Abstract
Histological and molecular evidence has led to a model of breast cancer progression in which cells from the terminal duct lobular unit give rise to atypical ductal hyperplasia or atypical lobular hyperplasia, which can progress to ductal carcinoma in situ or lobular carcinoma in situ, and eventually to invasive ductal carcinoma or invasive lobular carcinoma respectively. This review will present a histomorphological and epidemiological overview of the pre-invasive stages of breast cancer progression. As there is mounting evidence that these stages are likely rough phenotypes of underlying molecular changes, current knowledge regarding changes in genetic and epigenetic features of breast cancer progression will also be discussed. Microarray and CGH-based studies will be described, which suggest that low- and high-grade breast cancers can arise from normal terminal ducts through two distinct molecular pathways. Various in vitro and in vivo models used to study the cellular and molecular changes involved in early breast cancer progression will be presented. Lastly, the specific transition from pre-invasive to invasive breast cancer will be addressed, including possible molecular predictors of the invasive phenotype and a contemporary view highlighting the involvement of the tumor microenvironment during the transition to invasive disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Basal-like subtype
- Breast cancer progression
- Ductal carcinoma in situ
- Invasive ductal carcinoma
- Predictive biomarker
- Pre-invasive breast cancer
- Tumor microenvironment
- Lobular carcinoma
- Flat epithelial atypia (FEA)
- In vivo models
- In vitro models
- Invasive phenotype
8.1 Introduction
Breast cancer continues to be a major health concern among women worldwide. In North America, there has been a decreasing trend in the mortality rate of breast cancer over the last several decades [1,2]. This is likely due to increased screening and improved diagnostic recognition of early curable stages. However, there will still be approximately 45,000 deaths due to metastatic breast cancer in North America in 2012 [1,2]. There continues to be a clinical need for molecular biomarkers that can predict which non-invasive breast cancers are likely to progress to malignancy. An important event in the progression of breast cancer is the transition from a pre-invasive lesion to an invasive phenotype. Upon diagnosis of an in situ lesion, 10–15 % of women develop subsequent invasive disease [3]; hence, there is a clinical problem of predicting which pre-invasive lesions are likely to progress to malignancy.
8.2 Histopathologic Description of Breast Cancer Progression
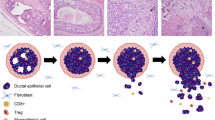
Evidence has led to a histological model of breast cancer progression in which cells from the terminal duct lobular unit give rise to atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH), which can in turn give rise to ductal carcinoma in situ (DCIS) or lobular carcinoma in situ (LCIS), and eventually to invasive ductal carcinoma (IDC) or lobular carcinoma (ILC) respectively (Fig. 8.1) [4–9]. In this chapter, breast cancer progression will first be discussed from histomorphological and epidemiological perspectives, followed by molecular evidence to support the view of this progression model.
Traditional linear model of breast cancer progression. Multiple lines of evidence (histomorphological, immunohistochemical, and molecular) support this model. Molecular alterations occurring in the normal terminal duct lobular unit (TDLU) can result in flat epithelial atypia (FEA). FEA may lead to additional changes that give rise to atypical ductal hyperplasia and ductal carcinoma in situ, upon which subsequent alterations in turn give rise to invasive ductal carcinoma (middle). Likewise, molecular alterations occurring in the normal TDLU result in atypical lobular hyperplasia, which can give rise to lobular carcinoma in situ, upon which subsequent alterations in turn give rise to invasive lobular carcinoma (top). There is some evidence that usual ductal hyperplasia may in some instances also be considered an early stage of breast cancer progression (bottom)
8.2.1 A Histopathological Overview of the Pre-invasive Stages of Breast Cancer
In order to provide a contemporary overview of the current molecular-based model of breast cancer progression, this section will build a conceptual framework of progression from normal breast tissue to the pre-invasive stages of breast cancer from a histomorphological perspective (Fig. 8.1).
The human breast is composed of thousands of small glands lined by epithelial cells that produce milk. These glands are composed of a single terminal duct with multiple end acini (terminal ductules in the non-functioning state) and are referred to as the terminal duct lobular unit (TDLU). Once milk is secreted from cells of the TDLU, it is propagated outward through a series of interconnecting and increasingly larger ducts. The TDLU is composed of two cell layers: (a) an inner luminal epithelial layer composed of low columnar cells in the terminal duct and cuboidal cells in the acini/terminal ductules, and (b) an outer myoepithelial layer directly adjacent to the basement membrane. Pre-invasive epithelial lesions are characterized by a neoplastic epithelial cell proliferation, which remains confined to the ductal-lobular network and does not penetrate the basement membrane or invade into the surrounding stroma.
The two most common histologic types of invasive breast cancer are known as infiltrating ductal (also known as “no special type, NOS”) and lobular carcinoma. These are matched by pre-invasive ductal and lobular neoplasias. Both types of breast cancers arise in the TDLU and the distinction between the two is based on morphological differences of the cells [10, 11]. Specifically, the lobular morphology consists of small, non-polarized cells that are discohesive, with vacuolated cytoplasm and a high nuclear to cytoplasmic ratio, resembling cuboidal cells of breast acini/terminal ductules. In contrast, the ductal morphology consists of larger, polarized cells in cohesive groups that resemble columnar cells of terminal ducts. The pre-invasive lobular lesions include atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS). Pre-invasive ductal lesions include atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS) and possibly some columnar cell lesions, such as flat epithelial atypia (FEA). In addition, although more controversial, there is some evidence that usual ductal hyperplasia (UDH) and an entity known as “unfolded lobules” may in some instances also be considered early stages (non-obligate precursors) of breast cancer progression [8, 12].
FEA, ADH, and DCIS are considered non-obligate precursors of invasive ductal carcinoma (IDC). FEA is characterized by a proliferation and replacement of luminal cells of the TDLU by one or more layers of columnar epithelial cells that exhibit low-grade cytological atypia [13]. The cells of FEA may form either a single cell layer or multiple cell layers [14], such that FEA by present definition is comprised of both columnar cell change with atypia (1-2 cell layers) and columnar cell hyperplasia with atypia (multiple cell layers). Like FEA, ADH is also characterized by low-grade cytological atypia, but differs from FEA in that it exhibits architectural abnormalities such as solid patterns with even cell placement, punched-out secondary lumena, rigid bridging and cribriform or micropapillary morphologies. The differences between ADH and DCIS are based upon the degree of atypia and the extent of the atypical epithelial proliferation [15, 16]. DCIS is further classified based on cytomorphological (low, intermediate, or high nuclear grade) and architectural features, as well as the presence or absence of luminal necrosis, all of which have been associated with outcome. Comedo-type DCIS consists of cells that show a high degree of nuclear atypia and is associated with abundant central luminal necrosis. Comedo-type DCIS is generally more aggressive in terms of both risk for recurrence (with narrow margins of excision) and risk for associated invasion. Specific architectural types of DCIS also have different implications in terms of clinical behavior. For example, micropapillary type DCIS tends to be very extensive in the breast [17], whereas a centrally located papillary carcinoma in situ is more commonly a localized lesion with lower risk for recurrence upon complete excision [18]. Lastly, with the transition to invasive disease, important distinguishing factors between DCIS and IDC are the complete loss of the outer myoepithelial layer in the latter, with extension of neoplastic cells into the surrounding stromal compartment, beyond the basement membrane [19].
Lobular neoplasias form a spectrum of diseases and include ALH and LCIS, both of which are considered non-obligate precursors of invasive lobular carcinoma (ILC) [20, 21]. The main histological distinction between ALH and LCIS is based on the degree to which the TDLU is filled with neoplastic cells and the amount the lobular unit becomes distended as a result [4]. In ALH, the TDLU is colonized by a homogenous cell population of small, round, non-polarized, loosely cohesive cells that have a high nuclear to cytoplasmic ratio. The proliferation of ALH is limited (by definition involves less than 50 % of acini of a lobular unit) and leaves the acini/terminal ductules somewhat intact (lack distension/distortion). Conversely, cells of classical LCIS are the same cytomorphologically compared to ALH, but proliferation is extensive enough to completely fill and distend/distort the acini/terminal ductules of the TDLU. The loss of expression of membrane E-cadherin is a hallmark feature of both ALH and LCIS [22]. Variants of LCIS have been described, including a pleomorphic variant, which consists of medium- to large-sized cells, with pleomorphic nuclei, and LCIS with central zonal (“comedo type”) necrosis [23, 24].
8.3 Epidemiological Evidence of Breast Cancer Progression
Epidemiological studies have provided support for a linear model of breast cancer progression. Through long term cohort studies it has been shown that having a previous ADH or DCIS diagnosis greatly increases the risk of developing invasive mammary carcinoma, up to 4–5 times for ADH and up to 8–10 times for DCIS compared to the general population [4, 5, 25]. In addition, the relative risk positively correlates with grade, extent and presence/absence of zonal necrosis. Similarly, the risk of invasive disease in women diagnosed with LCIS (classic type) is estimated at 8–10 times greater than women in the general population [25]. The relative risk associated with a finding of FEA is not yet well-established, but studies to date suggest the risk for developing DCIS or invasive mammary carcinoma varies from a slightly increased risk to an increase in risk similar to ADH [13, 14, 26, 27]. Although epidemiologic data would suggest possible precursor status of usual ductal epithelial hyperplasia as well (1.5–2 fold increased risk for mammary carcinoma), molecular (loss of heterozygosity) studies indicate that this is likely a rare event [28].
8.4 Molecular Evidence of Breast Cancer Progression
The histological patterns observed during breast cancer progression are likely rough phenotypic indications of underlying molecular changes. There is interest in identifying the cellular and molecular events involved to determine which lesions are more likely to progress. An important barrier in understanding these changes has been the inability to accurately assess the molecular events as they relate to progression. Highly specific tissue-microdissection technologies and rapidly evolving high-throughput genomic and transcriptomic analyses have combined to identify a number of genomic and gene expression correlates between different stages of breast cancer.
8.4.1 Molecular Features of Ductal Carcinoma Progression
DCIS forms a spectrum of neoplastic lesions, with some behaving more aggressively than others. These different behaviors are to some degree associated with morphologic characteristics, as described above; however, it has been further revealed that different morphological subtypes of DCIS reflect distinct genomic alterations (Fig. 8.2). For example, comparative genomic hybridization (CGH)-based studies of DCIS revealed frequent loss of 16q in low- and intermediate-grade DCIS and gain of 1q and loss of 11q in intermediate-grade DCIS [29, 30]. Additionally, high-grade DCIS has been characterized by frequent loss of 8p, 11q, 13q, and 14q; gains of 1q, 5p, 8q, and 17q; and amplifications of 17q12 and 11q13 [29]. Further CGH analysis comparing DCIS and IDC revealed an almost identical pattern of genetic variations [29, 31] and there is also a correlation between copy number variations and progression [32]. Together, this data supports the view that DCIS is a direct precursor of IDC and that distinct genetic abnormalities are reflected by nuclear grade within the morphological spectrum of DCIS.
A multistep model of human breast cancer progression based on immunohistochemical, genomic and gene-expression data. Molecular events that occur in the normal terminal duct lobular unit (TDLU) (blue rectangle) give rise to two distinct molecular pathways (low- and high-grade molecular pathways). Linear pathological progression occurs from normal TDLUs to invasive breast cancer (solid arrows) and intrastage progression occurs within ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) (dotted arrows). The low-grade molecular pathway is characterized by loss of 16q, gain of 1q, and estrogen receptor (ER) and progesterone receptor (PR) positivity and is observed during pre-invasive and invasive stages of both ductal and lobular lesions (yellow rectangles). The high-grade molecular pathway is characterized by amplification of 17q12 and 11q13, loss of 13q, and ER/PR negativity (red rectangles). Pleomorphic lobular lesions [atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), and invasive lobular carcinoma (ILC)] resemble high-grade tumors; however, immunohistochemical and genetic analyses support an association with the low-grade molecular pathway. The Luminal A and Luminal B subtypes of IDC constitute the majority of lesions in the low-grade molecular pathway. The human epidermal growth factor receptor 2 (HER2) and basal-like subtypes of IDC constitute the majority of the lesions in the high-grade molecular pathway. Abbreviations: ADH, atypical ductal hyperplasia; FEA, flat epithelial atypia [12]
A number of loss of heterozygosity-based and CGH-based studies support the hypothesis that ADH is a precursor to low-grade DCIS. For example, LOH in regions 16q and 17p in ADH is similar to the variations observed in low-grade DCIS [6, 33, 34]. Given that ADH and low-grade DCIS share many architectural and cytological features [15, 16], it makes sense that they share common chromosomal abnormalities. This supports the presumed sequence of progression of ADH to low-grade DCIS; however, the progression to high-grade DCIS is less clear. In terms of histological presentations and genetic aberrations, high-grade DCIS is more heterogeneous than low- and intermediate-grade DCIS. Despite the greater intricacy of the pattern of genetic aberrations found in high-grade DCIS (those with 17q12 amplifications), deletions of 16q are less frequent, suggesting that the majority of high-grade DCIS lesions arise de novo.
There is also molecular evidence suggesting that FEA is a precursor to ADH and/or low-grade DCIS. It has been shown that FEA has similar genetic alterations compared to ADH and both low-grade DCIS and low-grade invasive carcinoma [35]. There is an increase of loss of heterozygosity at chromosome 16q in FEA, low-grade DCIS, and low-grade IDC [33] and there are comparable chromosomal copy number gains and losses present in FEA, ADH, and low-grade DCIS [28]. A number of immunohistochemical approaches have also linked FEA, ADH, and low-grade DCIS. For example, the atypical/neoplastic cells of all three of these pre-invasive lesions show the same high-level expression of estrogen receptors, progesterone receptors and cytokeratin 19 [30, 36], an increase in expression of cyclin D1 [36], as well as identical negativity for cytokeratin 5/6 [30] and Human Epidermal Growth Factor Receptor 2 (HER2) [30, 37]. These data support the view that FEA may be a precursor to ADH and low-grade DCIS.
Much of the research on understanding the gene expression alterations that occur during the early pre-invasive stages of breast cancer have focused on the neoplastic epithelial cells of ADH and DCIS [38, 39]. For example, a patient-matched microdissection and microarray-based study showed that marked transcriptional alterations occur between normal TDLUs and ADH, which are sustained in DCIS and IDC [38]. However, in several studies, there were no major transcriptional profile changes between the pre-invasive and invasive stages [38–40]. This has led these authors to suggest that both pre-invasive and invasive stages of progression are clonal in origin and that genes expressed during ADH and DCIS may be responsible for progression. A number of studies have linked gene expression patterns during early stages of progression to the risk of developing IDC and metastasis [41–44]; however, there is a clinical need to further identify and characterize reliable markers of risk for progression.
Distinct differences in gene expression are also associated with grade [38, 45, 46]. For example, distinct gene expression patterns are present in low- and high-nuclear grade DCIS [38] similar to what is observed in IDC. Additionally, ADH and low-grade DCIS share gene expression patterns associated with ER expression, whereas high-grade DCIS has a gene expression pattern more associated with the cell-cycle and mitosis [38]. In a similar respect, gene expression analysis of intermediate-grade DCIS shows a combination of low- and high-grade characteristics [38, 46]. These gene expression analyses support the view that low- and high-grade breast cancers arise from normal TDLUs through distinct molecular pathways (Fig. 8.2). Defining distinct molecular pathways and breast cancer subtypes (see Sect. 8.6.1) continues to be an evolving field as stratification of breast cancer into distinct subgroups and their molecular drivers involves an integrated view of the both the genome and transcriptome [47].
8.4.2 Molecular Features of Lobular Carcinoma Progression
CGH-based analyses of ALH and classic LCIS have revealed a similar pattern of chromosomal variation—loss of 16p, 16q, 17p, and 22q [48] in both. Further studies have identified a common loss of 16q in ALH, LCIS, and classic ILC [49, 50]. This supports the view that ALH and LCIS are closely related lesions and that all three (ALH, LCIS, and classic ILC) represent a progression continuum. Additionally, gene expression analysis of LCIS and classic ILC shows a pattern that is correlated with low-grade DCIS and IDC [50]. Taken together, these studies support a common—16q, low-grade molecular pathway that includes ALH, LCIS, and classic ILC, as well as FEA, ADH, low-grade DCIS and low-grade IDC.
A small subset of ILCs shows a more aggressive clinical course, and consists of neoplastic lobular cells with more marked nuclear atypia (pleomorphic ILC). These cancers share common genetic variations with classic ILC—e.g., loss of 16q and gain of 1q; as well as common features of high-grade IDC—e.g., amplification of 17q12 [51, 52]. However, a CGH-based study revealed that overall genetic variations of pleomorphic ILC are more closely correlated to those observed in classic ILC compared to IDC [52]. This suggests that pleomorphic ILC has a common molecular pathway of progression to that of classic ILC, that later accumulates alterations more characteristic of a high-grade lesion (Fig. 8.2). Similarly, there is CGH evidence that variant LCIS (pleomorphic LCIS, LCIS with necrosis) is of a common molecular background to classic LCIS (loss of 16q, gain of 1q), but that it is also associated with numerous further genetic aberrations that are more characteristic of a high-grade lesion [53].
8.5 Models and Methods Used to Study Breast Cancer Progression
In order to study the pre-invasive stages of breast cancer progression, several in vitro and in vivo models have been developed. Most take advantage of established human breast epithelial cell lines, which have been altered with activated oncogenes which drive production of these pre-invasive phenotypes [54–57]. In vivo models take advantage of the short time interval required for murine mammary progression and the high incidence of pre-malignant lesions in certain genetic backgrounds [58].
One such model system is the HMT-3522 series cell lines [54, 57], which consists of three cell lines derived from a single patient presenting with fibrocystic change. The HMT-3522/S1 cell line was produced during in vitro culture of the explant and was shown to be non-tumorigenic in a mouse xenograft model; whereas the HMT-3522/S2 cell line was established after an EGF-independent growth selection of the HMT-3522/S1 cell line and was shown to be tumorigenic. The third cell line, HMT-3522/T4-2, was derived from a HMT-3522/S2 tumor and is considered to be the most tumorigenic of the three cell lines. The HMT-3522 cell lines have undergone malignant transformation in vitro without being exposed to known carcinogenic agents and this transformation resembles some aspects of progression during pre-invasive breast disease [57]. Similarly, the MCF10AT cell lines represent a range of pre-invasive breast lesions [55, 56]. The MCF10A cells, also derived from a patient with fibrocystic change, are benign, immortalized breast epithelial cells. The MCF10AT cell line was derived from these cells by ras transformation. Subclones of the MCF10AT cells have generated a number of pre-invasive lesions including ADH and DCIS [55, 56]. Both the HMT-3522 and MCF10AT cell lines have proven useful; however, both model systems suffer from disadvantages. Both show mixed phenotypes and lack of stability of the phenotypes after culture. Additionally, the HMT-3522 cell lines lack a pre-DCIS stage, while the MCF10AT series is ras-transformation dependent, an uncommon event in spontaneous human breast cancers.
The 21T cell lines, derived from a single patient with metastatic breast cancer, represent a human breast cancer progression series [59, 60]. When grown in the mammary fat pad of nude mice, each cell line can reproduce a distinct stage of progression. For example, 21PT cells are non-tumorigenic and generate lesions of ADH, 21NT cells form lesions with the morphology of DCIS, and 21MT-1 cells generate IDC and are both tumorigenic and metastatic [60].
In vitro systems are very useful for high throughput studies. However, it has been shown that when grown in 2D in vitro culture, cell lines can have distinctly different morphology and genetic profiles compared to in vivo growth [61–66]. Also, important signals released by the extracellular matrix, which control normal homeostasis and tissue phenotypes, are lost when cells are cultured in 2D. When cells are cultured in a laminin-rich extracellular matrix, many of these signals remain intact [64]. By allowing cells to grow in a 3D conformation in contact with extracellular matrix proteins, certain characteristics of cell morphogenesis, proliferation, apoptosis and invasiveness may be studied in a highly controlled 3D environment. In fact, there have been many studies using 3D systems to examine molecular controls of morphogenesis in normal and neoplastic breast epithelial cells [65, 67–70]. There has been limited use of 3D in vitro systems to directly study progression through the pre-invasive to invasive stages of breast cancer; however, use of the HMT-3522 cell lines [71], the MCF10A-derived cell lines [72] and the 21T series cell lines [60] in 3D systems have proven useful in identifying potential regulators of progression.
In vivo breast cancer progression models have often made use of genetically engineered mice that have been designed to develop atypical lesions that mimic some pre-invasive lesions in humans [73]. In addition to genetic manipulation, other murine models make use of viral, chemical or hormonal agents that induce pre-malignant lesions [58]. However, since these model systems are mouse-derived, they fail to mimic exactly human breast cancer progression, especially from a molecular perspective. Therefore, in order to study the molecular events underlying the pre-invasive stages of human breast cancer progression, researchers often make use of human cell lines in xenograft model systems. One such model system makes use of genetically engineered human breast organoids and activated human breast stromal cell xenografts. This approach has been useful in defining genetic events that are required to drive progression from pre-invasive stages to invasive carcinoma [74].
Breast cancer tissues are comprised of a complex mixture of healthy epithelial cells, invasive or in situ tumor cells, surrounding stroma, infiltrating immune cells, blood vessels, and capillaries. As a consequence, whole tissue lysates represent a variety of cell types, making analysis of tumor cell-specific signals very difficult. Laser capture microdissection technology has, however, proven useful in identifying different gene expression signatures of progression [29, 30, 32, 49–52, 75] that are representative of the different tissue components of a tumor or precursor lesion.
8.6 The Transition from Pre-invasive to Invasive Breast Cancer
One of the most important events in the progression of breast cancer is the transition from pre-invasive, in situ lesions, to an invasive phenotype, in which neoplastic cells of DCIS (or LCIS) gain the ability to break through the basement membrane and invade into the surrounding stromal tissue. First, to address the clinical problem of predicting which in situ lesions are likely to progress to malignancy, molecular markers of the invasive phenotype will be discussed. This will be followed by a discussion of the traditional epithelial centric view of progression, as well as a more contemporary view that includes involvement of the tumor microenvironment.
8.6.1 Molecular Predictors of the Invasive Phenotype
Microarray analysis has been used to identify gene expression patterns that are associated with clinical outcome of invasive breast cancers [41, 76–78]. These invasive breast cancers have been commonly categorized into four major subtypes: luminal A, luminal B, HER2 overexpressing/ER-, and basal-like. The basal-like subtype is typically ER-/PR- and HER2-, has high proliferation rates and is associated with a poor prognosis [77, 78]. There has been emerging refinement of these subtypes using paired DNA-RNA profiles that has revealed 10 novel subgroups based on clinical outcome [47].
In women diagnosed with DCIS, 15–30 % will develop subsequent DCIS or IDC within 10 years after lumpectomy and radiation [3]. Of the 70–85 % that do not recur, it is likely that some are being overtreated. Conversely, since a majority of DCIS lesions are treated with lumpectomy (usually with accompanying radiation), some women are still prone to recurrence and/or subsequent invasive disease and require more aggressive treatment (mastectomies). Therefore, there is a clinical need for accurate markers that will predict if and when DCIS will progress to an invasive phenotype. Recently, expression profiling and immunohistochemical studies confirm the presence of molecular subtypes in DCIS [79–81] that parallel subtypes of invasive breast cancers, which may help to address this clinical problem. For example, it has been proposed that DCIS with high p16 and COX-2 expression in the absence of the cell proliferation marker Ki67 produces a normal stress-activation response that is protective against progression to an invasive phenotype [80]. In contrast, DCIS expressing high p16, high COX-2, and high Ki67 is interpreted as an abnormal response to cellular stress, and has been said to be associated with progression to a basal-like subtype of invasive breast cancer [80] (Table 8.1). In one study, DCIS with high p16, high COX-2, and high Ki67 was a better predictor for invasive breast cancer than nuclear grade [81]. In ADH, expression of p16, either alone or in combination with COX-2 and Ki67, was not found to be associated with progression to malignancy, although the combination of high COX-2 and Ki67 was found to convey stronger risk of breast cancer within 10 years [82] (Table 8.1). In DCIS at least, the expression signature of high p16, COX-2 and Ki67 may define a progression pathway of basal-like breast cancers to invasive disease, and could prove useful in the management of patients with high-grade DCIS. Identification of biomarkers indicating probability of progression to other subtypes of invasive cancer is ongoing and could further improve the clinical management of patients diagnosed with pre-invasive disease.
8.6.2 The Transition to the Invasive Phenotype: “Escape” versus “Release”
The transition from a pre-invasive to an invasive phenotype occurs when cells of DCIS (or LCIS) invade through the basement membrane and into the surrounding stromal tissue, thus representing a key event in the progression of breast cancer. Work such as that described above has yielded a rudimentary understanding of the stage-specific molecular changes within the neoplastic epithelial cells themselves. However, there is evidence that the tumor microenvironment is important during progression and that molecular changes in non-neoplastic cells [39, 83, 84], in addition to neoplastic epithelial cells, have the potential to drive progression [72, 85–87]. For example, in a cell line model for DCIS, the transition from DCIS to IDC did not require additional molecular alterations within the neoplastic epithelial cells, but rather progression to IDC was promoted by fibroblasts and suppressed by myoepithelial cells that make up the stromal and periductal microenvironment of DCIS. Molecular profiling of isolated epithelial and myoepithelial cells identified a signaling interaction network involving transforming growth factor β (TGF-β), hedgehog, cell adhesion molecules and p63, which was required for the differentiation of myoepithelial cells. Elimination of this signalling network resulted in loss of the myoepithelial cells and progression to an invasive phenotype [72]. Similarly, the establishment of the self-sustaining TGF-β and stromal cell-derived factor 1 (SDF-1) autocrine-signaling loops in resident mammary myofibroblasts can give rise to carcinoma-associated myofibroblasts that promote progression to invasive mammary carcinoma [88]. In addition, carcinoma-associated fibroblasts may mediate tumor growth and angiogenesis through the secretion of SDF-1 by acting directly on neoplastic epithelial cells via the CXCR4 receptor and by recruiting endothelial progenitor cells respectively [85]. Additionally, tumor-associated macrophages can have progression-promoting effects through the secretion of immunosuppressive cytokines, the release of free radicals such as nitric oxide and hydrogen peroxide, and the secretion of angiogenic factors. It has been suggested that these signaling mechanisms may be useful as therapeutic targets to block the development of tumor-promoting stromal cells [89].
Studies such as these have changed our view of breast cancer progression as solely an epithelial/tumor cell-driven process. Two possible models of the DCIS-to-IDC transition (“escape” vs. “release”) have been suggested [90]. The “escape” model proposes that genetic alterations accumulate in a subpopulation of neoplastic epithelial cells, which provides them with the ability to disrupt the myoepithelial layer and invade through the basement membrane into the surrounding stromal compartment. In contrast, the “release” model proposes that degradation of the basement membrane and subsequent invasion is due to alterations in the tumor microenvironment, particularly in the myoepithelial cells, myofibroblasts, fibroblasts, and tumor-infiltrating inflammatory cells. What is actually occurring is most likely a combination of both models whereby changes in neoplastic epithelial cells and non-neoplastic cells of the tumor microenvironment both contribute to the transition from pre-invasive to invasive disease.
8.7 Conclusion
Histological and molecular evidence has led to a model of breast cancer progression in which cells from the TDLU give rise to ADH or ALH, which can progress to DCIS or LCIS, and eventually to IDC or ILC respectively. Gene expression analyses suggest that low- and high-grade breast cancers can arise from normal TDLUs through two distinct gene expression pathways. The low-grade molecular pathway is characterized by loss of 16q, gain of 1q, and ER/PR positivity; whereas the high-grade molecular pathway is characterized by amplification of 17q12 and 11q13, loss of 13q, and ER/PR negativity. In addition, gene expression profiling has revealed distinct subtypes of invasive breast cancer based on clinical outcome. There is a clinical need to identify markers that will predict which pre-invasive lesions will progress, some of which may be unique to a particular subtype of IDC. Identification of such biomarkers is currently ongoing, which could improve the management of patients diagnosed with DCIS. It is important to bear in mind that the transition to invasive disease likely involves an interplay between the neoplastic cells themselves, as well as cells of the surrounding tumor microenvironment, such that both may be important in the future development of biomarkers and potential therapeutic targets.
References
American Cancer Society (2012) Cancer Facts & Figures 2012. American Cancer Society, Atlanta GA
Canadian Cancer Society’s Steering Committee on Cancer Statistics (2012) Canadian Cancer Statistics 2012. Canadian Cancer Society, Toronto ON
Kerlikowske K, Molinaro A, Cha I et al (2003) Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst 95:1692–1702
Page DL, Dupont WD, Rogers LW et al (1985) Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55:2698–2708
Page DL, Dupont WD (1993) Anatomic indicators (histologic and cytologic) of increased breast cancer risk. Breast Cancer Res Treat 28:157–166
Lakhani SR, Collins N, Stratton MR et al (1995) Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol 48:611–615
Allred DC, Mohsin SK, Fuqua SA (2001) Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 8:47–61
Arpino G, Laucirica R, Elledge RM (2005) Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med 143:446–457
Allred DC, Wu Y, Mao S et al (2008) Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res 14:370–378
Wellings SR, Jensen HM (1973) On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst 50:1111–1118
Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 55:231–273
Sgroi DC (2010) Preinvasive breast cancer. Annu Rev Pathol 5:193–221
Schnitt SJ (2003) The diagnosis and management of pre-invasive breast disease: flat epithelial atypia–classification, pathologic features and clinical significance. Breast Cancer Res 5:263–268
Lerwill MF (2008) Flat epithelial atypia of the breast. Arch Pathol Lab Med 132:615–621
Tavassoli FA, Norris HJ (1990) A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer 65:518–529
Page DL, Rogers LW (1992) Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol 23:1095–1097
Bellamy CO, McDonald C, Salter DM et al (1993) Noninvasive ductal carcinoma of the breast: the relevance of histologic categorization. Hum Pathol 24:16–23
Ueng SH, Mezzetti T, Tavassoli FA (2009) Papillary neoplasms of the breast: a review. Arch Pathol Lab Med 133:893–907
Pinder SE, Ellis IO (2003) The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)–current definitions and classification. Breast Cancer Res 5:254–257
Marshall LM, Hunter DJ, Connolly JL et al (1997) Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev 6:297–301
Venkitaraman R (2010) Lobular neoplasia of the breast. Breast J 16:519–528
Vos CB, Cleton-Jansen AM, Berx G et al (1997) E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer 76:1131–1133
Eusebi V, Magalhaes F, Azzopardi JG (1992) Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol 23:655–662
Weidner N, Semple JP (1992) Pleomorphic variant of invasive lobular carcinoma of the breast. Hum Pathol 23:1167–1171
Fitzgibbons PL, Henson DE, Hutter RV (1998) Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med 122:1053–1055
Martel M, Barron-Rodriguez P, Tolgay Ocal I et al (2007) Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992–1999). Virchows Arch 451:883–891
Kunju LP, Kleer CG (2007) Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol 38:35–41
Ellis IO (2010) Intraductal proliferative lesions of the breast: morphology, associated risk and molecular biology. Mod Pathol 23(Suppl 2):1–7
Buerger H, Otterbach F, Simon R et al (1999) Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol 187:396–402
Buerger H, Mommers EC, Littmann R et al (2001) Ductal invasive G2 and G3 carcinomas of the breast are the end stages of at least two different lines of genetic evolution. J Pathol 194:165–170
Simpson PT, Gale T, Reis-Filho JS et al (2005) Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol 29:734–746
Yao J, Weremowicz S, Feng B et al (2006) Combined cDNA array comparative genomic hybridization and serial analysis of gene expression analysis of breast tumor progression. Cancer Res 66:4065–4078
O’Connell P, Pekkel V, Fuqua SA et al (1998) Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst 90:697–703
Amari M, Suzuki A, Moriya T et al (1999) LOH analyses of premalignant and malignant lesions of human breast: frequent LOH in 8p, 16q, and 17q in atypical ductal hyperplasia. Oncol Rep 6:1277–1280
Moinfar F, Man YG, Bratthauer GL et al (2000) Genetic abnormalities in mammary ductal intraepithelial neoplasia-flat type (“clinging ductal carcinoma in situ”): a simulator of normal mammary epithelium. Cancer 88:2072–2081
Oyama T, Iijima K, Takei H et al (2000) Atypical cystic lobule of the breast: an early stage of low-grade ductal carcinoma in situ. Breast Cancer 7:326–331
Kusama R, Fujimori M, Matsuyama I et al (2000) Clinicopathological characteristics of atypical cystic duct (ACD) of the breast: assessment of ACD as a precancerous lesion. Pathol Int 50:793–800
Ma XJ, Salunga R, Tuggle JT et al (2003) Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A 100:5974–5979
Ma XJ, Dahiya S, Richardson E et al (2009) Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res 11:7
Porter D, Lahti-Domenici J, Keshaviah A et al (2003) Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res 1:362–375
van de Vijver MJ, He YD, van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Goetz MP, Suman VJ, Ingle JN et al (2006) A two-gene expression ratio of homeobox 13 and interleukin-17B receptor for prediction of recurrence and survival in women receiving adjuvant tamoxifen. Clin Cancer Res 12:2080–2087
Jerevall PL, Brommesson S, Strand C et al (2008) Exploring the two-gene ratio in breast cancer–independent roles for HOXB13 and IL17BR in prediction of clinical outcome. Breast Cancer Res Treat 107:225–234
Desmedt C, Sotiriou C (2006) Proliferation: the most prominent predictor of clinical outcome in breast cancer. Cell Cycle 5:2198–2202
Sotiriou C, Wirapati P, Loi S et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98:262–272
Curtis C, Shah SP, Chin SF et al (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486(7403):346–352
Lu YJ, Osin P, Lakhani SR et al (1998) Comparative genomic hybridization analysis of lobular carcinoma in situ and atypical lobular hyperplasia and potential roles for gains and losses of genetic material in breast neoplasia. Cancer Res 58:4721–4727
Mastracci TL, Shadeo A, Colby SM et al (2006) Genomic alterations in lobular neoplasia: a microarray comparative genomic hybridization signature for early neoplastic proliferationin the breast. Genes Chromosomes Cancer 45:1007–1017
Morandi L, Marucci G, Foschini MP et al (2006) Genetic similarities and differences between lobular in situ neoplasia (LN) and invasive lobular carcinoma of the breast. Virchows Arch 449:14–23
Middleton LP, Palacios DM, Bryant BR et al (2000) Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am J Surg Pathol 24:1650–1656
Simpson PT, Reis-Filho JS, Lambros MB et al (2008) Molecular profiling pleomorphic lobular carcinomas of the breast: evidence for a common molecular genetic pathway with classic lobular carcinomas. J Pathol 215:231–244
Boldt V, Stacher E, Halbwedl I et al (2010) Positioning of necrotic lobular intraepithelial neoplasias (LIN, grade 3) within the sequence of breast carcinoma progression. Genes Chromosomes Cancer 49:463–470
Weaver VM, Howlett AR, Langton-Webster B et al (1995) The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol 6:175–184
Miller FR, Santner SJ, Tait L et al (2000) MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 92:1185–1186
Stampfer MR, Yaswen P (2000) Culture models of human mammary epithelial cell transformation. J Mammary Gland Biol Neoplasia 5:365–378
Briand P, Lykkesfeldt AE (2001) An in vitro model of human breast carcinogenesis: epigenetic aspects. Breast Cancer Res Treat 65:179–187
Medina D (2000) The preneoplastic phenotype in murine mammary tumorigenesis. J Mammary Gland Biol Neoplasia 5:393–407
Band V, Zajchowski D, Swisshelm K et al (1990) Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res 50:7351–7357
Souter LH, Andrews JD, Zhang G et al (2010) Human 21T breast epithelial cell lines mimic breast cancer progression in vivo and in vitro and show stage-specific gene expression patterns. Lab Invest 90:1247–1258
Shaw KR, Wrobel CN, Brugge JS (2004) Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J Mammary Gland Biol Neoplasia 9:297–310
Fournier MV, Martin KJ (2006) Transcriptome profiling in clinical breast cancer: from 3D culture models to prognostic signatures. J Cell Physiol 209:625–630
Kenny PA, Lee GY, Myers CA et al (2007) The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 1:84–96
Lee GY, Kenny PA, Lee EH et al (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4:359–365
Hebner C, Weaver VM, Debnath J (2008) Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol 3:313–339
Martin KJ, Patrick DR, Bissell MJ et al (2008) Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS ONE 3:e2994
Fauquette W, Dong-Le Bourhis X, Delannoy-Courdent A et al (1997) Characterization of morphogenetic and invasive abilities of human mammary epithelial cells: correlation with variations of urokinase-type plasminogen activator activity and type-1 plasminogen activator inhibitor level. Biol Cell 89:453–465
Weaver VM, Petersen OW, Wang F et al (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137:231–245
Wang F, Weaver VM, Petersen OW et al (1998) Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA 95:14821–14826
Debnath J, Mills KR, Collins NL et al (2002) The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111:29–40
Rizki A, Weaver VM, Lee SY et al (2008) A human breast cell model of preinvasive to invasive transition. Cancer Res 68:1378–1387
Hu M, Yao J, Carroll DK et al (2008) Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13:394–406
Howe LR, Chang SH, Tolle KC et al (2005) HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res 65:10113–10119
Wu M, Jung L, Cooper AB et al (2009) Dissecting genetic requirements of human breast tumorigenesis in a tissue transgenic model of human breast cancer in mice. Proc Natl Acad Sci U S A 106:7022–7027
Cao D, Polyak K, Halushka MK et al (2008) Serial analysis of gene expression of lobular carcinoma in situ identifies down regulation of claudin 4 and overexpression of matrix metalloproteinase 9. Breast Cancer Res 10:91
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Livasy CA, Karaca G, Nanda R et al (2006) Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol 19:264–271
Gauthier ML, Berman HK, Miller C et al (2007) Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell 12:479–491
Kerlikowske K, Molinaro AM, Gauthier ML et al (2010) Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 102:627–637
Radisky DC, Santisteban M, Berman HK et al (2011) p16(INK4a) expression and breast cancer risk in women with atypical hyperplasia. Cancer Prev Res 4:1953–1960
Allinen M, Beroukhim R, Cai L et al (2004) Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6:17–32
Hu M, Yao J, Cai L et al (2005) Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet 37:899–905
Orimo A, Gupta PB, Sgroi DC et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Sung KE, Yang N, Pehlke C et al (2011) Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol (Camb) 3:439–450
Kojima Y, Acar A, Eaton EN et al (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 107:20009–20014
Malmberg KJ (2004) Effective immunotherapy against cancer: a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother 53:879–892
Polyak K, Hu M (2005) Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia 10:231–247
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
MacMillan, C.D., Chambers, A.F., Tuck, A.B. (2013). Progression of Early Breast Cancer to an Invasive Phenotype. In: Ahmad, A. (eds) Breast Cancer Metastasis and Drug Resistance. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5647-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5647-6_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5646-9
Online ISBN: 978-1-4614-5647-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)