Abstract
A critical function of the thymus is to help enforce tolerance to self. The importance of central tolerance in preventing autoimmunity has been enlightened by a deeper understanding of the interactions of developing T cells with a diverse population of thymic antigen presenting cell populations. Furthermore, there has been rapid progress in our understanding of how autoreactive T cell specificities are diverted into the T regulatory lineage. Here we review and highlight the recent progress in how tolerance is imposed on the developing thymocyte repertoire.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Central Tolerance Induction
Immune tolerance is an essential process in the immune system to prevent untoward responses to self. The thymus not only provides the proper selecting environment for the positive selection of T cells, but also plays a critical role in promoting tolerance of the developing T cell repertoire to self. Central tolerance plays an integral role in immune tolerance along with a net of other peripheral tolerance mechanisms that together maintain an immune repertoire that is fit for response to a diverse array of potential foreign antigens but is unable to respond to self-antigens.
It has now become widely appreciated that during thymic selection, conventional αβ chain-expressing T cells with significant autoreactivity are tolerized mainly by deletion or induction into the Foxp3+ T regulatory lineage. The rules and mechanisms that lead to one fate over another are still being elucidated, however, there has been recent rapid progress in this area. Furthermore, the selecting environment present in the thymic medulla has also been an area of recent intensive investigation. Here in this review, we highlight some of the recent progress in our understanding of how central tolerance is imposed on the conventional αβ T cell repertoire.
1.1 Central Tolerance Through Deletion
Developing thymocytes are exposed to a wide array of self-antigens within the thymus and those T cells that can bind to self-antigen peptide/MHC complexes with high affinity are removed from the immune repertoire through deletional mechanisms. Thus, there are a number of important factors involved for this process to play out. First, is the timing and expression of a functional TCR during development, second is the timing and expression of self-antigen peptide/MHC ligands by APCs present in the thymus, and finally are the factors and pathways that allow for a deletional/apoptotic death to occur in autoreactive thymocytes.
As outlined by Allen and colleagues elsewhere in this book, developing thymocytes go through a coordinated series of developmental steps that involve the generation of a unique αβ chain TCR complex. Pre-T cells are recruited into the thymus and enter the thymic cortex and rearrangement of the TCR β chain occurs through RAG-mediated recombination (Schatz et al. 1989; Oettinger et al. 1990). If a functional β chain is formed, it complexes with a pre-T α chain, migrates to the cell surface, and this helps instruct the rearrangement of the TCR α chain (Fehling et al. 1995). If such an α chain can complex with the rearranged TCR β chain, a potentially functional TCR is then expressed at the cell surface. At this stage of development such T cells co-express both CD4 and CD8 and are termed Double Positives (DPs). Such DPs then proceed through an important step of selection termed positive selection where individual clones are exposed to self-peptide/MHC complexes present in the cortex. Individual T cell clones that have relatively low affinity for these complexes are allowed to survive, whereas those clones with no affinity for self-peptide/MHC die by neglect at this selection step. T cells surviving beyond this stage develop into CD4 or CD8 Single Positive cells (SPs) that then migrate into the thymic medulla, where they are further selected and those clones with high affinity for self-peptide/MHC complexes present on APCs present in this compartment are deleted or diverted into the Treg lineage (discussed later).
Although it had long been postulated that deletion of autoreactive clones was a major mechanism of central tolerance (Burnet 1958), clonal deletion was only first clearly demonstrated in vivo by examining superantigen reactive T cells (Kappler et al. 1987). Part of the reason for this was the lack of sophisticated tools to detect deletion of autoreactive thymocytes in the polyclonal repertoire. The development of T cell receptor transgenic mice in the late 1980s led to the development of experimental tools that allowed a more refined assessment of thymic deletion for single individual TCR clones. For example, TCR transgenic mice with specificity for the male HY self-antigen again confirmed the existence of a thymic deletional mechanism when thymocytes were exposed to their cognate self-antigen (Kisielow et al. 1988). Furthermore, injection of antigens that individual TCR transgenic were specific for demonstrated again the existence of thymocyte deletion when antigens were present in the thymus (Murphy et al. 1990; Fowlkes et al. 1988).
The mechanisms that lead to thymocyte death after antigen encounter are generally thought to involve controlled apoptotic mechanisms (Sohn et al. 2007). The orphan nuclear hormone receptor, Nur77 appears to be a key regulator of such apoptotic death. Nur77 is upregulated in thymocytes when they are exposed to high affinity ligands (Calnan et al. 1995; Cho et al. 2003) and although not completely worked out, it appears to exert an effect on pro-apoptotic mitochondrial proteins (Thompson and Winoto 2008; Fassett et al. 2012). In addition, to Nur77, the pro-apoptotic BH3-only protein Bim has been linked to thymic deletion (Bouillet et al. 2002) in that Bim-deficient T cells have shown to be resistant to thymic deletion in a number of models. Recently, a more thorough screen of other BH3-only proteins for their role in thymic deletion was explored and the BH3-only protein PUMA was also found to contribute to this process (Gray et al. 2012). Interestingly, Bim/PUMA double deficient mice demonstrate a more robust defect in thymic deletion than single deficient mice of either genotype and thus suggest that these molecules work in a complementary fashion to drive deletion.
As outlined above, thymocytes require low affinity TCR signals to be positively selected, yet high affinity TCR signals appear to drive deletion. Thus, an interesting question remains as to the differences in the TCR signaling cascade that promote positive versus negative selection. Work by Palmer and colleagues with a panel of Ovalbumin peptide mimotopes with a range of affinities for the OT-I TCR has demonstrated an exquisite line of TCR affinity for peptide/MHC that demarcates positive selection from negative selection (Daniels et al. 2006). Downstream of this affinity, there appears to be a number of differential signaling cascades that have been implicated in the distinction between positive and negative selection. For example, it has been suggested that high affinity TCR ligation leads to a conformational change in the tail of the CD3 epsilon chain that allows for the adapter molecule Lck to bind and interact with the chain (Gil et al. 2005), however, there remains conflicting data on this model (Nika et al. 2010). Differential activation of the proximal TCR signaling molecules JNK and ERK have also been implicated in positive versus negative selection signals (McNeil et al. 2005). In addition, a recent report demonstrated differential intracellular localization of Ras and MAP-kinase signaling components during positive versus negative selection signaling (Daniels et al. 2006), again reinforcing differences in TCR signaling cascades.
Thymic APC Subsets and tolerance. There are multiple Antigen Presenting Cell (APC) types that the developing thymocyte repertoire interacts with during maturation and each of these cell types likely play a role in imparting central tolerance. These major APC cell types included Cortical Thymic Epithelial Cells (cTECs), Medullary Epithelial Cells (mTECs), and Dendritic Cells (DCs) (see Fig. 1). All three of these major subsets display specialized expression of MHC Class II, antigen processing and presentation machinery, and costimulatory ligand expression (i.e., CD80 and/or CD86). Each subset appears to have sub-specialized properties with cTECs being a critical regulator of positive selection and mTECs and DCs being critical regulators of negative selection.
Antigen presenting cells involved in negative selection of αβ Tcells in the thymus. The two major types of antigen presenting cells that mediate deletion of autoreactive T cells include dendritic cells (DCs) and medullary thymic epithelial cells (mTECs). High affinity interactions between MHC-antigen complexes with T cell receptors (TCRs) on single positive T cells lead to deletion. Among the thymic DC populations, Sirpa+ DCs and plasmacytoid DCs (pDCs) have been shown to have migratory capacities and are likely to be sources of peripheral antigen. A subset of mTECs express the transcriptional regulator Aire which allows them to express an array of tissue-specific antigens (TSAs). Conventional DCs and mTECs express MHCI and MHCII molecules on their surface and can present TSAs, as cDCs have been shown to present TSAs through antigen transfer from mTECs. Lastly, cortical epithelial cells (cTECs) are critical for positive selection of thymocytes
After pre-T cells enter the thymus at the Cortico Medullary Junction (CMJ), they migrate into the cortex where they interact with cTECs. cTECs are epithelial cells derived from the endoderm of the third brachial pouch that express both MHC Class II and Class I molecules complexed with a broad array of self-peptides and interaction of T cells with these ligands is critical for positive selection (Starr et al. 2003). cTECs are also capable of mediating negative selection, however, the timing of when high levels of TCR expression are occurring during T cell development in this compartment also play into whether cTECs drive negative selection. Early work with the HY-TCR transgenic demonstrated that HY-specific T cells were likely being deleted by HY expressing cTECs, however, subsequent studies revealed that this deletion may have been an artifact of early expression of the TCR due to the transgene utilized (Baldwin et al. 2005). Nonetheless, for certain ubiquitously expressed antigens like HY or superantigen, it remains likely that cTECs can delete T cells with specificity for these antigens. Recently, a specialized property of cTECs was demonstrated showing that they express a specialized subunit of the immunoproteasome called β5t (Murata et al. 2007). The proteasome helps load MHC class I molecules with peptides and the existence of specialized subunit uniquely expressed in cTECs suggests that a unique array of MHC class I associated peptides are generated in cTECs that are not present elsewhere. In addition, the presence of β5t appears to generate an array of peptides that are less stable in the MHC class I binding groove, which may be ideal for low affinity MHC/peptide complexes for positive selection. In support of this notion, β5t-deficient mice display a defect in the positive selection of CD8+ T cells. In addition, the lack of peripheral expression of β5t may be part of a fail-safe mechanism for maintaining tolerance in that there is no way to generate the identical MHC/peptide complex in the immunological periphery which could provoke an autoimmune response.
After thymocytes receive positively selecting signals, they then migrate into the thymic medulla, in part, by upregulating the chemokine receptor CCR7 (Kwan and Killeen 2004; Ueno et al. 2004; Yin et al. 2007), where they further interact with the mTEC and DC APC populations that are present in this compartment. Interestingly, there has been recent rapid progress in demonstrating the capacity of mTECs to have the unique property to express a broad array of Tissue-Specific self-Antigens (TSAs) that is in part under the control of the Autoimmune Regulator (Aire) (Metzger and Anderson 2011). During the 1990s, Hanahan and colleagues have suggested that there were relatively rare thymic medullary cells that had the capacity to express the pancreatic islet-specific protein, insulin at detectable levels (Smith et al. 1997). Subsequent to this, Derbinski and Kyewski demonstrated that mTECs had the unique capacity to express a broad array of TSAs when compared to other thymic APC populations (Derbinski et al. 2001). Part of the molecular answer of how such TSA expression could occur in mTECs came with the discovery that the Aire protein was highly expressed in mTECs and that TSA expression was in part, dependent on Aire (Anderson et al. 2002). AIRE was originally identified as the defective gene in patients with the rare autosomal recessive Mendelian syndrome called APS1 (for Autoimmune Polyglandular Syndrome Type 1) (Aaltonen 1997). APS1 patients develop an array of organ-specific autoimmune diseases that frequently target endocrine organs. Studies on the mouse model of APS1, have demonstrated that Aire-deficient mTECs fail to express many TSAs and a direct link between this and a failure in deletion of an autoreactive T cell specific for this type of TSA has now been established (Su et al. 2008; Shum et al. 2009; DeVoss et al. 2010; Taniguchi et al. 2012). Thus, it appears that TSA expression by mTECs is an essential component of tolerance as a defect in properly driving this process leads to an organ-specific autoimmune process.
The mTEC compartment has also been shown to be phenotypically diverse and dynamic. mTECs can be broadly segregated into MHC class IIlo/CD80lo (mTEC lo) and MHC class IIhi/CD80hi (mTEC hi) subsets (Derbinski et al. 2005). Interestingly, AIRE-expression is restricted to the mTEC hi subset and mTEC hi cells demonstrate a broader array of TSA expression than mTEC lo cells (Derbinski et al. 2005). In BrdU labeling experiments, mTECs appear to have a turnover rate of two weeks and mTEC lo cells have been shown to give rise to mTEC hi cells in fetal thymic organ culture experiments (Gray et al. 2007; Rossi et al. 2007). Thus, it appears that mTECs are in a state of constant turnover and replacement. On an individual cell basis, it appears that TSA expression is stochastic in that there is heterogeneity between individual cells in which TSAs are expressed (Derbinski et al. 2008; Villasenor et al. 2008) and this may help ensure that there is a broad array of self-antigen present across the thymic medulla at all times. It also appears that thymocytes reside in the medulla for an extended period of time with recent estimates being around a four-day transit time (McCaughtry et al. 2007). Thus, thymocytes in the medulla may go through extensive scanning of mTECs and DCs to also help ensure exposure to self-antigens.
The maturation and development of mTECs has now been linked to signaling through the TNF-like receptors RANK and CD40 (Akiyama et al. 2008; Rossi et al. 2007; Hikosaka et al. 2008) and to a lesser extent, signaling through the lymphotoxin beta receptor (Venanzi et al. 2007; Mouri et al. 2011; Boehm et al. 2003). Mice with deficiencies in RANK/RANK-ligand or CD40/CD40 ligand have been shown to have a defect in the generation of mTEC hi cells in the medulla. In addition, intracellular signaling molecules downstream of these receptors like TRAF6 and Nik have also been implicated in the mTEC maturation process (Akiyama et al. 2005; Kajiura et al. 2004).
The molecular mechanisms by which Aire helps promote TSA expression are also an area of intense investigation. The PHD1 domain of Aire has been demonstrated by multiple groups to bind specifically to H3 histone tails that have the fourth lysine position unmethylated (H3K4Me0) (Org et al. 2008; Koh et al. 2010). This recognition links Aire to an epigenetic mechanism for identifying TSA genes to target. An extensive survey of proteins that are pulled down with Aire in transfected cells has demonstrated that AIRE interacts with proteins involved in DNA repair, particularly DNA-PK, and this may be another clue as to how TSA genes are targeted for induction of gene expression (Abramson et al. 2010). Finally, Aire has also been implicated in promoting transcriptional elongation of stalled RNA polymerase and this may be yet another component of how TSA expression is induced in mTECs (Oven et al. 2007; Giraud et al. 2012). Interestingly, it is important to note that Aire-deficient mTECs still display the ability of expressing some TSAs. This suggests that there may be other yet to be identified factors that allow mTECs to express TSAs.
Another important APC population resident to the thymic medulla are bone marrow-derived DCs. Thymic DCs can be broadly segregated into three distinct subsets: (1) plasmacytoid DCs (pDCs), which are CD11c Intermediate, B220+, (2) conventional DCs (cDCs), which are CD11c+, CD8−, Sirpα+, and (3) CD8+ DCs, which are CD11c+, CD8+, and Sirpα−. Interestingly, the CD8+ DC subset is thought to be derived from a pre-T lymphoid progenitor recruited into the thymus, while the other two subsets likely migrate in from the periphery through alternative pathways. All three subsets are equipped with MHC class II and co-stimulatory ligand expression although this is somewhat less in the pDC subset. There has been evidence that the cDC subset can pick up peripheral antigens and bring them to the thymus for the induction of tolerance (Li et al. 2009; Bonasio et al. 2006). How abundant this process is for the induction of central tolerance remains to be determined, however, it is important to note that if extensive migration of peripheral self-antigens to thymus via DC pick up and migration was a dominant mechanism, it would make little sense for the generation of the TSA expression system to exist in the mTEC compartment. Thymic DCs likely play a critical role in the display of blood-borne and ubiquitously expressed self-antigens in the thymic medulla given that they are professionally equipped for this process. It is also important to note that although thymic DCs preferentially home to the medulla, there are also detectable DCs in the cortex and thus, DCs are likely contributing to negative selection in both compartments.
DCs exhibit the ability to endocytose or phagocytose nearby antigens for presentation in the MHC class II pathway and also in the MHC Class I pathway via cross-presentation. Thus, one interesting prevailing model regarding the display of TSAs in the medulla is that mTECs may “hand off” such antigens for display to thymocytes. In fact, there is experimental data to support this notion, particularly for MHC class II restricted display (Gallegos and Bevan 2004; Taniguchi et al. 2012; Koble and Kyewski 2009), however, there is some conflicting data on this model (Hubert et al. 2011). Interestingly, mTECs are equipped with MHC class II expression and antigen processing and presentation machinery. This begs the question as to if and how TSAs are displayed directly on mTEC MHC class II since the preferred pathway for presentation is through exogenous acquisition of antigen. Recently, Klein and colleagues have demonstrated that at least some mTECs display evidence of autophagosomes and this may be a method for mTECs to have mTEC-derived TSAs crossover into the MHC Class II antigen presentation pathway (Nedjic et al. 2008). Taken together, a picture is beginning to emerge for central tolerance whereby specialized activities of various APC populations resident to the thymus work in concert to drive the delicate balance of positive and negative selection. The thymic medulla appears to be a dynamic compartment characterized by mTEC turnover and diverse self-antigen expression and display which helps promote the deletion of autoreactive specificities.
1.2 Tolerance Through Treg Selection
Regulatory T cells (Treg) are a subpopulation of T cells necessary for suppression of self-reactive T cells in the periphery. In addition to negative selection of autoreactive T cells, selection of Tregs occurs in the thymus. Both of these processes are critical for immune tolerance. An initial piece of evidence to suggest the presence of a suppressive T cell population that was derived from the thymus came from the observation of autoimmune pathology in neonatal thymectomized mice. Early studies by Nishizuka and Sakakura first described “ovarian dysgenesis” after thymectomy day 2 after birth but not day 4 (Nishizuka and Sakakura 1969). Although these results were initially thought to be due to a hormone deficiency, later experiments found it to be an autoimmune lesion and thus, first implicated the thymus as a critical organ for prevention of self-reactivity. The thymic emigrants necessary to prevent the pathology were later named suppressor T cells as introduction of T cells from adult mice prevented the autoimmunity caused by early thymectomy (Sakaguchi et al. 1982). This work and others suggested the presence of a T cell population present in the periphery with self-reactive specificity that was responsible for the autoimmune pathology observed in the thymectomized mice and another thymic-derived T cell subset that could suppress these cells. It is now accepted that conventional T cells also have the ability to differentiate into FoxP3-expressing Tregs in the periphery but this section of the review will focus on the differentiation of natural or thymic Tregs.
Tregs were later defined as a population of CD4+ as well as CD25+ (IL-2Rα chain) cells (Sakaguchi et al. 1995). In these studies, depletion of the CD25+ Treg subset from CD4+ cells and subsequent transfer into nude mice resulted in autoimmune pathology. Further insight into the molecular determinants of Treg differentiation came from identifying a frameshift mutation in FoxP3 as the cause of the lymphoproliferative disorder in the Scurfy mouse phenotype (Brunkow et al. 2001). In parallel, human studies in Immunodysregulation Polyendocrinopathy Enteropathy X-linked syndrome (IPEX) patients linked the Scurfy mouse phenotype with the pathologies observed in those patients and ultimately the FoxP3 mutation (Chatila et al. 2000; Wildin et al. 2001; Bennett et al. 2001). Soon after, FoxP3 was identified as the master regulator of Treg differentiation as deletion of FoxP3 in T cells caused autoimmune pathology and transfer of CD4+ CD25+ cells into FoxP3-deficient mice rescues from disease (Fontenot et al. 2005; Fontenot et al. 2003; Hori 2003; Khattri et al. 2003).
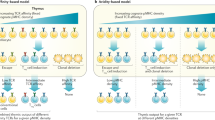
Treg TCR and self-antigen specificity . Evidence that TCR specificity is important for Treg differentiation came from the observation that not all specificities can generate Tregs. For example, Tregs were only detected in D011.10 TCR transgenic mice when there were endogenous TCR alpha chains present and not in the RAG knockout background (Itoh et al. 1999). The hypothesis that Tregs have specificity for self-antigen was supported by elegant studies where preferential selection of Tregs was observed only when both a TCR transgene and its cognate neo-self-antigen were present (Jordan et al. 2001). These and other studies using TCR transgenics and a second transgene as a source of cognate endogenous self-antigen formed the basis for the idea that the TCR avidity for self-antigen needed for Treg selection is intermediate between what drives positive and negative selection (Fig. 2a) (Knoechel et al. 2005). In support of the notion that the antigen specificity for Tregs is distinct from that of conventional T cells, TCR repertoire analysis of both thymic T cell populations showed that they do indeed have different repertoires although some overlap was observed (Hsieh et al. 2004; Pacholczyk et al. 2006; Wong et al. 2007). Retroviral expression in RAG-deficient TCR transgenic T cells of TCRa chains derived from CD25+ but not CD25− T cells led to a high frequency of expansion in lymphopenic hosts (Hsieh et al. 2004). Again, these data provide further evidence for the self-antigen specificity of thymic Tregs.
a Strength of TCR stimulation leads to differential T cell fate. Recent data suggests that strong TCR stimulation induces negative selection, whereas moderate and lower affinity interactions lead to survival and/or regulatory T cell (Treg) differentiation. b Model for Treg development. Following a high/moderate affinity MHCII/self-antigen interaction with the Treg TCR, the Treg precursor then upregulates CD25. CD25 allows responsiveness to IL-2- mediated signals which induce expression of FoxP3 and lead to the generation of a mature regulatory T cell
Discovery of the Niche . Transgenic expression of TCRs derived from naturally arising Tregs led to the unexpected result of the lack of thymic Tregs (Bautista et al. 2009; Leung et al. 2009). Further analyses demonstrated that the frequency of Tregs was inversely proportional to the clonal frequency and the most efficient Treg generation with observed with very low precursor numbers. In addition, these studies showed that the number of Tregs plateaued suggesting that there is an intraclonal competition for limiting factors in the thymus. Retrogenic mice expressing thymic Treg TCRs proliferate only under lymphopenic conditions presumably when antigen is no longer limiting (Hsieh et al. 2004, 2006). This ‘niche’ model suggests that whether or not a T cell becomes a Treg depends on what interactions it has in the thymus, which is distinct from clonal deletion.
TCR signal strength . Contrary to Brunet’s clonal selection theory, encounter with self-antigen in the thymus has two outcomes: negative selection and Treg selection. An avidity- dependent selection process for Tregs was suggested by studies where lower affinity TCR transgenic thymocytes were not selected to become Tregs, even with expression of cognate antigen expressed on a second transgene (Jordan et al. 2001). Through the use of Nur77 GFP transgenic mice where GFP levels reflect TCR signal strength, higher GFP levels were detected in thymic Tregs. In a Treg-derived TCR transgenic also containing the Nur77 reporter, higher GFP levels were only detected with lower precursor frequency (Moran et al. 2011). The importance of signal strength may explain why certain Treg TCR transgenics may result in negative selection as transgene expression levels may favor deletion (DiPaolo and Shevach 2009). Another scenario that suggests that moderate affinity is important for Treg induction is the reduction of antigen presentation in mTECs through synthetic miRNA-mediated knockdown of CIITA. In these studies, T cell fate was redirected from deletion to Treg cell selection (Hinterberger et al. 2010). Recent studies using the RIP-mOva and TCR retrogenics that recognize Ova with varying affinity suggest that the affinity needed for negative selection is about 100- fold higher than that found to promote Treg differentiation (Lee et al. 2012). In addition, those TCRs that led to Treg generation ranged in affinity within a 1000- fold range.
Cytokine plus self-antigen As mentioned earlier, Tregs express the marker CD25 on their cell surface, which also suggests continued stimulation from antigen encounter in the thymus. Aside from potentially indicating thymic Treg activation, expression of CD25 or the alpha chain of the high affinity IL-2 receptor on Tregs implicated IL-2 signaling in Treg maintenance. Evidence of the importance of IL-2 in Treg generation was shown through the observed autoimmunity in IL-2 deficient mice (Sadlack et al. 1993; Schorle et al. 1991). Similarly, the autoimmune phenotype of CD25 or CD122 (IL-2R beta chain) knockout mice reinforces the idea that IL-2 signaling is necessary for thymic Treg maintenance (Suzuki et al. 1995; Willerford et al. 1995). Although the phenotype of IL-2 deficient mice is less severe than what has been observed in STAT5-deficient animals, it is possible that other cytokines that share the common gamma chain such as IL-7 and IL-15 also contribute to Treg maintenance in the absence of IL-2 (Yao et al. 2007). From these studies and others, the following two-step model has been proposed where TCR engagement leads to the upregulation of IL-2R on thymic Tregs and consequently FoxP3 expression (Fig. 2b) (Lio and Hsieh 2008). A model in which STAT5 was constitutively expressed resulted in a larger Treg compartment (Burchill et al. 2008). IL-2 signaling may also influence Tregs through shaping the repertoire composition and size or by providing survival signals for Tregs (Burchill et al. 2008; D’Cruz and Klein 2005).
Additionally, TGFβ signaling has been implicated in thymic Treg development in that both TGFβRI and TGFβRII deficient mice had a reduction in the first wave of thymic Tregs (Liu et al. 2008; Marie et al. 2006). Although initial studies in TGFβRII knockout mice had no observed difference in thymic Tregs, it is thought that perhaps IL-2 induced proliferation of the remaining Tregs was responsible for the recovery in Treg numbers (Ouyang et al. 2010).
Cell types that induce Treg differentiation . The antigen presenting cells in the thymus also contribute to Treg selection. Various groups have shown the importance of CD28/B7.1 and B7.2 signaling for nTreg induction and all thymic APC types can contribute to this costimulatory signal. Some of the first evidence in support of mTECs being important in nTreg selection came from studies showing that a self-antigen expression by the thymic stroma induced the generation of Tregs (Jordan et al. 2001; Apostolou et al. 2002). Transgenic expression of hemagglutinin (HA) under the control of Aire regulatory elements along with an HA-specific TCR showed that expression of antigen specifically in the thymic epithelium resulted in Treg generation (Aschenbrenner et al. 2007). Furthermore, studies showing that lowering MHCII levels specifically in mTECs using transgenic expression of a microRNA specific to CIITA leads to enhanced Treg selection suggest that direct antigen presentation by mTECs leads to efficient Treg selection (Hinterberger et al. 2010). mTECs may also serve as a self-antigen reservoir for DCs that influence Treg differentiation. Both pDCs and cDCs can be found in the thymus. DCs have been shown to have a strong capacity to induce in vitro Treg differentiation with the CD8lo Sirpa+ subset being most robust (Watanabe et al. 2005; Proietto et al. 2008). Conversely, depletion of DCs using the CD11cDTR transgenic mouse led to a reduction in Treg numbers (Darrasse-Jeze et al. 2009). It has been proposed that both DCs and mTECs cooperate to eliminate autoreactive T cells and similarly, this may be the case during Treg differentiation (Gallegos and Bevan 2004; Spence and Green 2008). Further, epithelial cells may provide cues for thymic DCs like XCL-1 for DC migration or TSLP for tDC expression of costimulatory molecules (Watanabe et al. 2005; Lei et al. 2011). Aside from being involved in the differentiation of Tregs, it remains to be tested if mTECs influence Tregs in other aspects such as TCR repertoire.
2 Concluding Remarks
As the site critical for the education of T cells, the thymus is necessary to limit the escape of self-reactive conventional CD4+ T cells to the periphery. The thymus is also essential to generate regulatory T cells that suppress conventional CD4+ T cells and thus impose additional tolerance mechanisms. Remarkably, the interactions of thymic APCs and T cells likely follows a complex series of events that include positive selection in early T cell development with subsequent negative selection or positive Treg selection of T cell clones with autoreactive specificities and this has been an area that has seen rapid progress in recent years. This elegant selection process thus allows for a diverse T cell repertoire that is tolerant to self, yet poised for immune responses against diverse pathogens. Moving forward, future challenges lie on how this selection process could be manipulated to either enhance tolerance as in autoimmune disease settings or break tolerance as in cancer immunotherapy.
References
Aaltonen J (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17(4):399–403. doi:10.1038/ng1297-399
Abramson J, Giraud M, Benoist C, Mathis D (2010) Aire’s partners in the molecular control of immunological tolerance. Cell 140(1):123–135. doi:10.1016/j.cell.2009.12.030, S0092-8674(09)01616-X [pii]
Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J-I (2005) Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science 308:248–251
Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J-I (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29:423–437
Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395–1401
Apostolou I, Sarukhan A, Klein L, von Boehmer H (2002) Origin of regulatory T cells with known specificity for antigen. Nat Immunol 3:756–763
Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8:351–358
Baldwin TA, Sandau MM, Jameson SC, Hogquist KA (2005) The timing of TCR alpha expression critically influences T cell development and selection. J Exp Med 202(1):111–121. doi:10.1084/jem.20050359, jem.20050359 [pii]
Bautista JL, Lio C-WJ, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh C-S (2009) Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol 10:610–617
Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27(1):20–21. doi:10.1038/83713
Boehm T, Scheu S, Pfeffer K, Bleul CC (2003) Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med 198:757–769
Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH (2006) Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol 7:1092–1100
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415(6874):922–926. doi:10.1038/415922a, 415922a [pii]
Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27:68–73
Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio C-WJ, Vegoe AL, Hsieh C-S, Jenkins MK, Farrar MA (2008) Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28:112–121
Burnet F (1958) The clonal selection theory of acquired immunity. Vanderbilt University Press, Nashville
Calnan BJ, Szychowski S, Chan FK, Cado D, Winoto A (1995) A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity 3(3):273–282
Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM (2000) JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 106:75–81
Cho HJ, Edmondson SG, Miller AD, Sellars M, Alexander ST, Somersan S, Punt JA (2003) Cutting edge: identification of the targets of clonal deletion in an unmanipulated thymus. J Immunol 170(1):10–13
D’Cruz LM, Klein L (2005) Development and function of agonist-induced CD25+ Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6:1152–1159
Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E (2006) Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444(7120):724–729. doi:10.1038/nature05269, nature05269 [pii]
Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao Kh, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC (2009) Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med 206:1853–1862
Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B (2005) Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 202(1):33–45. doi:10.1084/jem.20050471, jem.20050471 [pii]
Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B (2008) Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci USA 105(2):657–662. doi:10.1073/pnas.0707486105, 0707486105 [pii]
Derbinski J, Schulte A, Kyewski B, Klein L (2001) Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2(11):1032–1039. doi:10.1038/ni723ni723, ni723 [pii]
DeVoss JJ, LeClair NP, Hou Y, Grewal NK, Johannes KP, Lu W, Yang T, Meagher C, Fong L, Strauss EC, Anderson MS (2010) An autoimmune response to odorant binding protein 1a is associated with dry eye in the Aire-deficient mouse. J Immunol 184:4236–4246
DiPaolo RJ, Shevach EM (2009) CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur J Immunol 39:234–240
Fassett MS, Jiang W, D’Alise AM, Mathis D, Benoist C (2012) Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci USA 109(10):3891–3896. doi:10.1073/pnas.1200090109, 1200090109 [pii]
Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H (1995) Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature 375(6534):795–798. doi:10.1038/375795a0
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol 4(4):330–336. doi:10.1038/ni904, ni904 [pii]
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY (2005) Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22:329–341
Fowlkes BJ, Schwartz RH, Pardoll DM (1988) Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature 334(6183):620–623. doi:10.1038/334620a0
Gallegos AM, Bevan MJ (2004) Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med 200:1039–1049
Gil D, Schrum AG, Alarcon B, Palmer E (2005) T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J Exp Med 201(4):517–522. doi:10.1084/jem.20042036, jem.20042036 [pii]
Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C (2012) Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci USA 109:535–540
Gray D, Abramson J, Benoist C, Mathis D (2007) Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med 204:2521–2528
Gray DHD, Kupresanin F, Berzins SP, Herold MJ, O′Reilly LA, Bouillet P, Strasser A (2012) The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity 37:451–462
Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J-I, Akiyama T, Takahama Y (2008) The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29:438–450
Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L (2010) Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol 11:512–519
Hori S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Hsieh C-S, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY (2004) Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 21:267–277
Hsieh C-S, Zheng Y, Liang Y, Fontenot JD, Rudensky AY (2006) An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol 7:401–410
Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZF, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR (2011) Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 118:2462–2472
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S (1999) Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 162:5317–5326
Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ (2001) Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2:301–306
Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M (2004) NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol 172:2067–2075
Kappler JW, Roehm N, Marrack P (1987) T cell tolerance by clonal elimination in the thymus. Cell 49(2):273–280, 0092-8674(87)90568-X [pii]
Khattri R, Cox T, Yasayko S-A, Ramsdell F (2003) An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol 4:337–342
Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H (1988) Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+ 8+ thymocytes. Nature 333(6175):742–746. doi:10.1038/333742a0
Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK (2005) Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med 202:1375–1386
Koble C, Kyewski B (2009) The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med 206:1505–1513
Koh AS, Kingston RE, Benoist C, Mathis D (2010) Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci USA 107(29):13016–13021. doi:10.1073/pnas.1004436107, 1004436107 [pii]
Kwan J, Killeen N (2004) CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol 172(7):3999–4007
Lee H-M, Bautista JL, Scott-Browne J, Mohan JF, Hsieh C-S (2012) A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 37:475–486
Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, De Waal Malefyt R, Nitta T, Takahama Y (2011) Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 208:383–394
Leung MWL, Shen S, Lafaille JJ (2009) TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med 206:2121–2130
Li J, Park J, Foss D, Goldschneider I (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med 206(3):607–622. doi:10.1084/jem.20082232, jem.20082232 [pii]
Lio C-WJ, Hsieh C-S (2008) A two-step process for thymic regulatory T cell development. Immunity 28:100–111
Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W (2008) A critical function for TGF-beta signaling in the development of natural CD4+ CD25+ Foxp3+ regulatory T cells. Nat Immunol 9:632–640
Marie JC, Liggitt D, Rudensky AY (2006) Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25(3):441–454. doi:10.1016/j.immuni.2006.07.012, S1074-7613(06)00388-8 [pii]
McCaughtry TM, Wilken MS, Hogquist KA (2007) Thymic emigration revisited. J Exp Med 204(11):2513–2520. doi:10.1084/jem.20070601, S1074-7613(06)00388-8 [pii]
McNeil LK, Starr TK, Hogquist KA (2005) A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA 102(38):13574–13579. doi:10.1073/pnas.0505110102, 0505110102 [pii]
Metzger TC, Anderson MS (2011) Control of central and peripheral tolerance by Aire. Immunol Rev 241:89–103
Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA (2011) T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208:1279–1289
Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H, Izumi K, Tamada K, Chen L, Penninger JM, Inoue J-I, Akiyama T, Matsumoto M (2011) Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol 186:5047–5057
Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K (2007) Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316(5829):1349–1353. doi:10.1126/science.1141915, 316/5829/1349 [pii]
Murphy KM, Heimberger AB, Loh DY (1990) Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science 250(4988):1720–1723
Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L (2008) Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455:396–400
Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O (2010) Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32(6):766–777. doi:10.1016/j.immuni.2010.05.011, S1074-7613(10)00203-7 [pii]
Nishizuka Y, Sakakura T (1969) Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 166(3906):753–755
Oettinger MA, Schatz DG, Gorka C, Baltimore D (1990) RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248(4962):1517–1523
Org T, Chignola F, Hetenyi C, Gaetani M, Rebane A, Liiv I, Maran U, Mollica L, Bottomley MJ, Musco G, Peterson P (2008) The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep 9(4):370–376. doi:10.1038/sj.embor.2008.11 embor200811 [pii]
Ouyang W, Beckett O, Ma Q, Li MO (2010) Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32: 642–653
Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM (2007) AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol 27(24):8815–8823. doi:10.1128/MCB.01085-07, MCB.01085-07 [pii]
Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L (2006) Origin and T cell receptor diversity of Foxp3+ CD4+ CD25+ T cells. Immunity 25:249–259
Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L (2008) Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA 105:19869–19874
Rossi SW, Kim M-Y, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJL, Anderson G (2007) RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204:1267–1272
Sadlack B, Merz H, Schorle H, Schorle H, Schimpl A, Feller AC, Horak I (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75(2):253–261, 0092-8674(93)80067-O [pii]
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151–1164
Sakaguchi S, Takahashi T, Nishizuka Y (1982) Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med 156:1577–1586
Schatz DG, Oettinger MA, Baltimore D (1989) The V(D)J recombination activating gene, RAG-1. Cell 59(6):1035–1048. 0092-8674(89)90760-5 [pii]
Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I (1991) Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352(6336):621–624. doi:10.1038/352621a0
Shum AK, DeVoss J, Tan CL, Hou Y, Johannes K, O’Gorman CS, Jones KD, Sochett EB, Fong L, Anderson MS (2009) Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med 1(9):9ra20. doi:10.1126/scitranslmed.3000284, 1/9/9ra20 [pii]
Smith KM, Olson DC, Hirose R, Hanahan D (1997) Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol 9(9):1355–1365
Sohn SJ, Thompson J, Winoto A (2007) Apoptosis during negative selection of autoreactive thymocytes. Curr Opin Immunol 19(5):510–515. doi:10.1016/j.coi.2007.06.001, S0952-7915(07)00104-5 [pii]
Spence PJ, Green EA (2008) Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci USA 105:973–978
Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21:139–176. doi:10.1146/annurev.immunol.21.120601.141107, 120601.141107 [pii]
Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, DeVoss JJ, Johannes KPA, Lu W, Gardner J, Chang A, Bubulya P, Chang HY, Peterlin BM, Anderson MS (2008) Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest 118:1712–1726
Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H (1995) Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 268(5216):1472–1476
Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS (2012) Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci USA 109:7847–7852
Thompson J, Winoto A (2008) During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med 205(5):1029–1036. doi:10.1084/jem.20080101, jem.20080101 [pii]
Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y (2004) CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med 200(4):493–505. doi:10.1084/jem.20040643 jem.20040643 [pii]
Venanzi ES, Gray DH, Benoist C, Mathis D (2007) Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J Immunol 179(9):5693–5700, 179/9/5693 [pii]
Villasenor J, Besse W, Benoist C, Mathis D (2008) Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci USA 105(41):15854–15859. doi:10.1073/pnas.0808069105, 0808069105 [pii]
Watanabe N, Wang Y-H, Lee HK, Ito T, Wang Y-H, Cao W, Liu Y-J (2005) Hassall’s corpuscles instruct dendritic cells to induce CD4+ CD25+ regulatory T cells in human thymus. Nature 436:1181–1185
Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME (2001) X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20
Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW (1995) Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3(4):521–530, 1074-7613(95)90180-9 [pii]
Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C (2007) Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol 178:7032–7041
Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ (2007) Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109:4368–4375
Yin X, Ladi E, Chan SW, Li O, Killeen N, Kappes DJ, Robey EA (2007) CCR7 expression in developing thymocytes is linked to the CD4 versus CD8 lineage decision. J Immunol 179(11):7358–7364, 179/11/7358 [pii]
Acknowledgments
This work was supported by the NIH, The Helmsley Charitable Trust, and The Burroughs Wellcome Fund. The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mouchess, M.L., Anderson, M. (2013). Central Tolerance Induction. In: Boehm, T., Takahama, Y. (eds) Thymic Development and Selection of T Lymphocytes. Current Topics in Microbiology and Immunology, vol 373. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2013_321
Download citation
DOI: https://doi.org/10.1007/82_2013_321
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40251-7
Online ISBN: 978-3-642-40252-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)