Abstract
Soil salinization is one of the most important factors which limit plant productivity. About 3.6 billion of the world’s 5.2 billion hectares of dryland used for agriculture have already suffered erosion, soil degradation, and salinization. Global climate change caused by rising atmospheric trace gases such as CO2 and forced migration add to the urgency of this global problem. Therefore, solutions are desperately needed, such as the improvement of drought and salinity resistance of crops or the use of (xero-) halophytes instead of glycophytic crops. As photosynthesis is a prerequisite for biomass production, this chapter focuses on information related to this essential sequence of reactions, thereby discussing the different levels of photosynthesis. At first, there are primary reactions of photosynthesis, namely absorption of light energy and (1) its conversion to redox energy, conserved in the coenzyme NADPH, and (2) energy of chemical bounds, conserved in the coenzyme ATP. On the second level, we find reactions of the Calvin cycle, nitrate and sulfate reduction as well as sugar, lipid, and amino acid metabolism. Typical reactions on the third level are transmembrane and inter tissues transport of metabolites. The fourth level of photosynthesis relates to physiological aspects of gas exchange and water relations.
Apart from these general effects of salinity on photosynthesis, we will review the probable photosynthetic performance of salt stressed plants under future atmospheric conditions, namely under elevated CO2 concentration. Special emphasis will be put on gas exchange and photosynthesis of C3 and C4 plants because these two photosynthesis types show different responses to elevated CO2, leading to different interactions with salinity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Feeding the rapidly growing human population is one of the biggest challenges we face on our planet today. Especially in arid and semiarid regions, the increasing population density is collaterally catalysed by the consequences of global climate change which is caused by anthropogenic emissions of trace gases such as CO2 (IPCC 2007). Thus salinity – already affecting 7% of the global total land area (Glenn et al. 1998; Millennium Ecosystem Assessment 2005) and 20–50% of the global irrigated farmland (Tanji 2002; Hu and Schmidhalter 2005; Koyro et al. 2008) – will pose a more and more serious threat to agriculture and human nutrition in future. Therefore, solutions are desperately needed, such as the improvement of salt resistance of crops or the use of (xero-) halophytes instead of glycophytic crops, which in turn requires a detailed knowledge about salt and drought resistance mechanisms in plants.
The viability of plants in saline habitats depends on their ability to cope with (1) water deficit due to a low water potential of the soil, (2) restriction of CO2 uptake, (3) ion toxicity, and (4) nutient imbalance. The first two constraints are directly related to photosynthesis which is a prerequisite for biomass and crop production, respectively. So this chapter focuses on information related to these constraints, thereby discussing the different levels of photosynthesis as described in the next chapter.
In the face of global climate change, one has also to consider the influence of elevated atmospheric CO2 concentration on photosynthesis, which we will do in the last part of this chapter. In C3 plants, CO2 often improves assimilation while reducing stomatal resistance, thus increasing water use efficiency, but decreasing photorespiration and oxidative stress (Urban 2003; Kirschbaum 2004; Rogers et al. 2004). As these effects are much less pronounced in C4 plants, it will be differentiated between these two photosynthesis types.
Effects of Salt Stress on Photosynthesis Under Ambient Atmospheric CO2 Concentration
Levels of Photosynthesis to Be Considered
Regarding crop production and food and feed supply, limitation of plant growth by environmental factors is a matter of general concern. As photosynthesis is dominating plant growth and production of biomass, the sequence of reactions leading to the phenomenon named photosynthesis is in the focus of interest when breeding for high crop yield. In order to allow a detailed analysis of salt effects, several individual steps have to be distinguished (Fig. 15.1).
-
At first, there are primary reactions of photosynthesis, namely absorption of light energy and (1) its conversion to redox energy, conserved in the coenzyme NADPH, and (2) energy of chemical bounds, conserved in the coenzyme ATP.
-
On the second level, we find reactions of the Calvin cycle, nitrate and sulfate reduction as well as sugar, lipid, and amino acid metabolism.
-
Typical reactions on the third level are transmembrane and inter tissues transport of metabolites.
-
The fourth level of photosynthesis relates to physiological aspects of gas exchange and water relations.
Most papers deal on salt effects on physiological aspects of biomass production rather than partial reactions of photosynthesis as defined above. As there are cross reactions as well as cell signaling involved in regulation of photosynthetic metabolic pathways, there are no strict correlations between biomass production and individual gene activities. As a consequence, predicted correlations, though they had been observed in laboratory experiments, could not be shown in subsequent field experiments. In this chapter we try a more differentiated approach and review analysis of salt stress effects at different levels of photosynthesis.
Regulation of Gas Exchange and Water Relations
According to Munns, plants show a two-phase growth response to salinity (Munns 1993, 2002; Munns et al. 2002). During the first phase of growth reduction water or osmotic stress prevails, and plant responses are presumably regulated by hormonal signals coming from the roots.
Terrestrial plants growing in saline habitats are often surrounded by low water potentials in the soil solution and atmosphere. As a part of resistance mechanisms, water flow through the soil–plant–atmosphere continuum must be ensured, so that a gradient of decreasing water potentials (Ψ) must be established. The Ψ of pure water is defined as 0 MPa; increasing salinity or concentrations of other solutes will decrease Ψ. Thus, any sharp rise in salinity could effectively hold water osmotically away from plants that lack any physiological or morphological modifications (Larcher 2003). To limit restrictions on water uptake, plants must generate increasingly lower Ψ to allow continued water flux into belowground structures (Touchette et al. 2009).
If plants cannot escape salinity (such as geophytes or pluviotherophytes), it is also important to prevent water loss by transpiration from being higher than the influx rate. This is necessary to avoid a negative tissue water balance and is only possible if the water potential remains lower in the plant than in the soil. However, experiments demonstrated that the leaf water potential of halophytes does not correlate alone as a single factor with salinity resistance. Plant species with different levels of salt resistance such as Aster tripolium, Avicennia marina, Innula critmoides, and Sesuvium verrucosum have a sufficient adjustment mechanism even at high salinities. Clearly, resistance in the form of osmotic adjustment plays an important role in halophytes residing in saline environments (Flowers and Colmer 2008). However, in addition were the osmotic potentials of all four halophytes (and many others) up to sea water salinity level sufficiently low to explain the full turgescence of the leaves (results not shown). Except for differences in concentration and type of osmotica used in plant tissues (in halophytes inorganic ions are more prevalent than organic substances), physiological responses in plants to salt stress were remarkably similar to those employed during drought (Touchette et al. 2009). For plants with limited water availability, physiological adjustments often involve avoidance and tolerance, with most plants using some combination of the two (Yue et al. 2006; Romanello et al. 2008).
Assuming there is no interruption of the water supply, water can flow passively from the root to the shoot and there seems to be no reason for growth reduction by water deficit for any of the studied species. However, by regulating the extent of apoplastic barriers and their chemical composition (long-distance response coordination), plants can effectively regulate the uptake or loss of water and solutes (by structures such as barriers in the hypo- or exodermis). This appears to be an additional or compensatory strategy of plants to acquire water and solutes (Hose et al. 2001). At the extremes of growth under saline or dry conditions even cell layers such as the exodermis can become an absolute barrier for water and ions in the strict sense (Azaizeh and Steudle 1991; North and Nobel 1991; Nublat et al. 2001).
Thus, the rate of water supply to the shoot can be restricted due to the coupling between the flows of water and solutes (Na and Cl) even if the leaf water potential is low. Therefore, the balance between water flow (sum of water accumulation and transpiration) and the decrease in the amounts of nutrients or unfavorable nutrient ratios (e.g. Na+/K+) are important factors for impaired leaf elongation and plant growth (Lynch et al. 1988; Munns et al. 1989; Neves-Piestun and Bernstein 2001; Cramer 2003).
In any case, plant water loss has to be minimized at low soil water potentials. However, biomass production depends mainly on the ability to keep a high net photosynthesis and a low transpiration simultaneously. In this field of tension, biomass production has always to be seen in connection with CO2/H2O-gas exchange, which can be estimated by the water use efficiency (WUE) of photosynthesis. It needs to be considered that anyway a critical point for the plant is reached when CO2 fixation (apparent photosynthesis) falls below CO2 production (compensation point).
Several halophytic plants such as Aster tripolium, Beta vulgaris ssp. maritima, Chenopodium quinoa, or Spartina townsendii reveal a combination of low (but positive) net photosynthesis, minimum transpiration, high stomatal resistance, and minimum internal CO2 concentration at their threshold salinity resistance (Koyro 2000; Koyro and Huchzermeyer 2004). However, there is a big bandwidth among halophytes, especially for succulent species such as Sesuvium portulacastrum or Avicennia marina, which have alternatives if the water balance (water uptake minus water loss) is still positive and not the limiting factor for photosynthesis. In case of S. portulacastrum, net photosynthesis and WUE increase but stomatal resistance decreases. These results show that it is quite important to describe the regulation of gas exchange at high salinity in strong reliance with other parameters (such as water relations).
Water deficit is one major constraint at high salinity and can lead to a restriction of CO2 uptake and to the development of reactive oxygen species (ROS). The balance between water loss and CO2 uptake helps to find weak spots in the mechanism of adjustment (of photosynthesis) to high salinity (Badawi et al. 2004).
Primary Reactions of Photosynthesis
Photosynthetic Conversion of Energy
In plants active in photosynthesis, energy of light quanta is absorbed by chlorophyll. Just like all other pigments, activated chlorophyll can return from its activated state to the stable, non activated state by emitting heat. Other than most pigments, activated chlorophyll is sufficiently stable to allow transfer of energy to acceptor molecules of biological relevance after having absorbed energy of a red light quantum (Strasser et al. 2004; Fig. 15.2). In principle, there are two options of biological relevance in addition to a third one we can use to monitor plant performance:
-
1.
Resonance energy transfer to activate other pigments and
-
2.
Transfer of an electron to an acceptor. The electron acceptor can be a component of the photosynthetic electron transport chain or any other molecule having a less negative potential as compared to activated chlorophyll. This second option requires a “refill” of electrons, and it is well documented that in chloroplasts the water splitting system fulfills this function, so that chlorophyll is recycled to its ground state (Strasser et al. 2004).
-
3.
Another option, competing for energy with the already mentioned ones, is the emission of fluorescence energy (Schreiber 1997; Schreiber et al. 2002; Strasser et al. 2004). As will be discussed later, any inhibition of an individual pathway will enhance the possibility of the other pathways to occur.
In thylakoid membranes, photosystems I and II (PSI and PSII) can be distinguished from other chlorophyll containing protein complexes, namely the light-harvesting complexes. Only the special pairs of chlorophyll a located in the active center of the two photosystems are involved in electron transport. Energy transfer among other pigments occurs via resonance energy transfer. In this section, we focus on electron transfer reactions.
As known from the literature, half lifetime of activated state of chlorophyll is very short and depends on the environment of the pigment (Rees et al. 1990; Laible et al. 1994; Ma et al. 2009). It is obvious that photosynthetic efficiency depends on the probability that activated chlorophyll will transfer electrons to an acceptor of the photosynthetic electron transport chain rather than “wasting” energy by using one of the other pathways mentioned above (Ruban et al. 1993; Horton et al. 1996; Horton 2000; Allen and Forsberg 2001). In order to meet this requirement, reaction partners are arranged in ideal neighbourhood within protein complexes located in the thylakoid membranes. Moreover, it has been demonstrated that this structure undergoes permanent adjustment to match the requirement of chloroplast metabolism and to adapt to changes in the environment, i.e. changes of light quality and intensity, for instance (Allen and Bennett 1981; Allen 1992; Allen and Forsberg 2001).
By means of photosynthetic electron transport, energy of absorbed light quanta is converted to redox energy stored in the coenzyme NADPH and proton motive force (Mitchell 1967) stored in a proton gradient across the thylakoid membranes. This proton gradient is the driving force for ATP synthesis catalyzed by the chloroplast F-type ATPase, called the CFOCF1-complex or the chloroplast coupling factor (Strotmann et al. 1976; Huchzermeyer and Strotmann 1977; Boyer 2000). The number of coenzyme molecules (NADP+/NADPH and ADP/ATP) is limited, and there is no exchange of coenzymes among cell compartments. Therefore, this machinery will work efficiently only if acceptor forms of coenzymes (NADP+ and ADP, respectively) are permanently recycled by subsequent metabolic pathways. Otherwise, energy turnover by the electron transport would be inhibited, and this inhibition finally would lead to an inhibition of electron release from activated chlorophyll, enhancing the probability of alternative routes mentioned above (Fig. 15.2).
Photosynthetic CO2 assimilation is the major consumer recycling both coenzymes in the reaction sequence of the Calvin cycle. Under physiological conditions, as a rule of thumb, in chloroplasts of non-woody plants two-thirds of the electrons from the noncyclic electron transport pathway finally will be consumed by CO2 fixation, while one-third will be used for nitrate reduction (see top part of Fig. 15.3) (Schmidt and Jäger 1992). But it has to be mentioned here that a significant portion of the absorbed light energy will be “wasted” in futile reaction sequences rather than be used for biomass synthesis.
Linking metabolic pathways of cell compartments. Biochemical pathways of chloroplasts, peroxisomes, and mitochondria are linked via shuttle systems. With respect to energy flow, photorespiration and nitrate reduction are of focal interest. Like the Calvin cycle, nitrate reduction is consuming electrons released from photosystem I. Thus, nitrate reduction is recycling the cofactors ferredoxin and NADP+. Photorespiration, on the other hand, is transferring redox power to the mitochondria and is involved in shuttling of ammonia
Salt Effects on Photosynthetic Energy Conversion
In order to understand individual photosynthetic reactions and get the principles of their interaction, thylakoid membranes and protein complexes have been isolated and analyzed with respect to their structure and function. During preparation and subsequent tests of enzyme activities, salt concentrations up to 50 mM NaCl have been applied without any inhibitory effect on individual enzyme activities (Strotmann et al. 1976). Such high salt concentrations are not found inside chloroplasts, neither under physiological conditions nor under salt stress. It therefore can be concluded that primary reactions of photosynthesis are not directly inhibited by ionic or ion specific stress (Richter et al. 2000; Huchzermeyer et al. 2004; Huchzermeyer and Koyro 2005; Koyro and Huchzermeyer 2005). However, this conclusion disagrees with an apparent inhibition of photosynthesis observed in whole plant experiments (Lawlor and Fock 1978; Lawlor 2002a). Therefore, it has to be analyzed in more detail to what extent the observed inhibition may be attributed to salt-dependent changes in thylakoid structure (Hesse et al. 1976) or to physiological effects (e.g. ion transport). One first approach to answer this question could be a detailed analysis of the kinetics of fluorescence light emission subsequent to chlorophyll activation by light pulses. Application of the pulsed amplitude modulation technique (PAM) allowed a detailed analysis of the network of primary reactions in photosynthesis (Strasser et al. 2004). It became quite obvious that salt stress does not directly inhibit primary reactions of photosynthesis, but inhibits product export and fine-tuning of primary reactions by interfering with optimal arrangement of proteins and membranes (Koyro and Huchzermeyer 1999; Huchzermeyer and Heins 2000; Huchzermeyer 2000).
Accordingly, it was observed that maximal photochemical efficiency, indicated by high Fv/Fm values of chlorophyll fluorescence, remain high under tolerable salt stress, while the growth rate of turf grass, for instance, was reduced under the same salinity level (Lee et al. 2004). This can be seen as an evidence of a reduced real photochemical efficiency.
It will be discussed in subsequent paragraphs how salt can inhibit export of products of photosynthesis and why proper function of the phosphate translocator, located in the inner envelope membrane, is essential for photophosphorylation. At this stage, we can state that inhibition of product export feeds back to primary reactions and finally will inhibit photosynthetic electron transport. One option to release energy from its activated state is blocked and chlorophyll will increase activity of fluorescence light emission, heat production, energy transfer to other pigments, and ROS production. This latter option will be discussed later in this review (see Sect. Dissipation of Surplus Energy and Production of Potential Toxic Intermediates of Photosynthesis).
Salt Effects on Chloroplast Structure and Metabolite Transfer
Chloroplasts exhibit a very high content of proteins which tend to interact and form aggregates inside the chloroplast stroma (Süss et al. 1993). Süss et al. were able to isolate such “super complexes” and found out that they contain enzymes belonging to individual metabolic pathways. Such an arrangement helps to increase substrate turnover because diffusion distances among enzymes of a pathway are close to zero. For the same reason, formation of such aggregates prevents occurrence of side reactions because intermediates are not available for other enzymes. As will be discussed in Sect. Control of Mechanisms Improving Stress Resistance: Compatible Solutes, on the one hand salt can inhibit the turnover of substrates by destroying enzyme aggregates, while on the other hand intermediates of sugar metabolism can become available for other enzymes and alternative products such as compatible solutes can be formed. Though salt effects on metabolite patterns have been analyzed in several papers, no data linking these findings to the occurrence of protein aggregates have been available up to now.
The occurrence of thylakoid grana stacks has attracted a lot of interest for years (Huchzermeyer et al. 1986; Lam and Malkin 1989; Malkin and Braun 1993; Romanowska and Albertson 1994). It was calculated that about 70% of all PSII complexes are located within stacked regions of the thylakoid membranes, while most of the PSI complexes are found in un-stacked parts of the thylakoid membranes (Schmidt and Malkin 1993; Romanowska and Albertsson 1994). From this, it was concluded that 70% of the PSII complexes must be in an inactive state because the distance between PSII and PSI would be too far to allow sufficient turnover rates of photosynthetic electron transport (Malkin and Braun 1993). Moreover, it was found that grana are unstable, permanently folding and unfolding structures. Apparently CFOCF1-complexes are initiating the formation of membrane loops (Boekema et al. 1988), and grana are formed by the interaction of membrane proteins (Staehelin 1975). As active PSII has an extremely short half lifetime in the range of 20–30 min (Kuhn and Böger 1990; Trebst and Soll-Bracht 1996; Keren et al. 1997; Jansen et al. 2001), the function of this permanent modification of membrane structure may be to initiate interactions among membrane proteins and to support the formation of aggregates of protein complexes interacting in the photosynthetic electron transport chain. Such preferred neighbourhood of membrane proteins has been found, indeed (Laszlo et al. 1984; Huchzermeyer and Willms 1985). There are several publications indicating that such neighborhoods of protein complexes control the efficiency of energy conversion in primary reactions of photosynthesis (Laszlo et al. 1984; Löhr and Huchzermeyer 1985; Löhr et al. 1985; Allnutt et al. 1989) and photo-inhibition (Lu and Zhang 1999; Lu et al. 2002).
The facts presented above show that the formation of grana stacks is essential for optimal functioning and permanent repair of the photosynthetic electron transport rate. It was found that grana become destabilized if the ratio of monovalent and divalent cations is impaired (Hesse et al. 1976). Similarly, investigations on Aster tripolium showed that NaCl salinity leads to a decreased amount of grana stacks (Geissler et al. 2009b; Fig. 15.4). This was accompanied by an increased chlorophyll a/chlorophyll b ratio and may be an explanation for a reduced photosynthetic efficiency (Moorthy and Kathiresan 1999) because chlorophyll b is mainly located in the light harvesting complex which supplies PSII with energy and is located mainly in the grana stacks. So the disintegration of the latter is probably correlated with the increased chlorophyll a/chlorophyll b ratio and may be an adaptation to prevent an electron surplus in the photosynthetic electron transport chain.
Influence of salinity and elevated CO2 concentration on chloroplast structure in Aster tripolium. (a) In control plants under ambient CO2, both stroma thylakoids and grana stacks are well developed. (b), (c) In salt treatments (375 mM NaCl) under ambient CO2, dilations of the thylakoid membranes are clearly visible, and well-developed grana stacks are almost lacking. (d) In salt treatments (375 mM NaCl) under elevated CO2, the thylakoid membranes are less damaged. st stroma thylakoid, gs grana stacks, d dilations, s starch grain, pg plastoglobulus
Last but not least it should be mentioned that in various plant species salt stress often leads to dilations of the thylakoid membranes, so that the spaces between the membranes look swollen, and undulated thylakoid areas develop (Kurkova et al. 2002; Rahman et al. 2002; Mitsuya et al. 2003; Fidalgo et al. 2004; Paramanova et al. 2004; Geissler et al. 2009b; Zhen et al. 2011; Fig. 15.4). This structural damage has been discussed by many authors as a consequence of oxidative stress (Mitsuya et al. 2003; Fidalgo et al. 2004; Oksanen et al. 2005) and is likely to contribute to impaired photosynthetic rates under saline conditions.
Dissipation of Surplus Energy and Production of Potential Toxic Intermediates of Photosynthesis
Photorespiration
Stomatal closure and a subsequent inhibition of gas exchange is a secondary effect of salt stress, mostly brought about by ABA released from the plant roots. In the presence of light, the O2/CO2 ratio will increase inside the leaves and impair CO2 fixation, especially in C3 plants. This happens as the enzyme Rubisco can bind O2 instead of CO2 to its reaction center, thus catalyzing the synthesis of a C3 plus a C2 compound instead of two C3 compounds in the primary reaction of the Calvin cycle (Fig. 15.3). The C2 compound 2-phosphoglycolate will be converted to glycolate, a molecule that cannot be metabolized by chloroplasts and the concentration of which eventually would become toxic. Detoxification and recycling of the C3 compound 3-PGA are understood to be the major function of photorespiration which takes place by metabolite transfer between chloroplasts, peroxisomes, and mitochondria.
The photorespiratory reaction cycle we know from our textbooks is a simplification allowing general estimates. We know that amino acids (glycine, serine, glutamic acid, and glutamine) may be subtracted from or fed into the cycle in vivo. Such reactions modify nitrogen flow among cell compartments. But, with respect to equilibrium of carbon flow, another aspect may be more important: It has been shown by Niessen et al. that mitochondrial glycolate oxidation contributes to photorespiration in higher plants as well (Niessen et al. 2007). It has to be kept in mind that photorespiration is a major source of H2O2 in illuminated C3 leaves. On the other hand, H2O2 production and interaction with pyridine nucleotide coenzymes make photorespiration an important player in cellular redox homeostasis. Furthermore, H2O2 is an important second messenger controlling cell development and tuning effects of hormones like ABA, for instance. Any interference with this reaction will have effects not only on immediate energy status and phosphorylation efficiency, but also on plant hormonal responsiveness, thus on development and plant life cycle (Foyer et al. 2009).
There is evidence that glycolate can inhibit QA/QB electron transfer in PSII (Petrouleas et al. 1994). As stated above, this would lead to stimulated ROS production. This interpretation is in line with the observed bleaching of maize in presence of glycolate (s.a.). In several publications, it is suggested that the function of photorespiration is to serve as a sink to dissipate excess redox energy (Kozaki and Takebe 1996; Wingler et al. 2000). In terms of our arguments, this would mean photorespiration is recycling coenzymes functioning as acceptors of the photosynthetic electron transport chain. This would help preventing ROS production as well. But, to our understanding, this interpretation does not sufficiently take into account the compartmentation of coenzymes.
Non-photochemical Quenching of Energy
Under conditions that limit CO2 assimilation, the potential rate of NADPH production exceeds the actual rate of consumption of reductive power. In order to be able to grow under stressful conditions, plants have to be equipped with mechanisms preventing excess reducing power. But these futile mechanisms compete with photochemistry for absorbed energy. They lead to a decrease in quantum yield of photosystem II (Genty et al. 1989; Cha-um et al. 2012).
The photosystem II antenna is highly flexible in tuning delivery of excitation energy to the photosystem II reaction center (Horton et al. 1996). The principal adaptation mechanism in photosynthesis is the control of thermal dissipation of excess energy within the photosystem II antenna, thus matching physiological needs (Johnson et al. 2009). In C3 plants, losses by this mechanism, named non-photochemical energy quenching, may exceed the ones caused by photorespiration. Despite extensive investigations, the reaction mechanism of photochemical quenching is not yet completely understood because the turnover of intermediates is fast and the reaction depends on intact structures of protein complexes and their in vivo arrangement inside the thylakoid membranes; i.e. reaction partners may not be extracted and individually analyzed. But some insight was achieved by a combination of molecular biological and biophysical techniques (Johnson et al. 2009).
For experimental approaches investigating salt stress effects, it is important to know that non-photochemical quenching of excitation energy is comprised of a fast and a slow component, qE and qI, respectively. Both reactions are reversible. The trigger of qE is the ΔpH across the thylakoid membrane sensed by the PsbS subunit of the light-harvesting complex (Li et al. 2000, 2004). Full expression of qE is associated with the enzymatic de-epoxidation of violaxanthin to zeaxanthin. This reaction is part of the xanthophylls cycle (Havaux et al. 2007). Enzymes involved are pH controlled and function on the expense of NADPH (Demmig-Adams and Adams 1996). This makes the cycle a futile reversible reaction sequence on its own. The majority of photoactive xanthophylls is bound to the light-harvesting complex. In addition to the ones involved in the xanthophyll cycle, lutein and lutein epoxide are bound there as well and can be turned over in a cycle on their own (Matsubara et al. 2001). Depending on distances among pigments, these two cycles can interact with soluble xanthophylls and control non-photochemical energy quenching in a synergistic, but not well-understood way (Johnson et al. 2009).
Sensitivity of non-photochemical quenching to any experimental approach interfering with membrane structure and protein fine structure indicates that this reaction sequence of outstanding physiological importance will be highly sensitive to any reaction causing imbalance of ionic homeostasis. The threshold ion concentration resulting in significant inhibition of non-photochemical quenching will depend on availability of compatible solutes, for instance. Based on current understanding, it can be expected that such adverse effects can be monitored by measuring pigment shifts due to altered ratios of xanthophylls and by high-resolution chlorophyll fluorescence measurement. Thus, it should be possible to measure salt effects on non-photochemical quenching even in the field using noninvasive techniques.
Especially near the end of a leaf’s life cycle, masking of chlorophyll by anthocyanins becomes important. It prevents photooxidative damage and allows an efficient nutrient retrieval from leaves to storage organs (Field et al. 2001). Some plants use such mechanisms regularly under saline conditions. They can be identified because leaf color will vary depending on their growth conditions like it can be observed with Salicornia and Sempervivum, for instance.
ROS Production
As described above, any reduction of the electron transport rate, especially under high light conditions, will increase the risk of ROS production (Figs. 15.2 and 15.5). The redox potential of activated chlorophyll is more negative than the one of oxygen. Therefore electron transfer from activated chlorophyll can occur unless energy is abstracted from activated chlorophyll faster than electron transfer to oxygen can occur. There are further options of ROS production as several intermediates of the photosynthetic electron transport are radicals. On the other hand, ROS can spontaneously convert or can be turned over under the control of enzymes (Fig. 15.5). Accordingly, in the literature the occurrence of various forms of ROS is described (Halliwell and Gutteridge 1986; Blokhina et al. 2002). Cytotoxicity may be attributed to oxidative damage of membrane lipids (Fridovich 1986; Wise and Naylor 1987) as well as oxidation of proteins and nucleic acids (Fridovich 1986; Imlay and Linn 1988).
ROS production and detoxification. Detoxification of ROS by sequential ascorbate and glutathione cycles will consume NADPH and, thus, result in a relief of NADP+ shortage in high light. A prerequisite is that (1) enzymes involved are available at ample concentrations and (2) are positioned in ideal neighbourhood to allow high turnover rates. SOD superoxide dismutase, Fd ferredoxin, CAT catalase, APX ascorbate peroxidase, MDAR monodehydroascorbate reductase, DHAR dehydroascorbate reductase, GR glutathione reductase, MDHA monodehydroascorbate, DHA dehydroascorbate, GSSG oxidated glutathione, GSH reduced glutathione
In the field, salt stress results in severe damage especially in situations when its inhibitory effects occur in the presence of high light intensity. Then PSII activity will result in high oxygen concentrations, especially if stomata are closed under stress. Concomitantly, chlorophyll will remain in its active state for a prolonged period of time, as the electron transport rate is reduced by inhibited off flow of products. Thus the probability for a transfer of electrons from activated chlorophyll to molecular oxygen to form O2 •− will increase. O2 •− ill rapidly dismutate to yield O2 and the less reactive ROS H2O2. But in the presence of some cations such as Cu and Fe, highly reactive OH•− may be formed (Imlay and Linn 1988; Fig. 15.5).
H2O2 is one of the most important secondary messengers in plant tissues, modulating effects of hormones and involved in developmental control of cells and tissues (van Breusegem and Dat 2006; van Breusegem et al. 2008). It has been shown that mitogen-activated protein kinases (MAP kinases) are involved in transduction of H2O2 signaling on cellular level (Pitschke and Hirt 2009). Apparently, MAPK3 and MAPK6 are integrating stress signals that regulate stomatal development (Wang et al. 2008a, b).
Ascorbic acid is a major antioxidant in plants. It detoxifies reactive oxygen species and maintains photosynthetic function (Fig. 15.5). Through its ascorbate recycling function, dehydroascorbate reductase affects the level of foliar reactive oxygen species and photosynthetic activity during leaf development. As a consequence, this enzyme influences the rate of plant growth and leaf aging (Chen and Gallie 2006).
ABA-induced closure of stomata and ABA-mediated inhibition of stomata opening are two ABA effects based on different reaction sequences (Mishra et al. 2006). Nevertheless, both processes are fine-tuned by ROS signaling, and it appears to be clear that ROS signals are transduced via a cascade of MAP kinase reactions (Gudesblat et al. 2007).
ROS Detoxification
As stated above, ROS production occurs permanently at a certain probability. It is well documented that ROS as well as nitrogen radicals are involved in cell signaling (Wilken and Huchzermeyer 1999) in plant cells like in cells of most other organisms. But as high ROS concentrations are toxic, plants are equipped at varying degrees with several systems to detoxify ROS. In this respect, molecules having antioxidative potential can be discriminated from enzyme-catalyzed reaction sequences.
Alpha-tocopherol is synthesized in the chloroplast (Schultz et al. 1976) and can be found in high concentrations in thylakoid membranes. Alpha-tocopherol disrupts lipid peroxidation cascades, reacts with O2 •−, and is capable of scavenging hydroxyl-, peroxyl-, and alkoxyl-radicals (Halliwell 1987). Oxidation of alpha-tocopherol leads to the formation of an alpha-chromoxyl radical, which can be reduced by ascorbic acid.
Ascorbic acid and several redox-active tri-peptides, called glutathiones, can be found in chloroplasts in millimolar concentrations (Halliwell 1982). Several different types are known from plants (Schmidt and Jäger 1992). This finding suggests that they are involved in different pathways controlled by specific enzymes. But there is only little information available to date.
In chloroplasts, H2O2 can be detoxified by an ascorbate-specific peroxidase (Chen and Asada 1989) involved in the ascorbate–glutathione cycle (Halliwell and Guteridge 1986) (Fig. 15.5) while in the cytosol H2O2 detoxification is catalyzed in a catalase-dependent reaction. Other enzymes involved in detoxification of ROS are superoxide dismutase, which converts O2 •− to H2O2, and several peroxidases (Chang et al. 1984).
Antioxidants as well as enzymes capable of detoxifying ROS are present in all plants and plant tissues. But their concentrations and catalytic activities, respectively, as well as their patterns differ a lot (Streenivasulu et al. 2000). Therefore plants differ in their capacities to immediately detoxify ROS upon their occurrence and to build up a detoxification potential under stress. If the balance between ROS production and quenching capacity of the respective tissues is upset, oxidative damage will be produced (Dhindsa and Matowe 1981; Wise and Naylor 1987; Spychalla and Desborough 1990). In experimental approaches, it was demonstrated that enzyme activities of antioxidative pathways increase as a salt stress response (Verma and Mishra 2005) and that the maximal level of salt resistance correlates with maximal respective enzyme activities (Kennedy and De Fillippis 1999; Benavides et al. 2000; Lee et al. 2001; Mittova et al. 2002, 2003; Stepien and Klobus 2005; Chang et al. 2012; Mittal et al. 2012).
Physiological Importance of ROS Production as an Electron Sink
Although O2 uptake which could not be accounted for Rubisco oxygenation or mitochondrial respiration has been observed in the light (Gerbaud and André 1980; Osmond et al. 1997), evidence of significant extra electron transport was not always found (Genty et al. 1989; Cornic and Briantais 1991). It is thought that O2 photoreduction increases with increasing reduction of the ferredoxin pool, thus allowing linear electron flow to continue when NADP is scarce. It has been suggested that O2 photoreduction can assist in maintaining a high trans-thylakoid pH gradient, which in turn enhances nonradiative dissipation of light energy and protects light reactions from photodamage (Lu et al. 2003).
In this context it has to be considered that a slight alkalization of the chloroplast stroma in the light is a prerequisite for the functioning of most plastidic metabolic pathways. The phosphorylation potential of ATP, like the group transfer potential of many other metabolites, depends on pH (and cation concentrations, especially Mg), and reaction equilibria within pathways would not allow metabolism to take place in all other cell compartments because of their lower pH values compared to illuminated chloroplasts. Additionally, the stoichiometries of NADPH and ATP production and consumption in chloroplasts are different and the alternative sinks are considered necessary to enable ATP production without NADP reduction (Noctor and Foyer 1998).
Dark Reaction of Photosynthesis
Rubisco Limitation
Rubisco is the most abundant protein in the leaves, contributing to up to 50% of the total protein. It has been discussed that a part of the total Rubisco protein is not catalytically active, but functions as a CO2 buffer. Different functions of Rubisco, CO2 binding, and CO2 turnover, are in accordance with the observation that for full catalytic activity in the light, Rubisco needs to be activated, which in turn requires Rubisco activase and ATP (Robinson and Portis 1988; Salvucci and Ogren 1996). Inhibitors are generally analogues of the enzyme’s substrate ribulose-1,5-bis phosphate (RuBP) (Edmonson et al. 1990) and bind to the enzyme in the absence of RuBP. Activase releases tight-binding inhibitors from the Rubisco active sites, thus increasing specific activity. This reaction requires ATP (Robinson and Portis 1988), so a decreased activity and activation state of Rubisco at low relative water content may be related to inadequate ATP supply to the protein complex (Tezara et al. 1999; Parry et al. 2002).
The amount of Rubisco protein is generally little affected by moderate or severe salt stress (Flexas et al. 2002), even if experienced over a period of many days (Tezara et al. 1999; Gunasekera and Berkowitz 1993; Medrano et al. 1997). This means that specific Rubisco activity rather than protein concentration is decreased under saline conditions. Restoration of the assimilation potential by rehydration also suggests that Rubisco is not irreversibly impaired (Medrano et al. 1997; Parry et al. 2002).
Calvin Cycle Enzymes
The reduction of RuBP content at low relative water content could result from a limitation in one or more enzymes of the Calvin cycle. There is little direct evidence regarding the response of the individual enzymes of the regenerative part of the Calvin cycle to increasing cytosolic mineral content subsequent to increased salinity. There do not seem to be significant differences between enzymes from glycophytes and halophytes, respectively (Huchzermeyer and Heins 2000; Huchzermeyer 2000). Furthermore, in most cases isolated enzymes did not show a degree of salt inhibition sufficient to explain the extent of inhibition of the respective metabolic reaction in in vivo experiments. Therefore it is doubtful that the high salt resistance of halophytes is due to changed properties of their Calvin cycle enzymes. Thus, there is a strong evidence that the Calvin cycle per se is not the cause of the decreased assimilation rate under osmotic stress.
RuBP Concentration and Rubisco Activity
The net assimilation rate depends on the synthesis of RuBP and Rubisco activity. Therefore, the decrease in leaf Rubisco content at low relative water content is significant (Giménez et al. 1992; Gunasekera and Berkowitz 1993; Tezara et al. 1999). In stressed sunflower plants, the photosynthetic rate correlated with RuBP concentration (Giménez et al. 1992), which suggests that the assimilation potential in these experiments was determined by RuBP content, but not by CO2.
Under salt stress, there is a general decline in Calvin cycle intermediates during the phase of stress adaptation. Decreased RuBP concentration might be caused by the general rundown of the Cavin cycle and decrease in assimilation rate. The high ratio of 3PGA/RuBP suggests a limitation in the RuBP regeneration part of the Calvin cycle, either caused by enzyme limitations or inadequate ATP, although it was interpreted by Tezara et al. (1999) as evidence of 3PGA production by mitochondria. In laboratory experiments with halophytes such as Aster tripolium however, the metabolite pattern recovered within less than two or three days under constant stress conditions.
Interaction between Rubisco activity and RuBP supply is well illustrated by studies on unstressed tobacco leaves with normal amounts of Rubisco (Laisk and Oja 1974; von Caemmerer 2000). The RuBP pool increased in leaves in bright light when the CO2 concentration was transiently decreased, so that when returning to normal CO2 concentrations, assimilation rate was much higher than the steady-state concentrations for a short time until the RuBP was consumed. Thus, under steady-state conditions, RuBP supply limited the CO2 assimilation rate.
Limitation of RuBP Regeneration by NADPH and ATP Availability
Synthesis of RuBP depends on ATP and NADPH concentration and on the Calvin cycle activity, or more specifically on PRK activity, and concentration of the substrates ATP and ribose 5-phosphate (Lawlor 2001). In the Calvin cycle, NADPH serves as substrate of the glyceraldehyde 3-phosphate dehydrogenase. If NADPH were limiting at low relative water content, it would decrease glyceraldehyde 3-phosphate and thus ribose 5-phosphate, the same effect as decreasing the activity of an enzyme in that part of the cycle. In salt stressed leaf mesophyll cells NADPH content remained constant (Lawlor and Khanna-Chopra 1984; Tezara et al. 1999), while NADH increased (Lawlor and Khanna-Chopra 1984), indicating that the electron transport capacity is sufficient to maintain and increase the reduction state of these pyridine nucleotides. Thus, with respect to high salinity resistance of the photosynthetic electron transport system in both glycophytes and halophytes, the availability of NADPH to the Calvin cycle is unlikely to limit its capacity to form RuBP (Koyro and Huchzermeyer 1999; Huchzermeyer and Heins 2000).
The rate of ATP synthesis depends on the light reactions, generation of the trans-thylakoid pH gradient (ΔpH), availabiliy of ADP and Pi, and activity of the chloroplast coupling factor (CF1) (Löhr and Huchzermeyer 1985; Löhr et al. 1985; Huchzermeyer 1988a, b; Richter et al. 2000). Inadequate ATP concentration would decrease the ability of the Calvin cycle to regenerate RuBP by PRK, so glyceraldehyde-3-phosphate would increase and RuBP decrease. This was the effect of reduced PRK activity (Paul et al. 1995), but ATP increased in the transgenics, in contrast to the case of water stress in some studies (Lawlor 2002a, b; Lawlor and Cornic 2002).
Triose Export
In the presence of light, sugars are exported as triose phosphates (DHAP and GAP) in exchange of free phosphate via the phosphate translocator (Riesmeier et al. 1993). Vmax of sugar phosphate export is too low to keep up with sugar phosphate synthesis under average light conditions. This would cause shortage of phosphate inside the chloroplast stroma, thus inhibiting the primary reactions of photosynthesis and increasing the risk of ROS production. Starch production inside the chloroplast is a relief, because phosphate will be released and becomes available for the chloroplast coupling factor to produce ATP. Vmax of starch production has not been measured yet as physiological sugar concentrations are too low. The capacity to produce starch is obviously high enough to turn over any sugar concentration that might become available under physiological conditions. There is an equilibrium between starch synthesis and hydrolysis, respectively. Therefore, starch will be degraded at night and allow a permanent supplementation of the cytosol of the host cell. This system allows plant cells to grow permanently on a continuous flow of incoming sugar phosphates.
In the cytosol of green leaf parenchyma cells, sugar phosphates are used to fuel cell metabolism. In a competing metabolic pathway sucrose is formed from glucose and fructose and is exported into the phloem to feed sink organs.
The description above shows that there are several alternatives how incoming salt may affect the functioning of cellular sugar metabolism. In principle, the situations in source and sink tissues are comparable. But under moderate salt stress, salt concentrations have been found to differ a lot between root, leaf, and fruit tissues. Therefore, the observed effect of salt stress also differs among tissues of plant organs.
Salt effects on sugar metabolism and sugar export from the chloroplasts into the cytosol can be explained on the basis of a competition between Cl− and H2PO4 − for binding sites on enzymes and receptors, respectively. This competition is due to similar diameters of these two ions when hydrated. This would mean that under salt stress the chloroplast stroma is at risk to run out of free phosphate. As mentioned above, this would inhibit ATP synthesis at the chloroplast coupling factor (Groth et al. 2000). The observed effect would be quite similar to the one observed after energy transfer inhibitors like nitrofen (Huchzermeyer and Löhr 1990). Nitrofen is a herbicide used in rice cultures and it is known to stimulate light-dependent ROS production in plants. By the way, this observation again is a proof for the tight coupling between primary reactions of photosynthesis.
Protection of Proteins and Biomembranes from Salt Effects
Control of Mechanisms Improving Stress Resistance: Compatible Solutes
Sequestration of ions to the vacuole is a strategy leading to enhanced salt stress resistance. Some low molecular weight nontoxic compounds have been identified to significantly contribute to ionic and osmotic balance inside cells. They are called “compatible solutes” (Ford 1984; Ashihara et al. 1997; Hasegawa et al. 2000; Zhifang and Loescher 2003; Lee et al. 2008). Chemically they can be described to be poly-amines or poly-hydroxyls. In addition to their function in ionic and osmotic homeostasis, their important function is that they can replace water in its function to stabilize aggregates of soluble proteins and membrane–protein interactions, respectively (Yancey et al. 1982; Crowe et al. 1992; Ashraf and Foolad 2007).
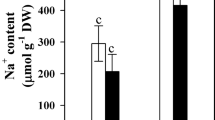
Depending on enzyme patterns and metabolic pathways preferred in individual plants, different compatible solutes have been found in plants (Fig. 15.6). Among the compounds described in the literature are proline (Singh et al. 2000; Kavi Kishore et al. 2005; Koca et al. 2007; Alla et al. 2012), glycine betaine (Khan et al. 1998; Wang and Nil 2000; Türkan and Demiral 2009; Zhu et al. 2011), sugars (Bohnert and Jensen 1996; Kerpesi and Galiba 2000; Gil et al. 2011), di-, tri-saccharides and other sugar derived compounds (Hagemann and Murata 2003), and polyols (Popp et al. 1985; Orthen et al. 1994; Bohnert et al. 1995; Gil et al. 2011). Overexpression of genes of metabolic pathways leading to production of compatible solutes has been shown to improve salt resistance. (Parida et al. 2002; Parida and Das 2005). Apparently, there are three ways to explain the mechanism how compatible solutes become overproduced under stress. Under optimal growth conditions, these compounds are produced at low rates, so that their concentration is found to be low. Therefore, the question is whether overproduction under stress is due to activation of already existing enzymes or de novo synthesis of such enzymes.
Overview on metabolism of compatible solutes. Glucose can function as a substrate for synthesis of compatible solutes. Cytosolic synthesis often occurs at the expense of NAD(P)H. Thus, synthesis of compatible solutes is a relief under conditions, when production of redox power and glucose are exceeding consumption of electrons and export of sugar. Cytosolic pattern of compatible solutes varies among plant species depending on respective enzyme activities. In addition to regulation of respective gene expression, synthesis rates are controlled by availability of precursors
It was observed that many stress conditions like drought and salt stress, for instance, initially inhibit export of products of photosynthesis from their source tissues. This will result in enhanced concentrations of primary products of the Calvin cycle in leaf cells. As shown by Koyro and Huchzermeyer (2005) the most compatible solutes derive from primary products of sugar metabolism. Therefore, increased concentrations of metabolites of photosynthesis will stimulate synthesis rate of compatible solutes. Such an increase will become significant if metabolite concentrations under optimal growth conditions are below the Km values of enzymes catalyzing the initial reactions of the pathways leading to compatible solutes. This interpretation agrees with the findings of Soussi et al. (1998), who attributed enhanced concentrations of proline and carbohydrates under salt stress in chick pea to damage of metabolic pathways rather than to protective mechanisms.
A second working hypothesis is based on findings of Süss et al. (1993). They found that enzymes of individual pathways in chloroplasts tend to form clusters. Such conditions would contradict free mobility of intermediates. Thus, substrate concentrations localized to catalytic centers of enzymes may significantly differ from respective bulk phase concentrations. Calvin cycle enzymes, for instance, tend to bind to one another and thus form aggregates of proteins. This allows substrates as well as products formed to be shuttled from one enzyme to the next one of the respective pathway. Süss postulated that modulation of pathways in this model is brought about by modulation of enzyme neighbourhood. From analysis of internal signalling within cells, it is well documented that such modulations can be brought about by protein phosphorylation and de-phosphorylation, for instance. Indeed, such phosphorylation-dependent variations in protein–protein interactions have been observed by Allen and Horton, when analyzing protein localization in thylakoid membranes (Horton and Foyer 1983; Pursiheimo et al. 2001; Allen 2002).
A third model refers to the observation that salt stress response of plants has been found to be under the control of hormones (ABA, for instance) and second messengers (sugar signalling, for instance). This implies that modification of enzyme patterns will result in modified metabolic activity of cells and tissues under stress. Such argumentation would explain the above observations on the level of gene activities.
These latter arguments may suggest not to discuss three different models of regulation of metabolic pathways leading to the synthesis of compatible solutes. It rather appears to us that hormone- and secondary messenger actions explain how formation of protein aggregates may be controlled in plants.
Biochemical reaction sequences leading to improved salt resistance are likely to act synergistically (Iyengar and Reddy 1996). The reactions include the synthesis of compatible solutes, the stimulation of antioxidative enzyme activities, and modifications of the photosynthetic pathway.
Energy Consumption by N- and S-Pathways
As a rule, in non-woody plants nitrate is reduced in chloroplasts, while it is reduced in root cell plastids of trees. Nitrate competes with chloride (due to similar radii of the hydrated ions) for uptake via specific translocators. These translocators are regulated via the plant’s sugar pool and their activity thus matches the photosynthetic activity of source leaves. In this chapter, we focus on coupling of nitrate reduction to photosynthesis in chloroplasts of weeds.
About one-third of the electrons released by PSII finally will be used for nitrate reduction. Therefore, nitrate reduction is a major sink for electrons and sufficient N fertilization contributes to prevention of primary reactions of photosynthesis from over-reduction in high light.
Nitrate reduction and incorporation of reduced nitrogen into glutamate is shown in Fig. 15.3. It is obvious that the first intermediates of nitrate reduction are toxic. Like in primary reactions of photosynthesis, building up of concentrations and occurrence of side reactions that eventually might be toxic for the cell are prohibited by fast turnover of products.
It has been shown in the literature that the affinity for nitrate uptake from the soil can be improved by means of a molecular biological approach (Matt et al. 2001; Wang et al. 2003; Lillo 2004). Furthermore, nitrate fertilization helps to reduce salt stress effects (Syvertsen et al. 1989; Murillo-Amador et al. 2006).
It is obvious that salt stress can have direct as well as indirect effects on nitrate reduction and amino acid biosynthesis. Direct effects mostly are due to competition among nitrate and chloride ions. Indirect effects are based on the dependence of nitrate reduction on electrons released by photosynthetic electron transport, an intact structure of enzymes and enzyme aggregates, and the dependence of amino acid biosynthesis on substrates that derive from sugar metabolism (i.e. sugar supply from photosynthesis). We have to keep in mind that sugars, sugar phosphates, and ROS are functioning as secondary messengers regulating the expression of enzymes. Therefore, there will be another level of regulatory effects. Though a lot information on sugar signaling has been analyzed and a list of participants has been outlined in pretty much detail, the complex pathway structure of second messenger signaling has not been well characterized (Gibson 2000; Sheen 2002; Baena-González and Sheen 2008).
As nitrate reduction and subsequent amino acid biosynthesis depend on photosynthetic activity, there has to be some buffer capacity for intermediates to allow continuous metabolic activity of plants. In principle, there are two storage compartments in leaf cells: plastids and the vacuole. In storage organs, plastids can differentiate to protein storage compartments. In chloroplasts, only low amounts of storage proteins typical for storage plastids are found. But in some papers it is discussed whether the huge amounts of Rubisco accumulating in chloroplasts may function as storage proteins as well. In vacuoles, free amino acids and other low molecular weight molecules are stored rather than macro molecules like proteins. It has been shown that vacuoles from tissues active in photosynthesis can actively import amino acids at final concentrations several fold exceeding the ones in the cytosol (Homeyer et al. 1989). Amino acid import occurs at the expense of a transmembrane pH gradient. Both v-Type ATPase and PPase of the tonoplast build this gradient at the expense of ATP and pyrophosphate, respectively (Homeyer et al. 1989). It is well known that the activity of these two enzymes is regulated (Taiz 1992; Davies 1997; Han et al. 2005; Park et al. 2005), and that amino acid content is affected by salt stress (Pahlich et al. 1983).
In contrast to nitrogen that is found exclusively in the reduced form in organic molecules, sulfur occurs in metabolites of biological relevance in several redox states. Only a minor portion of the photosynthetic electrons are used for the sulfate reduction pathway. As outlined by Schmidt and Jäger (1992, and citations therein), sulfate reduction pathway in chloroplasts differs from the one in bacteria that had been identified earlier.
Inhibition of sulfur metabolism has major secondary effects, because SH bounds are essential for functioning of catalytic centers of many enzymes and reduced sulfur is found in coenzymes like CoA and liponic acid, for instance. Moreover, a pool of various glutathiones forms the dominant redox buffer of plant cells, and glutathiones are involved in detoxification of heavy metals and ROS. Therefore, enhanced salt stress resistance of sulfur metabolism always goes along with improved metabolic activity and apparent resistance of other essential functions as well.
Photosynthesis of Salt Stressed Plants Under Future Elevated Atmospheric CO2 Concentration
C3 Plants
Elevated atmospheric CO2 concentration leads to a higher CO2 concentration gradient between the outside air and the intercellular spaces of the leaves, so that the diffusion of CO2 into the leaves and the pCO2/pO2 ratio at the sites of photoreduction is increased (Robredo et al. 2007). Therefore, photorespiration and the rates of oxygen activation and ROS formation are usually reduced in C3 plants due to an increased NADPH utilisation, whereas the net photosynthetic rate and thus the carbon supply is enhanced (Polle 1996; Sgherri et al. 2000; Urban 2003; Kirschbaum 2004; Long et al. 2004; Hikosaka et al. 2005; Ignatova et al. 2005; Fig. 15.7). Furthermore, we often find a higher stomatal resistance (Hsiao and Jackson 1999; Sgherri et al. 2000; Li et al. 2003; Marchi et al. 2004; Rogers et al. 2004), which – together with the higher net assimilation – also leads to a better water use efficiency of photosynthesis (Amthor 1999; Morgan et al. 2001; Urban 2003). Results from free-air CO2 enrichment (FACE) experiments showed that elevated CO2 stimulated the light-saturated photosynthesis of C3 plants by an average of 31% and reduced stomatal conductance by an average of 22% (Ainsworth and Rogers 2007).
Generalized and simplified model of the influence of NaCl salinity and elevated CO2 concentration on the photosynthesis of (a) C3 and (b) C4 plants.  = influence of NaCl;
= influence of NaCl;  = influence of elevated CO2 and salinity;
= influence of elevated CO2 and salinity;  = positive influence;
= positive influence;  = negative influence;
= negative influence;  = major influence;
= major influence;  = minor influence. The changing quantity of a parameter is taken into account when assessing its influence on the next one (Except for energy metabolism, following
= minor influence. The changing quantity of a parameter is taken into account when assessing its influence on the next one (Except for energy metabolism, following  ). R
s
stomatal resistance, WUE water use efficiency of photosynthesis
). R
s
stomatal resistance, WUE water use efficiency of photosynthesis
However, responses of gas exchange to elevated CO2 concentration are variable and depend on plant species and abiotic factors such as salinity and humidity (Drake et al. 1997; Ball and Munns 1992; Arp et al. 1993). So stomatal resistance of salt stressed C3 plants may even decrease under elevated CO2 if the maximization of photosynthesis and energy gain is given priority over a reduction of water loss in order to reduce oxidative stress. This is the case e.g. in Aster tripolium, which increases its WUE only by an improved photosynthesis, but not by a reduced transpiration. This is a reasonable strategy because the salt resistance of this species is mainly limited by an impaired net assimilation rate and accompanying ROS formation (Geissler et al. 2009a, b). Elevated atmospheric CO2 concentration therefore ameliorates metabolic processes in A. tripolium which are typically associated with C3 metabolism and are disadvantageous on saline habitats compared to C4 plants (see Sect. C4 Plants).
As a consequence of the enhanced plant water relations and/or net photosynthetic rate, there might be less need for antioxidants as elevated CO2 ameliorates oxidative stress (Schwanz et al. 1996), such as in barley (Pérez-López et al. 2009) and Solanum lycopersicum (Takagi et al. 2009). On the other hand elevated CO2 can induce carbon partitioning to various pathways associated with stress defence/response (Bokhari et al. 2007; Cseke et al. 2009; Jin et al. 2009), i.e. more energy can be provided for stress resistance mechanisms: The amount and/or activities of antioxidants can be increased, e.g. the activities of catalase (CAT), ascorbate peroxidase (APX) and superoxide dismutase (SOD) in Quercus (Marabottini et al. 2001; Schwanz and Polle 2001) or the expression and activities of APX, SOD and glutathione-S-transferase (GST) in Aster tripolium (Geissler et al. 2009b, 2010). Some species show also a higher amount of compatible solutes such as soluble carbohydrates (Carthamus mareoticus: Abdel-Nasser and Abdel-Aal 2002; Aster tripolium: Geissler et al. 2009a) or proline (Aster tripolium: Geissler et al. 2009a). Additionally, the larger number of carbon skeletons available due to an improved photosynthesis can increase the thickness of the outer epidermal cell walls and the cuticle (Tipping and Murray 1999; Tingey et al. 2003; Oksanen et al. 2005), which decreases transpiration on the one hand and light reflection and thus ROS production on the other hand (Thomas 2005; Geissler et al. 2009b). Reduced oxidative stress under elevated CO2 concentration in turn leads to less structural damage to the thylakoid membranes of the chloroplasts (see Sect. Salt Effects on Chloroplast Structure and Metabolite Transfer) with only minor dilations of the thylakoid membranes (Oksanen et al. 2001; Geissler et al. 2009b; Fig. 15.4). This fact is of major importance because the chloroplasts – as the site of photosynthesis – fulfil a key function in adaptation to salinity, and chloroplast integrity correlates with a higher assimilation rate.
All the factors discussed above can contribute to an increased survival of salt stressed plant under elevated CO2, which has been reported by various authors (Ball and Munns 1992; Rozema 1993; Drake et al. 1997; Fangmeier and Jäger 2001; Wullschleger et al. 2002; Urban 2003; Geissler et al. 2009a; Fig. 15.7).
C4 Plants
While elevated CO2 concentration generally has a positive effect on the water relations as well as on the net photosynthesis of salt stressed C3 plants, the situation in C4 plants is more ambiguous.
In C4 plants, CO2 is bound to PEP and pre-fixed by the formation of a C4 compound (that can be reduced to malate, for instance) in the mesophyll cells which do not contain any Rubisco. The final carbon fixation step takes place in the bundle sheath cells surrounding the mesophyll cells coronally. It releases CO2 from the C4 compound, and assimilation will be catalyzed by Rubisco in an environment characterized by low oxygen partial pressure. This highly efficient CO2 enrichment mechanism at the carboxylation sites in the bundle sheath cells and thus an equally efficient CO2 fixation enable C4 plants to maintain photosynthesis with almost closed stomata, which minimizes transpiration. Therefore they can use water more efficiently, which is of high advantage under saline conditions.
While it was proved in various studies that elevated CO2 can ameliorate drought stress in C4 plants similarly to C3 plants due to its effect on stomatal conductance (Mateos-Naranjo et al. 2010; Leakey 2009; Lopes et al. 2011), it has usually only small or no positive direct effects on the photosynthesis of C4 plants (Leakey et al. 2006; Ainsworth and Rogers 2007; Leakey 2009; Wang et al. 2012; Fig. 15.7). The latter are adapted to low atmospheric CO2 concentrations due to their efficient CO2 enrichment mechanism (see above), so their photosynthesis is saturated at the current atmospheric CO2 concentration (Fangmeier and Jäger 2001). In consistence with these facts, Polley et al. (1996) predicted that the productivity of various C4 species will probably show only a small increase under elevated CO2 because the intrinsic water use efficiency was stimulated proportionally more by a given relative increase in CO2 over sub-ambient than by elevated concentrations. So we expect the salt resistance of C4 species to be less enhanced by elevated CO2 concentration than the one of C3 species (Fig. 15.7). This could already be confirmed by several studies which have shown that C4 plants show less growth stimulation than C3 species on saline soils under elevated CO2 and/or are likely to be disadvantaged in competition (Schwarz and Gale 1984; Curtis et al. 1989; Rozema et al. 1991; Arp et al. 1993; Lenssen et al. 1993; Erickson et al. 2007; Jaggard et al. 2010).
However, this theory is not supported by Wand et al. (1999) and Ward et al. (1999), so the situation is a bit ambiguous. This is also true for the issue of photorespiration which leads to CO2 loss and ROS production in C3 plants (Ainsworth and Rogers 2007; Lüttge et al. 2010; Sect. ROS Production), thus limits their salt resistance and can be ameliorated by elevated CO2. In most textbooks, it is stated that C4 plants can overcome this problem on the expense of extra energy consumption and that the formation of significant concentrations of glycolate is not a problem of these plants. If salt stress would interfere with aggregate formation of chloroplasts, mitochondria, and peroxisomes, thus inhibiting by glycolate toxicity proper performance of C3 plants; such effects should not be observed in C4 species. Nevertheless, salt stress might interfere with intermediate transfer among cells and cell compartments in C4 plants. This would inhibit photosynthetic activity but would not result in “classical” glycolate toxicity.
But again the situation is not as simple as suggested in textbooks. Several studies have pointed out that a low rate of photorespiration takes place in C4 plants as well. In maize leaves grown at normal CO2 concentration, photorespiration may reach 5% of the rate found in tobacco grown under identical conditions (Zelitch 1973). Though such a rate may appear to be low, glycolate oxidase activity obviously is essential for C4 plants. Maize plants showing less than 10% of glycolate oxidase activity of the wild type could grow at high CO2 concentrations, they became necrotic in normal air, and died within 2 weeks (Zelitch et al. 2009). When considering these facts, the salt resistance of C4 plants may indeed be increased by elevated CO2 concentration.
Conclusions and Future Perspectives
Abiotic stresses such as soil salinity are responsible for a decrease in yield especially in arid and semiarid regions. It is estimated that 45% of the world’s agricultural land experience drought and 19.5% of the irrigated land are affected by salinization. This problem will be further catalyzed by global climate change. Salt stress directly affects the primary and secondary reactions of photosynthesis in numerous ways and consequently has also far reaching consequences on ROS production, on sugar, lipid, and amino acid metabolism, trans membrane and inter tissues transport of metabolites and on water relations and gas exchange.
Elevated atmospheric CO2 concentration can improve photosynthesis and water relations and reduce oxidative stress in C3 plants, so that it can enhance their salt resistance and thus their suitability as crops in a future world of climate change. In contrast, the effect of CO2 on the salt resistance of C4 plants is less beneficial.
The major challenge to modern plant scientists is to develop stress resistant and high-yield plants. Without doubt halophytic model plants will have to be developed as they are invaluable in precisely assessing the role which e.g. the complex photosynthetic reactions, ROS, antioxidants, or osmolytes play in the functional network which controls stress resistance. They can be of major importance not only for the breeding of halophytes as alternative crop plants, but also for increasing the salt resistance of conventional crops via genetic engineering. Halophytes as naturally salt resistant plants constitute especially valuable genetic models for understanding resistance mechanisms (Flowers and Colmer 2008). They are much more suited than classical glycophytic model plants such as Arabidopsis thaliana because genes which are expressed in halophytes but not in glycophytes indicate specific traits which enable survival under saline conditions.
In the face of climate change, it would be very desirable to develop model plants also regarding the interaction of salt stress with other abiotic factors associated with global change, such as elevated atmospheric CO2 concentration. In this regard, C3 and C4 plants should be compared as they respond differently to elevated CO2. Suited species for such a comparison would be Chenopodium quinoa (C3) and Atriplex spec. (C4) because they are related (both belong to the Chenopodiaceae), and show some similarities regarding their salt resistance mechanisms.
References
Abdel-Nasser LE, Abdel-Aal AE (2002) Effect of elevated CO2 and drought on proline metabolism and growth of saf-flower (Carthamus mareoticus L.) seedlings without improving water status. Pak J Biol Sci 5:523–528
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Alla MMN, Khedr A-HA, Serag MM, Abu-Alnaga AZ, Nada RM (2012) Regulation of metabolomics in Atriplex halimus growth under salt and drought stress. Plant Growth Regul 67:281–304
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Allen JF (2002) Plastoquinone redox control of chloroplast thylakoid protein phosphorylation and distribution of excitation energy between photosystems: discovery, background, implications. Photosynth Res 73:139–148
Allen JF, Bennett J (1981) Photosynthetic protein phosphorylation in intact chloroplasts Inhibition by DCMU and by the onset of CO2-fixation. FEBS Lett 123:67–70
Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6:317–326
Allnutt FCT, Dilley RA, Kelly T (1989) Effect of high KCl concentrations on membrane localized metastable proton buffering domains in thylakoids. Photosynth Res 20:161–172
Amthor JS (1999) Increasing atmospheric CO2 concentration, water use and water stress: scaling up from the plant to the landscape. In: Mooney HA, Luo Y (eds) Carbon dioxide and environmental stress. Academic, San Diego, pp 33–59
Arp WJ, Drake BG, Pockman WT, Curtis PS, Whigham DF (1993) Interaction between C3 and C4 salt marsh species during four years of exposure to elevated atmospheric CO2. Vegetation 104(105):133–143
Ashihara H, Adachi K, Otawa M, Yasumoto E, Fukushima Y, Kato M, Sano H, Sasamoto H, Baba S (1997) Compatible solutes and inorganic ions in the mangrove plant Avicennia marina and their effects on activities of enzymes. Z Naturforsch 52c:433–440
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Azaizeh H, Steudle E (1991) Effects of salinity on water transport of excised maize (Zea mays L.) roots. Plant Physiol 97:1136–1145
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Baena-González E, Sheen J (2008) Convergent energy and stress signaling. Trends Plant Sci 13:474–482
Ball MC, Munns R (1992) Plant responses to salinity under elevated atmospheric concentrations of CO2. Aust J Bot 40:515–525
Benavides MP, Marconi PL, Gallego SM, Comba ME, Tomaro ML (2000) Relationship between antioxidant defence systems and salt tolerance in Solanum tuberosum. Aust J Plant Physiol 27:273–278
Blokhina O, Virolainen E, Fagerstedt KV (2002) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Boekema E, Fromme P, Gräber P (1988) On the structure of the ATP-synthase from chloroplasts. Ber Bunsenges Phys Chem 92:1031–1036
Bohnert HJ, Jensen RG (1996) Strategies for engineering water stress tolerance in plants. Trends Biotechnol 14:89–97
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Bokhari SA, Wan X-Y, Yang YW, Zhou L, Tang WL, Liu JY (2007) Proteomic response of rice seedling leaves to elevated CO2 levels. J Proteome Res 6:4624–4633
Boyer PD (2000) Catalytic site forms and controls in ATP synthase catalysis. Biochim Biophys Acta 1458:252–262
Chang H, Siegel BZ, Siegel SM (1984) Salinity induced changes in isoperoxidase in taro, Colocasia esculenta. Phytochemistry 23:233–235
Chang I-H, Cheng K-T, Huang P-C, Lin Y-Y, Cheng L-J, Cheng T-S (2012) Oxidative stress in greater duckweed (Spirodela polyrhiza) caused by long-term NaCl exposure. Acta Physiol Plant 34:1165–1176
Cha-um S, Chuencharoen S, Mongkolsiriwatana C, Ashraf M, Kirdmanee C (2012) Screening sugarcane (Saccharum sp.) genotypes for salt tolerance using multivariate cluster analysis. Plant Cell Tiss Organ Cult 110:23–33
Chen G, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isoenzymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–787
Cornic G, Briantais J-M (1991) Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris) at different CO2 concentrations and during water stress. Planta 183:178–184
Cramer GR (2003) Differential effects of salinity on leaf elongation kinetics of three grass species. Plant Soil 253:233–244
Crowe JH, Hoekstra FA, Crowe CM (1992) Anhydrobiosis. Annu Rev Plant Physiol 54:579–599
Cseke LJ, Tsai CJ, Rogers A, Nelsen MP, White HL, Karnosky DF, Podila GK (2009) Transcriptomic comparison in the leaves of two aspen genotypes having similar carbon assimilation rates but different partitioning patterns under elevated [CO2]. New Phytol 182:891–911
Curtis PS, Drake BG, Whigham DF (1989) Nitrogen and carbon dynamics in C3 and C4 estuarine marsh plants grown under elevated CO2 in situ. Oecologia 78:297–301
Davies JM (1997) Vacuolar energization: pumps, shunts and stress. J Exp Bot 48:633–641
Demmig-Adams B, Adams WW III (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants. a consequence of rising atmospheric CO2? Annu Rev Plant Physiol 48:609–639
Edmonson DL, Kahne HJ, Andrews TJ (1990) Substrate isomerization inhibits ribulosebisphosphate carboxylase/oxygenase during catalysis. FEBS Lett 260:62–66
Erickson JE, Megonigal JP, Peresta G, Drake BG (2007) Salinity and sea level mediate elevated CO2 effects on C3–C4 plant interactions and tissue nitrogen in a Chesapeake Bay tidal wetland. Glob Change Biol 13:202–215
Fangmeier A, Jäger HJ (2001) Wirkungen erhöhter CO2-Konzentrationen. In: Guderian R (ed) Handbuch der Umweltveränderungen und Ökotoxikologie, vol 2a, Terrestrische Ökosysteme: Immissionsökologische Grundlagen – Wirkungen auf Boden – Wirkungen auf Pflanzen. Springer, Berlin, pp 382–433
Fidalgo F, Santos A, Santos I, Salema R (2004) Effects of long-term salt stress on antioxidant defense systems, leaf water relations and chloroplast ultrastructure of potato plants. Ann Appl Biol 145:185–192
Field TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn: the role of anthocyanins in senescing leaves of Red-Osier Dogwood. Plant Physiol 127:566–574
Flexas J, Escalona JM, Evain S, Gulías J, Moya I, Osmond CB, Medrano H (2002) Steady-state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C3 plants. Physiol Plant 114:231–240
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Ford CW (1984) Accumulation of low molecular solutes in water stressed tropical legumes. Phytochemistry 23:1007–1015
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60:455–488
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247:1–11
Geissler N, Hussin S, Koyro H-W (2009a) Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ Exp Bot 65:220–231
Geissler N, Hussin S, Koyro H-W (2009b) Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. J Exp Bot 60:137–151
Geissler N, Hussin S, Koyro H-W (2010) Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta 231:583–594
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gerbaud A, André M (1980) Effect of CO2, O2 and light on photosynthesis and photorespiration in wheat. Plant Physiol 66:1032–1036
Gibson SI (2000) Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol 124:1532–1539
Gil R, Lull C, Boscaiu M, Bautista I, Lidon A, Vicente O (2011) Soluble carbohydrates as osmolytes in several halophytes from a mediterranean salt marsh. Not Bot Horti Agrobo 39:9–17
Giménez C, Mitchell VJ, Lawlor DW (1992) Regulation of photosynthetic rate of two sunflower hybrids under water stress. Plant Physiol 98:516–524
Glenn EP, Brown JJ, O´Leary JW (1998) Irrigating crops with sea water. Sci Am 279:76–81
Groth G, Mills DA, Christiansen E, Richter ML, Huchzermeyer B (2000) Characterization of a phosphate binding domain on the α subunit of chloroplast ATP synthase using the photoaffinity phosphate analog 4-azido-2-nitrophenyl phosphate. Biochemistry 39:13781–13787
Gudesblat GE, Iusem ND, Morris PC (2007) Guard cell-specific inhibition of Arabidopsis MAPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 173:713–721
Gunasekera D, Berkowitz GA (1993) Use of transgenic plants with ribulose 1,5-bisphosphate carboxylase/oxygenase antisense DNA to evaluate the rate limitation of photosynthesis under water stress. Plant Physiol 103:629–635
Hagemann M, Murata N (2003) Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol 131:1628–1637
Halliwell B (1982) The toxic effects of oxygen on plant tissues. In: Oberly LW (ed) Superoxide dismutase, vol I. CRC Press, Boca Raton, pp 89–123
Halliwell B (1987) Oxidative damage, lipid peroxidation, and antioxidant protection in chloroplasts. Chem Phys Lipids 44:327–340
Halliwell B, Gutteridge JMC (1986) Free radicals in biology and medicine. Oxford University Press, London
Han N, Shao Q, Lu C-M, Wang B-S (2005) The leaf tonoplast V-H+-ATPase activity of a C3 halophyte Suaeda salsa is enhanced by salt stress in a Ca-dependent mode. J Plant Physiol 162:267–274
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145:1506–1520
Hesse H, Jank-Ladwig R, Strotmann H (1976) On the reconstitution of photophosphorylation in CF1- extracted chloroplasts. Z Naturforsch 31c:445–451
Hikosaka K, Onoda Y, Kinugasa T, Nagashima H, Anten NPR, Hirose T (2005) Plant responses to elevated CO2 concentration at different scales: leaf, whole plant, canopy, and population. Ecol Res 20:243–253
Homeyer U, Litek K, Huchzermeyer B, Schultz G (1989) Uptake of phenylalanine into isolated barley vacuoles is driven by both tonoplast adenosine triphosphatase and pyrophosphatase: evidence for a hydrophobic L-amino acid carrier system. Plant Physiol 89:1388–1393
Horton P (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51:475–485
Horton P, Foyer C (1983) Relationship between protein phosphorylation and electron transport in the reconstituted chloroplast system. Biochem J 210:517–521
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:665–684
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52:2245–2264
Hsiao TC, Jackson RB (1999) Interactive effects of water stress and elevated CO2 on growth, photosynthesis, and water use efficiency. In: Luo Y, Mooney HA (eds) Carbon dioxide and environmental stress. Academic, San Diego, pp 3–31
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Huchzermeyer B (1988a) Phosphate binding to isolated chloroplast coupling factor (CF1). Z Naturforsch 43c:213–216
Huchzermeyer B (1988b) Nucleotide binding and ATPase activity of membrane bound chloroplast coupling factor (CF1). Z Naturforsch 43c:133–139
Huchzermeyer B (2000) Biochemical principles of salt tolerance. In: Lieth H (ed) Sustainable halophyte utilization in the mediterranean and subtropical dry regions. University of Osnabrück Publication, Osnabrück, pp 130–133
Huchzermeyer B, Heins T (2000) Energy metabolism and salt stress. In: Lieth H, Moschenko M (eds) INCO-DC annual report. University of Osnabrück Publication, Osnabrück, pp 48–73
Huchzermeyer B, Koyro H-W (2005) Salt and drought stress effects on photosynthesis. Enzyme cohesion and high turn over metabolite shuttling, essential for functioning of pathways, is impaired by changes in cytosolic water potential. In: Pessarakli M (ed) Handbook of photosynthesis, 2nd edn. CRC Press/Taylor and Francis, Boca Raton, pp 751–777
Huchzermeyer B, Löhr A (1990) Diphenylether herbicides, a tool in elucidating the mechanism of photophosphorylation. Z Naturforsch 45c:552–557
Huchzermeyer B, Strotmann H (1977) Acid/base-induced exchange of adenine nucleotides on chloroplast coupling factor (CF1). Z Naturforsch 32c:803–809
Huchzermeyer B, Willms I (1985) Regulation of coupling factor activities by some component of chloroplast electron transport system. ICSU Short Rep 3:318–319
Huchzermeyer B, Löhr A, Willms I (1986) A direct interaction between photosystem I and the chloroplast coupling factor. Biochem J 234:217–220
Huchzermeyer B, Hausmann N, Paquet-Durant F, Koyro H-W (2004) Biochemical and physiological mechanisms leading to salt tolerance. Trop Ecol 45:141–150
Ignatova LK, Novichkova NS, Mudrik VA, Lyubimov VY, Ivanov BN, Romanova AK (2005) Growth, photosynthesis, and metabolism of sugar beet at an early stage of exposure to elevated CO2. Russ J Plant Physiol 52:158–164
Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240:1302–1309
IPCC (2007) Climate change 2007. The physical science basis. contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge\New York
Iyengar ERR, Reddy MP (1996) Photosynthesis in highly salt tolerant plants. In: Pesserakli M (ed) Handbook of photosynthesis. Marcel Dekker, New York, pp 897–909
Jaggard KW, Qi A, Ober ES (2010) Possible changes to arable crop yields by 2050. Philos T Roy Soc B 365:2835–2851
Jansen MAK, Mattoo AK, Edelmann M (2001) D1-D2 protein degradation in the chloroplast. Eur J Biochem 260:527–532
Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ (2009) Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol 150:272–280
Johnson MP, Pérez-Bueno M, Zia A, Horton P, Ruban AV (2009) The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol 149:1061–1075
Kavi Kishore PB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Kennedy BF, De Fillippis LF (1999) Physiological and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and the salt sensitive Grevillea arenaria. J Plant Physiol 155:746–754
Keren N, Berg A, Van Kan PJM, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kerpesi I, Galiba G (2000) Osmotic and salt stress induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40:482–487
Khan MA, Ungar IA, Showalter AM, Dewald HD (1998) NaCl-induced accumulation of glycinebetaine in four subtropical halophytes from Pakistan. Physiol Plant 102:487–492
Kirschbaum MUF (2004) Direct and indirect climate change effects on photosynthesis and transpiration. Plant Biol 6:242–253
Koca H, Bor M, Özdemir F, Türkan I (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Koyro H-W (2000) Untersuchungen zur Anpassung der Wildrübe (Beta vulgaris ssp. maritima) an Trockenstreß oder NaCl-Salinität. Habilitation thesis, Justus Liebig University Giessen
Koyro H-W, Huchzermeyer B (1999) Salt and drought stress effects on metabolic regulation in maize. In: Pessarakli M (ed) Handbook of plant and crop stress, 2nd edn. Marcel Dekker, New York, pp 843–878
Koyro H-W, Huchzermeyer B (2004) Ecophysiological needs of the potential biomass crop Spartina townsendii GROV. Trop Ecol 45:123–139
Koyro H-W, Huchzermeyer B (2005) Recent developments in stress tolerance breeding in maize. In: Ashraf M, Harris PJC (eds) Abiotic stresses: plant resistance through breeding and molecular approaches. Food Products Press, the Haworth Press, New York, pp 545–576
Koyro H-W, Geißler N, Hussin S, Debez A, Huchzermeyer B (2008) Strategies of halophytes to survive in a salty environment. In: Khan NA, Singh S (eds) Abiotic stress and plant responses. I. K. International Publishing House, New Delhi, pp 83–104
Kozaki A, Takebe G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Kuhn M, Böger P (1990) Studies on the light-induced loss of the D1 protein in photosystem-II membrane fragments. Photosynth Res 23:291–296
Kurkova EB, Kalinkina LG, Baburina OK, Myasoedov NA, Naumova TG (2002) Responses of Seidlitzia rosmarinus to salt stress. Biol Bull 29:221–228
Laible PD, Zipfel W, Owens TG (1994) Excited state dynamics in chlorophyll-based antennae: the role of transfer equilibrium. Biophys J 66:844–860
Laisk A, Oja V (1974) Leaf photosynthesis in short pulses of CO2.The carboxylation reaction in vivo. Sov Plant Physiol 21:1123–1131
Lam E, Malkin R (1989) Lateral distribution and diffusion of plastocyanin in chloroplast thylakoids. J Cell Biol 108:1397–1405
Larcher W (2003) Physiological plant ecology, 4th edn. Springer, New York
Laszlo JA, Baker GM, Dilley RA (1984) Nonequilibration of membrane-associated protons with the internal aqueous space in dark-maintained chloroplast thylakoids. J Bioenerg Biomembr 1:37–51
Lawlor DW (2001) Photosynthesis. BIOS Scientific Publishers, Oxford
Lawlor DW (2002a) Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann Bot 89:871–885
Lawlor DW (2002b) Carbon and nitrogen assimilation in relation to yield: mechanisms are the keys to understanding production systems. J Exp Bot 53:773–787
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Lawlor DW, Fock H (1978) Photosynthesis, respiration and carbon assimilation in water-stressed maize at two oxygen concentrations. J Exp Bot 29:579–593
Lawlor DW, Khanna-Chopra R (1984) Regulation of photosynthesis during water stress. In: Sybesma C (ed) Advances in photosynthesis research, vol 4. Martinus-Nijhoff/Dr. W. Junk Publishers, The Hague, pp 379–382
Leakey ADB (2009) Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc Roy Soc Lond B 276:2333–2343
Leakey ADB, Uribelarrea M, Ainsworth EA (2006) Photosynthesis, productivity, and yield of maize are not affected by open air elevation of CO2 concentration in the absence of drought. Plant Physiol 140:779–790
Lee DH, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J Plant Physiol 158:737–745
Lee G, Carrow RN, Duncan RR (2004) Photosynthetic responses to salinity stress of halophytic seashore paspalum ecotypes. Plant Sci 166:1417–1425
Lee G, Carrow RN, Duncan RR, Eiteman MA, Rieger MW (2008) Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ Exp Bot 63:19–27
Lenssen GM, Lamers J, Stroetenga M, Rozema J (1993) Interactive effects of atmospheric CO2 enrichment, salinity and flooding on growth of C3 (Elymus athericus) and C4 (Spartina anglica) salt marsh species. Vegetatio 104(105):379–388
Li XP, Björlmann O, Shih C, Grossmann AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li JH, Dugas WA, Hymus GJ, Johnson DP, Hinkle CR, Drake BG, Hungate BA (2003) Direct and indirect effects of elevated CO2 on transpiration from Quercus myrtifolia in a scrub-oak ecosystem. Glob Change Biol 9:96–105
Li XP, Gilmore AM, Caffari S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Lillo C (2004) Light regulation of nitrate uptake, assimilation and metabolism. Plant Ecophysiol 3:149–184
Löhr A, Huchzermeyer B (1985) Regulation of photosynthetic efficiency by some component of chloroplast electron transport chain. ICSU Short Rep 3:332–333
Löhr A, Willms I, Huchzermeyer B (1985) A regulatory effect of the electron transport chain on the ATP synthase. Arch Biochem Biophys 236:832–840
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Lopes MS, Araus JL, van Heerden PDR, Foyer CH (2011) Enhancing drought tolerance in C4 crops. J Exp Bot 62:3135–3153
Lu CM, Zhang JH (1999) Effects of salt stress on PSII function and photoinhibition in the cyanobacterium Spirulina platensis. J Plant Physiol 155:740–745
Lu C, Qiu N, Lu Q, Wang B, Kuang T (2002) Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in the halophyte Suaeda salsa grown outdoors? Plant Sci 163:1063–1068
Lu Q, Wen X, Lu C, Zhang Q, Kuang T (2003) Photoinhibition and photoprotection in senescent leaves of field-grown wheat plants. Plant Physiol Biochem 41:749–754
Lüttge U, Kluge M, Thiel G (2010) Botanik. Die umfassende Biologie der Pflanzen. Wiley-VHC, Weinheim
Lynch J, Thiel G, Läuchli A (1988) Effects of salinity on the extensibility and Ca availability in the expanding region of growing barley leaves. Bot Acta 101:355–361
Ma F, Chen X-B, Sang M, Wang P, Zhang J-P, Li L-B, Kuang T-Y (2009) Singlet oxygen formation and chlorophyll a triplet excited state deactivation in cytochrome b6f complex from Bryopsis corticulans. Photosynth Res 100:19–28
Malkin S, Braun G (1993) The degree of functional separation between the two photosystems in isolated thylakoid membranes deduced from inhibition studies of the imbalance in photoactivities. Photosynth Res 36:89–94
Marabottini R, Schraml C, Paolacci AR, Sorgona A, Raschi A, Rennenberg H, Badiani M (2001) Foliar antioxidant status of adult Mediterranean oak species (Quercus ilex L. and Q. pubescens Willd.) exposed to permanent CO2-enrichment and to seasonal water stress. Environ Pollut 113:413–423
Marchi S, Tognetti R, Vaccari FP, Lanini M, Kaligarić M, Miglietta F, Raschi A (2004) Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Funct Plant Biol 31:181–194
Mateos-Naranjo E, Redondo-Gómez S, Andrades-Moreno L, Davy AJ (2010) Growth and photosynthetic responses of the cordgrass Spartina maritima to CO2 enrichment and salinity. Chemosphere 81:725–731
Matsubara S, Gilmore AM, Osmond CB (2001) Diurnal and acclimatory responses of violaxanthin and lutein epoxide in the Australian Mistletoe Amyema miquelii. Aust J Plant Physiol 28:793–800
Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammoniumnitrate. Plant Cell Environ 24:1119–1137
Medrano H, Parry MAJ, Socias X, Lawlor DW (1997) Long term water stress inactivates Rubisco in subterranean clover. Ann Appl Biol 131:491–501
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: desertification Synthesis. World Resources Institute, Washington, DC, http://www.maweb.org/documents/document.355.aspx.pdf
Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312:264–266
Mitchell P (1967) Proton current flow in mitochondrial systems. Nature 214:1327–1328
Mitsuya S, Kawasaki M, Taniguchi M, Miyake H (2003) Relationship between salinity-induced damages and aging in rice leaf tissues. Plant Prod Sci 6:213–218
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Mittova V, Tal M, Volokita M, Guy M (2002) Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt-tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiol Plant 115:393–400
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Moorthy P, Kathiresan K (1999) Effects of UV-B radiation on photosynthesis reactions in Rhizophora apiculata. Plant Growth Regul 28:49–54
Morgan JA, Lecain DR, Mosier AR, Milchunas DG (2001) Elevated CO2 enhances water relations and productivity and affects gas exchange in C3 and C4 grasses of the Colorado shortgrass steppe. Glob Change Biol 7:451–466
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Gardner PA, Tonnet ML, Rawson HM (1989) Growth and development in NaCl-treated plants. II Do Na+ or Cl− concentrations in dividing or expanding tissues determine growth in barley. Aust J Plant Physiol 15:529–540
Munns R, Husain S, Rivelli AR, James RA, Condon AG, Lindsay MP, Lagudah ES, Schachtman DP, Hare RA (2002) Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil 247:93–105
Murillo-Amador B, Jones HG, Kaya C, Aquilar RL, Garcia-Hermández JL, Toyo-Diéguez E, Àvilla-Serrano NY, Rueda-Puente E (2006) Effects of foliar application of calcium nitrate on growth and physiological attributes of cowpea (Vigna unguiculata L. Walp.) grown under salt stress. Environ Exp Bot 58:188–196
Neves-Piestun BG, Bernstein N (2001) Salinity-induced inhibition of leaf elongation in maize is not mediated by changes in cell wall acidification capacity. Plant Physiol 125:419–1428
Niessen M, Thiruveedhi K, Rosenkranz R, Kebeish R, Hirsch H-J, Kreuzaler F, Peterhänsel C (2007) Mitochondrial glycolate oxidation contributes to photorespiration in higher plants. J Exp Bot 58:2709–2715
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Mol Biol 49:249–272
North GB, Nobel PS (1991) Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave desertii, Agavaceae. Am J Bot 78:906–915
Nublat A, Desplans J, Cassea F, Berthomieua P (2001) sas1, an Arabidopsis mutant overaccumulating sodium in the shoot, shows deficiency in the control of the root radial transport of sodium. Plant Cell 13:125–137
Oksanen E, Sober S, Karnosky DF (2001) Impacts of elevated CO2 and/or O3 on leaf ultrastructure of aspen (Populus tremuloides) and birch (Betula papyrifera) in the Aspen FACE experiment. Environ Pollut 115:437–446
Oksanen E, Riikonen J, Kaakinen S, Holopainen T, Vapaavuori E (2005) Structural characteristics and chemical composition of birch (Betula pendula) leaves are modified by increasing CO2 and ozone. Glob Change Biol 11:732–748
Orthen B, Popp M, Smirnoff N (1994) Hydroxyl radical scavenging properties of cyclitols. Proc R Soc Edinb Sect B 102:269–272
Osmond CB, Bagder M, Maxwell K, Björkman O, Leegood R (1997) Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci 2:119–120
Pahlich E, Kerres R, Jäger H-J (1983) Influence of water stress on the vacuole/extravacuole distribution of proline in protoplasts of Nicotiana rustica. Plant Physiol 72:590–591
Paramanova NV, Shevyakova NI, VlV K (2004) Ultrastructure of chloroplasts and their storage inclusions in the primary leaves of Mesembryanthemum crystallinum affected by putrescine and NaCl. Russ J Plant Physiol 51:86–96
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Saf 60:324–349
Parida A, Das AB, Das P (2002) NaCl stress causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45:28–36
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835
Parry MAJ, Andolojc JP, Khan S, Lea PJ, Keys AJ (2002) Rubisco activity: effects of drought stress. Ann Bot 89:833–839
Paul MJ, Knight JS, Habash D, Parry MAJ, Lawlor DW, Barnes SA, Loynes A, Gray JC (1995) Reduction in phosphoribulokinase activity by antisense RNA in transgenic tobacco: effects on CO2 assimilation and growth in low irradiance. Plant J 7:535–542
Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Muňoz-Rueda A, Navari-Izzo F, Mena-Petite A (2009) The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant 135:29–42
Petrouleas V, Deligiannakis Y, Diner BA (1994) Binding of carboxylate anions at the non-heme Fe(II) of PSII. Competition with bicarbonate and effects on the QA/QB electron transfer rate. Biochim Biophys Acta 1188:271–277
Pitschke A, Hirt H (2009) Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol 149:606–615
Polle A (1996) Protection from oxidative stress in trees as affected by elevated CO2 and environmental stress. In: Koch G, Mooney H (eds) Terrestrial ecosystem response to elevated CO2. Academic, New York, pp 299–315
Polley HW, Johnson HB, Mayeux HS, Brown DA, White JWC (1996) Leaf and plant water use efficiency of C4 species grown at glacial to elevated CO2 concentrations. Int J Plant Sci 157:164–170
Popp M, Larther F, Weigel P (1985) Osmotic adaptation in Australian mangroves. Vegetation 61:247–254
Pursiheimo S, Mulo P, Rintamäki E, Aro E-M (2001) Coregulation of light-harvesting complex II phosphorylation and lhcb mRNA accumulation in winter rye. Plant J 26:317–327
Rahman MS, Miyake H, Takeoka Y (2002) Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.). Plant Prod Sci 5:33–44
Rees D, Noctor GD, Horton P (1990) The effect of high-energy-state excitation quenching on maximum and dark level chlorophyll fluorescence yield. Photosynth Res 25:199–211
Richter ML, Hein R, Huchzermeyer B (2000) Important subunit interactions in chloroplast ATP synthase. Biochim Biophys Acta 1458:326–342
Riesmeier JW, Flügge U-I, Schulz B, Heineke D, Heldt H-W, Willmitzer L, Frommer WB (1993) Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA 90:6160–6164
Robinson SP, Portis AR (1988) Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact chloroplasts. Plant Physiol 86:293–298
Robredo A, Pérez-López U, de la Maza HS, González-Moro B, Lacuesta M, Mena-Petite A, Muňos-Rueda A (2007) Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ Exp Bot 59:252–263
Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Dohleman FG, Heaton EA, Mahoney J, Zhu XG, Delucia EH, Ort DR, Long SP (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ 27:449–458
Romanello GA, Chuchra-Zbytniuk KL, Vandermer JL, Touchette BW (2008) Morphological adjustments promote drought avoidance in the wetland plant Acorus americanus. Aquat Bot 89:390–396
Romanowska E, Albertsson P-Å (1994) Isolation and characterization of the cytochrome bf complex from whole thylakoids, grana, and stroma lamellae vesicles from spinach chloroplasts. Plant Cell Physiol 35:557–568
Rozema J (1993) Plant responses to atmospheric carbon dioxide enrichment: interactions with some soil and atmospheric conditions. Vegetation 104(105):173–190
Rozema J, Dorel F, Janissen R, Lenssen G, Broekman R, Arp W, Drake BG (1991) Effect of elevated atmospheric CO2 on growth, photosynthesis and water relations of salt marsh grass species. Aquat Bot 39:45–55
Ruban AV, Young AJ, Horton P (1993) Induction of non-photochemical energy dissipation and absorbance changes in leaves. Evidence for changes in the state of the light-harvesting system of photosystem II in vivo. Plant Physiol 102:741–750
Salvucci ME, Ogren WL (1996) The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res 47:1–11
Schmidt A, Jäger K (1992) Open questions about sulfur metabolism in plants. Annu Rev Plant Physiol Mol Biol 43:325–349
Schmidt CL, Malkin R (1993) Low molecular weight subunits associated with the cytochrome b6f complexes from spinach and Chlamydomonas reinhardtii. Photosynth Res 38:73–81
Schreiber U (1997) Chlorophyll fluorescence and photosynthetic energy conversion: simple introductory experiments with the teaching-PAM chlorophyll fluorometer. H. Walz GmbH, Effeltrich
Schreiber U, Gademann R, Bird P, Ralph PJ, Larkum AWD, Kühl M (2002) Apparent light requirement for activation of photosynthesis upon rehydration of desiccated beachrock microbial mats. J Phycol 38:125–134
Schultz G, Huchzermeyer Y, Reupke B, Bickel H (1976) On the intra cellular site of biosynthesis of alpha tocopherol in Hordeum-Vulgare. Phytochemistry (Oxford) 15:1383–1386
Schwanz P, Polle A (2001) Differential stress responses of antioxidative systems to drought in pendunculate oak (Quercus robur) and maritime pine (Pinus pinaster) grown under high CO2 concentrations. J Exp Bot 52:133–143
Schwanz P, Picon C, Vivin P, Dreyer D, Guehl J, Polle A (1996) Responses of antioxidative systems to drought stress in pendunculate oak and maritime pine as modulated by elevated CO2. Plant Physiol 110:393–402
Schwarz M, Gale J (1984) Growth responses to salinity at high levels of carbon dioxide. J Exp Bot 35:193–196
Sgherri CLM, Salvateci P, Menconi M, Raschi A, Navari-Izzo F (2000) Interaction between drought and elevated CO2 in the response of alfalfa plants to oxidative stress. J Plant Physiol 156:360–366
Sheen J (2002) Phosphorelay and transcription control in cytokinin signal transduction. Science 296:1650–1652
Singh SK, Sharma HC, Goswami AM, Datta SP, Singh SP (2000) In vitro growth and leaf composition of grapevine cultivars as affected by sodium chloride. Biol Plant 43:283–286
Soussi M, Lluch C, Ocana A (1998) Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick pea (Cicer arietinum L.). J Exp Bot 49:1329–1337
Spychalla JP, Desborough SL (1990) Superoxide dismutase, catalase, and alpha-tocopherol content of stored potato tubers. Plant Physiol 94:1214–1218
Staehelin LA (1975) Chloroplast membrane structure. Intramembrane particles of different sizes make contact in stacked membrane regions. Biochim Biophys Acta 408:1–11
Stepien P, Klobus G (2005) Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 125:31–40
Strasser RJ, Tsimilli-Michael M, Srivaslava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll Fluorescence: a signature of photosynthesis. Kluwer, Amsterdam, pp 1–42
Streenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of fox-tail millet (Setaria italica). Physiol Plant 109:435–442
Strotmann H, Bickel S, Huchzermeyer B (1976) Energy-dependent release of adenine nucleotides tightly bound to chloroplast coupling factor CF1. FEBS Lett 61:194–198
Süss K-H, Arkona C, Manteuffel R, Adler K (1993) Calvin cycle multienzyme complexes are bound to chloroplast thylakoid membranes of higher plants in situ. Proc Natl Acad Sci USA 90:5514–5518
Syvertsen JP, Boman B, Tucker DPH (1989) Salinity in Florida citrus production. Proc Fla State Hort Soc 102:61–64
Taiz L (1992) The plant vacuole. J Exp Biol 172:113–122
Takagi M, El-Shemy H, Sasaki S, Toyama S, Kanai S, Saneoka H, Fujita K (2009) Elevated CO2 concentration alleviates salinity stress in tomato plant. Acta Agr Scand B-S P 59:87–96
Tanji KK (2002) Salinity in the soil environment. In: Läuchli A, Lüttge U (eds) Salinity: environment – plants – molecules. Kluwer, Dordrecht, pp 21–51
Tezara W, Mitchell VJ, Driscoll SP, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Thomas SC (2005) Increased leaf reflectance in tropical trees under elevated CO2. Glob Change Biol 11:197–202
Tingey DT, McKane RB, Olszyk DM, Johnson MG, Rygiewicz PT, Lee H (2003) Elevated CO2 and temperature alter nitrogen allocation in Douglas-fir. Glob Change Biol 9:1038–1050
Tipping C, Murray DR (1999) Effects of elevated atmospheric CO2 concentration on leaf anatomy and morphology in Panicum species representing different photosynthetic modes. Int J Plant Sci 160:1063–1073
Touchette BW, Smith GA, Rhodes KL, Poole M (2009) Tolerance and avoidance: two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. J Exp Mar Biol Ecol 380:106–112
Trebst A, Soll-Bracht E (1996) Cycloheximids retards high light driven D1 protein degradation in Chlamydomonas rheinhardtii. Plant Sci 115:191–197
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Urban O (2003) Physiological impacts of elevated CO2 concentration ranging from molecular to whole plant responses. Photosynthetica 41:9–20
van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
van Breusegem F, Bailey-Serves J, Miller R (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 147:978–984
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol 162:669–677
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. CSIRO Publishing, Collingwood
Wand SJE, Midgley GF, Jones MH, Curtis PS (1999) Response of wild C4 and C3 grass (Poaceae) to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Glob Change Biol 5:723–741
Wang Y, Nil N (2000) Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J Hort Sci Biotechnol 75:623–627
Wang XW, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wang S, Assmann SM, Fedoroff NV (2008a) Characterization of the Arabidopsis heterotrimeric G protein. J Biol Chem 283:13913–13922
Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, Zhang S (2008b) Haplo-isuffiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 20:602–613
Wang D, Heckathorn SA, Wang X, Philpott SM (2012) A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169:1–13
Ward JK, Tissue DT, Thomas RB, Strain BR (1999) Comparative responses of model C3 and C4 plants in low and elevated CO2. Glob Change Biol 5:857–867
Wilken M, Huchzermeyer B (1999) Suppression of mycelia formation by NO produced endogenously in Candida tropicalis. Eur J Cell Biol 78:209–213
Wingler A, Lea PJ, Quick P, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355:1517–1529
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and endogenous antioxidants. Plant Physiol 83:278–282
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2 – implications for water-limited environments. Plant Cell Environ 25:319–331
Yancey P, Clark ME, Had SC, Bowlus RD, Somero GN (1982) Living with the water stress: evolution of osmolyte system. Science 217:1214–1222
Yue B, Xue W, Xiong L, Yu X, Luo L, Cui K, Jin D, Xing Y, Zhang Q (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172:1213–1228
Zelitch I (1973) Alternate pathways of glycolate synthesis in tobacco and maize and its relation to rates of photorespiration. Plant Physiol 51:299–305
Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP (2009) High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol 149:195–204
Zhen A, Bie Z, Huang Y, Liu Z, Lei B (2011) Effects of salt-tolerant rootstock grafting on ultrastructure, photosynthetic capacity, and H2O2-scavenging system in chloroplasts of cucumber seedlings under NaCl stress. Acta Physiol Plant 33:2311–2319
Zhifang G, Loescher WH (2003) Expression of a celery mannose 6-phosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and a glucosyl-mannitol dimer. Plant Cell Environ 26:275–283
Zhu Z, Zhang R, Liu T, Zheng H (2011) Solute accumulation and osmotic adjustment characteristics of the mangrove Avicennia marina under NaCl-induced salinity stress. Bot Mar 54:335–341
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Geissler, N., Huchzermeyer, B., Koyro, HW. (2013). Effects of Salt Stress on Photosynthesis Under Ambient and Elevated Atmospheric CO2 Concentration. In: Ahmad, P., Azooz, M.M., Prasad, M.N.V. (eds) Salt Stress in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6108-1_15
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6108-1_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6107-4
Online ISBN: 978-1-4614-6108-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)