Abstract
Historically, mesenchymal stromal/stem cells (MSCs) have been characterized by their capacity to support hematopoiesis and differentiate into various connective tissue cell types. However, in the past decade, the field of MSC research has witnessed tremendous growth, spurred principally by studies showing that the cells are efficacious in treating a broad array of diseases. Renewed interest in MSC biology has also yielded new insights into their developmental origin, contribution to the hematopoietic stem cell niche, and mechanism of action in promoting tissue repair and regeneration. In the latter case, MSCs have been shown to secrete a bevy of proteins and other molecules that exhibit trophic, angiogenic, immunomodulatory, neuro-regulatory, anti-inflammatory, and anti-apoptotic activity and that function to restore homeostasis at sites of tissue injury and in response to disease. Herein, we provide an overview of the paracrine functions of MSCs by describing the different classes of proteins secreted by cells, the influence of the local microenvironment on their expression, and their therapeutic effects in various experimental animal models of disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatocyte Growth Factor
- Middle Cerebral Artery Occlusion
- Keratinocyte Growth Factor
- Human MSCs
- Paracrine Function
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Friedenstein and coworkers were the first to identify a cell population in bone marrow, distinct from hematopoietic stem cells (HSCs), with the ability to generate in vivo a heterotopic osseous tissue capable of supporting hematopoiesis ( Chap. 1). This population, now referred to as mesenchymal stromal cells (MSCs), was subsequently exploited to establish long-term bone marrow cultures in vitro, which provided a unique opportunity to dissect the cell-type specific interactions and soluble factors that regulate various aspects of hematopoiesis. These studies revealed much about the phenotype and function of MSCs and as such revealed for the first time insight into their unique paracrine functions [1]. Therefore, we will begin by examining the role of MSCs in hematopoiesis, which continues to evolve.
Role of MSCs in Hematopoiesis
A number of reviews have been published describing in detail the important role played by MSCs in regulating hematopoiesis [2, 3]. Therefore, the topic is discussed only briefly here. MSCs secrete several classes of proteins including cytokines, chemokines, growth factors, neuropeptides, and extracellular matrix proteins that modulate hematopoiesis. For example, the matrix proteins fibronectin, laminin, vitronectin, thrombospondin, haemonectin, thrombopoietin, tenascin, and collagens function as organ and lineage-specific binding proteins for hematopoietic cells [4–12]. These proteins also directly bind cytokines and growth factors and present them in biologically active forms to hematopoietic cells, which then stimulate growth and maturation [13–15]. Many growth factors including stem cell factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor, kit ligand, leukemia inhibitory factor, interleukin 1 beta (IL-1B), interleukin 3 (IL-3), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 8 (IL-8), interleukin 11 (IL-11), insulin-like growth factor 1 (IGF-1), and transforming growth factor beta 1 (TGFβ1) are also secreted by MSCs [16, 17]. In addition, their expression may be altered in response to external stimuli, thereby providing a mechanism to modulate hematopoiesis in response to stress, infection, and injury. For example, treatment of MSCs with IL-1β, IL-6, and lipopolysaccharides (LPS) stimulates, whereas treatment with interferon α suppresses expression of GM-CSF and G-CSF [18]. Activin A, a potent stimulator of erythroid differentiation and negative regulator of B cell lymphopoiesis, is also strongly upregulated in MSCs by inflammatory cytokines and suppressed by glucocorticoids [19]. Moreover, platelet-derived growth factor (PDGF) enhances the ability of MSCs to support growth of colony forming unit-granulocytes macrophages (CFU-GM) by inducing secretion of GM-CSF, IL-3, and IL-6 [20]. Other factors secreted by MSCs that have been shown to play a role in hematopoiesis include Flt-3 ligand [21], hepatocyte growth factor (HGF) [22], jagged1 [23], substance P [24], and calcitonin gene-related protein [25]. Additionally, secreted frizzled-related protein-1 (sFRP1) inhibits osteoclast formation [26] as well as maintains homeostasis of HSCs in marrow via extrinsic regulation of beta-catenin [27].
More recent studies have identified MSC subpopulations in marrow, discriminated based on secretion of specific cytokines, that bind to functionally distinct T and B cell lineages [28]. For example, MSCs expressing CXCL12 (chemokine, CXC motif, ligand 12) have been shown to interact specifically with pre-pro-B cells and memory plasma cells, while MSCs that express IL-7 but lack expression of CXCL12 interact specifically with memory CD4+ T cells. These MSC subpopulations also express vascular cell adhesion molecule 1 but lack expression of endothelial cell adhesion molecule 1. These studies indicate that the bone marrow reticular system may be inordinately complex and contain distinct stromal subtypes that specifically interact with different hematopoietic lineages to sustain hematopoiesis [1]. Various groups have also reported that MSCs and or MSC-derived osteoprogenitors physically interact with HSCs in bone marrow and as such contribute to the HSC niche (see Chap. 3).
It is important to note that human CD34 + HSCs as well as CFU-GM, CFU-megakaryocytes, and blast-forming unit erythrocytes also secrete various growth factors, cytokines, and chemokines that function via autocrine and paracrine mechanisms to regulate hematopoiesis [29, 30]. For example, human CD34+ HSCs stimulate secretion of G-CSF and IL-6 from MSCs [31]. Therefore, hematopoiesis is orchestrated by the concerted action of many secreted proteins, expression of which is controlled both by changes in the external environment and cross-talk between stromal and hematopoietic cell lineages within marrow.
Regeneration Versus Replacement: Shifting Paradigms of MSC Function
Early studies demonstrating the multi-potency of MSCs led to the assumption that the cells function in tissue homeostasis by serving as a reservoir of connective tissue progenitors and as such were first employed clinically to treat osteogenesis imperfecta [32]. The established role of MSCs in supporting hematopoiesis was also exploited in clinical trials to speed hematopoietic recovery following bone marrow transplantation [33]. Although results from these trials were encouraging, reports published in the early 2000s indicating that MSCs possessed unexpected plasticity as evidenced by their transdifferentiation into cardiomyocytes [34], astrocytes [35], hepatocytes [36], lung epithelium [37, 38], and other lineages sparked renewed interest in the therapeutic potential of the cells. While further research revealed that “transdifferentiation” occurred at a low frequency in vivo [39], MSCs continued to demonstrate measurable therapeutic effects in experimental animal models of disease. Consequently, this prompted a major paradigm shift with respect to the anticipated function of MSCs. Rather than contribute directly to tissue replacement via directed differentiation, the ability of MSCs to alter the tissue microenvironment via paracrine signaling rapidly established itself as the principle mechanism by which the cells affected tissue repair and regeneration. This new paradigm has rapidly gained acceptance following the identification of various proteins secreted by MSCs that have demonstrated angiogenic, anti-apoptotic, anti-inflammatory, and trophic effects in various disease models.
The MSC Transcriptome
The diverse array of paracrine-acting factors secreted by MSCs was predicted in large part by analysis of their transcriptome using genomics-based approaches. For example, our laboratory was the first to analyze the transcriptome of human [40, 41] and primary rodent MSCs [42] via serial analysis of gene expression (SAGE). Interrogation of these databases revealed expressed transcripts encoding proteins that regulate a variety of functions necessary to maintain homeostasis of bone and bone marrow including angiogenesis, hematopoiesis, cell migration and communication, neural activities, immunity, and defense. These findings were confirmed by other groups [43–45]. We also validated the biological activity in vitro and in vivo of a subset of secreted proteins identified by SAGE. For example, we demonstrated that primary mouse MSCs ameliorated bleomycin-induced lung injury in mice and that secretion by cells of interleukin receptor 1 antagonist (IL-1RN), a protein with potent anti-inflammatory activity, contributed significantly to this effect [46]. We also showed that neural regulatory factors secreted by MSCs promoted survival and neurite outgrowth from neuroblastoma cells and spinal nerves from the dorsal root [47]. Finally, we identified a large number of angiogenic factors secreted by MSCs that induce growth and branching morphogenesis of human vascular endothelial cells [48]. Results from genomics-based studies have been codified by proteomics-based analyses showing that MSCs secrete chemokines [49], chemoattractants [50], and angiogenins [51] that play roles in tissue injury and repair.
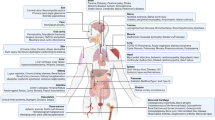
SAGE analysis also revealed that a single cell-derived colony of human MSCs simultaneously expressed a diverse array of lineage-specific mRNAs characteristic of skeletal and muscle tissue [40]. These data suggested that MSCs exist in a ground state with respect to mRNA expression, similar to that proposed for HSCs [52], and as such are poised for rapid differentiation in response to external stimuli. A similar ground state may exist for MSCs with respect to their paracrine function (Fig. 9.1). For example, MSCs constitutively secrete a number of mitogens including fibroblasts growth factor 2 (FGF-2), brain-derived neurotrophic factor (BDNF), IGF1, and HGF, angiogenins including vascular endothelial growth factor-A (VEGF-A), angiopoietin 1 (ANG1), and CRY61, as well as various cytokines and chemokines, such as GM-CSF, G-CSF, IL-6, CXCL12, and TGFβ1 that are important for bone homeostasis and hematopoiesis. However, a growing number of studies have shown that secreted levels of these proteins are altered and/or induced in MSCs following exposure to external stimuli, such as infection, inflammation, and changes in oxygen concentrations. Importantly, these conditions typify the microenvironments encountered by MSCs when transplanted ectopically to sites of tissue injury or disease. For example, MSCs express several toll-like receptors (TLRs) that allow cells to sense and respond to infectious agents [53]. TLR activation leads to secretion by MSCs of pro-inflammatory cytokines and chemokines [54], growth factors [55], and soluble mediators that regulate immune cell function [56]. MSCs also express receptors for tumor necrosis factor-alpha (TNF-α) and IL-1, which upon ligand binding induce expression of secondary mediators of inflammation, such as IL-6, and other proteins including HGF [57], monocyte chemotactic protein-1 (MCP1), cathepsin L, and several matrix metalloproteases [58]. Importantly, engagement of these receptors also leads to secretion of several potent anti-inflammatory proteins including IL-1RN [46] and TNF-α-induced protein 6 (TNF-αIP6) [59], which promote healing by limiting the extent of tissue inflammation. MSCs also induce mature dendritic cells to secrete interleukin 10 (IL-10), which has anti-inflammatory properties, and T helper 2 cells to secrete interleukin 4 (IL-4), which induces IL-10 secretion from macrophages [60]. Exposure of MSCs to interferon-gamma (IFN-γ) has also been shown to augment secretion of HGF, IL-10, TGFβ1, and indoleamine 2,3-dioxygenase, thereby enhancing their immunomodulatory effects [61] (Chap. 6).
MSCs are poised for rapid lineage specification and activation of paracrine signaling in response to external stimuli. (a) Genomics-based studies have shown that a single cell-derived colony of human MSCs expresses mRNAs characteristic of skeletal and muscle tissue [40], indicating that the cells are poised for rapid lineage specification in response to specific external stimuli as illustrated by the red (adipogenic), blue (chondrogenic) and yellow (osteogenic) arrows. (b) MSCs have also been shown to constitutively secrete a variety of cytokines/chemokines, angiogenins, and mitogens that play important roles in tissue homeostasis. Moreover, secreted levels of these proteins can be significantly altered in response to a variety of external stimuli, such as infection, inflammation, and hypoxia. Importantly, these conditions typify the microenvironments encountered by MSCs when transplanted ectopically to sites of tissue injury or disease. Therefore, these data suggest that the paracrine functions of MSCs also exist in a ground state and are poised for rapid activation in response to tissue injury and disease. Protein expression in MSCs is constitutive (black arrows), induced by infectious and inflammatory agents (red arrows) or by hypoxic conditions (blue arrows)
Similarly, hypoxia has been shown to elicit a pro-angiogenic program in human MSCs by stimulating expression of VEGF, IL-6, and IL-8 and suppressing expression of TNF-α, interleukin 12 (IL-12), and tissue inhibitor of metallo-protease 1 [62]. Others have confirmed and extended these studies by demonstrating that hypoxia also induces expression of FGF2, HGF, IGF1 [55] and IL-1β, TNFα, and IL-10 [63]. Consistent with these studies, MSCs derived from heme oxygenase-1 (HO1) knockout mice secreted lower levels of CXCL12, VEGF-A, and HGF and exhibited a lower angiogenic potential in vitro [64]. Therefore, HO1 also contributes to the paracrine response of MSCs following exposure to environmental stressors.
The ability of MSCs to respond to and modulate the inflammatory response has broad implications with respect to their use in clinical medicine. For example, although inflammation plays a central role in the elimination of infectious agents and reparation of tissues following injury, unremitting inflammation also is characteristic of many disease states, such as nonhealing wounds, interstitial lung disease, arthritis, psoriasis, inflammatory bowel disease, and others. Owing to their potent anti-inflammatory effects, MSCs are being evaluated in phase I/II clinical trials for the treatment of Crohn’s disease, osteoarthritis, muscle and skeletal trauma, and diabetes (www.clinicaltrials.gov). Moreover, inflammation also contributes prominently to other common maladies such as obesity and drug addiction and plays a role in allograft rejection. In the later case, MSCs may modulate regulatory T cell-dependent allograft acceptance by limiting the extent of tissue inflammation. Therefore, similar to their capacity to undergo multi-lineage differentiation, MSCs may also be primed to respond to a broad array of aberrant tissue microenvironments and restore homeostasis to these microenvironments via the secretion of paracrine-acting factors (Fig. 9.1). This capacity is exemplified by the broad therapeutic effect of MSCs demonstrated in the following disease models.
Paracrine Effects in Ischemic Diseases
Myocardial Infarction
From their onset, clinical studies have demonstrated the safety of intracoronary infusion of MSCs for the treatment of myocardial infarction and shown this yields a measurable improvement in overall left ventricular function [65] (Chap. 6). Factors implicated in contributing to the therapeutic effect of MSCs in myocardial infarction include IGF, HGF, VEGF, and FGF, and in some cases, MSCs have been genetically engineered to overexpress these factors to augment their therapeutic effect [66–69]. Importantly, the mechanism of MSC action in myocardial infarction is complex as the cells exhibit beneficial effects at various stages of disease progression. For example, MSCs exhibit anti-apoptotic activity and protect cardiomyocytes from hypoxia-induced death by downregulating expression of the pro-apoptotic protein Bax and augmenting expression of FGF, VEGF, and CXCL12 in heart tissue [70]. In addition, MSCs engineered to overexpress the murine thymoma viral oncogene homolog 1 (AKT1) were found to be superior to wild-type MSCs for cell therapy of acute myocardial infarction in a rat model [71]. Herein, genetic modification altered the repertoire of secreted paracrine factors in MSCs based on the finding that conditioned media from AKT1-modified but not wild-type MSCs exerted cardioprotective effects in vivo. A subsequent study identified soluble frizzled-related protein 2 as a key AKT1-regulated paracrine factor secreted by MSCs responsible for reparative effects and myocardial survival [72]. Similarly, overexpression in MSCs of GATA-binding protein 4 (GATA-4) has also been shown to augment secretion of IGF-1 and VEGF and enhance the cells’ cardioprotective effects in vivo [73].
MSCs also promote neovascularization in infracted myocardium, which is necessary to prevent cell death, promote tissue remodeling and improve overall cardiac function [74]. For example, autologous MSC administration in a rat model of myocardial ischemia significantly increased capillary density within the ischemic heart tissue [70]. Enhanced vasculogenesis appears to be a common outcome seen following MSC administration in other ischemic diseases. For example, in animal models of limb ischemia, local delivery of MSCs augments collateral perfusion. This effect is mediated, in part, via paracrine mechanisms since antibodies against VEGF and FGF-2 partially inhibit the capacity of MSC-conditioned media to promote proliferation of endothelial and smooth muscle cells [75]. Other factors secreted by MSCs, such as HGF and IGF-1, augment aortic endothelial cell growth and survival, a response not observed with fibroblast conditioned media [76]. MSCs modified to overexpress GATA-4 or preconditioned by exposure to hypoxic conditions in vitro also exhibit enhanced anti-apoptotic and angiogenic effects on endothelial cells [73, 77]. Hypoxic preconditioning enhances expression of VEGF, IL-6, MCP1 and CXCL12 in MSCs as well as other unidentified factor(s) that activate the phosphatidylinositol 3-kinase (PI3K)-AKT pathway in endothelial cells. The latter is consistent with the fact that PI3K signaling mediates angiogenesis in vascular endothelial cells [78].
The paracrine action of MSCs in myocardial infarction is exemplified by the observation that the cells exhibit cardioprotective effects when administered not only locally (transcardial and/or intraventricular) but also intravenously. In the latter case, most MSCs accumulate rapidly in lung tissue in the first few hours after administration and then are slowly released over a few days into the circulation in low numbers. Lee et al. [59] reported that MSCs trapped in emboli within lung tissue secrete high levels of TNF-αIP6, which antagonizes the function of TNF-α and ameliorates tissue inflammation that contributes to the pathogenesis of myocardial ischemia. These data are consistent with other studies showing that MSC administration attenuates increases in CD68-postiive inflammatory cells and expression levels of MCP-1in heart tissue in a rat model of acute myocarditis [79]. Nguyen et al. [80] further showed that intracoronary injection of MSC-conditioned media reduced cardiac troponin-T levels and improved cardiac output, stroke volume, and wall motion score index in a swine model of acute myocardial infarction.
Ventricular remodeling in response to ischemic injury is typically characterized by hypertrophy and apoptosis of cardiomyocytes and tissue fibrosis. Depending upon the size of the infarction, aberrant remodeling can lead to decreased cardiac output and increased susceptibility to a second heart attack. Paracrine factors produced by MSCs limit the extent of aberrant remodeling by supporting regeneration of cardiomyocytes [81]. However, the identity of factors responsible for this effect is indeterminate. MSCs may promote regeneration of myocardium by stimulating growth and survival of cardiac progenitor cells (CPC). For example, human growth hormone and IGF-1 are part of an autocrine loop that maintains muscle tissue integrity, but their expression declines rapidly with aging. Similarly, HGF is necessary for CPCs to migrate to areas of tissue damage and promote repair [82]. Therefore, IGF-1 and HGF secreted by MSC may promote myocardial regeneration by stimulating proliferation of CPCs resident in heart tissue.
Whether MSCs also prevent tissue fibrosis remains unclear based on the available data. We previously showed that MSCs prevent fibrosis in a mouse model of acute lung injury, but this effect was a consequence of their potent anti-inflammatory activity [46]. MSC administration has been shown to reduce fibrous tissue deposition in heart in rat models of ischemia/reperfusion injury [83] and dilated cardiac myopathy [84]. However, whether this outcome is secondary to the anti-apoptotic and angiogenic activity of the cells remains uncertain. It is anticipated that HGF secreted from MSCs has anti-fibrotic activity based on its ability to suppress expression of TGFβ-1 [85, 86]. Moreover, intramyocardial injection of IGF-1/HGF affinity bound alginate biomaterial has been shown to reduce fibrosis, attenuate infract expansion, and increase vessel formation at the site of infarct in a rat model of acute myocardial infarction [87]. Therefore, MSCs may limit the extent of tissue fibrosis via secretion of factors that antagonize TGFβ-1 activity. However, it is important to note that MSCs express an array of collagens, matrix proteins, and metalloproteases and under certain conditions can adopt a myofibrocyte phenotype [88]. Therefore, understanding how expression of these proteins is altered when MSCs encounter an ischemic or fibrotic milieu is necessary to better clarify their anti-fibrotic potential. Contradictory reports exist regarding the anti-fibrotic effects of MSCs in models of liver injury [89, 90], which may be related to differences in the timing of cell administration and extent of liver damage. MSCs have been reported to prevent renal fibrosis although this outcome may also be secondary to the anti-inflammatory effects of the cells in this model [91, 92].
In summary, evidence supporting a paracrine hypothesis of MSC action in myocardial infarction includes poor engraftment and retention of MSCs in heart tissue following transplantation, minimal capacity to transdifferentiate into cardiomyocytes in vivo, ectopic overexpression of genes that augment expression levels of secreted proteins enhances the therapeutic efficacy of MSCs in vivo, MSC-conditioned media has cardioprotective effects in vitro and in vivo, and neutralizing antibodies against secreted proteins diminishes the therapeutic effect of MSCs. Nevertheless, it is likely that the list of cardioprotective factors secreted by MSCs is incomplete. Other cardioprotective factors produced by MSCs may include insulin-like growth factor binding protein 7 (IGFBP7) [93], which stimulates prostacyclin production in cultured bovine endothelial cells [94]. Prostacyclin is both antithrombotic and a vasodilator. Therefore, IGFBP7 may play a beneficial role in myocardial infarction and peripheral vascular disease by inhibiting thrombosis and vasoconstriction. Mining of genomic databases is likely to facilitate discovery of additional paracrine-acting factors secreted by MSCs that contribute to their cardioprotective effects in vivo.
Stroke
Similar to effects in myocardial infarction, MSCs have also been shown to positively impact various stages of disease progression in stroke. Although studies defining the role of individual proteins are lacking, the prevailing data indicate that paracrine factors secreted by MSCs reduce ischemic damage [95] and apoptosis [96, 97], induce neurogenesis [98], angiogenesis, synaptogenesis [99], neurite outgrowth [100, 101], enhance neuroplasticity [102], and restore cognitive functions [103]. Proteins secreted by MSCs that have therapeutic effects in myocardial ischemia are also implicated in providing a therapeutic benefit in stroke. For example, the ability of MSCs to promote neuronal cell survival and ameliorate neurological deficits in a rat model of middle cerebral artery occlusion (MCAO) was partially attenuated when cells were transduced prior to injection with a VEGF-RNAi lentivirus [104]. Other studies have reported that IGF1 expression is upregulated in MSCs engrafted within the infarct border in the brains of rats subjected to MCAO, and endogenous levels of VEGF, epidermal growth factor (EGF), and FGF-2 are also upregulated in brain [105]. Lastly, exposure of human MSCs to extracts from ischemic brain tissue augments expression of BDNF, nerve growth factor (NGF), VEGF, and HGF [106]. Therefore, the ischemic brain microenvironment is capable of altering the paracrine activity of MSCs, similar to that seen by exposing cells to hypoxic conditions in vitro. Additionally, trophic factors produced by MSCs engrafted within the ischemic brain are anticipated to modulate the production and expression levels of autocrine and paracrine factors produced by the brain parenchyma [97, 107–110]. This feed forward affect may account for the potent therapeutic effects of MSCs in vivo.
Genetic modification of MSCs has also been used to enhance their therapeutic effects in experimental stroke models. For example, MCAO rats administered human MSCs engineered to overexpress ANG1 and VEGF showed enhanced structural and functional recovery as compared to untreated rats and those administered wild-type MSCs [111]. Placental growth factor gene-modified MSCs also elicited greater angiogenesis and a larger reduction in lesion volume compared to native MSCs [112]. Moreover, BDNF and glial cell line-derived neurotrophic factor (GDNF) but not ciliary neurotrophic factor (CNTF) or neurotrophin 3 (NT-3) gene-modified MSCs are reported to exhibit enhanced capacities to promote functional recovery and reduce infarct size in MCAO rats [95, 113, 114]. In addition to trophic factors, MSCs also secrete extracellular matrix proteins that support the growth of neurons, astrocytes, and oligodendrocytes in vitro by increasing their metabolic rate and protecting cells from nutrient and growth factor deprivation [115]. However, whether these proteins are secreted from cells engrafted within brain tissue has yet to be examined.
MSCs may also improve functional recovery after stroke by modulating cytokine expression in the brain. For example, MSC administration increases brain expression levels of IL10, which has anti-inflammatory and neuro-protective activity [97, 116] and decreases levels of the pro-inflammatory cytokine TNFα [116]. MSCs also increase bone morphogenetic protein 2/4 expression in ischemic astrocytes, which enhances subventricular progenitor cell gliogenesis by activating relevant signaling pathways [107]. The cells also increase tissue plasminogen activator activity and downregulate plasminogen activator inhibitor 1 activity within the ischemic boundary of MCAO mice and in astrocytes cultured in vitro. These changes resulted in enhanced neurite outgrowth form cortical neurons [101]. Importantly, behavioral recovery and neurogenesis in a rat stroke model was more pronounced when animals were administered early versus late passage MSCs. Moreover, endogenous levels of trophic factors, such as GDNF, NGF, VEGF, and HGF, were higher in early passage MSC-treated brains [117]. These findings illustrate that culture expansion and/or methods of isolation may significantly impact the paracrine functions of MSCs.
Paracrine Effects in Lung Disease
Pulmonary Fibrosis
Our lab was the first to show that MSC administration ameliorated inflammation and fibrosis in a mouse model of bleomycin-induced lung injury [37]. A critical result from these studies was the demonstration that MSC administration at the time of bleomycin exposure but not 1 week later significantly reduced the extent of neutrophil infiltration into lung tissue and upregulation of pro-inflammatory cytokine expression. Based on this result, it was apparent that the principal effect of MSCs was anti-inflammatory in nature and this was subsequently linked to secretion of high levels of IL-1RN [46]. Other studies have shown that MSC administration suppresses bleomycin-induced increases in TGFβ1, IGF-1, and PDGF in lung tissue and laminin and hyaluronan expression in bronco-alveolar lavage (BAL) in rats [118]. Similarly, MSCs derived from umbilical cord blood attenuate expression of TGFβ1, IL-10, IFN-γ, and macrophage migration inhibitory factor in mice exposed to bleomycin [119]. In related studies, coculture of polarized human alveolar epithelial type II cells after inflammatory insult with MSCs was shown to preserve their protein permeability. ANG1 secretion was responsible for this beneficial effect in part by preventing actin stress fiber formation and claudin 18 disorganization via suppression of nuclear factor kappa-B activity [120].
In lung as in heart, it remains unclear whether MSCs actually exhibit anti-fibrotic effects or if their capacity to block fibrosis is merely a consequence of their potent anti-inflammatory properties. Salazar et al. [121] recently showed that MSCs from mouse bone marrow and human umbilical cord blood secrete high levels of PDGF-AA and TGFβ1 and stimulated growth and collagen production of lung fibroblasts. Interestingly, antagonism of TGFβ1 reduced collagen expression in lung fibroblasts, but their growth was inhibited by the Wnt antagonist sFRP1. These data suggest that MSCs also secrete Wnt ligands that stimulate fibroblast proliferation. Lee et al. [88] also recently reported that treatment of MSCs with connective tissue growth factor upregulates collagen type 1 and tenacin-C expression as well as collagen type III, fibronectin, and matrix metallo-protease type I. Further exposure to TGFβ1 induced the cells to adopt a myofibroblast phenotype and undergo fibrogenesis instead of ectopic mineralization in vivo. These studies suggest that MSCs may be pro-fibrotic under specific conditions.
Endotoxin-Induced Lung Injury
MSCs from mouse bone marrow can also significantly reduce lung inflammation, edema and decrease levels of IFN-γ, IL-1B, IL-6, macrophage inflammatory protein 1-alpha, and IL-8 in peripheral blood in a mouse model of endotoxin-induced acute lung injury [122]. Human MSCs produce a similar effect that is paracrine in nature based on their capacity to downregulate expression of TNF-α by macrophages stimulated with LPS in vitro [123]. In related studies, Lee et al. [124] showed that human MSCs also restored alveolar epithelial fluid transport and lung fluid balance in an ex vivo perfused human lung preparation injured by E. coli endotoxin. Importantly, conditioned media from MSCs yielded a similar outcome and knockdown studies demonstrated that keratinocyte growth factor (KGF) secreted from MSCs contributed to their therapeutic effect in this model.
Asthma
In a ragweed-induced mouse asthma model, Nemeth et al. [125] demonstrated that MSCs administered i.v. at the time of antigen challenge significantly reduced the extent of eosinophil infiltration and mucus production in the lung; decreased expressed levels of IL-4, IL-5, and interleukin 13 (IL-13) in BAL; and also lowered serum levels of IgG1 and IgE. In this study, allogeneic and autologous MSCs exhibited similar therapeutic effects, while skin fibroblasts reduced the total number of cells in BAL but not the number of eosinophils compared to untreated mice. Skin fibroblasts also significantly reduced circulating levels of IL-13 but not IL-4. Treatment of mice with MSCs from TGFβ1 knockout mice failed to have a therapeutic effect, consistent with in vitro studies showing that IL4 within BAL from ragweed-challenged mice induced TGFB1 expression in MSCs. Similar results were obtained by Bonefield et al. [126] in an ovalbumin model of asthma. In the latter study, MSCs also reduced systemic levels of IL-1β, suppressed inducible nitric oxide synthase expression from lung infiltrating monocytes, and enhanced IFN-γ levels in BAL fluid.
Paracrine Effects in Wound Healing and Diabetes
Wound Healing
MSCs constitutively express a variety of growth factors important for wound healing including PDGF, EGF, TGFβ1, VEGF, KGF, FGF2, and HGF [127]. Moreover, expression of PDGF, EGF, KGF, and HGF is significantly elevated when MSCs are exposed to LPS or IL-1B. Similarly, treatment of MSCs with superfusates from wounded abdominal tissue upregulated expression of TGFB1, EGF, and VEGF. Human MSCs and adipose-derived cells also secrete appreciable levels of IGF1, VEGF, and HGF, the latter two of which were upregulated by exposure to TNF-α [128]. Therefore, exposure to the wound microenvironment appears to induce in MSCs expression of various angiogenins and mitogens that normally participate in the wound healing process. MSCs have also been reported to secrete significantly higher levels of ANG1, KGF, IGF-1, PDGF, and erythropoietin as compared to dermal fibroblasts [129, 130], which may explain their superior healing capacity [130]. They also secrete high levels of cysteine-rich angiogenic inducer 61 (CYR61), and depletion of this protein from MSC-conditioned media abolished their angiogenic activity [51]. This is consistent with studies showing that MSC-conditioned media also stimulates wound healing [129]. Therefore, paracrine signaling via release of angiogenins (VEGF, EPO, CYR61), growth factors (EGF, FGF2, IGF-1, KGF, PDGF, TGFβ1), and other soluble factors promote angiogenesis, keratinocyte proliferation, and migration and may also modulate the activity of inflammatory cells. MSCs also secrete a large array of extracellular matrix molecules including collagens, fibronectin, and various matrix metalloproteinases and as such may directly contribute to tissue repair by functioning akin to fibroblasts.
Diabetes
MSCs have been shown to normalize blood glucose levels when administered to streptozotocin-induced hyperglycemic mice [131, 132]. In these studies, MSCs were shown to increase the number of pancreatic islets and beta cells producing mouse insulin and also prevent renal damage. Consistent with these results, other studies have shown that islets from MSC-treated animals expressed high levels of pancreas/duodenum homeobox protein 1 and insulin and that peripheral T cells from these animals exhibited a shift toward IL10/IL13 production [133]. Coculture of human pancreatic islets with MSCs also improves their adenosine-5′-triphosphate/adenosine-5′-diphosphate ratio and insulin secretory function in vitro. MSC-conditioned media was shown to contain high levels of IL-6, VEGF-A, HGF, and TGFβ1, factors known to improve the survival, function, and angiogenesis/revascularization of islets [134]. Consistent with this result, Xu et al. [135] reported that exposure of MSCs to rat pancreatic extracts significantly upregulated secretion of IGF-1, VEGF, and FGF2 and conditioned media from pancreatic extract-treated MSCs was able to lower blood glucose levels when administered to diabetic rats. Finally, MSCs also reportedly restore normoglycemia in diabetic rats by promoting vascularization and enhancing survival of islet grafts via secretion of VEGF [136].
Paracrine Effects in Neurodegenerative Diseases
The anti-inflammatory and immunomodulatory affects of MSCs may be advantageous in the treatment of various neurodegenerative diseases since inflammation is thought to contribute significantly to their pathogenesis. For example, elevated levels of pro-inflammatory cytokines in brain tissue are detected in mouse models of lysosomal storage diseases, and the degree of inflammation has been shown to coincide with the onset of clinical symptoms in these models [137–139]. In most cases, microglia activation occurs in response to aberrant neural cell function or as part of a wider stress response in the brain and typically precedes neuronal cell loss. Inflammation is also a prominent feature in other neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease [140].
With respect to animal models of storage disease, injection of unmodified MSCs into the cerebellum markedly reduces the extent of microglial and astrocyte activation and reduces levels of macrophage colony-stimulating factor, a microglial activator, in a mouse model of Niemann-Pick type C disease [141]. Similar results were also obtained with adipose tissue-derived stem cells [142]. In the latter studies, cell transplantation directly to the cerebellum resulted in rescue of Purkinje neurons as evidenced by their enhanced electrical activity and suppression of neuro-inflammation based on decreased glial cell activation and decreased expression levels of IL-1B, IL-6, and TNF-α protein in the cerebellum.
MSCs administration also delays disease progression, improves motor performance, and decreases microglial activation and astrogliosis in the spinal cords of mice carrying a glycine 93 to alanine (G93A) mutation in the superoxide dismutase 1 gene (SOD1), a model of amyotrophic lateral sclerosis (ALS) [143, 144]. The therapeutic effect of MSCs in this model is also likely paracrine in nature based on studies showing that exposure of MSCs to extracts from the brains or spinal cords of SOD1 mutant transgenic rats significantly upregulated expression of VEGF-A, HGF, and NGF and suppressed expression of FGF2 and IGF1. Moreover, spinal cord but not brain extracts induced expression of BDNF and GDNF [145]. Other studies have shown that exposure to G5 supplement, FGF2, and CNTF induces mRNA and protein expression of the glutamate transporter 1 (GLT-1) in MSCs, which results in an enhanced ability of the cells to uptake aspartate. This result suggests that MSCs may be neuro-protective by restoring glutamate homeostasis in response to disease [146]. Importantly, MSCs from SOD1(G93A) mutant rats showed reduced aspartate uptake despite expressing higher level of GLT1 mRNA and protein. These results indicated that MSCs from mutant rats expressed a nonfunctional GLT1 receptor and therefore were unable to protect neurons from glutamate toxicity. This finding questions the suitability of autologous stem cell grafts for treatment of familial forms of ALS. Similarly, Cho et al. [147] reported that secreted levels of several trophic factors including FGF2, HGF, IGF-1, CXCL12, and VEGF-A are decreased in MSCs derived from the bone marrow of human ALS patients. Nevertheless, direct administration of MSCs from human ALS patients into the cistern magna of mice engineered to overexpress the human mutant SOD1(G93A) gene resulted in a dose-dependent increase in life span and survival of motor neurons in the ventral horn of the spinal cord [148]. Therefore, patient-specific MSCs may still provide some degree of therapeutic benefit when administered in vivo, despite their reduced paracrine activity.
Direct administration of MSCs into the cerebellum of newborn Lurcher mutant mice, a model characterized by the selective early postnatal death of Purkinje cells, resulted in significant improvement in motor function as evidenced by improvement in the rotarod test. At 2-month posttransplant, histological analysis demonstrated a significant increase in Purkinje cell numbers in treated versus control mice and revealed that many of the surviving MSCs in brain were juxtaposed to the Purkinje cell layer in the cerebellum. This outcome was attributed to secretion by MSCs of BDNF, NT-3, and GDNF, neurotrophins important for Purkinje cell survival [149]. MSCs also decreased the extent of glial activation, oxidative stress, and apoptosis within the hippocampus and improved memory function and learning in a mouse model of acute Aβ-induced Alzheimer’s disease [150].
Collectively, these studies indicate that paracrine mechanisms also contribute significantly to the therapeutic effect of MSCs in a variety of neurodegenerative diseases. This is consistent with genomics-based studies showing that MSCs secrete various neurotrophins as well as other factors that promote neural cell survival and neurite outgrowth under stressful conditions and following injury in vivo [47, 151, 152]. This capacity of MSCs is likely related to the fact that bone and marrow are innervated by nervous tissue, providing a means by which sympathetic efferent input can modulate hematopoiesis. Despite the benefits afforded by MSCs in the aforementioned disease models, it should be noted that one study has shown that MSC-conditioned medium promotes glial cell activation and upregulates expression of TNF-α and IL-6 in organotypic cultures of the hippocampus, leading to neuronal cell death [153]. These results should be carefully assessed as they suggest that MSC-based therapies may yield adverse or unforeseen outcomes that may exacerbate the disease state. The latter necessitates incorporating dose response studies into translational and clinical trials and metrics to measure potential adverse side effects.
Conclusions
Only a decade ago, MSCs were thought to function specifically as a reservoir of progenitor cells to maintain homeostasis and facilitate repair of connective tissues following injury. While exploring their potential use in tissue engineering remains an avid area of research, the field in general has witnessed a major paradigm shift with respect to the function and potential clinical applications of MSCs. Specifically, it is now believed that the principal mechanism by which MSCs affect tissue repair is by paracrine signaling. This paradigm shift has gained support from genomics and proteomics-based studies, which revealed that MSCs secrete an array of proteins that exhibit angiogenic, anti-inflammatory, immunomodulatory, and trophic activity, and studies demonstrating that MSCs or MSC-conditioned media ameliorate disease and promote tissue repair in a broad array of experimental animal models of disease and human clinical trials. Moreover, evidence is mounting that MSCs express a large repertoire of surface receptors that coordinately regulate paracrine function in response to changing environmental conditions, such as hypoxia, inflammation, and infection. Therefore, it appears the pendulum has swung full circle. In the 1970s, cytokine secretion by MSCs was exploited to establish long-term bone marrow cultures, which led to the identification of the HSC. Most MSC-based therapies currently being pursued today also exploit the paracrine activity of MSCs. Despite these advances, aspects of MSC biology remain indeterminate including their origin during development, the molecular mechanisms that regulate self-renewal and lineage specification, and how paracrine functions are specified within populations. Efforts aimed at addressing these questions will likely lead to the procurement of even more potent cell-based vectors for clinical therapies. Therefore, MSC research likely will remain an exciting and rewarding enterprise for some time to come.
References
Phinney DG (2008) Marrow stem cells. In: Polak J, Mantalaris S, Harding S (eds) Advances in tissue engineering. Imperial College Press, London, pp 95–122
Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I (2006) The role of mesenchymal stem cells in haemopoiesis. Blood Rev 20(3):161–171
Clark BR, Keating A (1995) Biology of bone marrow stroma. Ann N Y Acad Sci 770:70–78
Anklesaria P, Greenberger JS, Fitzgerald TJ, Sullenbarger B, Wicha M, Campbell A (1991) Hemonectin mediates adhesion of engrafted murine progenitors to a clonal bone marrow stromal cell line from Sl/Sld mice. Blood 77(8):1691–1698
Campbell AD, Long MW, Wicha MS (1987) Haemonectin, a bone marrow adhesion protein specific for cells of granulocyte lineage. Nature 329(6141):744–746
Zuckerman KS, Rhodes RK, Goodrum DD, Patel VR, Sparks B, Wells J et al (1985) Inhibition of collagen deposition in the extracellular matrix prevents the establishment of a stroma supportive of hematopoiesis in long-term murine bone marrow cultures. J Clin Invest 75(3):970–975
Fernandez M, Minguell JJ (1996) The role of collagen in hematopoiesis. Braz J Med Biol Res 29(9):1201–1207
Klein G (1995) The extracellular matrix of the hematopoietic microenvironment. Experientia 51(9–10):914–926
Siczkowski M, Clarke D, Gordon MY (1992) Binding of primitive hematopoietic progenitor cells to marrow stromal cells involves heparan sulfate. Blood 80(4):912–919
Talts JF, Falk M, Ekblom M (1998) Expansion of the nonadherent myeloid cell population by monoclonal antibodies against tenascin-C in murine long-term bone marrow cultures. Exp Hematol 26(7):552–561
Nagahisa H, Nagata Y, Ohnuki T, Osada M, Nagasawa T, Abe T et al (1996) Bone marrow stromal cells produce thrombopoietin and stimulate megakaryocyte growth and maturation but suppress proplatelet formation. Blood 87(4):1309–1316
Seki M, Kameoka J, Takahashi S, Harigae H, Yanai N, Obinata M et al (2006) Identification of tenascin-C as a key molecule determining stromal cell-dependent erythropoiesis. Exp Hematol 34(4):519–527
Gordon MY, Riley GP, Watt SM, Greaves MF (1987) Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 326(6111):403–405
Roberts R, Gallagher J, Spooncer E, Allen TD, Bloomfield F, Dexter TM (1988) Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature 332(6162):376–378
Gupta P, Oegema TR Jr, Brazil JJ, Dudek AZ, Slungaard A, Verfaillie CM (1998) Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood 92(12):4641–4651
Kittler EL, McGrath H, Temeles D, Crittenden RB, Kister VK, Quesenberry PJ (1992) Biologic significance of constitutive and subliminal growth factor production by bone marrow stroma. Blood 79(12):3168–3178
Deryugina EI, Muller-Sieburg CE (1993) Stromal cells in long-term cultures: keys to the elucidation of hematopoietic development? Crit Rev Immunol 13(2):115–150
Nagao T (1995) Significance of bone marrow stromal cells in hematopoiesis and hematological disorders. Tokai J Exp Clin Med 20(2):121–130
Shao LE, Frigon NL, Yu A, Palyash J, Yu J (1998) Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin A in human marrow stromal cells and their implications. Cytokine 10(3):227–235
Yang M, Li K, Lam AC, Yuen PMP, Fok TF, Chesterman CN et al (2001) Platelet-derived growth factor enhances granulopoiesis via bone marrow stromal cells. Int J Hematol 73(3):327–334
Lisovsky M, Braun SE, Ge Y, Takahira H, Lu L, Savchenko VG et al (1996) Flt3-ligand production by human bone marrow stromal cells. Leukemia 10(6):1012–1018
Weimar IS, Miranda N, Muller EJ, Hekman A, Kerst JM, de Gast GC et al (1998) Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+). Exp Hematol 26(9):885–894
Li L, Milner LA, Deng Y, Iwata M, Banta A, Graf L et al (1998) The human homolog of rat jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity 8(1):43–55
Manske JM, Sullivan EL, Andersen SM (1995) Substance P mediated stimulation of cytokine levels in cultured murine bone marrow stromal cells. Adv Exp Med Biol 383:53–64
Sakagami Y, Girasole G, Yu XP, Boswell HS, Manolagas SC (1993) Stimulation of interleukin-6 production by either calcitonin gene-related peptide or parathyroid hormone in two phenotypically distinct bone marrow-derived murine stromal cell lines. J Bone Miner Res 8(7):811–816
Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ et al (2004) Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res 19(11):1873–1881
Renstrom J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A et al (2009) Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell 5(2):157–167
Tokoyoda K, Hauser AE, Nakayama T, Radbruch A (2010) Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10(3):193–200
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA et al (2001) Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 97(10):3075–3085
Bodo M, Baroni T, Tabilio A (2009) Haematopoietic and stromal stem cell regulation by extracellular matrix components and growth factors. J Stem Cells 4(1):57–69
Gupta P, Blazar BR, Gupta K, Verfaillie CM (1998) Human CD34(+) bone marrow cells regulate stromal production of interleukin-6 and granulocyte colony-stimulating factor and increase the colony-stimulating activity of stroma. Blood 91(10):3724–3733
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M et al (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5(3):309–313
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18(2):307–316
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105(1):93–98
Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 96(19):10711–10716
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T et al (2002) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 109(10):1291–1302
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N et al (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100(14):8407–8411
Anjos-Afonso F, Siapati EK, Bonnet D (2004) In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci 117 (Pt 23): 5655–5664
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells 25(11):2896–2902
Tremain N, Korkko J, Ibberson D, Kopen GC, DiGirolamo C, Phinney DG (2001) MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells 19(5):408–418
Phinney DG (2006) Gene expression profiles of mesenchymal stem cells. In: Nolta JA (ed) Genetic engineering of mesenchymal stem cells. Springer, Dordrecht, pp 59–80
Phinney DG, Hill K, Michelson C, DuTreil M, Hughes C, Humphries S et al (2006) Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells 24(1):186–198
Wieczorek G, Steinhoff C, Schulz R, Scheller M, Vingron M, Ropers HH et al (2003) Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res 311(2):227–237
Silva WA Jr, Covas DT, Panepucci RA, Proto-Siqueira R, Siufi JL, Zanette DL et al (2003) The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 21(6):661–669
Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE et al (2005) Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine 31(2):119–126
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K et al (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104(26):11002–11007
Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG (2006) Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 198(1):54–64
Phinney DG (2007) Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle 6(23):2884–2889
Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E et al (2008) Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112(13):4991–4998
Sarojini H, Estrada R, Lu H, Dekova S, Lee MJ, Gray RD et al (2008) PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Biochem 104(5):1793–1802
Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E (2009) Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol 219(3):563–571
Zipori D (2004) The nature of stem cells: state rather than entity. Nat Rev Genet 5(11):873–878
Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S et al (2007) Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109(4):1422–1432
Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB (2008) Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 26(1):99–107
Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR (2008) Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B - but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294(3):C675–C682
Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A et al (2009) Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 27(4):909–919
Zhang A, Wang Y, Ye Z, Xie H, Zhou L, Zheng S (2010) Mechanism of TNF-alpha-induced migration and hepatocyte growth factor production in human mesenchymal stem cells. J Cell Biochem 111(2):469–475
Lee MJ, Kim J, Kim MY, Bae YS, Ryu SH, Lee TG et al (2010) Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res 9(4):1754–1762
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822
Ryan JM, Barry F, Murphy JM, Mahon BP (2007) Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 149(2):353–363
Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu J, Phinney DG, LaMarca HL et al (2007) Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol 35(4):640–652
Li Z, Wei H, Deng L, Cong X, Chen X (2010) Expression and secretion of interleukin-1beta, tumour necrosis factor-alpha and interleukin-10 by hypoxia- and serum-deprivation-stimulated mesenchymal stem cells. FEBS J 277(18):3688–3698
Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A et al (2011) Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 300(1):F254–F262
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ et al (2004) Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94(1):92–95
Haider H, Jiang S, Idris NM, Ashraf M (2008) IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103(11):1300–1308
Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ (2003) Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem 278(7):4705–4712
Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M et al (1998) Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 98(25):2800–2804
Suzuki G, Lee TC, Fallavollita JA, Canty JM Jr (2005) Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96(7):767–775
Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J et al (2005) Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80(1):229–236; discussion 36–37
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H et al (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11(4):367–368
Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N et al (2007) Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104(5):1643–1648
Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y et al (2010) Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol 299(6):H1772–H1781
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J et al (2001) Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7(4):430–436
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S et al (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109(12):1543–1549
Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C (2007) Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292(5):F1626–F1635
Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ (2007) Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 25(9):2363–2370
Jiang BH, Zheng JZ, Aoki M, Vogt PK (2000) Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA 97(4):1749–1753
Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M et al (2007) Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol 42(1):88–97
Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM et al (2010) Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res 3(5):547–558
Uemura R, Xu M, Ahmad N, Ashraf M (2006) Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98(11):1414–1421
Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C et al (2008) Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res 102(5):597–606
Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V et al (2006) Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290(6):H2196–H2203
Zhang N, Li J, Luo R, Jiang J, Wang JA (2008) Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes 116(2):104–111
Mizuno S, Kurosawa T, Matsumoto K, Mizuno-Horikawa Y, Okamoto M, Nakamura T (1998) Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J Clin Invest 101(9):1827–1834
Yang J, Dai C, Liu Y (2003) Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol 163(2):621–632
Ruvinov E, Leor J, Cohen S (2011) The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 32(2):565–578
Lee CH, Shah B, Moioli EK, Mao JJ (2010) CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120(9):3340–3349
Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC (2004) Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation 78(1):83–88
Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG et al (2008) Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells 26(5):1307–1314
Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A et al (2006) Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int 70(1):121–129
Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH et al (2009) Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 27(12):3063–3073
Igarashi A, Segoshi K, Sakai Y, Pan H, Kanawa M, Higashi Y et al (2007) Selection of common markers for bone marrow stromal cells from various bones using real-time RT-PCR: effects of passage number and donor age. Tissue Eng 13(10):2405–2417
Yamauchi T, Umeda F, Masakado M, Isaji M, Mizushima S, Nawata H (1994) Purification and molecular cloning of prostacyclin-stimulating factor from serum-free conditioned medium of human diploid fibroblast cells. Biochem J 303(Pt 2):591–598
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M et al (2005) Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11(1):96–104
Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M et al (2004) Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res 1030(1):19–27
Li J, Zhu H, Liu Y, Li Q, Lu S, Feng M et al (2010) Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macaca fascicularis. Brain Res 1334:65–72
Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD et al (2008) Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med 40(4):387–397
Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A et al (2007) One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke 38(7):2150–2156
Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M (2008) Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke 39(9):2571–2577
Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J et al (2010) Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One 5(2):e9027
Andrews EM, Tsai SY, Johnson SC, Farrer JR, Wagner JP, Kopen GC et al (2008) Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol 211(2):588–592
Sokolova IB, Fedotova OR, Zin’kova NN, Kruglyakov PV, Polyntsev DG (2006) Effect of mesenchymal stem cell transplantation on cognitive functions in rats with ischemic stroke. Bull Exp Biol Med 142(4):511–514
Deng YB, Ye WB, Hu ZZ, Yan Y, Wang Y, Takon BF et al (2010) Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol Res 32(2):148–156
Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T et al (2010) Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 88(5):1017–1025
Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J et al (2002) Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 22(4):275–279
Xin H, Li Y, Chen X, Chopp M (2006) Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res 83(8):1485–1493
Lu W, Tsirka SE (2002) Partial rescue of neural apoptosis in the Lurcher mutant mouse through elimination of tissue plasminogen activator. Development 129(8):2043–2050
Chopp M, Li Y (2006) Transplantation of bone marrow stromal cells for treatment of central nervous system diseases. Adv Exp Med Biol 585:49–64
Chopp M, Li Y, Zhang ZG (2009) Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40(3 Suppl):S143–S145
Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2008) Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab 28(2):329–340
Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H et al (2006) Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129(Pt 10):2734–2745
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S et al (2004) BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther 9(2):189–197
Huang D, Zhang Z, Chen B, Wu X, Wang N, Zhang Y (2008) Therapeutic efficacy of lentiviral vector mediated BDNF gene-modified MSCs in cerebral infarction. Sheng Wu Gong Cheng Xue Bao 24(7):1174–1179
Aizman I, Tate CC, McGrogan M, Case CC (2009) Extracellular matrix produced by bone marrow stromal cells and by their derivative, SB623 cells, supports neural cell growth. J Neurosci Res 87(14):3198–3206
Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J (2009) Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol 6(3):207–213
Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS et al (2008) Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant 17(9):1045–1059
Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG et al (2008) Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc 40(5):1700–1705
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S et al (2009) Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 175(1):303–313
Fang X, Neyrinck AP, Matthay MA, Lee JW (2010) Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 285(34):26211–26222
Salazar KD, Lankford SM, Brody AR (2009) Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 297(5):L1002–L1011
Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S et al (2007) Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 293(1):L131–L141
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179(3):1855–1863
Lee JW, Fang X, Gupta N, Serikov V, Matthay MA (2009) Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106(38):16357–16362
Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG et al (2010) Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA 107(12):5652–5657
Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI (2010) Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 299(6):L760–L770
Liu Y, Dulchavsky DS, Gao X, Kwon D, Chopp M, Dulchavsky S et al (2006) Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 136(2):336–341
Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR (2006) Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 291(4):R880–R884
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886
Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25(10):2648–2659
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD et al (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 103(46):17438–17443
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14(6):631–640
Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A et al (2009) Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 32(1):33–42
Park KS, Kim YS, Kim JH, Choi BK, Kim SH, Oh SH et al (2009) Influence of human allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5′-triphosphate)/ADP (adenosine-5′-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplant Proc 41(9):3813–3818
Xu YX, Chen L, Hou WK, Lin P, Sun L, Sun Y et al (2009) Mesenchymal stem cells treated with rat pancreatic extract secrete cytokines that improve the glycometabolism of diabetic rats. Transplant Proc 41(5):1878–1884
Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G et al (2009) Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc 41(5):1797–1800
Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF (2003) Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA 100(4):1902–1907
Hong YB, Kim EY, Jung SC (2006) Upregulation of proinflammatory cytokines in the fetal brain of the Gaucher mouse. J Korean Med Sci 21(4):733–738
Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d’Azzo A et al (2003) Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain 126(Pt 4):974–987
Das S, Basu A (2008) Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res 86(6):1199–1208
Bae JS, Furuya S, Ahn SJ, Yi SJ, Hirabayashi Y, Jin HK (2005) Neuroglial activation in Niemann-Pick Type C mice is suppressed by intracerebral transplantation of bone marrow-derived mesenchymal stem cells. Neurosci Lett 381(3):234–236
Bae JS, Carter JE, Jin HK (2010) Adipose tissue-derived stem cells rescue Purkinje neurons and alleviate inflammatory responses in Niemann-Pick disease type C mice. Cell Tissue Res 340(2):357–369
Zhao CP, Zhang C, Zhou SN, Xie YM, Wang YH, Huang H et al (2007) Human mesenchymal stromal cells ameliorate the phenotype of SOD1-G93A ALS mice. Cytotherapy 9(5):414–426
Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D et al (2008) Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 31(3):395–405
Nicaise C, Mitrecic D, Pochet R (2011) Brain and spinal cord affected by amyotrophic lateral sclerosis induce differential growth factors expression in rat mesenchymal and neural stem cells. Neuropathol Appl Neurobiol 37(2):179–188
Boucherie C, Caumont AS, Maloteaux JM, Hermans E (2008) In vitro evidence for impaired neuroprotective capacities of adult mesenchymal stem cells derived from a rat model of familial amyotrophic lateral sclerosis (hSOD1(G93A)). Exp Neurol 212(2):557–561
Cho GW, Noh MY, Kim HY, Koh SH, Kim KS, Kim SH (2010) Bone marrow-derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cells Dev 19(7):1035–1042
Kim H, Kim HY, Choi MR, Hwang S, Nam KH, Kim HC et al (2010) Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett 468(3):190–194
Jones J, Jaramillo-Merchan J, Bueno C, Pastor D, Viso-Leon M, Martinez S (2010) Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis 40(2):415–423
Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T et al (2010) The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett 481(1):30–35
Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M et al (2007) Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant 16(1):41–55
Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z et al (2007) Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology 27(4):355–363
Horn AP, Bernardi A, Luiz Frozza R, Grudzinski PB, Hoppe JB, de Souza LF et al (2011) Mesenchymal stem cell-conditioned medium triggers neuroinflammation and reactive species generation in organotypic cultures of rat hippocampus. Stem Cells Dev 20(7):1171–1181
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Boregowda, S.V., Phinney, D.G. (2013). MSCs: Paracrine Effects. In: Hematti, P., Keating, A. (eds) Mesenchymal Stromal Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-5711-4_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5711-4_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-5710-7
Online ISBN: 978-1-4614-5711-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)