Abstract
Low level laser therapy (LLLT) that refers to 1–1000 mW in the range of red or infrared laser or light alters cellular behavior in the absence of heat. LLLT is being used to treat various diseases involving musculoskeletal and neurologic structures. It has become a popular treatment option and finding a variety of uses in medical practice. LLLT acts on mitochondria to increase ATP and modulation of reactive oxygen species. Near-infrared light penetrates more deeply than red. Pulsed lasers may penetrate more deeply into tissue. LLLT has been known to be effective to treat hearing loss induced by noise and ototoxicity, tinnitus, vestibular dysfunction, nasal allergy, oral mucositis, and cervical pain. Its clinical applications are expected to become more popular in the near future as the LLLT-related researches are carried out very actively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Low level laser (light) therapy
- LLLT
- Hearing loss
- Tinnitus
- Vestibular dysfunction
- Oral mucositis
- Cervical pain
Low Level Laser (Light) Therapy
Recently, biotechnology has provided various new medical knowledge, tools, and techniques to generate treatments for diseases that were previously incurable or difficult to treat. Low level laser (light) therapy (LLLT) is one of these kinds of new medical technique that made it possible to treat many medical conditions that were difficult to treat previously.
LLLT, also known as photobiomodulation , has a wavelength-dependent capability to alter cellular behavior in the absence of significant heat. LLLT involves exposing lesions to low levels of red and near infrared laser or light in the range of 1–1000 mW and referred to low level because its light energy density is low compared to other forms of high energy laser that are used to cut, ablate, or thermally coagulate tissues. Traditionally, low power laser has been referred for LLLT, but recently, the light-emitting diode (LED) also has been used for LLLT in place of laser. LED produces lights that are similar to those of lasers, but its wavelength has broader output peaks and lacks the coherence that is a particular feature of laser light. LED has the advantage of being less expensive [1], safer to use, and easier to manufacture than laser.
Phototherapy (light therapy) was practiced in ancient Egypt, Greece, China, and India. The Egyptians utilized sunlight as well as color for healing [2]. Color has been investigated as medicine since 2000 BC [3]. LLLT was noticed to stimulate hair growth in 1967, wound healing in mice in 1971, and the wound healing effect was soon applied to human patients [4–7]. LLLT is being used to treat musculoskeletal injury, pain, inflammatory arthritis, tendinitis, neuropathic pain, orofacial pain, sports injuries, Buerger’s disease, headache, nerve repair, sympathetic nervous system dysfunction, hemangiomas, immune modulation, bacterial infections, inflammation, and tinnitus [8]. LLLT has become a popular treatment modality and is finding a variety of uses in medical practice. In the past decade, LLLT has been approved by the United States Food and Drug Administration (FDA) in treating diseases such as carpal tunnel syndrome [9] and alopecia [10].
Mechanisms of LLLT
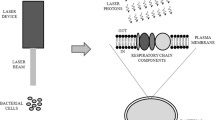
LLLT effects are due to photochemical effects unlike high powered laser. The photons of light must be absorbed by some molecular photoreceptors on chromophores for photochemistry to occur [11] as chlorophyll in plants responding to light and activating photosynthesis. Within the cells, there is strong evidence to suggest that LLLT acts on the mitochondria to increase adenosine triphosphate (ATP) production, modulation of reactive oxygen species (ROS), and induction of transcription factors. Several transcription factors are regulated by changes in cellular redox state. Among them, redox factor-1 (Ref-1) dependent activator protein-1 (AP-1) (a heterodimer of c-Fos and c-Jun), nuclear factor kappa B (NF-κ(kappa)B), p53 activating transcription factor/cAMP-response element-binding protein (ATF/CREB), hypoxia-inducible factor (HIF)-1, and HIF-like factor are those regulated [12]. These transcription factors then cause protein synthesis that triggers further effects downstream, such as increased cell proliferation and migration, modulation in the levels of cytokines, growth factors, and inflammatory mediators, and increased tissue oxygenation [13]. Figure 1 shows the proposed cellular and molecular mechanisms of LLLT [12].
Cellular mechanisms of LLLT . Schematic diagram showing the absorption of red or near infrared (NIR) light by specific cellular chromophores or photoacceptors localized in the mitochondrial. During this process in mitochondria respiration chain ATP production will increase and reactive oxygen species (ROS) are generated; nitric oxide is released or generated. These cytosolic responses may in turn induce transcriptional changes via activation of transcription factors (e.g., NF-κ(kappa) B and AP1) (from Ann Biomed Eng. 2012 40: 516–33, with permission from Springer)
Biphasic Dose Response
It is well established that if the light applied is not of sufficient irradiance or the irradiation time is too short then there is no response. If the irradiance is too high or irradiation time is too long then the response may be inhibited [14–16]. Somewhere in between is the optimal combination of irradiance and time for stimulation [12]. At present there has been no convincing report of biphasic dose responses occurring in patients, but several systematic reviews and meta analyses of randomized controlled trials in LLLT have found that some ineffective trials may be explained by over-dosing, in that the guidelines set by World Association for Laser Therapy were exceeded. Moreover, it is unknown to what extent the parameters are needed for the onset of the biphasic dose response, and it will vary in a highly heterogeneous patient population as compared with a highly uniform population of experimental animals [16].
Pulsing in LLLT
Pulsed light offers numerous potential benefits. Because there are “quench periods” (pulse OFF times) following the pulse ON times, pulsed lasers can generate less tissue heating. In instances where it is desirable to deliver light to deeper tissues, increased powers are needed to provide adequate energy at the target tissue. This increased power can cause tissue heating at the surface layers and in this instance, pulsed light could be very useful. Whereas continuous wave (CW) causes an increase in temperature of the intervening and target tissues or organ, pulsed light has been shown to cause no measurable change in the temperature of the irradiated area for the same delivered energy density.
Aside from safety advantages, pulsed light might simply be more effective than CW. The “quench period” (pulse OFF times) reduces tissue heating, thereby allowing the use of potentially much higher peak power densities than those that could be safely used in CW. For example, when CW power densities at the skin of ≥2 W/cm2 are used, doubling the CW power density would only marginally increase the treatment depth while potentially significantly increasing the risk of thermal damage; in contrast, peak powers of ≥5 W/cm2 pulsed using appropriate ON and OFF times might produce little or no tissue heating. The higher peak powers that can be safely used by pulsing light can overcome tissue heating problems and improve the ability of the laser to penetrate into deeper tissues achieving greater treatment depths. The majority of the pulsed light sources used for LLLT have frequencies in the 2.5–10,000 Hz range and pulse durations are commonly in the range of a few millisecond [1].
Penetration Depth
The most important parameter that governs the depth of penetration of laser light into tissue is wavelength. Both the absorption and scattering coefficients of living tissues are higher at lower wavelength, so near-infrared light penetrates more deeply than red. It is often claimed that pulsed lasers penetrate more deeply into tissue than CW lasers with the same average power. There is no consensus on the effects of different frequencies and pulse parameters on the physiologic and therapeutic response of the various disease states that are often treated with laser therapy. This has allowed manufacturers to claim advantages of pulsing without hard evidence to back up their claims. CW light is the gold standard and has been used for most of LLLT applications. However, review of the literature indicates that overall pulsed light may be superior to CW light with everything else being equal. This seemed to be particularly true for wound healing and post-stroke management. On the other hand, pulsed laser as a solo treatment may be less beneficial than CW in patients requiring nerve regeneration. Reviews of literatures indicate that pulsing will continue to play an important role in LLLT especially for applications where deep tissue penetration is required [1].
Transcanal LLLT on Noise-Induced Hearing Loss, Ototoxic Hearing Loss, Tinnitus, and Vestibular Dysfunction
Transcanal Penetration Rate and Side Effects of Transcanal LLLT
The penetration rate of low level laser (LLL) into perilymphatic space of cochlea through an external ear canal, tympanic membrane, and cochlear wall has been measured using 830 nm laser diode, and side effects of LLL on ear canal, tympanic membrane, and cochlea have been studied. Laser diode of 80 mW was irradiated through an external ear canal of guinea pigs once daily for 2 weeks. Histopathologic study was done, and hearing tests were measured using auditory brain response (ABR) . Transcanal penetration rates in guinea pigs were 6.4 mW (8 %) at the middle ear behind the tympanic membrane and 4 mW (5 %) at the perilymphatic space of cochlea. Histopathology studies of ear canal skin and tympanic membrane showed all normal findings. ABR hearing tests revealed all normal hearing after transcanal LLLT for 2 weeks. Separate penetration study using human cadaver temporal bones showed that transcanal penetration rate of LLL was 4 mW (5 %) at the middle ear and 1.6 mW (2 %) at the perilymphatic space. No laser penetration was measurable through mastoid bone (Table 1).
This study indicates that transcanal LLLT with 80 mW of 830 nm LD does not induce any side effects to ear canal skin, drum, and cochlea [8].
LLLT on Noise-Induced Hearing Loss
One of the most common factors that cause hearing disorders is noise trauma. Noise is an increasing hazard and it is pervasive, which makes it difficult to take precautions and prevent noise-induced hearing loss (NIHL) . Many researches have been carried out to find ways to restore hearing, but no definite treatment has been established yet. The effects of lasers on hearing recovery have been investigated, and several studies using animals reported that LLLT using 830 nm infrared laser diode may improve NIHL, ototoxic hearing loss, tinnitus, and vestibular dysfunction [17, 18].
NIHL was induced by exposing rats to 116 dB noise centered at 16 kHz for 6 h. The rats were treated with transcanal LLLT using 830 nm diode laser with 165 mW for 60 min/day (594 J/day) for 12 days. Hearing has been evaluated by auditory brainstem response, and hair cells of cochlea were examined by SEM. The results are shown in Figs. 2 and 3 [17]. This study indicated that LLLT may have a positive effect on cochlear hair-cell recovery after acute acoustic trauma. The hearing threshold became lower (improved) after repeated laser irradiation, and the final hearing result was significantly better than that of the untreated ears. Considering that there is no definitive treatment for acute acoustic trauma in humans, LLLT evolves as a new treatment modality for noise-induced acute hearing loss once a human study is completed with positive result [17].
Hearing threshold changes after repeated transcanal LLLT, 24 h after noise exposure (after the first LLLT), the ABR thresholds were increased markedly to 50–80 dB SPL for both N (noise only) and NL (noise and laser) ears. The hearing threshold between N and NL ears were almost the same at this time point. Signs of change were observed after 5 days of irradiation. After the 8th to 10th irradiation, a significant difference was found at all frequencies. After the 12th irradiation, the hearing threshold was significantly improved on the NL ears when compared to the N ears at all five frequencies ( p < 0.05) (from J of Biomed Opt 17, 068002, 2012, with permission from SPIE)
Number of hair cells observed by scanning electron microscopy . The number of hair cells of the NL (noise and laser) ears was larger than that of the N (noise only) ears and this difference was statistically significant in the middle turn ( p < 0.05).The number of hair cells of the C (control) ears was significantly larger than that of the N ears in the apical, middle, and the basal turn. The number of hair cells of the C ears was also significantly larger than that of the NL ears in the basal turn. But the number of hair cells of the C ear in the apical and middle turn was similar to that of the NL ears (from J of Biomed Opt 17, 068002, 2012, with permission from SPIE)
Before LLLT is applied to human ears, we need to consider two variations: the first one is the different penetration rate of humans; it is thought to be lower than that of rodents [8] (Rhee 2006 spie), therefore, more power needs to be delivered to the cochlea in humans, but without causing complications. The next consideration is local heating; this is presumed to be the only adverse effect [18]. Previous study showed that there is no damage with laser of power 200 mW, but there may be significant damages in human ears with irradiation of higher power than 200 mW [19].
LLLT on Tinnitus
Tinnitus is one of the most frequently encountered and the most enigmatic ear symptoms in otolaryngology clinic. Tinnitus has been known to develop from noise, aging, and many drugs such as salicylates, aminoglycoside, antibiotics, quinine, and cisplatin [20]. Even though many basic and clinical researches have been conducted to elucidate the mechanisms and to find ways to cure for decades, there is still no silver bullet for this bothersome ear problem. Since early 1990s, LLLT has been used to treat patients with tinnitus by several investigators. Significant reduction of tinnitus intensity was reported by different authors in a range of 15–80 % of patients [21–24].
A clinical study on tinnitus by transcanal LLLT applying 830 nm LD, 80.4 J/cm2, three times per week for 4 weeks, demonstrated significant reductions of loudness and the degree of annoyance, while the duration of tinnitus was not significantly decreased in laser group. This study used VAS and tinnitus handicap inventory (THI) questionnaire for evaluation that is well accepted method to study tinnitus [8]. The results of clinical studies on tinnitus utilizing transcanal LLLT were mixed. Four previous studies reported positive result [23–26] and five studies showed negative result [21, 22, 27–29]. An optimal dosage of the LLLT for tinnitus or other various inner diseases needs to be established by further studies involving patients with tinnitus and other inner ear diseases.
Recently, phototherapy with transcanal LLLT on animal models has shown possible role of lasers in inner ear pathology [17, 30]. But, how laser irradiation is acting on inner ear hair cells and auditory nerves after various insults is yet to be elucidated, urging further mechanism study with animal model.
A study to quantify the effect of LLLT on the treatment of tinnitus in animal model has been carried out studying the effect of LLLT on salicylate-induced tinnitus in the rat model by means of Gap Prepulse Inhibition of Acoustic Startle (GPIAS) . Tinnitus was elicited with salicylate intravenous injection daily. GPIAS was used to monitor tinnitus perception. Rats received transcanal LLLT, showed significantly higher GPIAS values throughout the experiment, indicating transcanal LLLT reduced tinnitus perception. The results of this study suggest that transcanal LLLT may provide a feasible therapeutic approach to control tinnitus. This is the first animal experiment to evaluate laser irradiation effects on tinnitus perception [31].
Effect of LLLT on Hearing and Cochlea Hair Cell Recovery After Ototoxic Hearing Loss
Gentamicin/furosemide-induced hearing loss animal models were established in rats using the modified method previously described [32]. The animals with gentamicin/furosemide-induced hearing loss were treated with transcanal LLLT using 830 nm diode laser at the fluence of 72 J (200 mW × 60 min) once a day for 10 days. Only 4.32 J (6 % of 72 J) is expected to reach into the cochlea. Hearing was measured with ABR, and quantitative scanning electron microscopic (SEM) observations were done by counting remaining hair cells. On SEM images, LLLT significantly increased the number of hair cells in middle and basal turn of the cochlea. Hearing was significantly improved by laser irradiation (from 57 to 44 dB). This study showed that LLLT improved gentamicin/furosemide-induced hearing loss and recovery of damaged cochlear hair cells. As for safety issues, transcanal LLLT in rats using the 830 nm laser irradiating 200 mW for 60 min has not induced any side effects [30].
Effects of Transcanal LLLT on Vestibular System After Gentamicin Ototoxicity
Vestibular disorders display high prevalence and can severely impact the daily life. Vertigo and dizziness rank among the most common reasons for consultation and referral to specialist care [33, 34]. However, pharmacological options that would efficiently relieve the vertigo symptoms without side effects are still lacking.
A bilateral vestibulopathy animal model using adult rats was developed by gentamicin (GM) intravenous injection once daily for 3 days. Bilateral vestibulopathy was confirmed by sinusoidal oscillation tests. Transcanal LLLT was irradiated to left ear canal for 7 days, starting 1 day post-GM injections for 3 days. The gain of LLLT left ear was decreased in 3 days post-LLLT but the decreased gain was improved significantly comparing to that of control right ear , and the improved gain of LLLT left ear was much closer to that of the pre-GM injection level. The average number of hair cells in the cupula of the laser treated left ear was significantly higher comparing to that of the control right ear and it was comparable to the cupula hair cells of the pre-GM injection level. This study demonstrated that LLLT restores vestibular dysfunction and damaged vestibular hair cells in rats post-gentamicin ototoxicity. Transcanal LLLT may have clinical implications in the treatment of various vestibular dysfunctions [35].
A study inducing unilateral vestibulopathy by GM injection into the middle ears of guinea pigs reported therapeutic effect of transcanal LLLT [8]. Unilateral left vestibulopathy was induced by injecting GM into left middle ears of both control and treated groups. Unilateral left vestibular dysfunction was confirmed by animal rotator in both groups. Transcanal LLLT was performed into the left ear canals of the treated-group, while the left ear of the control group was not treated. Unilateral vestibular dysfunction of the LLLT treated left ear was improved significantly, while the unilateral vestibular dysfunction in the left ear of the control group was not improved.
These two studies indicate that transcanal LLLT may be able to treat various vestibular dysfunctions of human patients.
Transnasal LLLT on Nasal Allergy
The number of patients with allergic rhinitis is still increasing, especially in the well-developed, industrialized countries. Although it is not associated with severe morbidity and mortality, allergic rhinitis has a major effect on the quality of life. Its increasing prevalence, its impact on the individual quality of life and social costs [36] and its role as a risk factor for asthma [37], underline the need for improved treatment options for this disorder.
Treatments for allergic rhinitis comprise allergen avoidance, pharmacotherapy, and immunotherapy. Although allergen avoidance may be the preferred treatment, total allergen avoidance may be an unrealistic approach, as it may require limited time spent outdoors. Thus, pharmacotherapy is preferable to allergen avoidance for symptom relief. Nonselective antihistamines can cause sedation and potentially cause other adverse effects such as dry mouth, dry eyes, urinary retention, constipation, and tachycardia. Nonselective antihistamines are associated with impaired sleep, learning, and work performance and with motor vehicle, boating, and aviation accidents [38]. Corticosteroids are recommended as first-line treatment for moderate/severe or persistent allergic rhinitis [39]. Adverse local effects may include increased intraocular pressure and nasal stinging, burning, bleeding, and dryness. Decongestants can be used only on healthy young patients for a limited period of time.
Since conventional therapy with antiallergic medications carries significant notable side effects and limitations, it would be worth to try LLLT to treat allergic rhinitis.
LLLT reduced delayed hypersensitivity reaction to ovalbumin in Balb/C mice in an animal study, and in a study with footpad histopathology, levels of TNF-α (alpha), INF-γ (gamma), and IL-10 analyses between control and hypersensitized animals. This study indicates that treatment with LLLT has an immunomodulatory effect on delayed hypersensitivity reaction to OVA [40].
The effects of intranasal LLLT on allergic rhinitis are not well established. The effects of intranasal LLLT on nasal allergy have been studied using rat allergy models [40] and patients with allergic rhinitis [42, 43].
The effect of LLLT in an experimental rat model of delayed hypersensitivity reaction in nasal cavity has been studied. Rats were sensitized with ovalbumin (OVA) and alum and challenged intranasally with OVA . The nasal rubbing symptom score was counted, spleen was emulsified, and cytokines IL-4, IL-5, IL-6, IL-10, IL-17, and IFN-γ in the splenocytes were assayed. Using 830 nm LD laser, 10 mW intranasal LLLT for 15 min daily for 10 days reduced allergic symptom and suppressed systemic cytokine production by splenocytes, while they were not decreased in no laser treated positive control group. This study demonstrated that intranasal LLLT with 10 mW LD laser into nasal cavity induced antiallergic effects by decreasing allergy symptoms and systemic cytokines production in an allergic rhinitis rat model. Intranasal LLLT with 50 mW was not effective to reduce allergy symptoms and cytokines. Biphasic dose response was applied here. Nasal LLLT may be considered as a potential therapeutic modality in treating allergic rhinitis [41].
In an open study, groups of patients with severe allergic rhinitis received intranasal LLLT with a 308 nm XeCl UVB excimer laser for 2 weeks. In the low-dose group, treatment was given twice weekly, starting with 0.25× the individual minimal erythema dose (MED) , whereas patients in the medium-dose group were treated four times weekly, starting with 0.4× MED. In each group, the dosage was gradually increased. Evaluation was based on the symptom scores. The effect of the XeCl laser on the skin prick test reaction was also studied. In the low-dose group, there was no improvement in the nasal symptoms. In the medium-dose group, the XeCl UVB irradiation significantly inhibited the rhinorrhoea, the sneezing, the nasal obstruction, and the total nasal score (p < 0.05). The XeCl UVB excimer laser also inhibited the allergen-induced skin prick test in a dose-dependent manner. These results suggest that the XeCl UVB excimer laser might serve as a new therapeutic tool in the treatment of allergic rhinitis [42].
As these animal and human studies demonstrated, transnasal LLLT appears to be effective to improve status of allergic rhinitis without notable side effects, while most of the antiallergic medications may carry significant side effects.
LLLT on Oral Mucositis
Oral mucositis (OM) refers to erythematous and ulcerative lesions of the oral mucosa observed in patients with cancer being treated with chemotherapy and/or with radiation therapy to fields involving the oral cavity. OM can be very painful and can significantly affect nutritional intake, mouth care, increase risk for local and systemic infection, and quality of life [44, 45]. At the same time, OM is a major dose-limiting toxicity of chemotherapy and radiation therapy to the head and neck region. It was reported that 303 of 599 patients (51 %) receiving chemotherapy for solid tumors or lymphoma developed oral and/or GI mucositis [45]. OM developed in 22 % of 1236 cycles of chemotherapy and even higher percentage (approximately 75–80 %) of patients who receive high-dose chemotherapy prior to hematopoietic cell transplantation developed clinically significant OM [47]. Almost all patients treated with radiation therapy for head and neck cancer will develop some degree of OM. In the recent studies, severe OM occurred in 29–66 % of all patients receiving radiation therapy for head and neck cancer [48, 49].
Management of OM has been largely palliative to date. Management of OM is divided into the following sections: nutritional support, pain control, oral decontamination, palliation of dry mouth, management of oral bleeding, and therapeutic interventions for OM. Several agents have been tested to reduce the severity of, or prevent, mucositis. Conventional treatments of OM include cryotherapy, administration of growth factors, anti-inflammatory agents, and antioxidants [50]. LLLT has been tried by many clinicians and investigators for preventive and therapeutic purposes . Multiple studies have indicated that LLLT can reduce the severity of chemotherapy and radiation-induced OM [51–53]. Studies are difficult to compare due to varying laser types and parameters (such as wavelength and fluence). Nevertheless, success rate of 81 % when LLLT was given as a preventive treatment, and 83 % of therapeutic success rate have been reported [53].
Previous study revealed that no heterogeneity between trials with optimal doses for the red (630–670 nm) and the infrared (780–830 nm) subgroups. The optimal dose ranges for red and infrared wavelengths on OM are usually 1–8 J. The laser applications are usually performed daily, perpendicular to the lesions intraorally. LLLT needs to be performed at least every other day for the duration of chemoradiotherapy regimens or as long as OM ulcers are present. The trials which aimed at the prevention of OM started LLLT at 7 days before chemoradiotherapy regimens [50]. The newly available blue LED has potential for the management of OM, and research is warranted based on the known effects of this light therapy in wound healing [54].
Although the literatures suggest that lasers with wavelengths varying between 632 and 830 nm can have beneficial effects on preventing and treating OM, no specific protocols that investigated other parameters such as tissue fluency (energy density), ideal time of laser application, variations in cancer type, and cancer treatment regimens are available [55]. Extraoral application of LED , 4 J (total 12 J/treatment) to the extraoral bilateral cheeks and anterior throat tissues, was shown to have a significant reduction in pain but not for other mucositis scoring scales [56].
LLLT improved quality of life by reducing oral hygiene, difficulty of drinking, swallowing, speaking, and secondary infection [52, 57]. LLLT appears to be effective in improving OM, in controlling the intensity of mucositis, in relieving the OM related pain, and in improving the quality of life . All the studies investigated possible side effects, but none found side effects or adverse effects. LLLT was well tolerated among all patients with OM [51].
LLLT in the Management of Neck Pain
Chronic neck pain is a highly prevalent and costly condition affecting 10–24 % of the population [58–60] and for which pharmacological management has limited evidence of efficacy. LLLT is noninvasive treatment for neck pain, in which nonthermal laser irradiation is applied to sites of pain. The presenting neck pain can have several concurrent sources of pain from joints, muscles, and ligaments.
Transcutaneous application results in laser-energy scattering and spreading into a three-dimensional volume of tissue up to 5 cm for infrared laser [61]. Previous studies suggest that trigger points in the neck coincide with the location of acupuncture points in 70–80 % of patients [62, 63]. Since trigger points and acupuncture points are characterized by tenderness, the treatment effect of laser irradiation to tender points, trigger points, or acupuncture points is likely to be the same. Thus, when treating neck pain with LLLT, irradiation of known trigger points, acupuncture points, tender points, and symptomatic zygapophyseal joints is advisable [64]. A meta-analysis reported that at 820–830 nm, doses are most effective in the range of 0.8–9.0 J per point, with irradiation times of 15–180 s. At 904 nm, doses are slightly smaller (0.8–4.2 J per point) with slightly longer irradiation times (100–600 s) than at 820–830 nm [64]. The optimum mean dose per point for 820–830 nm was 5.9 J, with an irradiation time of 39.8 s, and for 904 nm, 2.2 J delivered with an irradiation time of 238 s. The same meta-analysis reported moderate statistical evidence for efficacy of LLLT in the treatment of acute and chronic neck pain in the short and medium term [64].
This positive relieving effect was maintained for 3 months after the treatment ended, while the effect of NSAIDs ends rapidly when drug is discontinued [65]. Another study of LLLT on acute neck pain with radiculopathy reported significant improvement for intensity of arm pain and neck extension. LLLT was applied to the skin projection at the anatomical site of the spinal segment involved with the following parameters : 905 nm at 5000 Hz, power density of 12 mW/cm2, and dose of 2 J/cm2, for 120 s, at whole dose of 12 J/cm2 [66]. Side effects of tiredness, nausea, and stiffness have been reported post-LLLT [67]. LLLT does not generate any heat and safety issue relating mainly to potential eye damage, and safety glasses are required for the use of LLLT.
Mechanisms for LLLT-mediated pain relief are not fully understood. Anti-inflammatory effects of red and infrared laser irradiation have been shown by reduction in specific inflammatory markers (prostaglandin E, interleukin 1β (beta), TNF α in vivo and in vitro studies in animal and man [68]. Second possible mechanism is to reduce oxidative stress and skeletal muscle fatigue that has been reported in animal and human studies [69, 70]. Another mechanism for LLLT effects on myofascial pain and trigger points could be an inhibition of transmission at the neuromuscular junction [71]. Such effects could mediate the clinical finding that LLLT decreases tenderness in trigger points within 15 min of LLLT application [72]. LLLT studies on cervical pain compare favorable with other widely used therapies and especially with pharmacological interventions for which evidence is spare and side effects are common.
References
Hashmi JT, Huang YY, Osmani BZ, Shama SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2 Suppl:S292–305.
Hansen HJ, Thoroe U. Low power laser biostimulation of chronic oro-facial pain: a double-blind placebo controlled cross-over study in 40 patients. Pain. 1990;43:169–79.
Basford JR. Low intensity laser therapy: still not an established clinical tool. Lasers Surg Med. 1995;16:331–42.
Mester E, Szende B, Tota JG. Effect of laser on hair growth of mice. Kiserl Orvostud. 1967;19:628–31.
Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–5.
Mester E, Szende B, Spiry T, Scher A. Stimulation of wound healing by laser rays. Acta Chir Acad Sci Hung. 1972;13:315–24.
Mester E, Nagylucskay S, Doklen A, Tisza S. Laser stimulation of wound healing. Acta Chir Acad Sci Hung. 1976;17:49–55.
Rhee CK, Lim ES, Kim YS, Chung YW, Jung JY, Chung P. Effect of low-level laser (LLL) on cochlear and vestibular inner ear including tinnitus. Proc SPIE. 2006;6078:60781K-1-12.
Melkerson MN. K081166. Rockville: U.S. Food and Drug Administration; 2009.
Melkerson MN. K091496. Silver Spring: U.S. Food and Drug Administration; 2010.
Sutherland JC. Biological effects of polychromatic light. Photochem Photobiol. 2002;76:164–70.
Chung H, Dai T, Sharma SK, Huang YY, Carrol JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–33.
Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–61.
Castano AP, Dai T, Yaroslavsky I, Cohen R, Apruzzese WA, Smotrich MH, Hamblin MR. Low-level laser therapy for zymosan-induced arthritis in rats: importance of illumination time. Lasers Surg Med. 2007;39:543–50.
Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter Sr TA, Brondon P, Olson D. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg Med. 2007;39:534–42.
Huang YY, Sharma SK, Carroll J, Hamblin M. Biphasic dose response in low level light therapy—an update. Dose Response. 2011;9(4):602–18.
Rhee CK, Bahk CW, Kim SH, Ahn JC, Jung JY, Chung PS, Suh MW. Effect of low-level laser treatment on cochlea hair-cell recovery after acute acoustic trauma. J Biomed Opt. 2012;17:068002–6.
Rhee CK, He P, Jung JY, Ahn JC, Chung PS, Suh MW. Effect of low-level laser therapy on cochlear hair cell recovery after gentamicin-induced ototoxicity. Laser Med Sci. 2012;27:987–92.
Suh MW, Jung JY, Ahn JC, Jung PS, Moon TH. External, middle and inner ear safety of trans-canal low level laser therapy. J Intern Adv Otol. 2013;9(3):105.
Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–54.
Mirz F, Zachariae R, Andersen SE, Nielsen AG, Johansen LV, Bjerring P, Pedersen CB. The low-power laser in the treatment of tinnitus. Clin Otolaryngol Allied Sci. 1999;24:346–54.
Partheniadis-Stumpf M, Maurer J, Mann W. Soft laser therapy in combination with tebonin i.v. in tinnitus. Laryngorhinootologie. 1993;72:28–31.
Plath P, Olivier J. Results of combined low-power laser therapy and extracts of Ginkgo biloba in cases of sensorineural hearing loss and tinnitus. Adv Otorhi-nolaryngol. 1995;49:101–4.
Shiomi Y, Takahashi H, Honjo I, Kojima H, Naito Y, Fujiki N. Efficacy of trans-meatal low power laser irradiation on tinnitus: a preliminary report. Auris Nasus Larynx. 1997;24:39–42.
Hahn A, Sejna I, Stolbova K, Cocek A. Combined laser-Egb 761 tinnitus therapy. Acta Otolaryngol Suppl. 2001;545:92–3.
Tauber S, Beyer W, Schorn K, Baumgartner R. Transmeatal cochlear laser treatment of cochlear dysfunction : a feasibility study for chronic tinnitus. Lasers Med Sci. 2003;18:154–61.
von Wedel H, Calero L, Walger M, Hoenen S, Rutwalt D. Soft-laser/Ginkgo therapy in chronic tinnitus. A placebo-controlled study. Adv Otorhinolaryngol. 1995;49:105–8.
Rogowski M, Munich S, Gindzienska E, Lazarczyk B. Low-power laser in the treatment of tinnitus—a placebo-controlled study. Otolaryngol Pol. 1999;53:315–20.
Nakashima T, Ueda H, Misawa H, Suzuki T, Tominaga M, Ito A, Numata S, Kasai S, Asahi K, Vernon JA, Meikle MB. Transmeatal low-power laser irradiation for tinnitus. Otol Neurotol. 2002;23:296–300.
Rhee CK, He P, Jung JY, Ahn JC, Chung PS, Lee MY, Suh MW. Effect of low-level laser treatment on cochlea hair cell recovery after ototoxic hearing loss. J Biomed Opt. 2013;18(12):128003.
Park YM, Na WS, Park IY, Suh MW, Rhee CK, Chung PS, Jung JY. Trans-canal laser irradiation reduces tinnitus perception of salicylate treated rat. Neurosci Lett. 2013;7(544):131–5.
Liu HY, Chi FL, Gao WY. Taurine attenuates aminoglycoside ototoxicity by inhibiting inducible nitric oxide synthase expression in the cochlea. Neuroreport. 2008;19:117–20.
Nakashima K, Yokoyama Y, Shimoyama R, Saito H, Kuno N, Sano K. Prevalence of neurological disorders in a Japanese town. Neuroepidemiology. 1996;15:208–13.
Moulin T, Sablot D, Vidry E, Belahsen F, Berger E, Lemounaud P. Impact of emergency room neurologists on patient management and outcome. Eur Neurol. 2003;50:207–14.
Rhee CK, Hyun JH, Suh MH, Ahn JC, Jung JY. Effect of low level laser therapy (LLLT) on vestibular system after gentamicin ototoxicity. Proc SPIE. 2013;8565:85651S.
Law AW, Reed SD, Sundy JS, Schulman KA. Direct costs of allergic rhinitis in the United States: estimates from the 1996 Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2003;111:296–300.
Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–83.
Church MK, Maurer M, Simons FE, et al. Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy. 2010;65(4):459–66.
Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA, Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1–84.
Oliveira RG, Ferreira AP, Côrtes AJ, Aarestrup BJ, Andrade LC, Aarestrup FM. Low-level laser reduces the production of TNF-α, IFN-γ, and IL-10 induced by OVA. Lasers Med Sci. 2013;28(6):1519–25.
Rhee CK, Kim JH, Ahn JC, Mo JH. The effects of low level laser therapy on nasal allergy in SD rat allergy model on nasal allergy. Lasers Surg Med. 2012;44 Suppl 24:52.
Csoma Z, Ignacz F, Bor Z, Szabo G, Bodai L, Dobozy A, Kemeny L. Intranasal irradiation with the xenon chloride ultraviolet B laser improves allergic rhinitis. J Photochem Photobiol B. 2004;75:137–44.
Krespi YP, Kizhner V. Phototherapy for chronic rhinosinusitis. Lasers Surg Med. 2011;43:187–91.
Lalla RV, Peterson DE. Oral mucositis. Dent Clin North Am. 2005;49(1):167–84.
Duncan GG, Epstein JB, Tu D, El Sayed S, Bezjak A, Ottaway J, Pater J, National Cancer Institute of Canada Clinical Trials Group. Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: a report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head Neck. 2005;27(5):421–8.
Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–9.
Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer. 2007;15(5):491–6.
Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–36.
Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–20.
Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(1):61–77.
Bjordal JM, Bensadoun RJ, Tuner J, Frigo L, Gjerde K, Lopes-Martins RAB. A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer. 2011;19(8):1069–77.
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy—a randomized controlled trial. Support Care Cancer. 2012;21(5):1421–8.
Genot-Klastersky MT, Klastersky J, Awada F, Awada A, Crombez P, Martinez MD, Jaivenois MF, Delmelle M, Vogt G, Meuleman N, Paesmans M. The use of low-energy laser (LEL) for the prevention of chemotherapy- and/or radiotherapy-induced oral mucositis in cancer patients: results from two prospective studies. Support Care Cancer. 2008;16(12):1381–7.
Adamskaya N, Dungel P, Mittermayr R. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42:917–21.
Migliorati C, Hewson I, Lalla RV, Antunes HS, Estilo CL, Hodgson B, Lopes NN, Schubert MM, Bowen J, Elad S. Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(1):333–41.
Hodgson BD, Margolis DM, Salzman DE, Eastwood D, Tarima S, Williams LD, Sande JE, Vaughan WP, Whelan HT. Amelioration of oral mucositis pain by NASA near-infrared light-emitting diodes in bone marrow transplant patients. Support Care Cancer. 2012;20(7):1405–15.
Oton-Leite AF, Corrêa de Castro AC, Morais MO, Pinezi JC, Leles CR, Mendonça EF. Effect of intraoral low-level laser therapy on quality of life of patients with head and neck cancer undergoing radiotherapy. Head Neck. 2012;34(3):398–404.
Borghouts J, Koes B, Vondeling H, Bouter L. Cost-of-illness of neck pain in the Netherlands in 1996. Pain. 1999;80:629–36.
Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D. Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general population. Spine. 2003;28:1195–202.
Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834–48.
Oshiro T. The laser apple: a new graphic representation of medical laser applications. Laser Ther. 1996;8:185–90.
Melzack R, Stillwell DM, Fox EJ. Trigger points and acupuncture points for pain: correlations and implications. Pain. 1977;3:3–23.
Dorsher PT. Can classical acupuncture points and trigger points be compared in the treatment of pain disorders? Birch’s analysis revisited. J Altern Complement Med. 2008;14:353–9.
Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–908.
Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124:201–10.
Konstantinovic LM, Cutovic MR, Milovanovic AN, Jovic SJ, Dragin AS, Letic MDj, Miler VM. Low-level laser therapy for acute neck pain with radiculopathy: a double-blind placebo-controlled randomized study. Pain Med. 2010;11(8):1169–78.
Gur A, Sarac AJ, Cevik R, Altindag O, Sarac S. Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial. Lasers Surg Med. 2004;35(3):229–35.
Soriano F, Rios R. Gallium arsenide laser treatment of chronic low back pain: a prospective randomized and double blind study. Laser Ther. 1998;10:175–80.
Lopes-Martins RA, Marcos RL, Leonardo PS. Effect of low-level laser (Ga-Al-As 655nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol. 2006;101:283–8.
Leal Junior EC, Lopes-Martins RA, Vanin AA. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24:425–31.
Nicolau RA, Martinez MS, Rigau J, Tomas J. Effect of low power 655nm diode laser irradiation on the neuromuscular junctions of the mouse diaphragm. Lasers Surg Med. 2004;34:277–84.
Olavi A, Pekka R, Pertti K, Pekka P. Effects of the infrared laser therapy at treated and non-treated trigger points. Acupunct Electrother Res. 1989;14:9–14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rhee, CK. (2016). Low Level Laser (Light) Therapy (LLLT) in Otolaryngology. In: Wong, BF., Ilgner, J. (eds) Biomedical Optics in Otorhinolaryngology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1758-7_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1758-7_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1757-0
Online ISBN: 978-1-4939-1758-7
eBook Packages: MedicineMedicine (R0)