. Instead of recovering LA after the fermentation, in situ extraction removes LA from the fermentation broth during the fermentation, thereby decreasing product inhibition and enabling higher product yields. Gao et al. (2011), for example, increased the LA yield in pure culture fermentations by 50% using in situ extraction with activated carbon. But their glucose-based fermentation is not comparable to a “dirty” food waste fermentation because the latter contains particulate matter that potentially clogs the pores of the activated carbon and reduces its performance.
Figure 5 shows the results of the batch fermentations with additional activated carbon after 7 days of fermentation. The LA yield could be increased by 35% (gCOD/gTSfood waste fed) by the use of activated carbon (batch 20) compared to the control without activated carbon (batch 17). Note that the adsorption experiment has no duplicates. The LA adsorpted to activated carbon (and desorpted with acetone) was 18.6 mgLA/gactivated carbon. Yousuf et al. (2016) tested the adsorption of carboxylic acids to activated carbon in fermentation broths, containing LA with a concentration of 12.53 gCOD/L and found a very similar adsorption of 18.63 mgLA/gactivated carbon. These numbers are not directly comparable to the present values, since Yousuf et al. (2016) used a different kind of activated carbon, had a different fermentation broth and removed particles from the fermentation broth by centrifugation. Nevertheless, our results suggest that in situ extraction with activated carbon can be achieved even in a “dirty” fermentation broth with particulate matter. This is not an obvious result, because it could also be that the activated carbon pores get blocked with food waste particles and microorganisms, limiting the mass transfer. It could be that such effects appear after recycling activated carbon several times. It remains uncertain if all lactic acid was desorpted from the activated carbon. In batch experiment No. 19, it was tested a second acetone extraction of LA from activated carbon, and additional 18% LA were desorpted, indicating that most LA was already desorpted in the first extraction.
In the future, the use of in situ (or online) extraction of LA from a pH-uncontrolled fermentation of food waste in a percolation system can be a promising waste management option. In such a scheme, pure, and potentially isomerically pure, LA could be gained via in situ extraction with positive effects on the product yield. The LA remaining in the fermentation broth can be flexibly used depending on the market situation, for example, as a substrate for other bioprocesses, as final product after separation from the fermentation broth, or used for energy production (e.g., by bioelectrochemical systems or via converting LA to biomethane in an anaerobic digester).
In conclusion, in situ extraction has the advantage to remove carboxylic acids already during the fermentation, thereby reducing product inhibition. For a practicable application, activated carbon and acetone need to be recycled several times. This needs to be studied further.
4 Conclusions
Simple and practicable process configurations for lactic acid production in pH-uncontrolled food waste fermentations were successfully tested, in particular batch fermentation without mixing and a percolation system. The percolation system appeared to be techno-economically feasible but product recovery remained a major obstacle. In situ extraction using activated carbon was successfully tested as a simple way of product recovery that additionally increases lactic acid yield. In a future food waste biorefinery scheme, some of the lactic acid could be recovered by in situ extraction, while the remaining lactic acid in the fermentation broth could be fed to another bioreactor, e.g., for bioenergy production.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Aceves-Lara CA, Trably E, Bastidas-Oyanedel JR, Ramirez I, Latrille E, Steyer JP (2008) Bioenergy production from waste: examples of biomethane and biohydrogen. J Soc Biol 202:177–189
Angelidaki I, Alves M, Bolzonella D, Borzacconi L, Campos JL, Guwy AJ, Kalyuzhnyi S, Jenicek P, van Lier JB (2009) Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol 59:927–934
Bastidas-Oyanedel JR, Mohd-Zaki Z, Pratt S, Steyer JP, Batstones DJ (2010) Development of membrane inlet mass spectrometry for examination of fermentation processes. Talanta 83:482–492
Bastidas-Oyanedel JR, Bonk F, Thomsen MH, Schmidt JE (2015) Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Biotechnol 14:473–498
Bastidas-Oyanedel JR, Fang C, Almardeai S, Javid U, Yousuf A, Schmidt JE (2016) Waste biorefinery in arid/semi-arid regions. Bioresour Technol 215:21–28
Bonk F, Bastidas-Oyanedel J, Schmidt J (2015) Converting the organic fraction of solid waste from the city of Abu Dhabi to valuable products via dark fermentation – economic and energy assessment. Waste Manag 40:82–91
Castillo Martinez FA, Balciunas EM, Salgado JM, Domínguez González JM, Converti A, Oliveira RPDS (2013) Lactic acid properties, applications and production: a review. Trends Food Sci Technol 30:70–83
Diltz RA, Marolla TV, Henley MV, Li L (2007) Reverse osmosis processing of organic model compounds and fermentation broths. Bioresour Technol 98:686–695
Gao C, Ma C, Xu P (2011) Biotechnological routes based on lactic acid production from biomass. Biotechnol Adv 29:930–939
Grootscholten TIM, Kinsky dal Borgo F, Hamelers HVM, Buisman CJN (2013) Promoting chain elongation in mixed culture acidification reactors by addition of ethanol. Biomass Bioenergy 48:10–16
Hoelzle RD, Virdis B, Batstone DJ (2014) Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol Bioeng 111:2139–2154
Ju F, Zhang T (2015) Experimental design and bioinformatics analysis for the application of metagenomics in environmental sciences and biotechnology. Environ Sci Technol 49(21):12628–12640
Kim DH, Lim WT, Lee MK, Kim MS (2012) Effect of temperature on continuous fermentative lactic acid (LA) production and bacterial community, and development of LA-producing UASB reactor. Bioresour Technol 119:335–361
Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R (2012) Using QIIME to analyze 16 rRNA gene sequences from microbial communities. Curr Protoc Microbiol 27(1):1E.5.1-1E.5.20
Liang S, Gliniewicz K, Gerritsen AT, McDonald AG (2016) Analysis of microbial community variation during the mixed culture fermentation of agricultural peel wastes to produce lactic acid. Bioresour Technol 208:7–12
López-Garzón CS, Straathof AJJ (2014) Recovery of carboxylic acids produced by fermentation. Biotechnol Adv 32:873–904
Rodríguez J, Lema JM, van Loosdrecht MCM, Kleerebezem R (2006) Variable stoichiometry with thermodynamic control in ADM1. Water Sci Technol 54:101–110
Tabatabaei M, Zakaria MR, Rahim RA, Abdullah N, Wright ADG, Shirai Y, Shamsara M, Sakai K, Hassan MA (2010) Comparative study of methods for extraction and purification of environmental DNA from high-strength wastewater sludge. African Journal of Biotechnology 9(31):4926–4937
Taian Health Chemical Co. Ltd (2016) Factory price tech grade 50-21-5 l-lactic acid price [WWW Document]. http://www.alibaba.com/product-detail/food-grade-L-Lactic-acid-CAS_60464833300.html?spm=a2700.7724838.0.0.7BlTBV. Accessed 19 June 2016
Thang VH, Novalin S (2008) Green biorefinery: separation of lactic acid from grass silage juice by chromatography using neutral polymeric resin. Bioresour Technol 99:4368–4379
Wang X, Wang Y, Zhang X, Feng H, Xu T (2013) In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: continuous operation. Bioresour Technol 147:442–448
Wu Y, Ma H, Zheng M, Wang K (2015) Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour Technol 191:53–58
Wu Q-L, Guo W-Q, Zheng H-S, Luo H-C, Feng X-C, Yin R-L, Ren N-Q (2016) Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: the mechanism and microbial community analyses. Bioresour Technol 216:653–660
Yousuf A, Bonk F, Bastidas-Oyanedel J-R, Schmidt JE (2016) Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour Technol 217:137–140
Zanella O, Tessaro IC, Feris LA (2014) Desorption- and decomposition-based techniques for the regeneration of activated carbon. Chem Eng Technol 37:1447–1459
Zhang B, He P-J, Ye N-F, Shao L-M (2008) Enhanced isomer purity of lactic acid from the non-sterile fermentation of kitchen wastes. Bioresour Technol 99:855–862
Zhang F, Zhang Y, Ding J, Dai K, van Loosdrecht MCM, Zeng RJ (2014) Stable acetate production in extreme-thermophilic (70°C) mixed culture fermentation by selective enrichment of hydrogenotrophic methanogens. Sci Rep 4:5268
Acknowledgments
The author would like to acknowledge the financial support from Masdar Institute (Project 2BIONRG, 12KAMA4, and BIOREF, 13KAMA1), to help fulfill the vision of the late President Sheikh Zayed Bin Sultan Al Nahyan for sustainable development and empowerment of the United Arab Emirates and humankind.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bonk, F., Bastidas-Oyanedel, JR., Yousef, A.F., Schmidt, J.E. (2019). Exploring the Selective Lactic Acid Production from Food Waste in Uncontrolled pH Mixed Culture Fermentations Using Different Reactor Configurations. In: Bastidas-Oyanedel, JR., Schmidt, J. (eds) Biorefinery. Springer, Cham. https://doi.org/10.1007/978-3-030-10961-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-10961-5_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10960-8
Online ISBN: 978-3-030-10961-5
eBook Packages: EnergyEnergy (R0)