Abstract
The aim of this study was to evaluate the effects of elevated CO2 concentration on acclimation mechanisms related to gas exchange, photochemical activity, photorespiration, and oxidative protection in cashew plants exposed to salinity. Thirty-day-old cashew plants were irrigated with nutrient solution without (control) or with supplemental NaCl (100 mM) for 2 weeks in the greenhouse. Afterward, control and salt-stressed plants were transferred to the growth chamber and supplied with atmospheric (380 µmol mol−1) or high CO2 (760 µmol mol−1) concentrations for 15 days. The results show that elevated CO2 alone reduced the CO2 net assimilation rate (PN) without affecting stomatal conductance (gS) and transpiration rate (E), whereas salinity and NaCl + high CO2 reduced the PN associated with a decrease in gS and E. The potential quantum yield of photosystem II (Fv/Fm) was not altered, but a slight reduction in electron transport rate and photochemical quenching (qP) in response to high CO2 alone or combined with NaCl occurred. However, non-photochemical quenching increased due to the effects of high CO2 and NaCl alone and by their combination. High CO2 alleviated the toxic effects of Na+ favoring the K+/Na+ ratio under salinity. High CO2 coupled with salinity decreased glycolate oxidase activity and the contents of hydrogen peroxide (H2O2), NH4+, and glyoxylate. Furthermore, we observed increase in membrane damage associated with increased thiobarbituric acid-reactive substances levels under high CO2. High CO2 also decreased ascorbate peroxidase activity, but did not affect superoxide dismutase activity. In general, our data suggest that high CO2 could induce acclimation processes in plants independent of salinity, revealing a set of responses that are more associated with acclimation than with protective responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated CO2 acclimation processes in plants are not well understood, especially when associated with other adverse environmental factors such as salinity. Under abiotic stress, such as water deficit and salinity, down-regulation of photosynthesis can occur due to reduced carbon substrate (Golldack et al. 2014). Decreases in CO2 diffusion in the leaf mesophyll have been studied and are responsible for inducing important photosynthesis alterations. Following salinity-induced stomatal closure and a decrease in CO2 pressure in the leaf mesophyll, de-activation of Rubisco carboxylase has been observed, indicating a metabolic limitation of CO2 assimilation under these conditions. Thus, continuous CO2 supply for the carboxylation of Rubisco is required for maintaining photosynthesis at normal conditions and especially under stress as salinity (Xu et al. 2015).
Several experimental evidences have shown that CO2 enrichment in atmosphere, especially throughout long term, can trigger an increase in photosynthesis in plants exposed to salt stress (Geissler et al. 2015; Pérez-López et al. 2012, 2014). These effects of elevated CO2 pressure on increased photosynthetic activity (“photosynthetic acclimation”) are attributed mainly to high CO2 availability for carboxylation reactions in the chloroplast stroma (Xu et al. 2015). However, beneficial effects of high atmospheric CO2 on the photosynthesis in plants subjected to salt stress are still controversial. In fact, while some studies have reported beneficial effects of high CO2 pressure on carbon assimilation in plants under salinity (Geissler et al. 2015; Yu et al. 2015; Pérez-López et al. 2014; Yi et al. 2015), others have reported low (Ball et al. 1997) or even no effects on photosynthetic activity (Bowman and Strain 1987).
These conflicting results regarding the photosynthetic responses of plants exposed to high CO2 pressure are dependent on species and exposure time to elevated CO2 as well as the development stage of the plants exposed to this condition (Leakey et al. 2006). Short-term exposure (days, weeks) to high CO2 pressure can positively affect plant metabolism, but under increased exposure time, these beneficial effects can be lost (Darbah et al. 2010). Besides, the photosynthesis responses to high CO2 are dependent on type of carbon dioxide exposure such as closed growth chamber, “open top” in greenhouse, and in the field by FACE system. Indeed, plant acclimation and physiological responses will be dependent on all these conditions.
Photosynthetic restriction under salt stress conditions may be associated with an increase in the NADPH/NADP ratio in the chloroplast stroma due to a decrease in the reducing equivalent consumption by the Calvin cycle. In addition to the excess energy produced due to lower CO2 pressure in photosynthetic cells under salinity, an increase in the photorespiratory process can occur due to a lower CO2/O2 ratio in the leaf and the stimulation of the Rubisco oxygenase activity (Xu et al. 2015). The photorespiratory pathway in C3 plants has, as the main role, the recovery of the carbon lost by the Rubisco oxygenase activity. However, this process represents a loss of approximately 25% of the CO2 fixed during photosynthesis in C3 plants under normal environmental condition. Moreover, this response might be increased in plants submitted to abiotic stress, as salinity (Foyer et al. 2009).
In addition to the carbon loss under stress conditions associated with photorespiration, this process represents the main pathway responsible to produce hydrogen peroxide (H2O2) in the peroxisomes of plant cells (Foyer and Noctor 2003). On the other hand, superoxide radicals (O ·−2 ) are produced by photosynthesis by the direct electron transfer to oxygen, producing H2O2 through the Mehler reaction. However, this reaction consumes less than 10% of the photosynthetic electron flow (Heber 2002). The glycolate oxidase (GO) reaction, which generates glyoxylate in the peroxisomes, can produce similar H2O2 amounts (Foyer et al. 2009). Thus, the increase in CO2 pressure under restrictive conditions to photosynthesis, such as salinity, might reduce the photorespiratory activity and attenuate oxidative damage due to less H2O2 generation (Geissler et al. 2015).
Photorespiration and the photosynthetic metabolism are major sources of reactive oxygen species (ROS). These excess ROS can cause oxidative damage to proteins, lipids, and nucleic acids, which can lead to cell death. To avoid oxidative damage, plant cells have developed a complex array of enzymatic and non-enzymatic antioxidants. Catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX) are the main enzymatic antioxidants. SOD is an important enzyme avoiding ROS accumulation; this enzyme is present in various cell organelles scavenging O ·−2 and generating H2O2 (Sharma et al. 2012). Together, these antioxidants can avert damage and promote efficient cell protection, constituting a set of responses that is species and genotype dependent.
Cashew plants (Anacardium occidentale) are commonly cultivated in Brazil, primarily in the semi-arid coastal regions. This species is an important crop in Brazil, India, and East Africa due to their economical importance (Ferreira-Silva et al. 2010). The two main commercial products are the nuts and the juice extracted from the pseudo-fruit, generating a productive chain responsible for the generation of employment and income. As a wild species, it has evolved in environments with combined stresses promoting specific development related with acclimation to these conditions, as salinity, drought, and high temperature (Silveira et al. 2003; Ferreira-Silva et al. 2011). Recently, our group showed that high temperature was essential to increase the oxidative protection under salinity in cashew leaves (Ferreira-Silva et al. 2011). The data suggest that these plants were able to trigger efficient physiological mechanisms to acclimate to combined stress conditions. These protective mechanisms are related with the modulation of enzymatic and non-enzymatic antioxidants.
Some studies have demonstrated the harmful effects of salinity and high CO2 on plant metabolism disturbances, especially photosynthesis. However, to date, none work has been reported regarding integrative studies involving the combined effects of these factors (salinity + high CO2 concentration) under short-term exposure in cashew plants. This species, although a tropical fruit crop widely exploited in semi-arid regions, still needs further studies related to the impacts of abiotic factors on its production performance. In this study, we aimed to evaluate the acclimation mechanisms related to the processes of gas exchange, photochemical activity, photorespiration, and oxidative protection in cashew plants exposed to high CO2 and salinity.
Materials and methods
Plants material and treatments
Seeds of cashew (Anacardium occidentale L.) plants, genotype CCP 09, were provided by Empresa Basileira de Pesquisa Agropecuária (EMBRAPA), Ceará, Brazil. The seeds were sown in a 1:1 mixture of sand and vermiculite (v/v) in 3.0-l pots and irrigated daily with distilled water under greenhouse conditions. Afterward, the plants were watered every 2 days with 300 ml of nutrient solution (Hoagland and Arnon 1950) containing 3 mM Ca(NO3)2, 2 mM NH4Cl, 1 mM K2HPO4, 1 mM MgSO4, 6 mM KNO3, 100 μM Fe-EDTA, 40 μM HBO3, 9 μM MnCl2, 3 μM CuSO4, 7 μM ZnSO4, and 0.1 μM Na2MoO4, for 15 days. For salt acclimation, 30-day-old plants were irrigated every 2 days with 300 ml of full-strength nutrient solution without (0 mM) or with NaCl (100 mM) for 15 days. During the experiments in the greenhouse, the air temperature was between 25 and 35 °C. The relative humidity was approximately 65%. The maximum photosynthetic photon flux density (PPFD) was 1350 µmol m−2 s−1 with a photoperiod of 12 h.
For CO2 treatments, plants were placed in a growth chamber, with controlled conditions (photoperiod of 12/12 h light/dark; day temperature of 28 ± 2 °C; night temperature of 25 ± 2 °C; 65 ± 5% of relative humidity; and PPFD of 400 µmol m−2 s−1). In these conditions, plants exposed to salinity or without salt stress were exposed to ambient atmospheric (380 µmol mol−1) or high (760 µmol mol−1) CO2 concentration for 15 days. Both CO2 treatments were applied using the same growth chamber. In addition, using the same growth chamber minimized the inter-chamber effect (Alonso et al. 2009). The environmental gradients effects inside the chamber were also minimized by the random repositioning of the pots inside the chamber every week.
The full salt treatment lasted 30 days: during the first 15 days, the plants were subjected only to salt stress in greenhouse, whereas for the latest 15 days, plants were exposed to CO2 treatments. The moderate luminosity (400 µmol m−2 s−1) was used to mitigate the effect of excess light on oxidative damage, isolating the effects of salinity on ROS generation. At the end of the experiment, gas exchange and chlorophyll fluorescence parameters were assessed in mature leaves, and leaf discs (10 mm diameter) were harvested for the estimation of membrane damage. Leaves were then frozen in liquid N2 and stored at − 80 °C for further analyses.
Gas exchanges and chlorophyll fluorescence analyses
Gas exchange and chlorophyll fluorescence parameters were measured with an IRGA with a coupled LED source on the leaf chamber (IRGA LI-6400XT, LI-COR, Lincoln, USA). Measurements of net photosynthesis (PN), transpiration rate (E), stomatal conductance (gS), and intercellular CO2 concentration (CI) were carried between 10:00 and 11:00 h, on the 6th leaf from the basis in the cashew plants at 60 days after planting. The instantaneous carboxylation efficiency (PN/CI ratio) was calculated. During gas exchange measurements, the PPFD was set to 1000 μmol m−2 s−1, the concentrations of CO2 as treatments (380 µmol mol−1 CO2 and 760 µmol mol−1 CO2, respectively), 1.0 ± 0.2 kPa VPD at 28 °C. To maximize the stomatal aperture, blue light was set to 10% of the PPFD (Flexas et al. 2007).
In vivo chlorophyll a fluorescence was measured using an LI-6400-40 fluorometer (LI-COR, Lincoln, NE, USA) coupled with the IRGA. The actinic light to measure both gas exchange and chlorophyll a fluorescence was set to 1000 μmol m−2 s−1 PPFD, a saturating light for cashew according to previous studies in our lab. The fluorescence parameters were measured by the saturation pulse method in leaves exposed to light and 30-min dark-adapted conditions (Klughammer and Schreiber 1994).
The intensity of the saturation light pulse was 8000 μmol m−2 s−1 and its duration of 0.7 s. The maximum quantum yield of photosystem II (PSII) was calculated as [Fv/Fm = (Fm − Fo)/Fm] and measured in dark-adapted condition. The effective quantum yield of PSII was calculated as [ΔF/Fm′ = (Fm′ − Fs)/Fm′] which was measured in leaves adapted to actinic light at 1000 μmol m−2 s−1 PPFD for at least 30 min. The photochemical quenching coefficient was calculated by [qP = (Fm′ − Fs)/(Fm′ − Fo′)], the non-photochemical quenching was calculated by [NPQ = (Fm − Fm′)/Fm′], and the electron flow at PSII was calculated as [ETR = (ΔF/Fm′ × PPFD × 0.5 × 0.84)].
To ETR calculation, 0.5 was used assuming the equal partition of the excitation energy fraction distributed to PSII and 0.84 as the fraction of absorbed light by the leaves. Fm and Fo are the maximum and minimum fluorescence in dark-adapted leaves, respectively; Fm′ and Fs are the maximum and steady state fluorescence in leaves adapted to light and Fo′ is the minimum fluorescence after far-red illumination. All measurements were assessed in plants exposed to 30 days of salinity, as 15 days only exposed to salinity (0 or 100 mmol l−1 NaCl) and the other 15 days exposed to salt-stress coupled to ambient (380 µmol mol−1) or high (760 µmol mol−1) concentration of CO2.
Na+ and K+ concentration, K+/Na+ ratio, chlorophyll, carotenoids, and anthocyanin contents in leaves
For extraction of Na+ and K+ ions, the leaves were initially lyophilized and dry samples (200 mg) were transferred to tubes with deionized water and heated in a 100 °C water bath for 1 h. The extracts were filtered and used for Na+ and K+ determination by flame photometry (Micronal B462) as described by Silva et al. (2010). Chlorophylls and carotenoids contents were extracted in acetone (80%) and read with a spectrophotometer (biochrom Libra S60) at 665 and 649 nm (Lichtenthaler and Wellburn 1983). The anthocyanin contents were measured in accordance with Lees and Francis (1972).
NH3, N total, total free amino acids, and total soluble sugar content
Dry leaf samples were extracted in deionized water in closed tubes and heated in a water bath at 100 °C for 1 h. The concentration of ammonium ion (NH4+) and total nitrogen in leaves was performed according to Silveira et al. (2003). In addition, the total free amino acids, sucrose, and soluble sugar contents were determinated as previously described by Silva et al. (2010).
Electrolyte leakage (EL), hydrogen peroxide, glyoxylate content, and lipid peroxidation
The membrane damage, an indicator of cellular integrity, was estimated by the electrolyte leakage (Silva et al. 2015). Twenty leaf discs were extracted in 10 ml of deionized water and incubated at 25 °C in a water bath for 6 h. After that the electrical conductivity (L1) was measured. The samples were boiled at 100 °C for 1 h, and after cooling to 25 °C, a second measurement (L2) was obtained. The electrolyte leakage (EL) was calculated as: EL (%) = (L1/L2) × 100.
The hydrogen peroxide content was measured according to Cheeseman (2006). Samples of fresh leaves (0.1 g) were powdered in liquid nitrogen and were extracted with 100 mM potassium phosphate buffer (pH 6.4) with 5 mM KCN. The reaction was assessed for 30 min at 25 °C and the absorbance was read at 560 nm. A standard curve was performed and the H2O2 concentration was expressed in µmol (g FW)−1.
Lipid peroxidation was determined by measuring the content of the thiobarbituric acid-reactive substances (TBARS) in accordance with the Heath and Packer (1968). Leaf samples (0.1 g) were powdered in liquid N2 nitrogen and were homogenized with 1 ml of 6% (w/v) trichloroacetic acid (TCA). The samples were centrifuged at 12,000×g for 15 min at 4 °C. Aliquots of the supernatant were mixed with 2 ml of 20% (w/v) TCA with 0.5% (w/v) thiobarbituric acid (TBA). The mixture was heated for 30 min at 95 °C in tubes and then quickly cooled in an ice bath. The absorbance was read at 532 and 660 nm. The complex MDA-TBA was calculated using the molar extinction coefficient of 155 mM−1.
To determinate the glyoxylate content, samples of frozen leaves (0.1 g) were powdered in 1 ml of 100 mM HCl as described by Baker and Tolbert (1966). The extract was centrifuged at 12,000 rpm for 10 min at 8 °C, and the supernatant was used to determinate the glyoxylate content. The reaction was started by adding 200 µl of extract, 300 µl of 1% (v/v) phenylhydrazine in 100 mM HCl. The mixture was heated in water bath at 95 °C for 2 min. The reaction was stopped in ice bath and the absorbance was read at 324 nm. The glyoxylate content was calculated using the molar extinction coefficient of the glyoxylate–phenylhydrazine complex (17 mM−1 cm−1) and expressed as µmol g−1 FW.
Protein extraction and activity determination
Frozen leaf samples (0.1 g) were powdered in the presence of liquid N2 in a mortar and pestle and extracted in 100 mM Tris–HCl buffer (pH 8.0) with 30 mM DTT, 20% (v/v) glycerol (Ferreira-Silva et al. 2011). The pH of the buffer was adjusted to 7.0 for (SOD) and APX extraction and 1 mM of ascorbate was added. The protein content was measured by Bradford (1976) with bovine serum albumin (BSA) as standard.
The activity of ascorbate peroxidase (APX; EC: 1.11.1.11) was assayed by the addition of the leaf tissue extract (described above) a 0.1 ml of 30 mM H2O2. The absorbance was monitored for 300 s at 290 nm (Nakano and Asada 1981). APX activity was calculated by the molar extinction coefficient of ascorbate (2.8 mM−1 cm−1) and expressed as µmol ASA g−1 FW min−1.
The activity superoxide dismutase (SOD; EC: 1.15.1.1) was determined by the addition of 50 µL of the leaf extract, 13 mM l-methionine, 75 mM p-nitro blue tetrazolium chloride (NBT), 2 mM riboflavin, and 100 mM EDTA into 50 mM potassium phosphate buffer, pH 7.8. The reaction was illuminated (30 W fluorescent lamp) at 25 °C (Gianopolitics and Ries 1977). SOD activity unit was expressed as AU g−1 FW min−1. An AU was defined as the amount of enzyme required to inhibit 50% NBT photoreduction.
The glycolate oxidase activity (GO; EC: 1.1.3.15) was assayed by the formation of the complex glyoxylate–phenylhydrazone read at 324 nm (Baker and Tolbert 1966). The GO assay mixture was a 100 mM phosphate buffer (pH 8.3), 100 mM l-cysteine, 40 mM glycolic acid, and 100 mM phenylhydrazine. The reaction started by adding FMN 1 mM. The GO activity was calculated by the molar extinction coefficient of the glyoxylate–phenylhydrazone complex (17 mM−1 cm−1) and expressed as µmol glycolate g−1 FW min−1.
The glutamine synthetase (GS; EC: 6.3.1.2) was extracted in a 100 mM potassium phosphate buffer pH 7.4, 1 mM EDTA. The GS activity was assessed by the addition of 500 µl of the enzymatic extract to a solution containing Tris–HCl 0.25 M buffer pH 7.0, sodium glutamate 0.3 M, ATP 30 mM, and MgSO4 0.5 M. Hydroxylamine solution (1:1) was used at substrate and the γ-glutamyl hydroxamate (GGH), following method of Elliott (1953). The activity glutamine synthetase isoenzymes (GS2) were measured from extracts obtained under the same conditions activity GS described to increase total glucosamine-6-phosphate 1 mM. The GS2 activity is sensitive to feedback inhibition by amino acids or glucosamine-6-phosphate, and then being inhibited in the reaction. The readings at 540 nm provided activity of GS1, and subsequently calculated the activity of GS2 by the difference between GS activity and GS1.
Experimental design and data analysis
The experiment was performed in a completely randomized design. Here, the effects of salinity (0 and 100 mM) and CO2 concentration (380 and 760 μmol mol−1) were investigated. Data were subjected to ANOVA procedures and the mean values (four replicates) were compared using Tukey’s teste at a confidence level of 0.05. The standard deviation was plotted in all tables and figures. In addition, the data were analyzed in a factorial arrangement involving two NaCl levels (0 and 100 mM) and two CO2 concentrations, for evaluating the interactive effects among the factors on the different variables.
Results and discussion
Changes in gas exchange and photochemical parameters in response to salinity followed by high and normal CO2 concentrations in cashew plants
To analyze the potential protective role of high CO2 on disturbances caused by salt stress on gas exchange and photochemical activity, cashew plants were initially grown in the absence (0 mM) or presence of salinity (100 mM NaCl) for 15 days. Afterward, the plants were exposed to ambient (380 µmol mol−1) or high (760 µmol mol−1) CO2 concentrations for 15 days more under salinity. The data show that gas exchange measurements and chlorophyll a fluorescence parameters were differentially affected by salinity and high CO2 treatments (Tables 1, 2). Plants exposed to isolated high CO2 showed a reduction in leaf CO2 net assimilation rate (PN) compared with reference plants (0 mM NaCl + 380 µmol mol−1). In contrast, isolated salinity or in combination with high CO2 induced a higher reduction (by 65%) in photosynthesis, demonstrated by a significant interactive effect between these two factors (Table 4), and this effect was associated with restrictions in gS (Table 1).
The intercellular CO2 concentration (CI) was significantly higher (~ 210%) in plants subjected to high CO2 alone or in combination with NaCl than in reference plants. The instantaneous carboxylation efficiency (PN/CI) was significantly reduced in all the studied treatments, mostly under high CO2 concentrations alone as well as under high CO2 + NaCl conditions (Table 1). The gas exchange data showed that reduced CO2 assimilation under high CO2 was not attributed to stomatal closure, but under salinity, the stomatal limitation should be a determinant for the restriction of CO2 fixation in cashew. Moreover, the data indicate that high CO2 did not benefit PN under salinity conditions. These results suggest that PN restriction under high CO2 alone is not attributed to stomatal limitation and also indicates that salt stress may affect CO2 assimilation by both stomatal and non-stomatal factors.
The decrease in gS under high CO2 may limit the CO2 fixation, but it can lead to increase in water use efficiency, promoting plant growth under adverse conditions that limit stomatal opening (Xu et al. 2016). However, plant responses to high CO2 are relatively complex and still little characterized, and these effects are influenced by plant species, exposure time and interactions with other environmental factors. Stomata have a key function in controlling gas exchange through leaves to the atmosphere. Carbon dioxide can reach the carboxylation site of Rubisco after CO2 gas diffusion via boundary layer, stomata, and intercellular air spaces near to chloroplast (Aranjuelo et al. 2009).
In the present study, the CI of plants under high CO2 was much higher than the atmospheric CO2-treated plants under both saline and non-saline conditions. This suggests that non-stomatal limitations (e.g., photosystem II [PSII] activity and/or lower carboxylation efficiency) are the main cause of the reduced PN observed under high CO2 treatments. Together, these data indicate that both stomatal and non-stomatal limitations could occur under salinity and these responses indicate a potential negative interactive effect between high CO2 and salinity involving CO2 assimilation in cashew plants, possibly due to enhancement in stomatal closure as demonstrated by Souza et al. (2005).
It has been amply reported that elevated atmospheric CO2 should potentially benefit net CO2 assimilation under conditions of restrictive photosynthesis, such as water deficit and salinity, due to CO2 concentration in the leaf mesophyll (Yu et al. 2015; Xu et al. 2015). On the other hand, biochemical processes that inhibit carbon assimilation may be determining factors to limit CO2 fixation despite high carbon pressure near to the carboxylation sites. The obtained data in this current study suggest that despite the increased CI values, biochemical factors should be contributing to the reduction in carbon fixation. Although this parameter (PN/CI) is not conclusive regarding the presence of possible non-stomatal limitations, some authors have used this parameter (carboxylation efficiency) as an indicator to show that factors other than the stomata can reduce photosynthesis (Ribeiro et al. 2009).
C3 plants exposed to short-term high CO2 are unable to saturate Rubisco, which can lead to a transient increase in net photosynthesis (Aranjuelo et al. 2009). This increase in photosynthesis could be related with CO2 been the main substrate of photosynthesis reaction, and high CO2 can attenuate the Rubisco´s oxygenase. Due to that, high CO2 could alleviate photorespiration (Xu et al. 2015). However, longer exposure to high CO2 can result in an acclimation in some species. This acclimation in photosynthesis is characterized by decreases in gas exchange and photochemical activity (Long et al. 2004).
Although the initial increase in photosynthesis associated with high CO2 is occasionally retained during long-term exposure, the high rate is often reversed during the photosynthetic acclimation process (Aranjuelo et al. 2009). The down-regulation of photosynthesis by high CO2 is accompanied by changes in gas exchange that can indicate reduced carboxylation capacity (Ainsworth and Rogers 2007). Indeed, in the present study, exposure to high CO2 alone decreased the CO2 assimilation rate. This response in plants exposed to elevated CO2 may be attributed to carbohydrate production that could have exceeded the capacity to generate new sinks. It is common for plants to reduce their photosynthesis to adequate the balance in source-sink activity, which can be explained by a possible feedback regulatory mechanism due to excess sugars (Aranjuelo et al. 2009).
This hypothesis for the down-regulation of PN attributed to sugar accumulation is compatible with the results obtained here, since elevated CO2 induced an increase in soluble sugar content associated with reduced PN in both non-salinized and salinized plants (Table 1, Fig. 2). High CO2 can increase the rate of carboxylation of ribulose-1,5-bisphosphate relative to the rate of the oxygenation reaction in C3 plants, resulting in a higher PN and lower photorespiration (Foyer et al. 2009). However, long-term (weeks and months) exposure to elevated CO2 could lead to reductions in both Rubisco content and the maximum rate of the carboxylation reaction, which are thought to represent an acclimation process to high-CO2 conditions (Long et al. 2004).
Although seemingly associated with elevated CO2 and sugar content, the reduction of PN in these conditions should also be influenced by other metabolic disturbances, such as lower photochemical efficiency. This is particularly possible to occur in plants exposed to salinity alone, as these plants presented unaltered sugar levels associated with reduced PN. Regarding chlorophyll fluorescence parameters analyzed in this study, our data showed that cashew plants did not exhibit photo-damage [evidenced by the maintaining of maximal photochemical efficiency (Fv/Fm) values], as shown in Table 2. In addition, the electron transport rate (ETR) and photochemical quenching (qP) were slightly lower under high CO2 and high CO2 + NaCl treatments than in the reference plants. In contrast, the non-photochemical quenching (NPQ) increased by 39%, 78%, and 165% in response to high CO2, NaCl, and high CO2 + NaCl treatments, respectively, compared with those of the reference plants (Table 2). The ETR/PN ratio (an indicator of alternative electron sinks) was strongly influenced by the salinity and high CO2 + NaCl treatments, as both reached values nearly 2.5 times greater than those of the plants under reference conditions.
In general, chlorophyll a fluorescence data suggest that photosynthetic acclimation mechanisms related to lower CO2 assimilation under salinity, high CO2, and high CO2 + salinity were not caused by photoinhibition, as indicated by the maintaining of the Fv/Fm ratio. However, the PSII activity was decreased in these plants in association with induction of excess energy dissipation, possibly as heat (indicate by increase in NPQ), evidencing that regulation of these processes could have been important for photo-damage protection in cashew leaves. The decrease in photochemical activity and the increases in ETR/PN have been observed in cashew plants under similar stress conditions as studied here (Souza et al. 2005).
Such responses suggest that these changes could be part of acclimation mechanisms instead of an indicator of harmful effects on chloroplast (Ribeiro et al. 2009). Thus, these photoprotective mechanisms are important to maintain the primary electron acceptor of PSII in an oxidized state, limiting the probability of photo-damage and photo-oxidative stress in chloroplasts, as evidenced by the maintaining of Fv/Fm values (Silva et al. 2010). This apparent photoprotection also may be associated with other alternative mechanisms for electron flux, such as water–water cycle and cyclic transport around PSII and PSI (Lima Neto et al. 2017). Moreover, we have shown that down-regulation of PSII activity can also mitigate photo-oxidative damage induced by drought associate with high light in cashew plants (Lima et al. 2018). Together, the results exhibited by cashew plants demonstrate that in the conditions studied here, elevated CO2 did not benefit photosynthetic CO2 assimilation as in the absence as well as in the presence of salinity.
Effects of salt stress and high CO2 on the chlorophylls, carotenoids, anthocyanin, and sucrose contents; K+ and Na+ concentrations; and the K+/Na+ ratios of cashew leaves
In this study, we showed that salt stress and high CO2 could induce significant alterations in photosynthetic pigment contents as well a strong antagonism between K+ and Na+ ions. The leaf chlorophyll content was slightly enhanced (~ 15%) under all treatments compared with the reference plants (Table 3). On the other hand, the carotenoid content was similar in both stressed and reference plants. The anthocyanin content increased by 108% and 58% in response to high CO2 and high CO2 + NaCl treatments, respectively, compared to reference plants (Table 3). The sucrose content decreased by 28% only in the NaCl treatment, but remained at control levels under high CO2 and high CO2 + NaCl treatments (Table 3).
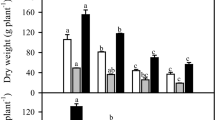
The leaf K+ content decreased by approximately 35% under salinity, whereas that in plants subjected to salinity + high CO2 it decreased by only 20% in comparison with the reference plants (Fig. 1a). Moreover, salinity alone induced an increase in leaf Na+ content by 73%, but when combined with high CO2, this increase was only 40% compared to reference plants (Fig. 1b). The leaf K+/Na+ ratio was increased by high CO2 alone (by 50%), but this ratio decreased by 67% and 50% under salinity and salinity + high CO2 treatments, respectively, compared to reference plants (Fig. 1c). These data suggest that high CO2 might be benefit to the K+/Na+ balance under salinity in cashew, partially mitigating the negative effects of salinity on the ionic homeostasis.
K+ (a) and Na+ (b) contents and the K+/Na+ ratio (c) in cashew plant leaves exposed to salt stress for 15 days followed by high (760 µmol mol−1) (black bars) or atmospheric (380 µmol mol−1) (white bars) CO2 concentrations for 15 more days under salinity. Bars represent the mean values (n = 4) ± SD. Values followed by different capital letters show statistical differences at 5% probability among salt treatments. Values followed by different letters show statistical differences at 5% probability by Tukey’s test
Regarding the accumulation of salt ions, our results show that the high CO2 treatment could alleviate in part the dangerous effects of salinity favoring the K+ nutrition, which increased the K+ content associate to lower the Na+ content in leaves. However, this beneficial effect of high CO2 on the ionic homeostasis was not related to the PN efficiency, suggesting that CO2 assimilation was more limited by osmotic effect compared to ionic. According to Britto et al. (2010), adequate ionic K+/Na+ homeostasis is necessary for salt tolerance, allowing K+/Na+ ratios greater than 1.0. Plants subjected to salinity alone presented a strong increase in Na+ levels coupled with a decrease in K+ content, resulting in a lower K+/Na+ ratio (Niazi et al. 2005; Britto et al. 2010). However, high CO2 reduced the leaf Na+ levels in the absence and presence of salinity and increased the K+ content under salinity, favoring the K+/Na+ ratio under both conditions. Thus, these results indicate that elevated CO2 could promote salt tolerance by involvement of ionic homeostasis in cashew plants involving interactive mechanisms (Table 4).
Similarly, Pérez-López et al. (2014) showed that under combined conditions of salt stress and high CO2, barley seedlings maintained increased uptake and translocation rates of Na+ and K+. This ability permitted the seedlings to adapt to a higher demand under elevated CO2 and to grow more rapidly by allocating more photoassimilates to the roots, promoting root growth and nutrient uptake and translocation.
Effects of salinity and high CO2 on nitrogen metabolism, carbohydrate content, and photorespiratory activity in cashew leaves
Salinity and high CO2 promoted significant changes in the levels of some nitrogenous compounds, sugar content and some indicators of photorespiratory activity. The total N content was not altered by stress conditions compared with control (Fig. 2a). High CO2 induced a slight reduction in the leaf ammonium (NH4+) content (~ 18%), but the plants subjected to NaCl and NaCl + high CO2 presented an increase in NH4+ content by 83% and 41%, respectively, compared with reference plants (Fig. 2b). The total free amino acid content was significantly increased by salt stress, but this N fraction was strongly higher when cashew plants were exposed to combined stress (high CO2 + NaCl) compared with reference plants (Fig. 2c). High CO2 pressure attenuates the reduction on N content salt-induce in barley plants (Pérez-López et al. 2013). However, the free amino acid content in barley was reduced by salinity and high CO2 pressure did not restored this salt effect, while that the salinized plants exposure to high CO2 resulted in an increase of leaf carbohydrate (Pérez-López et al. 2010).
Total nitrogen (a), ammonium (NH4+) (b), total free amino acid (c), and soluble sugar (d) contents of cashew plant leaves exposed to salt stress for 15 days followed by high (760 µmol mol−1) (black bars) or atmospheric (380 µmol mol−1) (white bars) CO2 concentrations for 15 more days under salinity. Bars represent the mean values (n = 4) ± SD. Values followed by different capital letters show statistical differences at 5% probability among salt treatments. Values followed by different letters show statistical differences at 5% probability by Tukey’s test
The total soluble sugar content slightly increased under high CO2 (~ 17%) in the absence of salt, but decreased under salinity by approximately 19%, whereas plants under salinity combined with high CO2 presented an increase of 39% in soluble sugars, all compared to references plants (Fig. 2d). GO activity was strongly increased under salinity (~ 57%), but was unaffected by the high CO2 and salinity + high CO2 treatments (Fig. 3a). The glyoxylate content decreased (~ 23%) under the enriched CO2 treatment compared with the control conditions (Fig. 3b). On the other hand, plants exposed to high CO2 presented an increase in glutamine synthetase (GS2 isoform) activity by 63% and 31% NaCl-free and NaCl-treated plants, respectively, compared to reference plants (Fig. 3c).
Glycolate oxidase (GO) activity (a), glyoxylate content (b), and glutamine synthetase (GS2) activity (c) in cashew plant leaves exposed to salt stress for 15 days followed by high (760 µmol mol−1) (black bars) or atmospheric (380 µmol mol−1) (white bars) CO2 concentrations for 15 more days under salinity. Bars represent the mean values (n = 4) ± SD. Values followed by different capital letters show statistical differences at 5% probability among salt treatments. Values followed by different letters show statistical differences at 5% probability by Tukey’s test
Higher salinity tolerance in plants exposed to high CO2, indicated by a favorable K+/Na+ homeostasis, contrasts with disturbances attributed to high contents of total free amino acids and sugars. On the other hand, these two C and N fractions are well known to act in the osmotic adjustment mechanism in plants under salinity (Silva et al. 2015). Bermudagrass plants grown under both elevated CO2 and salt stress exhibited a significant increase in osmotic adjustment mechanisms due to the accumulation of osmo-solutes, including soluble sugars, proline, and glycinebetaine (Yu et al. 2015). In fact, some C3 glycophyte plants, which have the ability to adjust osmotically to salt stress, can improve their salt tolerance when cultivated under high CO2 environments (Singh et al. 2015).
Similar results were found by Pérez-López et al. (2010) that demonstrated in barley cultivars an efficient osmotic adjustment under salt stress associated with elevated CO2. In these stressful conditions, the osmotic adjustment was greater than at ambient CO2. In fact, this response was positively correlated with the contribution of sugars and some inorganic solutes each as: Na+, Cl−, and K+. Thus, barley plants were likely to be successful in more salinized soils due to its capacity for adjust osmotically under elevated CO2.
In cashew plants, the salt-induced increase in the contents of amino acids, NH4+, and soluble protein is associated more with nitrogen recycling than with effective osmotic adjustment (Silveira et al. 2003), suggesting metabolic disturbances related to acclimation to salinity. In the present study, we observed an expressive increase in soluble protein content that was induced by salinity and high CO2 and an increase in NH4+ and amino acid levels in response to salinity alone. These results reinforce the salt-induced disturbance of nitrogen metabolism in cashew plants, as the increase in these nitrogenous compounds did not favor stomatal opening, which is a characteristic benefit of osmotic adjustment (Singh et al. 2015). On the other hand, the increase in NH4+ content in salinized plants exposed to high CO2 was lower than that in plants under salinity alone, suggesting that the salt-induced disturbance of NH4+ metabolism was attenuated by elevated CO2.
Salinity and high CO2 might modulate oxidative damage and ROS-scavenging systems in cashew leaves
In addition to the results mentioned above, we also investigated the occurrence of membrane damage, ROS accumulation, and oxidative protection under salt stress and high CO2. Electrolyte leakage (EL) was significantly higher only in the high CO2 + NaCl treatment and remained at control levels in the isolated treatments (high CO2 or salinity), as shown in Fig. 4a. However, lipid peroxidation [thiobarbituric acid-reactive substances (TBARS) accumulation] was 37% higher in plants under high CO2, salinity and salinity + high CO2 than in references plants (Fig. 4b). On the other hand, the high CO2 treatment induced a significant decrease (by 41%) in leaf H2O2 content under both salinity and NaCl-free conditions (Fig. 4c).
Electrolyte leakage (EL) (a) and thiobarbituric acid-reactive substance (TBARS) (b) and hydrogen peroxide (H2O2) (c) contents in cashew plant leaves exposed to salt stress for 15 days followed by high (760 µmol mol−1) (black bars) or atmospheric (380 µmol mol−1) (white bars) CO2 concentrations for 15 more days under salinity. Bars represent the mean values (n = 4) ± SD. Values followed by different capital letters show statistical differences at 5% probability among salt treatments. Values followed by different letters show statistical differences at 5% probability by Tukey’s test
Although treatment with high CO2 induced damage at the membrane level and resulted in lipid peroxidation in salt-stressed plants, high CO2 was able to avert an increase in H2O2 content. This response corroborates with the decreased GO activity and NH4+ and glyoxylate contents. These results taken together indicate that the CO2 concentration used in this study attenuated photorespiration. Indeed, this reduced photorespiratory activity under high CO2 may be also be corroborated by the unaltered SOD activity as well as by the reduced APX activity, both of which indicate the apparent absence of oxidative damage. APX is a peroxidase that acts in the removal of excess H2O2 generated during photorespiration, and APX isoforms are present in the peroxisomes and cytosol and are stimulated by increased ROS generation (Foyer et al. 2009).
High CO2 concentration induced an increase in total soluble protein content under all stress conditions compared to control (Fig. 5a). In this context, SOD activity was not affected by high CO2 or salinity, whereas APX activity was significantly reduced only under high CO2 concentrations (Fig. 5c). These indicators of redox change suggest that salinity could not induce decrease in cellular integrity despite the increase in lipid peroxidation under salinity and high CO2. In addition, our results show that high CO2 avoided H2O2 accumulation despite this treatment has induced low APX activity.
Total soluble protein content (a), superoxide dismutase (SOD) (b), and ascorbate peroxidase (APX) (c) activities in cashew plant leaves exposed to salt stress for 15 days followed by high (760 µmol mol−1) (black bars) or atmospheric (380 µmol mol−1) (white bars) CO2 concentrations for 15 more days under salinity. Bars represent the mean values (n = 4) ± SD. Values followed by different capital letters show statistical differences at 5% probability among salt treatments. Values followed by different letters show statistical differences at 5% probability by Tukey’s test
These beneficial effects of high CO2 on reduced photorespiration under saline and non-saline conditions can be attributed to an increase in the CO2/O2 ratio in the leaf mesophyll, as suggested by a higher CI. Plants exposed to high CO2 avert the occurrence of photorespiration by stimulating the CO2/O2 ratio, which reduces the oxygenase activity of Rubisco (Aranjuelo et al. 2009). In the present study, lower GO activity and H2O2 and glyoxylate contents were observed, which were also associated with reduced NH3 levels under elevated CO2. This response can be attributed to a reduction in the rate of conversion of glycine to serine in mitochondria, a reaction of the photorespiratory cycle (Foyer et al. 2009). Thus, this response might be closely associated with lower photorespiratory activity caused by high CO2 pressure. On the other hand, lower NH3 content under high CO2 may also have occurred due to increased GS2 activity in response to elevated CO2. This glutamine synthetase isoform is presented in chloroplasts and is responsible for the assimilation of NH3 generated during photorespiration into glutamine, averting the toxic effects of NH3 as well as promoting amino acid synthesis (Thomsen et al. 2014).
Conclusion
In summary, our data suggest that elevated CO2 can induce strong physiological and metabolic changes related to photosynthetic acclimation in cashew plants independent of salinity. This process involves a reduction in CO2 assimilation, preservation of PSII, and lower photorespiratory activity. Furthermore, high CO2 also favors ionic balance, which may contribute to the amelioration of salt tolerance. The accumulation of soluble sugars, amino acids, and proteins is apparently associated with metabolic disturbances but the involvement of these compounds in osmotic adjustment should not be ruled. Together, our results reveal a set of responses induced by high CO2, in the presence and absence of salinity, that are more associated with acclimatize metabolic processes than with stressful effects.
Author contribution statement
NCSS conducted experiments and performed biochemical measurements. JAGS interpreted data and contributed with writing. ENS performed photosynthesis measurements. MCLN performed photochemical determinations. CSL conducted experiments and performed biochemical measurements. RMA helped with the redrawing figures with Sigma Plot and revising the manuscript. SLFS designed and carried out the research, analyzed the data, and wrote the paper. All of the authors read and approved the final manuscript.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomacal conductance to rising (CO2): mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Alonso A, Pérez P, Martínez-Carrasco R (2009) Growth in elevated CO2 enhances temperature response of photosynthesis in wheat. Physiol Plant 135:109–120
Aranjuelo I, Irigoyen JJ, Nogués S, Sánchez-Díaz M (2009) Elevated CO2 and water availability effect on gas exchange and nodule development in N2 fixing alfalfa plants. Environ Exp Bot 65:18–26
Baker AL, Tolbert NE (1966) Glycolate oxidase (ferredoxin-containing form). Methods Enzymol 9:339–340
Ball MC, Cochrane M, Rawson HM (1997) Growth and water use of the mangroves Rhizophora apiculate and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant Cell Environ 20:1158–1166
Bowman WD, Strain BR (1987) Interaction between CO2 enrichment and salinity stress in the C4non-halophyte Andropogon glomeratus (Walter) BSP. Plant Cell Environ 10:267–270
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ (2010) 42K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytol 186:373–384
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57:2435–2444
Darbah JNT, Sharkey TD, Calfapietra C, Karnosky DF (2010) Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environ Pollut 158:1008–1014
Elliott WH (1953) Isolation of glutamine synthetase and glutamotransferase from green peas. J Biol Chem 201:661–672
Ferreira-Silva SL, Silva EN, Carvalho FEL, Lima CS, Alves FAL, Silveira JAG (2010) Physiological alterations modulated by rootstock and scion combination in cashew under salinity. Sci Hortic 127:39–45
Ferreira-Silva SL, Voigt EL, Silva EN, Maia JM, Fontenele AV, Silveira JAG (2011) High temperature positively modulates oxidative protection in salt-stressed cashew plants. Environ Exp Bot 74:162–170
Flexas J, Ortuño MF, Ribas-Carbo M, Diaz-Espejo A, Fórz-Sarasa D, Medrano H (2007) Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol 175:501–511
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60:455–484
Geissler N, Hussin S, El-Far MMM, Koyro H-W (2015) Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ Exp Bot 118:67–77
Gianopolitics CN, Ries SK (1977) Superoxide dismutase occurrence in higher plants. Plant Physiol 59:309–314
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:1–10
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heber U (2002) Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosynth Res 73:223–231
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ 347:1–32
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192(2):261–268
Leakey ADB, Bernacchi CJ, Ort DR, Long SP (2006) Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open air conditions. Plant Cell Environ 29:1794–1800
Lees DH, Francis FJ (1972) Standardization of pigment analyses in cranberries. Hortic Sci 7:83–84
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lima Neto MC, Cerqueira JVA, Cunha JR, Ribeiro RV, Silveira JAG (2017) Cyclic electron flow, NPQ and photorespiration are crucial for the establishment of young plants of Ricinus communis and Jatropha curcas exposed to drought. Plant Biol 19:650–659
Lima CS, Ferreira-Silva SL, Carvalho FEL, Neto MCL, Aragão RM, Silva EM, Sousa RMJ, Silveira JAG (2018) Antioxidant protection and PSII regulation mitigate photo-oxidative stress induced by drought followed by high light in cashew plants. Environ Exp Bot 149:59–69
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide. Plants FACE the future. Annu Rev Plant Biol 55:591–628
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Niazi BH, Athar M, Salim M, Rozema J (2005) Growth and ionic relations of fodderbeet and seabeet under saline environments. Int J Environ Sci Technol 2:113–120
Pérez-López U, Robredo A, Lacuesta M, Munõz-Rueda A, Mena-Petite A (2010) Atmospheric CO2 concentration influences the contributions of osmolyte accumulation and cell wall elasticity to salt tolerance in barley cultivars. J Plant Physiol 167:15–22
Pérez-López U, Robredo A, Lacuesta M, Munõz-Rueda A, Munõz-Rueda A (2012) Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth Res 111:269–283
Pérez-López U, Robredo A, Miranda-Apodacaa J, Lacuesta M, Munõz-Rueda A, Mena-Petite A (2013) Carbon dioxide enrichment moderates salinity-induced effects on nitrogen acquisition and assimilation and their impact on growth in barley plants. Environ Exp Bot 87:148–158
Pérez-López U, Miranda-Apodaca J, Mena-Petite A, Munoz-Rueda A (2014) Responses of nutrient dynamics in barley seedlings to the interaction of salinity and carbon dioxide enrichment. Environ Exp Bot 99:86–99
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009) Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 47:215–222
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:26. https://doi.org/10.1155/2012/217037
Silva EN, Ferreira-Silva SL, Viegas RA, Silveira JAG (2010) The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ Exp Bot 69:279–285
Silva EN, Silveira JAG, Rodrigues CRF, Viegas RA (2015) Physiological adjustment to salt stress in Jatropha curcas is associated with accumulation of salt ions, transport and selectivity of K+, osmotic adjustment and K+/Na+ homeostasis. Plant Biol 17:1023–1029
Silveira JAG, Viegas RA, Rocha IMA, Oliveira TJA (2003) Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J Plant Physiol 160:115–123
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol 14:407–426
Souza RP, Ribeiro RV, Machado EC, Oliveira RF, Silveira JAG (2005) Photosynthetic responses of young cashew plants to varying environmental conditions. Pesquisa Agropecuária Brasileira 40:735–744
Thomsen HC, Eriksson D, Møller IS, Schjoerring JK (2014) Cytosolic glutamine synthetase: a target for improvement of crop nitrogen use efficiency? Trends Plant Sci 19:656–663
Xu Z, Jiang Y, Zhou G (2015) Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front Plant Sci 6:1–17
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7:657
Yi C, Yao K, Cai S, Li H, Zhou J, Xia X, Shi K, Yu J, Foyer CH, Zhiou Y (2015) High atmospheric carbon dioxide-dependent alleviation of salt stress is linked to RESPIRATORY BURST OXIDASE 1 (RBOH1)-dependent H2O2 production in tomato (Solanum lycopersicum). J Exp Bot 66:7391–7404
Yu J, Sun L, Fan N, Yang Z, Huang B (2015) Physiological factors involved in positive effects of elevated carbon dioxide concentration on Bermudagrass tolerance to salinity stress. Environ Exp Bot 115:20–27
Acknowledgements
We thank the Conselho Nacional de Desenvolvimento Cientıfico e Tecnológico (CNPq), Fundação Cearense de Apoio ao Desenvolvimento Cientıfico e Tecnológico (FUNCAP) and Grant #2018/04258-6 São Paulo Research Foundation (FAPESP) for financial support, and the Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) for providing the cashew seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Montanaro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, N.C.S., Silveira, J.A.G., Silva, E.N. et al. High CO2 favors ionic homeostasis, photoprotection, and lower photorespiration in salt-stressed cashew plants. Acta Physiol Plant 41, 158 (2019). https://doi.org/10.1007/s11738-019-2947-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2947-1