Abstract
The management of nonunions can be very difficult. They have a significant impact on one’s function and can be debilitating. It requires a thorough understanding of the factors that can result in a nonunion. This includes patient factors as well as surgical factors. Nonunions can occur because of biological factors, mechanical factors, or both. Smoking , diabetes, and vitamin D deficiency all have been linked to potential risk factors for nonunions. It is important also to understand how nonunions are classified which can guide treatment principles. Evaluation requires a history of the original injury including the treatment rendered, medical history of the patient to include a social history with regard to habits and a careful physical examination. Appropriate radiographs, laboratory studies, and advanced imaging may be warranted. Infection must always be suspected and ruled out. An analysis of the original treatment is required. If operative intervention was performed, the implants used should be critically evaluated as to the technique and appropriateness of the fixation construct. If there was inadequate stability, a hypertrophic nonunion can develop. If the soft tissue envelope is not respected or there was significant soft tissue injury as in open fractures, an inadequate biological environment may occur leading to a nonunion. The etiology must be addressed whether it is improving the mechanical stability, providing a biological stimulus with bone grafts or other biologic adjuncts or both.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Fracture healing is a very unique process in the human body. Bone is a unique tissue in that it can regenerate itself during the process of healing. This requires a very complex process which is regulated by various metabolic and hormonal factors to include various growth factors. These biological processes occur at the cellular level requiring recruitment proliferation and differentiation of many cells including endothelial cells, osteoprogenitor cells, platelets, macrophages, mesenchymal stem cells (MSCs) , and monocytes. These cells secrete various biologically active molecules at the site of injury to facilitate fracture repair. The bone morphogenetic proteins (BMPs) are osteoinductive agents which promote the proliferation and differentiation of undifferentiated cells to become either osteoprogenitor or chondroprogenitor cells. Although our bodies have the inherent capability to repair the fracture, the fracture healing process can be impaired for numerous reasons.
When the fracture healing cascade stalls, a delayed union may develop, but the process may altogether cease. In a delayed union , both clinical evidence and radiographic evidence of healing do progress, but it lags behind what the normal healing time should be for a particular bone. There are however many factors to take into consideration such as the particular bone involved, the specific anatomic regions of the particular bone, the fracture pattern, as well as the method of treatment. There are certainly specific areas within the skeleton that already have a predisposition to impaired healing due to both biologic and mechanical factors such as the subtrochanteric femoral region . Additionally, the treatment method may contribute to a nonunion due to the inadequate mechanical environment provided by the choice of fixation. Often times the diagnosis is more retrospective in nature then prospective. Nonoperative interventions such as various noninvasive stimulation devices or medications can potentially augment the slow fracture healing process.
A delayed union may eventually heal or eventually may become a nonunion. Often times it is difficult to diagnose a nonunion in real time, and much of the time the diagnosis is made retrospectively. If the process stops altogether, a nonunion has developed which may require intervention. The US Food and Drug Administration (FDA) has defined a nonunion as a fracture that is at least nine months old and has not shown any signs of healing progression for at least three consecutive months [1]. From a clinical perspective, we define a nonunion as one in which the normal fracture healing process has ceased, to the extent that, without further treatment, healing will not progress. Thus, the nine-month rule should not be applied to all fractures and be based more upon the clinical presentation and the individual patient [2]. In addition to a lack of clear-cut “time” guidelines for a nonunion, there is difficulty in assessing a fracture for a nonunion based upon radiological findings and a wide disparity exists in orthopedic surgeons’ perceptions of nonunion criteria and time points for nonunions [3]. Additionally, it is well known that there are certain bones that are at a greater risk to go on to a nonunion. This may be due to the location on a certain bone due to vascularity issues or the whole bone itself, e.g., scaphoid. In certain situations, the associated bone loss that occurs clearly exceeds any critical size defect and will not heal with fixation alone, and thus, a nonunion is the expected result. It would be inappropriate to delay intervention in these patients until 9 months per the FDA definition. One can clearly see that the details of each case must be taken into consideration when deeming it a nonunion.
There has been considerable discussion regarding the costly burden of nonunions financially, but the affects on functional outcome and the quality of life can be devastating. In a study of tibia nonunions, the authors found that these patients had high per patient costs overall with increased healthcare resource usage [4]. In a study by Kanakaris and Giannoudis [5], the increased costs were also associated with humeral and femoral nonunions in addition to tibia nonunions. Not only are there direct costs associated with the treatment, but also significant indirect costs associated with losses in productivity [6]. Earlier treatment based on earlier diagnosis could result in significant financial savings to the healthcare system and society. In addition to the additional cost, there are significant impacts to the quality of life and functional outcome of these patients. In a study evaluating patients that have tibial shaft nonunion s with functional outcome scores, Brinker et al. [7] found that the SF-12 scores (physical and mental) indicated an extremely disabling effect on physical and mental health. The impact on physical health was comparable to that of end-stage hip arthrosis and worse than congestive heart failure. In a follow-up study, Schottel et al. [8] found that all longbone nonunions had a very low health-related quality of life based upon Time Trade-off direct measures to determine utility scores. Long-bone nonunions had a utility score of 0.68 that was well below that of type-1 diabetes (0.88), stroke (0.81), and HIV (0.79). Those with forearm nonunions had the worst quality of life. Unfortunately, even with successful treatment of the nonunion, it has been shown that, at least in respect to tibial nonunions, there is a long-term negative impact on one’s quality of life [9]. The indirect burden to society remains unanswered.
It has been estimated that between 5 and 10% all patients will have some difficulty in healing their fracture [6, 7]. It has also been reported that 1 out of 6 fractures that have delayed healing will go onto a nonunion [10]. Additionally, the incidence is also variable depending upon the anatomic area in question. Unfortunately, the overall incidence of delayed union and nonunion following fractures has been thought to be increasing due to various factors including an aging population, increased obesity, diabetes, smoking , vitamin D deficiency , as well as improved survival rates of patients with multiple injuries. These aforementioned factors certainly affect the biological aspect of fracture healing; however, the mechanical aspects of fracture healing can also be problematic. The mechanical factors are often dependent upon the type of treatment method chosen by the surgeon in discussion with the patient. The mechanical stability that can be achieved at the fracture site is dependent upon the type of stabilization method used whether it be nonoperative or operative means. Cast stabilization of the fracture has the least amount of stability, but can be effective in many fractures that are amenable to nonoperative management. Methods of surgical fixation include open reduction and internal fixation, external fixation, and intramedullary nailing. This multitude of options can lead to a vast spectrum of stability. This affects the type of fracture healing that can occur, either primary or secondary fracture healing, in which callous formation occurs in the latter type. The interplay of biologic factors, including osteogenic cells and the extracellular matrix, which acts as a natural scaffold, and growth factors inherent to fracture hematoma along with the mechanical environment forms the basis of the diamond concept of fracture healing introduced by Giannoudis et al. [11]. All of these factors should be taken into consideration in the management of nonunions as well. Neglect of one of these key cornerstones of fracture healing can doom the treatment of the nonunion.
Many people have tried to elucidate factors, biological markers , or other aspects of the fracture or treatment that could contribute to a nonunion allowing one to potentially predict which fractures or which patients may progress on to a nonunion [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The establishment of a nonunion on radiographs does not necessarily imply the need for operative intervention. Nonunions maybe asymptomatic, and therefore, both clinical and radiological findings as well as the patient’s current function and wishes are necessary to determine the best course of action in the management of a nonunion. Surgical intervention of the original fracture can often times make the diagnosis of a nonunion difficult especially in the absence of associated hardware failure. Thus, the evaluation, diagnosis, and the treatment of a nonunion can be very complicated [10, 31]. It requires a thorough understanding of the original injury and treatment, subsequent treatments as well as patient comorbidities, which may have contributed to the development of the nonunion.
1.2 History
Evaluation of a nonunion should begin, first and foremost, with an evaluation of the patient and their medical history. A thorough evaluation and review of the patient’s past medical and surgical history including medications are very important in helping to elucidate the etiology of the nonunion. It is important to take a medical history and assess for vascular disease, malnutrition, diabetes, social history, and metabolic bone disease such as osteoporosis, endocrine disorders, vitamin D deficiency , hepatic and renal disorders, steroid use, and rheumatologic disorders. Many of these comorbidities will be discussed below under “etiology.” Social issues such as smoking or illicit drug use are important to note as these things may prevent healing or increase the risk of complications. A thorough and complete physical examination should be performed on all patients presenting with a nonunion. The physical examination should include a general physical which may point to other underlying disorders that may have been overlooked. Detailed examination of the extremity involved should be performed to include an evaluation of the neurovascular status, looking for open wounds (draining sinuses), healed lacerations (indicative of perhaps an open injury), healed incisions, clinical alignment, joint motion, and examination of the presumed nonunion site for motion. Any open wound or draining sinus in proximity to the fracture should lead one to suspect a septic nonunion and is so until proven otherwise. Such open wounds must be taken into consideration, and a soft tissue reconstruction plan will need to be integral to the overall bony reconstruction. Previous incisions may limit options and may dictate how previous hardware is removed. Alternative approaches may need to be employed if the existing soft tissues are scarred in or suboptimal for further surgical intervention. If there is a deformity, correction of the malalignment has to be taken into consideration as well. This includes any leg length discrepancy that may need to be addressed. Joint motion may be limited from arthrofibrosis or a result from a false joint at the nonunion site, or patients may have developed contractures. Any surgical plan must take into consideration the need for lysis of adhesions, soft tissue releases, etc., to insure the best possible overall outcome. In short, preoperative planning taking all these factors into consideration before going down the reconstructive pathway is paramount.
It is extremely important to obtain an accurate history of the original injury mechanism as well as other fracture characteristics. It is important to determine whether or not the fracture was from a high-energy or low-energy injury. The extent of the initial soft tissue injury as well as the amount of periosteal stripping that may have been encountered at the time of surgery or because of the surgery may shed light on the potential cause of the development of the nonunion. It has been recently suggested that compartment syndrome and associated fasciotomy may be a risk factor for the development of nonunion in tibia fractures [12]. Open fractures obviously have much more soft tissue damage, and the potential for an occult infection and septic nonunion must also be taken into consideration.
A careful evaluation of all previous surgeries is critical, especially the index operation. Review of the operative reports and/or injury radiographs along with the immediate postoperative films can be crucial to understanding the underlying cause. Subsequent interventions should also be evaluated in a similar manner, taking into consideration the pre- and post-op radiographs and the details of the surgical procedure. If bone grafting or biologic adjuncts had been done or used at any time, the type of bone graft or adjunct, the location of harvest of the autogenous bone graft, should be noted. Previous sites of harvest may limit future options. Inadequate fixation or extensive surgical exposures can be large determinants in the development of a nonunion. In fractures treated with intramedullary nails, external fixation , cast stabilization , or bridge plating, a relatively stable construct has been created allowing for callous formation. In cases of open reduction and internal fixation (ORIF) , an environment with absolute stability often is created allowing for primary bone healing without callous formation. The surgical assault obviously affects the amount of soft tissue stripping which can affect the amount of blood supply to the fracture site. Additionally, past surgical interventions and hardware that is present can certainly affect future treatment options for the management of the nonunion.
A thorough evaluation of prior complications should be performed. Any history of infection should increase one’s suspicions for continued infection even in the absence of clinical signs or symptoms. Nerve injuries should be assessed as this may limit the overall outcome of any nonunion reconstruction and may lean one toward a more definitive intervention such as amputation. Previous vascular injuries may require further assessment in terms of viability of the previous repair and a thorough assessment of the vascular status of the limb.
1.3 Risk Factors for Nonunion
Biological factors and mechanical factors can contribute to the development of a nonunion. These can be related to the patient or the intervention performed by the surgeon. If the patient has been referred in, as mentioned previously, it is helpful to obtain previous injury radiographs, computed tomography (CT) scans, and other imaging studies as well as operative reports to understand what was done and why it was done. If you are the index surgeon, it is important to critically asses your own surgical intervention to determine whether things that were done may have contributed to the nonunion. Decision errors can always occur, and what is successful in one patient may not be so in another patient. In any event, risk factors for the development of a nonunion can be classified as patient dependent or independent [10, 25]. Many of the independent factors are more surgeon-dependent factors or injury characteristics.
The injury characteristics unique to a specific fracture location will be discussed in each specific anatomic section, but some generalities can be made. Areas that are known to have tenuous blood supplies have been shown to be at risk of nonunion [10, 28, 32]. Such areas include the femoral neck, subtrochanteric region of the femur, the scaphoid, the talus, the metadiaphyseal region of the fifth metatarsal, and tarsal navicular body. Open fractures with their significant soft tissue stripping clearly have increased risks of nonunion as well as infection [23, 25, 26, 28, 29]. The associated soft tissue injury and muscle loss in severe open injuries can result in loss of the blood supply to the bone resulting in a detrimental effect on the healing process and increasing the risk of infection. Lin showed that functional outcomes in patients with open tibia fractures were worse than those with closed fractures [33]. Westgeest et al. [29] found that fractures which were classified as open grade IIIA injuries were associated with delayed healing and nonunion. Additionally, in this prospective cohort of 736 subjects, all with open long bone fractures, deep infection was associated with delayed healing and nonunions. In a retrospective study of long-bone fractures treated with intramedullary nailing, Malik et al. [23] found that open fractures had a significant association with the development of deep infection which also was associated with the development of a nonunion. In the same study, they alluded that opening of a closed fracture also was a significant contributor to the development of a nonunion, and therefore, opening of the fracture, in cases of intramedullary nailing, be avoided if possible. In the study by Blair et al. [12], fasciotomy for compartment syndrome in tibia fractures, which in essence is opening of the fracture, was also associated with significant increase in both infection and nonunion. In an effort to prevent infection in open fractures, it is well established that antibiotics be administered as rapidly as possible and hopefully within an hour of the fracture presenting [34]. Often times the open fractures are also associated with significant bone loss and in most cases such defects cannot heal on their own and are expected to become nonunions if left alone. These eventually will require bony reconstruction. The type of reconstruction, timing of bone graft placement, and the source of bone graft is highly variable among orthopedic trauma surgeons [35]. Determining the amount of bone graft for such defects can be problematic, and some have tried to develop quantitative models to determine the amount needed [36]. Other fracture characteristics that need to be assessed include the degree of displacement, the extent of comminution, the amount of cortical apposition at final fixation, and the stability of fixation [24, 25, 28, 32, 37, 38].
Surgeon factors can contribute to either biological reasons for the development of a nonunion or a mechanical one [23, 25, 28, 32]. Contributions to a biological cause include excessive stripping of soft tissues, failure to bone graft at the appropriate time, and inadequate debridement of devitalized/dead bone, which can lead to infection, which then may prevent union. Mechanical factors introduced by the surgeon are related to the method of treatment and/or implant for the original fracture. Fracture stabilization has significant affects on fracture healing. In a literature review by Hildebrand et al. [37], the type and timing of fracture stabilization can alter the systemic inflammatory response after trauma and can affect fracture healing. They also found that the type and stability of the fracture stabilization affects gene expression involved in fracture healing. Relative stability constructs such as intramedullary nailing, cast immobilization, and external fixation allow the fracture to heal by callus formation; however, excessive motion could lead to a hypertrophic nonunion. The rigidity of the fracture fixation has been shown to improve the process of healing [37]. Reaming of the canal in intramedullary nailing can increase the size of the nail and enhance the mechanical stability. The effect of reaming has been looked at extensively [39]. It has been well established that reaming enhances fracture healing and that there is a higher incidence of delayed union and nonunions in unreamed nails with more secondary procedures to obtain union [23]. This is true despite a recent study showing that the functional outcomes in tibia fractures were not affected by reaming [33]. Inadequate internal fixation when one is trying to achieve absolute stability to create an environment for primary bone healing can also lead to excessive motion and a subsequent nonunion. Niikura et al. [25] reviewed 102 nonunions of which almost 80% were related to or solely caused by inadequate stability or reduction. Conversely, rigidly fixing fracture fragments with gaps or without proper internal fixation techniques such as obtaining compression across fracture planes may delay or even prevent healing [31]. Fixation can be too rigid leading to a failure in healing. If the patient had undergone what was felt to be appropriate fixation with appropriate surgical technique for the fracture in question, then it is important to investigate patient-related factors, both biological and mechanical, that may have contributed to the development of the nonunion. Brinker et al. [13] created an algorithm on when to refer patients for endocrine workups in relation to their nonunion. When evaluating the nonunion, the technical aspects of the fracture fixation should be assessed. If there was no technical error, then it was suggested that perhaps there was a metabolic etiology to the nonunion, and thus, the patient should be referred to an endocrinologist. If technical error was a crucial factor in the etiology, referral was not indicated. However, it is important to still assess metabolic issues even in light of inadequate fixation as many patients still have some deficiencies in bone metabolism [13].
Patient factors contributing to mechanical problems can be related to noncompliance with weight-bearing restrictions or an error in allowing the patient to weight bear too early. The healing process is always a race between hardware failure and fracture healing, and thus, when patients present with a nonunion in conjunction with hardware failure, the time from the original surgery is important in determining what came first—the hardware failure or nonunion, as each one can lead to the other. Often times, with plate failure there is an associated deformity through the nonunion site (Fig. 1.1). In cases of early hardware failure, often times the patient has started weight bearing too early or was allowed to do so. This is more common in cases of plate fixation. In these situations, the fracture has not healed sufficiently to handle the body weight and the implant is taking all the stress leading to early failure. Failure can be in the form of screw loosening, implant breakage, or bending. Depending on the fracture pattern and amount of comminution as well as the location, it may still unite. In the lower extremity more so than the upper, the alignment may gradually worsen as stability is lost and a mal-aligned nonunion can develop. In some instances, especially where there is comminution, as the angulation worsens resulting in more bony contact, the fracture may unite resulting in a malunion. In late cases of hardware failure, the fracture may have healed sufficiently to handle some weight in addition to the implant and may have maintained the alignment. After a while, the implant undergoes fatigue failure as the micromotion from the loading leads to failure of the implant at a stress riser such as a hole in the plate. The alignment is often times maintained, but the patient has pain and discomfort which necessitates surgical intervention. Loss of fixation can also occur without weight-bearing issues. This is often the case in patients with poor bone quality such as in those with comorbidities such as diabetes or osteoporosis. It is important to know whether patients have these conditions as special surgical and fixation techniques may need to be employed to obtain improved fixation by the judicious use of locked, fixed angle, or load-sharing devices such as intramedullary nails when appropriate.
Patient with right ankle injury treated with open reduction and internal fixation of fibula and closed treatment of distal tibia fracture. Referred for nonunion after progressive deformity developed. a–c Three views (anteroposterior [AP], mortise, lateral) of the right ankle show failure of the fibula hardware and mal-alignment with nonunion of both the tibia and fibula. Patient underwent hardware removal and cultures. d–f Three views (AP, lateral, and mortise) of the right ankle after hardware removal. Due to the malalignment and stiff nonunion, a Taylor spatial frame (TSF) was applied to allow correction and healing of the nonunion. g–h AP and lateral after TSF applied to right ankle prior to correction. i–j AP and lateral with TSF showing full correction of the deformity and realignment of the limb. k–m Three views (AP, lateral, and mortise) of the right ankle 1 year after consolidation of nonunion and removal of TSF
Patient medical factors contributing to a biological cause for the nonunion are many and can be problematic not only from the original fracture standpoint but also for the treatment of an established nonunion [10, 13, 25, 32, 40]. Established diseases such as vascular disease, rheumatologic disease, and s/p organ transplantation cannot be affected, but their effects on fracture healing and subsequent management of the nonunion need to be taken into consideration. Perhaps their steroids or immunosuppressive agents can be held for short time period which would allow for surgical intervention and healing, and such decisions should be made in conjunction with the patients’ appropriate other physicians. A multidisciplinary approach is needed to get many of these patients healed.
Although there are many endocrine abnormalities that can affect the musculoskeletal system, such as thyroid and parathyroid disorders, hypogonadism, and calcium imbalances to name a few [13], diabetes has had the most attention due to the high prevalence in the population. Diabetes has been shown to prolong healing times for fractures [40, 41]. It is also well documented that patients with diabetes have increased complications when dealing with musculoskeletal conditions, especially with fractures [32, 42, 43]. In a nationwide population based study out of Taiwan, diabetics were found to have an increased incidence of fractures as well as more adverse events and a higher mortality after fractures [42]. The addition of neuropathic complications can make even simple fractures that require surgery end up being disastrous for the patient. Wukich et al. [43] showed that patients with ankle fractures that had complicated diabetes had a 3.8 times increased risk of overall complications and a 3.4 times increased risk of malunion and nonunion compared to uncomplicated diabetic patients. These patients were also 5 times more likely to require revision surgery or arthrodesis. Diabetics need to understand that glucose control is extremely important for them to avoid diabetic complications of end organ damage, neuropathy, nephropathy, and peripheral arterial disease to minimize further musculoskeletal complications [32]. Diabetics should be treated with prolonged immobilization and delayed weight bearing compared to the nondiabetic to aid in avoiding complications. Additionally, many of these patients require additional fixation for otherwise straightforward fractures to try and prevent the late complications that occur with these injuries.
Vitamin D deficiency or insufficiency has been linked to nonunions, but a clear causal link is difficult to establish [13, 40]. Both the 25-OH vitamin D and 1, 25 OH2 vitamin D levels can be monitored, but the 25-OH level is the one that is important. Patients with 25-OH levels <20 are considered insufficient and between 20 and 30 deficient. It is not clear however whether higher levels than simply above the 30 level are needed in patients with fractures. Brinker et al. [13] showed that a preponderance of their nonunion patients had vitamin D deficiency. They had 37 patients that were evaluated for a metabolic or endocrine abnormality of which 68% (25 of 37) had vitamin D deficiency . It has become increasingly clear that many patients are vitamin D deficient or insufficient. In a meta-analysis of the literature, it was found that the pooled prevalence of hypovitaminosis was 77.5% in young trauma patients and 73% in geriatric fragility fracture patients [44]. In a follow-up study, the same authors showed that there is a lack of consensus in prescribing vitamin D to fracture patients. They found that 66% of surgeons tended to prescribe vitamin D to fragility fracture patients compared to 25.7% to nonfragility fracture patients [45]. The lack of prescribing in this population needs to be re-examined since the prevalence of low vitamin D in young trauma patients is high. Low vitamin D is more prevalent than previously thought and is widespread in patients of all orthopedic subspecialties and not just orthopedic trauma [46]. Management of vitamin D is easily done via replacement therapy and has been shown to be successful in raising serum levels [44]. In a study to evaluate the cost benefit of both calcium and vitamin D supplementation in all fracture patients, the cost of an 8-week course of treatment was determined and compared to the cost savings assuming just a 5% reduction in nonunions. This would result in a potential cost savings of $65,866 annually [47]. Many dosages of replacement therapy are available, but the authors’ preference is for high-dose (50,000 IU) vitamin D weekly for six months along with calcium supplementation. The target is to obtain a 25-OH level in the 40–60 range. Patients with low vitamin D can also develop secondary hyperparathyroidism and should also have a parathyroid hormone (PTH) level drawn when evaluating for a nonunion. The high PTH can contribute to the development of a nonunion [13]. In most cases, the high PTH will resolve with appropriate vitamin D replacement therapy.
Osteoporosis has also been linked to the development of nonunions [32]. The issues with osteoporotic bone healing are both biologic and mechanical [48]. By definition, osteoporotic bone is bone with less bone mass and as such is at an increased risk for fracture. The biologic changes that occur with osteoporosis, including a diminished level of mesenchymal stem cells and thus osteoblasts, a decrease in the chondrogenic potential of the periosteum and other alterations in the fracture healing pathway results in a less than robust fracture healing process [32, 40, 48]. Additionally, because of the lower bone mass, the fixation in such bone can be problematic and as such can lead to inadequate fixation and fracture stability. The result can be a nonunion. Many specialized techniques have been described in the management of osteoporotic fractures and should be employed when dealing with nonunions especially if mechanical failure was a significant contributor to the development of the nonunion. Locked plating, use of load-sharing devices, use of fixed angle devices, augmentation of fixation with cement or bone graft substitutes, adjunctive use of structural bone grafts, and preservation of soft tissue can assist in the management of these fractures and nonunions [48]. Although most osteoporotic individuals are elderly, age is an independent factor which can negatively affect fracture healing also resulting in delayed unions or nonunions [49]. This decline in healing potential can be attributed to hormonal changes, changes at the cellular level of fracture healing signaling, and diminished mesenchymal stem cells which all may also occur with osteoporosis. The true etiology still requires much more investigation due to the complex interplay that occurs in fracture healing and the overlap in physiology with aging and osteoporosis. Another confounding factor is that patients with osteoporosis are often being pharmacologically treated for their osteoporosis. The most common are the bisphosphonates , which are anti-resorptive agents and inhibit osteoclast function. The interference with remodeling of the bone has resulted in an unwanted side effect and resultant “atypical” femoral fractures. It is advised that these medications be discontinued during the fracture healing process [32, 48]. It is also important to evaluate all their medications and the potential effects that they may have on bone metabolism.
One of the most common class of medications that many patients take, both prescription and over the counter, are the nonsteroidal anti-inflammatory drugs (NSAIDs) . Recently, the use of NSAIDs during fracture healing has come under intense scrutiny [49,50,51]. Early reports of the use of NSAIDs in animal fracture healing models showed a clear deleterious effect [32, 49,50,51]. The doses required were very high. The mechanisms by which they are theorized to inhibit fracture healing include inhibition of prostaglandin synthesis and reduction of osteoblast activity, both of which result in an impaired fracture healing response [32]. Prostaglandins are needed during the inflammatory phase of fracture healing and help start the osteogenic response [6, 31, 38, 49]. Although a few clinical studies have shown a loose association between the use of NSAIDs and nonunions, it is controversial [50]. Kurmis et al. [51] performed a systematic analysis of over 300 relevant papers and concluded that there was not significant evidence to indicate a negative effect on fracture healing from the short-term use of NSAIDs after a fracture. Most of the clinical studies published in relation to NSAIDs in fracture healing were Level 5 evidence or expert opinion only [28]. Therefore, it is hard to make a recommendation on the use of NSAIDs both in terms of timing and duration immediately after a fracture. Due to the lack of guidelines and unknown true effects on fracture healing, the author’s practice is to avoid NSAIDS for the first 4–6 weeks after a fracture. This is especially true of Indomethacin. Additionally, we do not use Toradol (intravenous or per os) intra-operatively or immediately postoperatively for acute fracture cases. NSAID use after repair of nonunions has not been investigated to our knowledge.
Since inflammation is one of the initiating factors for bone healing, it has been suggested that perhaps healing may be altered in the polytrauma patient as well [32, 37, 40, 50]. These patients undergo a prolonged state of inflammation [40]. It is thought that the increased inflammation could delay fracture healing through a variety of cellular responses [20, 50]. Additionally, many of these patients also have multiple fractures that may require operative intervention. The post-op rehab protocol on one fracture may result in delayed stimulation of another fracture with resultant delayed healing or even nonunion. Other system injuries also may have an effect on fracture healing as well. A literature review by Hildebrand et al. [37] found that isolated hemorrhagic shock, chest trauma, severe soft tissue injury, and systemic inflammation can all affect fracture healing. Finally, it has been suggested that the American Society of Anesthesiologist (ASA) classification, which indicates overall health, was associated with nonunion development—the higher the ASA, the increase in probability of a nonunion [23].
Smoking has been clearly shown to inhibit fracture healing and result in both delayed unions and nonunions as well as increase the overall complications in the management of fractures [10, 21, 24, 28, 29, 32, 46, 49, 52,53,54]. Smoking has also been linked to an increase in fracture rates of the hip, distal radius, spine, and other osteoporotic fractures [52]. The exact mechanism and offending agent has not been clearly elucidated. Nicotine is one of more than 4000 chemicals that exist in cigarette smoke [46]. It has been shown to cause vasoconstriction (resultant hypoxia), platelet adhesion, and reduced cell proliferation for healing. All of these physiologic changes result in a negative effect on both wound and fracture healing [52]. However, it is not clear exactly which chemical is responsible for all the negative effects. Animal studies have had conflicting results with some studies, with nicotine alone, not showing the negative effects that are seen with smoking, whereas others have shown deleterious effects [49]. Nevertheless, the clinical literature overwhelmingly supports the increased risk of delayed unions, nonunions, and wound complications seen with smoking [21, 24, 52,53,54]. Scolaro et al. [54], in a systematic review of the literature, showed that smokers were over 2 times more likely (statistically significant) to develop a nonunion than nonsmokers. This was especially true in open fractures and tibia fractures. There was also a trend for longer healing times and infections (deep and superficial) in the smoking group. In a separate systematic review of the literature, Patel et al. [53] also found a negative effect of smoking on bone healing. They also looked at each study in relation to the bone or procedure in question. All the tibia fracture studies, except for one treated with external fixation, showed a clear increased risk of nonunion from smoking. This was also true in distraction osteogenesis, fibula fractures, ulna osteotomy healing, subtalar and ankle arthrodesis, and elective foot surgery. Fractures of the femoral diaphysis were not statistically significantly affected by smoking. In contrast, Hernigou and Schuind [21], in their retrospective study looking at diaphyseal fractures, found that smoking was significantly associated with nonunions (OR 8.25) in the femur, as well as the tibia and humerus. Westgeest et al. [29] found that in a prospective cohort study of open long-bone fractures, smoking (OR 1.73) was significantly associated with developing a nonunion. Murray et al. [24] looked at their series of diaphyseal clavicular fractures. They found that smoking was the strongest predictor of a nonunion (OR 3.76) and recommended that smoking cessation be an integral part of any treatment. However, getting patients to stop smoking is extremely difficult. The first step is acknowledging that smoking is bad for one’s health. Matuszewski et al. [55] performed a cross-sectional cohort survey study and found that smokers did not understand the negative effects of smoking on their general health or on fracture care. On a positive note, the orthopedic trauma patients surveyed seemed interested in smoking cessation more so than what was expected. They recommended formal education for smoking cessation. It is well accepted and has been shown that preoperative smoking cessation can reduce both pulmonary and wound complications postoperatively [46]. Educating the patients on the ill effects of smoking on fracture healing is part of our “discussion” with the patient being evaluated for nonunions. It is the author’s policy to not perform nonunion surgery on active smokers as long as the management can be done on an elective basis (aseptic nonunions). Both serum and urinary levels of cotine and nicotine are monitored to insure patient compliance. Although many feel that smoking cessation is the primary care physician’s responsibility, as an orthopedist it behooves us to play an active role to help maximize the patient’s outcome and minimize complications from any surgical intervention.
When evaluating a patient for a nonunion management, one must assess for the presence of the risk factors above. There are certainly more comorbidities than can affect fracture healing, but these are the most prevalent. These risk factors and/or co-morbidities should be improved upon or corrected if feasible. Many are injury or treatment related, but knowing those details can help devise an appropriate treatment plan for the nonunion.
1.4 General Principles
1.4.1 Diagnosis
The diagnosis of a nonunion is highly controversial because no gold standard exists for healing assessment [6, 15]. In a multinational survey of orthopedic surgeons, there was a 73 and 53% consensus that a lack of standardization in the definition for a delayed union and nonunion, respectively, existed. However, they did agree (88%) that the diagnosis should be done based on clinical evaluation and plain radiographs [3]. Pain on weight bearing was felt to be the most consistent predictor of delayed union and nonunion.
The diagnosis of a nonunion should be made on a series of radiographs in addition to the clinical picture. Often the fracture healing may be delayed, but critical evaluation of radiographs 6–8 weeks apart may show some improvement indicating progress. If the X-rays show no progress on two sets of consecutive images and the patient is having pain, then nonunion has probably been established assuming sufficient time has initially passed. The problem arises in the patient without symptoms but clear radiographic evidence of a nonunion. Many of these patients, because they lack symptoms, may not return in fear of needing surgery. The problem occurs when they return after hardware failure with new-onset pain and/or deformity. The time passed based on the FDA definition may not have been reached, but if cessation of all healing is indicated by plain radiographs and the patient is symptomatic, then intervention is probably warranted.
1.4.2 Radiographic Evaluation and Scoring
After the history and physical, evaluation should always begin with plain radiographs. It still remains the most common method of assessing for fracture union. However, just as in the lack of standardization of definitions, there is a lack of consensus on radiographic criteria as well. Dijkman et al. [56] reviewed the literature to look at radiographic criteria used in studies. They found that bridging of the fracture by bone, callus, or trabeculae was used 53% of the time. Bridging of the fracture across three cortices 27% of the time and loss of fracture lines was 18% of the time. The best interobserver reliability was found to be the number of cortices bridged by callus.
Despite the issues with radiological criteria , standard orthogonal views (anteroposterior and lateral) of the bone in question should be obtained. If the patient is referred in, previous studies are desired for comparison. In some cases, the fracture is actually progressing and reassurance is all that is needed. They may have a delayed union, but radiographic evidence of healing is occurring. The length, alignment, and rotation of the limb should be appropriately evaluated. In the lower extremity, if there is an associated deformity, then additional full-length radiographs (± ruler) from the hip to the knee are obtained to assess the mechanical axis of the limb (Fig. 1.2). Restoring the mechanical axis of the limb can aid in healing of the nonunion and should be part of the preoperative plan. If it appears to be short, then a scannogram (Fig. 1.3) or full-length radiographs with a ruler should be obtained (see Fig. 1.2 ). Oblique radiographs can aid in the diagnosis as well, if the standard anteroposterior and lateral do not clearly show the nonunion due to the obliquity of the original fracture or because of overlying hardware (internal or external fixation). Such views can also better define the plane of maximum deformity when that plane is not in the usual sagittal or coronal plane. Rotation can be assessed clinically in some situations; otherwise, a CT scan may be needed (Fig. 1.4).
When looking at the plain radiographs, the absence of bridging bone or callous at the fracture site, sclerotic fracture edges, bone resorption, or persistent fracture lines all may indicate a nonunion. It is imperative to also critically assess the implants and the initial fixation strategy to insure that the original type of healing wanted—primary versus secondary—was being achieved. Often times absolute stability was desired, yet there is callus formation on the radiographs (Fig. 1.5). This may indicate either excessive motion suggesting hardware failure or that the fixation was not as rigid as one wanted, allowing sufficient motion for callous formation. The fracture however may go on to heal. In other situations, it may go on to a nonunion with or without hardware loosening or breakage. Additionally, the radiographs should be assessed for periosteal reaction, loosening/lysis around hardware, and broken implants. Comparison to previous radiographs cannot be overemphasized.
a, b. Injury radiographs (anteroposterior [AP] and lateral) of patient with left humerus fracture after a motor vehicle collision. c, d Postoperative radiographs (AP and lateral) after open reduction and internal fixation performed in an effort to obtain absolute stability. e, f Follow-up radiographs (AP and lateral) showing unintended callus formation due to micromotion despite attempt at rigid fixation—infection workup was negative and patient went on to consolidate
As mentioned above, plain radiography alone is often times not a reliable tool for assessing fracture healing due to the lack of consistency among surgeons and interpretation of the films. It is clear that better ways of assessing fracture healing are needed [6]. Several clinical trials all have shown poor agreement between surgeons [15, 56]. Many have proposed criteria to standardize fracture healing assessment [57, 58]. One such assessment tool is the Radiographic Union Scale for Tibial (RUST) developed by Koolstra and his colleagues [58]. This scoring system assesses the presence or absence of fracture callus and the visibility of the fracture line on each of the four cortices. The scale is from 1 to 3 and based on callus and fracture line visibility at each cortex. A one is the absence of callus and a visible fracture line. A two is the presence of callus, but the fracture line is still visible. A three is for callus and the absence of a fracture line. The minimum score is 4, and the maximum is 12. This has shown to improve agreement for assessing union only in tibia fractures treated with intramedullary nails. Whelan et al. [59] showed an overall inter-observer reliability of 86% and intra-observer reliability of 88%. The RUST score has not been correlated with functional outcomes to date.
A similar scoring system was developed by Bhandari et al. [57] for use in hip fractures. The Radiographic Union Score in Hip Fractures or RUSH was developed to improve agreement in the assessment of femoral neck fractures. In a similar manner to the RUST, the RUSH evaluates cortical bridging on each of the four cortices as well as disappearance of the fracture line and an independent score is given. A one is given for no cortical bridging, two for some cortical bridging, and a three for complete cortical bridging. If the fracture line is visible, a one is given, a two for some evidence of the fracture line, and a three for no evidence of the fracture line. Two other aspects of femoral neck fractures are scored, the trabecular index based on consolidation and the disappearance of the fracture line. A score of 1–3 is assigned as well to each component. The overall minimum is 10 and maximum is 30. Their initial study showed that the RUSH improved agreement among reviewers regardless of subspecialty, but their agreement did not improve over time. A very important shortcoming was that the reviewer’s assessment was found to be potentially inaccurate without information regarding the time of the radiograph. They had 6 of 7 patients deemed as being healed at 2 weeks, which is not possible. Chiavaras et al. [60] extended the RUSH score to evaluate intertrochanteric hip fractures and evaluate agreement between radiologists and orthopedic surgeons. They found that the RUSH score did improve the overall agreement regarding fracture healing from fair to substantial between the two specialties.
Although scoring systems can be beneficial in determining union and providing a more objective measurement over time, the real issue is their use in predicting a nonunion. Recently, Frank et al. [19] did a study to assess the utility of the RUSH score to help define femoral neck fracture nonunion . They retrospectively pulled 250 cases from the FAITH hip fracture trial all of which had 6-month hip radiographs. They determined the RUSH score at 6 months for each case. They found that if the RUSH score at 6 months was <18, it had 100% specificity and a positive predictive value of 100% for a nonunion. They all had a 10 times greater risk of undergoing reoperation for a nonunion. If the patient does develop a nonunion of the femoral neck, a valgus intertrochanteric osteotomy is an option to obtain union. Varghese et al. [61] evaluated a group of 40 patients who underwent the procedure for a femoral neck nonunion developing after neglected fractures. They evaluated the presenting nonunion film for a radiographic index they called the neck resorption ratio (NRR) to determine whether that could predict nonunion of the valgus intertrochanteric osteotomy. The NRR is determined by measuring the length of the fractured head and neck fragment and comparing it to the length of the intact neck on the contralateral side (measured from the tip of the head to the intertrochanteric line). The NRR was found to be the most important factor in predicting union in their series. All patients that had a pre-op NRR of >0.52 had union. Taking this parameter into consideration before making treatment decisions in femoral neck nonunions may allow one to consider a more definitive treatment and avoid a repeat nonunion situation.
Although utilizing a score to predict nonunion after a reconstructive procedure can be useful, a score to predict nonunions for acute fractures would have greater applicability. The Nonunion Risk Determination (NURD) Score was developed by O’Halloran et al. [26]. The authors retrospectively reviewed all tibial shaft fractures at their institution over a 7-year period treated with an intramedullary nail. They had 382 patients with 56 nonunions. Factors were evaluated and they developed a logistic regression model to include seven of these factors. They assigned points to these seven factors. The NURD score gave 1 point for male gender, 2 points for open fractures, 3 points for chronic conditions, 4 points for compartment syndrome, and 5 points for flaps. Additionally, 1 point per ASA grade was given as well as for each 25% reduction of cortical contact (100% = 0; 75% = 1; 50% = 2; 25% = 3). If the injury was low energy or spiral, one point was subtracted for each factor. They found that a NURD score of 0–5 had a 2% chance of nonunion versus a 61% chance if the score was >12. The score was felt to be a potential nonunion prediction model that clinicians could utilize to determine which patients had a higher risk of nonunion. If such scores could be developed and validated for other bones, prediction of nonunions could be commonplace and allow for earlier intervention.
In our practice, comparative plain radiographs over time, and the clinical picture and evaluation of the patient are sufficient to diagnose a nonunion. However, in some situations, plain radiographs may not allow complete evaluation of the nonunion site because of the hardware. In these cases, a CT scan, with metal suppression if hardware is present, can be obtained to further evaluate the nonunion site as well as look for areas of sequestered dead bone or areas of bone deficits that may require bone grafting. CT scans have been shown to have high sensitivity but moderate specificity with about a 90% accuracy for the detection of nonunions [62]. Sagittal and coronal reconstructions to include 3D reconstructions can help with visualization (Fig. 1.6). Many times, these fractures are “clinically” healed, but patients have symptomatic hardware. A CT scan can also aid in looking at the integrity of the bony consolidation and for defects within the “healed” construct. In some situations, the patient can be considered as having an implant dependent union; e.g., there is some central bone loss but sufficient bridging that the bone has healed around these deficiencies, but the strength of the bone may be reliant upon the associated hardware.
Patient referred for nonunion 9 months after treatment for tibial plateau and tibia shaft fracture treated with open reduction and internal fixation. a, b Anteroposterior (AP) and lateral radiographs of the tibia show consolidation of the plateau component. There is hardware failure and nonunion of the tibial shaft. c–f Computed tomography scan images (axial, coronal, sagittal, and 3D reconstructions) which show the subtle hypertrophic nature of the nonunion. g, h One year after treatment of hypertrophic nonunion with hardware removal and subsequent reamed nailing (AP and lateral)
Ultrasonography (US) has been shown to have some utility in diagnosing nonunions [63]. In a study by Moed et al. [64] in which tibia fractures treated with an intramedullary nail were evaluated, the authors showed a sensitivity of 100% and a positive predictive value of 97% in detecting healing of the fracture site. They also could predict healing of these injuries much earlier (38 days versus 127 days) than plain radiography. Chachan et al. [14] in their prospective diagnostic follow-up study showed that ultrasound was able to predict fracture healing 2 weeks earlier than plain radiographs. More importantly, it was able to predict nonunions 8.5 weeks earlier. Despite the earlier detection for a nonunion, US has not become widespread in its use. The benefits of no radiation have not outweighed the primary issues of user dependency, time required for the study and additional cost. Three-dimensional ultrasound is a newer technology that may have added benefits of being able to measure the vascularity not only in the surrounding soft tissue but the fracture itself, as well as providing more information on the progression of healing [63].
Fluoroscopy is another imaging modality that can be used primarily to assess motion at a fracture site to determine healing. This is most useful in the patient treated without internal fixation and when there is a question of the healed status of the injury. It can also be useful in cases where external fixation has been used since the external fixation can be loosened without complete removal and the fracture site stressed. If there is motion, the external fixation can easily be “tightened” and “reset.” In our practice, this is usually done in conjunction with anticipated external fixation removal after definitive management of a fracture or in reconstructive cases, where determining the “laxity” of a nonunion can guide treatment.
Magnetic resonance imaging (MRI) with gadolinium can be used to assess the nonunion site for infection and more importantly for devascularized bone or a sequestrum [31, 65]. Additionally, because of its ability to detect marrow changes, it is very sensitive for osteomyelitis. Osteomyelitis usually shows decreased marrow signal on T-1 images but increased signal on T-2 images (Fig. 1.7a–d). The MRI also allows one to determine the extent of bony involvement [31] in such cases because of the marrow changes which is crucial in determining the best reconstructive option based upon the anticipated length of resection required to eradicate the osteomyelitis.
a, b Anteroposterior and lateral radiographs of patient with infected nonunion of right tibia. c, d Magnetic resonnance images of tibia showing increased signal on T2 image indicating osteomyelitis. e Nuclear medicine studies showing increased uptake on indium study suggesting the presence of infection
Nuclear medicine studies (Fig. 1.7e) have been historically used to aid in the detection of infection as well, but over time their utility has been questioned [66]. They are still of use in evaluating the nonunion site for infection and/or biologic activity [31]. Leukocyte-labeled studies have been shown to have appropriate diagnostic accuracy for osteomyelitis in the peripheral skeleton [67]. The traditional technetium bone scan will have increased signal on any biological bone activity, and hence, any fracture site that is biologically active should have uptake. Thus, it really is not used for the evaluation of healing although, in cases of avascular or nonviable fractures sites, e.g., the atrophic nonunion , decreased or no uptake may be the case. Our use is usually for the suspected infected cases when the clinical signs of an infection are absent but laboratory markers—erythrocyte sedimentation rate (ESR) , C-reactive protein (CRP) , or white blood cell (WBC) count —may be elevated. In these cases, a bone scan is obtained which is usually positive. If by chance it is negative for uptake, then no other imaging is done and concern becomes for an atrophic nonunion. After a positive bone scan, an indium (tagged WBC) scan is performed. If this is positive at the fracture/nonunion site, then there is increased suspicion for infection. If it is negative for uptake at the site, then infection is less likely but unfortunately never completely ruled out. The final study done after a positive Indium scan, is the sulfa colloid marrow scan. The areas of uptake are then compared to the indium scan. If the areas of uptake are concordant with the indium scan , the uptake is deemed to be secondary to the associated marrow changes and not infection. Conversely, if the areas of uptake on the indium scan do not coincide with uptake on the sulfa colloid (discordant), then it is thought to be suggestive of an infection [31]. The specificity and sensitivity of such imaging studies has been controversial. Stucken et al. [66] showed that not utilizing the nuclear medicine tests actually improved their predicted probabilities of infection based on laboratory studies alone. The latest imaging modality, which has shown some promise to aid in the detection of infection or osteomyelitis, has been the positron emission tomography (PET) scan ± CT scan. A fluorodeoxyglucose PET scan has been shown to have the highest diagnostic accuracy for excluding or confirming the diagnosis of chronic osteomyelitis [31, 67]. This could aid in the evaluation of the presence of infection in a nonunion.
1.4.3 Laboratory Evaluation
Laboratory studies can assist in determining the etiology of the nonunion or at least look at conditions that may have contributed to the development of the nonunion. All patients should be evaluated with a CBC with differential, ESR, and CRP. These are utilized to evaluate for infection but realizing that the ESR and CRP are simply indicators of inflammation and can be elevated in the absence of an infected nonunion. Conversely, normal markers do not necessarily rule out an infection either and are usually the case in indolent infections. A standardized protocol to rule out infection was assessed by Stucken et al. [66] to evaluate the efficacy of laboratory studies (WBC, CRP, ESR) and nuclear medicine studies. They found that the ESR and the CRP were both independently accurate predictors of infection. With all three tests being positive, the predicted probability of an infection was 100%. If the nuclear medicine studies were included, the probability went down to 86% for three positive tests.
As mentioned before, in cases where the original surgery was deemed to be highly contributory to the development of the nonunion, more extensive laboratory studies may not be needed. In the cases where the technical aspects seemed to be sound and the reason for the nonunion unclear, other laboratory studies may point to an underlying metabolic abnormality as the etiology [13]. These patients would probably benefit from an endocrinology workup if feasible. Often times in our practice, these are unfunded trauma patients and the workup is often left to the orthopedic trauma surgeon to do the full evaluation. Many of these patients may also have sustained fragility fractures which also warrant laboratory workup. These underlying metabolic disorders include vitamin D deficiency, hypothyroidism, hypogonadism, hypocalcemia, and overall poor nutritional status. Brinker et al. [13] showed that 31 of 37 of their patients with a nonunion had some type of metabolic abnormality with vitamin D deficiency being the most common. The laboratory studies, in addition to the above, should include serum 25-hydroxy-vitamin D, calcium, phosphorus, alkaline phosphatase, thyroid function tests, parathyroid hormone level, hormone levels (testosterone, estrogen, and follicle stimulating hormone), and albumin and cortisol levels [13]. Vitamin D deficiency has been set at 20–30 mg/dl, and <20 are considered insufficient.
In cases of infected nonunions, it is helpful to obtain results of previous cultures if available to determine the previous organism(s). At the time of surgery, especially in cases of staged procedures, which is often the situation in dealing with infected nonunions [66], deep tissue cultures and bone biopsies can help determine the presence or absence of an infection as well as the offending organism. Preoperative antibiotics should be withheld until after intra-operative cultures are obtained. It is also recommended to cease any antibiotics for at least two weeks, if possible, to maximize the chance of identifying the organism. The first stage is usually to remove previous hardware, to obtain a better idea of the nonunion site, and to get biopsies and cultures. Antibiotic beads can be placed in the interim prior to the second stage.
There has been an increasing interest in looking for serologic markers that may help to predict fracture healing and therefore potentially predict nonunions [16, 20, 22, 27, 30, 68]. Although a full review and discussion of these markers is beyond the scope of this chapter, it is important to mention that they exist and have future implications in predicting fracture healing. These biomarkers are either factors that regulate the healing process itself or bone turnover markers that are extracellular matrix components related to degradation or production during the repair process [15]. The local or systemic factors regulating the healing process include vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-β) . Serum TGF-β has been found to be an indicator of healing versus nonhealing with 4 week levels being much lower in a group of patients that had a delayed union [20]. The bone turnover markers can be divided into one of three categories: 1. bone formation markers, 2. bone resorption markers, and 3. osteoclast regulatory proteins [16, 20, 22]. The bone formation markers indicate osteoblastic activity and as such are fragments of type-I and type-III pro-collagen that are released during the formation of type-III collagen (PIIINP, PICP, PIIINP). Osteocalcin (OC) and bone-specific alkaline phosphatase (BSAP) are also measures of osteoblastic activity. Bone resorption markers include those that measure the degradation of type-I collagen (CTX, NTX, ICTP, pyridinoline, deoxypyridinoline). Tartrate-resistant acid phosphatase (TRAcP) and cathepsin K (CK) are noncollagenous markers that also measure bone resorption but are osteoclast regulatory proteins. Other osteoclast regulatory proteins include receptor activator of nuclear factor NF-kB ligand (RANKL) and osteoprotegerin (OPG). The marker activity of only a handful of these have been evaluated in various fractures and shown some promise in predicting fracture healing [30]. Fischer et al. [18] evaluated a number of cytokines—TGF-β, platelet-derived growth factor (PDGF-AB), insulin-like growth factor 1 (IGF-1)—in patients with long-bone nonunions treated both successfully and unsuccessfully with the Masquelet technique and compared them to a group with normal bone healing. They found temporal variations of these cytokines in the three groups, with high expressions of IGF-1 corresponding to a successful Masquelet treatment. They demonstrated significant differences in cytokine expression between normal fracture healing and the nonunion treatment groups. If the time profiles of each of these markers can be fully understood, then perhaps variations in these markers from what may be considered the normal in fracture healing may provide insight into which fractures will go on to a nonunion [27]. Earlier detection and subsequent earlier treatment could result in substantial cost savings [20].
1.5 Definitions and Classification
As mentioned previously, the US FDA defined a nonunion as a fracture that is at least nine months old and has not shown any signs of healing progression for at least three consecutive months [1]. This however cannot be applied to every fracture, and all nonunions are not the same. Harwood and Ferguson [31] proposed more sensible definitions. They suggested that a nonunion be defined as “a symptomatic fracture with no potential to heal without intervention.” A delayed union was defined as “a fracture in which healing has not occurred in the expected time and the outcome remains uncertain.”
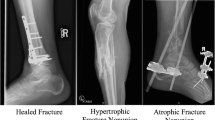
The most common classification was original described by Weber and Cech [69] in 1976 and has survived for 40 years. It was based on the viability and healing potential of the nonunion. From a vascular viewpoint, that corresponds to either a hypervascular or avascular environment [2, 31, 65, 69, 70]. This is based on the appearance of the fracture site on plain radiographs after a period of time when improvement in the fracture healing has ceased. The hypervascular nonunions have been further subdivided into a descriptive classification as an “elephant foot,” “horse hoof/foot,” and oligotrophic nonunion. The avascular nonunions have been further subdivided into the torsion wedge, comminuted, defect, or atrophic nonunion. In addition, the pseudarthrosis has been described [2, 31, 69]. Any of these types of nonunions can be aseptic or septic. If septic, the infection has to be eradicated and any osteomyelitis addressed usually with bone resection. Additionally, these may or may not have a deformity that is associated with it, and if present, any management needs to address the malalignment.
The hypervascular “elephant foot” nonunion (Fig. 1.8) is based on the appearance of the bone ends. These hypertrophic nonunion s exhibit abundant callus formation and are due to excessive motion at the fracture site from inadequate stability [31, 65]. The motion precludes union of the fracture ends. These are well vascularized and generally do not require a bone graft. These require enhanced mechanical stability, which may involve revision of the hardware or additional fixation. The “horse hoof/foot” nonunion is also hypertrophic but much less so. It usually occurs in a situation of inadequate or unstable plate fixation constructs but can occur with nails [2] (see Figs. 1.6 and 1.9). The “oligotrophic” nonunion albeit hypervascular is not hypertrophic on radiographic appearance (Fig. 1.10). The callus is absent, and some absorption occurs but the ends are viable [2]. It is often times due to inadequate reduction or distraction at the fracture site. Revision of the fixation is dependent upon the integrity of the hardware and need for cortical apposition. All three of these nonunions generally require revision fixation with the aim of improving stability. Bone grafts and other biologic adjuncts are not needed except possibly in the case of the oligotrophic nonunion [70].
Patient with a right grade I open tibia fracture treated initially with irrigation and debridement and reamed intrameduallary (IM) nailing. a, b Injury, anteroposterior (AP) and lateral. c, d Postop AP and lateral, follow-up after 10 months showing development of a hypertrophic nonunion and subsequent exchange nailing with union. e, f Nonunion AP and lateral. g, h Exchange IM nail, AP and lateral. i, j Healed AP and lateral
Patient with a segmental tibial shaft treated with an intramedullary nail but with distraction noted at the proximal fracture. a, b Anteroposterior (AP) and lateral. Patient developed an oligotrophic nonunion at 8 months at both sites. c, d Nonunion, AP, and lateral. Patient underwent dynamization with removal of both distal locking screws and subsequently healed. e, f Healed, AP and lateral
All of the avascular nonunion subtypes can be considered as having atrophic ends as all are deficient in callus formation, have undergone some resorption, or have significant bone loss at the time of injury [2, 31, 65, 69, 70] (Fig. 1.11). These generally require a biologic stimulus to heal the nonunion with varying degrees of fixation (or revision fixation) and/or soft tissue reconstruction [70]. If the hardware placed appears to be intact and appropriate, then a biologic stimulus may be all that is needed. This is usually in the form of autogenous bone grafting although various bone graft substitutes have been used. Other adjunctive treatments have also been described [70] and will be discussed later.
Patient with left clavicle fracture without shortening and minimal elevation initially treated non-operatively. a Injury radiograph. b Nonunion radiograph after 3 months showing resorption and established atrophic nonunion. c Healed clavicle fracture after treatment with open reduction and internal fixation and bone graft
A pseudarthrosis is a nonunion that chronically develops into a joint-like appearance with a hypertrophic callus or can be atrophic on radiographs with gross motion [2] (Fig. 1.12). Despite the obvious instability, these are surprisingly nonpainful. In fact, this was defined by Harwood and Ferguson [31] as “a painless fracture that has failed to unite and has no potential to do so without intervention.” These all require surgery. The cavity at the fracture site is usually filled with a synovial lining creating a “false joint.” These require resection of this cavity along with stabilization and bone grafting.
Patient with left tibia fracture treated with closed management and development of pseudarthrosis of tibia but healed fibula. (a, b) Pseudarthrosis anteroposterior (AP) and lateral; Patient treated with reamed intramedullary nailing of pseudarthrosis after resection synovial cavity with subsequent union.(c, d) Healed AP and lateral
Other classification schemes have been reported as well [71,72,73]. The classical Ilizarov description has been to define nonunions based upon the amount of motion at the site, as stiff, slack, or lax [71]. These correspond to the previously described radiographic appearances as well. It is however important to take into consideration that motion can only be assessed in the absence of intact hardware or adjacent intact structures, e.g., an intact fibula in the case of the tibia. The stiff nonunion (hypertrophic) generally has no detectable motion on stress examination. The slack nonunion (oligotrophic-hypertrophic) has some motion hinging at the fracture site. The lax nonunion (atrophic) has free movement at the fracture site. This classification is often used in the management of nonunions with external fixation [71, 74,75,76].
Biasibetti et al. [72] reported their classification based on radiographic evaluation. Their preference is for the use of external fixation in the management of these nonunions. They defined nonunions as types 1–4. The type 1 nonunions are the classic hypertrophic nonunion s that require mechanical stabilization by compression. The type 2 nonunions are those with large oblique fragments where axial compression would result in shear and torsion with negative affects on consolidation. Type 3 nonunions are those that were comminuted injuries, have significant defects, or are atrophic. These require both mechanical stability and biologic stimulation. The type 4 is the infected nonunion.
The management of nonunions is extremely complicated, and failure rates have been reported around the 20% level. Despite classification schemes and scoring systems to better provide improved agreement on when to diagnose a nonunion, treatment guidelines are lacking. In an effort to provide such guidelines, Calori et al. [73] in 2008 proposed a new scoring system to classify nonunions and dictate the level of care that the nonunion requires. This scoring system takes into consideration the bone, soft tissues, and the patient to determine the best course of action. The maximum score would be 100 (scored points × 2). The scoring system is very comprehensive and looks at all the issues previously mentioned including the fracture characteristics, adequacy of original treatment, defects, alignment, soft tissue integrity, and patient risk factors. The higher the score, the more difficult it was felt to obtain union. Those with a score up to 25 were felt to have a straightforward nonunion that could be managed by standard techniques. Those with scores from 26 to 50 should have more specialized care. In addition to specialized care, specialized treatment was also required if the score was 51–75. They recommended consideration for amputation for any score above 75. Although this score looks to have some promise, it has not been validated to our knowledge.
Careful assessment of the radiographs over time can help classify the type of nonunion. The type of nonunion can then help determine the cause of the nonunion suggesting either a biologic or mechanical etiology. Taking all of these previous factors that have been discussed can help determine the best course of action to take in managing the nonunion. Classification and scoring systems can certainly be helpful.
1.6 Management Principles
In general, the management principles for the treatment of the nonunion are common to all sites and are based on the classification. The goals in treatment of the nonunion are universal: 1. healing the nonunion, 2. restoring function, and 3. eliminating pain. There are two basic tenets in accomplishing these goals—maximize the biology and re-establish appropriate mechanical integrity of the nonunion environment. Maximizing the biology of the environment can be looked at from two perspectives: local and systemic. Locally, it is important to enhance the biology at the nonunion site and eradicate infection if present. Systemically, the patient’s comorbidities must be minimized or corrected if feasible. The mechanical integrity of the nonunion environment can be looked at from a local point of view as well as from the entire limb point of view if u will. Keep in mind that improvement of the local mechanical stability also improves the local biology at the nonunion site to promote union. It has also been suggested that nonunions should be treated with polytherapy, insuring the nonunion site is enhanced with osteoprogenitor cells, growth factors, and an adequate osteoconductive scaffold in cases of adequate stability [77]. This is certainly an aggressive approach and may be warranted if these three aspects of the diamond concept [11] are lacking.
1.6.1 Biological Environment: Systemic
After a careful evaluation of the patient and causes for the nonunion, any metabolic abnormalities should be addressed. Vitamin D deficiency or insufficiency should be corrected with replacement vitamin D therapy. Our preference is to start patients at 50,000 units of vitamin D weekly for at least 6 months. Levels should be obtained after 4–6 weeks to insure a proper response. In patients with associated secondary hyperparathyroidism, vitamin D replacement should solve the high PTH level. Patients should also be given calcium supplementation along with the vitamin D. Smoking cessation counseling should be initiated in efforts to minimize or even stop smoking to aid in the healing process after reconstruction. Diabetes should be as well controlled as feasible. All comorbidities should be optimized prior to intervention if time allows.
1.6.2 Biological Environment: Local
Infection should be ruled in or out prior to any definitive management. If infection is present, the decision between a one-stage and two-stage treatment plan must be made [78]. If a two-stage approach is deemed necessary, the first step is to remove existing hardware, evaluate the nonunion site, and obtain cultures and/or biopsies to determine the presence and extent of the infection. The presence of osteomyelitis must be determined as well as the extent of bony involvement. If there is an obvious infection, the infection must be cleared up prior to wound closure and certainly before definitive management for the nonunion including bone grafting [78]. There are varied opinions on when timing of the bone graft should occur from immediate to 6 weeks after the resection [35]. If osteomyelitis is present or suspected and confirmed by biopsy, the amount of bony resection that needs to be performed to eradicate the infection has to be determined. As mentioned before, MRI is quite useful since marrow changes can help delineate the extent of osteomyelitis. Reconstruction options for bony defects are discussed in Chap. 15, but include the Masquelet technique with massive bone grafting, distraction osteogenesis (bone transport) [71, 74], and vascularized or nonvascularized bone grafts (Figs. 1.13 and 1.14). Struijs et al. [79] reviewed the literature on the management of infected long-bone nonunions. The majority were case series, and definitive conclusions and recommendations could not be made. However, it was clear that appropriate debridement is universally required as a basis for any further treatment. The majority of the first-stage treatment methodologies, when significant bone resection is required for associated osteomyelitis, included bone transport techniques with the Ilizarov fixator after the debridement with 70–100% union results. In a retrospective review of utilizing a single-stage treatment protocol for “presumptive” aseptic nonunions, the authors had success in preventing secondary surgery in 72% of culture positive cases [80]. If preoperatively, the history and clinical examination do not indicate an infection, a single-stage protocol of withholding antibiotics, removing the implant, open debridement or canal reaming, sending cultures followed by antibiotics and revision ORIF or exchange nailing was performed. They had positive cultures in 28.7% and out of those 28% needed secondary surgery. Overall, they felt that a single-stage protocol is warranted in cases where the nonunion is considered aseptic preoperatively. In obvious cases of infected nonunions, a single-stage protocol is not utilized. The best two-stage results (93–100% union with recurrent infection rate up to 18%) seemed to be with debridement, antibiotic beads, and planned secondary fixation. In a study by Obremskey et al. [35] where they surveyed members of the Orthopedic Trauma Association (OTA), almost 90% of surgeons used some sort of antibiotic cement spacer before bone grafting in a segmental defect. In any event, treatment needs to be individualized for the patient. Such specialized techniques as using antibiotic impregnated cement-coated nails for the interim stabilization in infected long-bone nonunions are mainstay of treatment. Recently, Scolaro and Mehta [81] described the use of antibiotic impregnated cement-coated locking plates in the use of infected peri-articular nonunions. In a similar fashion to utilizing the antibiotic impregnated cement-coated nail to stabilize the nonunion temporarily while the infection is cleared, a plate was used in peri-articular nonunions for the same reason with success, albeit a small case series. In addition, the infection must be addressed with appropriate antibiotics. The duration of and mode of delivery (intravenous and per os) also should be based on the organism, bone penetration of the antibiotics, and the retention of potentially contaminated hardware. Much of the treatment plan is based on the surgeon’s experience since each case can be unique. Amputation should always be discussed with patients that have infected nonunions [71, 73]. If infection is present, the reconstructive path can be long and difficult. Many patients have already undergone numerous surgeries over several years to no avail in resolving the infection or nonunion.
Patient presented with a year history of draining sinus tracts after open reduction and internal fixation (ORIF) of grade IIIB right open tibia fracture concerning for infected nonunion. a, b Infected Nonunion anteroposterior (AP) and lateral. Patient underwent infection workup, which was consistent with osteomyelitis. The patient then had hardware removal with evaluation and biopsy of the bone. c, d AP and lateral after hardware removal. Patient then underwent resection of osteomyelitis with plating and cement spacer placement to create membrane in anticipation of Masquelet procedure. e, f AP and lateral after ORIF and cement placement. Bone grafting into membrane, which was obtained from ipsilateral femur, using reamer-irrigator-aspirator system (RIATM). g, h AP and lateral after cement removal and bone grafting. Patient went on to heal with complete consolidation of the bone graft. i, j. One-year follow-up AP and lateral of tibia
Patient referred after sustaining right Grade IIIB open femur fracture with massive bone loss. Initial treatment was irrigation and debridement and retrograde intramedullary nail with placement of bone cement in defect in anticipation of Masquelet technique. a, b Presenting anteroposterior (AP) and lateral showing bone defect with cement. Patient underwent bone harvesting from contra-lateral femur using the reamer-irrigator-aspirator system and then placed into defect after cement removed. c, d Initial postop AP and lateral radiographs after bone grafting. Patient went on to consolidate after the bone grafting with complete healing across the defect. e, f AP and lateral showing consolidation and incorporation of bone graft into defect
Atrophic or oligotrophic nonunions require a biologic stimulus to reinitiate the healing process. This is usually in the form of a bone graft. Autogenous bone graft remains the gold standard [31, 78, 82,83,84] because it is osteogenic, osteoinductive, and osteoconductive, with the iliac crest (ICBG) as the most common site of harvest historically [31, 84, 85]. In a retrospective study of long-bone nonunions by Flierl et al. [86], they compared the success rates of 5 different groups: autograft, allograft, autograft + allograft, recombinant human bone morphogenetic protein-2 (rhBMP-2) ± adjunctive bone grafting. The autograft was superior in union time, had the lowest rates of surgical revisions and revision bone grafting, and had a lower new-onset postoperative infection rate. Obremskey et al. [35] surveyed members of the OTA and 92% use autograft for bone grafting procedures in nonunions. The site from where the bone graft was obtained varied, however, with 50.9% of the respondents picking the reamer-irrigator-aspirator system (RIATM) , 49.9% chose anterior crest, and 24.8% for posterior crest (more than 1 choice was allowed). Only 20.8% of surgeons used allograft and BMP as an alternative bone graft. Since the invention of the RIATM system, it has been used more and more for harvesting autogenous bone graft. Those that have used it cite lower complications and lower comorbidity than ICBG harvesting.
Dimitrou et al. [85] performed a systematic review of the literature and found that the overall complication rate for RIATM was 6% compared to 19.4% for ICBG harvesting. They also showed that there were differences between the anterior and posterior crest harvest sites. The anterior crest had significantly higher rates of infection, hematoma formation, fracture, and hypertrophic scar formation but significantly lower rates of chronic donor site pain and sensory disturbances. In a separate clinical study by Loeffler et al. [87], they prospectively enrolled 92 patients undergoing anterior ICBG for nonunions. They had a 3% infection rate and only 2% rate of chronic pain. They felt that anterior ICBG harvesting was well tolerated. In addition to comparing complication rates between RIATM and ICBG, there has been concern that the bone graft quality (cellular constituents and biochemical characteristics) from the intramedullary canal is not as good. Sagi et al. [88], in a prospective study, harvested bone graft from both the medullary canal (RIATM) and the iliac crest from the same individual for nonunion procedures. They evaluated the graft histologically and performed transcriptional profiling for biochemical markers that are known to be expressed during fracture healing. The transcriptional profiles were found to be very similar. The RIATM graft was found to have greater regenerative characteristics as well as mesenchymal stem cells. This suggests that RIATM bone graft may actually be better.
Dawson et al. [89] looked at the union rates between RIATM and ICBG in a prospective randomized study for nonunions or a post-traumatic defect that required operative intervention. They had 113 patients for the final statistics, 57 patients received ICBG, and 56 had RIATM grafting. The union rates were similar as were the rates of donor site complications, but the RIATM had larger volumes of graft (anterior ICBG SS ≪ than posterior ICBG NSS < RIATM) and had significantly less donor site pain. Autogenous bone graft remains the gold standard, but the choice of harvest site is still at the surgeons’ discretion. Adjunctive bone substitutes are sometimes required for recalcitrant nonunions or if more volume is needed. The author prefers to use RIATM especially if large volumes of bone graft are required or the canal is being accessed due to the implants being used (intramedullary nails). Other local donor sites have been utilized for small amounts of graft depending on the site of the nonunion, e.g., distal radius metaphysis for forearm nonunions or proximal tibia metaphysis for distal tibia/fibula nonunions. If the patient is obese, the preference is also for RIATM to avoid wound complications with the large soft tissue envelope.
Another alternative for large bone defects besides massive autogenous cancellous bone grafts are the vascularized bone grafts [90]. In certain situations, a vascularized graft may be the best option. Not only can they provide better structural support but can promote healing due to the added blood supply. Numerous types of vascularized bone grafts exist [91], but it does require a surgeon with microvascular skills and they tend to have more issues. Historically, it was felt that if large structural grafts were used (>6 cm), then it should be vascularized. Allsopp et al. [90] in reviewing the literature found no evidence to support this perception nor did it support that the success was superior to nonvascularized grafts. The technique is still useful and a valuable part of one’s armamentarium. It may be beneficial in particular situations such as nonunions complicated with osteonecrosis, e.g., the femoral neck or scaphoid [91].
One of the advantages of autogenous bone grafts is that it contains the patient’s own osteoprogenitor cells which aid in its osteogenic potential. Other sources that can provide osteogenesis are bone marrow and peripheral blood. Bone marrow (BMA) can be aspirated providing a source of osteoprogenitor cells that have been shown to provide a biologic stimulus to aid healing in nonunions [31, 70, 82,83,84, 92,93,94,95]. It is considered both osteogenic and osteoinductive. Peripheral blood can be obtained and then centrifuged by a variety of commercially available proprietary systems that separate out the platelet rich plasma (PRP), which has shown mixed results in aiding facture healing [31, 50, 84]. The PRP is only considered osteoinductive.
A large bore needle is inserted into the iliac crest in order to aspirate the bone marrow. The aspirate can then be directly injected into the nonunion site under fluoroscopic guidance [70, 94]. The technique is useful when the retained hardware is intact and stable. Braly et al. [92] published their case series in eleven consecutive patients that presented with delayed union or a nonunion of the distal tibia metaphysis that were initially treated with ORIF. They had 9 of 11 patients heal within six months. They found it to be a safe and inexpensive, minimally invasive treatment. However, the use of BMA is limited because of the small number of stem cells obtained [82, 84]. In an effort to increase the number of stem cells, multiple aspirations and cell concentration techniques have been described [83]. Other future concepts include culturing aspirated cells to increase the numbers and combining these cultured cells with specific scaffolds during surgery or in the laboratory creating hybrid constructs for implantation [83, 93, 95].
To obtain PRP, peripheral blood is obtained from the patient. The amount of blood required depends on the commercial PRP concentration centrifuge systems available. The blood is placed in the centrifuge and the PRP separates out. It can then be drawn into a syringe and injected at the nonunion site [31]. The PRP contains numerous growth factors but in low concentrations [84]. The clinical outcome in the use of PRP has been extremely varied and thus has not gained wide acceptance.
Bone morphogenetic proteins (BMPs) have been considered the most important growth factor in bone formation and healing. Although there are many BMPs that have been described, only three have been shown to stimulate stem cell differentiation into the osteoblast lineage in vitro—BMP 2, 4, and 7 [96, 97]. Only recombinant BMP 2 (rhBMP-2:Infuse; Medtronic Sofamor Danek, Memphis, TN) and 7 (rhBMP-7: Osteogenic Protein-1 [OP-1]; Stryker, Kalamazoo, MI) have been cleared by the FDA for clinical use, with rhBMP-2 indicated for open tibia fractures and rhBMP-7 for recalcitrant long bone nonunions [98, 99]. There has been extensive research looking at these molecules as a way to repair nonunions and accelerate the fracture healing process [96, 97, 99]. Friedlaender et al. [100] reported on their prospective, randomized control multicenter study utilizing OP-1 in the management of tibial nonunions. All patients had an intramedullary rod and either OP-1 or autograft. At 9-month follow-up, there was a clinical success rate of 85% and a radiographically healed rate of 84% in the autogenous bone graft group compared to 81% and 75%, respectively, in the OP-1 group. There was no statistically significant difference between the two patients, although 20% in the autograft group had chronic pain at the donor site. OP-1 was felt to be safe and effective for tibial nonunions. Dimitriou et al. [101] utilized rhBMP-7 in 25 consecutive patients with 26 nonunions in various locations. They had success in 24 of the 26 nonunions; however, 16 of these successes also had autograft in addition to the BMP. Only 8 cases had rhBMP-7 alone. In an observational retrospective, nonrandomized study by Ronga et al. [102], they also looked at the use of rhBMP-7 in long-bone nonunions. They had an 88.8% success rate with an average healing time of 7.9 months. Their group was mixed in that 38 cases had the BMP alone, 11 cases were BMP with an osteoconductive agent, 50 cases with an autograft, and a composite graft in 6. In both these studies, the only conclusion that could be made was that the use of rhBMP-7 was safe and effective and could be utilized with autograft. Giannoudis et al. [103] specifically looked at the effect of BMP-7 with autograft. They retrospectively reviewed their prospective database of patients treated for atrophic nonunions in which both BMP-7 and autograft were used in all patients at different anatomic sites. Revision of the fixation was also performed in 77.8% of cases. They had a 100% union rate. They concluded that although autograft was the gold standard, the BMP-7 could enhance the osteoinductive capacity of the graft. Morison et al. [104] looked at the use of rhBMP-7 in atrophic long-bone nonunions in the upper extremity. They used BMP alone but with plate fixation. However, they did state that if local autogenous bone was available, it was morselized and added but autogenous bone graft was not harvested. They had an 89% success rate.
As mentioned before, the other BMP that is available is rhBMP-2. This has been primarily studied in open tibia fractures with little to no data in nonunions to our knowledge. Jones et al. [105] in the BESTT-ALL trial used BMP-2 with allograft versus autograft in open tibia fracture bone defects. The average size of the defect was 4 cm (1–7 cm). The success rates were not statistically significantly different with a 67% union rate in the autograft group and 87% in the BMP-2+ allograft group. In a study by Aro et al. [106], rhBMP-2 was used in conjunction with reamed intramedullary nail fixation of open tibia fractures and compared to reamed nailing alone. They found that the use of BMP-2 did not accelerate the rate of fracture healing, despite the trend toward faster healing at the 13-week mark in the BMP-2 group. This difference normalized at 20 weeks, where 68% of the BMP-2 group and 67% of the nail alone were healed.
Multiple reviews of the literature [97, 98, 107] have all concluded that although there was good clinical data on the effectiveness of BMPs, it was as good but not better than autogenous bone graft. The use of BMPs can be expensive, but a cost–benefit analysis has shown that their use can potentially provide a cost savings in both nonunions and open fractures [97,98,99, 103]. More prospective, randomized clinical studies are needed to determine the true effectiveness of BMPs in both nonunions and acute fractures.
There are many other bone graft substitutes, either derived from human sources or manmade, that are commercially available [31, 82, 84]. They include the calcium phosphate substances, bioactive glass, coral, allograft, and demineralized bone matrix (DBM). The synthetic substitutes and allograft are strictly osteoconductive, whereas the DBM is both osteoconductive and osteoinductive, although the osteoconductive potential is highly variable based on the company [84]. Most of these materials are best used as graft extenders in the management of nonunions. No good clinical studies exist evaluating these materials in the treatment of nonunions.
It is also important to note that in cases of open injuries, soft tissue management is integral to the initial treatment. Poor soft tissues and inadequate vascularity may contribute to the development of a nonunion. In all cases of nonunions, the local soft tissue environment should be appropriately assessed. If the soft tissues are deficient or damaged, it is important to obtain good soft tissue coverage through the use of either local or free tissue flaps [31].
1.6.3 Mechanical Environment: Local
The fixation may or may not need revision depending on the technical considerations with respect to the management of the initial fracture and its integrity. If appropriate and intact, then the biologic stimulus may be insufficient. If the original fixation was inadequate or has failed, the construct should be appropriately revised and the need for bone graft assessed based upon the initial healing response. The radiographic appearance of the nonunion should be used to classify the site, which can aid in the management and determination of the need for bone graft.
In cases of hypertrophic nonunion s, stability is needed (see Figs. 1.6g, h and 1.15). The best treatment is based largely in part due to the initial implant used for the original fracture. In cases of fractures previously treated with intramedullary nails, exchange nailing is regarded as the method of choice for both the femur [65, 108,109,110] and tibia [107, 108, 111] (see Fig. 1.9g–j). Nail dynamization is best reserved for cases of static locking and oligotrophic nonunions to stimulate the healing response [65] (see Fig. 1.10e, f). Care must be taken in cases of comminuted or oblique fractures, where dynamization could lead to unacceptable shortening or loss of rotation. In a review of the literature on aseptic tibial nonunions, Kanakaris et al. [107] in 2007 concluded that exchange nailing was the method of choice based upon better than 90% union rates. In looking at the literature on femoral nonunions, Crowley et al. [109] also found excellent rates with exchange nailing and felt that it remained the gold standard despite good results with adjunctive plate fixation. Swanson et al. [110, 111] reported their excellent results utilizing a systematic approach in both femoral and tibial nonunions regardless of classification. All patients had correction of any metabolic or endocrine abnormalities. The atrophic nonunions did not have open bone grafting. The femurs underwent secondary dynamization in 28% of the cases and the tibias in 7% of cases. There were 4 cases (9%) that had partial fibulectomy at the same time as the exchange nailing in the tibia cases. They had a 100% union rate in femurs and 98% union rate in tibias. In both studies, they routinely exchanged nails with a size at least 2 mm larger in diameter in static mode but used a different manufacturer’s nail. The use of a different manufacturer’s nail was felt to be important to optimize screw purchase since the screw locations/trajectories would be different. Other more recalcitrant long bone hypertrophic nonunions that have failed exchange nailing may be better off with adjunct plate fixation, which has been shown to be effective in these situations especially for the femur [65, 108, 109] (Fig. 1.16). In cases of plate fixation and hypertrophic nonunion s, often times the entire construct needs to be removed and completely revised. In these cases, the hardware has often failed with resultant mal-alignment. The healing actually may continue because of the excessive motion causing increased callus and a stiff nonunion. Many times, these can be managed with distraction osteogenesis utilizing external fixation, which also allows for correction of the deformity at the same time and subsequent healing of the nonunion [74,75,76, 112] (Fig. 1.1d–m). The frames can provide an excellent mechanically stable environment to provide healing. Distraction osteogenesis, by applying an Ilizarov circular fixator, was used in a case series of 16 hypertrophic mal-aligned nonunions [75]. They had complete correction of the deformity and 100% union. Feldman et al. [112] used the Taylor spatial frame (TSF) in conjunction with bone grafting to heal 5 atrophic nonunions in addition to two hypertrophic nonunions with 100% success. Schoenleber and Hutson [76] reported their results on eight patients utilizing either an Ilizarov fixator or the TSF for distraction osteogenesis also with 100% success. Other times, revision of the internal fixation is warranted to either a new plate construct, or in some cases depending upon the anatomic site in question, intramedullary devices can be successful in obtaining union if the intramedullary canal is still patent or can be re-established.
Patient sustained a “nightstick” fracture to right ulna after an assault and was treated with cast immobilization and subsequent bracing but developed painful nonunion (a, b) anteroposterior (AP) and lateral of established hypertrophic nonunion. Patient required stability and underwent open reduction and internal fixation (ORIF) with plate fixation. c, d AP and lateral after ORIF. The fracture healed once stability was obtained. e, f One-year follow-up AP and lateral showing the healed ulna
Patient referred for nonunion of left femur that had undergone four prior surgeries over a two year period. a, b Presenting anteroposterior (AP) and lateral radiographs showing oligotrophic nonunion. Patient underwent exchange nailing with bone graft harvesting from the same femur using the reamer-irrigator-aspirator (RIATM) system and placement of bone graft at the fracture site. c, d Postop AP and lateral radiographs after exchange intramedullary nail and bone grafting. Patient continued with pain and now a hypertrophic nonunion 8 months later. e, f AP and lateral showing development/conversion into a hypertrophic nonunion. Patient with persistent pain and instability at fracture site now with recalcitrant nonunion. Adjunctive plating was performed to provide increased stability. g, h Postoperative AP and lateral radiographs after plating; Patient subsequently had resolution of his pain with complete healing of the nonunion. i, j One-year follow-up AP and lateral radiographs showing complete consolidation of the nonunion site
In cases of atrophic nonunions with hardware failure, both the local mechanical and biological environment (see above) need to be addressed. Revision fixation is warranted if the hardware has failed or was inadequate at the outset. Anticipation of the need for early bone grafting in cases of bone loss can help prevent hardware failure and can lead to a successful outcome.
1.6.4 Mechanical Environment: Limb
As mentioned, any associated deformity must be addressed when dealing with the nonunion. Repair of the nonunion without correction of any deformity, especially the mechanical alignment in the lower extremity, will often fail to restore the proper biomechanics and result in persistence of the nonunion [31]. Correction of the biomechanics is crucial for many nonunions such as the femoral neck, where valgus intertrochanteric osteotomy can be successful as long as excessive valgus alignment is avoided [61]. The length, alignment, and rotation should always be assessed in patients and addressed at the time the nonunion is if there are issues. If the nonunion does heal despite ignoring the malalignment, a malunion will be created which can in and of itself be problematic for the patient [61]. It is imperative to fully evaluate the associated deformity with the appropriate radiographs (see previous section), scannogram for length, and CT scan for rotational issues if warranted. All bones in the particular limb should be assessed for any mal-alignment with long limb standing films. A detailed physical examination should be performed to assess for any compensatory changes in the adjacent joints.
1.6.5 Adjunct Therapies
In addition to the surgical management of nonunions, noninvasive interventions in the form of bone stimulators have been used to help facilitate fracture healing acutely as well in cases of delayed unions or nonunions [108]. They come in three forms: 1. ultrasound , 2. extracorporeal shock waves (ESWT), and 3. electrical stimulation [70]. The clinical data are varied.
Low-intensity pulsed ultrasound (LIPUS) has the most clinical data and has been shown to enhance bone healing safely [113,114,115,116,117,118,119]. The literature has shown that ultrasound can reduce the healing times of fresh fractures of the radius and tibia, can offset the negative effects of smoking and age on fracture healing, and can be effective in the treatment of delayed unions and nonunions [114,115,116,117,118,119] (Fig. 1.17). Heckman et al. [115] evaluated the use of LIPUS in acute tibia fractures. They had 67 fractures that were all treated with long leg immobilization. They showed that LIPUS significantly decreased the time to clinical union and overall union (clinical and radiographic) compared to the nontreatment group. Cook et al. [116] looked at patients that had either tibia fractures or distal radius fractures and that smoked to see whether LIPUS showed a difference in healing with smokers. In the tibia fracture groups, they showed a significant 41% reduction in healing time in the smokers and a 26% significant reduction in healing time in the nonsmokers. Smokers had a significant 51% reduction in healing time and nonsmokers a significant 34% reduction in healing time for the patients with distal radius fractures. Nolte et al. [118] reported their results in 29 cases of nonunions treated with LIPUS . They had an 86% success rate with an average treatment time of 22 weeks. This was a heterogeneous group of nonunions including both a variety of anatomic locations and types of nonunion. In a review of the literature, Watanabe et al. [119] showed that the reported success rates seemed to be better for more subcutaneous bones than the deeper bones in both delayed unions and nonunions. Overall rates in prospective cohort studies were reported as anywhere from 55 to 100%. It has also been suggested that LIPUS has utility in distraction osteogenesis and can reduce the time required for maturation of the callus [114, 119].
Elderly male presented with left humeral shaft fracture after low energy fall. Patient had significant medical comorbidities and decision was made to manage the patient with bracing. a, b Injury anteroposterior (AP) and lateral radiographs in brace showing excellent alignment. Patient continued with mild discomfort and radiographs showed persistent nonunion. c, d AP and lateral radiographs showing nonunion. Discussion with patient and family was to try alternative methods due to high surgical risks. Ultrasound bone stimulator (ExogenTM) was started and patient went on to heal the fracture. e, f AP and lateral radiographs 6 months later showing healed fracture
Another form of ultrasound therapy is ESWT. In ESWT, shock waves that are single high-amplitude sound waves which are generated by various means. It has been evaluated in the treatment of delayed unions and nonunions. Zelle et al. [120] in a systematic review of the literature found ten-level 4 studies using ESWT for this purpose. The overall union rate was 76% and was found to be significantly higher in hypertrophic nonunions (76%) than atrophic nonunions (29%). They concluded that the cumulative data suggests that ESWT can stimulate the healing process; however, further studies are warranted due to the level of evidence in these studies.
There are several types of electrical stimulation available: (1) capacitively coupled electric field (CCEF) , (2) pulsed electromagnetic fields (PEMF) , (3) direct current (DC) (more invasive), and 4. combined magnetic fields (CMF) [70, 121]. In 2008, Mollon et al. [122] performed a meta-analysis of randomized control trials looking at the use of electrical stimulation in long bone fracture healing. They could not show a benefit of its use in improving the rate of union in fresh fractures, delayed unions or nonunions. They did cite the heterogeneity in the studies as a reason for the lack of recommendations either way. In a subsequent review of the literature in 2010, Goldstein et al. [123] reviewed 4 separate meta-analyses on electrical stimulation in fracture healing. That review also concluded that no clear benefit to the use of electrical stimulation was seen. They felt that the meta-analysis by Mollon et al. [122] was the most methodologically rigorous. It was clear that better studies were needed. Adie et al. [124] published their multicenter, double-blind randomized trial on the use of PEMF stimulation for acute tibial shaft fractures. They showed that the use of PEMF did not reduce the number of secondary procedures needed for delayed unions or nonunions. Additionally, it did not improve union rates or patient-reported functional outcomes in acute tibial shaft fractures.
The studies available on adjunct therapies indicate that LIPUS has a much more positive response in delayed unions and nonunions as well as in certain fresh fractures. However, none of the studies provide guidelines as to when it should or should not be used. They can be of benefit in patients that may not be in the best health to undergo surgical procedures. The clinical decision-making should be based on one’s experience, patient’s needs and wants, and the type of nonunion.
1.7 Summary
The best management in treating nonunions is their prevention. Adhering to basic AO principles of fracture fixation and limiting the soft tissue dissection are paramount to a good result. Iatrogenic causes have been shown to be a significant contributor to nonunion development [25]. The soft fracture callus that begins to form right away has healing potential as shown by Danoff et al. [125] in an animal model. They created a mid-shaft femoral shaft fracture in rats and stabilized it with intramedullary nailing. They exposed the fracture site at seven days and created three study groups. In the first group, none of the soft callus was removed. In the second, the soft callus was removed. The final group had the callus removed and then replaced. The callus removal group showed significant evidence of delayed healing. Replacing the callus mitigated the negative effect on the healing. They recommended replacing the soft callus on all ORIF procedures. In addition to limiting the biologic insult of surgery, all fracture patients should be critically evaluated for comorbidities that may also contribute to nonunion development as mentioned before. Early bone grafting when appropriate should be performed to aid fracture healing when defects are present and promote healing and prevent hardware failure. If a nonunion presents, reassessment of the patient is required. Critical evaluation of the initial treatment should be performed. If there clearly were issues with the mechanical environment, metabolic causes may not need to be sought after; however, vitamin D insufficiency and sufficiency are more prevalent than thought. If the initial fixation was appropriate, then a metabolic workup is warranted. There should be careful planning of the treatment for the nonunion. All patient aspects of the nonunion must be addressed to include deformities, metabolic issues, biology, and stability of the nonunion site.
References
Food and Drug Administration. U.S. Department of Health and Human Services. Guidance document for industry and CDRH staff for the preparation of investigational device exemptions and premarket approval applications for bone growth stimulator devices; draft; availability. [Docket No. 98D-0238]. Rockville, MD: U. S. Food and Drug Administration; 1998.
Frölke JP, Patka P., Patka P. Definition and classification of fracture non-unions. Injury. 2007;38(Suppl 21):S19–S22. Erratum in: Injury. 2007;38(10)1224.
Bhandari M, Fong K, Sprague S, Williams D, Petrisor B. Variability in the definition and perceived causes of delayed unions and nonunions. J Bone Joint Surg Am. 2012;94(15):e1091–6.
Antonova E, Le TK, Burge R, Mershon J. Tibia shaft fractures: costly burden of nonunions. BMC. 213:14:42.
Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38(Suppl 2):S77–84.
Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl2):S3–7.
Brinker MR, Hanus BD, Sen M, O’Connor P. The devastating effects of tibial nonunion on health-related quality of life. J Bone Joint Surg Am. 2013;95(24):2170–6.
Schottel PC, O’Connor P, Brinker MR. Time trade-off as a measure of health-related quality of life: long bone nonunions have a devastating impact. J Bone Joint Surg Am. 2015;97(17):1406–10.
Wichlas F, Tsitsilonis S, Disch AC, Haas NP, Hartmann C, Graef F, Schwabe P. Long-term functional outcome and quality of life after successful surgical treatment of tibial nonunions. Int Orthop. 2015;39(3):521–5.
Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of compromised fracture healing. J Amer Acad Orthop Surg. 2012;20(5):273–82.
Giannoudis PV, Einhorn TA, marsh D. Fracture healing: The diamond concept. Injury. 2007;38(Suppl 4):S3-S6400-7.
Blair JA, Stoops TK, Doarn MC, Kemper D, Erdogan M, Griffing R, Sagi HC. Infection and non-union following fasciotomy for compartment syndrome associated with tibia fractures: a matched cohort comparison. J Orthop Trauma. 2016;30(7):392–6.
Brinker MR, O’Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21(8):557–70.
Chachan S, Tudu B, Sahu B. Ultrasound monitoring of fracture healing: is this the end of radiography in fracture follow-ups? J Ortho Trauma. 2015;29(3):e133–8.
Cook, GE, Bates BD, Tornetta P, McKee MD, Morshed, Slobogean GP, Schemitsch EH. Assessment of fracture repair. J Orthop Trauma. 2015;29(Suppl 12):S57-61.
Cox G, Einhorn TA, Tzioupis C, Giannoudis PV. Bone-turnover markers in fracture healing. J Bone Joint Surg Br. 2010;92(3):329–34.
Dimitriou R, Kanakaris N, Soucacos PN, Giannoudis PV. Genetic predisposition to non-union: evidence today. Injury. 2013;44(Suppl 1):S50–3.
Fischer C, Doll J, Tanner M, Bruckner T, Zimmermann Helbig L, et al. Quantification of TGF-B1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury. 2016;47(2):342–9.
Frank T, Osterhoff G, Sprague S, Garibaldi A, Bhandari M. Slobogean GP; FAITH Investigators. The radiographic union score for hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin Orthop Relat Res. 2016;474(6):1396–404.
Hankenson KD, Zimmerman G, Marcucio R. Biological perspectives of delayed fracture healing. Injury. 2014;45(Suppl 2):S8–15.
Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883–7.
Kurdy NM. Serology of abnormal fracture healing: the role of PIIINP, PICP, and BsALP. J Orthop Trauma. 2000;14(1):48–53.
Malik MH, Harwood P, Diggle P, Khan SA. Factors affecting rates of infection and nonunion in intramedullary nailing. J Bone Joint Surg Am. 2004;86(4):556–60.
Murray IR, Foster CJ, Eros A, Robinson CM. Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. J Bone Joint Surg Am. 2013;95(13):1153–8.
Niikura T, Lee SY, Sakai Y, Nishida K, Kuroda R, Kurosaka M. Causative factors of fracture nonunion: the proportions of mechanical, biological, patient-dependent, and patient-independent factors. J Ortho Sci. 2014;19(1):120–4.
O’Halloran K, Coale M, Costales T, Zerhusen T Jr, Castillo RC, Nascone JW, O’Toole RV. Will my tibial fracture heal? Predicting nonunion at the time of definitive fixation based on commonly available variables. Clin Orthop Relat Res. 2016;474(6):1385–95.
Pountos I, Georgouli T, Pneumaticos S, Giannoudis PV. Fracture non-union: can biomarkers predict outcome? Injury. 2013;44(12):1725–32.
Santolini E, West R, Giannoudis P. Risk factors for long bone fracture non-union: a stratification approach based on the level of existing scientific evidence. Injury. 2015;46(Suppl 8):S8–19.
Westgeest J, Kin BSC, Weber D, Dulai SK, Bergman JW, Buckley R, Beaupre LA. Factors associated with development of nonunion or delayed healing after an open long bone fracture: a prospective cohort study of 736 subjects. J Orthop Trauma. 2016;30(3):149–55.
Zimmermann G, Muller U, Wentzensen A. The value of laboratory and imaging studies in the evaluation of long-bone non-unions. Injury. 2007;38(Suppl 2):S33–7.
Harwood PJ, Ferguson DO. An update on fracture healing and non-union. Orthop Trauma. 2015;29(4):228–42.
Calori, GM, Albisetti W, Agus A, Iori S, Tagliabue L. Risk factors contributing to fracture non-unions. Injury. 2007;38S:S11–S18. THERE IS NO CITATION 19.
Lin CA, Swiontkowski M, Bhandari M, Walter SD, Schemitsch EH, Sanders D, Tornetta P 3rd. Reaming does not affect functional outcomes after open and closed tibial shaft fractures: the results of a randomized controlled trial. J Orthop Trauma. 2016;30(3):142–8.
Obremskey W, Molina C, Collinge C, Nana A, Tornetta P 3rd, Sagi C, et al. Evidence-based quality value and safety committee orthopaedic trauma association, writing committee. Current practice in the management of open fractures among orthopaedic trauma surgeons. Part A: Initial management. A survey of orthopaedic trauma surgeons. J Orthop Trauma. 2014;28(8):e198–202.
Obremskey W, Molina C, Collinge C, Tornetta P III, Sagi C, Schmidt A, et al. Evidence-based quality value and safety committee orthopaedic trauma association, writing committee current practice in the management of open fractures among orthopaedic trauma surgeons: Part B: Management of segmental long bone defects. A survey of Orthopaedic Trauma Association members. J Orthop Trauma. 2014;28(8):e203–7.
Dye NB, Vagts C, Manson TT, O’Toole RV. Estimation of tibial shaft defect volume using standard radiographs: development and validation of a novel technique. Injury. 2015;46(2):299–307.
Hildebrand F, Griensven MV, Huber-Lang, M, Flohe SB, Andruszkow H, Marzi I, Pape HC; Trauma research network for the German Society of Trauma, DGU. Is there an impact of concomitant injuries and timing of fixation of major fractures on fracture healing? A focused review of clinical and experimental evidence. J Orthop Trauma. 2016;30(3):104–112.
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5.
Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38(Suppl 2):S3-9. Erratum in Injury. 2007;38(10):1224.
Borrelli J Jr, Pape C, Hak D, Hsu J, Lin S, Giannoudis P, Lane J. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708–11.
Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1988;232:210–6.
Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, Chen TL. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diab Care. 2014;37(8):2246–52.
Wukich DK, Joseph A, Ryan M, Ramirez C, Irrgang J. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011;32(2):120–30.
Sprague S, Petrisor B, Scott T, Devji T, Phillips M, Spurr H, et al. What is the role of vitamin D supplementation in acute fracture patients? A systematic review and meta-analysis of the prevalence of hypovitaminosis D and supplementation efficacy. J Orthop Trauma. 2016;30(2):53–63.
Sprague S, Bhandari M, Devji T, Scott T, Petrisor B, McKay P, Slobogean GP. Prescription of vitamin D to fracture patients: a lack of consensus and evidence. J Orthop Trauma. 2016;30(2):e64–9.
Stephens BF, Murphy A, Mihalko WM. The effects of nutritional deficiencies, smoking, and systemic disease on orthopaedic outcomes. J Bone Joint Surg Am. 2013;95(23):2153–7.
Childs BR, Andres BA, Vallier HA. Economic benefit of calcium and vitamin D supplementation: does it outweigh the cost of nonunions? J Orthop Trauma. 2016;30(8):e285–8.
Hobby B, Lee MA. Managing atrophic nonunion in the geriatric population: incidence, distribution, and causes. Orthop Clin North Am. 2013;44(2):251–6.
Kwong FNK, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg. 2008;16(11):619–25.
Giannoudis PV, Hak D, Sanders D, Donohoe E, Tosounidis T, Bahney C. Inflammation, bone healing, and anti-inflammatory drugs: an update. J Orthop Trauma. 2015;29(Suppl):S6–9.
Kurmis AP, Kurmis TP, O’Brien JX, Dalen T. The effect of nonsteroidal anti-inflammatory drug administration on acute phase fracture-healing: a review. J Bone Joint Surg Am. 2012;94(9):815–23.
Lee JJ, Patel R, Biermann S, Dougherty PJ. The musculoskeletal effects of cigarette smoking. J Bone Joint Surg Am. 2013;95(9):850–9.
Patel RA, Wilson RF, Patel PA, Palmer RM. The effect of smoking on bone healing. Bone Joint Res. 2013;2(6):102–11.
Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture. J Bone Joint Surg Am. 2014;96(8):674–81.
Matuszewski PE, Boulton CL, O’Toole RV. Orthopaedic trauma patients and smoking: knowledge deficits and interest in quitting. Injury. 2016;47(6):1206–11.
Dijkman BG, Sprague S, Schemitsch EH, Bhandari M. When is a fracture healed? Radiographic and clinical criteria revisited. J Orthop Trauma. 2010;24(Suppl 3):S76–80.
Bhandari M, Chiavaras M, Ayeni O, Chakraverrty R, Parasu N, Choudur H, Bains S, Sprague S, Petrisor B. Assessment of radiographic fracture healing in patients with operatively treated femoral neck fractures. J Orthop Trauma. 2013;27(9):e213–9.
Koolstra BW, Dijkman BG, Sprague S, Schemitsch EH, Bhandari M. The radiographic union scale in tibia fractures: reliability and validity. J Orthop Trauma. 2010;24(Suppl 1):S81–6.
Whelan DB, Bhandari M, Stephen D, Kreder H, McKee MD, Zdero R, Schemitsch EH. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68(3):629–32.
Chiavaras MM, Bains S, Choudur H, Parasu N, Jacobson J, Ayeni O, et al. The radiographic union score for hip (RUSH): the use of a checklist to evaluate hip fracture healing improves agreement between radiologists and orthopaedic surgeons. Skeletal Radiol. 2013;42(8):1079–88.
Varghese VD, Boopalan PR, Titus VTK, Oommen AT, Jepegnanam TS. Indices affecting outcome of neglected femoral neck fractures after valgus intertrochanteric osteotomy. J Orthop Trauma. 2014;28(7):410–6.
Bhattacharyya T, Bouchard KA, Phadke A, Meigs JB, Kassarjian A, Salamipour H. The accuracy of computed tomography for the diagnosis of tibial nonunion. J Bone Joint Surg Am. 2006;88(4):692–7.
Augat P, Morgan EF, Lujan TJ, MacGillivray TJ, Cheung WH. Imaging techniques for the assessment of fracture repair. Injury. 2014;45(Suppl 2):S16–22.
Moed BR, Subramanian S, van Holsbeeck M, Watson JT, Cramer KE, Karges DE, et al. Ultrasound for the diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma. 1998;12(3):206–13.
Gelalis ID, Politis AN, Arnaoutoglou CM, Korompilias AV, Pakos EE, Vekris MD, et al. Diagnostic and treatment modalities in nonunions of the femoral shaft. A review. Injury. 2012;43(7):980–8.
Stucken C, Olszewski DC, Creevy WR, Murakami AM, Tornetta P. Preoperative diagnosis of infection in patients with nonunions. J Bone Joint Surg Am. 2013;95(15):1409–12.
Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ. The accuracy of diagnostic imaging for the assessmemt of chronic osteomyelitis: a systemic review and meta-analysis. J Bone Joint Surg Am. 2005;87(11):2464–71.
Stoffel K, Engler H, Kuster M, Riesen W. Changes in biochemical markers after lower limb fractures. Clin Chem. 2007;53(1):131–4.
Weber BG, Cech O. Pseudarthrosis, pathology, biomechanics, therapy, results. Bern: Hans Huber; 1976.
Panagiotis M. Classification of non-union. Injury. 2005;36(Suppl 4):S30–7.
Atkins RM. Principles of management of septic non-union of fracture. Injury. 2007;38(Suppl 2):S23–32.
Biasibetti A, Aloj D, Di Gregorio G, Masse A, Salomone C. Mechanical and biological treatment of long-bone non-unions. Injury. 2005;36(Suppl 4):S45–50.
Calori GM, Phillips M, Jeetle S, Tagliabue L, Giannoudis PV. Classification of non-union: need for new scoring system? Injury. 2008;39(Suppl 2):S59–63.
Kanellopoulos AD, Soucacos PN. Management of nonunion with distraction osteogenesis. Injury. 2006;37(Suppl 1):S51–5.
Kocaoglu M, Eralp L, Sen C, Cakmak M, Dincyurek H, Goksan SB. Management of stiff hypertrophic nonunions by distraction osteogenesis. J Orthop Trauma. 2003;17(8):543–8.
Schoenleber SJ, Hutson JJ Jr. Treatment of hypertrophic distal tibia nonunion and early malunion with callus distraction. Foot Ankle Int. 2015;36(4):400–7.
Calori GM, Mazza E, Colombo M, Ripamonti C, Tagliabue L. Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury. 2011;42(6):587–90.
Egol KA, Nauth A, Lee M, Pape HC, Watson JT, Borrelli J Jr. Bone grafting: sourcing, timing, strategies, and alternatives. J Orthop Trauma. 2015;29(Suppl 12):S10–4.
Struijs PAA, Poolman RW, Bhandari M. Infected nonunion of the long bones. J Orthop Trauma. 2007;21(7):507–11.
Amorosa LF, Buirs LD, Bexkens R, Wellman DS, Kloen P, Lorich DG, Helfet DL. A single-stage treatment protocol for presumptive aseptic diaphyseal nonunions: a review of outcomes. J Orthop Trauma. 2013;27(10):582–6.
Scolaro JA, Mehta S. Stabilisation of infected peri-articular nonunions with an antibiotic impregnated cement coated loaking plate: technique and indications. Injury. 2016;47(6):1353–6.
Mahendra A, Maclean AD. Available biological treatments for complex non-unions. Injury. 2007;38(Suppl 4):S7–12.
Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014;100(Suppl 1):S107–S112. Review.
Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl; 1):S75–S80.
Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–15.
Flierl MA, Smith WR, Mauffrey C, Irgit K, Williams AE, Ross E, et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Ortho Surg Res. 2013;8:33.
Loeffler BJ, Kellam JF, Sims SH, Bosse MJ. Prospective observational study of donor-site morbidity following anterior iliac crest bone-grafting in orthopaedic trauma reconstruction patients. J Bone Joint Surg Am. 2012;94(18):1649–54.
Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128–35.
Dawson J, Kiner D, Gardner W II, Swafford R, Nowotarski PJ. The reamer- irrigator-aspirator (RIA) as a device for harvesting bone graft compared with iliac crest bone graft (ICBG): Union rates and complications. 2014;28(10):586–90.
Allsopp BJ, Hunter-Smith DJ, Rozen WM. Vascularized versus nonvascularized bone grafts: what is the evidence? Clin Orthop Relat Res. 2016;474(5):1319–27.
Soucacos PN, Dailiana Z, Beris AE, Johnson EO. Vascularized bone grafts for the management of non-union. Injury. 2006;37(Suppl 1):S41–50.
Braly HL, O’Connor DP, Brinker MR. Percutaneous autologous bone marrow injection in the treatment of distal meta-diaphyseal tibial nonunions and delayed unions. J Orthop Trauma. 2013;27(9):527–34.
Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101.
Homma Y, Zimmermann G, Hernigou P. Cellular therapies for the treatment of non-union: the past, present and future. Injury. 2013;44(Suppl 1):S46–9.
Tseng SS, Lee MA, Reddi AH. Nonunions and the potential of stem cells in fracture healing. J Bone Joint Surg Am. 2008;90(Suppl 1):92–8.
De Biase P, Capanna R. Clinical applications of BMPs. Injury. 2005;36(Suppl 3):S43–6.
Giannoudis PV, Dinopoulos HT. BMPs: options, indications, and effectiveness. J Orthop Trauma. 2010;24(Suppl 3):S9–16.
Obert L, Deschaseaux F, Garbuio P. Critical analysis and efficacy of BMPs in long bones non-union. Injury. 2005;36(Suppl 3):S38–42.
Schmidmaier G, Schwabe P, Wildemann B, Haas NP. Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury. 2007;38(Suppl 4):S35–41.
Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic protein-1 (Bone morphogenetic protein-7) in the treatement of tibial nonunions. J Bone Joint Surg Am. 2001;83-A (Suppl 1 Pt 2):S151–S158.
Dimitriou R, Dahabreh Z, Katsoulis E, Matthews SJ, Branfoot T, Giannoudis PV. Application of recombinant BMP-7 on persistent upper and lower limb non-unions. Injury. 2005;36(Suppl 4):S51–9.
Ronga M, Baldo F, Zappala G. Cherubino P; BMP-7 Italian Observational Study (BIOS) Group. Recombinant human bone morphogenetic protein-7 for treatment of long bone non-union: an observational, retrospective, non-randomized study of 105 patients. Injury. 2006;37(Suppl 3):S51–6.
Giannoudis PV, Kanakaris NK, Dimitriou R, Gill I, Kolimarala V, Montgomery RJ. The synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop Relat Res. 2009;467(12):3239–48.
Morison Z, Vicente M, Schemitsch EH, McKee MD. The treatment of atrophic, recalcitrant long-bone nonunion in the upper extremity with human recombinant bone morphogenetic protein-7 (rhBMP-7) and plate fixation: a retrospective review. Injury. 2016;47(2):356–63.
Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, et al. BMP-2 evaluation in surgery for Tibial Trauma-Allograft (BESTT-ALL) Study Group. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(7):1431–41.
Aro HT, Govender S, Pate AD, Hernigou P, de Gregorio AP, Popescu GI, et al. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone J Surg Am. 2011;93(9):801–8.
Kanakaris NK, Paliobeis C, Manidakis N, Giannoudis PV. Biological enhancement of tibial diaphyseal aseptic non-unions: the efficacy of autologous bone grafting, BMPs and reaming by-products. Injury. 2007;38(Suppl 2):S65–S75. Erratum in Injuryl;207;28(10):1224.
Brinker MR, O’Connor DP. Management of aseptic tibial and femoral diaphyseal nonunions without bony defects. Orthop Clin N Am. 2016;47(1):67–75.
Crowley DJ, Kanakaris NK, Giannoudis PV. Femoral diaphyseal aseptic non-unions: is there an ideal method of treatment? Injury. 2007;38(Suppl 2):S55–63.
Swanson EA, Garrard EC, Bernstein DT, O’Connor DP, Brinker MR. Results of a systematic approach to exchange nailing for the treatment of aseptic femoral nonunions. J Orthop Trauma. 2015;29(1):21–7.
Swanson EA, Garrard EC, O’Connor DP, Brinker MR. Results of a systematic approach to exchange nailing for the treatment of aseptic tibial nonunions. J Orthop Trauma. 2015;29(1):28–35.
Feldman DS, Shin SS, Madan S, Koval KJ. Correction of tibial malunion and nonunion with six-axis analysis deformity correction using the Taylor spatial frame. J Orthop Trauma. 2003;17(8):549–54.
Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am. 2008;90(Suppl 1):138–44.
Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low intensity pulsed ultrasound for bone healing: an overview. Injury. 2006;37(Suppl 1):S56–62.
Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76(1):26–34.
Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orth Rel Res. 1997;337:198–207.
Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83(2):259–70.
Nolte PA, van der Krans A, Patka P, Janssen IM, Ryaby JP, Albers GH. Low-intenisty pulsed ultrasound in the treatment of nonunions. J Trauma. 2001;51(4):693–702.
Watanabe Y, Matsushita T, Bhandari M, Zdero R, Schemitsch EH. Ultrasound for fracture healing: current evidence. J Orthop Trauma. 2010;24(Suppl 3):S56–61.
Zelle BA, Gollwitzer H, Zlowodzki M, Buhren V. Extracorporeal shock wave therapy: current evidence. J Orthop Trauma. 2010;24(Suppl 3):S66–70.
Karamitros AE, Kalentzos VN, Soucacos PN. Electric stimulation and hyperbaric oxygen therapy in the treatment fo nounions. Injury. 2006;37(Suppl 1):S63–73.
Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90(11):2322–30.
Goldstein C, Sprague S, Petrisor BA. Electrical stimulation for fracture healing: clinical evidence. J Orthop Trauma. 2010;24(Suppl 3):S62–5.
Adie S, Harris IA, Naylor JM, Rae H, Dao A, Yong S, Ying V. Pulsed electromagnetic field stimulation for acute tibial shaft fractures. J Bone Joint Surg Am. 2011;93(7):1569–76.
Danoff JR, Aurégan JC, Coyle RM, Burky RE, Rosenwasser MP. Augmentation of fracture healing using soft callus. J Orthop Trauma. 2016;30(3):113–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Agarwal, A. (2018). Principles of Nonunions. In: Agarwal, A. (eds) Nonunions. Springer, Boston, MA. https://doi.org/10.1007/978-1-4939-7178-7_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7178-7_1
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4939-7176-3
Online ISBN: 978-1-4939-7178-7
eBook Packages: MedicineMedicine (R0)