Abstract
This chapter presents and discusses information on the application of ultraviolet light (UV) technology in continuous and pulse modes for processing whole and fresh-cut fruits, and fruit juices. It starts with a brief overview of the fundamentals of UV light generation and propagation in solid and fluid products and followed by the review of available UV sources. Recent reports are reviewed to illustrate the effect of UV light on fresh fruits to extend their shelf life as well as quality and nutritional aspects. The importance of fresh juices optical and physicochemical characteristics and design of effective UV light pasteurization system and processes are discussed. The analysis of reported results of UV inactivation of pathogenic and spoilage organisms in various static and flow-through UV systems is presented. The information on susceptibility of certain vitamins to degradation by UV light that may occur during treatments of fruits and fresh juices is presented. Finally, potential application of UV technology to improve toxicological and chemical safety of fruits are discussed and supported by the effect of UV light on degradation of patulin in buffer and apple juice. The prospective of UV technology as emerging technology in sustainable food production is presented.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
17.1 Introduction
During the last decade an increase of fresh fruit and fruit products production constantly grew due to fruits health properties. A large number of studies have associated the consumption of fruits and their products with decreased risks of development of diseases such as cancer and coronary heart disease (Hansen et al. 2003). This may be due to the presence of health promoting phytochemicals such as carotenoids, flavonoids, phenolic compounds, and vitamins (Gardner et al. 2000) which have in some cases been shown to have disease preventing properties.
Fruit products are consumed in raw, minimally processed or processed ready-to-eat or ready-to-drink forms as whole fresh fruits, fresh-cut fruits, and fruits as ingredients, beverages, juices, and jams. Processing of fruits starts after harvesting and four activities can be distinguished: stabilization or preservation, transformation, production of ingredients, and production of fabricated foods. The role of processing technology in each activity implies the control of microbiological, chemical, and biochemical changes occurred as a result of microbial and enzymatic activities, oxidation reactions that can lead to safety, color, flavor, taste, and texture problems. Processing technologies that do not significantly alter the organoleptic or nutritional qualities of the fruits and do not form any undesirable chemical compounds in the product would have obvious advantages in modern food production. The interest in so-called minimal processing technologies led to the broad development of nonthermal or mild heat high tech methods that have a potential to replace traditional thermal preservation techniques and also result not only in better quality and longer shelf life but potentially in higher nutritional value or products with health benefits. In this respect, it is of paramount importance to develop processing methods which preserve not only the safety of fruits but also sensorial and nutritional quality and bioactivity of the constituents present in fruits and their products.
UV light treatment of foods is a nonthermal physical method of processing that is cost effective, free of chemicals and waste effluents, which makes it an ecologically friendly and sustainable technology. It does not produce by-products and it is safe to use, although precautions must be taken to avoid human exposure to UV light and to evacuate ozone generated by vacuum and far UV wavelengths.
The discovery of UV inactivation of the chlorine-resistant parasites Cryptosporidium parvum and Giardia sp. has catalyzed the use of UV light in the drinking water industry (Hijnen et al. 2006) and treatment of waste and processing water. UV has been utilized similarly in the disinfection of air, nonfood contact and food contact surfaces, and recently was used for treatments of surfaces of solid foods, liquid foods, beverages and their ingredients. Based on engineering advances and new scientific data, ultraviolet (UV) light technology in continuous and pulsed modes (cUV and PL) offers promise of improved microbiological and chemical safety and enhanced functionality of whole fresh fruits, fresh-cut fruits, and juice products. Applications of UV treatments demonstrated better quality preservation of fruit products that have a freshness of flavor, color, texture, and nutritional value closer to non-treated products. Additionally, UV light not only minimally affects quality attributes but also has beneficial effects on foods functional properties such as content of bioactive compounds and has a potential for obtaining premium quality products that can lead to the faster commercialization. Reports are available that application of UV light can also improve toxicological safety of foods of plant origin through its ability to reduce levels of toxins such as patulin mycotoxin in fresh apple cider (Dong et al. 2010), and possibly to control browning through its effects on enzymes (Manzocco et al. 2009). The schematic diagram of potential areas of applications of UV light technology in fruit processing is shown in Fig. 17.1.
This chapter aims to review the latest applications of continuous and pulsed UV light for processing fresh fruits and fruits products. The fundamental principles and features of UV light generation, propagation, and evaluation of UV light parameters will be briefly reviewed. Prevention control measures where UV light can be utilized to improve safety during fruit production will be analyzed. A particular focus will be given to the effects of UV light on survival of pathogenic and spoilage microorganisms typical for fruits and fruit plants environment and essential for the establishment of UV preservation processes followed by the discussion of recent research of effects of UV light on quality and enhancement of bioactive compounds. The effects of UV light on the destruction of mycotoxins will be presented.
17.2 UV Light Technology Fundamentals
17.2.1 Basic Principles
The wavelength range for UV light for food processing varies from 100 to 400 nm. This range may be further subdivided into UV-A (315–400 nm) normally responsible for tanning in human skin; UV-B (280–315 nm) that causes skin burning and can lead to skin cancer; UV-C (200–280 nm) called the germicidal range since it effectively inactivates bacteria and viruses. Vacuum UV range (100–200 nm) can be absorbed by almost all substances and thus can be transmitted only in a vacuum. Radiation from UV light and the adjacent visible spectral range as well as other less energetic types are termed nonionizing radiation. In contrast, ionizing radiation which includes X-rays, gamma-rays, and ionizing particles (beta-rays, alpha-rays, protons) is capable of ionizing many atoms and molecules. The absorption of nonionizing radiation, however, leads to electronic excitation of atoms and molecules. Light is emitted from the gas discharge at wavelengths dependent upon its elemental composition and the excitation, ionization, and kinetic energy of those elements. The gas discharges are responsible for the light emitted from UV lamps.
17.2.2 UV Light Sources
Light is emitted from the gas discharge at wavelengths dependent upon its elemental composition and the excitation, ionization, and kinetic energy of those elements. The gas discharges are responsible for the light emitted from UV lamps. UV light transfer phenomenon is defined by the emission characteristics of the UV source along considering long-term lamp aging and absorbance/scattering of the product. Consequently, performance of UV system depends on the correct matching of the UV source parameters to the demands of the UV application. The commercially available UV sources include low and medium pressure mercury lamps (LPM and MPM), excimer (EL), pulsed lamps (PL), and light emitting diodes (LED). The LPM and excimer lamps are monochromatic sources whereas emission of MPM and PL is polychromatic. There are no reports on the application of EL in fruit processing so this UV source won’t be discussed in this chapter.
17.2.2.1 Mercury Lamps
The mercury vapor UV lamp sources have been successfully used in water treatment for nearly 50 years and well understood as reliable sources for other disinfection treatments that benefit from their performance, low cost, and quality. Typically three general types of mercury UV lamps are used: low-pressure (LPM) ; low-pressure high-output (LPHO) ; and medium-pressure (MPM) . These terms are based on the vapor pressure of mercury when the lamps are operating. LPM lamps are operated at nominal total gas pressures of 102–103 Pa that corresponds to the vapor pressure of mercury at temperature of 40 °C. The emission spectrum of LPM is concentrated at the resonance lines at 253.7 nm (85 % of total intensity) and 185 nm. The wavelength of 253.7 nm is most efficient in terms of germicidal effect since photons are absorbed most by the DNA of microorganisms at this specific wavelength. Light with a wavelength below 230 nm is most effective for the dissociation of chemical compounds. The photons with the wavelength of 185 nm are responsible for ozone production and the combination of both wavelengths is a very effective means for photochemical air treatment. The US FDA regulations approved the use of a LPM lamps for juice processing and they have already been successfully commercialized (US FDA 2000a).
MPM lamps are operated at a total gas pressure of 104–106 Pa. Compared to the LPM lamps, the coolest possible temperature of the MPM is about 400 °C, whereas it goes up to 600 and even 800 °C in a stable operation. The emission spectrum of MPM covers wavelengths from about 250 nm to almost 600 nm, which results from a series of emissions in the UV and in the visible ranges. MPM lamps are not considered to be useful for targeted germicidal treatment. However, their strong UV radiation flux results in high penetration depth. By varying the gas filling, doping, and the quartz material, the spectrum as well as the radiation flux of the UV lamps can be varied and matched to suit specific food processing applications, especially for oxidation or photo degradation.
Recently, LPHO amalgam lamps that contain a mercury amalgam were developed and incorporated into disinfection applications; however, LPM and MPM are the dominant sources for UV disinfection treatment.
17.2.2.2 Pulsed Lamps
The efficacy of pulsed flash lamps (PL) is potentially greater than continuous sources due to high intensity, broader spectrum, instant start, and robust packaging with no mercury in the lamp. In this technology, alternating current is stored in a capacitor and energy is discharged through a high-speed switch to form a pulse of intense emission of light within about 100 ms. The emission is similar in wavelength composition to the solar light. The UV pulsed devices can deliver high intensity UV which can both penetrate opaque fluids better than mercury lamps and provide enhanced treatment rates. More research is needed to establish them for fruit treatments applications.
Figure 17.2 shows the normalized spectra of continuous UV (cUV) sources such as LPM, MPM, and PL. Individual spectra are not comparable on a UV intensity basis but are comparable on a spectral basis regarding which wavelengths dominate the respective wavelength outputs.
17.2.2.3 Light Emitting Diodes
In recent years, UV-LEDs have been developed with the following advantages: low cost, energy-efficient, long life, easy control of emission, and no production of mercury waste. The wavelength of the commercial UV-LED is in the range 240–400 nm and enables new applications in existing markets as well as in new research areas. A LED is a semiconductor device that emits light when carriers of different polarities (electron and holes) combine generating a photon. The wavelength of the photon depends on the energy difference the carriers overcome in order to combine. The example of UV-LED system that operates between 210 and 365 nm is the one formed by aluminum nitride (AIN), gallium nitride (GaN), and intermediate alloys. Currently, UV-LEDs are commercially available at research grade in limited quantities and their lifetime reach on the order of 200 h. It is very likely that in the near future, many applications that today make use of mercury lamps will be carried out by UV-LEDs.
Table 17.1 provides a summary of some of the basic characteristics of common UV sources in commercial use and under development and can be used for comparison purposes. It is evident that no single lamp technology will represent the best source for all food applications. However, situation-specific requirements may dictate a clear advantage for a given process technology. For UV reactors containing LPM or LPHO mercury lamps, UV absorbance and transmittance at 253.7 nm are important design parameters. However, for broadband UV lamps, such as MPM or PL, it is important to measure the full scan of absorbance or transmittance in the germicidal region from 200 to 400 nm. Special technologies lamps as PL UV, LEDs are promising due to different spectral bands or specific wavelength that they can provide considering effects on quality attributes. More research is needed to establish their suitability for fruit processing applications.
17.2.3 UV Light Propagation
UV light emitted from the atoms and ions within the gas discharge of a UV source will propagate away from those atoms and ions. As UV light propagates, it interacts with the materials it encounters through absorption, reflection, refraction, and scattering. Each of these phenomenon influences the intensity and wavelength of the UV light reaching the bacteria or chemical compound on the surface or in the liquid.
Absorption (A) of light is the transformation of energy of light photons to other forms of energy as it travels through a substance. Reflection (R) is the change in the direction of propagation experienced by light deflected by an interface. Scattering is the phenomenon that includes any process that deflects electromagnetic radiation from a straight path through an absorber when photons interact with a particle. The scattering phenomenon plays an important role in disinfecting food liquids containing particles. Experimental measurements are usually made in terms of transmittance of a substance (T) or (UVT), which is defined as the ratio of the transmitted to the incident light irradiance. A convenient way of presenting information about UVT of materials is to give the values of their absorption coefficient at various wavelengths, over a given depth (e.g., 1 cm). Knowing this, the transmittance for any particular depth and the depth of the liquid which will absorb 90 % of the energy at 253.7 nm can be calculated.
Photochemical reactions proceed as a direct result of radiation energy (photons) being introduced to a system. In view of the wavelengths used in most UV-light treatments, the molecules (A) are primarily affected by energy absorption that results in photochemical reactions. In the general case, the process may be viewed as
The first step in this reaction is the absorbance of a photon by a reactant molecule (A), leading to the production of an electronically excited intermediate. The excited state can be for period of 10−10 to 10−8 s in which the energy of the electrons is increased by the amount of photon energy. Under some conditions, the intermediate state may undergo a chemical change to yield products that are relatively stable. For a photochemical reaction to proceed, photons must have sufficient energy to promote reactions to break or form a bond and photon energy must be absorbed to promote reactions. The extent of chemical reaction depends upon the quantum yield and fluence of incident photons. A quantum yield is the ratio of absorbed photons that cause a chemical change to the total absorbed photons. UV light at 253.7 nm has a radiant energy of 472.27 kJ/Einstein or 112.8 kcal/Einstein (1 Einstein represents 1 mole of photons). It is theoretically possible for 253.7 nm light to affect the O–H, C–C, C–H, C–N, H–N, and S–S bonds if it’s absorbed.
17.2.4 UV Fluence and Dose Definition and Determination
Fluence rate, fluence, and dose are other important terms to characterize UV light treatments in fruit processing. Fluence rate is the total radiant power incident from all directions onto an infinitesimally small sphere of cross-sectional area dA, divided by dA (Bolton and Linden 2003). Fluence is defined as the fluence rate multiplied by the exposure time. The term UV dose should be avoided as synonym of fluence because dose refers in other contexts to absorbed energy, but only a small fraction of all incident UV light is absorbed by microorganisms (Bolton and Linden 2003). In the case of PL , fluence is determined as energy per pulse multiplied by the number of pulses. The absorbed fluence indicates radiant energy is available for driving the solution reaction. However, when UV light is absorbed by the solution, it is no longer available for inactivating the microorganisms. The remaining interactions including reflection, refraction, and scattering change the direction of UV light but the light is still available for inactivation. The radiant energy delivered to the molecule or microorganism is called the effective or delivered germicidal UV dose. Microbial inactivation depends primarily on the effective dose.
UV fluence and consequently UV dose depends on the nature of media, the manner of radiation exposure, the target material to be irradiated, and the purpose of study. A general expression of UV fluence was given by Labas et al. (2006):
where Iλ,Ω(x,t) is the specific intensity for monochromatic radiation (λ) and for a particular direction (Ω). V is reaction volume. τ is residence time. Table 17.2 summarizes nomenclature used in Sect. 17.2. In order to apply the equation for specific calculation, many other equations were derived for various UV reactor and wavelength.
Bolton and Linden (2003) established a standard method of UV fluence determination in bench-scale collimated beam UV experiments for microbial inactivation. For a LPM lamp the UV fluence is calculated by Eq. (17.3) considering corrections of petri factor (PF), reflection factor (RF), divergence factor (DF), and water factor (WF). As only free photons transmitted through the media can be used to inactive the microbes, this UV fluence is also called as transmitted UV fluence .
where I0 is radiometer reading at the center of the dish and t is exposure time. The unit of transmitted UV fluence is mJ · cm−2.
The PF is defined as the ratio of the average of the incident irradiance over the area of the Petri dish to the irradiance at the center of the dish. The RF represents the decrease of a small fraction of beam due to the reflection between two different media. For finite distances of the cell suspension from the UV lamp, the beam is not perfectly collimated and diverges significantly, so the DF should be considered (Eq. 17.3a).
where l is UV path length of sample, L is a distance between UV source and sample surface.
If the water or other tested liquid absorbs UV at the wavelength of interest, then it is necessary to account for the decrease in irradiance arising from absorption as the beam passes through the sample. The WF is defined as Eq. (17.3b).
where α is absorption coefficient of total sample at 253.7 nm.
Equation (17.3) provides a method to calculate UV dose but it must be limited to collimated LPM UV lamp and microbial inactivation application. Other UV fluence and dose calculations may apply under different conditions and for various purposes.
Applied UV fluence is generated by an applied incident UV intensity modified by petri factor on the surface of sample in a certain exposure time. For a collimated beam UV lamp, it can be calculated based on Eq. (17.4) with unit of mJ · cm−2.
Applied fluence reflects the energy emission from the UV source and it is independent to the material to be irradiated. Knowledge of the applied fluence is important to select a correct power and type of UV source by taking into the account their UV efficiency as shown in Table 17.1 in order to achieve a targeted degradation or inactivation of material.
Absorbed UV fluence is the energy absorbed by the media and may result in the photochemical reaction (Eq. 17.1). For a collimated beam UV lamp, it can be calculated based on Eq. (17.5) with unit of mJ · cm−2.
If the absorption coefficient is constant, Eq. (17.5) can be rewritten as:
Absorbed UV fluence can be used to measure the degradation of chemicals in the liquid media. Totally absorbed energy may destroy the target chemical when liquid media itself does not absorb UV radiation. However, absorbed fluence is not suitable to measure the inactivation of microorganisms because the UV light is no longer available for the inactivation when it is absorbed by media.
Effective or delivered UV dose is the energy delivered and absorbed by the targeted component in the sample and result in the photochemical reaction, which can be calculated through chemical actinometry using Eq. (17.6)
where Φ is quantum yield of chemical compound, N is concentration of chemical compound, Uλ is energy per Einstein of photons, and t is UV exposure time. The unit of effective dose is mJ · cm−3. If the degradation reaction compliance with the first order reaction, Eq. (17.6) can be rewritten as following Eq. (17.6a).
where N0 is initial concentration of chemical compound, k1 is a first order reaction rate constant of photoreaction of chemical.
17.3 UV Light Based Control Measures in Fruits Processing Facilities
During manufacturing process, fruits can be exposed to microbiological cross contamination from the air, water, and surfaces. The traditional approach to controlling such contamination has been to target specific sites within the manufacturing environment with cleaning and disinfection regimes. UV light is an economical step towards improved hygiene control measures in the food industry. Sanitation, disinfection, and oxidation with UV light is a versatile, environmental-friendly technology, which can be used in the fruits processing and storage facilities to reduce microbial contamination and consequently to improve safety of fruits.
17.3.1 Air Treatment
Clean, fresh air is the basis in the industrial production of fruits. Microorganisms in the air, such as viruses, bacteria, yeasts, and fungi, can contaminate raw materials and intermediate products and spoil finished products during their processing and packaging. LPM sources are used very successfully in these applications, for disinfection in air intake ducting and store rooms and to ensure air of very low germ content in production areas. Short wave VUV radiation at 185 nm produces ozone from the oxygen in the ambient air so that this is activated for the oxidation process. UV oxidation breaks down pollutants in the exhaust air. For providing clean air in sensitive manufacturing food facilities, a combination of filters and UV light has been recommended. Basically two applications of UV are becoming common. In one, the moving air stream is disinfected in much the same manner as with a water system. In the other application, stationary components of the system such as air conditioning coils, drain pans, and filter surfaces are exposed to help prevent mold and bacteria growth or to disinfect the filter to aid in handling. The UVT in air is higher than in water and, therefore, the number of lamps required in a large duct is quite reasonable. Common airborne virus and bacteria are readily deactivated with UV. Fungi (molds and spores) require much higher doses. In the moving air stream, high wattage lamps are used, usually without a quartz sleeve. UV lamp fixtures are placed in such a manner as to completely irradiate surfaces where bacteria and mold might collect and grow. Mathematical modeling software and bioassay testing have been developed to allow efficient design and validation of these systems. Low operating costs and reasonable equipment costs can make UV very cost effective.
17.3.2 Water Treatment
Control of microorganisms in industrial process waters is often necessary to maintain the quality of the product or process. The fruit industry is a large volume consumer of water, and the potential for reuse or recycling of fruit processing water represents an attractive economic and sustainable benefit to the industry. A combination of UV light and ozone is a powerful oxidizing action to reduce microbial load and the organic content of water to very low levels.
17.3.3 Disinfection of Nonfood and Food Contact Surfaces
Mold and biofilms can develop on nonfood surfaces (ceilings, walls, floors) and equipment including tanks and vats, cooling coils, and food contact surfaces of equipment such as cutting equipment and conveyor belts (Kowalski 2006). In general, standard cleaning and disinfection procedures are adequate to contain these problems but alternatives are available, including antimicrobial coatings like copper and TiO2. UV irradiation of food processing equipment and surfaces, cooling coils disinfection systems, whole area UV disinfection, and after-hours irradiation of rooms when personnel are not present are all viable control options for maintaining high levels of sanitation and disinfection in fruit processing facilities (Kowalski and Dunn 2002). UV light kills up to 99.9 % of total germs on conveyor belts used for transporting fruits and vegetables.
17.3.4 Packaging
The packaging technologies play important role in extending the shelf life of fruits. UV light might be applied as pre- or post-packaging technology to reduce the microbial spoilage. As a pre-packaging control measure UV treatment of packaging in fruit filling plant, e.g., for lids, cups, sealing and packaging foils for drinks and beverages help to extend fruits shelf life. When using cUV and PL as post-packaging treatment for packaged fruits, the considerations about transparency are referred to the packaging materials. For example, materials such as glass, polystyrene, and PET, which allow visible light to penetrate through the container, are not transparent to the UV wavelengths that are essential for microbial inactivation and therefore they are not suitable for cUV and PL treatments. On the other hand, polymers such as polyethylene, polypropylene, polybutylene, EVA, nylon, Aclar, and EVOH transmit UV light and hence meet the requirements for PLT very well (Anonymous 2000). In addition, ink printed labels or drawings could interfere with the light absorption of the treated item and should be avoided on the surface of packaging materials. Besides the intrinsic transparency of the material, it is critical that the “condition” of the item to be treated is suitable for the penetration of the light. This means that the product surface should be smooth, clear and without roughness, pores and grooves which could “shadow” the microbial cells from the light, causing less complete light diffusion and thus reducing process effectiveness; for the same reason, the item to be treated should be clean and free of contaminating particulates. In addition, items having a complex geometry could have areas hidden from the light and could require a more accurate design of the treatment chamber in order for the light pulses to reach each point of the product surface.
17.4 UV Treatment of Whole Fresh Fruits to Enhance Functionality and Safety
17.4.1 Functional Foods and UV Hormesis
In the recent years, there has been an increasing interest by the consumers in functional food products that may help to maintain optimal health condition, performance, and well-being. Functional foods can be defined as foods that are clinically proven to provide health benefits and/or reduce the risk of chronic diseases beyond their basic nutritional value due to presence of physiologically bioactive compounds. Functional foods include natural foods (fruits, vegetables) and processed foods that have been enriched or fortified with nutrients, phytochemicals, or botanicals. The nutraceutical potential of plant foods can be also naturally enhanced through special growing conditions or postharvest exposure to abiotic stresses, such as UV light (Shama and Alderson 2005; Shama 2007). The latter treatment is known as “hormesis.” According to Shama (2007) “hormesis” involves the use of small levels of potentially harmful stressors directed against a living organism or living tissue in order to induce a beneficial or protective response. Recent studies on a variety of different fruits, such as berries (Baka et al. 1999; Allende et al. 2007; Pombo et al. 2011), apples (Ubi et al. 2006; Hagen et al. 2007), tropical fruits (Gonzalez-Aguilar et al. 2010; Srilaong et al. 2011), and mushrooms (Mau et al. 1998; Jasinghe and Perera 2006) proved that UV light can be successfully applied as a hormetic agent. In addition to enhanced levels of bioactive compounds, prolonged storability, delayed senescence, and microbial deterioration were observed in UV treated fruits.
17.4.2 UV Effects on Fruits Functionality
Fruits hormetic response is a sophisticated process, not fully understood yet. It has been shown that UV light stimulates cellular protective mechanisms that include changes in the metabolic activity with the activation of particular genes and enzymes. This includes: (1) the enzymes peroxidase and reductase that are responsible for the oxidative burst and formation of lignin polymers generating structural barriers against invading pathogens; (2) glucanases and chitinases that exhibit lytic activities towards major fungal cell wall components; and (3) l-phenylalanine ammonia lyase (PAL)—involved in biosynthesis of phenolics which are characterized by strong UV absorptive properties (Gonzalez-Aguilar et al. 2010). The exemplary UV absorbing plant phytochemicals, i.e., chlorogenic acid, gallic acid, epicatechin, and quercetin, are presented in Fig. 17.3.
Through the synthesis of phenolic compounds, plants primarily protect the DNA and also activate their antioxidant and antimicrobial defense system (El Ghaouth et al. 2003; Erkan et al. 2008; Interdonato et al. 2011; Pombo et al. 2011; Zhang et al. 2012). Bioactive compounds are formed mainly in the peel of treated fruits (Hagen et al. 2007). However, Bakhshi and Arakawa (2006) reported that fruit flesh has also the ability to accumulate phytochemicals. In post UV-B/visible treated apples, authors observed increased levels of phenolic acids, anthocyanin, and flavonols. Flavanols, procyanidins, and dihydrochalcones were not affected by the applied treatment.
Accumulation of antioxidants within plant tissues enhances nutritional quality of UV treated commodities. Phenolics, stilbenes, vitamins C and D, carotenoids, anthocyanins, and polyamines are essential ingredients in human diet due to health promoting activities, such as anticancer, anti-inflammatory, and antihistaminic. Table 17.3 summarizes data on the UV effects on functional fruit properties. In general, under optimal treatment conditions an increase in the levels of physiologically active compounds was observed.
17.4.3 Factors Affecting the Formation of Nutraceuticals
The overall effect of postharvest UV irradiation on the bioactive compounds depend on growing conditions, crop commodity and cultivar, temperature at which UV treatment is performed, applied UV bandwidth, and dose. Knowledge of these parameters allows optimizing the process in order to yield satisfactory nutritional, quality, and safety levels.
Growing conditions. The variable levels of sun light exposition during fruit growth can result in different postharvest fruits characteristics. Hagen et al. (2007) reported that apples grown in the shady side of the tree were characterized by ~40–50 % lower initial content of phytochemicals in comparison to those grown in sun-exposed canopies. The postharvest UV treatments of apples grown in shade resulted in higher yields of bioactive compounds than in apples grown in sun. Plant functional properties can be also modified by special growing conditions. Tsormpatsidis et al. (2011) cultivated “Elsanta” strawberry plants under UV opaque (blocked UV radiation up to 380 nm) and UV transparent film. UV radiation increased the rate of color development and resulted in higher levels of anthocyanins (14–31 %), flavonoids (9–21 %), and phenolics (9–20 %) content at strawberry harvesting. Moreover fruits ripened under UV transparent film were firmer, smaller but greater in number than fruit ripened under a UV opaque film. Authors also observed an increase in flavonoid (16 %) and phenolic (8 %) concentrations in plant leaves exposed to UV radiation.
Crop commodity and cultivar. In general, UV exposure results in enhanced antioxidant properties of treated fruits. However, different compounds that are characteristic for a given fruit will contribute to the antioxidant capacity of irradiated commodity. For example, resveratrol is characteristic to grapes and its remarkable accumulation was reported after UV-C exposure (Li et al. 2008). Citrus fruits are rich sources of flavonoids (naringin, tangeretin) and increased levels of those compounds were observed by Arcas et al. (2000) in UV-C treated bitter oranges. It is also necessary to mention that within a given specie the response to UV treatment may differ amongst cultivars. Ubi et al. (2006) noted different levels of anthocyanins induced by UV-B treatment at 17 °C in several tested apple cultivars. The highest levels of nutraceuticals were found in Tsugaru, whereas the lowest in Sansa apple cultivar (Tsugaru > Akane > Iwai > Sansa).
Temperature. Several studies were performed on the photo-stimulation of anthocyanins production in the fruits exposed to UV-B/visible light treatment at different temperature conditions. In the case of several apples cultivars (Iwai, Sansa, Tsugaru, and Akane) Ubi et al. (2006) found the treatment at 17 °C more effective, in comparison to that performed at 27 °C. On the contrary, Arakawa (1991) and Reay and Lancaster (2001) observed a higher yield of anthocyanins in “Jonathan,” “Gala,” and “Royal Gala” apples irradiated at higher temperatures (20–25 °C) than at lower temperature (10–15 °C) conditions. Similarly Zhang et al. (2012) reported UV-B/visible irradiation of Red Chinese sand pears to be more effective at 27 °C than at 17 °C. Postharvest exposure to UV-B/visible light at −0.5/−0.5 °C (day/night), 20/20, and 20/6 °C resulted in higher levels of anthocyanins in apples but not in European pears (Marais et al. 2001). Therefore, the choice of the optimal temperature conditions for postharvest UV treatment has to be experimentally defined for a given commodity and cultivar.
UV bandwidth. Effects of different UV bands, i.e., UV-C (200–280 nm), UV-B (280–315 nm), and UV-A (315–400 nm), alone and in combination with visible light on accumulation of physiologically active compounds in treated fruits were studied. Beneficial effects on the plant functional properties were observed in the case of combined UV-B–visible light treatments for apples (Arakawa 1991; Ubi et al. 2006; Hagen et al. 2007) and pears (Zhang et al. 2012). Ubi et al. (2006) and Hagen et al. (2007) found UV-B/visible light treatment to be more effective in the accumulation of apple phytochemicals in comparison to the application of UV-B (Ubi et al. 2006) or visible light (Hagen et al. 2007) treatment alone. Mau et al. (1998) studied the effects of UV-B and UV-C on the transformation of ergosterol to vitamin D2 in common (Agaricus bisporus) mushrooms. Both tested treatments yielded in vitamin D2 formation, however UV-B light was found to be more effective. The UV-B exposure (4.93 kJ∙m−2) resulted in the increase of vitamin D2 by 387 %, whereas UV-C (6.06 kJ∙m−2) by 173 %. In another studies, Jasinghe and Perera (2006) compared the effects of UV-C (23.0 kJ∙m−2) with the UV-A (25.2 kJ∙m−2) on the formation of vitamin D2 in edible mushrooms. The UV-C exposure resulted in higher levels of vitamin D2 in all tested mushrooms: Shiitake, Oyster, Abalone, and Button. UV-C light was also successfully applied to a variety of other fruits. As a result of UV-C irradiation, an increase and/or better maintenance of the phenolic compounds during storage was observed in the case of mangoes (González-Aguilar et al. 2001, 2007), blueberries (Wang et al. 2009), pepper fruits (Vicente et al. 2005), and green tomatoes fruits (Liu et al. 2011a).
UV dose and optimal treatment conditions. González-Aguilar et al. (2001) observed the highest accumulation of phytochemicals in mangoes exposed to 4.93 kJ∙m−2 whereas treatments at 2.46 or 9.86 kJ∙m−2 resulted in lower yield of phenols and polyamine compounds. Lammertyn et al. (2004) and Allende et al. (2007) recommended 1.0 kJ∙m−2 as optimal fluence for the UV-C processing of strawberries since at higher treatments browning and dehydration of the sepals occurred. Moreover, overdosing can result in accelerated ripening and senescence processes as well as lower resistance to microbial and/or fungal decay, leading to reduced fruit storability and economical losses (Nigro et al. 1998). Therefore in order to obtain the most satisfactory levels of nutraceuticals without affecting adversely appearance and shelf life of a given fruit commodity, the optimal UV treatment conditions must be applied.
17.4.4 Synergistic Antimicrobial Effects of UV Light and Hormetic Plant Response
The germicidal effects of UV light against naturally occurring pathogenic and nonpathogenic microflora on the surface of fresh produce can be synergistically enhanced by the hormetic response of irradiated fruits. For instance Li et al. (2010) reported a higher inhibition of Monilinia fructicola growth in the pears inoculated with the pathogen before the UV-C treatment than in those being inoculated after UV-C exposure. Similarly Pombo et al. (2011) observed a reduction in growth of Botrytis cinerea inoculated on the strawberries 8 h after UV-C treatment (4.1 kJ∙m−2). In other studies Obande et al. (2011) studied the shelf life of tomatoes that were first exposed to UV-C light at 8 kJ∙m−2 and then were inoculated with Penicillum digitatum. After 10 days of storage at 20 °C, the UV treated fruits were firmer and the diameter of fungal lesions was considerably smaller in comparison to controls. Therefore higher resistance to postharvest diseases of UV treated commodities can be partially attributed to the physiological changes stimulated by UV light. These include accumulation of phytochemicals, known to have antimicrobial and antifungal activities, and increased activities of lignifying enzymes that strengthen structural barriers against invading pathogens. Enhanced levels of phytoalexins (scoparone) and flavonoids (naringin, tangeretin) were associated with reduced fungal decay caused by P. digitatum in UV treated lemons (Ben-Yehosua et al. 1992), grapefruits (Lers et al. 1998), and oranges (Arcas et al. 2000). Lower susceptibility to grey mold rot (B. cinerea) was attributed to accumulation of rishitin in tomatoes (Charles et al. 2008) and resveratrol in grapes (Nigro et al. 1998) exposed to UV-C fluences of 3.7 kJ∙m−2 and 0.5 kJ∙m−2, respectively.
Besides the molds, pathogenic bacteria can be present on the surface of fresh produce, such as Salmonella spp., O157:H7 and non-O157 shiga toxin producing Escherichia coli that constitute a threat to human health and safety. It was presented by several authors that either UV-C or pulsed light (PL) treatments have the ability to reduce the population of these pathogens. For instance Yaun et al. (2004) reported a reduction of E. coli O157:H7 by approximately 3.3 log on apples exposed to UV-C light at 240 W∙m−2. The same UV irradiation conditions resulted in slightly lower log reduction of Salmonella spp. on tomatoes (2.19 log). Pulsed light (Xenon Corp.) with the emission spectrum in the UV/Visible range (100–1100 nm) was applied for 5, 10, 30, 45, and 60 s to raspberries inoculated with E. coli O157:H7 and Salmonella spp. Bialka et al. (2008) reported reductions between 0.7 and 3.0 log10 CFU/g of E. coli O157:H7 and 1.2 and 3.4 log10 CFU/g of Salmonella on treated berries. However, fruit processing with PL light was accompanied by temperature increase and therefore microbial reduction might result from the combined light-heat effects.
These examples demonstrated that the postharvest UV processing of variety of fresh produce can be effective against both pathogenic and nonpathogenic microflora. More cases of successful UV applications are presented in Table 17.4.
Fresh produce have tender skin that can be easily injured during harvesting and handling stages. The positive effects of UV treatments were also observed in the case of damaged fruits, which are normally characterized by higher susceptibility to the microbial decay. For instance, delayed decay development after UV-C treatments of artificially wounded pears and grapes was observed by Li et al. (2010) and Nigro et al. (1998), respectively.
17.4.5 UV Effects on Shelf Life
Fruits are highly perishable and after harvesting require appropriate handling that will delay their ripening and senescence during storage. The major symptoms of deterioration are quality loss, discoloration, tissue softening, weight loss, increased respiration rate, and ethylene production. Traditionally, through the manipulation of storage conditions, i.e., temperature and atmosphere, attempts were made to prolong the storability of fresh produce. However, these two factors must be optimized to avoid adverse effects. For example, very low temperatures can induce chilling injury in stored commodities. Application of hormetic UV doses can stimulate the expression of defense response genes, and decrease the expression of genes involved in wall degradation, lipid metabolism, and photosynthesis (Pombo et al. 2009; Liu et al. 2011b). These physiological and biochemical changes induced by UV treatments can help to maintain the overall quality and prolong the storability of harvested fresh produce. Better maintenance of nutritional and sensory qualities, delayed ripening, softening and electrolyte leakage, retarded chlorophyll degradation, higher resistance to chilling injury, reduced respiration rate, and weight loss were reported in the case of the variety of UV treated commodities, such as apples (Lu et al. 1991; Hagen et al. 2007), strawberries (Baka et al. 1999; Marquenie et al. 2002; Lammertyn et al. 2004; Allende et al. 2007), peaches (Lu et al. 1991; Gonzalez-Aguilar et al. 2004), limes (Kaewsuksaeng et al. 2011), bananas (Pongprasert et al. 2011), tomatoes (Barka et al. 2000), peppers (Vicente et al. 2005), and broccoli (Costa et al. 2006; Lemoine et al. 2007). Table 17.5 provides several examples of UV effects on the parameters attributed to the shelf life of irradiated fruits.
17.4.6 Factors Affecting the Delivery of UV Dose
Satisfactory microbial reduction can be achieved when the correct UV dose is delivered to the fruit surface. However, delivery of the UV dose to the fruit can be affected by the skin topography and applied procedure and so it needs to be carefully controlled.
Many varieties of fruits are characterized by rough surface and porous veins that allows the bacteria to attach tightly. Moreover, bacteria or pathogens of interest may become incorporated into biofilms with naturally existing microflora (Ukuku et al. 2001). As a consequence, bacteria can be shielded from the UV light and lower microbial reduction might be achieved.
In order to induce the host postharvest resistance to decay and reduce the microbial population, experimental procedures were developed allowing exposure of the entire fruit surface to UV light. This was achieved by the manual rotation of the treated commodities for two or four times during UV treatment (Stevens et al. 2005; Yang et al. 2009). However, as noticed by Stevens et al. (2005) such practices are rather impractical and can seriously affect the commercialization of the postharvest UV treatments of fresh produce. Authors verified if fruit rotating can have a major impact on the reduction of bitter rot (Colletotrichum gloeosporioides), brown rot (M. fructicola), and green mold (P. digitatum) in apples, peaches, and tangerines, respectively. Exposure to UV-C light in the stationary position of the stem ends of apples (7.5 kJ∙m−2), peaches (7.5 kJ∙m−2), and tangerines (1.3 kJ∙m−2) resulted in comparable or slightly better resistance to mold decay than when fruits were rotated four different times. The lowest resistance to the spoilage decay was induced when only one or two different sides of fruits were exposed to the UV light. The difference in fruit response to the applied treatment procedures were attributed by Stevens et al. (2005) to the sites of UV-C photoreception and possible transmission mechanisms of the transduction signal within the phloem vascular tissue of fruits. Recently Obande and Shama (2011) applied the biodosimetry in order to measure the UV-C dose delivered to a polystyrene sphere that could mimic the shape of fruits such as apples, peaches, and tomatoes. The spheres were inoculated with spores of Bacillus subtilis and exposed to UV-C light with applied static and rotary procedures. Authors reported that under UV irradiation conditions at the theoretical dose of 10.6 J, spore biodosimetry yielded 9.1 ± 0.9 J for a single exposure to UV-C for 80 s, 10.7 ± 1.0 J in case of two rotations by 180° (2 × 40 s), and 6.1 ± 0.6 J for a sphere rotated 4 times by 90° (4 × 20 s). The lowest UV dose, i.e., 3.5 J, was obtained in the case of continuously rotated sphere for 80 s. From the comparison of the results obtained by Stevens et al. (2005) and Obande and Shama (2011) it comes a small contradiction. The highest UV dose for the polystyrene sphere was obtained with the rotation for two times. Application of the same procedure in the case of fruits yielded in the lowest decay inhibition. Certainly, correct determination of the UV dose delivered to the fruits is very important for the future commercialization. However, more work has to be done in order to find the correlation between applied UV dose, its distribution over the fruit surface, and physiological mechanisms induced by the UV hormetic processing.
17.5 UV Preservation of Fruit Products
17.5.1 UV Pasteurization of Fruit Juices
Fresh fruit juices are popular beverages in the world market. They are perceived as wholesome, nutritious, all day beverages. For items such as juices or juice beverages, minimal processing techniques are expected to be used to retain fresh physical, chemical, and nutritional characteristics with extended refrigerated shelf life. The US FDA approval of UV-light as an alternative treatment to thermal pasteurization of fresh juice products (US FDA 2000b) led to the growing interest and research in UV technology. Key factors that influence the efficacy of UV treatment of fruit juices include optical properties, design of UV processing systems, and UV resistance of pathogenic and spoilage organisms. Chemical composition, pH, dissolved solids (oBrix), and water activity have to be considered as hurdles that can modify the efficacy of UV microbial inactivation. There are a number of studies recently published that examined the UV light not only as a potential means of alternative pasteurization by studying effects on microflora but also on enzymes, flavor, color, and nutrient content of fresh juices and nectars (Koutchma 2009).
17.5.1.1 UV Absorption of Fruit Juices
Fruit juices are characterized by a diverse range of chemical, physical, and optical properties. Optical properties (absorbance and scattering) are the major factors impacting UV light transmission and consequently microbial inactivation. UV absorbance and transmittance at 253.7 nm are important parameters to design UV preservation process using LPM or LPHO source. In the case of the broadband continuous UV and pulsed lamps it is important to measure the spectra of the absorbance or transmittance in the UV germicidal region from 200 to 400 nm. In terms of UV transmittance, fruit juices can be characterized as transparent fluids if 10 % < UVT < 100 %, opaque fluids if UVT ~0 %, and semitransparent fluids if 0 < UVT < 10 % for anything in between. In a majority of cases, juices will absorb UV radiation. For example, clear or clarified juices (apple, grape, or cranberry juices) can be considered as a case of semitransparent fluids. Juices with suspended solids or particles (apple cider, orange juice) are opaque fluids. Chemical composition such as vitamins content and concentration of dissolved and suspended solids determines the level of juices UVT.
The Beer–Lambert law (Eq. 17.1) is used to describe absorption behavior of fluids. In the case of Lambertian fluids, the relationship between absorbance (A) and concentration of an absorber of UV radiation (c, mol∙L−1), extinction coefficient (ε, L∙mol−1∙cm−1) or molar absorptivity of the absorbing species, and path length of light (d, cm) is linear.
In the case of fruit juices with suspended solids, the function of A = F(ε, c, d) can be nonlinear, which is typical for non-Lambertian fluids. Examples of the optical characteristics of some clarified fruit juices and opaque juices with particles are shown in Fig. 17.4a, b. Integrated sphere attachment to spectrophotometer and micro-cuvettes was used to measure total transmittance of juice samples due to their low UVT. Total transmittance measurement included both absorptive and scattering properties that contribute to how UV photons travel in juice matrixes.
As it can be noted in Fig. 17.4a, b clear juices including apple, cranberry, and white grape, and juices with particles such as apple cider and coconut water, followed linear behavior as Lambertian fluids, which is typical behavior for category of semitransparent juices. The majority of fruit juices with suspended particles did not follow the Beer–Lambert law. More research has to be done to separate absorptive and light scattering behavior of juices and understand their contribution to microbial inactivation. Knowledge of total absorption coefficients is necessary to calculate absorbed fluence of juices using Eqs. (17.2) and (17.5) from Sect. 17.2. The absorption coefficients of a few brands of freshly squeezed and commercial juices that are Lambertian liquids are summarized in Table 17.6.
Coconut water and coconut liquid were transparent at 0.1 cm liquid and semitransparent at 1 cm. Apple cider was a semitransparent fluid in 0.1 cm and opaque at 1 cm. All other clear juices were opaque at both path lengths. The absorption coefficient of fresh non-treated apple cider that contained suspended particles was approximately of 12 cm−1 which is lower than other fruit juices with particles as well as clarified brands. The higher absorbance of the clarified commercial brands can be probably due to contribution of added preservatives and vitamin C. From this prospective, the UV treatment of freshly pressed fruit juices looks more favorable.
17.5.1.2 UV Processing Systems for Juices
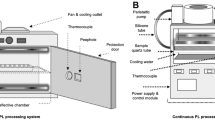
A number of continuous flow UV systems were developed and validated for a variety of fruit juices or other fruit beverages ranging from exotic tropical juices and nectars, to the more common apple cider and apple juice. The reactor designs include traditional annular, thin film, static and dynamic mixers (Taylor-Couette UV reactor), and coiled tube devices. Annular type laminar reactors were used for the treatment of apple juice and cider (Worobo 1998) and mango nectar (Guerrero-Beltran and Barbosa-Canovas 2006). The length and gap size can vary depending on the type of treated juice or flow rate. Thin film reactors are characterized by laminar flow with a parabolic velocity profile. Extensive research of the application of UV-light for fresh apple cider by Worobo (1998) yielded a design and production model of a thin film with 0.8 mm gap “CiderSure” UV reactor that was approved for a safe use to reduce microbial load of apple cider. UV treatment of orange juice was reported by Tran and Farid (2004) using a vertical single UV lamp thin film reactor. The thickness of the film was approximately 0.21–0.48 mm. Another commercial thin film reactor is the PureUV/SurePure reactor that was used for treatment of apple juice, guava-and-pineapple juice, mango nectar, strawberry nectar and two different orange and tropical juices (Keyser et al. 2008). This reactor is a single-lamp system with a thin fluid film formed between the lamp surface and a surrounding rippled or undulating outer wall. The reactor consisted of inlet, outlet chambers and a corrugated spiral tube between the chambers. Another type of static mixers is coiled tube UV reactors that are used to increase liquid delivery to UV source by more mixing due to Dean effect (Dean 1927). Salcor Inc. has promoted a UV reactor in which juice is pumped through the Teflon tubes coiled in a helix, with 12 LPM lamps inside and 12 lamps outside the helix (Anonymous 1999; Koutchma et al. 2007). The curved flow path can result in a pair of counter-rotating vortices with their axis along the length of the coil. Koutchma et al. (2007) validated the performance of a coiled UV module 420 model (Salcor Inc., Fallbrook, CA) for fresh tropical juices pasteurization. Geveke (2005) processed apple cider with a single lamp UV system surrounded by a coil of UV transparent Chemfluor tubing. Forney et al. (2004) used dynamic mixer Taylor-Coutte design to improve UV inactivation efficiency in apple juice.
17.5.1.3 Inactivation of Pathogenic, Nonpathogenic , and Spoilage Organisms
Table 17.7 summarizes the results of several reports on inactivation of pathogenic and nonpathogenic bacteria in fruit juices using continuous UV light sources. These data were obtained using static (collimated beam device) and continuous flow UV systems. The approaches to determine UV fluence also differed so reported results are not directly comparable.
Bobe et al. (2007) studied the presence and concentrations of pathogenic and indicator microorganisms in apple cider processed in Michigan. Neither E. coli O157:H7 nor Salmonella were detected in any tested cider samples, suggesting a very low frequency of pathogens in apple cider. The persistent and relatively high frequency of generic E. coli observed in samples indicated a continued risk of pathogen contamination in apple cider, especially when it is untreated. Basaran et al. (2004) compared log reductions among the E. coli strains in the apple cider made of different cultivars. The result failed to show any statistically significant relationship. However, the results of this study indicate that regardless of the apple cultivar used, a minimum 5-log reduction is achieved for all of the strains of E. coli O157:H7 tested. Gabriel and Nakano (2009) examined the UV resistance of strains of E. coli (K-12 and O157:H7), Salmonella (enteritidis and typhimurium), and Listeria monocytogenes (AS-1 and M24-1) that were individually suspended in phosphate-buffered saline (PBS) and apple juice prior exposure to UV radiation (220–300 nm). The AS-1 and M24-1 strains of L. monocytogenes were found to be most resistant to UV in PBS (0.28–0.29 min) while the AS-1 strain was most resistant in juice (1.26 min). The AS-1 strain of L. monocytogenes and E. coli O157:H7 were most heat resistant when suspended in PBS (4.41 min) and juice (4.43 min), respectively. Ye et al. (2007) reported that Yersinia pseudotuberculosis was less resistant to UV light than E. coli K12.
Table 17.8 summarizes results of reported studies in terms of inactivation of spoilage microorganisms in fresh juices. Variations in UV fluence levels can be accounted for due to limitations in dosimetry and fluid absorbance measurements. Mold spores are considered to be very UV resistant, with the resistance higher than of B. subtilis spores, followed by yeasts and lactic bacteria (Warriner et al. 2004, unpublished proprietary data). However, data on UV effectiveness against food borne pathogenic and spoilage microorganisms of high importance are limited or available in confidential reports and need to be generated. Data generated in air or water cannot be used for the calculation of UV process of low UVT food liquids. The results should be considered by juice processors in selecting appropriate surrogate organisms for UV light process lethality validations.
17.5.2 UV Surface Treatment of Fresh Fruit and Fresh-Cut Produce
cUV and PL treatments result in various levels of inactivation of spoilage and pathogenic microflora on the surface of a wide variety of foods. Comprehensive reviews of the literature in this field have been compiled by the US FDA (2000b) and by Woodling and Moraru (2005). The variability of the results (a 2- to 8-log reduction was generally reported) is most likely due to the different challenge microorganisms used in various studies, the intensity of the treatment, and the different properties of the treated substrates. Woodling and Moraru (2005) demonstrated that the efficacy of PL is affected by substrate properties such as topography and hydrophobicity, which affect both the distribution of microbial cells on the substrate surface and the interaction between light and the substrate (i.e., reflection and absorption of light). Surface disinfection of fresh and cut fruit products is a basis for longer shelf life. In designing a PL treatment for fruit items, both source (as light wavelength, energy density, duration and number of the pulses, interval between pulses) and target (as product transparency, color, size, smoothness, and cleanliness of surface) parameters are critical for process optimization, in order to maximize the effectiveness of product microbial inactivation and to minimize product alteration. Such alteration can be mainly determined by an excessive increase of temperature causing thermal damage to fruits but also by an excessive content of UV-C light which could result in some undesired photochemical damage to fruit itself or packaging materials.
17.5.2.1 Fresh-Cut Produce
Fresh-cut fruits became popular among consumers due to an increased preference for minimally processed fresh-like and ready-to-eat products. Mechanical operations of fresh-cut fruits production, such as peeling, slicing, and shredding, often result in enzymatic browning, off-flavors, texture breakdown, and lower resistance of fresh-cut produce to microbial spoilage in comparison with the unprocessed commodities (Lemoine et al. 2007) because of the presence of natural microflora on the surface of raw commodities. Therefore during operations of cutting and shredding, cross contamination may occur that might increase the risks of food-borne outbreaks.
To improve the hygiene and safety during the mechanical processing, sanitizing and dripping treatments are commonly applied. During washing and dipping steps, raw or fresh-cut material is immersed into tap water containing sanitizing agents (chlorine, sodium hypochlorite) to remove spoilage microorganisms, pesticide residues, and plant debris from product surface (Martin-Belloso et al. 2006). To reduce the usage of sanitizing chemicals, UV light alone or in combination with ozone or another preservative agent was explored as novel processing alternative. Fonseca and Rushing (2006) examined the effects of UV-C light (1.4–13.7 kJ∙m−2 at 253.7 nm) on the quality of fresh-cut watermelon compared to the common sanitizing solutions. Dipping cubes in chlorine (40 μL∙L−1) and ozone (0.4 μL∙L−1) was not effective in reducing microbial populations and cubes quality was lower after these aqueous treatments compared to UV-irradiated cubes or control. In commercial trials, exposure of packaged watermelons cubes to UV-C at 4.1 kJ∙m−2 produced more than 1-log reduction in microbial populations by the end of the product’s shelf life without affecting juice leakage, color, and overall visual quality. Higher UV doses did not show differences neither in microbial populations nor in quality deterioration (13.7 kJ∙m−2). Spray applications of hydrogen peroxide (2 %) and chlorine (40 μL∙L−1), without subsequent removal of excess water, failed to further decrease microbial load of cubes exposed to UV-C light at 4.1 kJ∙m−2. It was concluded that when properly utilized, UV-C light is the only method tested that could be potentially used for sanitizing fresh-cut watermelon. Similarly, exposure of sliced apples to UV-C resulted in higher (~1 log) reduction of Listeria innocua ATCC 33090, E. coli ATCC 11229, and Saccharomyces cerevisiae KE 162 in comparison to the apples pre-treated with anti-browning and sanitizing agent (1 % w/v ascorbic acid − 0.1 % w/v calcium chloride). The combination of UV-C with anti-browning pre-treatment better preserved color of sliced apples during storage at 5 °C for 7 days (Gómez et al. 2010). Other studies have shown that UV-C treatment applied alone was efficient in the reduction of a number of microbiological organisms present on the surface of fresh-cut crops. The examples of successful applications of UV-C light are given in Table 17.9.
Similarly to raw crops, the effectiveness of UV treatment on the reduction of microbial deterioration and quality retention was defined by the delivered UV dose and overall characteristics of the surface exposed to the UV light. Lamikanra et al. (2005) stressed out that the moment of application of UV light during the fruit processing is an important factor. In their studies the authors exposed the cantaloupe melon to UV-C at 254 nm during cutting and after cut of the fruits. Cutting of cantaloupe melon under the UV-C light was as effective as post-cut treatment in reduction of yeast, molds, and Pseudomonas spp. populations. However fruit cutting during simultaneous exposure to UV-C resulted in improved product quality, i.e., reduced rancidity and respiration rate, and also increased firmness retention, when compared to post-cut and control samples. Better preservation of fruits processed during the UV exposure can be related to the defence response of the wounded plant enhanced by the UV. Mechanical injury of the plant tissues activates the expression of wound-inducible genes. UV radiation is capable to induce the expression of plant defence-related proteins that are normally activated during wounding. For example, Lamikanra et al. (2005) reported a significant increase in ascorbate peroxidase enzyme activity during storage of cantaloupe melon processed under UV-C light. Peroxidases protect plant cells against oxidation. Higher levels of terpenoids (β-cyclocitral, cis- and trans- β-ionone, terpinyl acetate, geranylacetone, and dihydroactinidiolide) that can play important roles as phytoalexins in the disease resistance of a variety of plant families were found in cantaloupe tissues (Lamikanra et al. 2005; Beaulieu 2007). Significant increase of anti-oxidative compounds, such as phenolics and flavonoids, was also observed by Alothman et al. (2009) in UV treated fresh-cut banana, pineapple, and guava fruits. However a decrease in vitamin C was observed in all fruits.
In terms of UV effects on fruits flavor, Beaulieu (2007) and Lamikanra et al. (2005) reported that fruits processed with the UV light preserved their aroma to the same extent as non-treated control samples. Detailed studies of volatile compounds in thin-sliced cantaloupe tissues revealed that UV treatment is not responsible for the chemical transformations to ester bonds, esterase, and lipase decrease. However Beaulieu (2007) indicated that improper cutting, handling, sanitation treatment, and storage can radically alter the desirable volatile aroma profile in cut cantaloupe, and potentially leads to decreased consumer acceptance.
17.6 UV Effects on Chemicals in Fruit Products
17.6.1 Degradation of Patulin
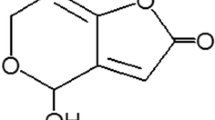
Patulin [4-hydroxy-4H-furo (3, 2-c)-pyran-2-(6H)-one] is a mycotoxin produced by a wide range of molds involved in fruit spoilage. Penicillium expansum is the predominant patulin producing fungus in naturally rotted apples (Lovett et al. 1974). Although cases of contamination were reported in various peaches, cherries, berries, and strawberries, patulin occurs most frequently in rot lesions of apples. Beretta et al. (2000) reported 21 patulin positive samples of rotten areas of apples in a total of 26 samples. The concentration of patulin has been detected up to 130 mg∙kg−1. As with the majority of mycotoxins, patulin is stable and can persist in juice over extended time periods. Although the washing and removal of rotten apples may reduce 90 % of the original patulin concentration (Leggott et al. 2000), patulin contamination in apple juice was detected up to 733 μg∙L−1 and reported by Ehlers (1986), Gökmen and Acar (1998), and Yurdun et al. (2001). Patulin is a health concern for both consumers and manufactures, which may cause acute but more frequently, chronic intoxications leading to nervousness, convulsion, lung congestion, oedema, hyperaemia, immunotoxic, immunosuppressive, and teratogenic effect (Roll et al. 1990). Because of the prevalence of patulin and possible accumulation of the toxin within the body over time, the Codex Alimentarius Commission (2003) and the US FDA (2005) have recommended a limit for patulin content on apple products intended for human consumption of 50 μg∙L−1 (50 ppb). The European Union has gone further and imposed a maximum limit of 10 μg∙L−1 (10 ppb) for baby food and formulae.
Although several methods for control and elimination of patulin have been proposed, there is no unifying method being commercially successful for reducing patulin while keeping produce quality. A few recent studies evaluated feasibility of UV radiation as a possible commercially alternative for the reduction of patulin and patulin producing Penicillium spores in fresh apple juice. Dong et al. (2010) used the CiderSure 3500 commercial UV system equipped with the 8 LPM lamps for patulin destruction. It was reported that UV exposure of 14.2–99.4 mJ∙cm−2 resulted in a significant and nearly linear decrease in patulin levels while producing no quantifiable changes in the chemical composition (i.e., pH, Brix, and total acids) or organoleptic properties of the cider.
Zhu et al. (2012) investigated UVC-light to control patulin content in model solution, apple cider, and apples juice by using R-52G MINERALIGHT® UV Lamp and studied the kinetics of degradation of patulin. It was shown that 56.5 %, 87.5 %, 94.8 %, and 98.6 % reduction of patulin can be achieved in the model solution, apple cider, apple juice without vitamin C addition, and apple juice with vitamin C addition, respectively. Sample (2-mm length) was initially spiked with 1 mg∙L−1 of patulin after UV exposure for 40 min at UV intensity of 3.00 mW∙cm−2. The effective UV doses which were directly absorbed by patulin for photochemical reaction were 430, 674, 724, and 763 mJ · cm−3, respectively (Fig. 17.5). Similar applied UV fluence of 7064 mJ∙cm−2 was adopted for all samples. The decimal reduction time (D-value) was estimated at 112.6, 44.2, 32.6, and 19.4 min, respectively. Degradation of patulin complied with the first-order reaction model. Both time-based and fluence-based reaction rate constants were determined for predict of patulin degradation. The fluence-based model should be more beneficial given that the uniform degradation rate constant in the same media can be obtained from one specific experiment but consequently to be adopted for further prediction with different UV intensity and sample thickness (UV path length). Yan’s work also compared the patulin degradation rate in dynamic system with well stirring during UV radiation and in static system without mixing. The study revealed the reaction rate constant of dynamic samples (model solution: 2.95E-4 s−1, juice: 4.31E-4 s−1) were significantly higher than static ones (model solution: 2.79E-4 s−1, juice: 3.49E-4 s−1, P < 0.05) when applied UV intensity and sample length were identical. Although the patulin solution is homogeneous, the intensity of UV light is not uniform along the volume of the solution. Based on Beer–Lambert Law, the UV intensity decreases exponentially when IV light enter the liquid sample. The stirring applied in the dynamic system increased the collision chance between patulin molecular and photons and consequently increased the reaction rate. The patulin degradation rate constant in apple juice was significantly higher than in model solution (P < 0.05). This suggests that apple juice constituents enhanced the degradation of patulin. Polyphenols and ascorbic acids contained in apple juice can be activated by UV light and produce free radicals that react with patulin molecules. However, further work will be required to confirm this hypothesis. This study provided strong evidence that UV radiation can become an effective method of reducing the patulin level in apple cider and apple juice.
17.6.2 Inactivation of Enzymes
Enzymatic activity actually depends on the native structure of the protein which, by principle, can be modified following photo-oxidation promoted by exposure to UV and visible light. Photo-oxidation of enzymes can occur via two major routes: (1) direct photo-oxidation arising from the absorption of radiation by the protein structure or bound chromophore and (2) indirect protein oxidation mediated by singlet oxygen generated by energy transfer by either protein bound, or other chromophores (Davies and Truscott 2001). The effect of UV light on the activity and structure of fruit enzymes is still a matter of speculation. Limited and controversial information is available in the literature.
Color is a very important quality parameter in fruit juices. It is related to non-enzymatic and enzymatic browning, due to polyphenol oxidase (PPO) activity. The effect of UV light on the inactivation of enzymes related to food quality is diverse. While Noci et al. (2008) reported no effect of UV on apple PPO activity, Manzocco et al. (2009) reported about 80 % inactivation of PPO at approximately of 1250 mJ∙cm−2 of UV fluence. Guerrero-Beltran and Barbosa-Canovas (2006) found that after UV treatment of mango nectar at 44,633 mJ∙cm−2 PPO reduced its activity to 19 %. Falguera et al. (2011) irradiated apple juices made from four different varieties (Golden, Starking, Fuji, and King David) during 120 min with a polychromatic mercury lamp of 400 W in a range of 250 and 740 nm with an incident energy of 3.88 × 10−1 Einstein∙min−1. The treatment was effective in the inactivation of PPO after 100 min, while peroxidase was completely destroyed in 15 min in all the four varieties. It should be noted that the major absorbance peak of PPO enzyme matched with the largest peak of the emission spectrum of the lamp.
One important factor in orange juice appearance is the “cloud” formed by pectin. Pectin methylesterase (PME) is an enzyme that tends to de-esterify pectin, and which inactivation is consequently pursued. Tran and Farid (2004) reported the results of UV treatment of reconstituted orange juice. In addition to the decimal reduction dose for the standard aerobic plate count, effects on shelf life, pH, color, vitamin C, and destruction of PME enzyme were studied. The shelf life of freshly squeezed orange juice was extended to 5 days as a result of limited exposure of UV light of 73.8 mJ∙cm−2. No destruction of PME (5 %), which is a major cause of cloud loss of juices, was reported whereas the activity of this enzyme was significantly decreased (70 %) by mild heat treatment at 70 °C for 2 s.
17.6.3 Effects on Essential Vitamins
Even though vitamins may be present in small amounts in fresh juices they are of concern because some vitamins are considered light sensitive. Water soluble light sensitive vitamins include C (ascorbic acid), B12 (cobalamin), B6 (pyridoxine), B2 (riboflavin), and folic acid. Fat soluble, light sensitive vitamins include A, K, E (alpha-tocopherol), and carotene. Most studies were conducted on the effects of light on vitamins in the wavelength range of 290–700 nm, which includes both UV and visible light. They have involved exposure to fluorescent lamps, but there are limited data available at 253.7 nm. Since vitamin C is characterized by high UV absorbance within the germicidal wavelength range (peak at approximately of 260 nm) but does not absorb light significantly above 300 nm, the content of vitamin C also affected the magnitude of absorption coefficient. The destruction of vitamin C during exposure to UV light may alter the absorption properties of the treated juice. Ye et al. (2007) measured vitamin C content before and after UV treatment. Two brands of packaged apple juice (pasteurized, no preservatives), Sahara Burst and Gordon Food Service, were enriched with Vitamin C. The UV system consisted of four chambers with varied lengths and a single LPM bulb at output power of 25 W at 253.7 nm. Approximately 50 % destruction of vitamin C was observed after one complete pass through the system at the slowest flow rate. The effect of vitamin C destruction on the value of the absorption coefficient in apple juice enriched with this vitamin was also measured. After three passes through the UV system at the flow rate of 4 mL∙s−1 the absorption coefficient of apple juice reduced to approximately 20 % of initial value. It was concluded that juices enriched with vitamin C require significantly higher doses of UV irradiation for pasteurization purposes. A comparison of vitamin C destruction and inactivation of E. coli K12, in commercial apple juice (Motts) exposed to UV at the fluence rate of 1.0 mW∙cm−2 showed that E. coli bacteria were more sensitive to UV light exposure with a destruction rate almost of 2.5 times higher compared to samples containing vitamin C. When destruction of vitamin C in apple juice was measured after processing using a commercial multiple lamp UV unit CiderSure1500, it was found that after three consecutive passes through the system at the slowest flow rate of 57 mL∙s−1, approximately 50–60 % of the initial concentration of vitamin C (25 mg/100 g) remained. Comparison of the destruction of vitamin C in clarified apple juice with absorption coefficient of 15 cm−1 and orange juice of 54 cm−1 after exposing both juices to the identical levels of UV fluence of 1.0 mW∙cm−2 in a Petri dish demonstrated that the destruction rate was 8 times faster in clarified apple juice due to greater levels of available absorbed energy (Koutchma et al. 2008). Falguera et al. (2011) studied the effect of a mercury lamp of 400 W in a range of 250 and 740 nm at incident energy of 3.88 × 10−1 Einstein min−1 on the content of vitamin C in juices from Golden, Starking, and Fuji. The loss in Golden juice after 120 min of UV irradiation was 5.7 %, while in Starking one was 5.6 %, and in Fuji one 4.0 %. In the juice from King David the loss was 70.0 %. This significant difference was attributed to the lack of pigmentation of this juice. In the three first cases, more vitamin C was damaged in the first 60 min than in the second hour, meaning that as pigments were degraded (and the juice color was lighter) its protective effect was less important. In the King David juice the loss after 0 min was 62.4 % of the initial content, and after 60 min it was 69.8 %. In recent years pulsed UV sources gained interest for their application for food processing due to potentially greater germicidal effectiveness and depth penetration. Orlowska et al. (2012) compared the effects of continuous (LPM and MPM) and pulsed UV (PUV) sources on the vitamin C content of fortified apple juice and milk. Applied PUV lamps were characterized by different emission spectra in the range of 200–350 nm, energy per pulse, and frequency (PUV-1: 31 J/pulse, 8 Hz; PUV-2: 344 J/pulse, 0.75 Hz; PUV-3: 644 J/pulse, 0.5 Hz). Comparison was made at the UV fluence that was determined based on 5-log microbial reduction requirement, i.e., 10 mJ∙cm−2 for LPM and MPM, and 5 mJ∙cm−2 for the PUV sources. The UV treatments with the MPM and PUV-2 induced significant (P < 0.05) reduction of vitamin C by −5.45 ± 0.27 % and −8.52 ± 0.50 % in apple juice, −61.73 ± 3.08 % and −35.80 ± 1.79 % in milk, respectively. The other two pulsed UV lamps didn’t affect significantly (P > 0.05) vitamin C in apple juice, and its reduction was on the same level as in the case of LPM, i.e., −1.30 ± 0. 07 %. Similarly PUV-1 and PUV-3 caused least changes in ascorbic acid content in milk, i.e., −12.31 ± 0.62 % and −21.66 ± 1.08 %, respectively, whereas treatment with the LPM lamp resulted in reduction of vitamin C by −35.13 ± 1.56 %. Results have shown that PUV-3 source can constitute a promising alternative for UV treatments as it offers deeper penetration in opaque liquids due to broader emission spectrum in comparison to LPM, and about 10 times shorter exposure times when compared with PUV-1. Authors also stressed out the importance of knowledge of the optical properties of ingredients and their chemical interactions in UV treated beverage and the emission spectra of applied UV sources. For instance, a significantly higher reduction of vitamin C in milk was observed, in comparison to apple juice (<10 %), which can be associated with the riboflavin, also known as vitamin B2. Riboflavin is a photosensitive compound characterized by four absorption peaks in the UV range (222, 266, 373 nm) and in visible light range (445 nm). As it can be seen in Fig. 17.6 the peaks of MPM emission spectrum overlap the broad riboflavin peak with its maximum of absorbance at 266 nm. This can lead to the occurrence of photochemical reactions if sufficient energy is delivered to the UV exposed system. From the literature (Gilmore and Dimick 1979; Bender 2003) it is known that riboflavin photolysis leads to the formation of lumiflavin and lumichrome, which catalyze the oxidation of other milk ingredients, such as vitamin C. Therefore in order to explore the full potential and applications of pulsed UV sources for specific food systems more studies have to be conducted.
Vitamin A is another vitamin of great importance in fresh juices because it contributes to more than 2 % of the nutritional value of the Recommended Daily Allowance (RDA) . After exposure of vitamin A in malate buffer to UV light at the fluence of 200 mJ∙cm−2, approximately 50 % of vitamin A initial concentration remained. Orange juice is an essential source of vitamin C and A. One 8 fluid ounce (3.69 mL) serving of orange juice contributes approximately to 210 % of RDA of vitamin C and 10 % RDA of vitamin A in the diet. The destruction of essential vitamins in orange juice was reported by Anonymous (1999) after treatment in the commercial Salcor UV module (Salcor Co, CA) at a flow rate of 7.5 gpm (28.39 L∙min−1) when total accumulative UV dose was 298.9 mJ∙cm−2. The highest destruction of riboflavin and beta carotene (~50 %) was observed. However, in terms of vitamins C, B6, and A only 16.6–11 % of those vitamins were destroyed after exposure to UV light.
17.6.4 Degradation of Herbicides
The use of agricultural pesticides has increased dramatically and has consequently led to increasing concerns related to their toxicity, stability, and pollution of soil, water, and air. Triazine herbicides are among the most commonly used herbicides in the world. A maximum admissible concentration of 0.1 μg∙L−1 per individual pesticide was set in the EEC Directive on the Quality of Water Intended for Human Consumption. Evgenidou and Fytianos (2002) studied the photodegradation of three triazines, atrazine, simazine, and prometryn, in aqueous solutions and natural waters using UV radiation (λ > 290 nm). Experimental results showed the rate of photodecomposition in aqueous solutions depends on the nature of the triazines and follows first-order kinetics. The half-lives of triazines in distilled water and surface waters ranged from 2.7 to 11.6 h with exposure of high-pressure mercury UV lamp. The work demonstrated the effects of photodegradation of triazines during direct UV exposure and indirect (UV with H2O2) irradiation and suggested the existence of various degradation routes resulting in complex and interconnected pathways.
17.7 Sustainability of UV Technology
Expected increase of world population up to 9 billion by 2050 brings the necessity to implement sustainable practices that will allow meeting the needs of the present without compromising the ability of future generations to meet their own needs. These include wiser management of the natural resources use, product stewardship, strengthening energy efficiency, development of new technologies that reduce the consumption of resources, and eradication of poverty.
UV light is an emerging nonthermal technology that has much to offer for the sustainable development of the society. Its application for the food processing is energy and cost-effective, and also was proven to yield fresh-like, safe and highly nutritional fruits and fruit products, such as juices. Moreover, UV light applied as a postharvest technology can significantly reduce the loss of fresh produce, which in the developed countries is of the order of 20 % and as high as 50 % in developing countries (Obande and Shama 2011). It was shown by many researchers that UV technology might be used as alternative method to control postharvest diseases caused by fungi. This in turn may substantially reduce the usage of fungicides as well as other chemicals that pose serious health hazard and environmental risks (Lu et al. 1991).
The major disadvantage of UV technology is the mercury content in UV sources. The potential mercury exposure due to lamp sleeve breakage is a health concern. Breakage of lamps can occur when lamps are in operation and during maintenance. The mercury contained within a UV lamp is isolated from exposure by the lamp envelope and surrounding lamp sleeve. For the mercury to be released, both the lamp and lamp sleeve must break. The mercury content in a single UV lamp used for water treatment typically ranges from 0.005 to 0.4 g (5–400 mg). LPM lamps have less mercury (5–50 mg/lamp) compared to LPHO (26–150 mg/lamp) and MPM lamps (200–400 mg/lamp). The EPA established a maximum contaminant level (MCL) for mercury at 0.002 mg∙L−1. The EPA has found mercury to potentially cause kidney damage from short-term exposures at levels above the 0.002 mg∙L−1 MCL (EPA 1995). The concern over the impact of mercury release into the food plant environment stimulated the development and validation of mercury-free special technologies lamps and LEDs.
17.8 Conclusions and Future Trends
Ultraviolet (UV) light technology using continuous and pulsed modes is a viable nonthermal alternative for fruits and fruit products processing. A large number of reviewed studies reported successful applications of UV light for eliminating or reducing the levels of undesirable pathogenic, nonpathogenic and spoilage microorganisms on the surfaces of fresh fruits and fruit products like juices. In order to achieve the required microbial reduction along with color, texture, and flavor preservation, optimal UV processing conditions and proper UV source have to be found for a given product. Moreover, UV light can be recommended as an effective mean to control microbial loads in the air, water, nonfood, and food contact surfaces in fruit processing facilities. A variety of UV sources are commercially available or currently under development that can be applied for specific fruit processing purposes whereas LPM lamps and xenon PL are currently the dominant sources for UV treatment of fruits since they were approved by the US FDA and Health Canada. A number of UV-light continuous flow systems that included annular laminar and turbulent flow reactors, thin film devices, static and dynamic mixers were developed and validated for a variety of fruit juices for pasteurization purposes. The correct UV design can reduce the interference of low UVT and viscosity associated with some juices and therefore improves the UV inactivation efficiency. More work is needed in regards of design of UV systems capable of delivering sufficient UV doses to all parts of the treated liquid with low UVT such as fruit juices.
Recent studies reported a potential of UV light for enhancement of health promoting compounds such as antioxidants, polyphenols, and flavonoids. Numerous studies cited here have shown the beneficial effects of the UV treatment on the preservation of many fruits, both raw and fresh-cut. However on the basis of the available literature data the mechanism that underlies the hormetic response in fresh produce is still under debate. In response to the exposure of UV light, plants activate different enzymes peroxidases, reductases, chitinases which differ by chemical structure and absorptive properties in UV-A, UV-B, and UV-C ranges. Therefore plant response varies depending on applied UV emission spectrum and UV dose. To improve the state of the current knowledge on UV processing on fresh produce, further studies are necessary that will measure and report conditions and parameters of the UV treatment, such as lamp characteristic, emitted wavelength, and UV fluence levels.
The effect of UV-light on quality of fruits and their products requires further studies. Despite the fact that UV is a pure nonthermal treatment, possible undesirable effects may include damage to vitamins and proteins, destruction of the antioxidants, changes in color and formation of off-flavors and aromas depending on UV spectra and applied dose. In addition, the effects of UV light on the potential formation of chemical compounds in foods that may present a health threat should be evaluated to determine if there is any toxicological or chemical safety concerns associated with products that have undergone UV treatment. Closer examination of UV light potential to destroy undesirable compounds or pollutants also deserves more attention. Due to low penetration of UV light, the combinations with other postharvest technologies (ozone, ultrasound, modified packaging atmosphere, sanitizing and anti-browning agents) might be attractive for processors and also more efficient. Limited data are available on UV processing combined with other treatments and further studies are necessary to undertake.
Abbreviations
- A :
-
Absorption
- AIN:
-
Aluminum nitride
- DF:
-
Divergence factor
- EL:
-
Excimer lamp
- EPA:
-
US Environmental Protection Agency
- EVA:
-
Ethylene vinyl acetate
- EVOH:
-
Ethyl vinyl alcohol copolymer
- FDA:
-
US Food and Drug Administration
- GaN:
-
Gallium nitride
- LED:
-
Light emitting diodes
- LPHO:
-
Low-pressure high-output lamp
- LPM:
-
Low-pressure mercury lamp
- MCL:
-
Maximum contaminant level
- MPM:
-
Medium pressure mercury lamp
- PBS:
-
Phosphate-buffered saline
- PET:
-
Polyethylene terephthalate
- PF:
-
Petri factor
- PL:
-
Pulsed lamp
- PLT:
-
Polyethylene
- PME:
-
Pectin methylesterase
- PPO:
-
Polyphenol oxidase
- R:
-
Reflection
- RDA:
-
Recommended Daily Allowance
- RF:
-
Reflection factor
- T or UVT:
-
Transmittance or transmittance of material in the ultraviolet range
- TiO2 :
-
Titanium dioxide
- UV:
-
Ultraviolet
- UV-A:
-
Ultraviolet light range: 315–400 nm
- UV-B:
-
Ultraviolet light range: 280–315 nm
- UV-C:
-
Ultraviolet light range: 200–280 nm
- cUV:
-
Continuous ultraviolet mode
- VUV:
-
Vacuum ultraviolet radiation (100–200 nm)
- WF:
-
Water factor
References
Allende, A., A. Marín, B. Buendía, F. Tomás-Barberán, and M.I. Gil. 2007. Impact of combined postharvest treatments (UV-C light, gaseous O3, superatmospheric O2 and high CO2) on health promoting compounds and shelf-life of strawberries. Postharvest Biology and Technology 46: 201–211.
Alothman, M., R. Bhat, and A.A. Karim. 2009. UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innovative Food Science and Emerging Technologies 10: 512–516.
Anonymous. 1999. A food additive petition for the use of ultraviolet light in the reduction of microorganisms on juice products. Submitted to FDA regarding CFR 21 179. Glendore, CA: California Day-Fresh Foods Inc. pp. 1–117.
———. 2000. Kinetics of microbial inactivation for alternative food processing technologies. Institute of Food Technologists. J. Food Sci. Supplement http//vm.cfsan.fda.gov/~comm/ift-pref.html. Accessed: 7 February, 2012
Arakawa, O. 1991. Effect of temperature on anthocyanin accumulation in apple fruit as affected by cultivar, stage of fruit ripening and bagging. The Journal of Horticultural Science & Biotechnology 66: 763–768.
Arcas, M.C., J.M. Botía, A.M. Ortuño, and J.A. Del Río. 2000. UV irradiation alters the levels of flavonoids involved in the defence mechanism of Citrus aurantium fruits against Penicillium digitatum. European Journal of Plant Pathology 106: 617–622.
Artés-Hernández, F., P.A. Robles, P.A. Gómez, A. Tomás-Callejas, and F. Artés. 2010. Low UV-C illumination for keeping overall quality of fresh-cut watermelon. Postharvest Biology and Technology 55: 114–120.
Baka, M., J. Mercier, R. Corcuff, F. Castaigne, and J. Arul. 1999. Photochemical treatment to improve storability of fresh strawberries. Journal of Food Science 64: 1068–1072.
Bakhshi, D., and O. Arakawa. 2006. Induction of phenolic compounds biosynthesis with light irradiation in the flesh of Red and yellow apples. Journal of Applied Horticulture 8: 101–104.
Barka, E.A., S. Kalantari, J. Makhlouf, and J. Arul. 2000. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. Journal of Agriculture and Food Chemistry 48: 667–671.
Basaran, N., A. Quintero-Ramos, M.M. Moake, J.J. Churey, and R.W. Worobo. 2004. Influence of apple cultivars on inactivation of different strains of Escherichia coli O157:H7 in apple cider by UV irradiation. Applied and Environmental Microbiology 70: 6061–6065.
Beaulieu, J.C. 2007. Effect of UV irradiation on cut cantaloupe: terpenoids and esters. Journal of Food Science 72: S272–S281.
Bender, D.A. 2003. Nutritional biochemistry of the vitamins. Cambridge: Cambridge University Press.
Ben-Yehosua, S., V. Rodov, J. Kim, and S. Carmeli. 1992. Preformed and induced antifungal materials of citrus fruit in relation to the enhancement of decay resistance by heat and ultraviolet treatment. Journal of Agricultural Food Chemistry 40: 1217–1221.
Beretta, B., A. Gaiaschi, C.L. Galli, and P. Restani. 2000. Patulin in apple-based foods: occurrence and safety evaluation. Food Additives & Contaminants 5: 399–406.
Bialka, K.L., and A. Demirci. 2007. Decontamination of Escherichia coli O157:H7 and salmonella enterica on blueberries using ozone and pulsed UV-light. Journal of Food Science 72: M391–M396.
Bialka, K.L., A. Demirci, and V.M. Puri. 2008. Efficacy of pulsed UV-light for the decontamination of Escherichia coli O157:H7 and salmonella spp. on raspberries and strawberries. Journal of Food Science 73: M201–M207.
Bobe, G., D. Thede, T.A.T. Eyck, and L.D. Bourquin. 2007. Microbial levels in Michigan apple cider and their association with manufacturing practices. Journal of Food Protection 70: 1187–1193.
Bolton, J.R., and K.G. Linden. 2003. Standardization of methods for fluence UV dose determination in bench-scale UV experiments. Journal of Environmental Engineering 129: 209–215.
Boue, S.M., T.E. Cleveland, C. Carter-Wientjes, B.Y. Shih, D. Bhatnagar, J.M. McLachlan, and M.E. Burow. 2009. Phytoalexin-enriched functional foods. Journal of Agricultural and Food Chemistry 57: 2614–2622.
Charles, M.T., J. Mercier, J. Makhlouf, and J. Arul. 2008. Physiological basis of UV-C-induced resistance to botrytis cinerea in tomato fruit I. Role of pre- and post-challenge accumulation of the phytoalexin-rishitin. Postharvest Biology and Technology 47: 10–20.
Codex Alimentarius Commission. 2003. Code of Practice for the Prevention and Reduction of Patulin Contamination in Apple Juice and Apple Juice Ingredients in Other Beverages, www.codexalimentarius.net/download/standards/405/CXC_050e.pdf (accessed: February 5, 2012)
Costa, L., A.R. Vicente, P.M. Civello, A.R. Chaves, and G.A. Martínez. 2006. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biology and Technology 39: 204–210.
D’hallewin, G., M. Schirra, E. Manueddu, A. Piga, and S. Ben-Yehoshua. 1999. Scoparone and scopoletin accumulation and ultraviolet-C induced resistance to postharvest decay in oranges as influenced by harvest date. Journal of the American Society for Horticultural Science 124: 702–707.
D’hallewin, G., M. Schirra, M. Pala, and S. Ben-Yehoshua. 2000. Ultraviolet C irradiation at 0.5 kJ · m−2 reduces decay without causing damage or affecting postharvest quality of star ruby grapefruit (C. paradisi Macf.). Journal of Agricultural and Food Chemistry 48: 4571–4575.
Davies, M.J., and R.J.W. Truscott. 2001. Photo-oxydation of proteins and its role in cataractogenesis. Journal of Photochemistry and Photobiology B: Biology 63: 114–125.
Dean, W.R. 1927. Note on the motion of fluid in a curved pipe. Philosophical Magazine Journal of Science 4: 208–223.
Dong, Q., D.C. Manns, G. Feng, T. Yue, J.J. Churet, and R.W. Worobo. 2010. Reduction of patulin in apple cider by UV radiation. Journal of food Protection 73: 69–74.
Ehlers, D. 1986. HPLC-Bestimmung von patulin in obstsäften-probenaufarbeitung mit einem modifizierten extraktions-und reinigungsverfahren. Lebensmittelchemie und Gerichtliche Chemie 40: 1–5.
El Ghaouth, A., C.L. Wilson, and A.M. Callahan. 2003. Induction of chitinase, β-1,3-glucanase and phenylalanine ammonia lyase in peach fruit by UV-C treatment. Phytopathology 93: 349–355.
EPA, Office of Water, 1995. National Primary Drinking Water Regulations Contaminant Fact Sheets Inorganic Chemicals—Technical Version. EPA 811-F-95-002-T, Washington, D.C.
Erkan, M., S.Y. Wang, and C.Y. Wang. 2008. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biology and Technology 48: 163–171.
Evgenidou, E., and K. Fytianos. 2002. Photodegradation of triazine herbicides in aqueous solutions and natural waters. Journal of Agricultural and Food Chemistry 50: 6423–6427.
Falguera, V., J. Pagán, and A. Ibarz. 2011. Effect of UV irradiation on enzymatic activities and physicochemical properties of apple juices from different varieties. LWT - Food Science and Technology 44: 115–119.
Fonseca, J.M., and J.W. Rushing. 2006. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biology and Technology 40: 256–261.
Forney, L., J.A. Pierson, and Z. Ye. 2004. Juice irradiation with Taylor-Coutte flow: UV inactivation of Escherichia coli. Journal of Food Protection 67: 2410–2415.
Gabriel, A.B., and H. Nakano. 2009. Inactivation of Salmonella, Escherichia coli and Listeria monocytogenes in phosphate-buffered saline and apple juice by ultraviolet and heat treatments. Food Control 20: 443–446.
Gardner, P.T., T.A.C. White, D.B. McPhail, and G.G. Duthie. 2000. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chemistry 68: 471–474.
Geveke, D. 2005. UV inactivation of bacteria in apple cider. Journal of Food Protection 68: 1739–1742.
Gilmore, T.M., and P.S. Dimick. 1979. Photochemical changes in major whey proteins of cow’s milk. Journal of Dairy Science 62: 189–194.
Gökmen, V., and J. Acar. 1998. Incidence of patulin in apple juice concentrates produced in Turkey. Journal of Chromatography A 815: 99–102.
Gómez, P.L., S.M. Alzamora, M.A. Castro, and D.M. Salvatori. 2010. Effect of ultraviolet-C light dose on quality of cut-apple: microorganism, color and compression behavior. Journal of Food Engineering 98: 60–70.
González-Aguilar, G.A., C.Y. Wang, J.G. Buta, and D.T. Krizek. 2001. Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe `Tommy Atkins' mangoes. International Journal of Food Science and Technology 36: 767–773.
Gonzalez-Aguilar, G., C.Y. Wang, and G.J. Buta. 2004. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. Journal of the Science of Food and Agriculture 84: 415–422.
González-Aguilar, G.A., R. Zavaleta-Gatica, and M.E. Tiznado-Hernández. 2007. Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biology and Technology 45: 108–116.
Gonzalez-Aguilar, G.A., J.A. Villa-Rodriguez, J.F. Ayala-Zavala, and E.M. Yahia. 2010. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends in Food Science & Technology 21: 475–482.
Guerrero-Beltran, J.A., and G.V. Barbosa-Canovas. 2005. Reduction of Saccharomyces cerevisiae, Escherichia coli and Listeria innocua in apple juice by ultraviolet light. Journal of Food Process Engineering 28: 437–452.
———. 2006. Inactivation of Saccharomyces cerevisiae and polyphenoloxidase in mango nectar treated with UV Light. Journal of Food Protection 69: 362–368.
Guerrero, R.F., B. Puertas, M.I. Fernández, M. Palma, and E. Cantos-Villar. 2010. Induction of stilbenes in grapes by UV-C: comparison of different subspecies of Vitis. Innovative Food Science and Emerging Technologies 11: 231–238.
Hagen, S.F., G.I.A. Borge, G.B. Bengtsson, W. Bilger, A. Berge, K. Haffner, and K.A. Solhaug. 2007. Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica Borkh., cv. Aroma): effect of postharvest UV-B irradiation. Postharvest Biology and Technology 45: 1–10.
Hanes, D.E., P.A. Orlandi, D.H. Burr, M.D. Miliotis, M.G. Robi, J.W. Bier, G.J. Jackson, M.J. Arrowood, J.J. Churey, and R.W. Worobo. 2002. Inactivation of Crytosporidium parvum oocysts in fresh apple cider using ultraviolet irradiation. Applied and Environmental Microbiology 68: 4168–4172.
Hansen, S.L., S. Purup, and L.P. Christensen. 2003. Bioactivity of falcarinol and its content in carrots. Journal of the Science of Food and Agriculture 83: 1010–1017.
Hijnen, W.A.M., E.F. Beerendonk, and G.J. Medema. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Research 40: 3–22.
Interdonato, R., M. Rosa, C.B. Nieva, J.A. González, M. Hilal, and F.E. Prado. 2011. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environmental and Experimental Botany 70: 204–211.
Jasinghe, V.J., and C.O. Perera. 2006. Ultraviolet irradiation: the generator of vitamin D2 in edible mushrooms. Food Chemistry 95: 638–643.
Kaewsuksaeng, S., Y. Urano, S. Aiamla-or, M. Shigyo, and N. Yamauchi. 2011. Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biology and Technology 61: 124–130.
Keyser, M., I. Műllera, F.P. Cilliersb, W. Nelb, and P.A. Gouwsa. 2008. UV radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juices. Innovative Food Science and Emerging Technologies 9: 348–354.
Konczak, I., and W. Zhang. 2004. Anthocyanins—More than nature’s colours. Journal of Biomedicine and Biotechnology 5: 239–240.
Koutchma, T., S. Keller, B. Parisi, and S. Chirtel. 2004. Ultraviolet disinfection of juice products in laminar and turbulent flow reactors. Innovative Food Science & Emerging Technologies 5: 179–189.
Koutchma, T., B. Parisi, and E. Patazca. 2007. Validation of UV coiled tube reactor for fresh fruit juices. Journal of Environmental Science and Engineering 6: 319–328.
Koutchma, T., L. Forney, and C. Moraru. 2008. Ultraviolet light in food technology: principles and applications. Boca Raton: CRS Press, Taylor and Francis Group.
Koutchma, T. 2009. Advances in UV light technology for non-thermal processing of liquid foods. Food and Bioprocess Technology 2: 138–155.
Kowalski, W.J., and C.E. Dunn. 2002. Current trends in UVGI air and surface disinfection. INvironment Professional 8: 4–6.
Kowalski, W.J. 2006. Aerobiological engineering handbook: A guide to airborne disease control technologies. New York: McGraw-Hill.
Labas, M.D., R.J. Brandi, C.A. Martin, and A.E. Cassano. 2006. A contribution to the UV dose concept for bacteria disinfection in well mixed photoreactors. Chemical Engineering Journal 116: 197–202.
Lamikanra, O., D. Kueneman, D. Ukuku, and K.L. Bett-Garber. 2005. Effect of processing under ultraviolet light on the shelf life of fresh-cut cantaloupe melon. Journal of Food Science 70: C534–C538.
Lammertyn, J., B. De Ketelaere, D. Marquenie, G. Molenberghs, and B.M. Nicolaï. 2004. Mixed models for multicategorical repeated response: modelling the time effect of physical treatments on strawberry sepal quality. Postharvest Biology and Technology 30: 195–207.
Leggott, N.L., H.F. Vismer, E.W. Sydenham, G.S. Shephard, P. Rheeder, and W.F.O. Marasas. 2000. Occurrence of patulin in the commercial processing of apple juice. South Africa Journal of Science 5: 241–243.
Lemoine, M.L., P.M. Civello, G.A. Martínez, and A.R. Chaves. 2007. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica). Journal of the Science of Food and Agriculture 87: 1132–1139.
Lers, A., S. Burd, E. Lomaniec, S. Droby, and E. Chalutz. 1998. The expression of a grapefruit gene encoding an isoflavone reductaselike protein is induced in response to UV irradiation. Plant Molecular Biology 36: 847–856.
Li, X., X. Zheng, S. Yan, and S. Li. 2008. Effects of salicylic acid (SA), ultraviolet radiation (UV-B and UV-C) on trans-resveratrol inducement in the skin of harvested grape berries. Frontiers of Agriculture in China 2: 77–81.
Li, J., Q. Zhang, Y. Cui, J. Yan, J. Cao, Y. Zhao, and W. Jiang. 2010. Use of UV-C treatment to inhibit the microbial growth and maintain the quality of Yali pear. Journal of Food Science 75: M503–M507.
Liu, C., X. Han, L. Cai, X. Lu, T. Ying, and Z. Jiang. 2011a. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant capacity in tomato fruit during storage. Postharvest Biology and Technology 59: 232–237.
Liu, C., L. Cai, X. Han, and T. Ying. 2011b. Temporary effect of postharvest UV-C irradiation on gene expression profile in tomato fruit. Gene 486: 56–64.
Lovett, J., B. Boutin, and R.G. Thompson. 1974. Patulin production in cherries by Penicillium and Aspergillus species. Journal of Milk Food Technology 37: 530.
Lu, J.Y., C. Stevens, V.A. Khan, M. Kabwe, and C.L. Wilson. 1991. The effect of ultraviolet irradiation on shelflife and ripening of peaches and apples. Journal of Food Quality 14: 299–305.
Maharaj, R. 1995. The effect of ultraviolet radiation (UV-C) on the postharvest storage behaviour of tomato (Lycopersicon esculentum Mill, cv. Capello). Ph.D. Dissertation. Québec, Canada: Université Laval.
Maharaj, R., J. Arul, and P. Nadeau. 1999. Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biology and Technology 15: 13–23.
Manzocco, L., B. Quarta, and A. Dri. 2009. Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innovative Food Science and Emerging Technologies 10: 506–511.
Marais, E., G. Jacobs, and D.M. Holcroft. 2001. Postharvest irradiation enhances anthocyanin synthesis in apples but not in pears. HortScience 36: 639–644.
Marquenie, D., C.W. Michiels, A.H. Geeraerd, A. Schenk, C. Soontjens, J.F. Van Impe, and B.M. Nicolaï. 2002. Using survival analysis to investigate the effect of UV-C and heat treatment on storage rot of strawberry and sweet cherry. International Journal of Food Microbiology 73: 187–196.
Martin-Belloso, O., R. Soliva-Fortuny, and G. Oms-Oliu. 2006. Fresh-cut fruits. In Handbook of fruits and fruit processing, ed. Y.H. Hui, 129–144. Ames: Blackwell Publishing.
Mau, J.-L., P.-R. Chen, and J.-H. Yang. 1998. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. Journal of Agricultural and Food Chemistry 46: 5269–5272.
Nigro, F., A. Ippolito, and G. Lima. 1998. Use of UV-C light to reduce botrytis storage rot of table grapes. Postharvest Biology and Technology 13: 171–181.
Noci, F., J. Riener, M. Walkling-Ribeiro, D.A. Cronin, D.J. Morgan, and J.G. Lyng. 2008. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. Journal of Food Engineering 85: 141–146.
Obande, M.A., and G. Shama. 2011. The use of biodosimetry to measure the UV-C dose delivered to a sphere, and implications for the commercial treatment of fruit. Journal of Food Engineering 104: 1–5.
Obande, M.A., G.A. Tucker, and G. Shama. 2011. Effect of preharvest UV-C treatment of tomatoes (Solanum lycopersicon Mill.) on ripening and pathogen resistance. Postharvest Biology and Technology 62: 188–192.
Orlowska, M., Koutchma, T., Grapperhaus, M., Gallagher, J., Schaefer, R., and Defelice, C. 2012. Continuous and pulsed ultraviolet light for non-thermal treatment of liquid foods. Part 1: Effects on quality of fructose solution, apple juice and milk. Food and Bioprocess Technology. Online first. DOI: 10.1007/s11947-012-0779-8.
Oteiza, J.M., L. Giannuzzi, and N. Zaritzky. 2010. Ultraviolet treatment of orange juice to inactivate E. coli O157:H7 as affected by native microflora. Food and Bioprocess Technology 3: 603–614.
Pombo, M.A., M.C. Dotto, G.A. Martínez, and P.M. Civello. 2009. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biology and Technology 51: 141–148.
Pombo, M.A., H.G. Rosli, G.A. Martínez, and P.M. Civello. 2011. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × Ananassa, Duch.). Postharvest Biology and Technology 59: 94–102.
Pongprasert, N., Y. Sekozawa, S. Sugaya, and H. Gemma. 2011. A novel postharvest UV-C treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Scientia Horticulturae 130: 73–77.
Reay, P.F., and J.E. Lancaster. 2001. Accumulation of anthocyanins and quercetin glycosides in ‘Gala’ and ‘Royal Gala’ apple fruit skin with UV-B—Visible irradiation: modifying effects of fruit maturity, fruit side, and temperature. Scientia Horticulturae 90: 57–68.
Rodov, V., S. BenYehoshua, J.J. Kim, B. Shapiro, and Y. Ittah. 1992. Ultraviolet illumination induces scoparone production in kumquat and orange fruits and improves decay resistance. Journal of the American Society for Horticultural Science 117: 788–791.
Roll, R., G. Matthiaschk, and A. Korte. 1990. Embryotoxicity and mutagenicity of mycotoxins. Journal of Environmental Pathology, Toxicology and Oncology 10: 1–7.
Schenk, M., S. Guerrero, and S. Maris Alzamora. 2007. Response of some microorganisms to ultraviolet treatment on fresh-cut pear. Food and Bioprocess Technology 1: 384–392.
Shama, G., and P. Alderson. 2005. UV hormesis in fruits: A concept ripe for commercialisation. Trends in Food Science and Technology 16: 128–136.
Shama, G. 2007. A new role for UV? extensions to the shelf life of plant foods by UV-induced Effects. Paper presented at the IOA-IUVA Joint World Congress, Los Angeles, California, USA, August 27–29.
Soda, K. 2011. Polyamines—The principal candidate substance of soybean-induced health. In Soybean and health, ed. H.A. El-Shemy, 489–502. Croatia: InTech.
Srilaong, V., S. Aiamla-or, A. Soontornwat, M. Shigyo, and N. Yamauchi. 2011. UV-B irradiation retards chlorophyll degradation in lime (Citrus latifolia Tan.) fruit. Postharvest Biology and Technology 59: 110–112.
Stevens, C., V.A. Khan, J.Y. Lu, C.L. Wilson, P.L. Pusey, M.K. Kabwe, E.C.K. Igwegbe, E. Chalutz, and S. Droby. 1998. The germicidal and hormetic effects of UV-C light on reducing brown rot disease and yeast microflora of peaches. Crop Protection 17: 75–84.
Stevens, C., V.A. Khan, C.L. Wilson, J.Y. Lu, E. Chalutz, and S. Droby. 2005. The effect of fruit orientation of postharvest commodities following low dose ultraviolet light-c treatment on host induced resistance to decay. Crop Protection 24: 756–759.
Tran, M.T., and M. Farid. 2004. Ultraviolet treatment of orange juice. Innovative Food Science and Emerging Technologies 5: 495–502.
Tsormpatsidis, E., M. Ordidge, R.G.C. Henbest, A. Wagstaffe, N.H. Battey, and P. Hadley. 2011. Harvesting fruit of equivalent chronological age and fruit position shows individual effects of UV radiation on aspects of the strawberry ripening process. Environmental and Experimental Botany 74: 178–185.
Ubi, B.E., C. Honda, H. Bessho, S. Kondo, M. Wada, S. Kobayashi, and T. Moriguchi. 2006. Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Science 170: 571–578.
Ukuku, D.O., V. Pilizota, and G.M. Sapers. 2001. Bioluminescence ATP assay for estimating total plate counts of surface microflora of whole cantaloupe and determining efficacy of washing treatment. Journal of Food Protection 64: 813–819.
U.S. Food and Drug Administration. 2000a. 21 CFR Part 179. Irradiation in the production, processing and handling of food. Federal Register 65:71056–71058.
———. 2000b. Kinetics of Microbial Inactivation for Alternative Food Processing Technologies: Pulsed Light Technology, http://www.fda.gov/Food/ScienceResearch/ResearchAreas/SafePracticesforFoodProcesses/ucm103058.htm (accessed March 1, 2011)
———. 2005. CPG Sec.510.150. Apple juice, apple juice concentrates, and apple juice products—adulteration with patulin, http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm074427.htm (accessed December 10, 2011).
Vicente, A.R., C. Pineda, L. Lemoine, P.M. Civello, G.A. Martinez, and A.R. Chaves. 2005. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biology and Technology 35: 69–78.
Wang, C.Y., C.-T. Chen, and S.Y. Wang. 2009. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chemistry 117: 426–431.
Warriner, K., S. Movahedi, and W.M. Waites. 2004. Laser-based packaging sterilization in aseptic processing. In Improving the thermal processing of foods, ed. P. Richardson, 277–303. Cambridge: Woodhead Publishing Limited.
Woodling, S.E., and C.I. Moraru. 2005. Influence of surface topography on the effectiveness of pulsed light treatment for the reduction of Listeria innocua on stainless steel surfaces. Journal of Food Science 70: 245–351.
Worobo, R.W., J.J. Churey, and O. Padilla-Zakour. 1998. Apple cider: Treatment options to comply with new regulations. Journal of the Association of Food and Drug Officials 62: 19–26.
Wright, H.B. 2000. Comparison and validation of UV dose calculations for low- and medium-pressure mercury arc lamps. Water Environment Research 72: 439–443.
Yang, D.S., R.R. Balandrán-Quintana, C.F. Ruiz, R.T. Toledo, and S.J. Kays. 2009. Effect of hyperbaric, controlled atmosphere, and UV treatments on peach volatiles. Postharvest Biology and Technology 51: 334–341.
Yaun, B.R., S.S. Sumner, J.D. Eifert, and J.E. Marcy. 2004. Inhibition of pathogens on fresh produce by ultraviolet energy. International Journal of Food Microbiology 90: 1–8.
Ye, Z., T. Koutchma, B. Parisi, J. Larkin, and L.J. Forney. 2007. Ultraviolet inactivation kinetics of E. coli and Y. pseudotuberculosis in annular reactors. Journal of Food Science 72: E271–E278.
Yurdun, T., G.Z. Omurtag, and Ö. Ersoy. 2001. Incidence of patulin in apple juices marketed in Turkey. Journal of Food Protection 11: 1851–1853.
Zhang, D., B. Yu, J. Bai, M. Qian, Q. Shu, J. Su, and Y. Teng. 2012. Effects of high temperatures on UV-B/visible irradiation induced postharvest anthocyanin accumulation in ‘Yunhongli No. 1’ (Pyrus pyrifolia Nakai) pears. Scientia Horticulturae 134: 53–59.
Zhu, Y., Wu, F., Koutchma, T., Warriner, K., and Zhou, T. 2012. Validation and Kinetics of Patulin Degradation in Model Solution, Apple Cider and Apple Juice by Ultraviolet Radiation. (manuscript was submitted.)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Koutchma, T., Orlowska, M., Zhu, Y. (2018). Fruits and Fruit Products Treated by UV Light. In: Rosenthal, A., Deliza, R., Welti-Chanes, J., Barbosa-Cánovas, G. (eds) Fruit Preservation. Food Engineering Series. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3311-2_17
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3311-2_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3309-9
Online ISBN: 978-1-4939-3311-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)